Abstract
Glioblastoma (GBM) displays diffusive invasion throughout the brain microenvironment, which is partially responsible for its short median survival rate (<15 months). Stem-like subpopulations (GBM stem-like cells, GSCs) are believed to play a central role in therapeutic resistance and poor patient prognosis. Given the extensive tissue remodeling and processes such as vessel co-option and regression that occur in the tumor microenvironment, it is essential to understand the role of metabolic constraint such as hypoxia on GBM cell populations. This work describes the use of a multidimensional gelatin hydrogel to culture patient-derived GBM cells, to evaluate the influence of hypoxia and the inclusion brain-mimetic hyaluronic acid on the relative activity of GSCs versus overall GBM cells. Notably, CD133+ GBM cell fraction is crucial for robust formation of tumor spheroids in multidimensional cultures. In addition, while the relative size of the CD133+ GBM subpopulation increased in response to both hypoxia and matrix-bound hyaluronan, we did not observe cell subtype-specific changes in invasion signaling pathway activation. Taken together, this study highlights the potential of biomimetic culture systems for resolving changes in the population dynamics and behavior of subsets of GBM specimens for the future development of precision medicine applications.
Impact Statement
This study describes a gelatin hydrogel platform to investigate the role of extracellular hyaluronic acid and hypoxia on the behavior of a CD133+ subset of cells within patient-derived glioblastoma (GBM) specimens. We report that the relative expansion of the CD133+ GBM stem cell-like population is strongly responsive to extracellular cues, highlighting the significance of biomimetic hydrogel models of the tumor microenvironment to investigate invasion and therapeutic response.
Keywords: glioblastoma, invasion, hypoxia, cancer stem cell
Introduction
Glioblastoma (GBM) is the most common and deadly form of primary brain cancer. Despite the current standard of care, which includes surgical debulking of the tumor mass followed by a combination of radiotherapy and chemotherapy, patients diagnosed with GBM display only a 15–18-month median survival rate and a <15% five-year survival rate. Patients display rapid rates of recurrence (6.9 months post-therapy) with new tumors typically reappearing within a few centimeters of the original tumor site.1–6 A major challenge to treatment is the invasive spreading of GBM cells within the brain. While surgical resection is a central feature of early therapeutic intervention, it requires a sharp surgical margin being made across a diffuse cellular margin. It is imperative to improve our understanding of invasive spreading of GBM cells from the tumor mass into the surrounding brain, a process marked by spatial transitions across a heterogeneous tumor microenvironment. While hyaluronic acid (HA) is the primary constituent of the brain extracellular matrix (ECM), the tumor microenvironment presents a wider diversity of matrix molecules, including collagen, fibronectin, laminin, and vitronectin.7–9
Tissue engineering platforms offer the opportunity to vary matrix features in defined increments, offering the ability to examine the role of biophysical (e.g., matrix stiffness) and biochemical (e.g., HA content vs. molecular weight) signals on GBM invasion or drug resistance.10,11 These platforms are also increasingly being adapted to study the role of additional features within the tumor microenvironment such as recent work from Munson et al. demonstrating interstitial flow significantly influences GBM proliferation, invasion, and stem cell activity.12,13
Biophysical features of the tumor microenvironment are not the only relevant axis of stimuli for GBM cell activity, invasion, and drug response. The intersection between metabolic constraint and the tissue environment offers opportunities to understand GBM progression. Hypoxia is hallmark of the GBM tumor microenvironment,14 where intratumoral oxygen availability can drop below 2% and alter key cellular metabolic processes.15 GBM invasion within perivascular niches is marked by exposure to regions of vascularized stroma and hypoxic foci (hypercellular pseudopalisades) resulting from proliferation-induced vessel co-option and regression.16 However, studies of the role of metabolic constraint on GBM activity are complicated by the heterogeneous nature of the cellular content of GBM tumors, which can include a mixture of dendritic and perivascular cells, macrophages, microglia, astrocytes, and a subpopulation of tumor-initiating cells (GBM stem-like cells, GSCs),17–19 each of which can play a central role in invasive spreading, therapeutic resistance, recurrence, and mortality.18,20–33 Evidence increasingly suggests GSC subpopulations are able to self-renew, generate a wide range of tumor-associated cells,20,34–36 and contribute to drug resistance,32,34,37–40 all critical features of GBM progression and poor prognosis. While the exact definition of GSCs remains cryptic, common markers include CD133+ along with Sox2+ and Nestin+ GBM fractions.32,41 While CD133 is a cancer stem cell marker widely matched with a range of stem-like characteristics,42–44 many immortalized GBM cell lines contain negligible fractions of GSCs. However, patient-derived cells (PDCs) contain (widely variable patient-to-patient) GSC subpopulations, making them ideally suited for the development of tissue engineering efforts to study the behavior of cohorts of GBM and GSC-like cells.45
The objective of this work is to examine the combined influence of hypoxia and matrix-immobilized HA on the activity and invasive phenotype of patient-derived GBM specimens with a particular focus on the behavior of a subpopulation of cells displaying markers (CD133+) associated with GSCs. These efforts build on recent work published by our group developing a gelatin-based hydrogel platform that used immortalized U87 GBM cell lines to show hypoxia increased GBM invasion, while suppressing proliferative phenotype.46 Investigation of the activation of hypoxia-associated signaling pathways for cells in hydrogel culture was motivated by recent studies that suggested even short-term activation (0–24 h) of hypoxia-inducible factor 1α was sufficient to trigger important downstream pathways, notably mitogen-activated protein kinase/extracellular regulated kinase (ERK), a known promoter of GBM malignancy and invasive behavior,47–49 as well as matrix metalloproteinases (MMPs), a central mediator of matrix remodeling and tumor progression.50 Our laboratory demonstrated expression of signal transducer and activator of transcription 3 (STAT3), a known mediator of tumor progression and related with tumor survival, is strongly connected to GBM resistance to repeated exposure to chemotherapies, such as tyrosine kinase inhibitor erlotinib, in the presence of matrix-immobilized HA.51 Together, these findings suggested not only that hypoxia and insoluble HA may be a strong mediator of patient-derived GBM cell invasion but also that tissue engineering platforms provide a powerful tool to identify potential pathways mediating the invasive capacity of GBM in response to hypoxia. As a result, in this study, we report the use of patient-derived xenograft GBM specimens and a gelatin hydrogel platform to define the role of hypoxia and the presence of matrix-bound HA on the activation of progression-associated signaling pathways, on the relative expansion of a GSC subpopulation, and on the contribution of a GSC subpopulation on overall GBM invasion capacity.
