Abstract
The emergence of the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) represents an unprecedented threat for the human population, necessitating rapid and effective intervention measures. Given the main infection route by airborne transmission, significant attention has been bestowed upon the use of antiseptic mouthrinses as a way to possibly reduce infectious viral titers. However, clinical evaluations are still sparse. Thus, we evaluated a wide variety of antiseptic agents that can be used as mouthrinses for their antiviral effects in vitro and their respective mode of action. One of the most promising antiseptic agents (benzalkoniumchloride, BAC) was used in a randomized placebo-controlled clinical trial with subsequent analysis of viral loads by RT-qPCR and virus rescue in cell culture. Mechanistic analysis revealed that treatment with BAC and other antiseptic agents efficiently inactivated SARS-CoV-2 in vitro by primarily disrupting the viral envelope, without affecting viral RNA integrity. However, the clinical application only resulted in a mild reduction of viral loads in the oral cavity. These results indicate that gargling with mouthrinses comprising single antiseptic agents may play a minor role towards a potential reduction of transmission rates and thus, these findings are of utmost importance when considering alternative COVID-19 prevention strategies.
Keywords: SARS-CoV-2, Antiseptic agents, Mouthrinse, Benzalkonium chloride, Capsid protection assay, Mouthwash
1. Introduction
The pandemic caused by the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) and its associated Coronavirus Disease 2019 (COVID-19) represents an unprecedented threat for the human population, causing millions of infections worldwide. Despite ongoing SARS-CoV-2 vaccination campaigns, current vaccines cannot reliably exclude infectious virus in the upper respiratory tract upon re-exposure, in particular with the currently circulating variants of concern (Buttenheim, 2020; Krammer, 2020). Therefore, effective measures preventing further global spread of the virus remain essential. SARS-CoV-2 is transmitted either by direct contact or by airborne transmission due to inhalation of aerosols and respiratory droplets (van Doremalen et al., 2020). Independent of the severity of clinical symptoms, patients can exhibit high viral loads in the oropharynx and the oral cavity (van Doremalen et al., 2020; Zou et al., 2020). Accordingly, Johansson et al. demanded that effective control of SARS-CoV-2 spread required a reduction of transmissions from individuals with subclinical course of COVID-19. It is estimated that the transmissions from such asymptomatic individuals account for more than half of SARS-CoV-2 transmissions (Johansson et al., 2021).
Besides other COVID-19 prevention measures such as facemasks, hand disinfection and social distancing, the use of throat sprays or mouthrinses containing antiseptic agents has been discussed for temporarily reducing viral titers in the oral cavity and oropharynx (Carrouel et al., 2021; O'Donnell et al., 2020). The use of oral antiseptics has already been recommended in the early stages of the pandemic for protecting healthcare professionals (HCPs) with close contact to patients, such as dentists, maxillofacial surgeons and otorhinolaryngologists, but the data basis behind these recommendations was scarce (Carrouel et al., 2021; Gottsauner et al., 2020; Krajewska et al., 2020; Peng et al., 2020; Zimmermann and Nkenke, 2020). In the meantime, some in vitro studies have reported antiviral effects against SARS-CoV-2 for several antiseptic agents (Bidra et al., 2020; Carrouel et al., 2021; Meister et al., 2020; Muñoz-Basagoiti et al., 2021). However, in vitro studies concurrently investigating a broader range of compounds and, in particular, the underlying mechanisms of antiviral effects on SARS-CoV-2 are still lacking (Carrouel et al., 2021). Furthermore, clinical translation of the promising results remains unclear (Carrouel et al., 2021; Gottsauner et al., 2020; Seneviratne et al., 2021). Importantly, the impact of a “false sense of security” in HCPs and the public should not be underestimated as this may lead to reduced use of protective gear or increased social interaction with potentially infected individuals (Carrouel et al., 2021; Gottsauner et al., 2020). Therefore, recommendations of oral antiseptics solely based on in vitro data need to be reflected critically until clinical studies are available, and current research needs to address this knowledge gap as soon as possible (Carrouel et al., 2021).
Thus, the aim of the present study was to evaluate a wide variety of antiseptic agents for their antiviral effects towards SARS-CoV-2 and the underlying mechanisms of action in vitro, and investigate the most effective compound in a randomized placebo-controlled clinical trial for its efficacy in terms of reducing viral loads and infectivity in the oral cavity of infected individuals.
2. Material and methods
2.1. In vitro experiments
2.1.1. Cell culture
For SARS-CoV-2 isolation VeroE6 cells, kindly provided by C. Drosten and M. Müller, were seeded in 25 cm2 flasks at a density of 7 × 105 cells in Dulbecco's modified Eagle's medium (DMEM, supplemented with 10% (v/v) fetal calf serum, 1% non-essential amino acids, 100 IU/mL penicillin, 100 µg/mL streptomycin and 2 mM L-Glutamine). The next day, medium was replaced by 2.5 μg/mL amphotericin B–containing medium. Subsequently, 100 μL of a SARS-CoV-2 positive throat swab was added to the cells and incubated until cytopathic effects were observed. After 6 days the supernatant was harvested and cell debris were removed by centrifugation. Afterwards, the supernatant was used to inoculate VeroE6 cells seeded in 75 cm2 flasks at 2 × 106 cells to amplify the virus. The SARS-CoV-2 was designated hCoV-19/Germany/BY-Bochum-1/2020 (GISAID ID: EPI_ISL_1118929; Meister et al., 2021). Supernatant was aliquoted and stored at -80 °C until further usage. Viral titers were determined by endpoint-dilution and the 50% tissue culture infective dose (TCID50/mL) was calculated according to Spearman and Kärber (Kärber, 1931; Spearman, 1908).
2.1.2. Quantitative suspension test and virus titration
Virucidal activity of a variety of commercially available mouthrinses and single antiseptic agents (Table 1 ) found in those mouthrinses was determined by a quantitative suspension test as published recently (Meister et al., 2020).
