Abstract
Significance:
Neutrophil behavior and function are altered by hyperglycemia associated with diabetes. Aberrant activation by hyperglycemia causes neutrophils to respond with increased production of reactive oxidative species (ROS). Excess ROS, a signature of primed neutrophils, can intracellularly induce neutrophils to undergo NETosis, flooding surrounding tissues with ROS and damage-associated molecular patterns such as S100 calcium binding proteins (S100A8/A9). The cargo associated with NETosis also attracts more immune cells to the site and signals for increased immune cell production. This inflammatory response to diabetes can accelerate other associated conditions such as atherosclerosis and thrombosis, increasing the risk of cardiovascular disease.
Recent Advances:
As the prevalence of diabetes continues to grow, more attention has been focused on developing effective treatment options. Currently, glucose-lowering medications and insulin injections are the most widely utilized treatments. As the disease progresses, medications are usually stacked to maintain glucose at desired target levels, but this approach often fails and does not effectively reduce cardiovascular risk, even with the latest drugs.
Critical Issues:
Despite advances in treatment options, diabetes remains a progressive disease as glucose lowering alone has failed to abolish the associated cardiovascular complications.
Future Directions:
Significant interest is being generated in developing treatments that do not solely focus on glucose control but rather mitigate glucotoxicity. Several therapies have been proposed that target cellular dysfunction downstream of hyperglycemia, such as using antioxidants to scavenge ROS, inhibiting ROS production from NOX, and suppressing neutrophil release of S100A8/A9 proteins. Antioxid. Redox Signal. 36, 652–666.
Keywords: diabetes, cardiovascular disease, DAMPS, oxidative stress, neutrophils, hyperglycemia
Introduction
Diabetes is a major risk factor for cardiovascular disease (CVD), which is driven by low-grade chronic inflammation and accelerated atherosclerosis (21, 41). Increased leukocyte count seen in diabetes is a major risk factor for coronary artery disease (52) and acute coronary syndrome (ACS) (72). In recent years, through many elegant preclinical studies, diabetes is recharacterized from a primarily metabolic disease to an inflammatory and immune-mediated disease.
Two major forms of diabetes exist, type 1 and type 2, with the latter being the most common, making up nearly 95% of diabetic individuals (1). The pancreas of individuals with type 1 diabetes is targeted by the immune system, destroying the beta cells that produce insulin, consequentially preventing cells from processing glucose normally. Individuals with type 2 diabetes develop hyperglycemia as a culmination of genetic predispositions, and environmental factors that lead to insulin resistance, hyperinsulinemia, and eventually reduced insulin production. Both types are subject to strikingly similar complications arising from hyperglycemia. However, glucose control alone may not be enough to reinstate a normal inflammatory environment in either type 1 or type 2 diabetes. For example, transient intermittent hyperglycemia (TIH) associated with regular high carbohydrate meals has the ability to sustain inflammation (21).
With the introduction of several continuous glucose monitoring devices, the occurrence and the impact of TIH are increasingly appreciated (40). Diabetic individuals who have relatively well-controlled glucose levels can continue to experience detrimental TIH, especially in the postprandial phase or when short-acting medication or insulin is ineffective or inadequate (21, 41). Despite ambient glucose levels being lowered overall, TIH spikes put individuals with insulin resistance at a greater risk of CVD (21). Exposure to TIH, even as brief as a few hours and uncapturable by HbA1c values (25), is associated with enhanced myelopoiesis, increased plasma levels of S100A8/A9, and atherosclerotic macrophage burden (21).
The pathways through which diabetes elicits an innate immune response are yet to be fully characterized, but numerous studies have identified that many of the complications associated with diabetes can be attributed to oxidative stress (8, 27, 60). Phagocytic immune cells such as neutrophils, eosinophils, monocytes, and macrophages produce large quantities of reactive oxidative species (ROS) and free radicals when activated. Glucose uptake is essential for phagocytes to produce ROS and carry out key immune functions (42). Hyperglycemia can cause dysregulation of metabolic processes and detrimentally increase ROS production in all these cells, but neutrophils are disproportionately affected due to their metabolic inflexibility (21).
It is important to note that studies characterizing neutrophil function in hyperglycemic conditions have produced conflicting reports, with some experiments showing both decreased neutrophil activity and ROS production, and others showing the opposite. One study delved into this phenomenon and found that glucose concentrations of 12 mM were sufficient to strongly activate neutrophils for a few hours, followed by a steady decline in neutrophil activation (42), which pairs well with the finding that acute glucose fluctuations induce oxidative stress, whereas chronic hyperglycemia does not (49). In addition, some studies suggest that chronic exposure of neutrophils to consistently high glucose levels can eventually induce a fatigued-like state in the cells, resulting in an attenuated immune response (42). This decrease in neutrophil immune activity could contribute to the higher incidence of infections seen in both type 1 and 2 diabetic patients with uncontrolled glucose levels (12). Paradoxically, patients with better controlled glucose levels who experience TIH may maintain their neutrophils in a perpetually active state.
Activation of neutrophils by diabetic hyperglycemia elicits an inflammatory immune response (6, 21, 65) through oxidative bursts and release of damage-associated molecular patterns (DAMPs) (52), notably S100A8/A9. Further investigation must be devoted to understanding this observation fully, as valuable therapeutic insights can still be gathered. Since glucose fluctuations are largely unavoidable, and can rapidly elicit detrimental immune responses, it may be more effective to develop therapeutics that target downstream meditators of hyperglycemia, for use in conjunction with glucose control treatments. Antioxidant-based therapies have seen some success in clinical applications, and drugs targeting S100A8/A9 release and signaling have shown promise in preclinical studies, but the full potential of targeting oxidative stress and its downstream pathways requires additional attention. In this review, we focus on the ROS pathways, specifically in neutrophils, that could be potentially exploited to target diabetes-associated CVD.
Hyperglycemia Causes Neutrophil-Mediated Oxidative Stress
Neutrophil-mediated oxidative stress affects several organ systems. The macrovasculature and microvasculature are both affected by ROS (60). Promoting a vicious feed-forward loop, the pancreas is one of the most sensitive tissues in the body to oxidative assault (58). Studies have also found a relationship between excess ROS and insulin resistance in type 2 diabetes (60). Hyperglycemic conditions associated with diabetes create an environment that enhances neutrophil ROS levels and oxidative bursts (6). It has been observed that oxidative damage begins when the cell's natural antioxidant defense systems are overwhelmed and decreased by the flux of oxidative species being produced (60).
Neutrophils metabolize primarily through glycolysis, making them particularly sensitive to glucose levels (41). Unable to downregulate glucose transporter-1 (GLUT-1) during hyperglycemia, neutrophils will continue to increase metabolism as more glucose becomes available, until they reach a maximum glycolytic rate (21). As neutrophils increase metabolism in response to hyperglycemia, production of ROS as a by-product through the mitochondrial electron transport chain increases. Mitochondrial overproduction of ROS is thought to be the initiator of several dysregulated ROS-mediated pathways (66). This is supported by the experimental observation of endothelial cells lacking mitochondrial function failing to increase ROS production in response to high glucose exposure (25). DNA damage caused by excess ROS activates repair mechanisms that decrease the activity of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). As an essential glycolytic enzyme, GAPDH inhibition leads to a buildup of glycolytic intermediates upstream, that divert into other cellular pathways and further contribute to ROS formation (24, 66, 88).
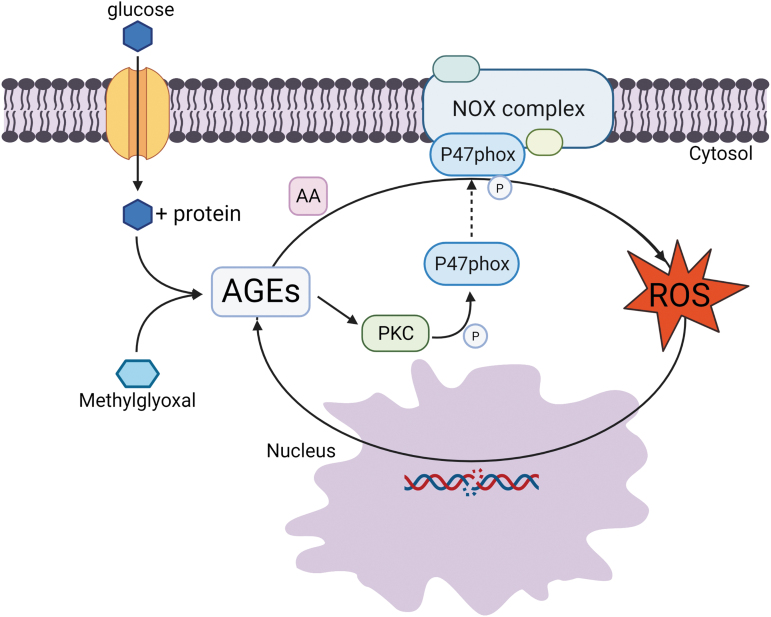
Glucose itself has the ability to auto-oxidate, which produces damage causing oxidative agents (60). Excess intracellular glucose and glycolytic intermediates lead to the accumulation of higher levels of advanced glycolytic end products (AGEs) (7, 24). Of these, methylglyoxal has been deemed the most reactive glycolytic by-product and the major source of AGEs (63). Methylglyoxal and AGEs mediate the generation of ROS, mainly through upregulating the NADPH oxidase (NOX). It has been suggested that AGEs can also contribute to enhanced protein kinase C (PKC) activity (24, 60). AGEs and PKCs both modulate the activity of the adaptor protein, p47-phox, that facilitates the assembly of NOX (27, 31). This is achieved by PKC phosphorylating several p47-phox serine residues located in the C-terminus (31). AGEs have been shown to induce arachidonic acid (AA), which interacts with p47-phox to enhance its activity (27), creating a feed-forward loop, augmenting ROS production (31). This feed-forward loop is further perpetuated as ROS can increase production of AGEs (60) through DNA damage as previously stated (Fig. 1). In addition, methylglyoxal can directly modify DNA, leading to formation of the modification N2-(1-carboxyethyl)-2′-deoxyguanosine (CEdG) (75), and has been linked to the activity of the histone-tail modifying enzyme SET7 (18). Therefore, the glycation pathways likely have durable effects, through long-lived protein modifications, as well as epigenetic pathways. Furthermore, the increased utilization of NADPH for the generation of ROS through the polyol pathway causes a shortage of the ROS scavenger, reduced glutathione (GSH), contributing to oxidative overload stress, with an inability of the cell to detoxify (24). As ROS production spirals out of control in the cell, oxidation damage to proteins can become prevalent enough to cause cellular function dysregulation, antioxidant overwhelm, and eventual cell death.