Materials and Methods
Fabrication and characterization of gelatin hydrogels
Methacrylamide-functionalized gelatin (GelMA) macromers were fabricated from gelatin (Type A, 300 bloom from porcine skin; Sigma-Aldrich, St. Louis, MO) and methacrylamide-functionalized HA (HAMA, 60 kDa; Lifecore Biomedical, Chaska, MN) was also fabricated as previously described.10,52 The degree of functionalization of both GelMA and HAMA compounds was confirmed (∼50% degree of functionalization, data not shown) by 1H NMR, matching previously reported GelMA and HAMA. Hydrogels were prepared as previously described.10,52 Briefly, a prepolymer solution of 4 wt% GelMA with 0 or 15 w/w% HAMA was suspended in PBS (Invitrogen) and then photopolymerized by 7.1 mW/cm2 UV for 30 s in the presence of 0.1 wt% LAP (lithium phenyl-2,4,6-trimethylbenzoylphosphinate) as a photoinitiator. The compressive modulus of hydrated GelMA +/− HAMA hydrogels was measured using an Instron 5943 mechanical tester under unconfined compression at the rate of 0.1 mm/min. The Young's modulus was obtained from the linear region of the stress-strain curve (15% region) using a customized MATLAB code53 and both hydrogel variants exhibited a physiologically (Supplementary Data S1) relevant Young's modulus (GelMA: 1.08 kPa, n = 6 and GelMA+HAMA: 1.10 kPa, n = 6; data not shown). Cell containing hydrogels were made by addition of 4 million cells/mL or by inclusion of cell spheroids into the prepolymer solution before being pipetted into Teflon molds (0.2 mm thick and 5 mm radius), photopolymerized, and then placed into culture (details below).
In vitro culture of PDCs encapsulated in gelatin hydrogels
Patient-derived xenograft GBM cells (PDX, GBM6) used in this study were obtained from Mayo Clinic (Rochester, Minnesota) as described in previous publications.46,51 All patients consented to the use of their tumor tissue in support of this research, and the use of the patient tissues received prior institutional review board authorization (Mayo Clinic). GBM6 was isolated from a male patient, was highly invasive, and displayed unmethylated MGMT (O[6]-methylguanine-DNA methyltransferase) and EGFR vIII mutation (epidermal growth factor receptor variant III) by the Mayo Clinic Brain Tumor Patient-Derived Xenograft National Resource. GBM6 is highly invasive in mouse xenograft models of GBM progression.54 GBM6 cells were shipped in media made with Dulbecco's modified Eagle's medium (Gibco) supplemented with 10% fetal bovine serum (Atlanta biologicals) and 1% penicillin/streptomycin (Lonza, Basel, Switzerland) and then used upon arrival. For studies of changes in cell number, cell metabolic activity, or relative fractions of CD133+ GSCs, GBM6 cells were homogeneously mixed into the prepolymerized GelMA +/− HAMA solution at a density of 100,000 cells per 25 μL hydrogel followed by photopolymerization as described above. Cell-seeded hydrogels were subsequently maintained at 37°C in low-adhesion well plates containing standard culture media; metabolic state (normoxia: 5% CO2, O2 naturally balanced and hypoxia: 5% CO2, 1% O2) was established using a computer-controlled hypoxia chamber (C-Chamber Hypoxia Chamber, Biospherix™, Parish, NY) within the cell culture incubator. Culture media were changed at days 3 and 5 for all cell-containing hydrogels. Media used for hypoxia studies were preconditioned in the hypoxia chamber overnight to allow for oxygen equilibration (1% O2) before adding to cell culture.
Quantifying number, metabolic, and signaling activity of encapsulated GBM cells
The number and overall metabolic activity of GBM6 PDXs encapsulated within hydrogel were traced throughout a 7-day culture period, with data gathered on days 0 (immediately postseeding), 3, 5 and 7. The total number of cells in each hydrogel specimen was quantified by a Hoechst 33528-based DNA quantification method,46,55,56 while total metabolic activity per hydrogel disk was determined using a commercial dimethylthiazol-diphenyltetrazolium bromide (MTT) assay (Molecular Probes, Eugene, OR). All methods were adapted from the manufacturer's protocol and as previously described.46,57 All results were reported as fold change, normalized to day 0 (immediately after seeding) values.
Protocols of protein isolation and Western blotting analysis were described in previous publications.46,58 Briefly, sample lysates were collected with RIPA (radioimmunoprecipitation assay) buffer on ice and total protein concentration was determined using the Pierce™ BCA Protein Assay Kit (ThermoFisher). Lysates (5 μg) were mixed (1:1) with 2 × Laemmli Sample Buffer (Bio-Rad) and 2-mercaptoethanol (Sigma-Aldrich), denatured by heating to 95°C for 10 min, and then loaded onto polyacrylamide gels (4–20% gradient; Bio-Rad). Gel electrophoresis was performed at 150 V for roughly 60 min before transferring proteins onto nitrocellulose membrane (GE Healthcare) under 300 mA for 2 h. Detailed information regarding primary and secondary antibodies as well as buffers is described in Table 1.
Table 1.
Buffers and Antibodies Used in Western Blotting
| Buffer | Composition | ||
|---|---|---|---|
| RIPA | Radioimmunoprecipitation assay buffer. 150 mM sodium chloride, 1% Triton X-100, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM Tris base in deionized water. |
||
| TBST | 150 mM sodium chloride, 20 mM Tris base, 0.1% (w/v) Tween 20 | ||
| 5% BSA | 5 wt% bovine serum albumin (Sigma-Aldrich, St. Louis, MO) in TBST | ||
| 5% NFDM | 5 wt% nonfat instant dry milk (Great Value) in TBST | ||
| Target name | Blocking buffer | Primary antibody | Secondary antibody |
|---|---|---|---|
| Stat3 (79, 86 kDa) |
5% NFDM | 1:1000 in 5% NFDM (Cell Signaling, 12640S) |
1:2500 in TBST (Cell Signaling Technology, 7074S) |
| Phospho-Stat3 (79, 86 kDa) |
1:1000 in 5% NFDM (Cell Signaling, 9131S) |
||
| Erk1/2 (42, 44 kDa) |
1:1000 in 5% NFDM (Abcam, ab138541) |
||
| Phospho-Erk1/2 (42, 44 kDa) |
1:1000 in 5% NFDM (Abcam, ab85375) |
||
| MMP-9 (84, 92 kDa) |
1:1000 in 5% NFDM (Cell Signaling, 3852S) |
||
| FAK (125 kDa) |
1:1000 in 5% NFDM (Cell Signaling, 3275S) |
||
| Phospho-FAK (125 kDa) |
1:1000 in 5% NFDM (Cell Signaling, 3283S) |
||
| CHCHD2 (16.7 kDa) |
0.4 μg/mL in 5% NFDM (Novus, NBP1–94106) |
||
| β-actin (48 kDa) | 1:1000 in 5% BSA (Cell Signaling, 4967S) |
BSA, bovine serum albumin; CHCHD2, coiled-coil-helix-coiled-coil-helix domain containing 2; FAK, focal adhesion kinase.
