Abstract
Purpose:
There are limited prospective data on predictors of patient-reported outcomes (PROs) after whole-breast irradiation (WBI) plus a boost. We sought to characterize longitudinal PROs and cosmesis in a randomized trial comparing conventionally fractionated (CF) versus hypofractionated (HF) WBI.
Methods and Materials:
From 2011 to 2014, women aged ≥40 years with Tis-T2 N0-N1a M0 breast cancer who underwent a lumpectomy with negative margins were randomized to CF-WBI (50 Gray [Gy]/25 fractions plus boost) versus HF-WBI (42.56 Gy/16 fractions plus boost). At baseline (pre-radiation), at 6 months, and yearly thereafter through 5 years, PROs included the Breast Cancer Treatment Outcome Scale (BCTOS), Functional Assessment of Cancer Therapy−Breast (FACTB), and Body Image Scale; cosmesis was reported by the treating physician using Radiation Therapy Oncology Group cosmesis values. Multivariable mixed-effects growth curve models evaluated associations of the treatment arm and patient factors with outcomes and tested for relevant interactions with the treatment arm.
Results:
A total of 287 patients were randomized, completing a total of 14,801 PRO assessments. The median age was 60 years, 37% of patients had a bra cup size ≥D, 44% were obese, and 30% received chemotherapy. Through 5 years, there were no significant differences in PROs or cosmesis by treatment arm. A bra cup size ≥D was associated with worse BCTOS cosmesis (P < .001), BCTOS pain (P = .001), FACT-B Trial Outcome Index (P = .03), FACT-B Emotional Well-being (P = .03), and Body Image Scale (P = .003) scores. Physician-rated cosmesis was worse in patients who were overweight (P = .02) or obese (P < .001). No patient subsets experienced better PROs or cosmesis with CF-WBI.
Conclusions:
Both CF-WBI and HF-WBI confer similar longitudinal PROs and physician-rated cosmesis through 5 years of follow-up, with no relevant subsets that fared better with CF-WBI. This evidence supports broad adoption of hypofractionation with boost, including in patients receiving chemotherapy and in a population with a high prevalence of obesity. The associations of large breast size and obesity with adverse outcomes across multiple domains highlight the opportunity to engage at-risk patients in lifestyle intervention strategies, as well as to consider alternative radiation treatment regimens.
Introduction
In 2018, the American Society for Radiation Oncology published clinical guidelines endorsing hypofractionated (HF) whole-breast irradiation (WBI) as the preferred fractionation schedule for women with early stage breast cancer.1 These recommendations were made based upon evidence from multiple randomized trials demonstrating the safety and efficacy of HF-WBI compared with conventionally fractionated (CF) WBI.2–4 We have previously reported 3year outcomes of our phase 3 randomized trial of CF-WBI versus HF-WBI followed by a boost, which supported the use of HF-WBI, including in patients who received chemotherapy or were obese.5
However, in our study, the rates of favorable cosmesis were unexpectedly low regardless of the treatment arm. Only 76% of patients experienced a physician-rated good-excellent cosmetic outcome, and 2.9% of patients experienced poor cosmesis at 3 years. Similarly, only 71% of patients experienced observer-rated good-excellent cosmesis at 10 years in the Canadian HF-WBI trial2 and only 60% of patients were without moderate to marked breast changes at 5 years in the Standardization of Breast Radiotherapy (START) trials.6 Given the unexpectedly suboptimal cosmetic results in our study and others, we sought to understand whether longer follow-up on our study may afford the opportunity to identify clinically relevant predictors of adverse cosmesis and to determine whether unique subsets of patients might experience better cosmesis with 1 treatment arm or the other. Therefore, the objective of this study was to characterize longitudinal patient- and physician-reported outcomes, including cosmesis, through 5 years of follow-up in our randomized trial; identify clinically relevant predictors of outcomes; and evaluate for possible interactions between outcomes and the randomization arm.
Methods and Materials
Enrollment
Patients were enrolled between February 2011 and February 2014 at MD Anderson Cancer Center and 4 community centers (Houston, TX), Orlando Health (formerly MD Anderson Orlando, Orlando, FL), and Banner MD Anderson Cancer Center (Gilbert, AZ). Detailed inclusion and exclusion criteria have been previously reported.7 The trial included women 40 years or older with ductal carcinoma in situ or early stage invasive breast cancer (stage Tis-T2, N0N1a, M0) who underwent a lumpectomy with negative margins. Exclusion criteria included a history of prior breast cancer, bilateral breast cancer, prior overlapping radiation, pregnancy, or lack of fluency in English or Spanish. This study was approved by the institutional review board at the MD Anderson Cancer Center.
Randomization
Patients were randomized to CF-WBI (50 Gray [Gy] in 25 fractions with a tumor bed boost) or HF-WBI (42.56 Gy in 16 fractions with a tumor bed boost). Boost doses were determined by resection margins. For resection margins greater than 2 mm, the CF-WBI and HF-WBI arms received 10 Gy in 5 fractions and 10 Gy in 4 fractions, respectively. For margins less than 2 mm, the CF-WBI and HF-WBI arms received 14 Gy in 7 fraction and 12.5 Gy in 5 fractions, respectively. The randomization was stratified by baseline physician-reported cosmesis (excellent/good vs fair/poor), bra cup size (C or lower vs D or higher), chemotherapy (yes vs no), margin status (<2 mm vs ≥2 mm), and treatment facility (Houston vs Orlando Health vs Banner MD Anderson).
Radiation therapy
Radiation was started within 12 weeks of surgery or the last infusion of chemotherapy. Supine or prone positioning was permitted. Patients were treated with megavoltage tangential beams with forward or inverse-planned segmental fields with the goal of limiting doses greater than 108% of the prescription. Treatment of the low axilla without the addition of a thirdfieldwaspermitted atthe discretionofthe treatingphysician. The boost was delivered by either photons or electrons.
