Abstract
Background:
Loop is an open-source automated insulin dosing system that allows users unrivaled control over system settings that affect future glucose prediction. Thousands use Loop, but little is known about those who discontinue.
Methods:
In a large observational study, 874 Loop participants completed surveys and provided glycemic data, 46 (5.3%) of those self-identified as discontinuing Loop use during the observation window, 45 completed a discontinued use survey, 22 provided system settings data, and 19 participated in semistructured interviews about their discontinuation. Qualitative data were transcribed, coded, and analyzed.
Results:
Older age and not trusting Loop were associated with discontinued use, although no other demographic or clinical characteristics were significant correlates. The most endorsed reasons were “I decided to try something else” (27.8%) followed by “It just didn't help as much as I thought it would” (22.2%). Qualitative analyses revealed prominent themes centered upon mental and emotional burden and adjusting settings. Other reasons for discontinued use included fear of disapproval of Loop use from diabetes provider, barriers to acquiring component devices, a desire to try new/different technologies, concerns that Loop could not accommodate specific exercise or low insulin dose regimens, and worry about Loop use during pregnancy. It was noted that burdens might be alleviated by enhanced technical assistance and expert guidance.
Conclusions:
Although the majority of individuals in the Loop observational study continued use, those who discontinued reported similar challenges. Technical support and education specific to setting calculations could expand Loop benefits, alleviate burden, and support sustained use among new Loop users. Clinical Trial Registration: clinicaltrials.gov (NCT03838900).
Keywords: Diabetes mellitus, Type 1/drug therapy, Insulin/administration and dosage, Automation
Introduction
Open-source automated insulin dosing systems are driven by communities with personal vested interests in advancing diabetes technology for people living with type 1 diabetes (T1D). One of these systems is “Loop,” an open-source iOS app. Loop has gained increased interest and popularity over time and is safe and efficacious for adults and children.1 As with other open-source initiatives, developers have effectively leveraged user feedback and suggestions for rapid iterative development. No automated insulin dosing system is perfect, and significant discontinuation has been reported for some commercial systems.2 However, little is known about those who discontinue Loop and their reasons for discontinuing use.
This study seeks to examine the demographic and clinical characteristics of discontinuers in a large observational study, as compared with those who continued Loop use, as well as identify the primary reasons for discontinued Loop use. Discontinuers were Loop users who self-identified as having stopped Loop use at any point during study participation. Understanding the correlates and reasons for discontinuing Loop use can uncover potential modifiable and unmodifiable barriers to automated insulin dosing and inform efforts to increase access to and further dissemination of these life-changing technologies.
Methods
The observational study was conducted under real-world circumstances rather than a controlled clinical environment with all data provided directly by study participants. The protocol was approved by the institutional review board of the Jaeb Center for Health Research (JCHR) and conducted in compliance with standards of good clinical practice (GCP). The protocol is available at (https://public.jaeb.org/datasets).
The study sample included 874 adults and children with T1D who were U.S. residents and were either already using Loop (n = 277, 31.7%) or had decided to initiate Loop (n = 597, 68.3%). Participants used both Medtronic and Omnipod insulin pumps. The existence of the study was publicized on websites and at the point of purchase for a Bluetooth-to-subGHz bridge (dubbed “RileyLink,” required for Loop operation). Interested individuals were directed to the study website for information about the study where electronic informed consent was obtained from participants ≥18 years old and the legally authorized representatives for participants <18 years old who provided assent. Enrollment was open between January 2019 and August 2019. The data collection ended in April 2020.
Survey data were collected by electronic questionnaires after study enrollment (baseline) and when participants indicated that they had discontinued using Loop.
Basal rates, carbohydrate-to-insulin ratio (CIR), and insulin sensitivity factor (ISF) were collected through Loop issue reports uploaded directly to the study coordinating center. Two weeks of continuous glucose monitoring (CGM), carbohydrate intake, and insulin delivery data surrounding each issue report upload were used to determine percentage time-in-range (TIR), median daily carbohydrates, and median total daily dose. These data were aggregated using Tidepool accounts associated with the study participants. The body mass index (BMI) data were calculated from height and weight provided by the participants.