FIG. 1.
ROS and AGE feedback loop through NOX. ROS and AGEs can form a feedback loop, initiated by glucose and glycolytic intermediates forming AGEs, which activate the NADPH oxidase complex (NOX complex) through PKC and AA. PKC, activated by AGEs, phosphorylates the P47phox subunit of NOX, allowing for assembly of the complex. AGEs then shuttle AA to P47phox, increasing the activity of the complex. Excess ROS created by this complex cause DNA damage in the nucleus that leads to a buildup of glycolytic intermediates upstream that then perpetuate the cycle by their conversion into AGEs. AA, arachidonic acid; AGEs, advanced glycolytic end products; NOX, NADPH oxidase; PKC, protein kinase C; ROS, reactive oxidative species. Color images are available online.
Accumulation of ROS is also thought to contribute to the phenomenon of innate training, a concept in which innate immune cells undergo epigenetic changes upon a challenge with microbial or metabolic triggers, altering their propensity to react to certain stimuli. This mechanism has likely evolved to reduce the risk of reinfections, and one of the best-known inducers of innate training is the BCG vaccine that not only offers partial protection against tuberculosis but also against influenza infection (53). A similar phenomenon may occur in the setting of atherosclerosis (20, 28), where prior exposure of cultured macrophages to oxidized low-density lipoproteins (ox-LDL) induces innate training in a mechanistic target of rapamycin and ROS-dependent pathway, inducing epigenetic changes that lead to increased cytokine production 5 days after initial ox-LDL challenge (69). In addition to inflammatory reprogramming, metabolic reconfiguration is a hallmark of innate training. Especially, activation of glycolytic pathways is a defining hallmark of the trained monocyte/macrophage in the setting of atherosclerosis (69). In particular, the rate limiting glycolytic enzyme pyruvate kinase M2 boosts the production of cytokines, the signal transducer and activator of transcription 3 (STAT3) (67). The interaction of this emerging mechanism with hyperglycemia constitutes a major knowledge gap.
The role of glucose-induced S100A8/A9 secretion may also be of interest, as the release of massive amounts of S100A8/A9 in the newborn may play a role in the counter-regulatory tolerance that prevents hyperinflammation. At least in neonates, a transient excess production of S100A8/A9 prevents hyperinflammation associated with bacterial infection (78), but this response does likely not translate to the setting of the hyperglycemic adult.
Oxidative Stress Induces Neutrophils to Upregulate Production and Release of S100A8/A9
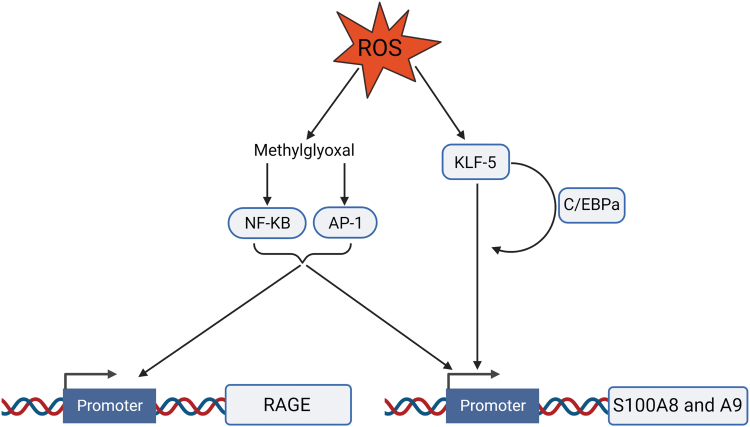
Neutrophils are the main source of the serum pool of S100A8/A9 in diabetes (21). Oxidative stress stemming from hyperglycemia can enhance neutrophil gene expression and secretion of S100A8 and S100A9 (24, 41). Methylglyoxal, an AGE precursor produced by ROS (24), increases redox-sensitive transcription factors activator protein 1 (AP-1) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) binding to the promoters of S100A8/A9 and receptor for advanced glycation end products (RAGE), respectively, upregulating their expression (66, 88). Experimental data have shown that overexpression of proteins that prevent hyperglycemia-stimulated mitochondrial production of ROS abrogated the upregulation of S100A8/A9 and RAGE (88). ROS induce expression of the transcription factor Kruppel-like factor 5 (KLF-5) that transactivates the promoters of S100A8 and S100A9 (23). KFL-5 also regulates CCAAT/enhancer-binding protein α (C/EBPα) expression and, together, these two factors interact to synergistically promote S100A8 and S100A9 expression (23) (Fig. 2).
FIG. 2.
ROS upregulate S100A8/A9 and RAGE expression. Through the production of methylglyoxal, ROS induce the redox-sensitive transcription factors, NF-κB and AP-1, to bind to the promoters of RAGE and S100A8/A9. ROS induce the expression of the transcription factor KLF-5, which signals to and synergizes with C/EBPa to transactivate S100A8/A9. AP-1, activator protein 1; KLF-5, Kruppel-like factor 5; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; RAGE, receptor for advanced glycation end products. Color images are available online.
Despite the well-known robust role for S100A8/A9 in the immune response to diabetes and other inflammatory diseases, the precise molecular mechanisms that trigger its secretion/release from neutrophils have remained somewhat elusive. It is established that S100A8/A9 is not secreted via the classical ER-Golgi pathway, due the lack of a leader sequence (74). Stimulation of neutrophils with phorbol myristate acetate (PMA), a potent NETosis inducer, has been shown to promote a robust secretion of S100A8/A9 (36, 64, 74). One of the studies found that the release of neutrophil extracellular traps (NETs), signatures of NETosis, correlates with the secretion of S100A8/A9 from PMA-stimulated neutrophils. To further investigate this relationship, neutrophils were treated with N-formyl-met-leu-phe (FMLP), an exocytosis stimulant that does not induce NET formation. In the absence of NET formation, S100A8/A9 secretion failed (64), strengthening the connection between NETosis and S100A8/A9 release. To link ROS production with NETosis and S100A8/A9 secretion, the study inhibited NOX activation in neutrophils using diphenyleneiodonium before stimulation with PMA. As a result, both NET formation and S100A8/A9 secretion were abolished (64), demonstrating the essential nature of ROS production in NOX-dependent NETosis (35) and S100A8/A9 release. Other studies have also explored this relationship through the lens of hyperglycemia, as shown by glucose-challenged neutrophils treated with an ROS scavenger and an NOX inhibitor, respectively, failing to release S100A8/A9 (21).
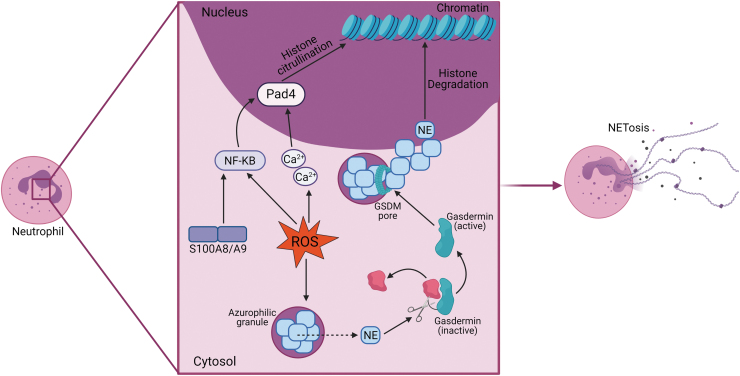
A possible mechanism by which ROS mediate neutrophil S100A8/A9 release could be through its important role in NET formation (16). ROS are known to mediate the movement of neutrophil elastase, a serine protease essential for NOX-dependent NETosis (55), from the azurophilic granules of neutrophils (10, 70) via activating the azurosome (35). Outside of the granules, elastase can cleave gasdermin-D (GSDMD) to form N-GSDM that localizes to and creates pores in azurophilic granules, releasing the contents into the neutrophil cytosol (32). As one of the most abundant proteins in azurophilic granules, the massive influx of elastase begins histone degradation, resulting in chromatin decondensation to form NETs (31, 32). Excess ROS accelerates elastase transportation (70), expediting NET formation consequentially. Adding to the powerful role in NETosis, ROS can influence the alternative NOX-independent NETosis pathway through indirect signaling on peptidyl arginine deiminase 4 (PAD4) (66). This protein, necessary for NOX-independent NETosis (37) in response to sterile inflammation, is upregulated in neutrophils in diabetes (77, 85). Both ROS and S100A8/A9 can activate NF-κB, which promotes the expression of PAD4, and ROS are capable of increasing intracellular calcium levels that activate PAD4 (66). When activated, PAD4 deiminates arginine residues on histones (37), reducing their positive charge and affinity to DNA. This leads to the same result as the NOX-dependent pathway—chromatin decondensation and the formation of NETs in anticipation of NETosis (Fig. 3).
FIG. 3.
