Abstract
We report that 2 μg of azithromycin/ml inhibits the quorum-sensing circuitry of Pseudomonas aeruginosa strain PAO1. Addition of synthetic autoinducers partially restored the expression of the trancriptional activator-encoding genes lasR and rhlR but not that of the autoinducer synthase-encoding gene lasI. We propose that azithromycin interferes with the synthesis of autoinducers, by an unknown mechanism, leading to a reduction of virulence factor production.
Pseudomonas aeruginosa is a major bacterial pathogen in patients suffering from cystic fibrosis (CF) or diffuse panbronchiolitis (DPB) (7). Macrolides, such as azithromycin, are normally not included in the antipseudomonal therapeutic arsenal because of the absence of bactericidal or bacteriostatic activity. However, several studies have highlighted the benefit of long-term macrolide treatment in patients suffering from DPB or CF (5, 10). The mechanisms by which macrolides affect the outcome of chronic infections with P. aeruginosa could include an anti-inflammatory activity and/or a modulation of the production of bacterial virulence factors (9). Macrolides inhibit the production of bacterial exoproteases; however, whether this is independent of a decrease in bacterial growth and total protein production is controversial (13, 14, 19, 24, 25).
In P. aeruginosa, the las and rhl quorum-sensing systems regulate the production of several extracellular virulence factors, including elastase and rhamnolipid (27). Each system is composed of a gene encoding a transcriptional activator, lasR and rhlR, and a gene encoding an autoinducer synthase, lasI and rhlI, required for the synthesis of the autoinducer molecules 3-oxo-C12-homoserine lactone (3-oxo-C12-HSL) (15) and C4-HSL (16), respectively. The importance of quorum sensing in the pathogenesis of chronic infections is unknown. P. aeruginosa isolates from CF patients produce autoinducers (6), and autoinducer production by CF sputum has been associated with P. aeruginosa biofilms (22). Autoinducers have also been found in lung tissue of mice infected with P. aeruginosa (29). A reduction of autoinducer production by 50 μg of erythromycin/ml has been suggested (23). However, the Chromobacterium violaceum bioassay used could only measure the C4-HSL autoinducer (11).
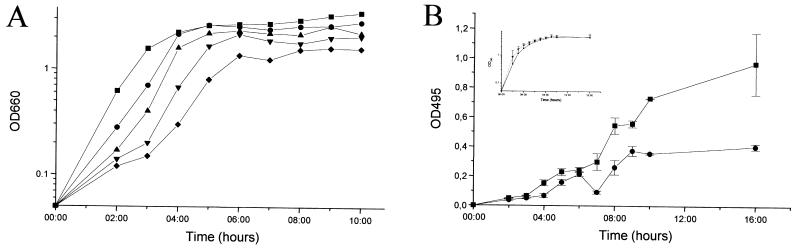
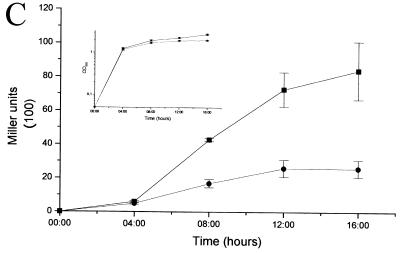
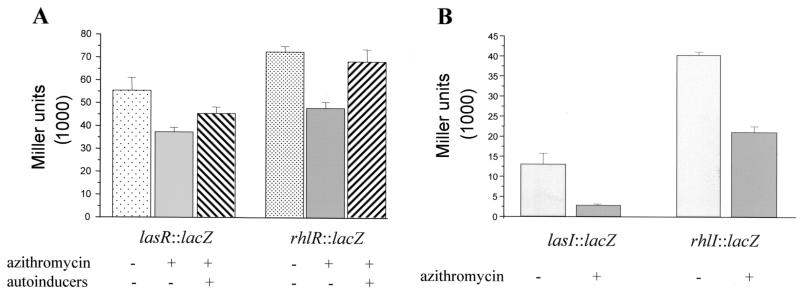
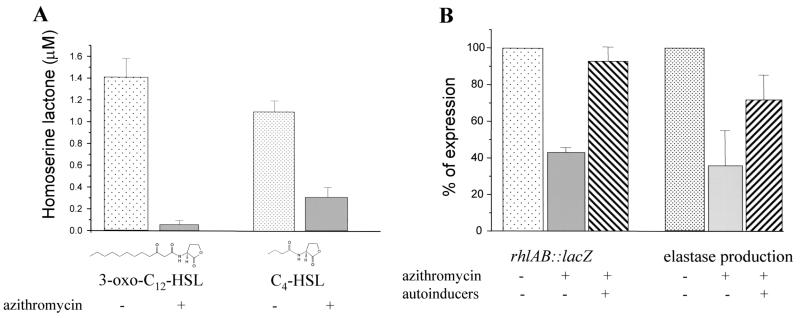
We wondered whether azithromycin interferes with the quorum-sensing circuitry. To differentiate the inhibition of virulence factor production from a nonspecific effect, we optimized our experimental conditions so that at the onset of stationary phase, when quorum sensing is active, growth was not notably affected. Figure 1A shows growth curves of wild-type PAO1 (8) in the presence of increasing concentrations of azithromycin. Exponential growth was slightly affected in the presence of 2 μg of azithromycin/ml, but no effect on the stationary growth phase was observed. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of total protein extracts of cells grown either in the absence or the presence of 2 μg of azithromycin/ml did not reveal major differences (data not shown). We next determined the effect of 2 μg of azithromycin/ml on elastase and rhamnolipid production in strain PAO1. Elastase production was monitored using elastin Congo red assays (17). In accordance with previous reports (13, 14), azithromycin inhibited the production of elastase (Fig. 1B). To determine the effect of azithromycin on rhamnolipid production, we used an azithromycin gradient incorporated into M9-based agar plates (21). The production