Flow cytometry analysis and sorting of CD133+ GBM cells
To examine the fraction of CD133+ GBM cells as a function of hydrogel composition and hypoxia, GBM6 containing hydrogels were dissociated using a combination of collagenase type IV (100 U/mL, Worthington Biochemical Corporation, Lakewood, NJ) and hyaluronidase (100 U/mL; Sigma-Aldrich) for 30 min at each culture time points (days 0, 3, 5, and 7). One million cells were separately collected upon receipt of GBM6 cells from Mayo Clinic to determine a baseline fraction of GBM6 cells. Cells were stained with propidium iodide (Thermo Fisher Scientific, Waltham, MA) to exclude dead cells as well as CD133/1(AC133) (Miltenyi Biotec, Bergisch Gladbach, Germany) following manufacturers' protocols. Flow cytometry was performed using a BD LSR II flow cytometer (BD Biosciences, Franklin Lakes, NJ) to quantify the fraction of CD133+ cells as a function of culture time and condition, with data analyzed using FCS Express 5.0 software (De Novo Software, Glendale, CA). For studies to examine the role of GSC fractions on GBM cell invasion, GBM6 cells were stained upon receipt with CD133/1(AC133) antibody (Miltenyi Biotec) and DAPI (Sigma-Aldrich), and then sorted using a fluorescence active sorter BD FACS ARIA II cytometer (BD Biosciences) to generate CD133+ and CD133− GBM6 cell fractions.
Analysis of patterns of GBM cell invasion through spheroid assay
We subsequently examined the contribution of CD133+ GSCs on GBM6 cell invasion using a spheroid-based invasion assay as previously described.11 We compared the invasion profile of spheroids created using CD133+ GSCs only versus a mixed GBM6 population comprising CD133+ and CD133- GBM cells. Disparate subfractions of GBM PDXs (CD133− GBM6; CD133+ GBM6; and mixed GBM6 cells) were resuspended at 25,000 cells/mL in culture media, with 200 μL (5000 cells) added into Corning spheroid microplate wells. Cell suspensions were cultured for 48 h in the incubator (37°C, 5% CO2) to form spheroids. Spheroids were then embedded into GelMA +/− HAMA hydrogels as previously described.11,46,59,60 Unfortunately, CD133− GBM6 cells did not form stable spheroids, so all comparisons were made for CD133+ GSC-only spheroids versus mixed GBM6 spheroids. GBM cell invasion was quantified over 7 days with images acquired using a Leica DMI 400B florescence microscope (Leica, Wetzlar, Germany). Invasion distance was calculated as fold change relative to the mean radius of each spheroid immediately after seeding through an Image J macro as previously described by our group.10,11 All invasion data were quantified with sample numbers at least n = 4.
Statistical analysis
OriginPro 2020 (Origin Lab) and RStudio were used for statistical analysis. Underlying assumptions for each statistical test were tested before analyzing data. All analyses were performed using one-way analysis of variance followed by Tukey's HSD post hoc test. Significance was defined as p < 0.05. Data are presented as mean ± standard deviation unless otherwise noted. Experimental group sizes were n = 3 unless otherwise noted.
Results
Matrix-immobilized HA promotes GBM6 proliferation and metabolic activity
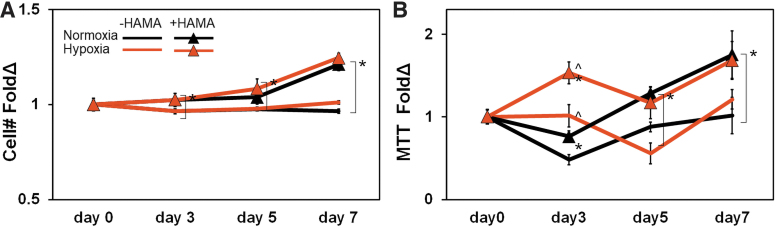
The number of GBM6 cells increased significantly with the presence of hydrogel-immobilized HA regardless of hypoxia/normoxia status (Fig. 1A). The metabolic activity of GBM-seeded hydrogels displayed a more complex temporal pattern in response to matrix-bound HA and extracellular hypoxia. Long-term metabolic activity data (day 7) aligned with overall cell numbers, with a significant increase in overall metabolic activity of GBM cells maintained in GelMA hydrogels containing matrix-immobilized HA (Fig. 1B). However, short-term responses (day 3) displayed a more complex response, with a significant increase in the measured metabolic activity of GBM6 cells in both GelMA and GelMA+HAMA hydrogel conditions in response to hypoxia (vs. normoxia). However, this effect was lost by 1 week in culture, suggesting a transient early response to hypoxia.
FIG. 1.
GBM6 cell expansion in response to matrix-bound HA and extracellular hypoxia. (A) Overall GBM6 cell number not affected by hypoxia, but increases significantly with the presence of matrix-immobilized HA. (B) GBM6 cell metabolic activity showed an early increase in response to hypoxia, but over extended cultures is significantly increased in response to matrix-immobilized hypoxia regardless of hypoxia versus normoxia. Sample numbers were all n = 3. ^p < 0.05 for +/− hypoxia; *p < 0.05 for +/− HAMA. GBM, glioblastoma; HA, hyaluronic acid; HAMA, methacrylamide-functionalized hyaluronic acid. Color images are available online.
GBM6 invasion was sensitive to matrix-bound HA, but not hypoxia
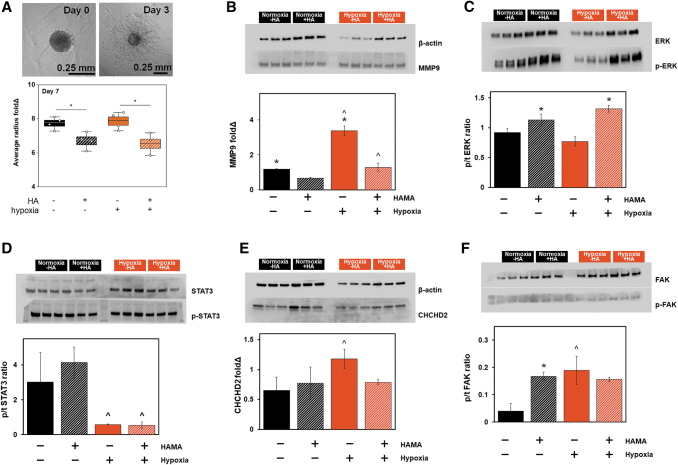
We subsequently examined GBM6 cell invasion in gelatin hydrogels in the presence or absence of matrix-immobilized HA and in the presence or absence of extracellular hypoxia (n = 4 for each group). Matrix-immobilized HA strongly inhibited GBM6 invasion (*p < 0.05), but extracellular hypoxia did not alter overall patterns of GBM6 invasion (Fig. 2A). We examined activation of signaling pathways involved in hypoxia response and matrix remodeling. MMP9 was significantly increased in response to extracellular hypoxia, but inhibited in gelatin hydrogels containing matrix-immobilized HA. Similarly, extracellular signal-regulated kinase 1/2 (ERK1/2) was strongly upregulated in response to matrix-bound HA, but showed no significant response to hypoxic challenge. Focal adhesion kinase (FAK) expression, involved in cellular adhesion and migration,61,62 was increased in response to both hypoxic challenge and the addition of matrix-immobilized HA, but the effects were not synergistic. STAT3 expression was strongly downregulated in response to extracellular hypoxia, but was not affected by the presence or absence of matrix-bound HA. Finally, we examined the expression of coiled-coil-helix-coiled-coil-helix domain containing 2 (CHCHD2), a mitochondria-to-nucleus signaling protein involved in hypoxia response and tied to proliferation, invasion, resistance to apoptosis, and stem cell differentiation.63–66 CHCHD2 expression was not affected by extracellular HA content under normoxia; however, CHCHD2 expression was significantly increased in response to extracellular hypoxia in GelMA-only hydrogels, an effect lost with the inclusion of matrix-bound HA.
