Highlights
-
•
Albumin, CRP, d-dimer, ferritin, hemoglobin, IL-2R, IL-6, LDH, and PCT were identified as commonly changed biomarkers in three different comparison groups.
-
•
Increased levels of CRP, d-dimer, ferritin, IL-2R, IL-6, LDH, and PCT are positively correlated to the COVID-19 severity and cancer surveillance in cancer patients with COVID-19.
-
•
High levels of CRP, ferritin, and LDH after immunotherapy for COVID-19 in cancer patients indicate a poor prognosis.
-
•
Cancer patients who had lower levels of CRP, ferritin, and LDH exhibit a good prognosis after antivirals/antibiotic treatment for SARS-CoV-2 infection.
Keywords: SARS-CoV-2, COVID-19, Cancer, Prognostic biomarkers, Immunotherapy
Abstract
Purpose
Cancer patients with COVID-19 likely express biomarker changes in circulation. However, the biomarkers used in SARS-CoV-2 infected cancer patients for COVID-19 severity and prognosis are largely unclear. Therefore, this systematic review aims to determine what biomarkers were measured in cancer patients with COVID-19 and their prognostic utility.
Methods
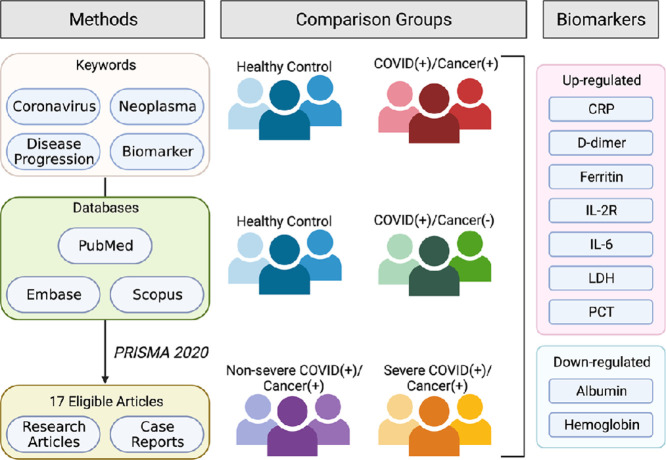
A systematic literature review in PubMed, Embase, and Scopus was performed on June 16th, 2021. The search keywords coronavirus, neoplasm, biomarkers, and disease progression were used to filter out 17 eligible studies, which were then carefully evaluated.
Results
A total of 4,168 patients, 16 types of cancer, and 60 biomarkers were included. Seven up-regulated markers, including CRP, d-dimer, ferritin, IL-2R, IL-6, LDH, and PCT, were identified in eligible studies. Albumin and hemoglobin were significantly down-regulated in cancer patients with COVID-19. Moreover, we observed that the SARS-CoV-2 infected cancer patients with lower CRP, ferritin, and LDH levels successfully survived from COVID-19 treatments.
Conclusion
Several important clinical biomarkers, such as CRP, ferritin, and LDH, may serve as the prognostic markers to predict the outcomes following COVID-19 treatment and monitor the deterioration of COVID-19 in cancer patients.
Graphical abstract
Introduction
Biomarkers refer to a subgroup of medical signs that can be tested and observed objectively from patients [1]. Monitoring biomarkers from relevant biological media (blood, stool, urine, or other body fluid) provides important information on medical conditions, effectiveness of interventions, disease progression, or recovery [2]. For example, cardiac troponin is a specific and sensitive biomarker for myocardial injury. It is the preferred serologic test to evaluate patients with suspected acute myocardial infarction [3]. Additionally, some biomarkers are used in cancer diagnosis or progression, such as carcinoembryonic antigen (CEA) that is commonly used for colorectal [4] and breast cancer [5] diagnosis, and alpha-fetoprotein (AFP), which is associated with the progression of hepatocellular carcinomas [6].
Coronavirus is an enveloped positive-sense single-strand RNA (+ssRNA) virus family transmitted in mammals and avians [7]. Seven coronaviruses are known to infect humans, and three of them led to outbreaks in recent years [8]. The severe acute respiratory syndrome coronavirus (SARS-CoV) gave rise to a SARS epidemic in 2003 [8]. Subsequently, the Middle East respiratory syndrome coronavirus (MERS-CoV) outbroke in South Korea in 2012 [8]. At present, coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2, has been a global pandemic since its first declaration in 2019 by WHO [9]. Compared with MERS-CoV and SARS-CoV, SARS-CoV-2 quickly spread worldwide, imposing a tremendous burden on the healthcare system and the global economy. Pneumonia, acute respiratory distress syndrome (ARDS), and cytokine release syndrome (CRS) are severe symptoms found in COVID-19. In addition, several up-regulated biomarkers in severe COVID-19 patients have been reported, including (i) inflammatory markers: C-reactive protein (CRP), ferritin, interleukin (IL)−2, IL-6, IL-10, lactate dehydrogenase (LDH), or procalcitonin (PCT); (ii) biomedical markers: aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatine kinase, and (iii) coagulant marker: d-dimer [10,11]. These identified biomarkers may serve as predictors of COVID-19 severity. Moreover, monitoring COVID-19 severity through these biomarkers may be imperative to reduce mortality.
Cancer is a disease caused by uncontrolled cell proliferation, which may metastasize to other tissues and organs through epithelial-mesenchymal transition (EMT) [12]. According to the National Cancer Institute, around 1,806,590 new cancer patients were diagnosed, and 606,520 people died from cancer in the United States in 2020 [13]. One of the hallmarks of cancers is inflammation, characterized by the release in the extracellular environment of several pro-inflammatory cytokines and chemokines, which facilitate communication between various cell types and regulate immune response, as well as cell migration [14]. For instance, overexpressed IL-6 within the tumor microenvironment promotes tumorigenesis by regulating multiple signaling pathways such as proliferation, invasiveness, metastasis, or metabolism [15]. Up-regulated levels of transforming growth factor (TGF)-β, IL-6, IL-20, and IL-23 could also act as predictors of poor survival in breast cancer [16].