Table 1.
Overview of antiseptic agents used in the study in regard to the concentration, cytotoxicity and calculated log10 reduction factors.
| Agent | Concentration (% in water) | Log10-RF | Log10-Cytotoxicity |
|---|---|---|---|
| Benzalkoniumchloride (BAC) | 0.025 | 1.03 | 2.2 |
| 0.05 | 3.11 | 2.2 | |
| 0.075 | ≥ 3.74 | 2.2 | |
| 0.1 | ≥ 3.74 | 2.2 | |
| Cetylpyridiniumchloride (CPC) | 0.025 | 0.37 | 3.2 |
| 0.05 a | 2.24 | 3.2 | |
| 0.075 a | 2.37 | 3.2 | |
| 0.1 | ≥ 2.74 | 3.2 | |
| Chlorhexidine digluconate (CHX) | 0.1 | 0.46 | 3.2 |
| 0.125 | 0.05 | 3.2 | |
| 0.2 a | 0.57 | 3.2 | |
| 0.5 | 1.22 | 3.2 | |
| Dequaliniumchloride (DQC) | 0.025 | 0.22 | 2.2 |
| 0.05 | 0.31 | 2.2 | |
| 0.075 | 0.48 | 2.2 | |
| 0.1 | 0.22 | 2.2 | |
| Hydrogen peroxide (H2O2) | 0.5 | 0.67 | 4.2 |
| 1 a | 0.63 | 4.2 | |
| 2 | 0.37 | 4.2 | |
| 3 | 0.68 | 4.2 | |
| Hydroxyapatite (HAP) | 0.1 | 0.42 | 3.2 |
| 0.5 | 0.01 | 3.2 | |
| 1 | 0 | 3.2 | |
| Octenidine-Dihydrochloride (Oct-DiHCl) | 0.05 | ≥ 2.97 | 3.2 |
| 0.1 a | ≥ 1.97 | 4.2 | |
| 0.5 | ≥ 0.97 | 5.2 | |
| 2 | 0 | 6.2 | |
| Polyaminopropyl-Biguanide (PAP) | 0.05 | 0.42 | 2.2 |
| 0.15 a | 0.41 | 2.2 | |
| 0.3 | 0.52 | 2.2 | |
| 0.5 | 0.86 | 2.2 | |
| Polyvenylpyrrolidone iodine (PVP-I) | 0.05 | 0.39 | 2.2 |
| 0.1 | 0.62 | 2.2 | |
| 0.5 | 3.91 | 2.2 | |
| 1 a | 3.8 | 2.2 | |
| Surfactants (Sodium Lauryl Sulfate, Sodium Methyl Cocoyl Taurate, Sodium Myristoyl Sarcosinate) | 0.05 | 1 | 2.2 |
| 0.1 | ≥ 3.77 | 2.2 | |
| 0.5 | ≥ 3.77 | 2.2 | |
| 1 | ≥ 3.77 | 2.2 |
Concentration that normally occur in commercially available mouthrinses.
To this end, eight-parts mouthrinse, active agent or medium were mixed with one-part nasal secrete (Eggers et al., 2009) mirroring respiratory secretion (100 μL of 4 mg/mL mucin type I-S, 25 μL of 50 mg/mL BSA Fraction V, and 35 μL of 50 mg/mL yeast extract), and one-part SARS-CoV-2 (200 µL in total). The suspension was immediately vortexed for 30 s to mimic gargling and subsequently titrated serially on VeroE6 cells. After an incubation at 37 °C for 72 h, the cells were fixed and stained by crystal violet and wells displaying cytopathic effects were counted to calculate TCID50 values. Reduction factors were calculated as described before (Meister et al., 2020). Additionally, cytotoxic effects were monitored by replacing SARS-CoV-2 with phosphate buffered saline (PBS) and visual inspection of the cell layer by crystal violet staining and are indicated as the lower limit of quantification (LLOQ). All experiments were executed in at least three independent biological replicates.
2.1.3. Density gradient ultracentrifugation (DGU)
A 0-40% step iodixanol (Optiprep; Sigma Aldrich) gradient was created. Therefore, a working solution of 40% iodixanol, 0.2% (w/v) sodium chloride and 10 mM tricine-NaOH (pH 7.4) was prepared. This solution was then further diluted with CSM (0.77% (w/v) sodium chloride and 10 mM tricine-NaOH) to create 10, 20 and 30% iodixanol solutions. From bottom to top of a 5 mL centrifugation tube 1 mL of each solution was layered with declining density. On top of the step gradient the total 200 µL from the quantitative suspension test mixed with 800 µL of CSM were layered. Gradients were centrifuged for 18 h at 100,000 × g at 4 °C. Ten fractions of 0.5 mL each were collected from the top and virus infectivity (as TCID50/mL) as well as the amount of viral RNA by RT-qPCR was determined. The density of each fraction was quantified by refractometry.
2.1.4. Reverse transcription quantitative PCR (RT-qPCR)
SARS-CoV-2 RNA was isolated using the QIAamp Viral RNA Kit (Qiagen) according to the manufacturer's instructions. RNA was directly subjected to a one-step quantitative PCR (RT-qPCR) running a GoTaq Probe RT-qPCR System (Promega). RT-qPCR was performed as described previously (Toptan et al., 2020) using a light cycler LC480 to quantify the M-Gene abundance.
