Abstract
Background:
IL-25 can function as an early signal for the respiratory Type 2 response characteristic of allergic asthma and chronic rhinosinusitis with nasal polyps (CRSwNP). In the mouse gut, tuft cells are the epithelial source of IL-25. However, the source of human airway epithelial IL-25 has remained elusive.
Objective:
In this study, we sought to determine if the solitary chemosensory cell (SCC) is the predominant source of IL-25 in the sinonasal epithelium.
Method:
Flow cytometry and immunofluorescence for SCCs and IL-25 were used to interrogate polyp and turbinate tissue from patients with chronic rhinosinusitis with nasal polyps (CRSwNP). Mucus was collected during acute inflammatory exacerbations from patients with CRSwNP or CRS without nasal polyps and IL-25 levels determined by Enzyme Linked Immuno-Sorbent Assay (ELISA). Lastly, sinonasal epithelial cultures derived from polyp and turbinate tissue were stimulated with IL-13 and analyzed for SCC proliferation and IL-25 production.
Results:
This study demonstrates that a discrete cell type, likely a solitary chemosensory cell (SCC), characterized by expression of the taste-associated G-protein, gustducin, and the intestinal tuft cell marker, doublecortin-like kinase 1, DCAMKL1, is the predominant source of IL-25 in the human upper airway. Additionally, we show that patients with CRSwNP have increased numbers of SCCs in nasal polyp tissue and in-vitro IL-13 exposure both increased proliferation and induced apical secretion of IL-25 into the mucus layer.
Conclusions:
Inflammatory sinus polyps but not adjacent turbinate tissue have an expansion of a solitary chemosensory cell population that is the source of epithelial IL-25.
Clinical Implications:
Nasal polyps have an expansion of a “taster” solitary chemosensory cell (SCC) population that apically secretes the pro-Type 2 cytokine IL-25. Over abundance of upper airway SCC’s together with prolonged epithelial barrier breakdown may be a driver towards Type 2 inflammation. Quantifying IL-25 from sinus mucus may be a useful biomarker for type-2 inflammatory status.
Keywords: IL-25, IL-13, solitary chemosensory cells, chronic rhinosinusitis with nasal polyps, Type 2 inflammation, gustducin, DCAMKL1
Introduction
Chronic rhinosinusitis (CRS) affects nearly 16% of the population and is comprised of upper respiratory pathology with both inflammatory and infectious components 1,2. Chronic rhinosinusitis with nasal polyps (CRSwNP) is dominated by Type 2 inflammation3,4 with 20–60% of CRSwNP patients having concomitant asthma with eosinophil rich pathology5,6. CRSwNP is marked by increased levels of Interleukins (ILs) 4 (IL-4), IL-5, and IL-134,7. In addition, innate Type 2 cytokines, such as IL-25 and IL-33, thought to be primarily released from epithelial cells, have also recently been implicated in CRSwNP8–10 as well as in asthma11.
Epithelial-derived IL-25 rather than IL-25 derived from hematopoietic cells appears to be required for the subsequent production of IL-13 during Th-2 inflammatory responses in a mouse asthma model12. Expression of IL-25 is upregulated in CRSwNP tissue with high eosinophilia compared to non-polyp CRS or control sinus tissue8,13. IL-25 regulated IL-13 leads to increased airway hyper-reactivity and subepithelial fibrosis in asthma models and changes to the respiratory epithelium resulting in increased mucus secretion14. Additionally, IL-13 affects epithelial differentiation leading to a decrease in ciliation and an increase in goblet cell metaplasia15,16 and participates in epithelial barrier regulation in both intestinal17 and in sinonasal epithelial cells18. However, the cellular sources of IL-25 in the human upper airway remain undefined. It is unknown if a specific cell type in the upper respiratory epithelium produces IL-25, or if there is a shift in epithelial composition that would bias toward IL-25 production in inflammatory sinonasal polyps.
Tuft cells, rare gut epithelial cells, were recently demonstrated to be the epithelial source of Interleukin-25 (IL-25) in the mouse gut, and these cells promote a Th-2 inflammatory response in an IL-13-dependent fashion through group 2 innate lymphoid cells (ILC2)19–21. Tuft cells are characterized in part by expression of DCAMKL1, and also express transient receptor cation channel subfamily M member 5 (TRPM5) and the taste-associated G-protein, gustducin (GNAT3)19–22. In the upper airway, solitary chemosensory cells (SCCs) are rare epithelial cells that express, gustducin, TRPM5, and sweet and bitter taste receptors23,24. SCCs have a role in the human innate immune response in the upper airway - bitter agonists stimulate SCCs propagating a calcium wave that releases stored antimicrobial peptides from the adjacent respiratory epithelium23.
Here we demonstrate that SCCs are the predominant source of sinonasal epithelial IL-25. We also find that IL-25 is apically secreted and can be detected in the mucus of patient with CRSwNP. We further demonstrate that an extended exposure of actively differentiating epithelial cells to IL-13 maintains the epithelium in a “leaky state” allowing IL-25 to move from the apical to basolateral surface. Our results suggest that SCC proliferation with subsequent IL-25 secretion may be an early event in the Th-2 inflammatory cascade.
Methods
Reagents and experimental solutions:
Cell culture materials were obtained as previously described23. ELISA for Interleukin-25 (Abexxa, Cambridge, UK) and total protein with bichinchonic acid (BCA) assay (Thermofisher Scientific, Waltham, MA) were all performed according to the manufacturer’s instructions. Carrier-free recombinant human IL-13 was obtained from Cell Signaling Technology (Danvers, MA). For immunofluorescence, see table E1 for list of primary antibodies, secondary antibodies, source, and dilution.
Tissue acquisition:
Tissue and/or secretions were obtained from patients recruited from the Department of Otorhinoloaryngology – Head and Neck Surgery, Division of Rhinology, University of Pennsylvania and the Philadelphia Veterans Affairs Medical Center, with informed consent and full approval of both Institutional Review Boards. Patients undergoing sinonasal surgery met the selection criteria for recruitment. Diagnosis of CRS or CRSwNP was per established clinical diagnostic standards25,26. Exclusion criteria included a history of systemic diseases (e.g., granulomatosis polyangitis, sarcoidosis, cystic fibrosis, immunodeficiency). Tissue for establishing air-liquid interface (ALI) cultures, submerged cultures or fixation was obtained from the anterior ethmoid sinus, middle turbinate, or inferior turbinate.