FIG. 2.
Profiling shifts in GBM6 invasion and signal transduction in response to extracellular hypoxia and matrix-bound HA. (A) Representative image of GelMA-only GBM6 invasion from day 0 to 3 visualized by spheroid invasion assay and the quantitative radius fold change results on day 7. (B–E) Proteomic activity quantified by Western blotting (n = 3; *p < 0.05 for −/+ HAMA; ^p < 0.05 for −/+ hypoxia). (B) Hypoxia increased MMP9 activity regardless of the presence or absence of matrix-bound HA. (C) ERK1/2 phosphorylation increased in response to matrix-bound HA, but not in response to hypoxic culture. (D) STAT3 phosphorylation significantly reduced in response to hypoxia. (E) CHCHD2 was elevated in hypoxia in the absence of matrix-bound HA. (F) FAK phosphorylation was increased in response to matrix-immobilized HA, hypoxia, and the combination of the two, although the effects were not additive or synergistic. ^p < 0.05 for +/− hypoxia; *p < 0.05 for +/− HAMA. CHCHD2, coiled-coil-helix-coiled-coil-helix domain containing 2; ERK, extracellular regulated kinase; FAK, focal adhesion kinase; MMP9, matrix metalloproteinase 9; STAT3, signal transducer and activator of transcription 3. Color images are available online.
The relative size of the CD133+ subpopulation increased in response to matrix-immobilized HA and hypoxia
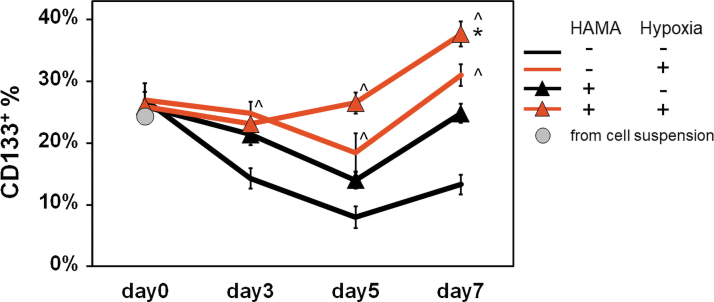
We subsequently quantified the change in the GSC-like subpopulation as a function of hypoxia and HA. We first compared the fraction of CD133+ GBM cells in the initial cell suspension versus immediately after hydrogel encapsulation to confirm hydrogel degradation and cell recovery do not affect analysis of cell CD133 expression. Flow cytometry confirmed roughly 25% (initial suspension 24.4%; GelMA: 27% ± 2.7% and GelMA+HAMA: 26% ± 2.3%) of GBM6 PDX cells were CD133 positive, suggesting hydrogel degradation and cell recovery do not influence CD133+ fraction results (Fig. 3). The relative fraction of CD133+ GBM cells was strongly responsive to matrix-bound HA and hypoxia over 7-day culture, with CD133+ fraction increasing significantly in response to both matrix-bound HA (regardless of hypoxia/normoxia status) or hypoxia (regardless of matrix HA status). Most notably, the combined effect of culture in matrix-bound HA and hypoxia showed the highest CD133+ fraction after 7 days along with the earliest response (between days 3 and 5 of culture).
FIG. 3.
Profiling the change in the relative size of the CD133+ GBM6 cell population by flow cytometry. There was no significant difference in CD133+ population before and immediately after encapsulation in the hydrogel on day 0. By 7 days of encapsulation, the fraction of CD133+ cells grew significantly in response to matrix-immobilized HA, hypoxia, and most significantly in response to the combination of the two. Hypoxia was the most significant driver of CD133+ cell expansion. n = 3. CD133+ fraction compared to nonstained blank sample. ^p < 0.05 for +/− hypoxia; *p < 0.05 for +/− HAMA. Color images are available online.
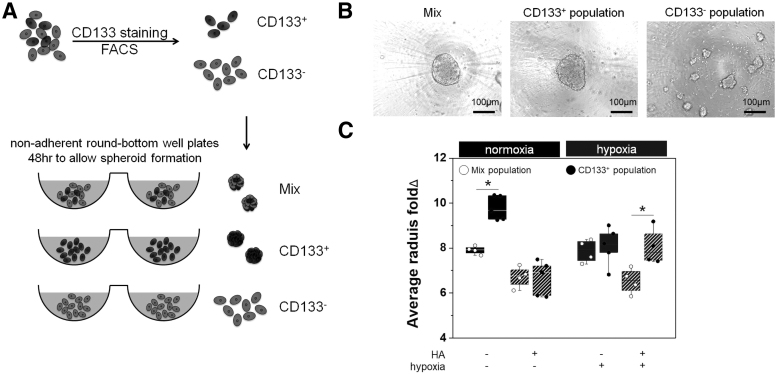
CD133-positive population is necessary to form GBM spheroids and modulated invasive phenotype in response to extracellular hypoxia and HA
Given the dynamic changes in the CD133+ subpopulation in PDX GBM cells in distributed hydrogel culture, we subsequently examined the effects of hypoxia and HA on GBM invasion and in particular how a CD133+ GBM population contributes to overall patterns of invasion. GBM6 PDX cells were sorted by FACS, based on their CD133 staining level, into CD133+ and CD133− populations and subsequently used to form spheroids (Fig. 4A and Supplementary Fig. S1). While CD133+ GBM cell fraction and the Mix population (that matched the original GBM6 specimen) robustly formed spheroids, all CD133- cell samples (n = 24) failed to form spheroids (conventional 48-h spheroid formation assay), but rather formed small, weakly adherent cell clusters (Fig. 4B). We subsequently characterized the invasive behavior of GBM cells using CD133+ and Mixed (CD133+/−) GBM populations, reporting invasion distance as fold change compared to their original radius immediately after encapsulation. In Mix (CD133+/−) GBM groups, GBM invasion was negatively affected by the presence of matrix-bound HA, but showed no response to hypoxia. Interestingly, while under normoxic conditions, CD133+ GBM cells showed significantly increased invasion in GelMA hydrogels, invasion for Mix (CD133+/−) and CD133+ GBM cells was both significantly reduced in GelMA+HAMA hydrogels containing matrix-bound HA (Fig. 4C). CD133+ GBM cells also showed significantly increased invasion versus Mix (CD133+/−) GBM spheroids in the presence of hypoxia and matrix-bound HA (Fig. 4C), the condition in which the most substantial CD133+ cell population expansion was observed.