2.1.5. Capsid protection assay and western blot
According to the quantitative suspension test antiseptic agents were mixed with organic load and SARS-CoV-2 in triplicates and vortexed for 30 s. The first replicate was directly inactivated by adding 50 µL of 5 × Laemmli buffer followed by a 10 min incubation at 95 °C. The second replicate was incubated with 50 µg/mL Proteinase K (Roche), while the third replicate was additionally treated with 5% Triton X-100. Both samples were incubated for 1 h on ice. Proteinase K was impeded by adding 5 mM Phenylmethylsulfonylfluorid (PMSF) for 10 min on ice. Subsequently, 5 × Laemmli buffer was added and the samples were heat inactivated for western blot analysis. To detect the nucleocapsid protein a sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed to separate viral proteins according to their size for 2 h at 100 V. In a wet blot, proteins were transferred on a nitrocellulose membrane for 1 h at 110 V. The primary antibody anti-SARS-CoV-2 nucleocapsid (antibodies-online, 1:1,000 dilution in 0.5% non-fat dry milk and 0.5% Tween-20 in PBS) was incubated over night at 4 °C with agitation. After washing the membrane, the secondary antibody (Jackson Immuno, anti-mouse HRP, 1:10,000) was applied for 1 h at room temperature with agitation. The protein signal was later detected by enhanced chemiluminescence (ECL, Thermo Fisher). Signal intensity was quantified using Fiji.
2.2. Randomized placebo-controlled clinical trial
For details, please see Supplementary Methods.
3. Results
3.1. Various agents of mouthrinses reduce SARS-CoV-2 infectivity in vitro
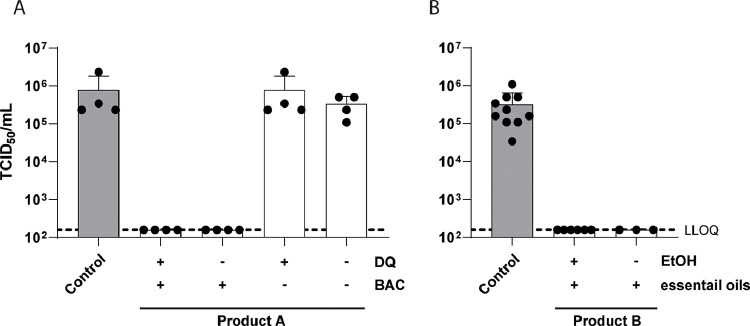
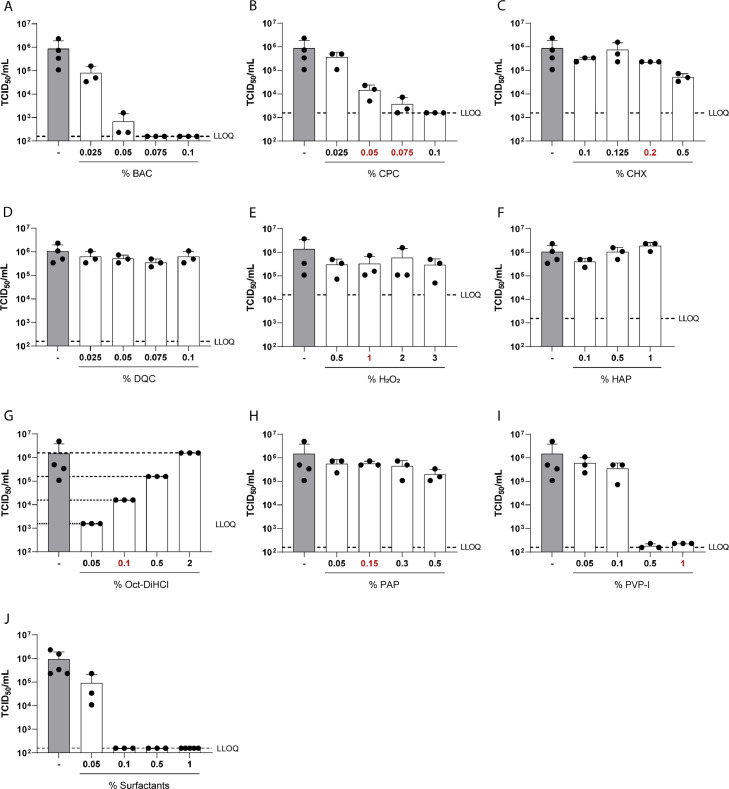
Several mouthrinses have been shown to effectively reduce SARS-CoV-2 infectivity in vitro, however, the antiseptic agent responsible for the reduction has not been elucidated in the majority of studies (Meister et al., 2020). Therefore, we investigated two mouthrinses with distinct component compositions regarding their antiviral activity towards SARS-CoV-2 in vitro by performing a quantitative suspension test (Fig. 1 ). Additionally, cytotoxic effects were monitored and are indicated as the lower limit of quantification (LLOQ). Whilst Product A contains dequaliniumchloride (DQ) and benzalkoniumchloride (BAC), Product B is a mixture of essential oils and ethanol. Testing their antiviral activity revealed that Product A containing both antiseptic agents, as sold by the manufacturer, efficiently inactivates SARS-CoV-2 to background levels within 30 seconds of exposure. As soon as BAC is removed from the composition, no antiviral activity was observed. Eliminating DQ from the original mouthrinse does not influence the inactivation capacity of Product A (Fig. 1A). Whereas Product A can only be purchased with both antiseptic agents present, Product B is commercially available with and without ethanol. Product B exhibited strong antiviral effects independent of the presence of ethanol, reducing viral titers to background levels (Fig. 1B). These results already indicated that not all antiseptic agents may display the same potential to inactivate SARS-CoV-2 in vitro. In order to address this issue, a total of ten different agents (Table 1) present in various commercially available mouthrinses were tested for their antiviral activity against SARS-CoV-2 in a similar experimental setup. Each agent was tested in up to four different concentrations covering a range that is usually found in mouthrinses (indicated in red, Fig. 2 ). Of the agents tested, benzalkoniumchloride (BAC; Fig. 2A), cetylpyridiniumchloride (CPC; Fig. 2B), polyvinylpyrrolidone iodine (PVP-I; Fig. 2I) and a mixture of surfactants (Fig. 2J) resulted in a strong dose-dependent reduction of SARS-CoV-2 infectivity, with log reduction factors between 2.74 and 3.91. Viral infectivity was completely abolished down to background levels (LLOQ) with only low cytotoxicity (Fig. 2, dashed line, Table 1). In contrast, exposure of the virus towards chlorhexidine digluconate (CHX) only moderately affected viral infectivity with log reduction factors of 1.22 in the highest concentration tested (0.5%) (Fig. 2C). Of note, CHX only slightly reduced viral infectivity (reduction factors of 0.57) at a concentration of 0.2%, which is commonly found in commercially available mouthrinses (Fig. 2C). Similarly, dequaliniumchloride (DQC, Fig. 2D), hydrogen peroxide (H2O2, Fig. 2E), hydroxyapatite (HAP, Fig. 2F) and polyaminopropyl-biguanide (PAP, Fig. 2H) did not show any significant reduction in viral titers (Table 1). Octenidine-Dihydrochloride reduced viral titers with a reduction factor of ≥ 2.97 at a concentration of 0.5%, but displayed a high cytotoxicity in higher concentrations (Oct-DiHCl, Fig. 2D, Table 1). These results demonstrate the high efficacy of various antiseptic agents in reducing SARS-CoV-2 infectivity in vitro.