HbA1c was measured through a fingerstick blood sample at baseline using a collection kit mailed directly to the study participant and returned through USPS mail to a central laboratory (University of Minnesota Advanced Research and Diagnostic Laboratory).
Discontinuers were invited to participate in semistructured interviews that were conducted and recorded through secure videoconferencing (Zoom) until saturation was reached concerning common discontinuation themes, which resulted in interviews with nearly half of all discontinuers. The audio recordings were transcribed and deidentified by the professional transcript service Medikin. Transcripts were then uploaded into NVivo, version 12, where they were coded and analyzed.
Measures
All participants provided self-/parent report of demographic, clinical, and socioeconomic information through online surveys, which also included psychosocial questionnaires. Parent report data were collected for participants below the age of 18 years, except for youth-specific measures on which the youth participants themselves provided self-report. Study variables included participants' current age in years, gender (woman, man, and nonbinary), race/ethnicity, education, annual household income, insurance type (private, public, and other), and age at diabetes diagnosis in years.
BMI (collected at baseline), median daily carbohydrates, and median total daily dose were used to derive calculated (optimal) settings.3 These data were used to calculate ratios of actual settings compared with calculated settings. The ratio of the user's settings to the calculated settings and the corresponding TIR was plotted in Matlab.
Psychosocial survey measures captured diabetes distress, fear of hypoglycemia, and attitudes toward diabetes technology. Diabetes distress was measured through the 4-item diabetes distress scale (DDS4). Participants were asked how often they were bothered by each item over the past month. A sample item includes “Feeling that I am not as skilled at managing diabetes as I should be.” Responses are averaged to create a total score with higher scores reflecting greater diabetes distress.4,5 Fear of hypoglycemia was measured using the worry subscale on the hypoglycemic fear survey, which includes 15 items to assess anxiety concerning possible hypoglycemia that are summed to create an overall score with higher scores indicating greater fear of hypoglycemia.6,7
Attitudes toward diabetes technology was assessed using a 5-item Diabetes Technology Attitudes scale that captures the use of and comfort with diabetes devices and technologies. Response scores for each item (e.g., “Diabetes technology has made my life better”) were used to create summary scores with higher scores indicating more positive attitude toward diabetes technology.8
Discontinued use specific measures were also administered to participants if/when they indicated discontinued use of Loop during the study. The quantitative measures included a survey about reasons for discontinuing, and qualitative data were captured through semistructured interviews conducted over secure videoconferencing. Interview questions largely focused on user experience with Loop, barriers, and reasons for discontinued use. The interview guide is provided as Supplementary Table S1.
Analytic approach
Quantitative analyses comprise generating descriptive statistics and conducting independent t-tests and chi-squares tests to compare the discontinuers subsample with study participants who did not self-identify as discontinuing use of Loop.
Qualitative data from interviews were transcribed and then coded by a team of five coders. Each transcript was coded by two independent coders. Qualitative data were analyzed and synthesized by the multidisciplinary coding team that included two qualitative experts and the remaining coding team members. Weekly meetings were used to reconcile problematic codes, review tricky coding examples, and add codes to help address conceptual gaps.
Given the current study's emphasis on discontinuation and reasons for discontinuation, the qualitative analysis was conducted within the quotes that were coded under the theme of “reasons for discontinuing” from the larger study qualitative analysis. These quotes were grouped into broad categories that were used to generate subthemes, which were then reviewed and verified by a second coder.
Results
Participant characteristics and other factors associated with discontinuing
A total of 46 (5.3% of the study sample) participants self-identified as discontinuers. Sample characteristics for discontinuers and the rest of the sample presumed to have continued Loop use (“continuers”) are presented in Table 1. Between-group comparisons revealed several statistically significant differences. Specifically, discontinuers were older, on average, than the continuers (35.09 vs. 27.26, P = 0.003).