Patient-reported evaluations
Participants completed the following PRO evaluations at baseline, at 6 months, and yearly thereafter through 5 years.
The Breast Cancer Treatment Outcome Scale (BCTOS)8 is divided into 3 subscales that assess patient-reported cosmetic outcomes, breast pain, and functional status (eg arm and shoulder mobility, arm and shoulder pain, and ability to lift objects). Each item on the subscales is scored on a scale of 1 to 4, with 1 indicating no difference between the treated and untreated breast, 2 indicating a slight difference, 3 indicating a moderate difference, and 4 indicating a large difference. The subscale score is calculated as the arithmetic mean of the answers for each item. For BCTOS cosmesis, we also report a dichotomized score at a threshold of <2.5 or ≥2.5, as this reflects an average difference that is moderate or greater.
The Functional Assessment of Cancer Therapy−Breast (FACT-B; version 4)9,10 is divided into subscales assessing physical well-being, social well-being, emotional well-being, and functional well-being, as well as breast cancer−specific quality of life (QOL). The subscale score is calculated as the sum of the individual items, with higher scores indicating a more favorable outcome. The FACT-B Trial Outcome Index is a composite scale summing the physical well-being, functional well-being, and breast cancer−specific subscales, with a score range of 0 to 92.
The Body Image Scale11 is composed of 10 items that assess body image−related distress in cancer patients. The score is calculated by summing the items, which are rated on a scale of 0 to 3, with lower scores reflecting a better outcome.
Physician-rated evaluations
Physician-rated cosmesis was evaluated using the Radiation Therapy Oncology Group scale.12 Cosmesis was scored on a scale of 1 to 4, with 1 indicating excellent, 2 indicating good, 3 indicating fair, and 4 indicating poor outcomes.
Statistical methods
Differences in baseline patient and clinical characteristics were evaluated by treatment arm using chi-square test statistics. PROs and physician-rated cosmesis outcomes were compared between each arm at each time point using the Student t-test.
A multivariable mixed-effects growth curve model was used to model longitudinal outcomes.13 Covariates for this model were initially selected based on a univariate analysis and included baseline characteristics with a P value less than .2. These potential covariates were then assessed using a likelihood ratio test to determine the final model. Each multivariable model also included treatment arm, baseline scores, and time as covariates. Interaction terms of the treatment arm with statistically significant covariables retained in the final models were evaluated.
To assess the influence of missing follow-up data, a dropout analysis was also performed. If a patient was not assessed for an outcome at year 5, they were defined as a “dropout.” Differences in dropout rates were compared by treatment arm using chi-square test statistics. The impact of dropouts on the longitudinal multivariable analysis was assessed using a pattern-mixture model.14 For longitudinal models with a significant dropout effect, separate longitudinal analyses were compared between patients with 5-year outcomes and those who had dropped out.
All comparisons were intention-to-treat analyses with a 2-sided alpha of 0.05. Statistical analyses were made usi0ng SAS (version 9.4; SAS Institute Inc.).
Results
There were 287 patients randomized, with 149 assigned to CF-WBI and 138 to HF-WBI. All patients received their assigned doses with the exception of 1 patient, who was randomized to HF-WBI but was treated with a conventionally fractionated schedule.
The median follow-up time was 48.3 months (interquartile range, 42.3–49.6 months). A total of 173 patients (60.3%) returned for their 5-year follow-up assessment, including 89 patients (59.7%) from the CF-WBI arm and 84 patients (60.9%) from the HF-WBI arm. Baseline patient characteristics have been reported previously.7 In brief, the median age was 60 years (interquartile range, 54–66 years). Approximately 75% of patients were non-Hispanic white, 12% were Hispanic, and 11% were non-Hispanic black. The majority of patients were overweight (28%) or obese (44%), 37% had a bra cup size of D or larger, and 25% had a central axis separation ≥25 cm. There were 43 patients (28.9%) in the CF-WBI arm and 42 patients (30.4%) in the HF-WBI arm who received chemotherapy. As previously reported, patient, tumor, and treatment characteristics were well balanced between the 2 arms.7
Over the course of the trial, patients completed a total of 14,801 PRO assessments. At 5 years, there were no significant differences in PROs or physician-rated outcomes by treatment arm. Outcome comparisons at baseline and each follow-up time point are listed in Table E1 and depicted in Figure E1.