Cancer patients who undergo chemotherapy have compromised immunity which may increase susceptibility to SARS-CoV-2 infection. In particular, cancer patients with COVID-19 have a higher mortality rate than COVID-19 patients without active cancer [17]. Moreover, our recent study found that SARS-CoV-2 may trigger breast cancer metastasis through Snail up-regulation [18]. Thus far, specific biomarkers in cancer patients infected with SARS-CoV-2 are unclear. This article aims to identify prognostic biomarkers in cancer patients at the early stage of COVID-19, which may help predict cancer or COVID-19 outcomes and assist in developing therapeutic strategies.
Methods
Search strategy
PubMed, Embase, and Scopus databases were used to identify studies related to cancer patients infected with the coronavirus. The hierarchical search strategies and keywords were (1) biomarker, (2) coronavirus, and (3) cancer progression (Table S1). Duplications were removed. Titles and abstracts of articles were reviewed for eligibility. All relevant articles from reference lists were identified. After screening the abstracts, final eligibility was determined based on the full content. The last search was conducted on June 16th, 2021.
Eligibility criteria
Inclusion criteria were: (1) primary source of quantitative research in a peer-reviewed journal published in English, including research articles, case reports, and correspondences; (2) adult cancer patients (all types of cancers) infected by a coronavirus; and (3) biomarkers data were reported. To maintain consistency, the severity of COVID-19 was defined by the National Institutes of Health (NIH) guidelines for COVID-19 treatment [19]. Articles that did not meet all the criteria were excluded. Unpublished theses, dissertations, review articles, conference proceedings, and studies using pediatric cancer patients or animal models were also excluded.
Data extraction
According to the inclusion and exclusion criteria, data extraction was completed by one author (T.A.L.) and verified by the other author (Y.J.L.). The following headlines were extracted from the articles: authors, type of study, study location, the total number of patients, patients' age (median), type of cancer, comparison groups, biomarker data, and terminal outcomes (Table S2).
Quality assessment
The quality of the studies was assessed using the modified REporting recommendations for tumor MARKers prognostic studies (REMARK) (Table S3) [20]. Two independent reviewers (T.A.L. and C.T.K.) verified the total scores (Table S4).
Results
Characteristics of the studies
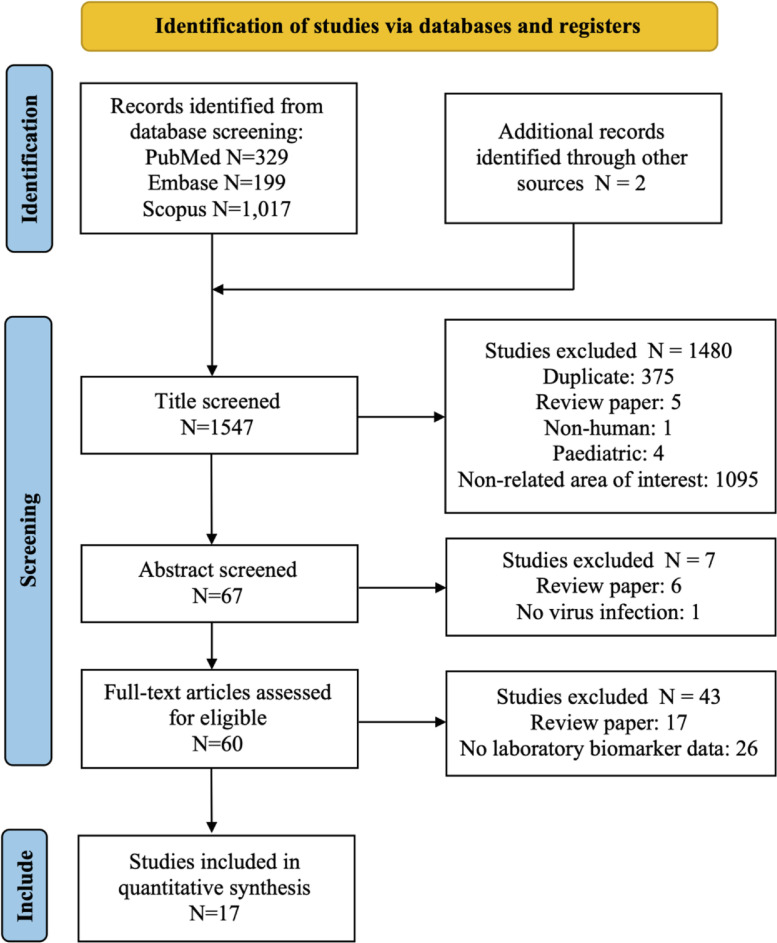
Through the database search, 1545 studies were identified from PubMed, Embase, and Scopus. Two more studies were found on Google Scholar. The search procedure followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram [21] as depicted in Fig. 1. After screening each article's title and abstract, 60 full-text studies were assessed for eligibility. Seventeen studies met the eligibility criteria and were included in the systematic review (Fig. 1). As shown in Table S2, the eligible articles included research articles (8, 47.1%) and case reports (9, 52.9%). The studies were conducted in China (4, 23.5%), Spain (3, 17.6%), Australia (2, 11.8%), Italy (2, 11.8%), and 1 (5.9%) each in Brazil, Egypt, France, Turkey, United Kingdom, and, United States. Among the 4168 subjects, there were 971 (23.2%) COVID-19 patients with cancer, 3162 (75.9%) COVID-19 patients without cancer, and 35 (0.8%) Non-COVID-19 patients with cancer. Sixteen types of cancers, including solid tumors and hematological malignancies, were found among the cancer patients. (Tables S2 and S5).
Fig. 1.
Flow diagram of the literature search. Adapted from Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi:10.1136/bmj.n71.