Table 1.
Discontinuer Sample Characteristics and Comparisons with Continuers Sample
| Discontinuers (n = 46) | Continuers (n = 828) | P | |
|---|---|---|---|
| Age | 35.09 (16.36) | 27.26 (17.36) | 0.003 |
| Adult (age 18+) | 37 (80.4%) | 492 (59.4%) | 0.005 |
| Gender | |||
| Male | 15 (45.4%) | 375 (46.1%) | 0.176 |
| Female | 33 (67.4%) | 443 (53.5%) | |
| Nonbinary | 0 (0%) | 3 (0.4%) | |
| White race/ethnicity (n = 859) | 42 (93.3%) | 765 (94.0%) | 0.859 |
| Education (graduate level; n = 873) | 20 (43.5%) | 371 (44.9%) | 0.854 |
| Income ($100,000+; n = 800) | 28 (65.1%) | 546 (72.1%) | 0.321 |
| Private insurance | 41 (89.1%) | 750 (90.6%) | 0.744 |
| Age at diabetes diagnosis | 14.29 (11.55) | 11.68 (9.79) | 0.138 |
| Percentage TIR (n = 761) | 71.39% (16.19) | 70.18% (16.59) | 0.649 |
| A1c (n = 519) | 6.58 (0.803) | 6.68 (0.946) | 0.490 |
| Psychosocial measures | |||
| Diabetes distress (DDS4; n = 522) | 1.83 (0.88) | 1.91 (0.94) | 0.624 |
| Hypoglycemia fear: adult | 18.76 (11.15) | 17.96 (10.82) | 0.665 |
| Hypoglycemia fear: parent | 26.67 (11.96) | 26.07 (10.94) | 0.873 |
| Hypoglycemia fear: child | 18.00 (6.24) | 18.04 (9.77) | 0.992 |
| Technology attitudes (n = 522) | 20.22 (2.48) | 20.20 (3.08) | 0.971 |
| Loop-specific measures | |||
| New Loop user | 27 (58.7%) | 570 (68.8%) | 0.150 |
| Hard to start Loop (1 = easy, 5 = hard; n = 787) | 2.54 (1.20) | 2.54 (1.05) | 0.933 |
| Need help starting Loop (yes/no; n = 787) | 21 (46.7%) | 274 (36.9%) | 0.190 |
| Trust Loop (yes/no; n = 787) | 39 (86.7%) | 715 (96.4%) | 0.002 |
| No. of weeks to trust Loop (n = 738) | 1.89 (1.67) | 2.34 (2.398) | 0.263 |
N = 874 for full sample. All measures are captured at baseline.
DDS4, 4-item diabetes distress scale; TIR, time-in-range.
This difference was also reflected in a larger proportion (80.4% vs. 59.4%, P = 0.005) of adult Loop users among discontinuers compared with continuers, suggesting that adults were more likely to discontinue use than child or adolescent users. Although both groups reported trusting Loop at generally high rates, a smaller proportion of discontinuers reported trusting Loop compared with continuers (86.7% vs. 96.4%, P = 0.002). No other statistically significant differences were found based on baseline characteristics, including gender, race/ethnicity, education, income, insurance type, as well as diabetes distress, fear of hypoglycemia, and attitudes toward diabetes technology or Loop-specific measures, including difficulty starting Loop, needing help starting Loop, and time it took to trust Loop (Table 1).
Regarding duration of Loop use before discontinuation, no statistically significant differences were found in discontinuation rate between participants who were initiating Loop when they enrolled in the study (new Loop users) and those who had had already been using Loop at the time of enrollment.
Reasons for discontinuing based on survey data
Discontinuer surveys were completed by 45 participants (97.8% of self-identified discontinuers). Discontinuer survey results showed varied endorsement of reasons for discontinued use across items (Table 2). The most endorsed reasons were “I decided to try something else” (33.3%) followed closely by “It just didn't help as much as I thought it would” (26.7%). The least endorsed items were “It cost too much” and “My (or my child's) version was not up to date,” each of which were only endorsed by one discontinuer.