of rhamnolipids progressively decreased with increasing azithromycin concentrations without a parallel drop in growth (data not shown). To reveal a possible effect on rhlAB transcription, we measured β-galactosidase (β-Gal) activity (12) using the rhlA′-lacZ reporter fusion pECP60 (18). The expression of rhlAB was strongly inhibited by the macrolide (Fig. 1C), thus confirming the results from the plate assays. Interestingly, no inhibition of virulence factor production was observed when the antibiotic was omitted in the overnight preculture, suggesting that prolonged exposure to the antibiotic is required. Subsequently, we determined the effect of azithromycin on the expression of the two transcriptional activator genes, lasR and rhlR, using the reporter fusions pPCS1001(lasR′-lacZ) (18) and pPCS1002(rhlR′-lacZ) (18). Azithromycin reduced the expression of both reporter fusions (Fig. 2A). We further investigated whether the macrolide also affects the expression of the two autoinducer synthase genes, lasI and rhlI, by using the reporter fusions pPCS223(lasI′-lacZ) (28) and pLPRI(rhlI′-lacZ) (28). Azithromycin reduced the transcription of lasI by 80% and of rhlI by 50% (Fig. 2B). To ensure that this inhibition was effectively associated with a decreased production of the 3-oxo-C12-HSL and C4-HSL signaling molecules, we extracted these two autoinducers from bacterial supernatants and measured their respective concentrations using specific bioassays (17, 20). In the presence of the macrolide, the concentrations of 3-oxo-C12-HSL and C4-HSL were reduced by 94 and 72%, respectively (Fig. 3A). We also measured the effect of azithromycin on the expression of the xcpR gene, which codes for a structural protein belonging to the type II secretion pathway (1, 2), by using the transcriptional reporter fusion pMPR(xcpR′-lacZ) (3). The transcription of xcpR was not affected by azithromycin (2,741 ± 37 Miller units versus 2,760 ± 25 Miller units, respectively; mean of three experiments ± standard deviation [SD], measured at an optical density at 660 nm of 4.8). These results seem to be in contradiction with a previous report suggesting that xcpR transcription is positively regulated by the las system (3). This apparent discrepancy could be explained by dissimilar experimental conditions and the use of different laboratory strains. Another explanation could be the compensation by other, already previously suspected (3), regulatory pathways maintaining xcpR expression despite the inhibition of the las quorum-sensing system by azithromycin. Since the expression of both lasR and rhlR genes is dependent on the presence of adequate autoinducer levels, we measured their transcription in the presence of exogenous synthetic 3-oxo-C12-HSL and C4-HSL, each provided at concentrations of 10 μM. The addition of both autoinducers completely restored the expression of rhlR and almost completely restored the expression of lasR (5,500 ± 600 Miller units in the absence of azithromycin versus 4,600 ± 300 Miller units in the presence of azithromycin and exogenous autoinducers) (Fig. 2A). In contrast, the addition of exogenous autoinducers could not restore the expression of the lasI autoinducer synthase gene (20% of initial value in the presence of azithromycin and 16% in the presence of both azithromycin and exogenous autoinducers; data not shown). The addition of exogenous autoinducers also partially restored the production of elastase and almost completely restored the expression of rhlAB (Fig. 3B). These results suggest that azithromycin might reduce the production of quorum-sensing-dependent virulence factors by inhibiting the synthesis of the autoinducer molecules.