Methodological assessment
All the eligible studies were further evaluated by the modified REMARK questionnaire (Table S3). The majority of studies defined patients’ enrollment period (70.6%) and were unbiased in selecting the patients (52.9%); 41.6% described the outcomes at the onset of the study and provided a list of candidate variables; 29.4% provided information on the measurement of biomarkers. Few articles were prospective reports (17.6%) and provided a rationale for the sample sizes (5.9%). Importantly, no studies blinded the measurements of biomarkers to patient outcomes (Table S4 and Fig.S1).
Biomarker findings
Of the 60 biomarkers assessed in the 17 studies, 29 (48.3%) biomarkers showed statistically significant changes (p-value ≤ 0.05) between different groups in the original cohort study, and 14 (23.3%) biomarkers without statistical analysis from case reports were determined based on the reference intervals [22]. The biomarkers were divided into four categories based on their biological functions: biochemical, inflammatory, hematological, and coagulant markers (Table 1). Additionally, according to patients’ cancer and COVID-19 conditions from the eligible articles, we classified them into the four main groups (COVID-19 (+) & cancer (+); COVID-19 (-) & cancer (-); COVID-19 (+) & cancer (-); and COVID-19 severity & cancer (+). The following comparisons were made between groups: (1) COVID-19 (-) & cancer (-) vs. COVID-19 (+) & cancer (+): patients’ clinical data were compared with reference intervals from case studies; (2) COVID-19 (+) & cancer (-) vs. COVID-19 (+) & cancer (+); and (3) non-severe COVID-19 & cancer (+) vs. severe COVID-19 & cancer (+) (Tables 1 and 2).
Table 1.
The categorized biomarkers from different comparison groups of patients.
| Category | Biomarkers | Group 1 | Group 2 | Group 3 |
|
COVID-19 (-) & Cancer (-)† vs. COVID-19 (+) & Cancer (+) |
COVID-19 (+) & Cancer (-) vs. COVID-19 (+) & Cancer (+) |
Non-severe COVID-19 & Cancer (+) vs. Severe COVID-19 & Cancer (+) |
||
| Clinical Biochemical | A/G ratio | + | ||
| ALT | + | |||
| AST | + | + | ||
| Bilirubin (Indirect) | + | |||
| Bilirubin (Total) | + | |||
| Creatinine | + | + | ||
| Globulin | + | |||
| hs-cTnI | + | + | ||
| Lactate | + | |||
| Myoglobin | + | |||
| NT-proBNP | + | |||
| Total protein | + | + | ||
| Urea | + | |||
| Albumin | + | + | + | |
| Subtotal (%) | 35.7 | 28 | 40 | |
| Inflammatory | CRP | + | + | + |
| Ferritin | + | + | + | |
| IL-10 | + | + | ||
| IL-1β | + | |||
| IL-2R | + | + | + | |
| IL-6 | + | + | + | |
| IL-8 | + | |||
| LDH | + | + | + | |
| PCT | + | + | + | |
| TNF-α | + | + | ||
| Subtotal (%) | 42.9 | 40 | 40 | |
| Hematological | ESR | + | ||
| Hb | + | + | + | |
| Subtotal (%) | 7.1 | 8 | 5 | |
| Coagulant | Antithrombin activity | + | ||
| aPTT | + | + | ||
| D-dimer | + | + | + | |
| Fibrinogen | + | |||
| INR | + | |||
| Prothrombin time activity | + | |||
| PT | + | + | ||
| Subtotal (%) | 14.3 | 24 | 15 | |
| References | Villegas et al. Baumann et al. Pasin et al. Bellmann-Weiler et al. da Costa et al. Yekedüz et al. Zhang et al. |
Abdul-Jawad et al. Cai et al. Martínez-López et al. Meng et al. Smith et al. Tian et al. Zahran et al. Gallo et al. |
Albiges et al. Smith et al. Tian et al. |
The biomarkers were identified from case reports, compromising the statistical power. +, with the data reported from the eligible studies. ALT, Alanine aminotransferase; aPTT, Activated partial thromboplastin time; AST, Aspartate aminotransferase; CRP, C-reactive protein; ESR, Erythrocyte sedimentation rate; Hb, Hemoglobin; hs-cTnI, High-sensitivity cardiac troponin I; IL-1β, Interleukin-1β; IL-2R, Interleukin-2 receptor; IL-6, Interleukin-6; IL-10, Interleukin-10; LDH, Lactate dehydrogenase; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PCT, Procalcitonin; PT, Prothrombin time; TNF-α, Tumor necrosis factor α.
Table 2.
The up- and down-regulated biomarkers from different comparison groups of patients.
| Groups | Up-regulated biomarkers | Down-regulated biomarkers | Refs. | |
| 1 |
COVID-19 (-) & Cancer (-)† vs. COVID-19 (+) & Cancer (+) |
Bilirubin (Indirect); CRP; d-dimer; Ferritin; Fibrinogen; IL-2R; IL-6; LDH; PCT; Urea | Albumin; Bilirubin (Total); Hb; Total protein | Villegas et al. Baumann et al. Pasin et al. Bellmann-Weiler et al. da Costa et al. Yekedüz et al. Zhang et al. |
| 2 |
COVID-19 (+) & Cancer (-) vs. COVID-19 (+) & Cancer (+) |
ALT; aPTT; AST; Creatinine; CRP; ESR; Ferritin; hs-cTnI; IL-10; IL-1β; IL-2R; IL-6; IL-8; INR; LDH; PCT; PT; TNF-α | Albumin; Albumin-globulin ratio; Antithrombin activity; Globulin; Hb; Prothrombin time activity; Total protein | Abdul-Jawad et al. Cai et al. Martínez-López et al. Meng et al. Smith et al. Tian et al. Zahran et al. Gallo et al. |
| 3 |
Non-severe COVID-19 & Cancer (+) vs. Severe COVID-19 & Cancer (+) |
aPTT; AST; Creatinine; CRP; d-dimer; Ferritin; hs-cTnI; IL-10; IL-2R; IL-6; Lactate; LDH; Myoglobin; NT-proBNP; PCT; PT; TNF-α | Albumin; Albumin-globulin ratio; Hb | Albiges et al. Smith et al. Tian et al. |
The biomarkers were identified from case reports, compromising the statistical power. ALT, Alanine aminotransferase; aPTT, Activated partial thromboplastin time; AST, Aspartate aminotransferase; CRP, C-reactive protein; ESR, Erythrocyte sedimentation rate; Hb, Hemoglobin; hs-cTnI, High-sensitivity cardiac troponin I; IL-1β, Interleukin-1β; IL-2R, Interleukin-2 receptor; IL-6, Interleukin-6; IL-10, Interleukin-10; INR, International normalized ratio; LDH, Lactate dehydrogenase; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PCT, Procalcitonin; PT, Prothrombin time; TNF-α, Tumor necrosis factor α.