Table 2.
Reasons for Discontinuing Based on Survey Response
| Discontinuer survey items | n (%) |
|---|---|
| 1. I did not like it | 7 (15.6) |
| 2. I decided to try something else | 15 (33.3) |
| 3. It cost too much | 1 (2.2) |
| 4. It was hard to get all the supplies | 2 (4.4) |
| 5. Too complicated/could not find resources to use it correctly | 6 (13.3) |
| 6. I did not see an improvement in my (or my child's) blood sugars | 9 (20.0) |
| 7. It was not working properly | 10 (22.2) |
| 8. My (or my child's) version was not up to date | 1 (2.2) |
| 9. I spent too much time on it | 10 (22.2) |
| 10. It just did not help as much as I thought it would | 12 (26.7) |
| 11. Another reason not listed above | 19 (42.2) |
n = 45 for discontinuer subsample with discontinuer survey data.
Nearly half (42.2%) of discontinuers indicated reasons outside of those listed in the survey instrument, which are likely reflected in the qualitative results from interview data (see “Reasons for discontinuing based on interview data” below). Of note, several who reported deciding to “try something else” uploaded subsequent data to the study through Loop issue reports after the supposed discontinuation date—indicating some returned to using Loop.
Posthoc analyses compared responses to discontinuer survey items based on the more established significant correlates to discontinuing: age and trusting loop. Although age as a continuous variable was not significantly related to item endorsement, lack of improvement in blood sugars was a more commonly endorsed reason for discontinuing among child/adolescent users compared with adult users (44.4% vs. 13.9%, P = 0.040). Compared with discontinuers who trusted Loop, discontinuers who did not trust Loop more frequently endorsed Loop not helping as much as expected (83.3% vs. 18.4%, P < 0.001) and not liking Loop (50.0% vs. 10.5%, P = 0.005) as reasons for discontinuing.
Reasons for discontinuing based on interview data
Nineteen discontinuers (41.3% of all discontinuers) completed semistructured interviews. Independent t-tests revealed no significant differences between discontinuers who were interviewed and those who were not interviewed on any demographic or clinical study variables (all P > 0.050). Varied endorsement of reasons for discontinued use across items is reported in Table 2. The current coding of interview data was conducted within a broader qualitative examination of Loop users' experiences (see Suttiratana et al., under review) and sought to more deeply examine the themes revealed by discontinuers' data, specifically, and focus on their reasons for discontinued use.
These analyses revealed two prominent themes largely centered on balancing the benefits and drawbacks of Loop use related to (1) mental and emotional burden and (2) adjusting settings. Within each of these themes, discontinuers weighed the stress, effort, and/or time required to effectively start up and run Loop against the potential benefits of the system while comparing the strengths and weaknesses of familiar management regimens. Example quotes by theme and participant type are presented in Table 3.
Table 3.