BCTOS cosmesis
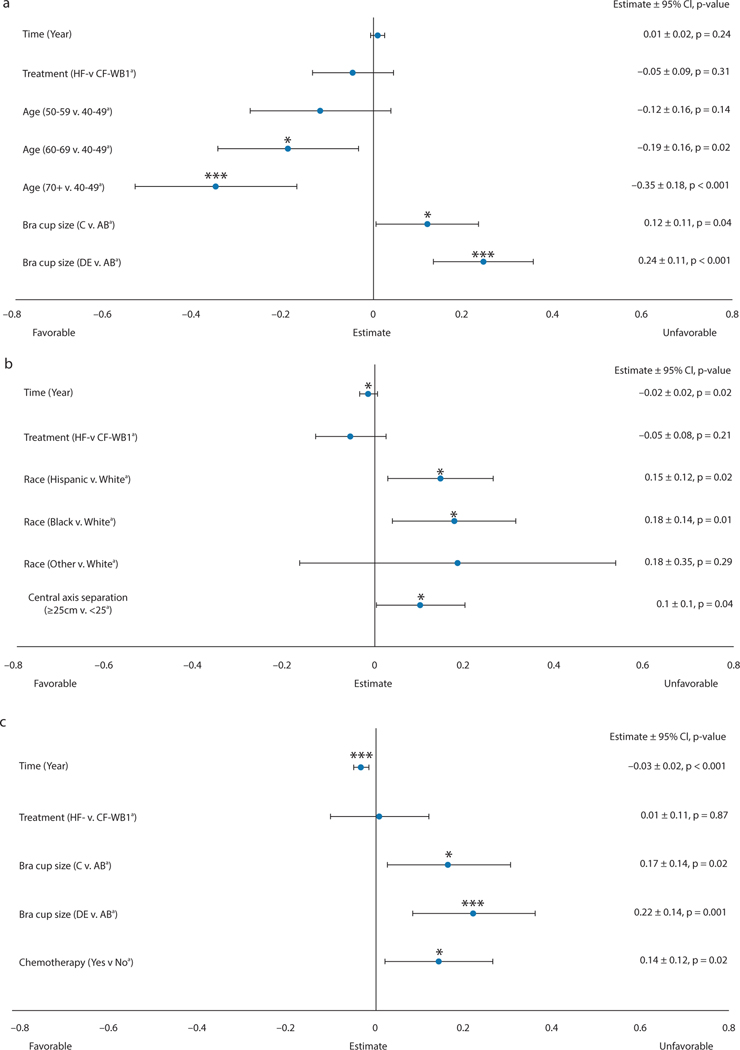
BCTOS cosmetic outcomes (Fig. 1A) did not vary significantly by treatment arm over the longitudinal 5-year follow-up (P = .31) and did not change over time (P = .24). BCTOS cosmetic outcomes were worse in patients with bra cup sizes C (effect size [E] = 0.12; P = .04) and ≥D (E = 0.24; P < .001) compared with sizes A/B. Older patients had more favorable cosmetic outcomes than younger patients, with a greater effect size with increasing age (age 60–69, E = −0.12 [P = .02]; age >70, E = −0.35 [P < .001]). Age did not have a differential effect on BCTOS cosmesis by treatment arm (Pinteraction = 0.10); however, bra cup size had a significant interaction with treatment arm (Pinteraction = 0.02). Specifically, in the subgroup of patients who had bra cup size A-C, BCTOS cosmetic outcomes were not significantly different in the 2 treatment arms (P = .28) after additional adjustments for time, baseline BCTOS cosmesis, and age. In the subgroup of patients who had bra cup size D or larger, BCTOS cosmetic outcomes were better in the HF-WBI arm than the CF-WBI arm (E = −0.18; P = .04). Age was not significantly associated with BCTOS cosmetic outcomes in this subgroup. The proportions of patients with an adverse BCTOS cosmesis (score ≥2.5) outcome at the 5-year time point were 18.9% in the CF-WBI arm and 17.9% in the HF-WBI arm (P = .86).
Fig. 1.
(a) Cosmetic, (b) Functional, (c) Pain. BCTOS longitudinal multivariable mixed-effects growth curve models. A lower score indicates a better outcome. aReferent group. *P value < .05; ***P value < .001. Abbreviations: BCTOS = Breast Cancer Treatment Outcome Scale; CF = conventionally fractionated; CI = confidence interval; HF = hypofractionated; WBI = whole-breast irradiation.
BCTOS functional
BCTOS functional outcomes (Fig. 1B) did not vary significantly by treatment arm over the longitudinal 5-year follow-up (P = .21) and improved over time (E = −0.02; P = .02). Non-Hispanic white patients had better functional outcomes than non-Hispanic black (E = −0.18; P = .01) and Hispanic (E = −0.15; P = .02) patients. Patients with a central axis separation <25 cm had better functional outcomes (E = −0.10; P = .04), regardless of their treatment arm (Pinteraction = 0.09).
BCTOS pain
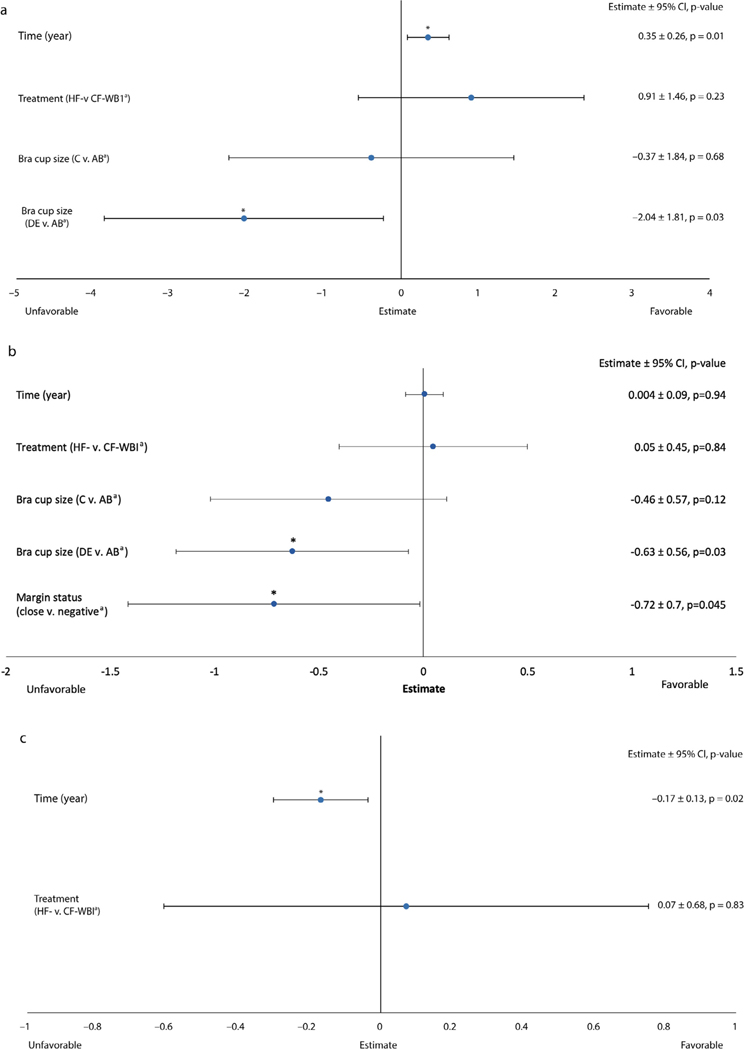
BCTOS pain outcomes (Fig. 1C) did not vary significantly by treatment arm over the longitudinal 5-year follow-up (P = .87) and improved over time (E = −0.03; P < .001). BCTOS pain outcomes were worse in patients with bra cup sizes C (E = 0.17; P = .02) and ≥D (E = 0.22; P = .001) compared with sizes A/B, regardless of treatment arm (Pinteraction = 0.93). BCTOS breast pain was worse in patients who received chemotherapy (E = 0.14; P = .02), but this did not vary by treatment arm (Pinteraction = 0.67). FACT-B Trial Outcome Index FACT-B Trial Outcome Index (Fig. 2A) scores did not vary significantly by treatment arm over the longitudinal 5-year follow-up (P = .23) and improved over time (E = 0.35; P = .01). Women with bra cup sizes D or larger had worse FACT-B trial outcome index scores (E = −2.04; P = .03), but this did not vary by treatment arm (Pinteraction = 0.69).