Biomarkers for cancer-related COVID-19 patients
Compared with reference intervals, cancer patients with COVID-19 exhibited higher levels of indirect bilirubin, CRP, d-dimer, ferritin, fibrinogen, glucose, IL-2R, IL-6, LDH, PCT, and urea, and lower levels of albumin, total bilirubin, hemoglobin (Hb), and total protein (Table 2). Notably, 35.7% (5 of 14) are biochemical biomarkers, and 42.9% (6 of 14) are inflammatory biomarkers (Table 1, Group 1). Next, we identified 25 cancer-related biomarkers in COVID-19 patients from the eligible cohort studies. As shown in Table 2, up-regulated ALT, activated partial thromboplastin time (aPTT), AST, creatinine, CRP, erythrocyte sedimentation rate (ESR), ferritin, high-sensitivity cardiac troponin I (hs-cTnI), IL-10, IL-1β, IL-2R, IL-6, IL-8, international normalized ratio (INR), LDH, PCT, prothrombin time (PT), and tumor necrosis factor (TNF)-α, and down-regulated albumin, albumin-globulin ratio, antithrombin activity, globulin, Hb, prothrombin time activity, and total protein were associated with COVID-19 patients with cancer. Similar to Group 1, 28% (7 of 25) are biochemical biomarkers, and 40% (10 of 25) are inflammatory biomarkers. Interestingly, 24% (6 of 25) are coagulation biomarkers, including PT, aPTT, and d-dimer (Table 1, Group 2). As a result, these significantly changed markers may serve as the biomarkers for monitoring the risk for cancer progression related to COVID-19 infection.
Biomarkers associated with COVID-19 severity in cancer patients
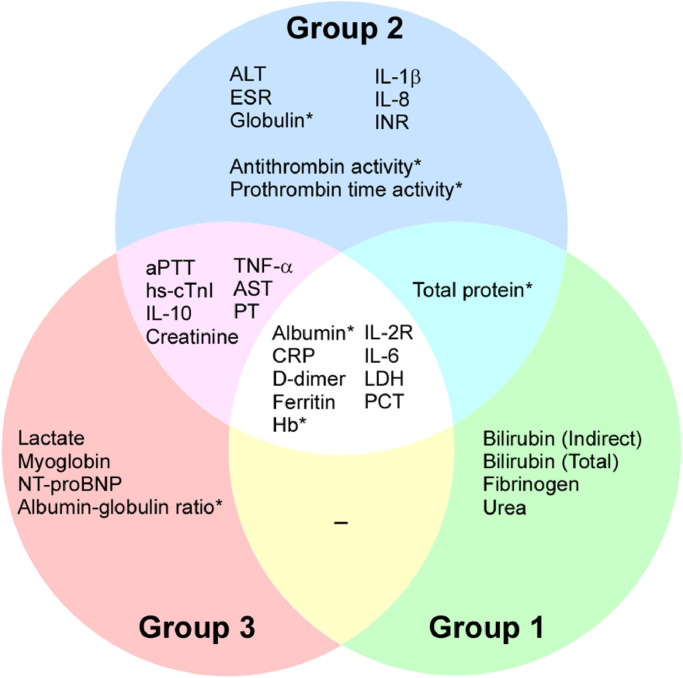
Among 20 biomarkers that were significantly associated with COVID-19 severity (Table 2), increased levels of aPTT, AST, creatinine, CRP, d-dimer, ferritin, hs-cTnI, IL-10, IL-2R, IL-6, lactate, LDH, myoglobin, NT-proBNP, PCT, PT, and TNF-α, and decreased levels of albumin, albumin-globulin ratio, and Hb were related to cancer patients with severe COVID-19. Biochemical and inflammatory biomarkers each account for 40% (8 of 20) in this group (Table 1, Group 3). Additionally, through the Venn diagram comparative analysis of these biomarkers, nine biomarkers were identified in the overlapping portion of these three groups (Fig. 2). 66.7% (6 of 9) are elevated inflammatory markers, suggesting that inflammation in SARS-CoV-2 infected cancer patients is closely related to the severity of COVID-19. With the identification of these COVID-19 severity and cancer-related biomarkers, we may be able to better manage COVID-19 progression in cancer patients by monitoring the changes of these biomarkers.
Fig. 2.
The biomarkers from three clinical comparison groups. There are 25 cancer-related biomarkers in the blue circle, 20 biomarkers correlated to COVID-19 in the red circle, and 14 biomarkers in the green circle were identified outside the reference internal from case reports. Different overlapped colors indicate distinct categories of the biomarkers in different groups of patients. The central overlapped area demonstrates the molecules identified in the cancer patients with severe COVID-19. *The biomarkers were significantly down-regulated. Those without a star were significantly up-regulated. ALT, Alanine aminotransferase; aPTT, Activated partial thromboplastin time; AST, Aspartate aminotransferase; CRP, C-reactive protein; ESR, Erythrocyte sedimentation rate; Hb, Hemoglobin; hs-cTnI, High-sensitivity cardiac troponin I; IL-1β, Interleukin-1β; IL-2R, Interleukin-2 receptor; IL-6, Interleukin-6; IL-10, Interleukin-10; INR, International normalized ratio; LDH, Lactate dehydrogenase; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PCT, Procalcitonin; PT, Prothrombin time; TNF-α, Tumor necrosis factor α.