Example Quotes by Theme and Participant Type
| Theme | Participant type | Example quotes |
|---|---|---|
| Mental and emotional burden | Adult | …Part of that anxiety was the, you know, ripping my hair out learning how to use the thing, but I didn't enjoy the anxiety, so that, that certainly was part of contributing to what, what caused me to just stop using it. …It's obviously the garbage in, garbage out problem when you're making predictive modeling, that the getting them dead-on matters a lot more, your prediction comes out way off. And then you wind up on the, I would wind up on a roller coaster of my, you know, my glucose soaring up and down, which made it very hard for me to work, because I'd be in a fog all day. And I had a pretty good A1c going in. I had a 6.3, more or less, starting, when we started the study at least, so there'd definitely be an improvement, I know I could get an improvement with it, but the problem was the cost of it was I couldn't, I couldn't concentrate anymore at work. It was just, it was just, really, it felt like fog in my brain. |
| Parent | We felt like it was a little more stressful for us to be on it than the advantages of it. There were a lot of technological issues that we were experiencing… So, it was just causing a lot of stress and it was a difficult decision, we felt like it was the best thing to do for him, it was to stop. Yeah, it was just, it was really frustrating. He just ran high a lot. Yeah, my wife and I were frustrated to see those. He actually said, we are doing terrible with this. I want to stop using it. |
|
| Adjusting settings | Adult | It did not meet my expectations as far as lessening of the burden of diabetes management. What I found was that there, as everybody says, you just—it's not set it and forget it. You've got to get the settings absolutely right. And those are all of the settings that have to do with the inputs for the algorithm to deal with. So that's insulin-to-carb ratios, insulin sensitivity factor, whatever else. And if the algorithm doesn't have those set just right, it's not going to be able to come up with appropriate dosage. So, getting those settings right entailed like months of constant reading of the document and just getting the whole application written. Well, my issue was for nine months I felt like I was constantly trying to adjust my settings and just could never figure out a good setting and my frustration was I had had such good control for so long that I just felt like my control was getting so much worse because I couldn't get the numbers right. I think if it would have taken less than five months to get my settings dialed in, somebody who had knowledge and was willing to work with me, somebody not just a Mentor but someone with credentials and understanding of the system. I know that there are a lot of people who are knowledgeable including the people who developed and programmed Loop and the Facebook group that exists, but I felt like a real roadblock in reaching out to them in particular because so many people are contacting them and bothering them. Yeah. They are just people like me, but there was nobody who…it was hard to sift through the available mentors between who had experience with people similar to me, there are a lot of parents with kids, there were people who have been on the system for a month who are mentoring people, well, I didn't want that. Umm…it was just hard to identify who would be a good Mentor and who had the same goals. |
| Parent | So, for us, kind of the driving factor was her going to school and needing to either get her settings dialed in before we send her off to school or having to put it on hold. So ultimately, we just couldn't get it dialed in where we wanted it to be. It felt like we had better control off of it than on it. | |
| Fear of disapproval | Parent | Well, one of the concerns that I had had when we started was, how our endocrinologist was going to respond, so that was. As that appointment was getting closer, I was getting more nervous about what she would say…I was worried about what the appointment would be like when we would take our devices for the download and there would be nothing to download because we are delivering the insulin through the phone instead of through the PDM, so I just wanted to avoid that conversation. |
| Specific circumstances or concerns | Adult | For myself personally, I am like afraid to bolus for meals…because I am afraid of lows. I didn't never have any like severe-severe lows, but I would be high and it would be and I will be kicking in an extra basal and then I would just get these double down arrows and then it would show me how it is going to be a negative number and I know that it is not always not accurate but like me and I am taking care of two small kids at home and I said why I cannot be risking having like a severe incidence when I am with my kiddos. |
Theme: mental and emotional burden
Participants described a sense of mental and emotional burden associated with Loop uptake and/or use that caused them to decide to discontinue use, including emotional frustration and undue cognitive toll. These burdens were associated with learning how to use Loop, observed glycemic outcomes while using Loop, and just general frustration with the Loop system.
Theme: adjusting settings
The second prominent theme that emerged centered on the difficulties of setting and changing Loop system parameters, including basal, CIR, ISF, glucose targets, and suspend threshold. Specifically, participants who discontinued described challenges with adjusting Loop settings to achieve optimal system performance such as taking prolonged periods of time, requiring extensive effort, emotional distress, and/or deterioration in glycemic control, all leading to their decision to discontinue use.