Fig. 1.
Quantitative suspension test of commercially available mouthrinses. (A) Product A and (B) Product B of different compositions (white bars) or medium (grey bar) were incubated with SARS-CoV-2 and an interfering substance for 30 s. Viral titers were obtained by end point dilution on Vero E6 cells. Cytotoxicity is indicated as lower limit of quantification (LLOQ, dotted line). 50% tissue culture infectious dose (TCID50/mL) was calculated according to Spearman and Kärber. Data are represented as mean ± SD of three independent experiments.
Fig. 2.
Quantitative suspension test of antiseptic agents. (A-J) Antiseptic agents in various concentrations (white bars) or medium (grey bar) were incubated with SARS-CoV-2 and an interfering substance for 30 s. Viral titers were obtained by end point dilution on Vero E6 cells. Cytotoxicity is indicated as lower limit of quantification (LLOQ, dotted line). 50% tissue culture infectious dose (TCID50/mL) was calculated according to Spearman and Kärber. Red numbers indicate concentrations that appear in commercially available mouthrinses. Data are represented as mean ± SD of three independent experiments.
3.2. Antiviral agents disrupt the viral envelope integrity
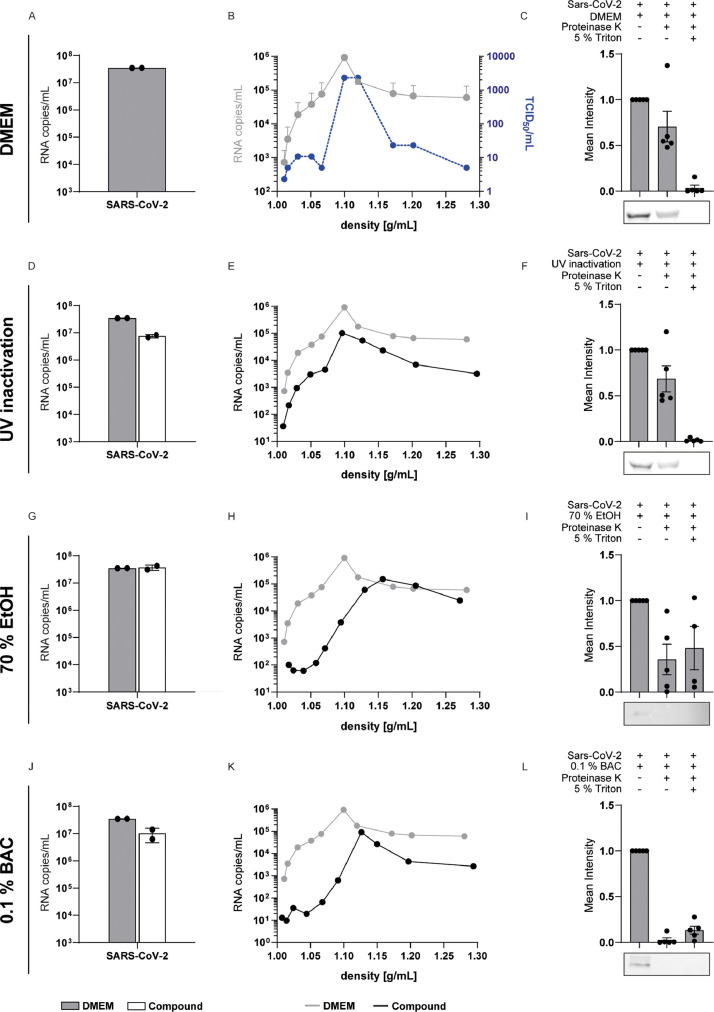
Due to strong antiviral effects found for some of the tested agents, we analyzed their respective modes of action. RT-qPCR revealed that RNA integrity remained unaffected following treatment for 30 s (Fig. 3 A, D, G, J; Supp. Fig. 1A, D, G, J, Supp. Fig. 2A, D, M). Of note, for some of the agents a slight reduction in viral RNA was observed (Supp. Fig. 2G, J). In order to evaluate a possible technical effect on the RNA extraction procedure or RT-qPCR reaction, we spiked an mRNA transcript into the respective agents, and extracted viral RNA. This revealed that the reduction of viral RNA correlated with the recovery of the mRNA transcript, indicating that RNA extraction and/or RT-qPCR can be affected by some of the agents (Supp. Fig. 2G, J).
Fig. 3.
Mode of action of antiseptic agents. SARS-CoV-2 was either incubated with DMEM (A-C), UV-inactivated (D-F), treated with 70% EtOH (G-I) or 0.1% BAC (J-L) and an interfering substance for 30 s. RNA integrity (A, D, G and J) for each treatment (white bar) was investigated by RT-qPCR and compared to DMEM (grey bar). Sucrose step gradient ultracentrifugation was performed to evaluate viral envelope integrity (B, E, H, K) and infectivity (blue line, B). RNA copy numbers in each fraction were determined by RT-qPCR (black line) and compared to DMEM (grey line). The viral envelope was further assessed by a capsid protection assay (C, F, I, L). Therefore, one replicate was left untreated, one part was treated with proteinase K for 1 h at 4 °C, and another part was lysed in 5% Triton X-100 prior to proteinase K treatment. The amount of protease-resistant nucleocapsid protein was quantified by Western blot. Data indicate averages.