Tissue homogenization:
Paired polyp, middle and inferior turbinate samples from 15 patients with CRSwNP were rinsed three times in DMEM:Ham’s F12 media containing 100 U/ml penicillin (Millipore Sigma), 100 μg /ml streptomycin (Millipore Sigma), and 250ng/ml amphotericin B (Millipore Sigma, St Louis, MO). Samples were placed into 1mL 1X PBS containing complete mini protease cocktail inhibitor (Roche, Indianapolis, IN) and finely minced. Samples were homogenized in a Dounce homogenizer on ice, transferred to an eppendorf tube, and centrifuged (14,000RPM, 5 minutes). The supernatant was transferred to a fresh eppendorf tube, centrifuged (14,000RPM, 5 minutes), and samples were stored at −20°C until assayed for IL-25 levels and total protein as described below.
Nasal secretion collection:
Nasal secretions were collected in a Luken’s Specimen trap (Busse, Hauppauge NY) from the ethmoid cavities of patients with clinical evidence of acute exacerbation of chronic sinusitis – all patients had previous surgery for CRS (N=12) or CRSwNP (N = 15). Samples were frozen at −20°C until processing. 2ml of 1X PBS plus complete mini-cocktail protease inhibitor (Roche) was added to each mucus sample and samples were homogenized by serial passage through an 18-gauge needle. Samples were incubated on ice for 30 minutes with intermittent vortexing, and then transferred to eppendorf tubes and centrifuged (1,000g; 5 minutes). Soluble fraction was transferred to a fresh eppendorf, centrifuged (1,000g; 5minutes) and stored at −20°C until analysis for IL-25 and total protein concentrations, respectively, as described below. Nasal secretions from those without a history of CRS with or without nasal polyps (control secretions) were obtained in the absence of any topical anesthetic or decongestant by placing a pre- expanded Pope ear wick (Medtronic ENT; Mineapolis, MN) in the nasal cavity under direct visualization between the nasal septum and inferior turbinate bilaterally. Wicks were removed after 5 minutes and placed into a 500μl tube. A hole was made in the bottom of the tube with a 20-gauge needle, and the 500μl tube was placed inside a 1.5ml tube and centrifuged (1,000 g; 5 minutes); secretions were collected in the 1.5ml tube and processed for IL-25 and total protein concentration as described below.
Sinonasal ALI culture:
Sinonasal mucosal specimens were acquired from residual clinical material obtained during sinonasal surgery as described above. ALI cultures were established from enzymatically dissociated HSECs (human sinonasal epithelial cells), as previously described 23,27,28, and grown to confluence with proliferation medium consisting of DMEM/Ham’s F-12 and bronchial epithelial basal medium [BEBM (Lonza; Basel, Switerzland)] supplemented with 100 U/ml penicillin and 100 μg /ml streptomycin for 7 days. Cells were then trypsinized and seeded on porous polyester membranes (about 6 X 104 cells per membrane) in cell culture inserts [Transwell-clear, 12-mm diameter, 0.4-μm pores; Corning (Corning, NY)] coated with 100 μl coating solution (Bovine Serum Albumin (BSA) [0.1 mg/ml; Sigma-Aldrich], type I bovine collagen [30 μg/ml; BD (Franklin Lakes, NJ)], and fibronectin [10 μg/ml; BD] in LHC basal medium (Thermofisher Scientific). After achieving confluence, the culture medium was removed from the upper compartment and the epithelium was allowed to differentiate for at least 3 weeks by using the differentiation medium consisting of 1:1 DMEM (Thermofisher Scientific) and BEBM (Lonza), with the Clonetics complements for hEGF (0.5 ng/ml), epinephrine (5 μg/ml), BPE (0.13 mg/ml), hydrocortisone (0.5 μg/ml), insulin (5 μg/ml), triiodothyronine (6.5 μg/ml), and transferrin (0.5 μg/ml), supplemented with 100 UI/ml penicillin, 100 μg/ml streptomycin, 0.1 nM retinoic acid (Millipore Sigma), and 2% Nu-Serum (Corning) in the basal compartment.
IL-13 experiments:
Fully differentiated ALI cultures derived from polyp tissue or turbinate tissue from patients with CRSwNP were left untreated (control), or treated by addition to the basal differentiation media of recombinant human IL-13 at 50ng/ml. The apical surface was washed with 100μl of 1X PBS (5minutes, 37°C) with each feeding, and cells were fed every other day for 14 days. Apical supernatant and basal media were collected at day 2, 6, 10 and 14.
Lysates of ALI cultures after 14 days of treatment were made using a lysis buffer containing 150 mM NaCl (Thermofisher Scientific), 1 mM EDTA (Thermofisher Scientific), 1% Triton X-100 (Thermofisher Scientific), and 100 mM Tris, pH 7.4 (Thermofisher Scientific), with Complete Mini Protease Inhibitor. For all experiments, there were at least three replicates per condition with tissue from a minimum of 3 patients.
Submerged Culture:
Sinonasal mucosal specimens of polyp tissue or turbinate tissue from at least 3 patients with CRSwNP were acquired as described above and enzymatically dissociated HSECs were obtained as previously described 23,27,28. Cells were filtered through 40μm filters (Corning) and plated into 24-well plates containing glass coverslips (Corning) coated with coating solution at a density of about 5 × 104 cells/well. Cultures were rinsed twice with 1X PBS on day 1 and fed daily for 14 days with proliferation media alone (untreated control) or proliferation media containing 50ng/ml IL-13. Supernatant was collected for IL-25 ELISA as described below.
IL-25 ELISA and total protein quantification:
Human IL-25 ELISA (Abexxa) was performed per manufacturer’s instruction with 100μl samples of homogenized sinonasal tissue (undiluted and diluted 1:5), nasal secretions (1:2 dilution), basal media, apical secretions, or ALI culture lysates. Total protein concentration for homogenized sinonasal tissue, nasal secretions and ALI cell culture lysates was determined using a BCA protein assay (ThermoFisher Scientific) in microwell format per manufacturer’s instructions. IL-25 levels were normalized to total protein levels for tissue and mucus specimens.