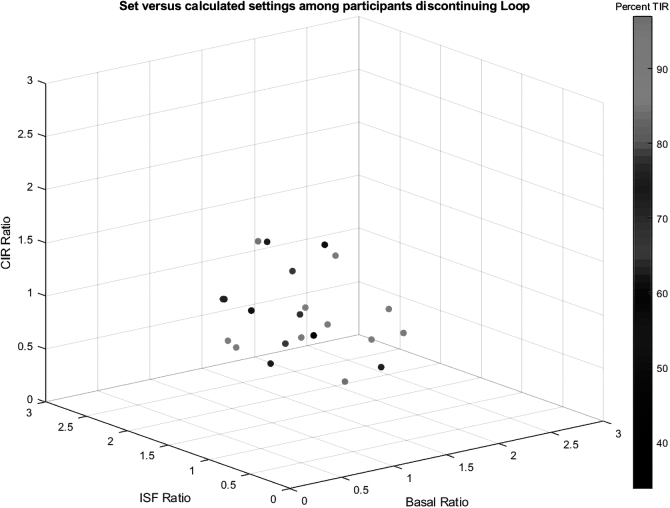
This theme aligned with the range in ratios of actual settings to calculated (optimal) settings,1 as given in Table 4, which were available for 22 discontinuers (47.8% of all discontinuers). These were the discontinuers who provided data through Loop issue report before their discontinuation. Of note, participants' settings frequently varied, sometimes widely, from the calculated settings. Interestingly, however, deviations from calculated settings were not consistent across setting types, meaning that the ratios of actual versus calculating settings were not correlated across setting type (basal, ISF, and CIR) and that the bivariate correlations between these variables were not statistically significant (all P > 0.05) and relatively small (all R < 0.35).
Table 4.
System Setting Ratios (Actual/Calculated) and Percentage Time-in-Range
| Variable | Mean (SD) | Min | Max |
|---|---|---|---|
| Basal ratio | 1.09 (0.272) | 0.58 | 1.62 |
| ISF ratio | 1.25 (0.528) | 0.03 | 2.23 |
| CIR | 1.07 (0.325) | 0.62 | 1.73 |
| TIR (%) | 75.74 (16.638) | 33.83 | 97.03 |
n = 22 for discontinuer subsample with system settings data. Ratios compare actual setting divided by calculated (optimal) setting, with values closer to 1 reflecting more congruency between actual and calculated settings.
CIR, carbohydrate-to-insulin ratio; ISF, insulin sensitivity factor; SD, standard deviation.
Figure 1 depicts the relationship between actual versus calculated setting ratios for basal, ISF, and CIR as well as with TIR.
FIG. 1.
Scatterplot of CIR, ISF, and basal ratios of actual settings versus calculated settings with TIR. CIR, carbohydrate-to-insulin ratio; ISF, insulin sensitivity factor; TIR, time-in-range.
Theme: fear of disapproval
Given that Loop was not FDA approved at the time of the study, the risk of provider disapproval and its consequences were of concern and identified as the reason for discontinuing by one parent.
Theme: technical and logistical barriers
Technical issues related to device connectivity, as well as component parts not necessarily specific to Loop, including issues with component parts of the insulin pump and continuous glucose monitor, were also reasons for discontinued use. Although technical issues with component parts were not caused by the Loop system, such issues did interfere with the operability and accuracy of the Loop system.
One participant noted cost as being a barrier to continued use. Although the Loop code is available at no cost, there are costs associated with acquiring specific insulin pumps and continuous glucose monitors, maintaining an Apple Developer account, and access to a computer capable of building a Loop app, all of which are necessary to build and use the Loop system. These devices and the accompanying supplies are not always covered by insurance and can be quite costly to purchase.
Specific circumstances or concerns
There were several reasons for discontinued use that were specific to participant's lifestyles, concerns, preferences, and personal contexts. For instance, some participants indicated that they discontinued Loop due to their desire to try out newer technologies that became available during the study, rather than any particular dissatisfaction with Loop itself.
Additional reasons included difficulties using Loop with exercise and with low insulin dose regimens. One participant expressed feeling that she could not “keep-up” with managing Loop and felt that continued use once she became pregnant was a “risk.” Another adult participant perceived an increased fear of hypoglycemia as a result of Loop use, due to its predictive algorithm suggesting steep declines in glucose levels or negative glucose projections (Table 3).