ROS stimulate NETosis. ROS in the neutrophil stimulate NETosis through both the NOX-dependent and NOX-independent pathways. Through the NOX-dependent pathway, ROS allow for the leakage of NE from azurophilic granules. Free from the granule, NE is free to cleave GSDM into its active form. Active GSDM forms pores in the azurophilic granules, releasing the contents into the cytosol, including NE. NE then migrates to the nuclease and degrades histones. ROS and S100A8/A9 indirectly stimulate NOX-independent NETosis through activating PAD4 through NF-κB. ROS also increase intracellular calcium, which also activates PAD4. PAD4 citrullinates arginine residues on histone tails, causing loosened DNA binding. Both pathways lead to chromatin decondensation and eventual NETosis. GSDM, gasdermin; NE, neutrophil elastase; PAD4, peptidyl arginine deiminase 4. Color images are available online.
Neutrophil release of S100A8/A9, stimulated by oxidative stress, recruits immune cells to the source of the signaling (62, 72, 74). This gives rise to an additional pathway for the release of S100A8/A9 from migrating neutrophils, via the so-called elongated neutrophil-derived structures (ENDS). Attracted by chemokine signaling to the site of acute inflammation, neutrophils roll along vessel walls using tethers to eventually arrest on the endothelium and subsequently extravasate. Some of these tethers break during the rolling process, forming ENDS. Interestingly, high levels of S100A8/A9 protein are present in ENDS and upon their breakdown, S100A8/A9 is released (47). ENDS were also found to contain myeloid leukocyte activation and glycolytic metabolism pathway proteins (47). While not experimentally examined, ENDS are another potential mechanism for local S100A8/A9 release in diabetes, as neutrophils are activated and tether along the activated endothelial cells. This mechanism may be more important for localized enrichment of S100A8/A9 to act as a further chemoattractant signal, amplifying sterile inflammation, and thus warrants further investigation.
S100A8/A9 Initiates an Inflammatory Immune Response on Its Release in Diabetes
S100A8/A9 demonstrates the ability to elicit the responses of various immune cell types. Having a complex role in immunity, S100A8/A9 acts in both a cell-type autocrine and paracrine manner (62). Being the most abundant cytosolic proteins found in neutrophils (74) and interacting with numerous neutrophil components intracellularly and extracellularly during activation, S100A8/A9 is suggested to have dominant effects in these cells. S100A8/A9 has been observed to attract and sequester neutrophils (and other leukocytes via alternative pathways) in a concentration gradient-dependent manner (45, 62). At lower concentrations, S100A8/A9 can act as a specific chemoattractant to neutrophils, by inducing the shedding of L-selectin (62, 74), however, at higher concentrations, S100A8/A9 induces neutrophil adhesion to fibrinogen through macrophage-1 antigen (Mac-1) (84), contributing to neutrophil retention at the site of inflammation. S100A8/A9 also regulates the polymerization and organization of microtubules in the presence of high calcium levels (26), which aids in facilitating neutrophil migration (74, 81). This reorganization of the cytoskeleton additionally aids phagocytic functions (84). S100A8/A9 contributes to neutrophil oxidative bursts as it can have a potentiating effect on ROS formation due to its binding of NOX components (45, 62, 73). This, in turn, increases its own secretion from the neutrophil.
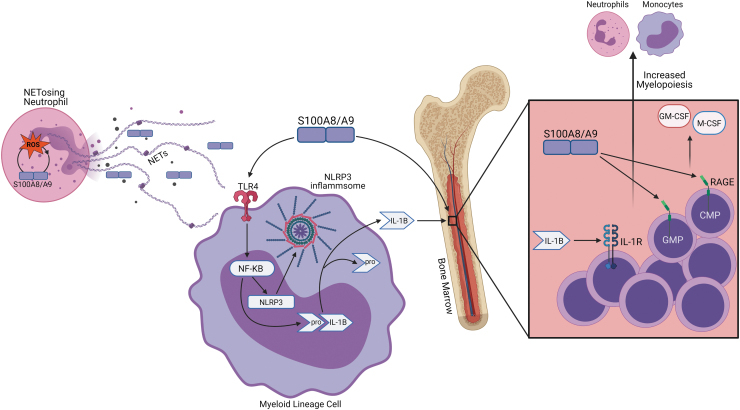
Upon release, S100A8/A9 interacts with its innate immune receptors, toll-like receptor 4 (TLR4) and RAGE (74). Expressed on endothelial cells and hematopoietic cells (21), RAGE is a promiscuous receptor that can bind to multiple DAMPs (41) and its expression is increased in diabetes by ROS (88). S100A8/A9-mediated RAGE signaling drives myelopoiesis in diabetes (21), contributing to leukocytosis (52). S100A8/A9 in the plasma interacts with RAGE on common myeloid progenitors (CMPs) and granulocyte macrophage progenitors (GMPs) in the bone marrow. This interaction activates NF-κB signaling to promote the secretion of monocyte colony stimulating factor and granulocyte macrophage colony stimulating factor, which enhances myelopoiesis of GMPs and CMPs (74), increasing neutrophil and monocyte numbers in the circulation (73) (Fig. 4).
FIG. 4.
S100A8/A9 signaling promotes myelopoiesis. S100A8/A9 released from NETosis interacts with its innate immune receptors RAGE and TLR4 on myeloid progenitor cells. S100A8/A9 signaling through TLR4 on myeloid lineage cells causes NF-κB to promote the expression of NLRP3 and pro-IL-1β. After NLRP3 inflammasome assembly, pro-IL-1β is cleaved to its mature form and secreted. Upon secretion IL-1β interacts with IL-1R on stem cells in the bone marrow. S100A8/A9 can directly signal through RAGE on GMPs and CMPs in the bone marrow to release GM-CSF and M-CSF. CMPs, common myeloid progenitors; GM-CSF, granulocyte macrophage colony stimulating factor; GMPs, granulocyte macrophage progenitors; IL-1β, interleukin 1 beta; IL-1R, interleukin-1 receptor; M-CSF, monocyte colony stimulating factor; NETs, neutrophil extracellular traps; NLRP3, NLR family pyrin domain containing 3; TLR4, toll-like receptor 4. Color images are available online.
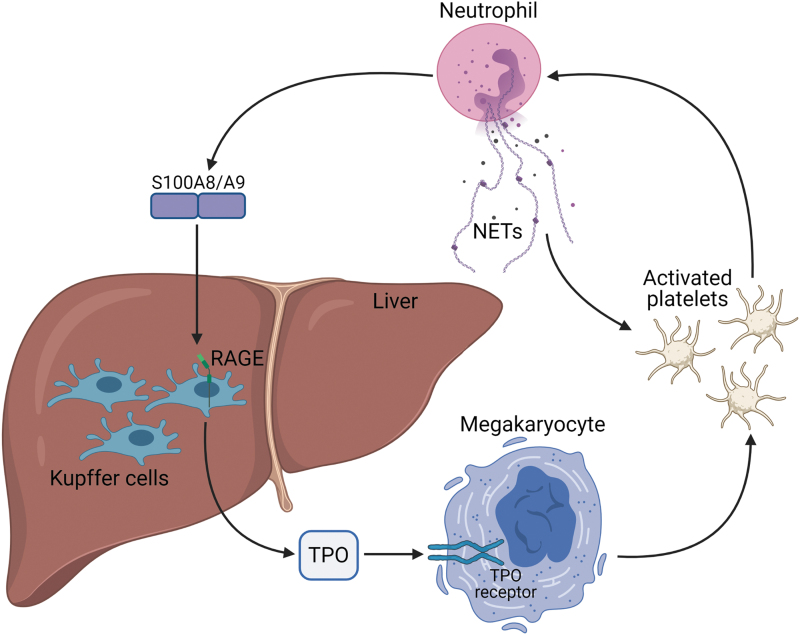
S100A8/A9-RAGE signaling axis also has a direct role in platelet production in diabetes (41). S100A8/A9 signals through RAGE on Kupffer cells in the liver to stimulate thrombopoietin (TPO) production (41, 44). TPO promotes the proliferation of megakaryocytes and bone marrow progenitor cells, giving rise to younger, more reactive platelets that are resistant to standard antiplatelet treatments in diabetic patients (44) (Fig. 5).
FIG. 5.
Neutrophils and platelets participate in an activation loop. Activated neutrophils release S100A8/A9, which then signals through RAGE on Kupffer cells in the liver for increased TPO production. TPO interacts with its receptors on megakaryocytes to upregulate platelet production. These platelets can be activated by NETs, and in turn, the platelets activate neutrophils, perpetuating the cycle. TPO, thrombopoietin. Color images are available online.
Cardiac fibroblasts (CFs), an abundant cell type in the heart (86) and one of the major mediators of diabetic cardiac fibrosis (61), are highly influenced by S100A8/9 via RAGE signaling (86). S100A8/A9 has been found to be chemotactic to CFs and can modulate the expression of over 200 CF genes, including stimulating chemokines and proinflammatory cytokines (86). S100A8/A9 activation of CFs through RAGE initiates angiotensin II-induced cardiac inflammation (86), part of the major inflammatory and fibrotic renin/angiotensin/aldosterone system (RAAS) that has been found to be upregulated in diabetes (9). RAAS activation was shown to increase RAGE ligand levels, including S100A8/A9 (56), initiating a positive feedback cycle. Normally, RAGE plays a role in acute inflammation, but persistent increased exposure to endogenous ligands, such as S100A8/A9, can cause chronic RAGE signaling, which can contribute to the development of diabetes-associated diseases (88).