FIG. 4.
Quantifying shifts in invasive phenotype of CD133+ GBM cells. (A) GBM6 cell specimens were sorted by FACS to generate CD133+ and CD133- GBM6 cell subpopulations to form cell spheroids for cell invasion assays. (B) While CD133+ and Mix (CD133+ + CD133− cells) groups formed spheroids, CD133− group was not able to form spheroids. (C) Invasion of overall GBM6 cells (Mix) decreased significantly in response to matrix-bound HA, but was not affected by extracellular hypoxia. CD133+ spheroids showed significantly increased invasion relative to Mix (CD133+ + CD133− cells) specimens in gelatin hydrogels under hypoxia. CD133+ spheroids showed significantly increased invasion (vs. Mix cells) in hydrogels containing matrix-immobilized HA and in response to hypoxia. Spheroid invasion assay performed for n = 4 (Mix-normoxia+HA, Mix-hypoxia-/+HA, CD133+hypoxia+HA) or n = 5 (Mix-normoxia-HA; CD133+ normoxia+HA; CD133+ hypoxia−/+HA) specimens. *p < 0.05.
CD133 status strongly influences protein expression patterns in response to extracellular hypoxia and HA
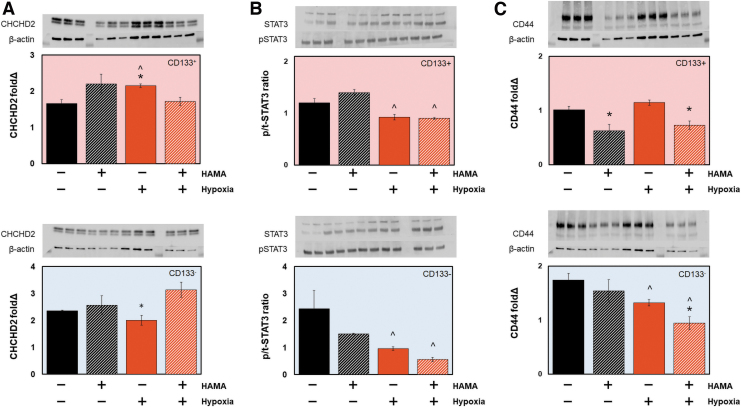
To examine how changes in signaling pathways within the CD133+ subpopulation may contribute to shifts in overall invasion, we examined expression profiles of CHCHD2, STAT3, and CD44 after 7 days in hydrogel culture for populations of sorted CD133+ or CD133− GBM6 cells (Fig. 5). Results were also compared to phenotypes observed for Mix (CD133+/−) cells (Fig. 2). CHCHD2 response in CD133+ GBM cells largely mimicked that seen for Mix populations, with increased CHCHD2 levels in response to hypoxia and in hydrogel environments lacking matrix-bound HA (Figs. 2E, 5A). The most striking difference between CD133+ and CD133− GBM6 cells came in hypoxic conditions, where CD133+ cells showed significantly decreased CHCHD2 expression, while CD133− cells showed significantly increased CHCHD2 expression in response to matrix-bound HA (Fig. 5A). STAT3 response in CD133+ and CD133− cell fractions largely mimicked that seen for Mix populations, with decreased STAT3 levels seen regardless of matrix HA content in response to extracellular hypoxia (Figs. 2D, 5B). CD44 expression levels in both CD133+ and CD133- cell fractions also showed significant decreases in GelMA+HAMA hydrogels containing matrix-bound HA and in response to hypoxia (Fig. 5C).
FIG. 5.
Western blot analysis of CD133+ versus CD133− GBM6 cell specimens in response to matrix-bound HA or extracellular hypoxia. (A) CHCHD2 expression in CD133+ cells was significantly increased in GelMA hydrogels under hypoxia (vs. GelMA normoxia; GelMA+HAMA hypoxia). CHCHD2 expression in CD133− cells was significantly increased in HA containing hydrogels in response to extracellular hypoxia. (B) STAT3 expression was largely similar in both CD133+ and CD133− GBM6, with significant decreases in STAT3 phosphorylation in response to extracellular hypoxia, regardless of the presence or absence of matrix-bound HA. (C) Both CD133+ and CD133− GBM6 cells showed decreased expression of CD44 with addition of matrix-bound HA and in the presence of hypoxia; CD133+ GBM6 cells also showed decreased expression of CD44 under normoxia in response to matrix-bound HA. ^p < 0.05 for +/− hypoxia; *p < 0.05 for +/− HAMA. Color images are available online.
Discussion
The tumor microenvironment presents a complex constellation of biophysical, biochemical, cellular, and metabolic cues that can impact processes such as cancer cell invasion and drug response. Tissue engineering technologies are increasingly offering the opportunity to evaluate cancer phenomena and pathophysiological processes linked to outcomes that are difficult to examine in vivo. In this study, we sought to build on recent efforts using gelatin hydrogels that identified both matrix-immobilized HA51 and hypoxia46 separately influenced patterns of GBM cell invasion, proliferation, and drug resistance. These findings aligned with findings related to spatial and temporal transitions within the tumor microenvironment where GBM are exposed to a diversity of cell-cell interactions and ECM proteins that accelerate invasion, drug resistance, and recurrence.38,39 However, future innovation in precision medicine applied toward GBM tumors requires a deeper consideration of GBM tumors as a heterogeneous mix of cells, notably the presence of a subpopulation of tumor-initiating cells (GSCs)17–19 critical for invasion, recurrence, and mortality.18,20–26 GSC-like cells are believed to vary temporally with progression and patient to patient, making it important to evaluate the role of features from the tumor microenvironment on GSC and cohorts of GBM cells containing defined fractions of GSCs.