Discussion
Loop has emerged as a popular open-source automated insulin dosing system. It has been developed and driven by passionate teams with personal stakes in system success who are tired of waiting for comparable commercial solutions to be made available in the market. The vast majority of Loop users saw glycemic benefit and continued use.1 Our mixed-method approach revealed that, among people who discontinued Loop, mental and emotional burden as well as overall effort required to get the system optimized outweighed the perceived benefits.
Technical issues, user efforts, and mental and emotional strain are clearly highly interrelated factors that tend to play a role in many discontinuers' decisions to end their Loop use. Several users conveyed a strong sense of uncertainty about the underlying cause of settings or technical issues (the system, the user, or both) and their ability to resolve these issues with the resources at hand. Such uncertainty seemed to exacerbate the mental and emotional burdens of starting up the system and drove their decisions to discontinue use.
The current mixed-methods investigation suggests that increased clinical and technical support related to settings could be beneficial and potentially prevent undue frustration with settings adjustments. For instance, a participant shared “…And let's say that I thought the setting were pretty spot on. But I need settings change all the time anyway. It's like, oh, what's going wrong today?” Settings data from this participant revealed relatively high deviations in all three settings from the calculated (optimal) values, including the greatest discrepancy between actual and recommended ISF settings within the discontinuer sample.
Intriguingly, many users who discontinued still had a TIR within target, but this likely required more work to maintain when settings deviated from predicted. Increased support has the potential to alleviate these burdens and potentially remedy settings issues. Prior research on open-source systems has identified peer support as a major facilitator in system use as it fills, and at times exceeds, the role traditionally played by device companies.9
The factors associated with discontinuing Loop seem to vary from other systems. A previous study of the Medtronic 670G hybrid closed-loop system among youth living with T1D discovered that the primary reasons for discontinuing the system were related to the workload required for use.10 However, in this study, we found that issues with workload were largely tied to setting adjustments within Loop, as opposed to ongoing maintenance tasks such as installing system updates and new branches.
Another discrepancy between studies is that the 670G study found higher HbA1c to be associated with increased risk for discontinuing compared with lower HbA1c, whereas this study found no association between HbA1c and discontinued use. Several participants expressed concerns about difficulties with settings impacting glycemic outcomes. For instance, one participant described the experience of adjusting setting as “I could just never quite figure it out… I just couldn't get it to work for me and then I just got so frustrated because I know my A1c was moving up and I didn't want to move in that direction…” However, we did not find an association between deviations from recommended settings and TIR.
Conversely, some participants may have experienced glycemic benefits that may have fallen short of their own expectations. One participant explained “There are people who used to have A1c's in the 8's or 9's and now it was 6 and they are happy, but that is not what I want. I want it in the 5's.” Such cases may have counter-balanced out the discontinuers who did struggle with TIR and settings. Helping users set appropriate expectations, providing reassurance, emotional support, specific technical support, and recommendations for Loop settings may be the keys to Loop uptake and sustained use over time.
This study had several strengths including a relatively large sample under real-world circumstances and mixed- and multimethod data (include self-report, physiological, and setting measures). Study limitations include reliance on self-identification of discontinuer status, which is likely an underestimate. Although 5.3% of the current sample of 874 new and continued Loop users self-identified as discontinuers, 14% of new Loop users were found to stop providing data within the first 6 months of the study.1 Further research is needed to determine the actual discontinuation rates of Loop and how they compare with the rates (36%–50%) reported by studies of other systems.2,10
Furthermore, data on elapsed time from discontinuation and self-report of discontinuation as well as duration of Loop use before discontinuation were not available. Such information could provide helpful context for the current results. Another limitation is a homogeneous sample that largely represented white and high socioeconomic status populations. Although the study sample may reflect early adopters of Loop, future research will need to explore similarities and differences in user and discontinuer experiences across more diverse demographic groups. Lastly, the current findings would be strengthened by comparative studies that include samples using other “Do-It-Yourself” (DIY) and/or commercially available systems.