S100A8/A9-TLR4 signaling contributes to diabetes-associated inflammation via the NLR family pyrin domain containing 3 (NLRP3) inflammasome in myeloid lineage cells. TLR4 expression can be induced by hyperglycemia through PKC interaction with NADPH-oxidase (3, 14, 83). S100A8/A9 and TLR4 exert control over the NLRP3 inflammasome through NF-κB (22, 59, 68), a redox-sensitive transcription factor that modulates the expression of a plethora of genes involved in inflammation (19). NF-κB primes the inflammasome by inducing the expression of NLRP3 and pro-interleukin 1 beta (IL-1β) (15). Classically, the inflammasome requires a second signal to assume full functionality through the assembly of the inflammasome complex; however, recent studies have demonstrated that TLR4 signaling can activate the NLRP3 inflammasome without the need for secondary stimuli (15). Initiation of the inflammasome complex assembly, which facilitates caspase-1 cleavage of pro-1L-1β to its mature form, allows for the subsequent secretion of this inflammatory cytokine. In the setting of obesity and insulin resistance, IL-1β release from myeloid cells in the adipose tissue interacts with the IL-1R1 in the bone marrow to induce myelopoiesis (72, 73) (Fig. 4). S100A8/9-induced signaling pathways and self-perpetuating activity can produce severe immune responses in the vasculature. Persistent immune activity leads to cardiovascular dysfunction and eventual disease.
Oxidative Stress and S100A8/9 Are Major Contributors to Diabetic Cardiovascular Complications
As ROS and S100A8/A9 are released through oxidative bursts and neutrophil death, they inflict oxidative stress and damage on and recruit immune cells to the vascular endothelium, leading to the formation of diabetes-associated cardiovascular complications. S100A8/A9 has been found to be a biomarker for predicting cardiovascular events as high levels of this protein correlate with atherosclerotic plaque rupture, platelet aggregation, and thrombosis (8, 13).
S100A8/A9 activates the endothelium, leading to cytokine/chemokine production, adhesion molecule expression (17), and increased endothelial platelet aggregation (65). S100A8/A9 signaling through RAGE and TLR4 has been implicated as contributing to the development of atherosclerosis (5). Both receptors exhibit atherogenic properties and deficiency in either of these receptors was found to slow the progression of atherosclerotic plaque formation (65). Several studies have linked the vascular and hematopoietic RAGE signaling axis and S100A8/A9 expression in diabetes to increases in the expression of adhesion molecules (21, 39, 65, 87). Both ROS and S100A8/A9 are known to increase the endothelial expression of intracellular adhesion molecule 1 (ICAM-1) and vascular adhesion molecule 1 (VCAM-1) (65, 76). S100A8/A9-mediated platelet production amplifies platelet/leukocyte interactions, found to activate the leukocytes and increase expression of ICAM-1 binding receptor Mac-1 (44). This is potentiated by S100A8/A9 augmenting leukocyte Mac-1 and ICAM-1 binding through a TLR4-mediated pathway (84). As the initiating step in atherogenesis (33), expression of these adhesion molecules slows leukocyte rolling and eventually leads to leukostasis (84, 87).
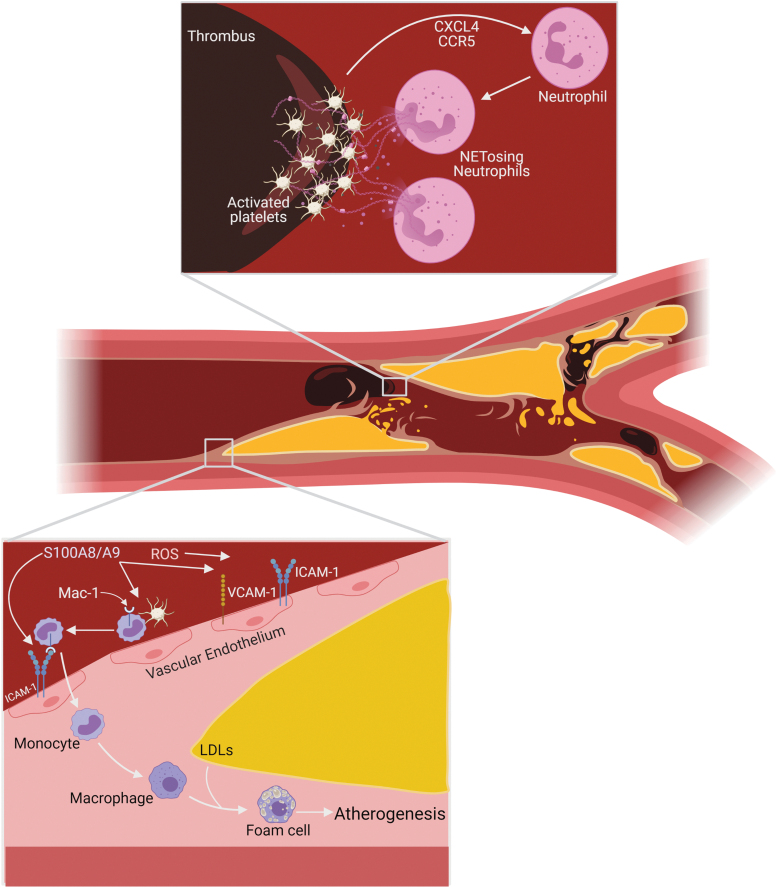
S100A8/A9, in a dose-dependent manner, can disrupt the monolayer integrity of the endothelium and increase its permeability (80), facilitating increased immune cell infiltration. ROS-induced PKC activation has also been linked to increased vascular endothelial permeability (66). Following extravasation through the vascular endothelium to the interior of the arterial wall, inflammatory leukocytes, specifically Ly6-Chi monocytes, mature into macrophages that are transformed into foam cells through the uptake of ox-LDL (22, 33), formed by ROS (34), and begin the atherogenic process. Interestingly, there are a higher percentage of S100A8/A9-positive macrophages in rupture-prone lesions than in stable lesions (29). Neutrophil infiltration in the atherogenic lesion can give rise to thrombogenic activity via NET release (5) and has been implicated as a factor in plaque rupture (16). Neutrophils and platelets in thrombosis are engaged in a vicious feedback cycle. Neutrophil NETs can activate platelets and these platelets, in turn, can activate neutrophils (43). Increased activated platelet count in diabetic individuals is correlated with thrombosis and accelerated atherosclerosis (44). Platelet-derived chemokine (C-X-C motif) ligand 4 (CXCL4) and C-C chemokine receptor type 5 (CCR5) can activate neutrophils to induce entry into the lesions (51) and NET release, explaining the presence of NETs in luminal atherosclerotic plaques (16) (Fig. 6). Upon further investigation, NETs were found to localize around apoptotic endothelial and smooth-muscle cell clusters in the lesions (16). Interestingly, excessive oxidative stress has been found to induce vascular smooth muscle cell apoptosis, which has been suggested as an avenue to plaque instability and rupture (19).
FIG. 6.
S100A8/A9, ROS, and NETs contribute to atherosclerosis and thrombosis. S100A8/A9 increases the expression of ICAM-1 and VCAM-1 on the vascular endothelium. S100A8/A9-augmented platelets interact with leukocytes to activate them and induce expression of the ICAM-1 binding molecule Mac-1. S100A8/A9 through a TLR4-mediated pathway can increase Mac-1 and ICAM-1 binding. Once bound the leukocytes can extravasate through the endothelium that has been made more permeable by ROS and S100A8/A9. The leukocytes, specifically monocytes, mature into macrophages and then foam cells through the uptake of low-density lipoproteins, leading to atherogenesis. Neutrophil NETs contribute to thrombogenesis through sequestering platelets in the DNA, aiding in clot formation. The platelets, activated by NETs, secrete CXCL4 and CCR5, activating more neutrophils and encouraging NETosis. CCR5, C-C chemokine receptor type 5; CXCL4, chemokine (C-X-C motif) ligand 4; ICAM-1, intracellular adhesion molecule 1; LDLs, low-density lipoproteins; Mac-1, macrophage-1 antigen; VCAM-1, vascular adhesion molecule 1. Color images are available online.
S100A8/A9 is not normally expressed in nonmyeloid cells, but under inflammatory conditions, such as that found in atherosclerosis, it has been found that S100A8/A9 expression can be induced in cardiovascular cells (5) and in endothelial cells after exposure to IL-1β (4). S100A8/A9 has been implicated as a key granulopoiesis stimulating molecule postmyocardial infarction (72). Amplification of myocardial inflammation by S100A8/A9 secreted from neutrophils and monocytes (74) in response to ischemia/reperfusion injury facilitates myocardial remodeling and heart failure (65). Platelets and megakaryocytes have also been suggested as a possible source of S100A8/A9 in ACS (50). In the setting of myocardial infarction (MI), monocytes are particularly sensitive to S100A8/A9-induced TLR4 upregulation and secrete increased amounts of cytokines up to 14 days post-MI (65). This if found to correlate with the development of heart failure (65).
S100A8/A9-RAGE signaling in cardiomyocytes results in decreased contractility through diminished calcium flux in the cells (74). S100A8/A9 also induces death in cardiomyocytes through facilitating mitochondrial respiratory dysfunction in these cells (74). Furthermore, S100A8/A9-RAGE signaling contributes to post-MI heart failure development through the phosphorylation of mitogen-activated protein kinases, which are implicated as having a crucial role in this process (82). Ablation of RAGE in mice provides protection from MI-induced heart failure via decreased phosphorylation of kinases (82), demonstrating the important role that S100A8/A9-RAGE signaling plays in mediating inflammatory cellular processes in the postischemic heart. Reduction of serum S100A8/A9 levels is a potential therapeutic avenue to reduce the severity of cardiac complications as elevated S100A8/A9 levels have been found in conditions, such as ACS, coronary and cardiac atherosclerosis, and associated thrombosis.