For this study, we focused on the individual and combined effect of matrix-immobilized HA and hypoxia on GBM cell activity, using a previously characterized GBM6 patient-derived xenograft line known to be highly invasive in vivo.54,67 While serum-free media are often used to maintain or increase the stem cell-like fraction in cancer specimens, in this study, we kept cultures in serum-containing media to investigating the explicit influence of hypoxia and matrix-immobilized HA on stem fraction and GBM cell activity. Notably, we observed matrix-bound HA and extracellular hypoxia both influence the activity of cohorts of GBM6 cells as well as the relative expansion of a CD133+ cell subpopulation. At the cell cohort level, the presence of matrix-bound HA significantly increased GBM6 proliferation and ERK phosphorylation, but inhibited GBM cell invasion and MMP9 expression. While ERK1/2 and FAK phosphorylation were upregulated in the presence of matrix-immobilized HA, and are often connected to heightened cell migration, we observed reduced cell invasion, perhaps linked to reduced MMP activity and reduced matrix remodeling required for cell invasion in a fully 3D environment. Future efforts are adapting recently reported nascent protein labeling68 and matrisome analysis69–71 to consider the dynamics of local matrix remodeling within the hydrogel platform. Interestingly, extracellular hypoxia did not significantly influence bulk GBM6 proliferation or invasion, but did significantly inhibit STAT3 activation. While STAT3 phosphorylation has been reported to be activated under hypoxia, hypoxia-induced suppressors of cytokine signaling 3 (SOC3) have been reported to suppress STAT3 phosphorylation,72 consistent with the inhibited p/t-STAT3 shown in this study. We observed increased activity of CHCHD2 in response to hypoxia in gelatin hydrogels, consistent with previous reports that CHCHD2 expression increased in response to hypoxia.66,73 However, future efforts will need to evaluate not only expression but also mitochondrial versus nuclear localization of CHCHD2.74 Increased FAK phosphorylation in response to matrix-immobilized HA, hypoxia, or both is consistent with previous reports.61,62
Overall GBM6 invasion was inhibited in the presence of matrix-bound HA, but was not responsive to hypoxia, consistent with our previous studies using U251 cells.10 In this study, we also considered the explicit role of a CD133+ cell subpopulation in GBM invasion. The CD133+ group showed increased invasion relative to the overall (Mix) GBM6 cell population both in gelatin hydrogels in response to normoxia, but more interestingly in matrix-immobilized HA and in response to hypoxia. Interestingly, compensatory production of soluble HA in gelatin environments has previously been identified as a significant feature in enhanced invasion, so parsing the role of HA remodeling in CD133+ versus CD133− cell specimens will be an important subject of future efforts using these patient-derived specimens. Notably, observed shifts in overall CHCHD2 expression in hypoxic environments and in the presence of matrix-bound HA for CD133+ versus CD133− cells should be complemented with examination of the nuclear versus mitochondrial compartmental localization of these factors. STAT3 phosphorylation remained consistent among CD133+ versus CD133− cells. Interestingly, STAT3 expression has been previously associated with invasion, and we observed decreased STAT3 phosphorylation in hypoxic environments that did not correlate to reduced GBM cell invasion in all cases, suggesting the need to examine a wider range of factors associated with CD133- cell invasion. Together, these responses suggested that a series of signaling cascades related to matrix-bound hyaluronan and extracellular hypoxia, as well as the presence of a subfraction of CD133+ GBM cells, all play a significant role in mediating an invasive phenotype. While this work was performed using a single patient-derived xenograft cell source, we demonstrate a workflow for rapidly examining GBM cell activation and invasive phenotype in an engineered tissue model. Integrating matrix composition and metabolic restrictions hold promise to identify signaling pathways and external stimuli simultaneously to achieve improved therapeutic outcomes.
Conclusions
Clinical progression of GBM has been suggested to be linked with the activity of a stem cell-like subpopulation. However, the dynamic nature of such subpopulations in response to the matrix and metabolic conditions within the tumor microenvironment makes it difficult to investigate GSC progression in vivo. We show that a multidimensional gelatin hydrogel platform can be used to examine the behavior of patient-derived GBM specimens in response to matrix and metabolic signals. The CD133+ GBM subpopulation expanded significantly in response to matrix-bound HA and hypoxia, contributed significantly to the ability to form cell spheroids, and showed increased invasive potential in environments containing matrix-bound HA and hypoxia versus the overall GBM cell population. These studies also motivate significant future work to refine our understanding of the signaling interactions between the size and activity of a GSC subpopulation with invasive phenotype and therapeutic response.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary Material
Acknowledgments
We acknowledge Dr. Jan Lumibao, Dr. H. Rex Gaskins, and Dr. Romana Nowak for access to the hypoxia chamber. We acknowledge Haw-Wen Hsiao for generating Matlab code for mechanical analysis.
Disclosure Statement
No competing financial interests exist.
Funding Information
Research reported in this publication was supported by the National Cancer Institute, National Institute of Diabetes and Digestive and Kidney Diseases, and the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Numbers R01 CA256481 (B.A.C.H.), R01CA197488 (B.A.C.H.), R01 DK099528 (B.A.C.H.), and T32EB019944 (J.-W.E.C.). The authors are also grateful for additional funding provided by the Department of Chemical and Biomolecular Engineering, the Cancer Center at Illinois, and the Carl R. Woese Institute for Genomic Biology at the University of Illinois at Urbana-Champaign. Development and maintenance of the GBM PDC models were supported by Mayo Clinic, the Mayo SPORE in Brain Cancer (P50 CA108961), and the Mayo Clinic Brain Tumor Patient-Derived Xenograft National Resource (R24 NS092940).
Supplementary Material
References
- 1. Anne, C., Catherine, G., Rogatien, F., et al. Glioblastoma-associated stromal cells (GASCs) from histologically normal surgical margins have a myofibroblast phenotype and angiogenic properties. J Pathol 233, 74, 2014. [DOI] [PubMed] [Google Scholar]
- 2. Paolillo, M., Boselli, C., and Schinelli, S.. Glioblastoma under siege: an overview of current therapeutic strategies. Brain Sci 8, 15, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnson, D.R., and O'Neill, B.P.. Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol 107, 359, 2012. [DOI] [PubMed] [Google Scholar]