Conclusion
Our findings support the Loop system as well accepted by Loop users, with a small portion who reported discontinuation for a variety of reasons. Survey, interview, glycemic, and settings data all suggest that difficulties with settings optimization can prompt discontinuation and that deviations in settings from optimal settings may not be as tightly linked to glycemic outcomes and mental and emotional burden as one might expect. Nevertheless, increased technical support and expert guidance related to setting adjustments as well as user expectations would likely improve user experience and support sustained Loop use over time.
At the time of this writing, an international consensus article for providers on the use of open-source automated insulin delivery (AID) is under review. The optimized formulas for predicting system settings were presented at Advanced Technologies & Treatments for Diabetes (ATTD) 2021. Future studies evaluating the effects of increased support for Loop use can provide critical information on how to optimize Loop use and minimize diabetes burdens in the future.
Supplementary Material
Authors' Contributions
J.J.W. wrote and revised this article and contributed toward data collection, coding, analysis, interpretation, and revisions. D.N. contributed toward conceptualization, study design, data collection, analysis, interpretation, and revisions. S.C.S. contributed toward revision, data analysis, and interpretation. M.S.L. was involved in data collection, coding, and analysis. A.D. was involved in coding and analysis. B.A., S.J.H., J.W.L., R.J.B., and K.K.H. contributed toward study design, data collection, interpretation, and revisions. R.A.L. contributed toward conceptualization, system settings data analysis and interpretation, and revisions.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported by The Leona M. and Harry B. Helmsley Charitable Trust and NIH grant K23-DK121771 and was coordinated by Jaeb Center for Health Research.
Supplementary Material
References
- 1. Lum J, Bailey R, Barnes-Lomen V, et al. : A real-world prospective study of the safety and effectiveness of the loop open source automated insulin delivery system. Diabetes Technol Ther 2021;23:367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lal RA, Basina M, Maahs DM, et al. : One year clinical experience of the First Commercial Hybrid Closed-Loop System. Diabetes Care 2019;42:2190–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lal RA, Quinlan A, Desborough L, Nykaza E: Optimizing formulas for basal, carb ratio and sensitivity factor for predictive controllers: lessons learned from loop. Oral Presentation presented at the: advanced Technologies & Treatments for Diabetes. https://www.liebertpub.com/doi/full/10.1089/dia.2021.2525.abstracts (accessed November 18, 2021).
- 4. Fisher L, Glasgow RE, Mullan JT, et al. : Development of a brief diabetes distress screening instrument. Ann Fam Med 2008;6:246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fisher L, Hessler DM, Polonsky WH, Mullan J: When Is diabetes distress clinically meaningful?: establishing cut points for the Diabetes Distress Scale. Diabetes Care 2012;35:259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gonder-Frederick L, Nyer M, Shepard JA, et al. : Assessing fear of hypoglycemia in children with type 1 diabetes and their parents. Diabetes Manag Lond Engl 2011;1:627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gonder-Frederick LA, Schmidt KM, Vajda KA, et al. : Psychometric properties of the Hypoglycemia Fear Survey-II for adults with type 1 diabetes. Diabetes Care 2011;34:801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tanenbaum ML, Hanes SJ, Miller KM, et al. : Diabetes device use in adults with type 1 diabetes: barriers to uptake and potential intervention targets. Diabetes Care 2017;40:181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schipp J, Skinner T, Holloway E, et al. : How adults with type 1 diabetes are navigating the challenges of Open-Source Artificial Pancreas Systems: a Qualitative Study. Diabetes Technol Ther 2021;23:546–554. [DOI] [PubMed] [Google Scholar]
- 10. Messer LH, Berget C, Vigers T, et al. : Real world hybrid closed-loop discontinuation: predictors and perceptions of youth discontinuing the 670G system in the first 6 months. Pediatr Diabetes 2020;21:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.