FIG. 1.
Effect of azithromycin on growth and elastase and rhamnolipid production. (A) Bacterial strains were grown in Luria-Bertani (LB) medium in the absence (squares) or in the presence (circles, 2 μg/ml; upside triangles, 3 μg/ml; downside triangles, 4 μg/ml; diamonds, 5 μg/ml) of azithromycin. Growth curve determinations were repeated five times and the graph shows results from one typical experiment. (B) Supernatants of cells, grown either in the absence (squares) or the presence (circles) of 2 μg of azithromycin/ml, were collected at regular intervals and elastase activity was determined by the elastin Congo red assay. (C) PAO1(pECP60) (rhlA′-lacZ) was grown in LB medium either in the absence (squares) or the presence (circles) of 2 μg of azithromycin/ml, and β-Gal activities were assayed at regular time intervals. Inserted graphs in panels B and C show the corresponding growth curves. Results are the mean ± SD of three independent experiments performed in duplicate.
FIG. 2.
Azithromycin affects transcription of quorum-sensing genes. The expression of the transcriptional activator and the autoinducer synthase genes was measured by β-Gal determinations in strain PAO1 cultures grown for 10 h in the absence (azithromycin −) or presence (azithromycin +) of 2 μg of azithromycin/ml, using plasmids pPCS1001(lasR′-lacZ) and pPCS1002(rhR′-lacZ) (A) and plasmids pPCS223(lasI′-lacZ) and pLPRI(rhlI′-lacZ) (B). Exogenous autoinducers were added to the cell culture as indicated (autoinducers +). Results are the mean ± SD of three independent experiments performed in duplicate.
FIG. 3.
Autoinducers are reduced in the presence of azithromycin and partially restore rhlAB expression and elastase production. (A) Strain PAO1 cultures were grown for 12 h in Luria-Bertanimedium, either in the absence (azithromycin −) or presence (azithromycin +) of 2 μg of azithromycin/ml. The supernatants were extracted with ethyl acetate, and the 3-oxo-C12-HSL and C4-HSL concentrations were measured using specific bioassays. Results are the mean ± SD of three independent experiments. (B) Cells were grown for 10 h in the absence (azithromycin −) or presence (azithromycin +) of 2 μg of azithromycin/ml and in the absence (autoinducers −) or presence (autoinducers +) of exogenous autoinducers. The expression of the rhlAB gene was determined by measuring the β-Gal activity of the rhlA′-lacZ reporter fusion pECP60. The production of elastase was determined by the elastin Congo red assay performed on culture supernatants. Results are expressed as the percentage of the maximum observed in each individual experiment and represent the mean ± SD of three independent experiments performed in duplicate.
How does azithromycin interfere with the transcription of genes belonging to the quorum-sensing circuitry? The absence of a reduction of xcpR transcription suggests that, in our experimental conditions, azithromycin does not inhibit the transcription of genes in a nonspecific manner. Azithromycin inhibits protein synthesis at the ribosomal level, and therefore a direct effect on gene transcription seems unlikely. The necessity of a prolonged exposure to azithromycin suggests an indirect effect on the quorum-sensing circuitry. As the expression of lasI could not be complemented by exogenous autoinducers, we hypothesize that azithromycin might interfere with the translation of a so-far-unidentified protein important for the transcription of the autoinducer synthase. A reduced level of autoinducer could explain the observed effects on the quorum-sensing circuitry.
Chronic infections by P. aeruginosa lead to serious deterioration of lung function in DPB and CF patients. The recent reports showing improvement in DPB and CF patients treated with macrolides have been encouraging (5, 10). Our data show a clear inhibition of the quorum-sensing circuitry of P. aeruginosa by azithromycin. Most of the quorum-sensing-regulated virulence factors cause tissue damage. Moreover, the 3-oxo-C12-HSL autoinducer has some immunomodulatory activity (26) and stimulates the production of interleukin-8 by respiratory epithelial cells (4). Therefore, this autoinducer might itself be responsible for a chronic inflammatory response. Administration of macrolides to reduce autoinducer synthesis might therefore partially prevent the tissue damage.