Single-cell tissue preparation
Nasal polyps (N = 10) or control mucosa (N = 8) from inferior turbinate or middle turbinate samples were cut into 5 mm pieces. Tissue pieces were incubated for 5 hours at 37 °C in Dulbecco’s Modified-Eagle Medium (Lonza) with 5μg/mL Brefeldin A (Sigma-Aldrich). Tissue was transferred and minced into 1–2 mm pieces in solution containing 0.26 Wurtsch units/mL Liberase TM (Sigma-Aldrich) with 100 μg/mL DNAse I (Sigma-Aldrich), 10 mM HEPES buffer (ThermoFisher), 5 mM KCl (Sigma-Aldrich), 1 mM MgCl2, (ThermoFisher), 1.8 mM CaCl2 (ThermoFisher). Tissue was incubated at 37 °C with intermittent vortexing for 45 minutes and passed through a 5 mL syringe to release cells into solution. EDTA (Sigma-Aldrich) at final concentration 2 mM was added to stop the digestion. Debris was removed using a 40 μm cell strainer and red blood cells were removed using a RBC lysis buffer (Miltenyi Biotech). Cells were serially washed in 2% bovine serum albumin in PBS (Sigma-Aldrich).
Cell Staining & Flow Cytometry
Single cell suspensions (each >1.0 million cells) were stained with viability dye Aqua at 1:1000 dilution (Thermofisher) for dead cell exclusion. Fc receptors were blocked with TruStain FcX TM (Biolegend). For each marker, fluorescence minus one (FMO) controls were also prepared. Cell surface markers were stained using anti-EpCAM-PECy7 (eBiosciences) to mark epithelial cellsand anti-CD45-eFlour450 (eBiosciences) to mark lymphocytes. After fixation and permeabilization, intracellular markers were stained overnight at 4 °C using anti-DCAMKL1-AF647 (AbCAM), anti-GNAT3-PE (LS Biosciences), anti-IL25-FITC (Invitrogen). Isotype control antibodies were used in FMOs for GNAT3 and IL-25 (Figure E4).
Cell suspensions were analyzed on an LSR Fortessa TM using FACSDiva Software 8 (BD Biosciences). At least 250,000 live events were acquired per sample. Data were analyzed using FlowJo 10 (Treestar). Samples were FSC-A/SSC-A gated to identify singlets and exclude debris. Positive gates were determined using <1% of events on FMO samples (Figure E4). Solitary chemosensory cells were identified as Aqua Live,EpCAM+CD45−GNAT3+DCAMKL1+IL-25+ events and represented as a frequency of Aqua Live, EpCAM+ events (gating strategy as in Figure E5). There is a high percentage of dead cells based on the Aqua Live staining. This may be related to the prolonged Brefeldin treatment that enriches for intracellular cytokines, together with the tissue homogenization process required to isolate single cells. The percent of IL-25+ cells that are SCCs were identified as live, EpCAM Mann-Whitney nonparametric t-test was used to compare polyp versus control samples with α=0.05.
Trans Epithelial Electrical Resistance (TEER):
TEER was measured using an EVOM ohm-voltmeter (World Precision Instruments; Sarasota, FL). Briefly, transwell cultures from 3 patients with nasal polyps were placed in a fresh chamber containing 1X PBS in the basal compartment and 150μl of 1XPBS in the apical compartment and TEER was measured using the chopstix adapter for the EVOM. Resistance was measured on at least 6 cultures for each experimental condition tested and converted to Ohms-cm2 by multiplying by the surface area of the transwell insert.
Paracellular Transport of FITC-Dextran:
Trans-epithelial transport of 20kDa FITC-dextran (Millipore Sigma) across sinus epithelial cultures grown in transwells was used as an additional estimate for transport of IL-25 (~18kDa protein). Briefly, 100μl of 20μg/ml of 20kDa FITC-dextran was placed on the apical surface of cultures and 500μl of Hank’s balanced salt solution (HBSS) (Thermo Fisher Scientific) with glucose in the basal chamber, and then cultures were incubated for 2-hours at 37°C, 5%CO2. Basal fluid was collected and assayed in duplicate for FITC-dextran using a fluorescent multi-well plate reader [Synergy H4 hybrid microplate reader, (Biotek, Winooski, VT)] Ex/Em (490/510). 6 biologic replicates per sample were run for each condition. The concentration of FITC-dextran was determined by calibration to a standard curve of FITC-dextran. Basal migration of FITC-dextran was determined using a transwell with no cells.
Immunofluorescence:
ALI cultures:
Fully differentiated sinus epithelial cell cultures on transwells were washed three times with 1X PBS and then fixed for 20 minutes at room temperature with 4% paraformaldehyde. For samples analyzed for Il-25, cultures were incubated with Brefeldin A at 5μg/ml (Millipore Sigma) for 4 hours prior to fixation to amplify cytokine staining. Transwells were rinsed three times in 1X PBS. Transwell membranes were cut out and then permeabilized for 1 hour at 4°C in block buffer [1X PBS, 5% Donkey Serum (Thermo Fisher Scientific), 1% Bovine Serum Albumin, 0.2% Saponin (Millipore Sigma)] containing 0.3% Triton X-100. Membranes were rinsed three times in 1X PBS and then incubated at 4°C overnight in block buffer plus 0.05% Tween20 and primary antibody(ies) (See Table E1 for antibody combinations). Membranes were rinsed three times in PBST (1X PBS + 0.05% Tween20), twice in 1X PBS, and then incubated for two hours in block buffer plus appropriate secondary antibody (See Table E1). Membranes were rinsed three times in PBST (1X PBS + 0.05% Tween20) and twice in 1X PBS and mounted on glass slides with Fluoroshield Mounting Media with DAPI (Abcam). Tissue samples were also individually stained with each primary antibody and with all three secondary antibodies and images acquired as described below demonstrating no false positive staining (Figure E2).
Sinonasal tissue sections:
Polyp, middle and inferior turbinate specimens were rinsed three times in DMEM:Ham’s F12 media containing 100 U/ml penicillin, 100 μg /ml streptomycin, and 250ng/ml amphotericin B. Large bone fragments were removed and samples were fixed in 4% paraformaldehyde at 4°C. Samples were embedded in paraffin wax. 5–7μm sections were obtained and tissue slices were mounted on positively charged slides (Thermo Fisher Scientific).
Immunofluorescence with tissue sections was performed after deparaffinization with xylene and graded ethanol washes. Samples were blocked for 1-hour at room temperature in 10% donkey serum, 2% BSA with 0.1% Triton X-100, and then incubated in 10% donkey serum, 2% BSA with primary antibody (Table E1) at 4°C overnight. Specimens were rinsed three times with 1X PBST, two times with 1X PBS, and incubated in 10% donkey serum, 2% BSA with secondary antibody at room temperature for 1 hour. Samples were rinsed three times with 1X PBST, two times with 1X PBS. Prior to mounting, the tissue sections were incubated in 1X trueblack autofluorescence quencher (Biotium; Fremont, CA) for 3 minutes at room temperature. Tissue sections were rinsed three times in 1XPBS, everbrite mounting media with DAPI (Biotium) and coverslips were applied.