Therapies Based on Antioxidants, ROS, and S100A8/A9 and Associated Pathway Inhibitors
Several new therapies have emerged recently that focus on targeting ROS and S100A8/A9 and its receptors/associated pathways in diabetes. Studies have looked into balancing the increased ROS production in diabetes with antioxidants. They suggest that preventing ROS-induced damage to beta cells in the pancreas with antioxidant therapy can prevent insulin resistance in both types of diabetes (60). Clinical studies have identified two antioxidants, α-lipoic acid and RRR-α-tocopherol, that have been promising in the treatment of diabetes and in the prevention of atherosclerosis (60). Possible limitations to the practicality of these antioxidant-based treatments arise from the fact that they react with ROS on a one-to-one basis, while ROS production is a continuous process (24), which would rapidly deplete the antioxidants. As a possible solution, a therapy has been proposed involving treatment with a superoxide dismutase/catalase mimetic, which prevents ROS inactivation of critical antiatherosclerosis enzymes and the subsequent activation of damaging metabolic pathways in diabetes (24). Clinical assessment of this treatment is still required. Inhibition of oxidative bursts from neutrophils has been explored using pyrazolone derivatives. It was found that aminopyrine and dipyrone prevent PMA-induced neutrophil bursts, but can contribute to agranulocytosis (11). The important signaling functions of ROS under normal physiological conditions imply that aggressive antioxidant and ROS scavenging based therapies should be approached with caution, as abolishing oxidizing action indiscriminately can be detrimental to cellular functions (76).
Direct NOX-inhibiting drugs have been studied in mice modeling diabetes-associated macrovascular disease with some success. GKT137831, a semispecific inhibitor of NOX, NOX1 and 4 specifically, has been shown to attenuate diabetes-induced atherosclerotic plaque progression at the onset of diabetes, and has been found to have the same effect, at a lower dosage, in established diabetes (79). A lower dosage of GKT137831 than was used in the early-onset model was required in the established diabetes model to halt atherosclerotic plaque progression. This difference in dosage effectiveness is speculated to stem from the differing functions NOX1 and NOX4 carry out in diabetes-associated atherosclerosis, with NOX1 having detrimental effects on plaque progression as opposed to the protective NOX4 activity (79). It should be stressed that developing isoform-specific NOX inhibitors is needed to selectively abolish the detrimental effects certain NOX isoforms confer in diabetes-related macrovascular disease, while the maintaining activity of the protective isoforms (79).
Alternatively, to or in conjunction with other therapies, the signaling mediators that drive both the upstream and downstream effects of S100A8/A9 are potential candidates for the risk reduction of diabetic CVD. Multiple studies have examined the efficacy of inhibiting RAGE activation in a plethora of disease models, including diabetes-related CVDs (56). RAGE signaling is postulated to be an outlet through which local RAAS activity exerts proinflammatory effects (56). An existing cross talk between the AGE/RAGE axis and RAAS has been shown to contribute to atherosclerotic plaque instability (30). In diabetic Rage−/−Apoe−/− mice, upregulation of NOX subunits was abolished, and atherosclerotic plaque size was reduced (71), showing that inhibition of RAGE signaling can produce protective effects, especially for the diabetic heart. In a recent study, inhibition of RAGE with a targeted small molecule capitulated similar results to RAAS blockade [decreased stroke, myocardial infarction, and death associated with diabetes (9)] with no effect on blood pressure, reigniting the idea that RAGE could be a therapy to reduce the risk of vascular complications in diabetes (56).
Targeting the NLRP3 inflammasome has shown potential as a therapeutic inhibitor of cardiac damage in diseases that present excess inflammation. As inflammation is a vital part of the immune system, inhibition of all inflammasome activity would prove detrimental, and therefore, the discovery of NLRP3 inflammasome-specific pharmaceutical agents is key to this therapy option. Targeted inhibition of several NLRP3 inflammasome components has been investigated in CVD, such as blockage of inflammasome assembly, inhibition of caspase-1, inhibition of NT-GSDMD-mediated pore formation, and neutralization of IL-1β (48). One such pharmaceutical agent studied in preclinical models of inflammatory diseases, OLT1177, selectively targets the NLRP3 inflammasome and has shown to be effective in numerous inhibitory activities, including inhibiting NLRP3 oligomerization, NLRP3-ASC interaction and thus inhibition of downstream activation, and release of IL-1β (48). Some studies have delved into exploring the use of anti-inflammatory compounds as potential treatments for cardiovascular complications stemming from inflammasome activity. Indeed, the recently concluded clinical trials, Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) and Low Dose Colchicine for Secondary Prevention of Cardiovascular Disease (LoDoCo), provide proof of principle that treatments involving anti-inflammatory compounds can attenuate CVD events (although with a nondiabetic focus) (54, 57). CANTOS demonstrated that canakinumab reduced the risk of CVD. Canakinumab is a therapeutic monoclonal antibody targeting IL-1β, a molecule involved in S100A8/A9 signaling, illustrating an example of how targeting S100A8/A9 or its downstream mediators may achieved in a clinical setting. However, since canakinumab also raised the risk of infections, a further refinement of the anti-inflammatory approach is needed. These recent findings only further underlie the importance of mechanistic studies into the role of inflammation in CVD.
Several studies have investigated the efficacy of direct S100A8/A9 inhibitors in preventing cardiovascular complications. In the setting of TIH, a small-molecule inhibitor of S100A8/A9, ABR-215757 (paquinimod) was tested to assess if blocking the RAGE-S100A8/A9 signaling axis reduces atherosclerosis in mice (21). The treatment, independent of hyperglycemic response, reduced myeloid progenitors, translating to reduced circulating neutrophils and monocytes (21). A separate study assessing the effectiveness of using paquinimod to reduce S100A8/A9 binding to RAGE and TLR4 found that it significantly reduced reticulated platelet formation and thus thrombocytosis (41). A similar study using a different small-molecule inhibitor of S100A8/A9, ABR-238901, found that short-term inhibition of S100A8/A9 early during MI blunted myelopoiesis and promoted an anti-inflammatory environment in the myocardium post-MI (46). However, long-term inhibition of S100A8/A9 with this drug, beyond the inflammatory phase, resulted in decreased stem cell proliferation in the bone marrow and eventual deterioration of cardiac function (73). It is important to fully understand the boundary between S100A8/A9's pro- and anti-inflammatory properties, and identify a correct window of opportunity for its targeted therapeutics (73).
Treatment of hyperglycemia in mice with a sodium/glucose cotransporter 2 inhibitor (SGLT2i) lowered levels of circulating S100A8/A9 and ROS accumulation, reducing the incidence of diabetes-related myelopoiesis, thrombopoiesis, and atherosclerosis (41, 74). SGLT2is have also been established as an inhibitor of NLRP3 inflammasome activity in diabetes and their usage has been found to abrogate diabetes-associated cardiac damage (38). In the landmark clinical trial, EMPA-REG OUTCOME, empagliflozin, an SGLT2i, showed significant differences from the placebo group in lowering rates of death from cardiovascular events and less hospitalizations for heart failure in type 2 diabetics (2, 89). Another SGLT2i clinical trial, DAPA-HF, demonstrated that treatment with dapagliflozin reduced the risk of worsened heart failure and cardiovascular death in patients, regardless of type 2 diabetes incidence (2). SGLT2i therapies have been overall effective at reducing cardiovascular mortality, and heart failure in particular, among the myriad of oral antidiabetic drugs, even though their effect on glycated hemoglobin is overall modest (76). However, as SGLT2i's have been shown to lower ROS and S100A8/A9 levels, more research should be devoted to developing treatments aimed at lowering the levels of both these factors and related pathways in diabetic complications to tease out whether similar treatment success may be achieved. The issue of S100A8/A9 release from glycemic spikes still remains however, thus, whether a safety net is required in the setting of diabetes to prevent the consequences of S100A8/A9 needs to be determined.
Summary
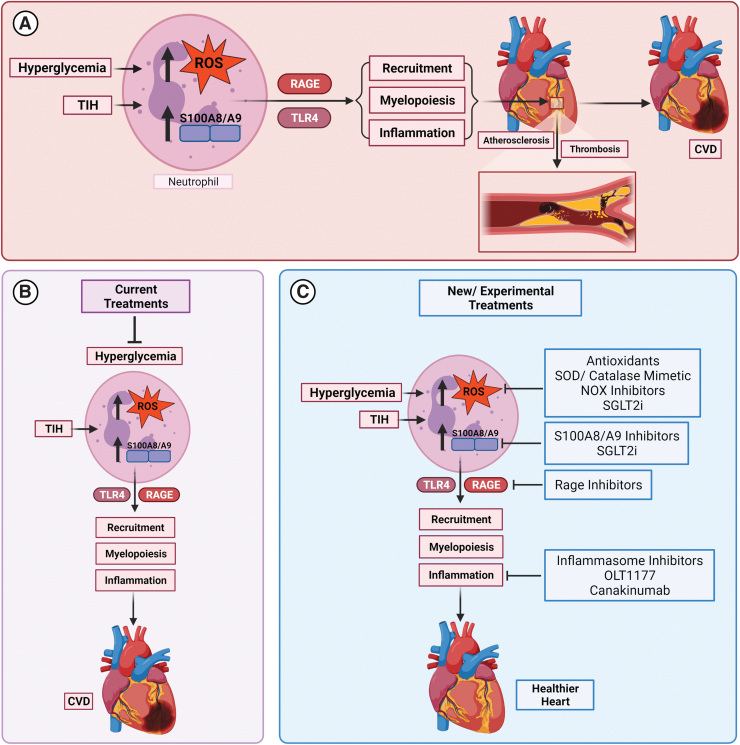
Current diabetes treatments are not extremely dimensional in their approach toward the disease. Expanding the scope of therapeutic targets to include factors that are highly affected by hyperglycemia and oxidative stress could advance the field of diabetic treatment options drastically. As shown by several clinical and experimental studies, knockdown or suppression of S100A8/A9 and its receptors has been shown to abrogate several of the complications associated with diabetes, especially pertaining to cardiovascular complications. Characterizing these pathways through which S100A8/A9 exerts damaging immune effects serves to provide a platform for the therapeutic research (Fig. 7).