Biomarkers related to different treatment outcomes in cancer patients with COVID-19
Based on the therapies utilized to treat SARS-CoV-2 infection, the case reports were divided into two groups: (1) immunotherapy combined with antiviral drugs and (2) antivirals/antibiotics only (Table 3). First, patients who received IL-6R blockade (tocilizumab) for COVID-19 [23] or PD-1 blockade (nivolumab & pembrolizumab) prior to the SARS-CoV-2 infection [24,25] had lower levels of CRP, LDH, or PCT eventually recovered from COVID-19. However, patients who
Table 3.
Biomarkers associated with different treatments in cancer patients with COVID-19 from case report studies.
| Cancer types | Treatment types | Biomarkers | Outcomes | Refs. |
| Hematological cancer (N = 5) |
IL-1R blockade (Anakinra) IL-6R blockade (Tocilizumab) Hydroxychloroquine Lopinavir/Ritonavir |
CCL2, CRP, CXCL10, d-dimer, Ferritin, IL-1, IL-6, IL-7, IL-10, LDH | The research team observed elevated pro-inflammatory and anti-inflammatory cytokines despite the use of anakinra. All patients died from severe respiratory failure a median of six days (range 1∼29 days) after anakinra was started. | Villegas et al. |
| Melanoma (N = 1) |
PD-1 blockade (Nivolumab) Piperacillin-Tazobactam Oseltamivir Clarithromycin Metronidazole Hydroxychloroquine Azithromycin |
Albumin, ALP, ALT, AST, Bilirubin (Total), Bilirubin (Direct), Creatinine, CRP, GGT, LDH, PCT, Total protein, Urea nitrogen, Uric acid | The patient had been gone through 27 cycles of nivolumab before the COVID-19 diagnosis, and the last dose was administered six days before the SARS-CoV-2 infection. The favipiravir and hydroxychloroquine were used for five days, and the patient was discharged. | Yekeduz et al. |
| Merkel cell caricinoma (N = 1) |
PD-1 blockade (Pembrolizumab) Hydroxychloroquine Azithromycin |
Creatinine, CRP, d-dimer, LDH, Urea | Hydroxychloroquine was administered for five days but stopped after supraventricular extrasystoles. However, the clinical improvement of the patient allowed him to discharge after 81 days. | da Costa et al. |
| Multiple myeloma (N = 1) |
IL-6R blockade (Tocilizumab) Moxifloxacin Umifenovir Thalidomide |
Albumin, ALT, AST, aPTT, Bilirubin (Total), CK, CK-MB, Creatinine, CRP, d-dimer, FDP, Fibrinogen, Hypersensitivity Tn I, IgA, IgG, IgM, IL-2, IL-4, IL-6, IL-10, LDH, PT, TNF-α, β2-microglobulin, κ light chain, λ light chain | The first case of COVID-19 in a patient with MM was successfully treated with the humanized anti-IL-6 receptor antibody tocilizumab. | Zhang et al. |
| Hematological cancer (N = 3) |
Rituximab Hydroxychloroquine Favipiravir Prednisone Azithromycin Betalactam antibiotic |
CRP, IL-6 | Hyperinflammation-associated organ failure may be less pronounced in hematological malignancies due to pre-existing or treatment-related immunosuppression (disease-associated in the patient 1 and 3; Bendamustin/rituximab-induced in the patient 2). | Bellmann-Weiler et al. |
| NK/T-cell lymphoma (N = 1) |
Rituximab Pembrolizumab SMILE DDGP CHOP Levofloxacin Supportive therapy |
CRP, Bilirubin (Indirect), LDH | The COVID-19 pneumonia signs and symptoms became stable after the transfusion, and steroid therapy was stopped. The hemoglobin level reached its peak on day 20. No antiviral or chloroquine drugs were used. | Pasin et al. |
| Chronic lymphocytic leukemia (CLL) (N = 4) |
Rituximab Lopinavir/Ritonavir Hydroxychloroquine Azithromycin Ceftriaxone Teicoplanin |
CRP, d-dimer, LDH, Ferritin, PCT, Troponin I | The disease course was mild, and no patient required admission to an ICU; three patients quickly recovered after 4–8 days and one after 24 days of experimental therapy for COVID-19. | Baumann et al. |
ALT, Alanine aminotransferase; aPTT, Activated partial thromboplastin time; AST, Aspartate aminotransferase; CCL2, Chemokine C—C motif ligand 2; CHOP, Cyclophosphamide, doxorubicin, vincristine, and prednisone; CK, Creatine kinase; CK-MB, Creatine kinase myocardial band; COVID-19, Coronavirus disease 2019; CRP, C-reactive protein; CXCL10, C-X-C motif chemokine ligand 10; DDGP, Dexamethasone, cisplatin, gemcitabine, and PEG-asparaginase; FDP, Fin degradation product; GGT, Gamma-glutamyl transferase; ICU, Intensive care unit; IgA, Immunoglobulin A; IgG, Immunoglobulin G; IgM, Immunoglobulin M; IL-2, Interleukin-2; IL-4, Interleukin-4; IL-6, Interleukin-6; IL-10, Interleukin-10; LDH, Lactate dehydrogenase; MM, Multiple myeloma; PCT, Procalcitonin; PD-1, Programmed cell death protein 1; PT, Prothrombin time; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SMILE, Dexamethasone, methotrexate, ifosfamide, l-asparaginase, and etoposide; TNF-α, Tumor necrosis factor α.
had consistently higher ferritin and LDH after administering IL-1R blocker (anakinra) all died from severe respiratory failure. The results found that, in cancer patients with COVID-19, the effectiveness of immunotherapy treatment varies, with some drugs are more effective than others, which may indicate that biomarker-based precision medicine may be the better therapy for cancer patients with COVID-19. Second, patients treated with antivirals/antibiotics exhibited lower CRP and LDH levels, together with a successful virus clearance [24], [25], [26]. In addition, patients with a normal PCT level at the early stage of COVID-19 had a better prognosis after giving the antiviral drug [27] or immunotherapy [23]. This result reflects that cancer patients with low levels of CRP, LDH, or PCT may indicate a good prognosis of COVID-19 after antiviral/antibiotic drug delivery.