All imaging was performed on an Olympus Fluoview confocal system attached to an Olympus IX81 confocal microscope as previously described 23. Images were acquired with a 60X oil objective using Type FF immersion oil (Cargill; Wayzata, MN) at 0.2μm intervals and image reconstruction was accomplished with FIJI. Tissue images were processed with a median filter, pixel size 2. All comparative polyp and turbinate images were taken with the same microscope settings.
Data Analysis:
Data were analyzed using Microsoft Excel and Prism (Graphpad) using non-parametric t-tests for IL-25 levels, TEER and FITC-dextran levels from ALI-based experiments. Median values for IL-25/total protein from nasal secretions and total protein were calculated using Prism software. Statistical significance was determined using Kruskall-Wallis test for nasal secretions, and using one-way ANOVA and Dunnett’s multiple comparisons test for paired tissue.
Results
We hypothesized that SCCs may function similarly to tuft cells as the source of airway epithelial IL-25. We first examined cultured sinus epithelial cells by immunofluorescence and found that gustducin (GNAT3)-positive upper respiratory epithelial cells co-express DCAMKL1 and the bitter taste receptor T2R8 (Figure 1A, Figure E1A). GNAT3-positive cells in unstimulated cultures are neither ciliated cells nor goblet cells, consistent with these cells as a possible undifferentiated cell, progenitor cell or solitary chemosensory cell (Figure E1B, C). We then examined polyp tissue via immunofluorescence and found that gustducin-positive cells co-localize with DCAMKL-1 in polyp epithelium (Figure 1C). In unstimulated primary human sinonasal epithelial cultures (Figure 1B) and in polyp tissue (Figure 1D), IL-25 also co-localizes with DCAMKL1-positive cells or gustducin-positive cells. These data suggest that gustducin-positive cells, likely SCCs, are indeed the source of IL-25 in the upper respiratory epithelium.
Fig. 1. Gustducin-positive cells express DCAMKL-1 and IL-25 in differentiated human sinus epithelial cultures and tissue.

(A) Hi-resolution image depicting overlap in fluorescence between DCAMKL1 (green) and gustducin (red). (B) Co-labeling of gustducin-positive cells with IL-25. (C) Overlap between gustducin-positive (red) and cytoplasm-positive DCAMKL-1 cells (green) in human sinus polyp epithelium. Note scattered DCAMKL-1 positive nuclei in discrete cell population (white arrow). White bracket denotes edges of epithelium. (D) Co-labeling of gustducin/IL-25 positive cells in human sinus polyp epithelium. Nuclei labeled with DAPI depicted in blue, and DIC overlay for tissue on merged images. Scale bar 10 μm.
We examined IL-25 levels by ELISA from homogenized tissue of patients with CRSwNP compared to adjacent middle and inferior turbinate tissue from the same patients. We found a significant (P = 0.021) increase in IL-25 in polyp tissue (191 pg/mg) compared to inferior turbinate tissue (82 pg/mg) (Figure 2A). We utilized flow cytometry and immunofluorescence to examine the GNAT3/DCAMKL-1/IL-25 positive epithelial cell population (Figure 2B, Figure E5) to determine if solitary chemosensory cells are enriched in polyp tissue, and to determine if these SCCs are the source of IL-25 in sinus tissue (Figure 2C–E). We found that there are significantly (P = 0.0006) more SCCs (39%) in polyp tissue compared to turbinate tissue (8%) (Figure 2B).
Fig. 2. SCCs and IL-25 are increased in polyp tissue compared to turbinate tissue.

(A) IL-25/total protein (pg/mg) for matching polyp and turbinate samples from 15 CRSwNP patients demonstrates a significant increase (P = 0.0395) in IL-25 in polyp tissue (122 pg/mg) compared to inferior turbinate (60 pg/mg). (B) Significant increase in the percent of SCCs (GNAT+DCAMKL1+IL-25+/EpCAM+) in sinus polyps [39% (95% confidence interval 33–53%), 10 patients] versus control sinus tissue [8%, 995% confidence interval 0.6–24%) 8 patients] as determined by flow cytometry. (C) Percent of IL-25-positive epithelial cells that are DCAMKL1+/GNAT+ in polyp tissue (89%, 10 patients) versus control tissue (81%, 8 patients) as determined by flow cytometry. Shown are the individual data points (filled circles), the median (bar graph) +/− 95% confidence interval. (D,E) Representative gating strategy to determine percent of IL-25-positive epithelial cells that are DCAMKL1+/GNAT+ in polyp (D) and control (E) tissue.
Polyp tissue has significantly more IL-25+ epithelial cells (44%) compared to control tissue (0.1%) (Figure 2D,E). In both control tissue and polyp tissue, 81% and 89%, respectively, of the IL-25+ epithelial cell population are DCAMKL1+/GNAT+ cells (Figure 2C). These results demonstrate that IL-25+ epithelial cells are likely SCCs and that SCCs are significantly enriched in polyp tissue compared to control tissue.
Immunofluoresence was consistent with the flow cytometry data, and demonstrated a marked increase in gustducin-positive and DCAMKL1-positive cells in polyp tissue (Figure 3A) compared to turbinate tissue that was devoid of gustducin, DCAMKL1, or IL-25 positive epithelial cells (Figure 3B). The increase in gustducin-positive cells in polyps was not uniform and occurred in clusters (Figure E2).
Fig. 3. IL-25-positive SCCs are enriched in polyp tissue.