FIG. 7.
Summary of graphic illustration. (A) The main pathways through which hyperglycemia inflicts its cardiovascular damaging effects and the eventual rise of CVD through ROS and S100A8/A9 are shown. (B) The current diabetes treatment of targeting hyperglycemia still allows for the progression of CVD in diabetic patients due to detrimental TIH, which involves glucose spikes, usually undetectable by blood glucose readings and A1C levels. (C) New and experimental diabetes treatments that target pathways downstream of hyperglycemia and TIH are shown in preliminary studies and trials to be effective at preventing/slowing the development of CVD. The implications of these studies give an optimistic view of the usefulness of these targeted therapies in conjunction with current treatment options for mitigating the damaging cardiovascular effects of diabetes. CVD, cardiovascular disease; TIH, transient intermittent hyperglycemia; SGLT2i, sodium/glucose cotransporter 2 inhibitor; SOD, superoxide dismutase. Color images are available online.
Conclusion
Diabetes is indeed a multifaceted disease, ruled by oxidative stress and the various inflammatory pathways it initiates (24). Neutrophils are among the most reactive and impacted cells in the body in response to dysregulated glucose levels in diabetes. Primed by oxidative stress from increased metabolism, neutrophils release DAMP molecules, such as S100A8/A9, that in turn trigger myelopoiesis and inflammatory responses through various pattern recognition receptors. Many of the processes are involved in feed-forward systems, where the presence of increased inflammatory signaling molecules such as ROS, S100A8/A9, and IL-1β begat heightened immune responses by directly or indirectly interacting with one another (84). Diabetes-induced increase in myelopoiesis promotes atherosclerosis and thrombosis, the major contributors of ischemic CVD (41). Platelets, also found to be elevated in diabetes, contribute to the formation and progression of atherosclerotic lesions and thrombi, adding to CVD mortality (41). Many of the factors involved in the formation of these diabetes-associated cardiovascular complications have a direct or indirect relationship with S100A8/A9, meaning S100A8/A9 may be an important link between diabetes and cardiovascular complications. It would be beneficial to further investigate the cellular and molecular mechanisms by which S100A8/A9 operates and its complex relationship with ROS, particularly in neutrophils. Delinking aberrant formation of ROS with S100A8/A9 release from neutrophils is key to developing potential strategies to reduce CVD, not only in diabetes but also in conditions of impaired glucose tolerance/insulin resistance where TIH/glucose spikes play a significant role in sustaining vascular inflammation. It is important to recognize that even if S100A8/A9-targeted candidate therapies are successful, they are unlikely to be used as a “stand-alone” therapeutic option in managing CVD, but rather as ancillary agents with the existing antidiabetic medications.
Acknowledgment
We thank Dr. Gaia Spinetti for her invitation to contribute to this forum issue on “Diabetes and Cardiovascular Complications.” All figures were generated with BioRender.
Abbreviations Used
- AA
arachidonic acid
- ACS
acute coronary syndrome
- AGEs
advanced glycolytic end products
- AP-1
activator protein 1
- C/EBPα
CCAAT/enhancer-binding protein α
- CEdG
N2-(1-carboxyethyl)-2′-deoxyguanosine
- CANTOS
Canakinumab Anti-inflammatory Thrombosis Outcomes Study
- CCR5
C-C chemokine receptor type 5
- CFs
cardiac fibroblasts
- CMPs
common myeloid progenitors
- CVD
cardiovascular disease
- CXCL4
chemokine (C-X-C motif) ligand 4
- DAMPs
damage-associated molecular patterns
- ENDS
elongated neutrophil-derived structures
- FMLP
N-formyl-met-leu-phe
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GLUT-1
glucose transporter-1
- GM-CSF
granulocyte macrophage-colony stimulating factor
- GMPs
granulocyte macrophage progenitors
- GSDM
gasdermin
- GSDMD
gasdermin-D
- GSH
reduced glutathione
- ICAM-1
intracellular adhesion molecule 1
- IL-1R
interleukin-1 receptor
- IL-1β
interleukin 1 beta
- KLF-5
Kruppel-like factor 5
- LDLs
low-density lipoproteins
- LoDoCo
Low Dose Colchicine for Secondary Prevention of Cardiovascular Disease
- Mac-1
macrophage-1 antigen
- M-CSF
monocyte colony stimulating factor
- MI
myocardial infarction
- NE
neutrophil elastase
- NETs
neutrophil extracellular traps
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NLRP3
NLR family pyrin domain containing 3
- NOX
NADPH oxidase
- ox-LDL
oxidized low-density lipoproteins
- PAD4
peptidyl arginine deiminase 4
- PKC
protein kinase C
- PMA
phorbol myristate acetate
- RAAS
renin/angiotensin/aldosterone system
- RAGE
receptor for advanced glycation end products
- ROS
reactive oxidative species
- SGLT2i
sodium/glucose cotransporter 2 inhibitor
- SOD
superoxide dismutase
- STAT3
signal transducer and activator of transcription 3
- TIH
transient intermittent hyperglycemia
- TLR4
toll-like receptor 4
- TPO
thrombopoietin
- VCAM-1
vascular adhesion molecule 1
Authors' Contributions
P.R.N. and J.J. conceptualized the main outline for the article. J.J. wrote the majority of the article and created figures. P.R.N., J.J., R.M.J., S.G., A.D., N.M.J.H., and A.J.M. contributed by writing specific sections and editing.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
P.R.N. is supported by grants from the NIH (R01HL137799, R21AG063197). NMJH is supported by a DFN- DON [2020.10.002]. A.J.M. is supported by a CSL Centenary Award and an NHMRC Investigator grant.
References
- 1. National Diabetes Statistics Report 2020. Centers for Disease Control and Prevention, 2020, p. 1.
- 2. American Diabetes Association. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2021. Diabetes Care 44: S125–S150, 2021. [DOI] [PubMed] [Google Scholar]
- 3. Asehnoune K, Strassheim D, Mitra S, Yeol Kim J, and Abraham E. Involvement of PKCalpha/beta in TLR4 and TLR2 dependent activation of NF-kappaB. Cell Signal 17: 385–394, 2005. [DOI] [PubMed] [Google Scholar]
- 4. Averill MM, Barnhart S, Becker L, Li X, Heinecke JW, Leboeuf RC, Hamerman JA, Sorg C, Kerkhoff C, and Bornfeldt KE. S100A9 differentially modifies phenotypic states of neutrophils, macrophages, and dendritic cells: implications for atherosclerosis and adipose tissue inflammation. Circulation 123: 1216–1226, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Averill MM, Kerkhoff C, and Bornfeldt KE. S100A8 and S100A9 in cardiovascular biology and disease. Arterioscler Thromb Vasc Biol 32: 223–229, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ayilavarapu S, Kantarci A, Fredman G, Turkoglu O, Omori K, Liu H, Iwata T, Yagi M, Hasturk H, and Van Dyke TE. Diabetes-induced oxidative stress is mediated by Ca2+-independent phospholipase A2 in neutrophils. J Immunol 184: 1507–1515, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bansal S, Siddarth M, Chawla D, Banerjee BD, Madhu SV, and Tripathi AK. Advanced glycation end products enhance reactive oxygen and nitrogen species generation in neutrophils in vitro. Mol Cell Biochem 361: 289–296, 2012. [DOI] [PubMed] [Google Scholar]
- 8. Berezin AE. The cardiovascular risk prognostication in diabetes mellitus: the role of myeloid-related protein complex calprotectin. Int J Pathol Clin Res 2: 026, 2016. [Google Scholar]
- 9. Bernardi S, Michelli A, Zuolo G, Candido R, and Fabris B. Update on RAAS modulation for the treatment of diabetic cardiovascular disease. J Diabetes Res 2016: 8917578, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen KW, Monteleone M, Boucher D, Sollberger G, Ramnath D, Condon ND, von Pein JB, Broz P, Sweet MJ, and Schroder K. Noncanonical inflammasome signaling elicits gasdermin D–dependent neutrophil extracellular traps. Sci Immunol 3: eaar6676, 2018. [DOI] [PubMed] [Google Scholar]
- 11. Costa D, Marques AP, Reis RL, Lima JL, and Fernandes E. Inhibition of human neutrophil oxidative burst by pyrazolone derivatives. Free Radic Biol Med 40: 632–640, 2006. [DOI] [PubMed] [Google Scholar]
- 12. Critchley JA, Carey IM, Harris T, DeWilde S, Hosking FJ, and Cook DG. Glycemic control and risk of infections among people with type 1 or type 2 diabetes in a large primary care cohort study. Diabetes Care 41: 2127–2135, 2018. [DOI] [PubMed] [Google Scholar]
- 13. Croce K, Gao H, Wang Y, Mooroka T, Sakuma M, Shi C, Sukhova GK, Packard RR, Hogg N, Libby P, and Simon DI. Myeloid-related protein-8/14 is critical for the biological response to vascular injury. Circulation 120: 427–436, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dasu MR, Devaraj S, Zhao L, Hwang DH, and Jialal I. High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes 57: 3090–3098, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ding S, Xu S, Ma Y, Liu G, Jang H, and Fang J. Modulatory mechanisms of the NLRP3 inflammasomes in diabetes. Biomolecules 9: 850, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doring Y, Libby P, and Soehnlein O. Neutrophil extracellular traps participate in cardiovascular diseases: recent experimental and clinical insights. Circ Res 126: 1228–1241, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ehrchen JM, Sunderkotter C, Foell D, Vogl T, and Roth J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol 86: 557–566, 2009. [DOI] [PubMed] [Google Scholar]