- 4. Jackson, C., Ruzevick, J., Phallen, J., Belcaid, Z., and Lim, M.. Challenges in immunotherapy presented by the glioblastoma multiforme microenvironment. Clin Dev Immunol 2011, 732413, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stupp, R., Mason, W.P., van den Bent, M.J., et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352, 987, 2005. [DOI] [PubMed] [Google Scholar]
- 6. Mehta, A.I., Linninger, A., Lesniak, M.S., and Engelhard, H.H.. Current status of intratumoral therapy for glioblastoma. J Neurooncol 125, 1, 2015. [DOI] [PubMed] [Google Scholar]
- 7. Wiranowska, M.R., and Rojiani, M.V.. Extracellular Matrix Microenvironment in Glioma Progression. Rijeka, Croatia: InTech, 2011. [Google Scholar]
- 8. Syková, E. Plasticity of extracellular space. In: Walz, W., ed. The Neuronal Environment: Brain Homeostasis in Health and Disease. Totowa, NJ: Humana Press, 2002, p. 57. [Google Scholar]
- 9. Quirico-Santos, T., Fonseca, C.O., and Lagrota-Candido, J.. Brain sweet brain: importance of sugars for the cerebral microenvironment and tumor development. Arq Neuro Psiquiatr 68, 799, 2010. [DOI] [PubMed] [Google Scholar]
- 10. Chen, J.-W.E., Pedron, S., and Harley, B.A.C.. The combined influence of hydrogel stiffness and matrix-bound hyaluronic acid content on glioblastoma invasion. Macromol Biosci 17, 1700018, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen, J.-W.E., Pedron, S., Shyu, P., Hu, Y., Sarkaria, J.N., and Harley, B.A.C.. Influence of hyaluronic acid transitions in tumor microenvironment on glioblastoma malignancy and invasive behavior. Front Mater 5, 39, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Munson, J.M., Bellamkonda, R.V., and Swartz, M.A.. Interstitial flow in a 3D microenvironment increases glioma invasion by a CXCR4-dependent mechanism. Cancer Res 73, 1536, 2013. [DOI] [PubMed] [Google Scholar]
- 13. Atay, N., Yuan, J., Cornelison, C., and Munson, J.. Tami-15. The effect of interstitial fluid flow and astrocytes/microglia on invasion, proliferation and stemness of patient-derived glioma stem cells. Neurooncology 22, ii216, 2020. [Google Scholar]
- 14. Ho, I.A.W., and Shim, W.S.N.. Contribution of the microenvironmental niche to glioblastoma heterogeneity. BioMed Res Int 2017, 13, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Michiels, C. Physiological and pathological responses to hypoxia. Am J Pathol 164, 1875, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vartanian, A., Singh, S.K., Agnihotri, S.,et al. GBM's multifaceted landscape: highlighting regional and microenvironmental heterogeneity. Neuro Oncol 16, 1167, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Charles, N.A., Holland, E.C., Gilbertson, R., Glass, R., and Kettenmann, H.. The brain tumor microenvironment. Glia 59, 1169, 2011. [DOI] [PubMed] [Google Scholar]
- 18. Binda, E., Visioli, A., Reynolds, B., and Vescovi, A.L.. Heterogeneity of cancer-initiating cells within glioblastoma. Front Biosci (Schol Ed) 4, 1235, 2012. [DOI] [PubMed] [Google Scholar]
- 19. Reynolds, B.A., and Vescovi, A.L.. Brain cancer stem cells: think twice before going flat. Cell Stem Cell 5, 466, 2009. [DOI] [PubMed] [Google Scholar]
- 20. Lathia, J.D., Mack, S.C., Mulkearns-Hubert, E.E., Valentim, C.L., and Rich, J.N.. Cancer stem cells in glioblastoma. Genes Dev 29, 1203, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Uemae, Y., Ishikawa, E., Osuka, S., et al. CXCL12 secreted from glioma stem cells regulates their proliferation. J Neurooncol 117, 43, 2014. [DOI] [PubMed] [Google Scholar]
- 22. Sun, L., Hui, A.M., Su, Q., et al. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell 9, 287, 2006. [DOI] [PubMed] [Google Scholar]
- 23. Baysan, M., Woolard, K., Bozdag, S., et al. Micro-environment causes reversible changes in DNA methylation and mRNA expression profiles in patient-derived glioma stem cells. PLoS One 9, e94045, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Binda, E., Reynolds, B.A., and Vescovi, A.L.. Glioma stem cells: turpis omen in nomen? (The evil in the name?). J Intern Med 276, 25, 2014. [DOI] [PubMed] [Google Scholar]
- 25. Lemke, D., Weiler, M., Blaes, J., et al. Primary glioblastoma cultures: can profiling of stem cell markers predict radiotherapy sensitivity? J Neurochem 131, 251, 2014. [DOI] [PubMed] [Google Scholar]
- 26. Hambardzumyan, D., Cheng, Y.-K., Haeno, H., Holland, E.C., and Michor, F.. The probable cell of origin of NF1- and PDGF-driven glioblastomas. PLoS One 6, e24454, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pranatharthi, A., Ross, C., and Srivastava, S.. Cancer stem cells and radioresistance: Rho/ROCK pathway plea attention. Stem Cells Int 2016, 5785786, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kalkan, R. Hypoxia is the driving force behind GBM and could be a new tool in GBM treatment. Crit Rev Eukaryot Gene Expr 25, 363, 2015. [DOI] [PubMed] [Google Scholar]
- 29. Hambardzumyan, D., and Bergers, G.. Glioblastoma: defining tumor niches. Trends Cancer 1, 252, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Persano, L., Rampazzo, E., Basso, G., and Viola, G.. Glioblastoma cancer stem cells: role of the microenvironment and therapeutic targeting. Biochem Pharmacol 85, 612, 2013. [DOI] [PubMed] [Google Scholar]
- 31. Sakariassen, P.Ø., Immervoll, H., and Chekenya, M.. Cancer stem cells as mediators of treatment resistance in brain tumors: status and controversies. Neoplasia 9, 882, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Medema, J.P. Cancer stem cells: the challenges ahead. Nat Cell Biol 15, 338, 2013. [DOI] [PubMed] [Google Scholar]
- 33. Clarke, M.F., Dick, J.E., Dirks, P.B., et al. Cancer stem cells—perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res 66, 9339, 2006. [DOI] [PubMed] [Google Scholar]
- 34. Mei, X., Chen, Y.-S., Chen, F.-R., Xi, S.-Y., and Chen, Z.-P.. Glioblastoma stem cell differentiation into endothelial cells evidenced through live-cell imaging. Neurooncology 19, 1109, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Filatova, A., Acker, T., and Garvalov, B.K.. The cancer stem cell niche(s): the crosstalk between glioma stem cells and their microenvironment. Biochim Biophys Acta 1830, 2496, 2013. [DOI] [PubMed] [Google Scholar]
- 36. Guelfi, S., Duffau, H., Bauchet, L., Rothhut, B., and Hugnot, J.P.. Vascular transdifferentiation in the CNS: a focus on neural and glioblastoma stem-like cells. Stem Cells Int 2016, 2759403, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dirks, P.B. Cancer: stem cells and brain tumours. Nature 444, 687, 2006. [DOI] [PubMed] [Google Scholar]
- 38. Gilbertson, R.J., and Rich, J.N.. Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer 7, 733, 2007. [DOI] [PubMed] [Google Scholar]
- 39. Vescovi, A.L., Galli, R., and Reynolds, B.A.. Brain tumour stem cells. Nat Rev Cancer 6, 425, 2006. [DOI] [PubMed] [Google Scholar]
- 40. Goffart, N., Kroonen, J., and Rogister, B.. Glioblastoma-initiating cells: relationship with neural stem cells and the micro-environment. Cancers (Basel) 5, 1049, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bayin, N.S., Modrek, A.S., and Placantonakis, D.G.. Glioblastoma stem cells: molecular characteristics and therapeutic implications. World J Stem Cells 6, 230, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Joo, K.M., Kim, S.Y., Jin, X., et al. Clinical and biological implications of CD133-positive and CD133-negative cells in glioblastomas. Lab Invest 88, 808, 2008. [DOI] [PubMed] [Google Scholar]