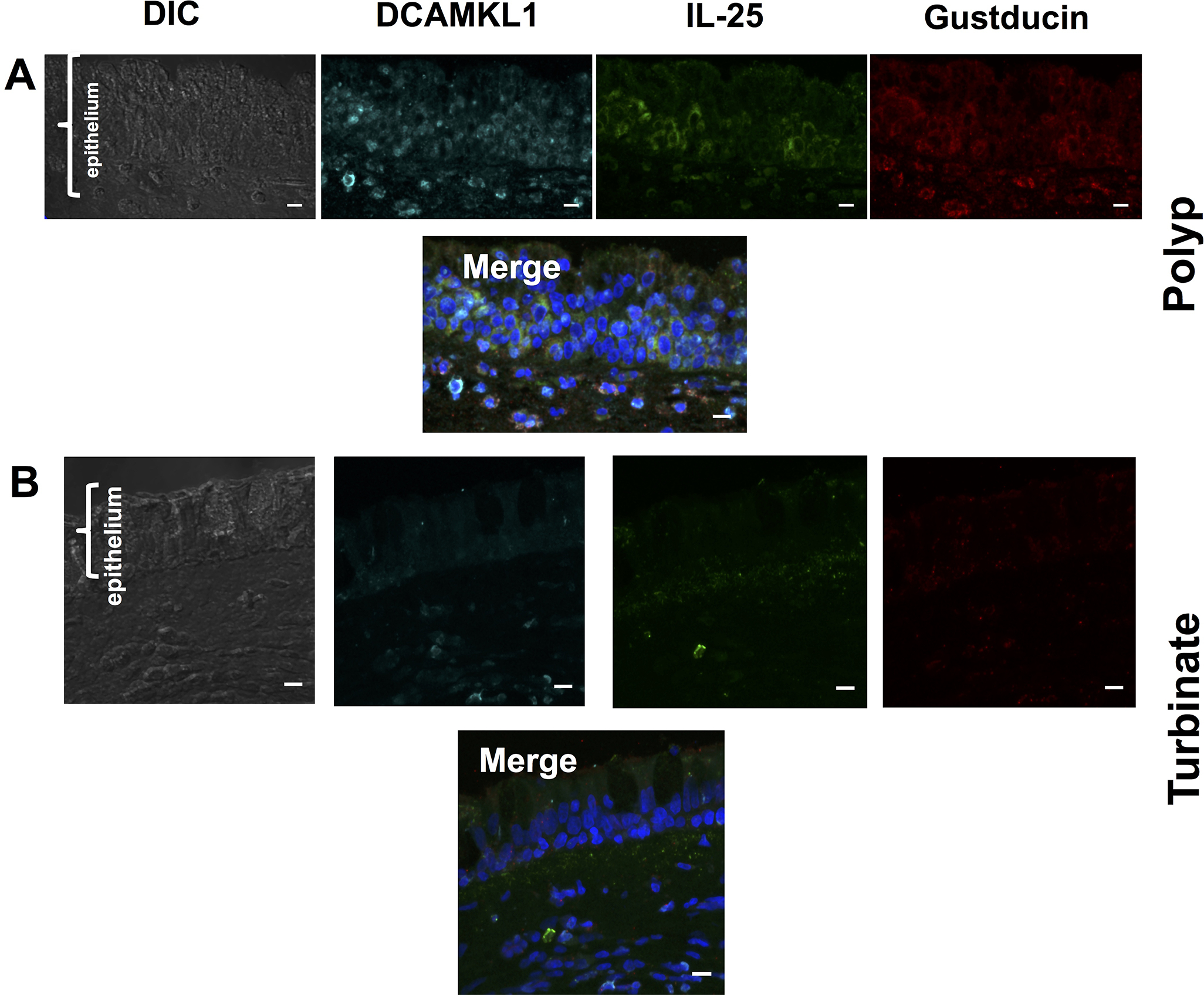
Immunofluorescence of pathology-confirmed, (A) eosinophil-rich polyp, and (B) turbinate tissue from a patient with inflammatory sinus polyps with DCAMKL1 (cyan), IL-25 (green), and Gustducin (red). Turbinate tissue is devoid of DCAMKL1, IL-25 and gustducin-positive cells. DAPI-stained nuclei depicted in blue in merged images. Scale bar 10μm.
We utilized primary human epithelial tissue culture models to examine the effect of IL-13 treatment on IL-25 secretion derived from sinonasal polyp or turbinate tissue simulating the feed-forward IL-25 – IL-13 loop demonstrated in the mouse gut19–21. Exogenous application of IL-13 causes changes in the epithelium, most notably increases in mucus production from goblet cells14. In animal models, changes occur within a few days, however, in isolated epithelial tissue culture models, goblet cell metaplasia, increased mucus production and decreased ciliation occur after 7 to 10 days of treatment with IL-1316. We examined human sinus epithelial cultures grown under submersed conditions to determine if epithelial polarization and ciliation are required for IL-25 secretion. We found that IL-25 levels increased in epithelial cultures derived from polyps after 10 days of IL-13 treatment (38pg/ml) from basal levels < 20pg/ml (Figure 4A). After 14 days of IL-13 treatment, we observed a significant (P < 0.0001) increase in IL-25 in cultures derived from polyps (110 pg/ml), whereas there were nominal levels of IL-25 in cultures derived from middle (8.2 pg/ml) or inferior turbinate (9 pg/ml) samples (Figure 4B,C). Of note, approximately 50% of the polyp cultures were able to secrete large amounts of IL-25 when stimulated with IL-13, which may be due to random sampling of the polyps for tissue culture in regions that may have a lower abundance of gustducin-positive cells as noted above (Figure E2).
Fig. 4. IL-25 secretion increases only in IL-13 treated Polyp Epithelial Cultures.