- 18. El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, Cooper ME, and Brownlee M. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med 205: 2409–2417, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fiorentino TV, Prioletta A, Zuo P, and Folli F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr Pharm Des 19: 5695–5703, 2013. [DOI] [PubMed] [Google Scholar]
- 20. Flores-Gomez D, Bekkering S, Netea MG, and Riksen NP. Trained immunity in atherosclerotic cardiovascular disease. Arterioscler Thromb Vasc Biol 41: 62–69, 2021. [DOI] [PubMed] [Google Scholar]
- 21. Flynn MC, Kraakman MJ, Tikellis C, Lee MKS, Hanssen NMJ, Kammoun HL, Pickering RJ, Dragoljevic D, Al-Sharea A, Barrett TJ, Hortle F, Byrne FL, Olzomer E, McCarthy DA, Schalkwijk CG, Forbes JM, Hoehn K, Makowski L, Lancaster GI, El-Osta A, Fisher EA, Goldberg IJ, Cooper ME, Nagareddy PR, Thomas MC, and Murphy AJ. Transient intermittent hyperglycemia accelerates atherosclerosis by promoting myelopoiesis. Circ Res 127: 877–892, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes 26: 77–82, 2008. [Google Scholar]
- 23. Fujiu K, Manabe I, and Nagai R. Renal collecting duct epithelial cells regulate inflammation in tubulointerstitial damage in mice. J Clin Invest 121: 3425–3441, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Giacco F and Brownlee M. Oxidative stress and diabetic complications. Circ Res 107: 1058–1070, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Giacco F, Du X, Carratu A, Gerfen GJ, D'Apolito M, Giardino I, Rasola A, Marin O, Divakaruni AS, Murphy AN, Shah MS, and Brownlee M. GLP-1 cleavage product reverses persistent ROS generation after transient hyperglycemia by disrupting an ROS-generating feedback loop. Diabetes 64: 3273–3284, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goyette J and Geczy CL. Inflammation-associated S100 proteins: new mechanisms that regulate function. Amino Acids 41: 821–842, 2011. [DOI] [PubMed] [Google Scholar]
- 27. Gupta A, Tripathi AK, Tripathi RL, Madhu SV, and Banerjee BD. Advanced glycosylated end products-mediated activation of polymorphonuclear neutrophils in diabetes mellitus and associated oxidative stress. Indian J Biochem Biophys 44: 373–378, 2007. [PubMed] [Google Scholar]
- 28. Hoogeveen RM, Nahrendorf M, Riksen NP, Netea MG, de Winther MPJ, Lutgens E, Nordestgaard BG, Neidhart M, Stroes ESG, Catapano AL, and Bekkering S. Monocyte and haematopoietic progenitor reprogramming as common mechanism underlying chronic inflammatory and cardiovascular diseases. Eur Heart J 39: 3521–3527, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ionita MG, Catanzariti LM, Bots ML, de Vries JP, Moll FL, Kwan Sze S, Pasterkamp G, and de Kleijn DP. High myeloid-related protein: 8/14 levels are related to an increased risk of cardiovascular events after carotid endarterectomy. Stroke 41: 2010–2015, 2010. [DOI] [PubMed] [Google Scholar]
- 30. Kamioka M, Ishibashi T, Sugimoto K, Uekita H, Nagai R, Sakamoto N, Ando K, Ohkawara H, Teramoto T, Maruyama Y, and Takeishi Y. Blockade of renin-angiotensin system attenuates advanced glycation end products-mediated signaling pathways. J Atheroscler Thromb 17: 590–600, 2010. [DOI] [PubMed] [Google Scholar]
- 31. Karima M, Kantarci A, Ohira T, Hasturk H, Jones VL, Nam B-H, Malabanan A, Trackman PC, Badwey JA, and Van Dyke TE. Enhanced superoxide release and elevated protein kinase C activity in neutrophils from diabetic patients: association with periodontitis. J Leukoc Biol 78: 862–870, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karmakar M, Minns M, Greenberg EN, Diaz-Aponte J, Pestonjamasp K, Johnson JL, Rathkey JK, Abbott DW, Wang K, Shao F, Catz SD, Dubyak GR, and Pearlman E. N-GSDMD trafficking to neutrophil organelles facilitates IL-1beta release independently of plasma membrane pores and pyroptosis. Nat Commun 11: 2212, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Katakami N. Mechanism of development of atherosclerosis and cardiovascular disease in diabetes mellitus. J Atheroscler Thromb 25: 27–39, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kayama Y, Raaz U, Jagger A, Adam M, Schellinger IN, Sakamoto M, Suzuki H, Toyama K, Spin JM, and Tsao PS. diabetic cardiovascular disease induced by oxidative stress. Int J Mol Sci 16: 25234–25263, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kenny EF, Herzig A, Kruger R, Muth A, Mondal S, Thompson PR, Brinkmann V, Bernuth HV, and Zychlinsky A. Diverse stimuli engage different neutrophil extracellular trap pathways. Elife 6: e24437, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kerkhoff C, Klempt M, Kaever V, and Sorg C. The two calcium-binding proteins, S100A8 and S100A9, are involved in the metabolism of arachidonic acid in human neutrophils. J Biol Chem 274: 32672–32679, 1999. [DOI] [PubMed] [Google Scholar]
- 37. Khan MA and Palaniyar N. Transcriptional firing helps to drive NETosis. Sci Rep 7: 41749, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim SR, Lee SG, Kim SH, Kim JH, Choi E, Cho W, Rim JH, Hwang I, Lee CJ, Lee M, Oh CM, Jeon JY, Gee HY, Kim JH, Lee BW, Kang ES, Cha BS, Lee MS, Yu JW, Cho JW, Kim JS, and Lee YH. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun 11: 2127, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koulis C, Kanellakis P, Pickering RJ, Tsorotes D, Murphy AJ, Gray SP, Thomas MC, Jandeleit-Dahm KA, Cooper ME, and Allen TJ. Role of bone-marrow- and non-bone-marrow-derived receptor for advanced glycation end-products (RAGE) in a mouse model of diabetes-associated atherosclerosis. Clin Sci (Lond) 127: 485–497, 2014. [DOI] [PubMed] [Google Scholar]
- 40. Kovatchev BP. Metrics for glycaemic control—from HbA1c to continuous glucose monitoring. Nat Rev Endocrinol 13: 425–436, 2017. [DOI] [PubMed] [Google Scholar]
- 41. Kraakman MJ, Lee MK, Al-Sharea A, Dragoljevic D, Barrett TJ, Montenont E, Basu D, Heywood S, Kammoun HL, Flynn M, Whillas A, Hanssen NM, Febbraio MA, Westein E, Fisher EA, Chin-Dusting J, Cooper ME, Berger JS, Goldberg IJ, Nagareddy PR, and Murphy AJ. Neutrophil-derived S100 calcium-binding proteins A8/A9 promote reticulated thrombocytosis and atherogenesis in diabetes. J Clin Invest 127: 2133–2147, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kummer U, Zobeley J, Brasen JC, Fahmy R, Kindzelskii AL, Petty AR, Clark AJ, and Petty HR. Elevated glucose concentrations promote receptor-independent activation of adherent human neutrophils: an experimental and computational approach. Biophys J 92: 2597–2607, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Laridan E, Martinod K, and De Meyer SF. Neutrophil extracellular traps in arterial and venous thrombosis. Semin Thromb Hemost 45: 86–93, 2019. [DOI] [PubMed] [Google Scholar]
- 44. Lee MKS, Kraakman MJ, Dragoljevic D, Hanssen NMJ, Flynn MC, Al-Sharea A, Sreejit G, Bertuzzo-Veiga C, Cooney OD, Baig F, Morriss E, Cooper ME, Josefsson EC, Kile BT, Nagareddy PR, and Murphy AJ. Apoptotic ablation of platelets reduces atherosclerosis in mice with diabetes. Arterioscler Thromb Vasc Biol 41: 1167–1178, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lim SY, Raftery MJ, and Geczy CL. Oxidative modifications of DAMPs suppress inflammation: the case for S100A8 and S100A9. Antioxid Redox Signal 15: 2235–2248, 2011. [DOI] [PubMed] [Google Scholar]
- 46. Marinkovic G, Grauen Larsen H, Yndigegn T, Szabo IA, Mares RG, de Camp L, Weiland M, Tomas L, Goncalves I, Nilsson J, Jovinge S, and Schiopu A. Inhibition of pro-inflammatory myeloid cell responses by short-term S100A9 blockade improves cardiac function after myocardial infarction. Eur Heart J 40: 2713–2723, 2019. [DOI] [PubMed] [Google Scholar]
- 47. Marki A, Buscher K, Lorenzini C, Meyer M, Saigusa R, Fan Z, Yeh YT, Hartmann N, Dan JM, Kiosses WB, Golden GJ, Ganesan R, Winkels H, Orecchioni M, McArdle S, Mikulski Z, Altman Y, Bui J, Kronenberg M, Chien S, Esko JD, Nizet V, Smalley D, Roth J, and Ley K. Elongated neutrophil-derived structures are blood-borne microparticles formed by rolling neutrophils during sepsis. J Exp Med 218: e20200551, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mezzaroma E, Abbate A, and Toldo S. NLRP3 inflammasome inhibitors in cardiovascular diseases. Molecules 26: 976, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miyoshi A, Yamada M, Shida H, Nakazawa D, Kusunoki Y, Nakamura A, Miyoshi H, Tomaru U, Atsumi T, and Ishizu A. Circulating neutrophil extracellular trap levels in well-controlled type 2 diabetes and pathway involved in their formation induced by high-dose glucose. Pathobiology 83: 243–251, 2016. [DOI] [PubMed] [Google Scholar]