Fig. 2.
(a) Trial outcome index, (b) emotional well-being, (c) social well-being. FACT-B longitudinal multivariable mixed-effects growth curve models. A higher score indicates a better outcome. aReferent group. *P value < .05; ***P value < .001. Abbreviations: CF = conventionally fractionated; CI = confidence interval; FACT-B = Functional Assessment of Cancer Therapy−Breast; HF = hypofractionated; WBI = whole-breast irradiation.
FACT-B Emotional Well-Being
FACT-B Emotional Well-Being outcomes (Fig. 2B) did not vary significantly by treatment arm over the longitudinal 5-year follow-up (P = .84) and did not change significantly over time (P = .94). Women with bra cup sizes D or larger had higher rates of worse emotional outcomes (E = −0.63; P = .03), but this did not vary by treatment arm (Pinteraction = 0.72).
FACT-B Social Well-Being
FACT-B Social Well-Being outcomes (Fig. 2C) did not vary significantly by treatment arm over the longitudinal 5-year follow-up (P = .83) and worsened over time (E = −0.17; P = .02). No clinical-pathologic variables were associated with this outcome.
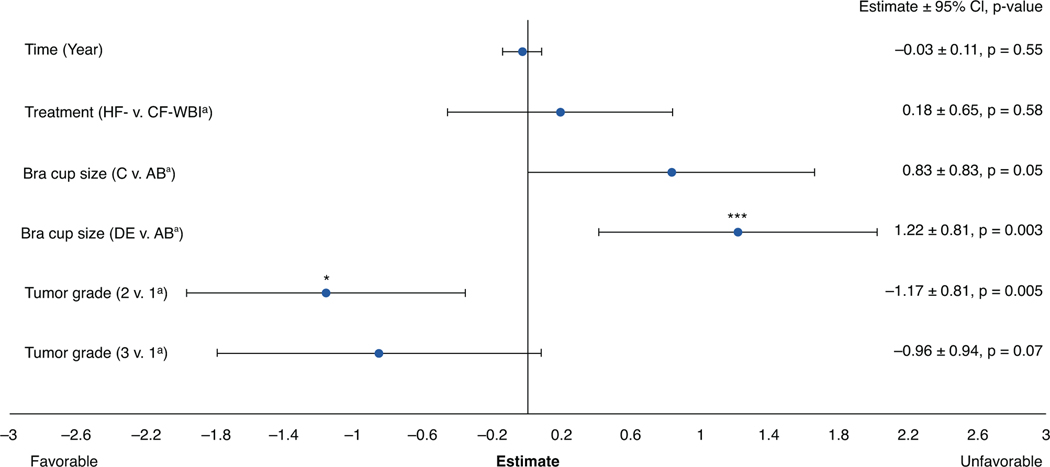
Body Image Scale
Body Image Scale outcomes (Fig. 3) did not vary significantly by treatment arm (P = .58) and did not change significantly over time (P = .55). Women with bra cup sizes D or larger had higher rates of worse Body Image Scale outcomes (E = 1.22; P = .003), but this did not vary by treatment arm (Pinteraction = 0.94).
Fig. 3.
Body Image Scale longitudinal multivariable mixed-effects growth curve model. A lower score indicates a better outcome. aReferent group. *P value < .05; ***P value < .001. Abbreviations: CF = conventionally fractionated; CI = confidence interval; HF = hypofractionated; WBI = whole-breast irradiation.
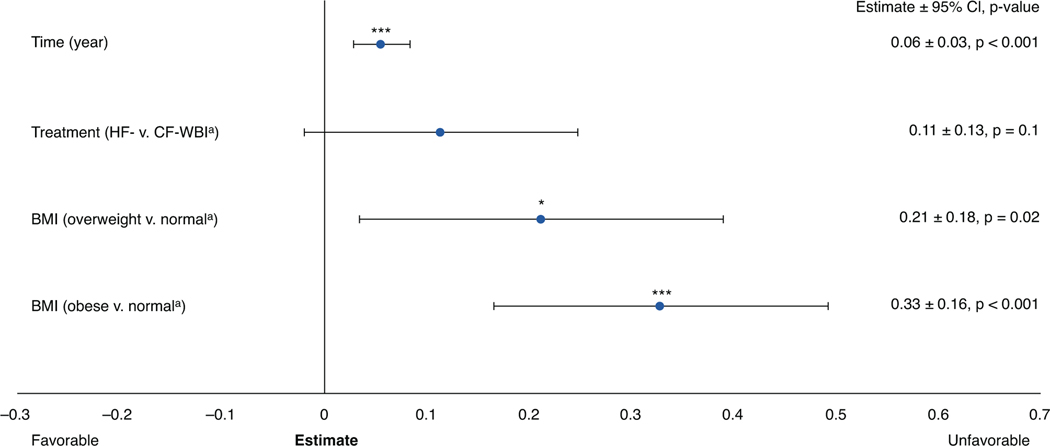
Physician-rated cosmesis
Physician-rated cosmetic outcomes (Fig. 4) did not vary significantly by treatment arm (P = .10). Physician-rated cosmesis scores worsened over time (E = 0.06; P < .001) and were worse in patients who were overweight (E = 0.21; P = .02) or obese (E = 0.33; P < .001). However, the effects of body mass index (BMI) did not vary by treatment arm (Pinteraction = 0.96).