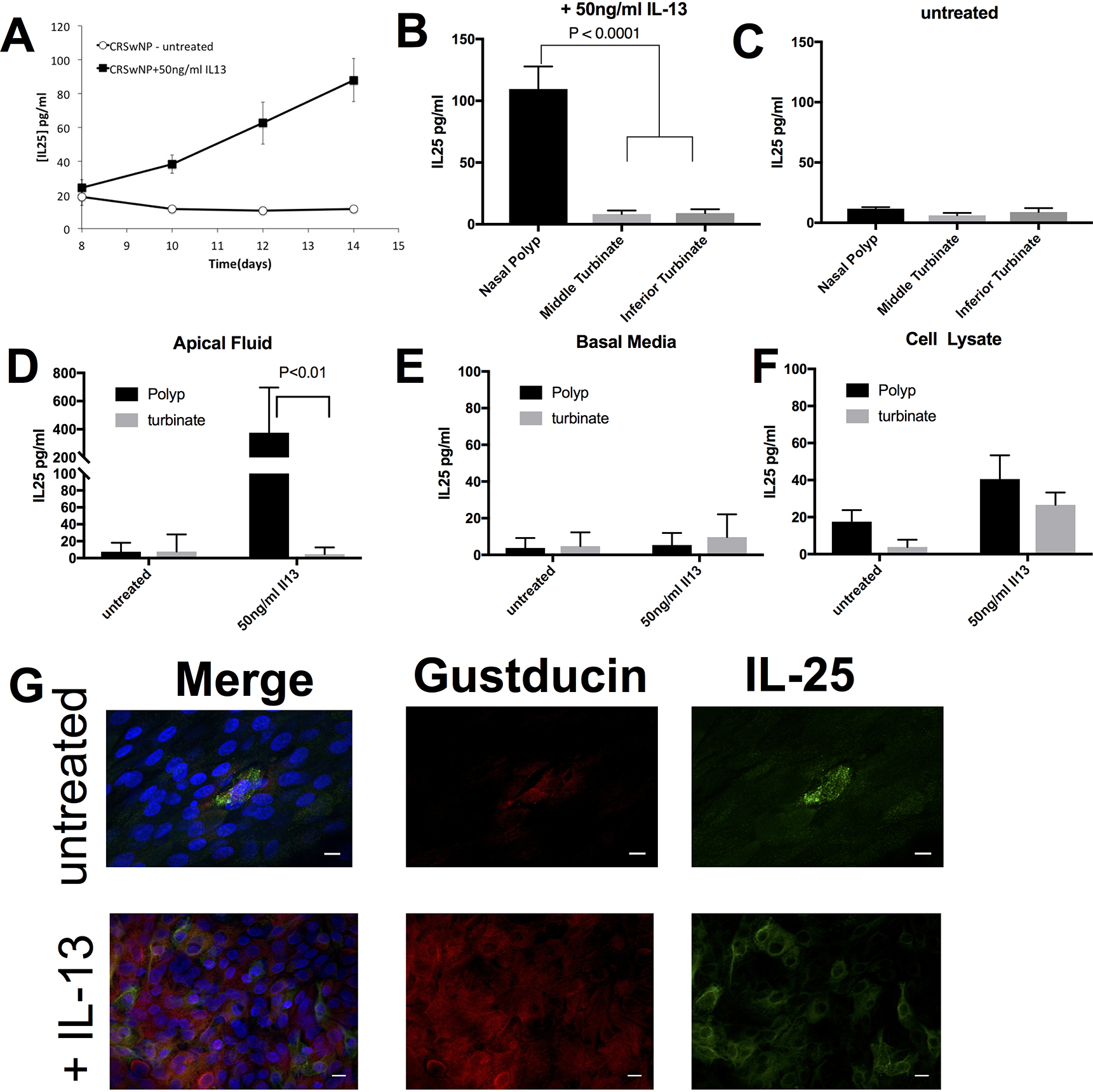
(A-C) IL-25 secretion in growth media +/− 50ng/ml IL-13. (A) IL-25 (pg/ml) secretion increases over time (days) for IL-13 treated CRSwNP epithelial cultures (filled black square) compared to untreated CRSwNP epithelial cultures (open squares) grown in submersion. IL-25 (pg/ml) after 14 days of treatment with (B) or without (C) 50ng/ml IL-13 in epithelial cultures derived from polyp tissue, middle turbinate or inferior turbinate grown in submersion. IL-25 (pg/ml) in (D) apical fluid, (E) basal media and (F) cell lysate of differentiated epithelial cells grown at an air-liquid interface derived from polyp or turbinate tissue that were untreated or treated with 50ng/ml IL-13 for 14 days. (G) Representative imaging of differentiated polyp cultures stained for gustducin and IL-25 +/−50ng/ml IL-13 for 14 days. DAPI staining of nuclei depicted in blue on merge image. Scale bar 10 microns.
Next, to assess whether biphasic culture conditions changed the cytokine profile, human sinonasal epithelial cell cultures from polyp or turbinate tissue were grown at an air-liquid interface in the presence or absence of IL-13 for 14 days. Consistent with our submerged culture data, only IL-13-treated polyp epithelial cultures demonstrated an increase in IL-25 (Figure 4D–F). IL-13 treatment in polyp-derived epithelial cultures significantly increased the frequency of gustducin and IL-25-positive cells (Figure 4G).
Evaluating the secretion of IL-25, we found that it was secreted onto the apical surface and not into the basal media (Figure 4D, E). There was minimal IL-25 buildup in the cell lysate consistent with an active secretion process as a result of IL-13 treatment (Figure 4F). Barrier function in inflammatory polyps may be abnormal compared to normal respiratory epithelium, and there is conflicting data on whether IL-13 can reduce transepithelial electrical resistance (TEER) in cultured sinus epithelium over a 24-hr period of exposure18,29. It is possible that IL-25 will cross the epithelium if the barrier is violated (e.g. injury) or altered by a chronic inflammatory response. Precedent for transition of IL-25 across the epithelium can be found with epithelial or endothelial-generated IL-33. IL-33 is released by the barrier layer following damage, thereby allowing the epithelial cytokine to interact with the underlying adaptive immune components30,31. We found that TEER began to decline on day 10 of treatment with IL-13 (Figure 5A). Although, TEER declined in this experimental setup, we did not observe IL-25 in the basal media (Figure 4B), nor did we observe movement of a 20kDa FITC-Dextran, which is of similar size to IL-25, from the apical to basal chamber (Figure 5B).
Fig. 5. IL-25 is apically secreted in culture and elevated in nasal secretions of patients with CRSwNP.

(A) Change in TEER (ohms-cm2) over time (days) for differentiated (mature ALI) or immature ALI polyp cultures +/− 50ng/ml IL-13. *= P<0.0005, ** = P<0.0001. mean +/−s.e.m. (B) Apical to basal transport of 20kDa FITC-Dextran (μg/ml) for differentiated (mature ALI) or immature ALI polyp cultures +/− 50ng/ml IL-13 for 14 days. (C) IL-25 (pg/ml) in apical secretions and basal media of undifferentiated polyp cultures (new ALI) +/− 50ng/ml IL-13 for 14 days, (## - below limit of detection). (D) IL-25 levels (pg/mg) in nasal secretions [CRSwNP (N=15), CRSsNP (n=12), control (N=7)] – shown are the median +/− 95% confidence interval plus all individual data points.
IL-13 alters the composition of the epithelium and it is possible that long-term exposure to IL-13 during the maturation phase of the epithelium, which could occur in-vivo with dynamic cell turnover, may have a greater affect on paracellular transport. We treated epithelial cultures derived from nasal polyps with IL-13 from the initial submerged phase and continued treatment through the first two weeks of transwell growth as an air-liquid interface (immature ALI) (see methods for additional details). Data show that untreated cultures had increased TEER on day 14, whereas IL-13 treated cultures did not generate a TEER above baseline (Figure 5A). We found that FITC-dextran was able to transit from the apical to basal chamber (Figure 5B) and we were able to detect significant levels of IL-25 in both the apical secretions and basal media (Figure 5C).