It has been suggested before that oral antiseptics exert their antiviral action by targeting the lipid composition on the viral envelope (O'Donnell et al., 2020). Thus, we developed a variety of assays scrutinizing the viral envelope integrity. Enveloped viruses are complexes of proteins, nucleic acids and lipids. Ultracentrifugation through a iodixanol step gradient can reveal changes in the buoyant density based on the sedimentation velocities of viral particles, which could change upon disruption of the viral lipid envelope. For this purpose, the mixture from the quantitative suspension test was subjected to a 0-40% step iodixanol gradient with subsequent determination of SARS-CoV-2 RNA copy numbers and virus infectivity in different fractions. This procedure allowed recovery of infectious virus in fractions 6-7, with a peak of viral RNA copies and infectious particles at a density of 1.10 g/mL (Fig. 3B, Supp. Figs. 1 and 2). Exposure towards UV irradiation, which targets mostly the viral RNA, or treatment with 70% ethanol, which causes swelling and leakage of membranes (Fig. 3D-I), served as controls. Indeed, treatment with 70% ethanol resulted in a clear shift of virus RNA towards higher densities, indicating changes in the buoyant density possibly via membrane disruption, which was not detected after UV irradiation (Fig. 3E and H). Importantly, exposure of virus towards BAC (Fig. 3K), CPC, Oct-DiHCl and PVP-I (Supp. Fig. 1B, E, H, K) similarly resulted in a shift of the buoyant density towards higher fractions, which could not be observed with the agents that were not found active in the quantitative suspension tests (Supp. Fig. 2B, E, H, K, N). To confirm that these changes are mediated by disruption of the viral envelope, we developed a proteolytic protection assay to determine the amount of protease K (PK)-resistant, enveloped nucleocapsid protein. Treatment-induced disruptions of the viral envelope permit access of the PK to the viral nucleocapsid resulting in a digestion of nucleocapsid protein, which can be quantified via western blot analysis. To control that the concentration of PK used was sufficient to cleave the nucleocapsid protein and not affected by the presence of the respective agent, we added a high dose of the detergent Triton X-100 as a positive control, which resolved all membranes. In accordance with our previous results, we could show that the viral nucleocapsid was susceptible towards proteolytic digestion after treatment with BAC (Fig. 3L) as well as CPC, Oct-DiHCl and a mixture of surfactants (Supp. Fig. 1C, F, I, L). Although the density gradient showed a clear shift to higher densities for PVP-I treated virus suggesting disruption of the viral envelope, a faint signal for the nucleocapsid was detectable upon PK treatment. In summary, these results clearly demonstrate that these agents resulted in a disruption of the viral envelope, thereby mediating their antiviral effects.
3.3. A randomized placebo-controlled clinical trial resulted in a mild reduction of viral infectivity
Based on the in vitro results reported above, we chose BAC as potent antiviral agent and performed a randomized placebo-controlled clinical trial to analyze potential antiviral effects in terms of reduction of viral loads and viral infectivity in COVID-19 patients. Altogether 24 SARS-CoV-2 positive patients (12 female, 12 male) were included in this study, where of 18 (8 female, 10 male) were randomly assigned to the BAC group and 6 (4 female, 2 male) to the placebo group (see Supp. Fig. 3 for the CONSORT flow chart). The median age was 28.5 years (range 20-62) in the BAC group and 31 years (range 22-73) in the placebo group. All but two patients from the placebo group presented rather mild COVID-19 related symptoms and were treated as outpatients.
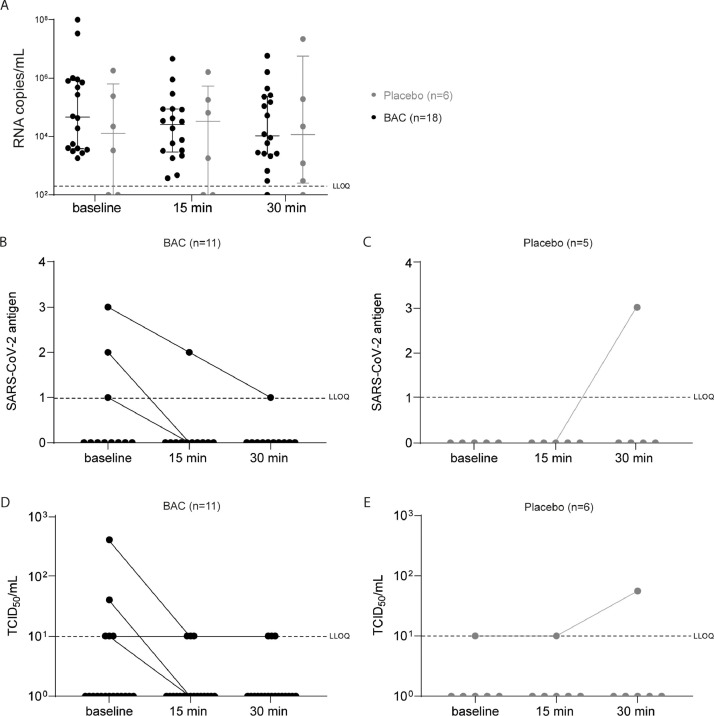
Viral loads were determined in the oropharyngeal specimens immediately before as well as 15 and 30 min after gargling with BAC using RT-qPCR. As expected, based on the mechanism of action, no significant reduction in viral RNA was observed at all time points tested (Fig. 4 A). Viral antigen was determined in the oropharyngeal specimens of 16 patients using a point-of-care test. Four patients harbored antigen-positive samples, mostly reflecting the high viral titers in these specimens. The amount of viral antigen decreased in three patients following BAC mouthrinse, whereas it increased in one patient with 0.9% NaCl rinse (Fig. 4B and C). Most importantly, we were able to rescue infectious virus from the respiratory specimens of 5 patients in the BAC group and one patient in the placebo group, respectively. Interestingly, we observed a mild reduction in viral infectivity after BAC treatment which remained constant for up to 30 min (Fig. 4D). In contrast, for the placebo group, in one patient with detectable virus, no reduction in viral infectivity could be observed (Fig. 4E). These results indicate that oral application of BAC resulted in a mild reduction of viral titers, however, did not consistently abolish viral rescue in cell culture, which indicates persisting infectiousness in patients which could lead to virus transmission.