- 50. Morrow DA, Wang Y, Croce K, Sakuma M, Sabatine MS, Gao H, Pradhan AD, Healy AM, Buros J, McCabe CH, Libby P, Cannon CP, Braunwald E, and Simon DI. Myeloid-related protein 8/14 and the risk of cardiovascular death or myocardial infarction after an acute coronary syndrome in the Pravastatin or Atorvastatin Evaluation and Infection Therapy: thrombolysis in Myocardial Infarction (PROVE IT-TIMI 22) trial. Am Heart J 155: 49–55, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Murphy AJ and Tall AR. Disordered haematopoiesis and athero-thrombosis. Eur Heart J 37: 1113–1121, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG, Ramkhelawon B, Distel E, Westerterp M, Huang LS, Schmidt AM, Orchard TJ, Fisher EA, Tall AR, and Goldberg IJ. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab 17: 695–708, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Netea MG, Quintin J, and van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe 9: 355–361, 2011. [DOI] [PubMed] [Google Scholar]
- 54. Nidorf SM, Eikelboom JW, Budgeon CA, and Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol 61: 404–410, 2013. [DOI] [PubMed] [Google Scholar]
- 55. Papayannopoulos V, Metzler KD, Hakkim A, and Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol 191: 677–691, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pickering RJ, Tikellis C, Rosado CJ, Tsorotes D, Dimitropoulos A, Smith M, Huet O, Seeber RM, Abhayawardana R, Johnstone EK, Golledge J, Wang Y, Jandeleit-Dahm KA, Cooper ME, Pfleger KD, and Thomas MC. Transactivation of RAGE mediates angiotensin-induced inflammation and atherogenesis. J Clin Invest 129: 406–421, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, and CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 377: 1119–1131, 2017. [DOI] [PubMed] [Google Scholar]
- 58. Ridzuan N, John CM, Sandrasaigaran P, Maqbool M, Liew LC, Lim J, and Ramasamy R. Preliminary study on overproduction of reactive oxygen species by neutrophils in diabetes mellitus. World J Diabetes 7: 271–278, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Riva M, Kallberg E, Bjork P, Hancz D, Vogl T, Roth J, Ivars F, and Leanderson T. Induction of nuclear factor-kappaB responses by the S100A9 protein is Toll-like receptor-4-dependent. Immunology 137: 172–182, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rosen P, Nawroth PP, King G, Moller W, Tritschler HJ, and Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev 17: 189–212, 2001. [DOI] [PubMed] [Google Scholar]
- 61. Russo I and Frangogiannis NG. Diabetes-associated cardiac fibrosis: cellular effectors, molecular mechanisms and therapeutic opportunities. J Mol Cell Cardiol 90: 84–93, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ryckman C, Vandal K, Rouleau P, Talbot M, and Tessier PA. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J Immunol 170: 3233–3242, 2003. [DOI] [PubMed] [Google Scholar]
- 63. Schalkwijk CG and Stehouwer CDA. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol Rev 100: 407–461, 2020. [DOI] [PubMed] [Google Scholar]
- 64. Schenten V, Plancon S, Jung N, Hann J, Bueb JL, Brechard S, Tschirhart EJ, and Tolle F. Secretion of the phosphorylated form of S100A9 from neutrophils is essential for the proinflammatory functions of extracellular S100A8/A9. Front Immunol 9: 447, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schiopu A and Cotoi OS. S100A8 and S100A9: DAMPs at the crossroads between innate immunity, traditional risk factors, and cardiovascular disease. Mediators Inflamm 2013: 828354, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shah MS and Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res 118: 1808–1829, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shirai T, Nazarewicz RR, Wallis BB, Yanes RE, Watanabe R, Hilhorst M, Tian L, Harrison DG, Giacomini JC, Assimes TL, Goronzy JJ, and Weyand CM. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J Exp Med 213: 337–354, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Simard JC, Cesaro A, Chapeton-Montes J, Tardif M, Antoine F, Girard D, and Tessier PA. S100A8 and S100A9 induce cytokine expression and regulate the NLRP3 inflammasome via ROS-dependent activation of NF-kappaB(1.). PLoS One 8: e72138, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sohrabi Y, Lagache SMM, Schnack L, Godfrey R, Kahles F, Bruemmer D, Waltenberger J, and Findeisen HM. mTOR-dependent oxidative stress regulates oxLDL-induced trained innate immunity in human monocytes. Front Immunol 9: 3155, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sollberger G, Choidas A, Burn GL, Habenberger P, Di Lucrezia R, Kordes S, Menninger S, Eickhoff J, Nussbaumer P, Klebl B, Krüger R, Herzig A, and Zychlinsky A. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci Immunol 3: eaar6689, 2018. [DOI] [PubMed] [Google Scholar]
- 71. Soro-Paavonen A, Watson AM, Li J, Paavonen K, Koitka A, Calkin AC, Barit D, Coughlan MT, Drew BG, Lancaster GI, Thomas M, Forbes JM, Nawroth PP, Bierhaus A, Cooper ME, and Jandeleit-Dahm KA. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes 57: 2461–2469, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sreejit G, Abdel-Latif A, Athmanathan B, Annabathula R, Dhyani A, Noothi SK, Quaife-Ryan GA, Al-Sharea A, Pernes G, Dragoljevic D, Lal H, Schroder K, Hanaoka BY, Raman C, Grant MB, Hudson JE, Smyth SS, Porrello ER, Murphy AJ, and Nagareddy PR. Neutrophil-derived S100A8/A9 amplify granulopoiesis after myocardial infarction. Circulation 141: 1080–1094, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sreejit G, Abdel Latif A, Murphy AJ, and Nagareddy PR. Emerging roles of neutrophil-borne S100A8/A9 in cardiovascular inflammation. Pharmacol Res 161: 105212, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sreejit G, Flynn MC, Patil M, Krishnamurthy P, Murphy AJ, and Nagareddy PR. S100 family proteins in inflammation and beyond. Adv Clin Chem 98: 173–231, 2020. [DOI] [PubMed] [Google Scholar]
- 75. Synold T, Xi B, Wuenschell GE, Tamae D, Figarola JL, Rahbar S, and Termini J. Advanced glycation end products of DNA: quantification of N2-(1-carboxyethyl)-2′-deoxyguanosine (CEdG) in biological samples by LC-ESI-MS/MS. Chem Res Toxicol 21: 2148–2155, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Teodoro JS, Nunes S, Rolo AP, Reis F, and Palmeira CM. Therapeutic options targeting oxidative stress, mitochondrial dysfunction and inflammation to hinder the progression of vascular complications of diabetes. Front Physiol 9: 1857, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Thiam HR, Wong SL, Wagner DD, and Waterman CM. Cellular mechanisms of NETosis. Annu Rev Cell Dev Biol 36: 191–218, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ulas T, Pirr S, Fehlhaber B, Bickes MS, Loof TG, Vogl T, Mellinger L, Heinemann AS, Burgmann J, Schoning J, Schreek S, Pfeifer S, Reuner F, Vollger L, Stanulla M, von Kockritz-Blickwede M, Glander S, Barczyk-Kahlert K, von Kaisenberg CS, Friesenhagen J, Fischer-Riepe L, Zenker S, Schultze JL, Roth J, and Viemann D. S100-alarmin-induced innate immune programming protects newborn infants from sepsis. Nat Immunol 18: 622–632, 2017. [DOI] [PubMed] [Google Scholar]
- 79. Urner S, Ho F, Jha JC, Ziegler D, and Jandeleit-Dahm K. NADPH oxidase inhibition: preclinical and clinical studies in diabetic complications. Antioxid Redox Signal 33: 415–434, 2020. [DOI] [PubMed] [Google Scholar]
- 80. Viemann D, Strey A, Janning A, Jurk K, Klimmek K, Vogl T, Hirono K, Ichida F, Foell D, Kehrel B, Gerke V, Sorg C, and Roth J. Myeloid-related proteins 8 and 14 induce a specific inflammatory response in human microvascular endothelial cells. Blood 105: 2955–2962, 2005. [DOI] [PubMed] [Google Scholar]
- 81. Vogl T, Ludwig S, Goebeler M, Strey A, Thorey IS, Reichelt R, Foell D, Gerke V, Manitz MP, Nacken W, Werner S, Sorg C, and Roth J. MRP8 and MRP14 control microtubule reorganization during transendothelial migration of phagocytes. Blood 104: 4260–4268, 2004. [DOI] [PubMed] [Google Scholar]
- 82. Volz HC, Laohachewin D, Seidel C, Lasitschka F, Keilbach K, Wienbrandt AR, Andrassy J, Bierhaus A, Kaya Z, Katus HA, and Andrassy M. S100A8/A9 aggravates post-ischemic heart failure through activation of RAGE-dependent NF-kappaB signaling. Basic Res Cardiol 107: 250, 2012. [DOI] [PubMed] [Google Scholar]
- 83. Wang L, Wang J, Fang J, Zhou H, Liu X, and Su SB. High glucose induces and activates Toll-like receptor 4 in endothelial cells of diabetic retinopathy. Diabetol Metab Syndr 7: 89, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wang S, Song R, Wang Z, Jing Z, Wang S, and Ma J. S100A8/A9 in inflammation. Front Immunol 9: 1298, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wong SL, Demers M, Martinod K, Gallant M, Wang Y, Goldfine AB, Kahn CR, and Wagner DD. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med 21: 815–819, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wu Y, Li Y, Zhang C, A X, Wang Y, Cui W, Li H, and Du J. S100a8/a9 released by CD11b+Gr1+ neutrophils activates cardiac fibroblasts to initiate angiotensin II-Induced cardiac inflammation and injury. Hypertension 63: 1241–1250, 2014. [DOI] [PubMed] [Google Scholar]
- 87. Xu J, Chen LJ, Yu J, Wang HJ, Zhang F, Liu Q, and Wu J. Involvement of advanced glycation end products in the pathogenesis of diabetic retinopathy. Cell Physiol Biochem 48: 705–717, 2018. [DOI] [PubMed] [Google Scholar]
- 88. Yao D and Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes 59: 249–255, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, and EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373: 2117–2128, 2015. [DOI] [PubMed] [Google Scholar]