The apical secretion of IL-25 suggested that IL-25 should be found within respiratory mucus from patients with Th-2 airway inflammation, i.e. with polyps. Indeed, there was a significant (P = 0.005) increase in the levels of IL-25 in mucus from CRSwNP patients (27pg/mg) compared to those with a history of CRS without nasal polyps (10pg/mg) or healthy control subjects (12pg/mg) (Figure 5D).
Discussion
Similar to tuft cells in the murine gut model19–21, DCAMKL1-positive, gustducin-positive upper respiratory cells, likely solitary chemosensory cells, appear to be the major source of epithelial IL-25 in the human upper airway. We demonstrate here that sinus inflammatory polyps but not adjacent turbinate tissue have an expansion of a gustducin/DCAMKL1-positive population. These gustducin/DCAMKL-1 positive cells are also the epithelial source of IL-25 (Figure 2C). Upper airway inflammatory polyp formation is regional within the confines of the sinonasal cavity with polypoid degeneration or polyp formation most common in the ethmoid sinus and less common on the middle turbinate, however, inflammatory polyps do not arise from the inferior turbinate32. The paucity of solitary chemosensory cells / IL-25 in non-polyp tissue suggests that these cells contribute to polyp formation and that perhaps IL-25 is an early marker of sinus polyp formation.
The increased abundance of IL-25-positive solitary chemosensory cells we have observed here in polyp tissue is significantly higher than the increased level of tuft cells seen in the mouse gut helminth infection model19–21. This may be related to the relatively short amount of time of infection in the mouse model (days to weeks) compared to our patient population with chronic rhinosinusitis where the underlying disease process may have been ongoing for years to decades prior to presentation. It is possible that increasing abundance of SCCs in the epithelium correlates with cumulative epithelial injury over time by infectious, allergic and/or environmental stimuli.
The feedback loop between tuft cells (IL-25) and ILC2s (IL-13) demonstrated in the gut has not been demonstrated to-date in the human upper respiratory mucosa. The ILC2 population is enriched in eosinophil-rich sinus polyp tissue33,34, and type-2 cytokines such as IL-5, IL-13 and eotaxin-2 are elevated in CRSwNP tissue4. This coupled with the increase in solitary chemosensory cells demonstrated here suggests that a similar relationship may exist between SCCs and ILC2s.
SCCs which are rare epithelial cells in normal sinus and turbinate tissue express bitter and sweet taste receptors and can modulate release of defensins by bitter agonists in the absence of sweet receptor-mediated suppression23. An abundance of cells that can “taste” the environment suggests that there could be an increased role for bitter or sweet agonist byproducts35 of native or infectious sinus flora or aerosolized allergens in the regulation of innate immunity in patients with Th-2 skewed airway inflammation.
DCAMKL1-positive cells also increase in abundance in human colorectal cancer polyps36, and in human non-small cell lung cancer specimens37. Suppression of DCAMKL1 can reduce polyp burden in a murine colon polyp model36, and DCAMKL1 may have a role in epithelial to mesenchymal transition38, suggesting that the population of nuclear-staining DCAMKL1 positive cells observed may be an undifferentiated or progenitor cell subtype. Both submerged sinus epithelial cultures as well as biphasic epithelial cultures from polyps were able to produce IL-25 following IL-13 stimulation. These data suggest that epithelial cells do not have to undergo terminal differentiation into predominantly polarized ciliated cells for IL-25 production. It is possible that the gustducin/DCAMKL-1-positive epithelial cell population may be a progenitor cell population with the ability to terminally differentiate into SCCs. Development of an exclusive panel of external flow cytometry markers will allow for better characterization and isolation of this cell population.
Precedent for transition of IL-25 across the epithelium can be found with epithelial or endothelial-generated IL-33. IL-33 is released by the barrier layer following damage, thereby allowing the epithelial cytokine to interact with the underlying adaptive immune components30,31. Epithelial IL-25 production can be triggered by helminth infection19,20 and possibly by the viral TLR agonist Poly(I:C)39 suggesting a broad array of triggers. It is possible that the duration and timing of movement of secreted IL-25 across the epithelium determines the severity of the adaptive immune system inflammatory response. Additionally, prolonged barrier breakdown allowing transit of luminal factors combined with epithelial remodeling or exposure to IL-13 may condemn the epithelium to a Th-2 dominated inflammatory cycle.
The mucus samples from all patients with CRS studied here have already undergone treatment with maximal medical therapy, surgery and post-surgical maintenance therapy. Thus, there may be a role for anti-IL25 or anti-IL-13 monoclonal antibodies in the setting of acute and chronic rhinosinusitis as well as in the post-operative setting in those patients with clinically elevated levels of mucus and/or tissue IL-25. Targeting these pathways may be a way to break the IL-25/IL-13 feedback loop that may be present in the respiratory tissue, thereby allowing the mucosa to “reset” with normal healing.
Supplementary Material
Acknowledgements
The authors have no relevant conflicts of interest. Research for D.R.H. supported by NIH grant R01 AI095289 and BWF Path award U01AI125940. Research for N.A.C. supported by NIH grant R01DC013588 and a philanthropic donation from the R.L.G. Foundation, Inc.