Fig. 4.
In vivo activity of BAC against SARS-CoV-2. BAC was applied in a randomized placebo-controlled clinical trial in COVID-19 patients. RNA copy numbers were determined before gargling (baseline) as well as 15 and 30 min after gargling (A) comparing the placebo group (grey) to the BAC group (black). At similar time points SARS-CoV-2 antigen (B and C) and infectivity (D and E) was assessed for the BAC and placebo group, respectively. Data are shown as medians including 1st and 3rd quartiles.
4. Discussion
SARS-CoV-2 is predominantly transmitted by virus-loaded droplets or aerosols released from the upper respiratory tract of infected individuals. Hence, it has been suggested that a reduction of viral loads in the oral cavity could provide an additional precautionary measure to potentially lower transmission rates (Carrouel et al., 2021; Gottsauner et al., 2020; Herrera et al., 2020; Peng et al., 2020). Recently, we and others could show that mouthrinses can rapidly inactivate SARS-CoV-2 in vitro (Bidra et al., 2020; Meister et al., 2020; Muñoz-Basagoiti et al., 2021; Steinhauer et al., 2021). Subsequently, there have been extensive discussions regarding utilization of mouthrinses to possibly complement current prevention measures such as facemasks, hand disinfection and social distancing in order to reduce the global spread of SARS-CoV-2 (Carrouel et al., 2021; O'Donnell et al., 2020). Especially, in dental or otorhinolaryngological clinical practices no face protection can be worn by the patient during examination and treatment, greatly increasing the risk for HCPs. A number of clinical trials are currently being performed to analyze the efficacy of oral antiseptics against SARS-CoV-2 (Carrouel et al., 2021; Gottsauner et al., 2020; Seneviratne et al., 2021; Stathis et al., 2021). In order to identify the antiviral agents responsible and understand their respective antiviral mode of action, we analyzed two commercially available mouthrinses and ten different antiseptic agents, commonly found in mouthrinses for their ability to reduce SARS-CoV-2 infectivity in vitro. Our experiments revealed four agents, namely BAC, CPC, PVP-I and a combination of surfactants to be highly effective against SARS-CoV-2 in vitro, whether applied by a commercially available mouthrinse (Fig. 1) or as an individual component (Fig. 2). This is in line with a recent study showing that CPC could reduce the infectivity of SARS-CoV-2 variants in vitro (Muñoz-Basagoiti et al., 2021). While CHX, DQ, H2O2, HAP and PAP had no effect on SARS-CoV-2 infectivity (Fig. 2), ethanol also did not contribute to virus inactivation as the final ethanol concentration within Product B does not reach 30% (Meister et al., 2021), implying essential oils as the crucial incredients responsible for the inactivation of SARS-CoV-2 as supported by recent findings (Fig. 1) (Davies et al., 2021; Meister et al., 2020; Meister et al., 2022). Combining two or more antiseptic agents could improve the antiviral capacity, however antagonistic effects can also not be excluded. It has been shown before that antiseptic agents such as CHX or quaternary ammonium compounds such as BAC or CPC may interact with lipid bilayers which can lead to a disturbance of cell permeability and subsequent leakage of cytoplasmic material and cell death (Cieplik et al., 2019; Mao et al., 2020; O'Donnell et al., 2020). It has been speculated early on that antiseptics in mouthrinses have the potential to target the SARS-CoV-2 envelope, which originates from the host cell, thereby reducing viral loads (O'Donnell et al., 2020). Data obtained by density gradient ultracentrifugation followed by RT-qPCR provide experimental evidence that the antiviral activity against coronaviruses is mostly exerted by disruption of the viral envelope (Figs. 3, Supp. Figs. 1, and 5).
One very potent antiviral agent we tested against SARS-CoV-2 was BAC, which is known for its broad-spectrum antimicrobial properties against a variety of pathogens, including viruses (Merchel Piovesan Pereira and Tagkopoulos, 2019). BAC is considered safe in intranasal products when used in concentrations up to 0.1% (Marple et al., 2004) and unlike PVP-I does not contribute to teeth discoloring when frequently administered. To further elucidate whether those in vitro findings are clinically relevant, we chose BAC to perform a randomized placebo-controlled clinical trial in COVID-19 patients. Surprisingly, in contrast to the strong in vitro effects, oral application of BAC resulted in only a mild reduction of viral infectivity, as determined in cell culture (Fig. 4). Viral loads, as determined by RT-qPCR, were not significantly affected, which is in accordance with the identified mechanism of action (Fig. 5 ). Importantly, despite a treatment-related mild reduction in viral loads (probably due to mechanical effects from gargling mouth and throat and not related to the active compound), we were still able to successfully isolate infectious virus in cell culture. As viral particles are being constantly released from infected cells, it is possible that a short exposure and inactivation of virus particles is not sufficient due to continuous viral shedding. Furthermore, the areas which can be reached upon “gargling” in the oral cavity and the pharynx are spatially limited, further compromising potential antiviral effects. The minimal infectious dose for SARS-CoV-2 is still being debated, however, it is speculated that it is only slightly higher than the hundreds of particles estimated for SARS-CoV-1 (Karimzadeh et al., 2021). This indicates that the mild reduction, as observed in our clinical trial, is likely not sufficient to effectively prevent transmission, unless viral titers in the patient are very low. There are some limitations to our study including a small cohort size, inclusion of patients with high viral loads only, so a potential benefit upon lower viral titers has not been addressed. Furthermore, in our clinical study we tested only one antiseptic compound, not combinations of antiseptics usually sold in commercial products. Future research is urgently needed analyzing different antiseptics and their combinations in clinical trials. Furthermore, putative effects on viral transmission, the use of mouthrinses for prophylaxis after exposure, and the possibility to diminish progression by reduction of the viral load in the early stages of infection still need to be explored.