Acknowledgments
We gratefully acknowledge B. H. Iglewski for providing plasmids and synthetic autoinducers. This work was supported by a Pfizer grant (to K.T.), a research grant from the Programme Commun de Recherche en Genie Biomedical Geneve-Lausanne 1999–2002 (to C.V.D.), and research grants 3231-051940.97 and 3200-052189.97 from the Swiss National Research Foundation (to C.V.D.).
REFERENCES
- 1.Akrim M, Bally M, Ball G, Tommassen J, Teerink H, Filloux A, Lazdunski A. Xcp-mediated protein secretion in Pseudomonas aeruginosa: identification of two additional genes and evidence for regulation of xcp gene expression. Mol Microbiol. 1993;10:431–443. doi: 10.1111/j.1365-2958.1993.tb02674.x. [DOI] [PubMed] [Google Scholar]
- 2.Bally M, Filloux A, Akrim M, Ball G, Lazdunski A, Tommassen J. Protein secretion in Pseudomonas aeruginosa: characterization of seven xcp genes and processing of secretory apparatus components by prepilin peptidase. Mol Microbiol. 1992;6:1121–1131. doi: 10.1111/j.1365-2958.1992.tb01550.x. [DOI] [PubMed] [Google Scholar]
- 3.Chapon V, Akrim M, Latifi A, Williams P, Lazdunski A, Bally M. Regulation of the xcp secretion pathway by multiple quorum-sensing modulons in Pseudomonas aeruginosa. Mol Microbiol. 1997;24:1169–1178. doi: 10.1046/j.1365-2958.1997.4271794.x. [DOI] [PubMed] [Google Scholar]
- 4.DiMango E, Zar H J, Bryan R, Prince A. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J Clin Investig. 1995;96:2204–2210. doi: 10.1172/JCI118275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujii T, Kadota J, Kawakami K, Iida K, Shirai R, Kaseda M, Kawamoto S, Kohno S. Long term effect of erythromycin therapy in patients with chronic Pseudomonas aeruginosa infection. Thorax. 1995;50:1246–1252. doi: 10.1136/thx.50.12.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geisenberger O, Givskov M, Riedel K, Hoiby N, Tummler B, Eberl L. Production of N-acyl-l-homoserine lactones by P. aeruginosa isolates from chronic lung infections associated with cystic fibrosis. FEMS Microbiol Lett. 2000;184:273–278. doi: 10.1111/j.1574-6968.2000.tb09026.x. [DOI] [PubMed] [Google Scholar]
- 7.Hoiby N. Diffuse panbronchiolitis and cystic fibrosis: East meets West. Thorax. 1994;49:531–532. doi: 10.1136/thx.49.6.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holloway B W, Krishnapillai V, Morgan A F. Chromosomal genetics of Pseudomonas. Microbiol Rev. 1979;43:73–102. doi: 10.1128/mr.43.1.73-102.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howe R A, Spencer R C. Macrolides for the treatment of Pseudomonas aeruginosa infections? J Antimicrob Chemother. 1997;40:153–155. doi: 10.1093/jac/40.2.153. [DOI] [PubMed] [Google Scholar]
- 10.Jaffe A, Francis J, Rosenthal M, Bush A. Long-term azithromycin may improve lung function in children with cystic fibrosis. Lancet. 1998;351:420. doi: 10.1016/S0140-6736(05)78360-4. [DOI] [PubMed] [Google Scholar]
- 11.McClean K H, Winson M K, Fish L, Taylor A, Chhabra S R, Camara M, Daykin M, Lamb J H, Swift S, Bycroft B W, Stewart G S, Williams P. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology. 1997;143:3703–3711. doi: 10.1099/00221287-143-12-3703. [DOI] [PubMed] [Google Scholar]
- 12.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 352–355. [Google Scholar]
- 13.Mizukane R, Hirakata Y, Kaku M, Ishii Y, Furuya N, Ishida K, Koga H, Kohno S, Yamaguchi K. Comparative in vitro exoenzyme-suppressing activities of azithromycin and other macrolide antibiotics against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1994;38:528–533. doi: 10.1128/aac.38.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molinari G, Guzman C A, Pesce A, Schito G C. Inhibition of Pseudomonas aeruginosa virulence factors by subinhibitory concentrations of azithromycin and other macrolide antibiotics. J Antimicrob Chemother. 1993;31:681–688. doi: 10.1093/jac/31.5.681. [DOI] [PubMed] [Google Scholar]