Discussion
Since 29 biomarkers were identified as significantly changed in cohort studies, we focused on the molecules in the overlapping portion of the three groups (Fig. 2). The levels of CRP, d-dimer, ferritin, IL-2R, IL-6, LDH, and PCT were found elevated in cancer patients with severe COVID-19. Also, the albumin and Hb levels were down-regulated in SARS-CoV-2 infected cancer patients. Of note, most of the molecules are known to be inflammatory markers, suggesting that the interaction of cancer and COVID-19 biology may promote highly inflammatory conditions and lead to the progression of both cancer and COVID-19.
Previous studies showed that SARS-CoV-2 infection might induce metastasis in breast cancer [18] and EMT in lung cancer [28], which could be one of the COVID-19 pathophysiologies in cancer patients with COVID-19. During pandemics, 20% of cancer patients might develop severe respiratory illness, and 12% die within 30 days because of COVID-19 [29]. Collectively, there was a large proportion of poor prognosis in cancer patients with COVID-19. Since the interaction between cancer and COVID-19 is not well understood, the implications for the care of cancer patients require more clinical focus and attention. Our findings of distinct panels of biomarkers of disease progression in cancer patients with COVID-19 provide an opportunity to monitor patient conditions and tailor treatments to avoid progression to severe illness.
Immunotherapy used in cancer patients with COVID-19
Immuno-checkpoint blockade (ICB) and cytokine immunotherapy have been considered effective therapies for multiple cancers, such as lung cancer [30], breast cancer [31], and renal cell cancer [32]. During the COVID-19 outbreak, many researchers investigated whether these therapies also benefit the infected patients. As shown in Table 3, the SARS-CoV-2 was eliminated in multiple myeloma (MM) patients undergoing treatment with tocilizumab [23]. IL-6R blockades (tocilizumab and sarilumab) could be one of the potential immunotherapies for severe COVID-19 patients [33]. Since IL-6 plays a critical role in the cytokine release syndrome, blocking IL-6 signal transduction may reduce COVID-19 severity [34]. Indeed, tocilizumab reduced serum CRP to a normal level in most COVID-19 patients [35].
In addition, PD-1 blockade could serve as supportive therapy for severe COVID-19 patients (Table 3) [24,25]. CD8+ T cells can be activated and exhausted by expressing PD-1 during chronic viral infection [36]. A higher percentage of PD-1+ CD8+ and CD4+ T cells was found in severe COVID-19 patients [37]. As a result, targeting PD-1 may expedite the viral clearance by eliciting the PD-1+ CD8+ and CD4+ T cells in COVID-19 patients. In animal models, blocking PD-1/PD-L1 during chronic lymphocytic choriomeningitis virus infection reactivated the antiviral T cell and reduced viral load [38]. These findings suggest that ICB may be a potential supportive therapy combined with antiviral drugs to reinforce patients’ immunity and reduce viral load.
Cancer and COVID-19
SARS-CoV-2 has high infectivity and mortality rate in immune-deficient patients, such as cancer patients [17]. A previous study showed that hematological malignancies, lung cancer, or metastatic cancer (stage IV) patients had the highest frequency of severe complications due to COVID-19 [39]. In addition, the angiotensin-converting enzyme 2 (ACE2), a receptor that mediates SARS-CoV-2 entering into the host cells, is overexpressed in some specific cancers such as pancreatic, cervical, and renal cancer [40,41]. ACE2 also positively correlated with the epithelial-mesenchymal transition (EMT) regulators, Zeb1 and AXL [28], which could be a risk factor of metastasis for cancer patients. Moreover, TMPRSS2, a critical protease of SARS-CoV-2 internalization, was also found significantly increased at the mRNA level in prostate adenocarcinomas [42]. In this regard, cancer patients may have a higher risk of SARS-CoV-2 infection due to elevated levels of ACE2 and TMPRSS2.
During the COVID-19 pandemic, delayed diagnosis and treatment also pose challenges to cancer patients. Shortage of medical supplies, scheduling issues, and reduced patient mobility have indirectly impacted patients’ treatment and management of cancers. The overwhelmed healthcare system, staff shortages, and restricted medication access were the most frequent barriers for cancer patients [43]. In the United States, the pandemic has reduced outpatient visits by 60–70% for either new or established patients [44]. Additionally, cancer-related hospitalizations declined by 30–41% in five months. These findings indicate that patients were reluctant to visit the hospital due to COVID-19, which may delay the diagnosis and treatment, as well as increase the risk in patients with potential cancers.
It remains unclear whether acute infection by SARS-CoV-2 may enhance cancer progression, relapse, drug resistance, or metastasis. Future research should focus on longitudinal analysis to investigate the impact of COVID-19 on cancer patients. In addition, a specific study should be designed to examine outcomes in different cancer types as the divergent characteristics of different types of neoplasms likely respond to viral infection differently. Our study found that CRP, ferritin, and LDH positively relate to COVID-19 prognosis and severity in cancer patients. Therapies that reduce the expression of CRP, ferritin, and LDH will be of interest to explore as these three markers may be the appropriate serum biomarkers connecting acute infection and chronic inflammation in cancer.
Strengths and weaknesses of the research
Our study highlights the first systematic analysis to determine potential prognostic biomarkers for cancer patients with COVID-19. The findings may guide clinicians and help predict either deterioration in COVID-19-related health condition or aid in the prognosis in cancer patients infected with SARS-CoV-2 after specific treatment for COVID-19 symptoms. This study focuses on the linkage of acute viral infection and chronic cancer inflammation and identifying potential serum biomarkers in cancer patients with virally mediated inflammation. The identified list of biomarkers can serve as candidates for long-term follow-up of COVID-19 convalescent cancer patients. Nevertheless, there are several limitations to our approach. There is a lack of eligible articles on cancer-type specific COVID-19 research in this review. COVID-19 patients with various types of cancer may contribute to distinct serum biomarkers. Additionally, some eligible studies are case reports, compromising the statistical power of the relationship between biomarkers and disease progression.