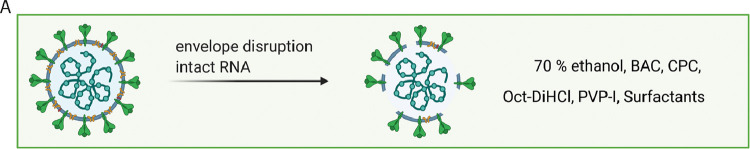
Fig. 5.
Schematic modes of action of antiseptic agents on SARS-CoV-2 particles. Selected agents disrupt the viral envelope without affecting RNA integrity. Figure created with BioRender.com.
5. Conclusions
Taken together, our results indicate that the oral application of BAC as antiseptic mouthrinse only mildly reduces viral infectivity in vivo, despite its high efficacy in vitro. This clearly shows that promising in vitro data on antiviral effects of a given antiseptic compound cannot be readily transferred to the clinical situation. These findings are of utmost importance when discussing COVID-19 prevention strategies in order to avoid the impact of a “false sense of security” due to the use of a mouthrinse and potential neglection of other protection measures. Further studies are required to study the clinical effects of combinations of antiseptics.
CRediT authorship contribution statement
Toni Luise Meister: Investigation, Methodology, Data curation, Formal analysis, Visualization, Writing – review & editing. Josef-Maximilian Gottsauner: Investigation, Writing – review & editing. Barbara Schmidt: Investigation, Data curation, Visualization, Writing – review & editing. Natalie Heinen: Investigation, Data curation, Writing – review & editing. Daniel Todt: Visualization, Methodology, Writing – review & editing. Franz Audebert: Investigation, Data curation, Writing – review & editing. Felix Buder: Investigation, Data curation, Writing – review & editing. Henriette Lang: Investigation, Data curation, Writing – review & editing. André Gessner: Resources, Writing – review & editing. Eike Steinmann: Supervision, Methodology, Resources, Writing – review & editing. Veronika Vielsmeier: Supervision, Methodology, Writing – review & editing. Stephanie Pfaender: Supervision, Methodology, Resources, Writing – original draft, Writing – review & editing. Fabian Cieplik: Supervision, Methodology, Resources, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
All authors declare that they have no conflicts of interest.
Acknowledgments
Acknowledgements
We would like to thank all members of the Molecular and Medical Virology at the Ruhr University Bochum for their support and fruitful discussions. In addition, we thank Anette Rohrhofer, Institute of Clinical Microbiology and Hygiene, Regensburg, for excellent technical assistance. Furthermore, we are grateful to the team of the Praxiszentrum Alte Mälzerei and to all patients who volunteered to participate in this study.
Funding
The authors acknowledge financial support through the pandemic responsiveness fund of the Bavarian Ministry of Science and Art (to B.S.), by the VIRus ALliance NRW (VIRAL) from the Ministry of Culture and Science of the State of North Rhine-Westphalia (323-8.03-151826; to E.S.), and in part by the Deutsche Forschungsgemeinschaft (DFG; grant CI 263/3-1 to F.C.).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.virusres.2022.198791.
Appendix. Supplementary materials
References
- Bidra A.S., Pelletier J.S., Westover J.B., Frank S., Brown S.M., Tessema B. Comparison of in vitro inactivation of SARS CoV-2 with hydrogen peroxide and povidone-iodine oral antiseptic rinses. J. Prosthodont. 2020;29(7):599–603. doi: 10.1111/jopr.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttenheim A.M. SARS-CoV-2 vaccine acceptance: we may need to choose our battles. Ann. Intern. Med. 2020;173(12):1018–1019. doi: 10.7326/M20-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrouel F., Gonçalves L.S., Conte M.P., Campus G., Fisher J., Fraticelli L., Gadea-Deschamps E., Ottolenghi L., Bourgeois D. Antiviral activity of reagents in mouth rinses against SARS-CoV-2. J. Dent. Res. 2021;100(2):124–132. doi: 10.1177/0022034520967933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieplik F., Jakubovics N.S., Buchalla W., Maisch T., Hellwig E., Al-Ahmad A. Resistance toward chlorhexidine in oral bacteria – is there cause for concern? Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies K., Buczkowski H., Welch S.R., Green N., Mawer D., Woodford N., Roberts A.D.G., Nixon P.J., Seymour D.W., Killip M.J. Effective in vitro inactivation of SARS-CoV-2 by commercially available mouthwashes. J. Gen. Virol. 2021;102(4) doi: 10.1099/jgv.0.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers M., Terletskaia-Ladwig E., Enders M. How effective is hand washing against influenza virus? 2009;34(12):492–498. [Google Scholar]
- Gottsauner M.J., Michaelides I., Schmidt B., Scholz K.J., Buchalla W., Widbiller M., Hitzenbichler F., Ettl T., Reichert T.E., Bohr C., Vielsmeier V., Cieplik F. A prospective clinical pilot study on the effects of a hydrogen peroxide mouthrinse on the intraoral viral load of SARS-CoV-2. Clin. Oral Investig. 2020;24(10):3707–3713. doi: 10.1007/s00784-020-03549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera D., Serrano J., Roldán S., Sanz M. Is the oral cavity relevant in SARS-CoV-2 pandemic? Clin. Oral Investig. 2020;24(8):2925–2930. doi: 10.1007/s00784-020-03413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M.A., Quandelacy T.M., Kada S., Prasad P.V., Steele M., Brooks J.T., Slayton R.B., Biggerstaff M., Butler J.C. SARS-CoV-2 transmission from people without COVID-19 symptoms. JAMA Netw. Open. 2021;4(1) doi: 10.1001/jamanetworkopen.2020.35057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärber G. Beitrag zur kollektiven behandlung pharmakologischer Reihenversuche. Arch. Exp. Path Pharmacol. 1931;(162):480–484. [Google Scholar]