References
- 1.Halawi AM, Smith SS & Chandra RK Chronic rhinosinusitis: epidemiology and cost. Allergy Asthma Proc 34, 328–334, doi: 10.2500/aap.2013.34.3675 (2013). [DOI] [PubMed] [Google Scholar]
- 2.DeConde AS & Soler ZM Chronic rhinosinusitis: Epidemiology and burden of disease. Am J Rhinol Allergy 30, 134–139, doi: 10.2500/ajra.2016.30.4297 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Daines SM & Orlandi RR Inflammatory cytokines in allergy and rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg 18, 187–190, doi: 10.1097/MOO.0b013e328338206a (2010). [DOI] [PubMed] [Google Scholar]
- 4.Stevens WW et al. Cytokines in Chronic Rhinosinusitis. Role in Eosinophilia and Aspirin-exacerbated Respiratory Disease. Am J Respir Crit Care Med 192, 682–694, doi: 10.1164/rccm.201412-2278OC (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarvis D et al. Asthma in adults and its association with chronic rhinosinusitis: the GA2LEN survey in Europe. Allergy 67, 91–98, doi: 10.1111/j.1398-9995.2011.02709.x (2012). [DOI] [PubMed] [Google Scholar]
- 6.Larsen K The clinical relationship of nasal polyps to asthma. Allergy Asthma Proc 17, 243–249 (1996). [DOI] [PubMed] [Google Scholar]
- 7.Otto BA & Wenzel SE The role of cytokines in chronic rhinosinusitis with nasal polyps. Curr Opin Otolaryngol Head Neck Surg 16, 270–274, doi: 10.1097/MOO.0b013e3282fb2885 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Lam M et al. Interleukin-25 and interleukin-33 as mediators of eosinophilic inflammation in chronic rhinosinusitis. Am J Rhinol Allergy 29, 175–181, doi: 10.2500/ajra.2015.29.4176 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Shin HW et al. IL-25 as a novel therapeutic target in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol 135, 1476–1485 e1477, doi: 10.1016/j.jaci.2015.01.003 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Reh DD, Wang Y, Ramanathan M Jr. & Lane AP Treatment-recalcitrant chronic rhinosinusitis with polyps is associated with altered epithelial cell expression of interleukin-33. Am J Rhinol Allergy 24, 105–109, doi: 10.2500/ajra.2010.24.3446 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salter BM et al. IL-25 and IL-33 induce Type 2 inflammation in basophils from subjects with allergic asthma. Respir Res 17, 5, doi: 10.1186/s12931-016-0321-z (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzukawa M et al. Epithelial cell-derived IL-25, but not Th17 cell-derived IL-17 or IL-17F, is crucial for murine asthma. J Immunol 189, 3641–3652, doi: 10.4049/jimmunol.1200461 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iinuma T et al. Interleukin-25 and mucosal T cells in noneosinophilic and eosinophilic chronic rhinosinusitis. Ann Allergy Asthma Immunol 114, 289–298, doi: 10.1016/j.anai.2015.01.013 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Kuperman DA et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med 8, 885–889, doi: 10.1038/nm734 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Kondo M, Tamaoki J, Takeyama K, Nakata J & Nagai A Interleukin-13 induces goblet cell differentiation in primary cell culture from Guinea pig tracheal epithelium. Am J Respir Cell Mol Biol 27, 536–541, doi: 10.1165/rcmb.4682 (2002). [DOI] [PubMed] [Google Scholar]
- 16.Laoukili J et al. IL-13 alters mucociliary differentiation and ciliary beating of human respiratory epithelial cells. J Clin Invest 108, 1817–1824, doi: 10.1172/JCI13557 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dames P et al. Interleukin-13 affects the epithelial sodium channel in the intestine by coordinated modulation of STAT6 and p38 MAPK activity. J Physiol 593, 5269–5282, doi: 10.1113/JP271156 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wise SK et al. Interleukin-4 and interleukin-13 compromise the sinonasal epithelial barrier and perturb intercellular junction protein expression. Int Forum Allergy Rhinol 4, 361–370, doi: 10.1002/alr.21298 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Moltke J, Ji M, Liang HE & Locksley RM Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529, 221–225, doi: 10.1038/nature16161 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerbe F et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529, 226–230, doi: 10.1038/nature16527 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howitt MR et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 351, 1329–1333, doi: 10.1126/science.aaf1648 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerbe F, Brulin B, Makrini L, Legraverend C & Jay P DCAMKL-1 expression identifies Tuft cells rather than stem cells in the adult mouse intestinal epithelium. Gastroenterology 137, 2179–2180; author reply 2180–2171, doi: 10.1053/j.gastro.2009.06.072 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Lee RJ et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest 124, 1393–1405, doi: 10.1172/JCI72094 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tizzano M, Cristofoletti M, Sbarbati A & Finger TE Expression of taste receptors in solitary chemosensory cells of rodent airways. BMC Pulm Med 11, 3, doi: 10.1186/1471-2466-11-3 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orlandi RR et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. Int Forum Allergy Rhinol 6 Suppl 1, S22–209, doi: 10.1002/alr.21695 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Fokkens WJ et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 50, 1–12, doi: 10.4193/Rhino50E2 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Ramanathan M Jr. & Lane AP A comparison of experimental methods in molecular chronic rhinosinusitis research. Am J Rhinol 21, 373–377 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Lai Y et al. Inflammation-mediated upregulation of centrosomal protein 110, a negative modulator of ciliogenesis, in patients with chronic rhinosinusitis. J Allergy Clin Immunol 128, 1207–1215 e1201, doi: 10.1016/j.jaci.2011.09.001 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Ramezanpour M, Moraitis S, Smith JL, Wormald PJ & Vreugde S Th17 Cytokines Disrupt the Airway Mucosal Barrier in Chronic Rhinosinusitis. Mediators Inflamm 2016, 9798206, doi: 10.1155/2016/9798206 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz C, O’Grady K, Lavelle EC & Fallon PG Interleukin 33: an innate alarm for adaptive responses beyond Th2 immunity-emerging roles in obesity, intestinal inflammation, and cancer. Eur J Immunol 46, 1091–1100, doi: 10.1002/eji.201545780 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Martin NT & Martin MU Interleukin 33 is a guardian of barriers and a local alarmin. Nat Immunol 17, 122–131, doi: 10.1038/ni.3370 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Larsen PL & Tos M Origin of nasal polyps: an endoscopic autopsy study. Laryngoscope 114, 710–719, doi: 10.1097/00005537-200404000-00022 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Walford HH et al. Increased ILC2s in the eosinophilic nasal polyp endotype are associated with corticosteroid responsiveness. Clin Immunol 155, 126–135, doi: 10.1016/j.clim.2014.09.007 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaw JL et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med 188, 432–439, doi: 10.1164/rccm.201212-2227OC (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee RJ et al. Bacterial d-amino acids suppress sinonasal innate immunity through sweet taste receptors in solitary chemosensory cells. Sci Signal 10, doi: 10.1126/scisignal.aam7703 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakanishi Y et al. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat Genet 45, 98–103, doi: 10.1038/ng.2481 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Tao H, Tanaka T & Okabe K Doublecortin and CaM kinase-like-1 expression in pathological stage I non-small cell lung cancer. J Cancer Res Clin Oncol, doi: 10.1007/s00432-017-2405-7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sureban SM et al. DCAMKL-1 regulates epithelial-mesenchymal transition in human pancreatic cells through a miR-200a-dependent mechanism. Cancer Res 71, 2328–2338, doi: 10.1158/0008-5472.CAN-10-2738 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boita M, Bucca C, Riva G, Heffler E & Rolla G Release of Type 2 Cytokines by Epithelial Cells of Nasal Polyps. J Immunol Res 2016, 2643297, doi: 10.1155/2016/2643297 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