Fig. 4.
Physician-rated cosmesis longitudinal multivariable mixed-effects growth curve model. A lower score indicates a better outcome. aReferent group. *P value < .05; ***P value < .001. Abbreviations: BMI = body mass index; CF = conventionally fractionated; CI = confidence interval; HF = hypofractionated; WBI = whole-breast irradiation.
Effect of missing data
There was no difference in the proportion of patients without 5-year follow-up data between the 2 treatment arms (P = .94). For all outcomes except BCTOS cosmetic, BCTOS pain, and Body Image Scale scores, dropouts did not have a significant impact on the outcomes (data not shown). For these outcomes, we estimated the effects of time and treatment arm averaging over the completers and dropouts, and the results were similar to the models without including dropouts as an additional covariate.
Discussion
In this randomized trial of CF-WBI versus HF-WBI followed by a boost, there was no clear effect of treatment arm on longitudinal PROs and physician-rated cosmesis over 5 years. We observed encouraging improvement over time in patient-reported functional status and breast pain outcomes. In contrast, we found that an elevated BMI and large bra cup size were risk factors for worse PROs across many domains, including cosmesis. Among patients with cup sizes of D or higher, HF-WBI was associated with better patient-reported cosmesis compared with CF-WBI. Conversely, there were no subgroups of patients experiencing worse outcomes when treated with HF-WBI.
Our finding of gradual resolution of patient-reported adverse outcomes is consistent with previously reported data of serial PRO assessments. The START A and B trials randomized early-stage breast cancer patients to HF-WBI versus CF-WBI and included a QOL substudy with 2208 patients.6 As in our study, they found that breast symptoms, including pain, swelling, oversensitivity, and skin problems, improved from 6 months to 5 years post-radiation, independent of the fractionation schedule. This was similarly demonstrated in a large, randomized trial of HF-WBI versus CF-WBI with or without a boost in patients with ductal carcinoma in situ.15 There was no difference between whole-breast dose-fractionation schedules, and QOL measures returned to baseline by 2 years with the exception of the adverse effect of the tumor bed boost on patient-reported cosmesis. Notably, ultrahypofractionation has recently emerged as an alternative WBI regimen. The FAST-Forward study randomized 4096 patients to 40 Gy in 15 fractions versus 27 Gy or 26 Gy in 5 daily fractions.16 At 5 years, the PROs were equivalent between the 40 Gy and 26 Gy arms, with approximately 32% of patients in both arms experiencing moderate or marked changes in the breast appearance. This was higher than the approximately 18% of patients reporting adverse cosmesis in both arms of our study. However, a direct comparison is difficult, as the FAST-Forward trial used a single question assessing changes in breast appearance compared with the composite BCTOS cosmesis scale used in our trial. In general, the data from the aforementioned studies support gradual improvement of PROs with time after HF-WBI and CF-WBI and indicate that these outcomes may be similar to those seen after ultrahypofractionation.
Although it is encouraging that patient-reported toxicity is transient for most women, our findings suggest that patients with a large cup size or elevated BMI experience worse outcomes across multiple domains, including patient-reported cosmesis, breast pain, functional status, emotional well-being, and body image, as well as physician-rated cosmesis. This increased risk of unfavorable outcomes was irrespective of the fractionation schedule, with the exception of patient-reported cosmesis, in which outcomes were better with HF-WBI, consistent with our previously reported primary outcome data at 3 years of follow-up.5
Large cup size and obesity present dosimetric challenges for WBI that appear to translate to adverse patient- and physician-reported outcomes. In a randomized trial of HF-WBI with intensity modulated radiation therapy (IMRT) versus standard radiation therapy with paired wedged tangents, large breast volume as a continuous variable was independently associated with unfavorable outcomes in patientreported cosmesis, breast hardness, breast pain, and skin symptoms.17 A similar randomized trial of CF-WBI with IMRT versus standard techniques found that increased BMI was correlated with decreased patient-reported physical and social well-being.18 In addition, a retrospective dosimetric review of HF-WBI in 502 women with macromastia demonstrated that the BMI and a whole-breast clinical target volume of >1500 cm3 were independent predictors of acute grade 3 dermatitis,19 which has been associated with late toxicity, including fibrosis and telangiectasia.17 Taken together, these studies and our data suggest that BMI and macromastia are important risk factors for adverse QOL outcomes.
Particularly for patients with large breasts and relatively small tumors, partial breast irradiation (PBI) is an attractive strategy to deescalate treatment and decrease toxicity by reducing the volume of breast tissue irradiated. The results of modern, randomized trials of various PBI fractionation regimens have suggested that local control is noninferior and that rates of adverse events with daily or every other day partial breast irradiation are lower than those with WBI. For example, the intensity modulated and partial organ radiotherapy (IMPORT) LOW trial randomized low risk early breast cancer patients to 40 Gy WBI, 36 Gy WBI with a simultaneous integrated boost to 40 Gy PBI, or 40 Gy PBI, all in 15 daily fractions. The average number of moderate to marked patient-reported adverse events was significantly lower with reduced-dose WBI plus PBI compared with standard WBI and was further reduced in the PBI-only arm.20,21 In addition, the randomized Florence IMRT PBI study investigated 30 Gy in 5 fractions given every other day compared with CF-WBI. This PBI regimen was found to have substantially lower rates of acute and late toxicity, with improved patient-reported cosmesis.22 The benefits of PBI may be of even larger magnitude in patients with large breasts, for whom a greater relative volume of breast tissue can be spared from radiation compared with WBI.