Summary
Cancer patients with high levels of CRP, d-dimer, ferritin, IL-2R, IL-6, LDH, and PCT may suffer from more severe COVID-19 than those with mild/moderate symptoms. Additionally, this panel of biomarkers may be used to monitor potential cancer risk in COVID-19 patients. Furthermore, immunotherapies targeting IL-6R or PD-1 and antiviral drug treatment may benefit cancer patients with COVID-19 who exhibit lower levels of CRP, ferritin, and LDH. Therefore, CRP, ferritin, and LDH could be potential biomarkers to track COVID-19 prognosis or drug efficacy in cancer patients with COVID-19.
CRediT authorship contribution statement
Te-An Lee: Investigation, Formal analysis, Writing – original draft. Shih-Han Wang: Writing – original draft. Chun-Tse Kuo: Formal analysis, Visualization. Chia-Wei Li: Writing – original draft. Louise D. McCullough: Writing – original draft. Dhimiter Bello: Writing – original draft. Yun-Ju Lai: Supervision, Visualization, Formal analysis, Writing – original draft.
Declaration of Competing Interest
The authors declare not to have any conflict of interest.
Acknowledgments
This work was funded in part by the following: University of Massachusetts Lowell (Faculty start-up D50210000000022 to Y.-J.Lai); Ministry of Science and Technology (MOST 109–2314-B-001–002 and MOST 109–2314-B-001–008 to C.-W.Li); Academia Sinica (AS-KPQ-111-KNT and AS-GC-110-05 to C.-W.Li); Ministry of Science and Technology (MOST 109–2813-C-010–029-B to T.-A.Lee).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2022.101443.
Appendix. Supplementary materials
References
- 1.Strimbu K., Tavel J.A. What are biomarkers? Curr. Opin. HIV AIDS. 2010;5:463–466. doi: 10.1097/COH.0b013e32833ed177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.G. Biomarkers Definitions Working Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 3.Daubert M.A., Jeremias A. The utility of troponin measurement to detect myocardial infarction: review of the current findings. Vasc. Health Risk Manag. 2010;6:691–699. doi: 10.2147/vhrm.s5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan E., Gouvas N., Nicholls R.J., Ziprin P., Xynos E., Tekkis P.P. Diagnostic precision of carcinoembryonic antigen in the detection of recurrence of colorectal cancer. Surg. Oncol. 2009;18:15–24. doi: 10.1016/j.suronc.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Brooks M. Breast cancer screening and biomarkers. Methods Mol. Biol. 2009;472:307–321. doi: 10.1007/978-1-60327-492-0_13. [DOI] [PubMed] [Google Scholar]
- 6.Sell S. Alpha-fetoprotein, stem cells and cancer: how study of the production of alpha-fetoprotein during chemical hepatocarcinogenesis led to reaffirmation of the stem cell theory of cancer. Tumour Biol. 2008;29:161–180. doi: 10.1159/000143402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peck K.M., Burch C.L., Heise M.T., Baric R.S. Coronavirus host range expansion and middle east respiratory syndrome coronavirus emergence: biochemical mechanisms and evolutionary perspectives. Ann. Rev. Virol. 2015;2:95–117. doi: 10.1146/annurev-virology-100114-055029. [DOI] [PubMed] [Google Scholar]
- 8.Abdelrahman Z., Li M., Wang X. Comparative review of SARS-CoV-2, SARS-CoV, MERS-CoV, and influenza a respiratory viruses. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.552909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen E., Koopmans M., Go U., Hamer D.H., Petrosillo N., Castelli F., Storgaard M., Al Khalili S., Simonsen L. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect. Dis. 2020;20:e238–e244. doi: 10.1016/S1473-3099(20)30484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ponti G., Maccaferri M., Ruini C., Tomasi A., Ozben T. Biomarkers associated with COVID-19 disease progression. Crit. Rev. Clin. Lab. Sci. 2020;57:389–399. doi: 10.1080/10408363.2020.1770685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Valle D.M., Kim-Schulze S., Huang H.H., Beckmann N.D., Nirenberg S., Wang B., Lavin Y., Swartz T.H., Madduri D., Stock A., Marron T.U., Xie H., Patel M., Tuballes K., Van Oekelen O., Rahman A., Kovatch P., Aberg J.A., Schadt E., Jagannath S., Mazumdar M., Charney A.W., Firpo-Betancourt A., Mendu D.R., Jhang J., Reich D., Sigel K., Cordon-Cardo C., Feldmann M., Parekh S., Merad M., Gnjatic S. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mittal V. Epithelial mesenchymal transition in tumor metastasis. Ann. Rev. Pathol. 2018;13:395–412. doi: 10.1146/annurev-pathol-020117-043854. [DOI] [PubMed] [Google Scholar]
- 13.National Cancer Institute (2020). "Cancer Statistics." Retrieved Octorber 26, 2021, from https://www.cancer.gov/about-cancer/understanding/statistics.
- 14.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Kumari N., Dwarakanath B.S., Das A., Bhatt A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016;37:11553–11572. doi: 10.1007/s13277-016-5098-7. [DOI] [PubMed] [Google Scholar]
- 16.Esquivel-Velazquez M., Ostoa-Saloma P., Palacios-Arreola M.I., Nava-Castro K.E., Castro J.I., Morales-Montor J. The role of cytokines in breast cancer development and progression. J. Interferon Cytokine Res. 2015;35:1–16. doi: 10.1089/jir.2014.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rugge M., Zorzi M., Guzzinati S. SARS-CoV-2 infection in the Italian Veneto region: adverse outcomes in patients with cancer. Nat. Cancer. 2020;1:784–788. doi: 10.1038/s43018-020-0104-9. [DOI] [PubMed] [Google Scholar]
- 18.Lai Y.J., Chao C.H., Liao C.C., Lee T.A., Hsu J.M., Chou W.C., Wang J., Huang H.C., Chang S.J., Lin Y.L., Li C.W. Epithelial-mesenchymal transition induced by SARS-CoV-2 required transcriptional upregulation of Snail. Am. J. Cancer Res. 2021;11:2278–2290. [PMC free article] [PubMed] [Google Scholar]
- 19.National Institutes of Health (2021). "COVID-19 Treatment Guideline: Clinical Spectrum of SARS-CoV-2 Infection." Retrieved October 26, 2021, from https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/.