- 43. Rappa, G., Fodstad, O., and Lorico, A.. The stem cell-associated antigen CD133 (Prominin-1) is a molecular therapeutic target for metastatic melanoma. Stem Cells 26, 3008, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yao, J., Zhang, T., Ren, J., Yu, M., and Wu, G.. Effect of CD133/prominin-1 antisense oligodeoxynucleotide on in vitro growth characteristics of Huh-7 human hepatocarcinoma cells and U251 human glioma cells. Oncol Rep 22, 781, 2009. [DOI] [PubMed] [Google Scholar]
- 45. Guvenc, H., Pavlyukov, M.S., Joshi, K., et al. Impairment of glioma stem cell survival and growth by a novel inhibitor for survivin–ran protein complex. Clin Cancer Res 19, 631, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen, J.-W.E., Lumibao, J., Blazek, A., Gaskins, H.R., and Harley, B.. Hypoxia activates enhanced invasive potential and endogenous hyaluronic acid production by glioblastoma cells. Biomater Sci 6, 854, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Minet, E., Arnould, T., Michel, G., et al. ERK activation upon hypoxia: involvement in HIF-1 activation. FEBS Lett 468, 53, 2000. [DOI] [PubMed] [Google Scholar]
- 48. Guo, G., Yao, W., Zhang, Q., and Bo, Y.. Oleanolic acid suppresses migration and invasion of malignant glioma cells by inactivating MAPK/ERK signaling pathway. PLoS One 8, e72079, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kim, J.-Y., Kim, Y.-J., Lee, S., and Park, J.-H.. The critical role of ERK in death resistance and invasiveness of hypoxia-selected glioblastoma cells. BMC Cancer 9, 27, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Du, R., Petritsch, C., Lu, K., et al. Matrix metalloproteinase-2 regulates vascular patterning and growth affecting tumor cell survival and invasion in GBM. Neurooncology 10, 254, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pedron, S., Wolter, G.L., Chen, J.-W.E., Laken, S., Sarkaria, J.N., and Harley, B.A.C.. Hyaluronic acid-functionalized gelatin hydrogels reveal extracellular matrix signals temper the efficacy of erlotinib against patient-derived glioblastoma specimens. Biomaterials 219, 119371, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pedron, S., Becka, E., and Harley, B.A.C.. Regulation of glioma cell phenotype in 3D matrices by hyaluronic acid. Biomaterials 34, 7408, 2013. [DOI] [PubMed] [Google Scholar]
- 53. Chen, J.-W.E., Lumibao, J., Leary, S., et al. Crosstalk between microglia and patient-derived glioblastoma cells inhibit invasion in a three-dimensional gelatin hydrogel model. J Neuroinflamm 17, 346, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carlson, B.L., Pokorny, J.L., Schroeder, M.A., and Sarkaria, J.N.. Establishment, maintenance and in vitro and in vivo applications of primary human glioblastoma multiforme (GBM) xenograft models for translational biology studies and drug discovery. Curr Protoc Pharmacol Chapter 14, Unit 16, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pence, J.C., Clancy, K.B.H., and Harley, B.A.C.. The induction of pro-angiogenic processes within a collagen scaffold via exogenous estradiol and endometrial epithelial cells. Biotechnol Bioeng 112, 2185, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Caliari, S.R., and Harley, B.A.C.. The effect of anisotropic collagen-GAG scaffolds and growth factor supplementation on tendon cell recruitment, alignment, and metabolic activity. Biomaterials 32, 5330, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pedron, S., Becka, E., and Harley, B.A.. Spatially gradated hydrogel platform as a 3D engineered tumor microenvironment. Adv Mater 27, 1567, 2015. [DOI] [PubMed] [Google Scholar]
- 58. Caliari, S.R., Weisgerber, D.W., Grier, W.K., Mahmassani, Z., Boppart, M.D., and Harley, B.A.C.. Collagen scaffolds incorporating coincident gradations of instructive structural and biochemical cues for osteotendinous junction engineering. Adv Healthcare Mater 4, 831, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zambuto, S.G., Clancy, K.B.H., and Harley, B.A.C.. Tuning trophoblast motility in a gelatin hydrogel via soluble cues from the maternal-fetal interface. Tissue Eng Part A 27, 1064, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zambuto, S., Clancy, K.B.H., and Harley, B.A.C.. A gelatin hydrogel to study endometrial angiogenesis and trophoblast invasion. Interface Focus 9, 20190016, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim, Y., Park, Y.W., Lee, Y.S., and Jeoung, D.. Hyaluronic acid induces transglutaminase II to enhance cell motility; role of Rac1 and FAK in the induction of transglutaminase II. Biotechnol Lett 30, 31, 2008. [DOI] [PubMed] [Google Scholar]
- 62. Lee, S.H., Lee, Y.J., Song, C.H., Ahn, Y.K., and Han, H.J.. Role of FAK phosphorylation in hypoxia-induced hMSCS migration: involvement of VEGF as well as MAPKS and eNOS pathways. Am J Physiol Cell Physiol 298, C847, 2010. [DOI] [PubMed] [Google Scholar]
- 63. Monaghan, R.M., and Whitmarsh, A.J.. Mitochondrial proteins moonlighting in the nucleus. Trends Biochem Sci 40, 728, 2015. [DOI] [PubMed] [Google Scholar]
- 64. Aras, S., Bai, M., Lee, I., Springett, R., Huttemann, M., and Grossman, L.I.. MNRR1 (formerly CHCHD2) is a bi-organellar regulator of mitochondrial metabolism. Mitochondrion 20, 43, 2015. [DOI] [PubMed] [Google Scholar]
- 65. Aras, S., Pak, O., Sommer, N., et al. Oxygen-dependent expression of cytochrome c oxidase subunit 4–2 gene expression is mediated by transcription factors RBPJ, CXXC5 and CHCHD2. Nucleic Acids Res 41, 2255, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Grossman, L.I., Purandare, N., Arshad, R., et al. MNRR1, a biorganellar regulator of mitochondria. Oxid Med Cell Longev 2017, 6739236, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cen, L., Carlson, B.L., Schroeder, M.A., et al. p16-Cdk4-Rb axis controls sensitivity to a cyclin-dependent kinase inhibitor PD0332991 in glioblastoma xenograft cells. Neuro Oncol 14, 870, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Loebel, C., Kwon, M.Y., Wang, C., Han, L., Mauck, R.L., and Burdick, J.A.. Metabolic labeling to probe the spatiotemporal accumulation of matrix at the chondrocyte–hydrogel interface. Adv Funct Mater 30, 1909802, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Izzi, V., Davis, M.N., and Naba, A.. Pan-cancer analysis of the genomic alterations and mutations of the matrisome. Cancers (Basel) 12, 2046, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Arteel, G.E., and Naba, A.. The liver matrisome—looking beyond collagens. JHEP Rep 2, 100115, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hynes, R.O., and Naba, A.. Overview of the matrisome—an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol 4, a004903, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bai, L., Yu, Z., Qian, G., et al. SOCS3 was induced by hypoxia and suppressed STAT3 phosphorylation in pulmonary arterial smooth muscle cells. Respir Physiol Neurobiol 152, 83, 2006. [DOI] [PubMed] [Google Scholar]
- 73. Seo, M., Lee, W.-H., and Suk, K.. Identification of novel cell migration-promoting genes by a functional genetic screen. FASEB J 24, 464, 2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.