In addition to alternative radiation regimens, bilateral reduction mammoplasty may also be an approach to improve PROs in breast cancer patients with macromastia. Oncoplastic surgery has become an increasingly common approach for breast-conserving surgery in the last decade.23,24 Oncoplastic surgery is typically used for patients at risk of poor cosmesis due to a large tumor-tobreast-volume ratio; however, patients with a small tumor and large or ptotic breasts may also be offered reduction mammoplasty.25 Limited, retrospective data support the theory that reduction mammoplasty results in favorable PROs,26,27 suggesting this approach warrants further study.
Concerningly, approximately 50% of breast cancer survivors worldwide are overweight or obese (BMI ≥25 kg/m2).28 Given that BMI and macromastia are potentially modifiable risk factors for breast-cancer mortality29 and quality-of-life outcomes, lifestyle interventions focused on improving overall health and specifically weight may be an important component of survivorship care. The National Comprehensive Cancer Network and American Society of Clinical Oncology guidelines include general recommendations to maintain a normal BMI, but lack specific suggestions on weight loss interventions.30,31 Studies including inperson32 and remote educational strategies33,34 have demonstrated that weight loss interventions are feasible and effective among breast cancer patients. The ongoing, randomized Breast Cancer Weight Loss trial (NCT02750826) is assessing the impact of a telephone-based weight loss program among overweight or obese breast cancer survivors.35 The results of this trial may provide definitive evidence on the benefits of weight loss on both oncological and patient-reported outcomes.
Our study also revealed that patients receiving chemotherapy were more likely to have worse breast pain, independent of the fractionation schedule. Chemotherapy-related neuropathy is a common toxicity of adjuvant systemic therapy and may be a risk factor for breast cancer treatment−related pain.36 A meta-analysis of 7 studies including 4810 patients demonstrated that the odds of chronic pain were 1.44 times greater for patients who received chemotherapy.37 In addition to chemotherapy, surgery and radiation have also been implicated in treatment-related pain. A systematic review of observational studies suggests that the prevalence of chronic pain triples after breast cancer treatments, with approximately 30% of patients reporting long-term pain.38,39 As breast pain is known to be detrimental to the long-term QOL of breast cancer survivors and may have a larger impact than cosmesis,8,40,41 there remains an unmet need for further strategies to address chemotherapy and other treatment-related risk factors for chronic pain.
We also found that black and Hispanic patients had worse functional outcomes compared with white patients. Although this was an isolated finding among the PROs assessed in this study, it is concerning given that racial disparities in oncologic outcomes and PROs are numerous and well known.42–45 The etiology of the specific functional disparity observed in our trial is unclear, but there is evidence to suggest that racial disparities are due to both differences in tumor biology and patterns of care.42 Our findings are concordant with other observational data demonstrating that black women reported worse physical function than white women, even after adjustments for the stage at diagnosis and breast cancer treatment type.44 A systematic review of Latina breast cancer patients’ QOL scores revealed that Latina patients were more likely to report poor mental, physical, and social QOL outcomes.45 There remains a need for further investigation of interventions tailored to ameliorate racial and socioeconomic disparities in breast cancer outcomes.
This study has several limitations. Foremost, the definition of a clinically significant difference in scores has not been well established for the PRO instruments used in this study, and there is a possibility that the differences observed are not clinically meaningful. However, given that in this study obesity and macromastia were consistently associated with unfavorable outcomes, we conjecture that a difference in scores of this magnitude would be clinically significant. Second, although the median follow-up was 4 years, 40% of patients did not return for a 5-year assessment. We found that a large proportion of these patients were unable to follow-up owing to a loss or change of insurance coverage. In a sensitivity analysis, the dropout rate was balanced between both arms and the missing data did not significantly impact the conclusions drawn from our analyses. Third, subgroup analyses were limited in power given that comparisons were made between small sample sizes. Lastly, patients and physicians were not blinded to the treatment arm, which could bias reporting of outcomes.
Conclusion
In this randomized clinical trial, we found that fractionation schedule was not associated with differences in longitudinal PROs or physician-rated outcomes. This supports broad adoption of hypofractionation with boost, including in patients receiving chemotherapy and in a population with a high prevalence of obesity. However, obesity and large cup size were associated with unfavorable QOL outcomes across multiple domains. This suggests that lifestyle intervention strategies focused on improving breast cancer patients’ overall health and weight may be an important aspect of survivorship care. Reassuringly, we did not identify any subset of patients with inferior outcomes when treated in the HF-WBI arm, which supports continued broad adoption of HF-WBI for early stage breast cancer. Future studies are warranted to determine whether patients at higher risk of unfavorable outcomes with WBI may derive a benefit from alternative radiation regimens, such as partial breast irradiation.
Supplementary Material
Acknowledgments—
The authors thank the Radiation Oncology Access to Data Services Team at MD Anderson Cancer Center for creating a custom database to facilitate data management for this study. They also thank the Clinical Research Support Center at MD Anderson Cancer Center for their assistance in conducting the study in the 4 Houston-Area Locations.