- 15.Pearson J P, Gray K M, Passador L, Tucker K D, Eberhard A, Iglewski B H, Greenberg E P. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci USA. 1994;91:197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearson J P, Passador L, Iglewski B H, Greenberg E P. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:1490–1494. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearson J P, Pesci E C, Iglewski B H. Role of Pseudomonas aeruginosa las and rhl quorum-sensing systems in the control of elastase and rhamnolipid biosynthesis genes. J Bacteriol. 1997;179:5756–5767. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pesci E C, Pearson J P, Seed P C, Iglewski B H. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 1997;179:3127–3132. doi: 10.1128/jb.179.10.3127-3132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakata K, Yajima H, Tanaka K, Sakamoto Y, Yamamoto K, Yoshida A, Dohi Y. Erythromycin inhibits the production of elastase by Pseudomonas aeruginosa without affecting its proliferation in vitro. Am Rev Respir Dis. 1993;148:1061–1065. doi: 10.1164/ajrccm/148.4_Pt_1.1061. [DOI] [PubMed] [Google Scholar]
- 20.Seed P C, Passador L, Iglewski B H. Activation of the Pseudomonas aeruginosa lasI gene by LasR and the Pseudomonas autoinducer PAI: an autoinduction regulatory hierarchy. J Bacteriol. 1995;177:654–659. doi: 10.1128/jb.177.3.654-659.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siegmund I, Wagner F. New method for detecting rhamnolipids excreted by Pseudomonas species during growth on mineral plates. Biotechnol Tech. 1991;5:265–268. [Google Scholar]
- 22.Singh P K, Schaefer A L, Parsek M R, Moninger T O, Welsh M J, Greenberg E P. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;407:762–764. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]
- 23.Sofer D, Gilboa-Garber N, Belz A, Garber N C. ‘Subinhibitory’ erythromycin represses production of Pseudomonas aeruginosa lectins, autoinducer and virulence factors. Chemotherapy. 1999;45:335–341. doi: 10.1159/000007224. [DOI] [PubMed] [Google Scholar]
- 24.Tateda K, Ishii Y, Matsumoto T, Kobayashi T, Miyakawa S, Yamaguchi K. Potential of macrolide antibiotics to inhibit protein synthesis of Pseudomonas aeruginosa: suppression of virulence factors and stress responses. J Infect Chemother. 2000;6:1–7. doi: 10.1007/s101560050042. [DOI] [PubMed] [Google Scholar]
- 25.Tateda K, Ishii Y, Matsumoto T, Furuya N, Nagashima M, Matsunaga T, Ohno A, Miyazaki S, Yamaguchi K. Direct evidence for antipseudomonal activity of macrolides: exposure-dependent bactericidal activity and inhibition of protein synthesis by erythromycin, clarithromycin, and azithromycin. Antimicrob Agents Chemother. 1996;40:2271–2275. doi: 10.1128/aac.40.10.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Telford G, Wheeler D, Williams P, Tomkins P T, Appleby P, Sewell H, Stewart G S, Bycroft B W, Pritchard D I. The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-l-homoserine lactone has immunomodulatory activity. Infect Immun. 1998;66:36–42. doi: 10.1128/iai.66.1.36-42.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Delden C, Iglewski B H. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg Infect Dis. 1998;4:551–560. doi: 10.3201/eid0404.980405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Delden C, Pesci E C, Pearson J P, Iglewski B H. Starvation selection restores elastase and rhamnolipid production in a Pseudomonas aeruginosa quorum-sensing mutant. Infect Immun. 1998;66:4499–4502. doi: 10.1128/iai.66.9.4499-4502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu H, Song Z, Hentzer M, Andersen J B, Heydorn A, Mathee K, Moser C, Eberl L, Molin S, Hoiby N, Givskov M. Detection of N-acylhomoserine lactones in lung tissues of mice infected with Pseudomonas aeruginosa. Microbiology. 2000;146:2481–2493. doi: 10.1099/00221287-146-10-2481. [DOI] [PubMed] [Google Scholar]