- Karimzadeh S., Bhopal R., Nguyen Tien H. Review of Infective Dose, Routes of Transmission, and Outcome of COVID-19 Caused by the SARS-CoV-2 Virus: Comparison with Other Respiratory Viruses. Epidemiol. Infect. 2021;149:e96. doi: 10.1017/S0950268821000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewska J., Krajewski W., Zub K., Zatoński T. COVID-19 in otolaryngologist practice: a review of current knowledge. Eur. Arch. Oto-Rhino-Laryngol. 2020;277(7):1885–1897. doi: 10.1007/s00405-020-05968-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- Mao X., Auer D.L., Buchalla W., Hiller K.-A., Maisch T., Hellwig E., Al-Ahmad A., Cieplik F. Cetylpyridinium chloride: mechanism of action, antimicrobial efficacy in biofilms, and potential risks of resistance. Antimicrob. Agents Chemother. 2020;64(8) doi: 10.1128/AAC.00576-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marple B., Roland P., Benninger M. Safety review of benzalkonium chloride used as a preservative in intranasal solutions: an overview of conflicting data and opinions. Otolaryngol. 2004;130(1):131–141. doi: 10.1016/j.otohns.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Meister T.L., Brüggemann Y., Todt D., Conzelmann C., Müller J.A., Groß R., Münch J., Krawczyk A., Steinmann J., Steinmann J., Pfaender S., Steinmann E. Virucidal efficacy of different oral rinses against severe acute respiratory syndrome coronavirus 2. J. Infect. Dis. 2020;222(8):1289–1292. doi: 10.1093/infdis/jiaa471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister T.L., Fortmann J., Todt D., Heinen N., Ludwig A., Brüggemann Y., Elsner C., Dittmer U., Steinmann J., Pfaender S., Steinmann E. Comparable environmental stability and disinfection profiles of the currently circulating SARS-CoV-2 variants of concern B.1.1.7 and B.1.351. J. Infect. Dis. 2021;224(3):420–424. doi: 10.1093/infdis/jiab260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister T.L., Todt D., Brüggemann Y., Steinmann J., Banava S., Brill F.H.H., Pfaender S., Steinmann E. Virucidal activity of nasal sprays against severe acute respiratory syndrome coronavirus-2. J. Hosp. Infect. 2022;120:9–13. doi: 10.1016/j.jhin.2021.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchel Piovesan Pereira B., Tagkopoulos I. Benzalkonium Chlorides: Uses, Regulatory Status, and Microbial Resistance. Appl. Environ. Microbiol. 2019;85(13) doi: 10.1128/AEM.00377-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Basagoiti J., Perez-Zsolt D., León R., Blanc V., Raïch-Regué D., Cano-Sarabia M., Trinité B., Pradenas E., Blanco J., Gispert J., Clotet B., Izquierdo-Useros N. Mouthwashes with CPC reduce the infectivity of SARS-CoV-2 variants in vitro. J. Dent. Res. 2021;100(11):1265–1272. doi: 10.1177/00220345211029269. [DOI] [PubMed] [Google Scholar]
- O'Donnell V.B., Thomas D., Stanton R., Maillard J.-Y., Murphy R.C., Jones S.A., Humphreys I., Wakelam M.J.O., Fegan C., Wise M.P., Bosch A., Sattar S.A. Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection. Function. 2020;1(1) doi: 10.1093/function/zqaa002. zqaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X., Xu X., Li Y., Cheng L., Zhou X., Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci. 2020;12(1):1–6. doi: 10.1038/s41368-020-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneviratne C.J., Balan P., Ko K.K.K., Udawatte N.S., Lai D., Ng D.H.L., Venkatachalam I., Lim K.S., Ling M.L., Oon L., Goh B.T., Sim X.Y.J. Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore. Infection. 2021;49(2):305–311. doi: 10.1007/s15010-020-01563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearman C. The method of right and wrong cases (constant stimuli) without gauss’s formulae. Br. J. Psychol. 1908;3(2):227. [Google Scholar]
- Stathis C., Victoria N., Loomis K., Nguyen S.A., Eggers M., Septimus E., Safdar N. Review of the use of nasal and oral antiseptics during a global pandemic. Fut. Microbiol. 2021;16:119–130. doi: 10.2217/fmb-2020-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer K., Meister T.L., Todt D., Krawczyk A., Paßvogel L., Becker B., Paulmann D., Bischoff B., Pfaender S., Brill F.H., Steinmann E. Comparison of the in vitro-efficacy of different mouthwash solutions targeting SARS-CoV-2 based on the European Standard EN 14476. J. Hosp. Infect. 2021;111:180–183. doi: 10.1016/j.jhin.2021.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toptan T., Hoehl S., Westhaus S., Bojkova D., Berger A., Rotter B., Hoffmeier K., Cinatl J., Ciesek S., Widera M. Optimized qRT-PCR approach for the detection of intra- and extra-cellular SARS-CoV-2 RNAs. Int. J. Mol. Sci. 2020;21(12) doi: 10.3390/ijms21124396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., Wit E.de, Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M., Nkenke E. Approaches to the management of patients in oral and maxillofacial surgery during COVID-19 pandemic. J. Cranio-Maxillo-Facial Surg. 2020;48(5):521–526. doi: 10.1016/j.jcms.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J., Guo Q., Song T., He J., Yen H.-L., Peiris M., Wu J. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N. Engl. J. Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.