This work was supported by a Career Development Award from the American Society of Clinical Oncology Conquer Cancer Foundation, which is funded by the Breast Cancer Research Foundation (to B.D.S.). Additional support was provided by the Cancer Prevention and Research Institute of Texas Grant RP160674 (to B.D.S.) and the National Cancer Institute Grants R01 CA207216 (to B.D.S.) and R01 1CA225646 (to B.D. S.). Support was provided, in part, by the Biostatistics Shared Resource and the Assessment, Intervention and Measurement (AIM) Shared Resource through a Cancer Center Support Grant (CA16672, PI: P. Pisters, MD Anderson Cancer Center) from the National Cancer Institute, National Institutes of Health, and through the Duncan Family Institute for Cancer Prevention and Risk Assessment. Additional support was provided by a philanthropic gift from Ann and Clarence Cazalot. The funders had no role in the trial design, statistical analyses, decision to publish, or manuscript preparation.
S.F.S. reports grants from NIH R21 CA252411, grants from Emerson Collective, and grants from NIH R01 CA201487, outside the submitted work. G.P. reports grants from Blue Health Intelligence/Blue Cross Blue Shield of Texas/Blue Cross Blue Shield of Illinois/Health Care Service Corporation, outside the submitted work. V.K.R. is an uncompensated consultant for Varian. K.K.H. is on the medical advisory board of Armada Health and Merck & Co. and reports grants from Lumicell and OncoNano, outside the submitted work. T.A.B. is a board member of a new company, Empyrean, that makes radiation oncology equipment. B.D.S. reports royalty and equity interest in Oncora Medical and grants from Varian Medical Systems, outside the submitted work.
Footnotes
This protocol is registered with ClinicalTrials.gov (NCT01266642), and may be viewed online at https://clinicaltrials.gov/ct2/show/NCT01266642
Research data are not available at this time.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijrobp.2021.05.004.
Disclosures:
All other authors report no conflicts of interest.
References
- 1.Smith BD, Bellon JR, Blitzblau R, et al. Radiation therapy for the whole breast: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol 2018;8:145–152. [DOI] [PubMed] [Google Scholar]
- 2.Whelan TJ, Pignol J-P, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med 2010;362:513–520. [DOI] [PubMed] [Google Scholar]
- 3.Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol 2013;14:1086–1094. [DOI] [PubMed] [Google Scholar]
- 4.Owen JR, Ashton A, Bliss JM, et al. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: Long-term results of a randomised trial. Lancet Oncol 2006;7:467–471. [DOI] [PubMed] [Google Scholar]
- 5.Shaitelman SF, Lei X, Thompson A, et al. Three-year outcomes with hypofractionated versus conventionally fractionated whole-breast irradiation: Results of a randomized, noninferiority clinical trial. J Clin Oncol 2018;JCO1800317. [DOI] [PMC free article] [PubMed]
- 6.Hopwood P, Haviland JS, Sumo G, et al. Comparison of patientreported breast, arm, and shoulder symptoms and body image after radiotherapy for early breast cancer: 5-year follow-up in the randomised Standardisation of Breast Radiotherapy (START) trials. Lancet Oncol 2010;11:231–240. [DOI] [PubMed] [Google Scholar]
- 7.Shaitelman SF, Schlembach PJ, Arzu I, et al. Acute and short-term toxic effects of conventionally fractionated vs hypofractionated whole-breast irradiation: A randomized clinical trial. JAMA Oncol 2015;1 931–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanton AL, Krishnan L, Collins CA. Form or function? Part 1. Subjective cosmetic and functional correlates of quality of life in women treated with breast-conserving surgical procedures and radiotherapy. Cancer 2001;91:2273–2281. [PubMed] [Google Scholar]
- 9.Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol 1997;15:974–986. [DOI] [PubMed] [Google Scholar]
- 10.Hahn EA, Segawa E, Kaiser K, Cella D, Smith BD. Health-related quality of life among women with ductal carcinoma in situ or early invasive breast cancer: Validation of the FACT-B (version 4). Expert Rev Qual Life Cancer Care 2016;1:99–109. [Google Scholar]
- 11.Hopwood P, Fletcher I, Lee A, Al Ghazal S. A body image scale for use with cancer patients. Eur J Cancer 2001;37:189–197. [DOI] [PubMed] [Google Scholar]
- 12.Harris JR, Levene MB, Svensson G, Hellman S. Analysis of cosmetic results following primary radiation therapy for stages I and II carcinoma of the breast. Int J Radiat Oncol Biol Phys 1979;5:257–261. [DOI] [PubMed] [Google Scholar]
- 13.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. Springer series in statistics. New York, NY: Springer; 2000. [Google Scholar]
- 14.Little RJA. Modeling the drop-out mechanism in repeated-measures studies. J Am Stat Assoc 1995;90:1112–1121. [Google Scholar]
- 15.King MT, Link EK, Whelan TJ, et al. Quality of life after breast-conserving therapy and adjuvant radiotherapy for non-low-risk ductal carcinoma in situ (BIG 3–07/TROG 07.01): 2-year results of a randomised, controlled, phase 3 trial. Lancet Oncol 2020;21:685–698. [DOI] [PubMed] [Google Scholar]
- 16.Murray Brunt A, Haviland JS, Wheatley DA, et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, noninferiority, randomised, phase 3 trial. Lancet 2020;395:1613–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukesh MB, Qian W, Wilkinson JS, et al. Patient reported outcome measures (PROMs) following forward planned field-in field IMRT: Results from the Cambridge Breast IMRT trial. Radiother Oncol 2014;111:270–275. [DOI] [PubMed] [Google Scholar]
- 18.Pignol J-P, Truong P, Rakovitch E, Sattler MG, Whelan TJ, Olivotto IA. Ten years results of the Canadian breast intensity modulated radiation therapy (IMRT) randomized controlled trial. Radiother Oncol 2016;121:414–419. [DOI] [PubMed] [Google Scholar]
- 19.Patel AK, Ling DC, Richman AH, et al. Hypofractionated whole-breast irradiation in large-breasted women−Is there a dosimetric predictor for acute skin toxicities? Int J Radiat Oncol Biol Phys 2019;103:71–77. [DOI] [PubMed] [Google Scholar]
- 20.Coles CE, Griffin CL, Kirby AM, et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet 2017;390:1048–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhattacharya IS, Haviland JS, Kirby AM, et al. Patient-reported outcomes over 5 years after whole- or partial-breast radiotherapy: Longitudinal analysis of the IMPORT LOW (CRUK/06/003) phase III randomized controlled trial. J Clin Oncol 2019;37:305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meattini I, Marrazzo L, Saieva C, et al. Accelerated partial-breast irradiation compared with whole-breast irradiation for early breast cancer: Long-term results of the randomized phase III APBI-IMRT-Florence trial. J Clin Oncol 2020;38:4175–4183. [DOI] [PubMed] [Google Scholar]
- 23.Kimball CC, Nichols CI, Vose JG, Peled AW. Trends in lumpectomy and oncoplastic breast-conserving surgery in the US, 2011–2016. Ann Surg Oncol 2018;25:3867–3873. [DOI] [PubMed] [Google Scholar]
- 24.Carter SA, Lyons GR, Kuerer HM, et al. Operative and oncologic outcomes in 9861 patients with operable breast cancer: Single-institution analysis of breast conservation with oncoplastic reconstruction. Ann Surg Oncol 2016;23:3190–3198. [DOI] [PubMed] [Google Scholar]
- 25.Iwuchukwu OC, Harvey JR, Dordea M, Critchley AC, Drew PJ. The role of oncoplastic therapeutic mammoplasty in breast cancer surgery −A review. Surg Oncol 2012;21:133–141. [DOI] [PubMed] [Google Scholar]
- 26.Di Micco R, O’Connell RL, Barry PA, Roche N, MacNeill FA, Rusby JE. Standard wide local excision or bilateral reduction mammoplasty in large-breasted women with small tumours: Surgical and patientreported outcomes. Eur J Surg Oncol 2017;43:636–641. [DOI] [PubMed] [Google Scholar]
- 27.Di Micco R, O’Connell RL, Barry PA, Roche N, MacNeill FA, Rusby JE. Bilateral mammoplasty for cancer: Surgical, oncological and patient-reported outcomes. Eur J Surg Oncol 2017;43:68–75. [DOI] [PubMed] [Google Scholar]
- 28.Shaikh H, Bradhurst P, Ma L, et al. Body weight management in overweight and obese breast cancer survivors. Cochrane Database Syst. Rev. 2020;12 CD012110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan DSM, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 2014;25:1901–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.NCCN Clinical Practice Guidelines. Survivorship (version 2.2020). Available at: https://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf. Accessed October 14, 2020.
- 31.Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/ American Society of Clinical Oncology breast cancer survivorship care guideline. J Clin Oncol 2016;34:611–635. [DOI] [PubMed] [Google Scholar]
- 32.Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: Interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst 2006;98:1767–1776. [DOI] [PubMed] [Google Scholar]
- 33.Santa-Maria CA, Coughlin JW, Sharma D, et al. The effects of a remote-based weight loss program on adipocytokines, metabolic markers, and telomere length in breast cancer survivors: The POWER-Remote trial. Clin Cancer Res 2020;26:3024–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janni W, Rack BK, Friedl TW, et al. Lifestyle intervention and effect on disease-free survival in early breast cancer pts: Interim analysis from the randomized SUCCESS C study. Cancer Res 2019;79(4 Suppl) Abstract nr GS5–03 GS5–03-GS5–03. [Google Scholar]
- 35.Ligibel JA, Barry WT, Alfano C, et al. Randomized phase III trial evaluating the role of weight loss in adjuvant treatment of overweight and obese women with early breast cancer (Alliance A011401): Study design. NPJ Breast Cancer 2017;3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bao T, Basal C, Seluzicki C, Li SQ, Seidman AD, Mao JJ. Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: Prevalence, risk factors, and fall risk. Breast Cancer Res Treat 2016;159:327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leysen L, Beckwee D, Nijs J, et al. Risk factors of pain in breast cancer survivors: A systematic review and meta-analysis. Support Care Cancer 2017;25:3607–3643. [DOI] [PubMed] [Google Scholar]
- 38.Wang K, Yee C, Tam S, et al. Prevalence of pain in patients with breast cancer post-treatment: A systematic review. Breast 2018;42:113–127. [DOI] [PubMed] [Google Scholar]
- 39.Bao T, Seidman A, Li Q, et al. Living with chronic pain: Perceptions of breast cancer survivors. Breast Cancer Res Treat 2018;169:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Casso D, Buist DSM, Taplin S. Quality of life of 5–10 year breast cancer survivors diagnosed between age 40 and 49. Health Qual Life Outcomes 2004;2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krishnan L, Stanton AL, Collins CA, Liston VE, Jewell WR. Form or function? Part 2. Objective cosmetic and functional correlates of quality of life in women treated with breast-conserving surgical procedures and radiotherapy. Cancer 2001;91:2282–2287. [PubMed] [Google Scholar]
- 42.Daly B, Olopade OI. A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin 2015;65:221–238. [DOI] [PubMed] [Google Scholar]
- 43.Bowen DJ, Alfano CM, McGregor BA, et al. Possible socioeconomic and ethnic disparities in quality of life in a cohort of breast cancer survivors. Breast Cancer Res Treat 2007;106:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanez B, Thompson EH, Stanton AL. Quality of life among Latina breast cancer patients: A systematic review of the literature. J Cancer Surviv 2011;5:191–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samuel CA, Mbah OM, Elkins W, et al. Calidad de Vida: A systematic review of quality of life in Latino cancer survivors in the USA. Qual Life Res 2020;29:2615–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.