- 20.Lai Y.J., Hanneman S.K., Casarez R.L., Wang J., McCullough L.D. Blood biomarkers for physical recovery in ischemic stroke: a systematic review. Am. J. Transl. Res. 2019;11:4603–4613. [PMC free article] [PubMed] [Google Scholar]
- 21.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American College of Physicians (2018). "ACP Internal Medicine Meeting: Reference Ranges." Retrieved October 26, 2021, from https://annualmeeting.acponline.org/educational-program/handouts/reference-ranges-table.
- 23.Zhang X., Song K., Tong F., Fei M., Guo H., Lu Z., Wang J., Zheng C. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. 2020;4:1307–1310. doi: 10.1182/bloodadvances.2020001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yekeduz E., Dursun B., Aydin G.C., Yazgan S.C., Ozturk H.H., Azap A., Utkan G., Urun Y. Clinical course of COVID-19 infection in elderly patient with melanoma on nivolumab. J. Oncol. Pharm. Pract. 2020;26:1289–1294. doi: 10.1177/1078155220924084. [DOI] [PubMed] [Google Scholar]
- 25.da Costa C.M., de Souza Z.S., Real Salgues A.C., Harada G., Marino Rodrigues Ayres P.P., Vieira Nunes D.B., Katz A., Munhoz R.R. COVID-19 in a patient with advanced Merkel cell carcinoma receiving immunotherapy. Immunotherapy. 2020;12:1133–1138. doi: 10.2217/imt-2020-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bellmann-Weiler R., Burkert F., Schwaiger T., Schmidt S., Ludescher C., Oexle H., Wolf D., Weiss G. Janus-faced course of COVID-19 infection in patients with hematological malignancies. Eur. J. Haematol. 2020;105:502–504. doi: 10.1111/ejh.13470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumann T., Delgado J., Montserrat E. CLL and COVID-19 at the hospital clinic of Barcelona: an interim report. Leukemia. 2020;34:1954–1956. doi: 10.1038/s41375-020-0870-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart C.A., Gay C.M., Ramkumar K., Cargill K.R., Cardnell R.J., Nilsson M.B., Heeke S., Park E.M., Kundu S.T., Diao L., Wang Q., Shen L., Xi Y., Maria Della Corte C., Fan Y., Kundu K., Pickering C.R., Johnson F.M., Zhang J., Kadara H., Minna J.D., Gibbons D.L., Wang J., Heymach J.V., Byers L.A. SARS-CoV-2 infection induces EMT-like molecular changes, including ZEB1-mediated repression of the viral receptor ACE2, in lung cancer models. J. Thorac. Oncol. 2021;16:1821–1839. doi: 10.1016/j.jtho.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robilotti E.V., Babady N.E., Mead P.A., Rolling T., Perez-Johnston R., Bernardes M., Bogler Y., Caldararo M., Figueroa C.J., Glickman M.S., Joanow A., Kaltsas A., Lee Y.J., Lucca A., Mariano A., Morjaria S., Nawar T., Papanicolaou G.A., Predmore J., Redelman-Sidi G., Schmidt E., Seo S.K., Sepkowitz K., Shah M.K., Wolchok J.D., Hohl T.M., Taur Y., Kamboj M. Determinants of COVID-19 disease severity in patients with cancer. Nat. Med. 2020;26:1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ackermann C.J., Reck M., Paz-Ares L., Barlesi F., Califano R. First-line immune checkpoint blockade for advanced non-small-cell lung cancer: travelling at the speed of light. Lung Cancer. 2019;134:245–253. doi: 10.1016/j.lungcan.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Emens L.A. Breast cancer immunotherapy: facts and hopes. Clin. Cancer Res. 2018;24:511–520. doi: 10.1158/1078-0432.CCR-16-3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossi J.F., Negrier S., James N.D., Kocak I., Hawkins R., Davis H., Prabhakar U., Qin X., Mulders P., Berns B. A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer. Br. J. Cancer. 2010;103:1154–1162. doi: 10.1038/sj.bjc.6605872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.M. Masoomikarimi, B. Garmabi, J. Alizadeh, E. Kazemi, A. Azari Jafari, S. Mirmoeeni, M. Dargahi, N. Taheri, R. Jafari, Advances in immunotherapy for COVID-19: a comprehensive review, Int. Immunopharmacol., 93 (2021) 107409. [DOI] [PMC free article] [PubMed]
- 34.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X., Pan A., Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. U. S. A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yi J.S., Cox M.A., Zajac A.J. T-cell exhaustion: characteristics, causes and conversion. Immunology. 2010;129:474–481. doi: 10.1111/j.1365-2567.2010.03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G., Yuan Z., Feng Z., Zhang Y., Wu Y., Chen Y. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schonrich G., Raftery M.J. The PD-1/PD-L1 Axis and virus infections: a delicate balance. Front. Cell Infect. Microbiol. 2019;9:207. doi: 10.3389/fcimb.2019.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Addeo A., Friedlaender A. Cancer and COVID-19: unmasking their ties. Cancer Treat. Rev. 2020;88 doi: 10.1016/j.ctrv.2020.102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allegra A., Pioggia G., Tonacci A., Musolino C., Gangemi S. Cancer and SARS-CoV-2 Infection: diagnostic and Therapeutic Challenges. Cancers. 2020;12:1581. doi: 10.3390/cancers12061581. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.X. Jia, C. Yin, S. Lu, Y. Chen, Q. Liu, J. Bai, Y. Lu, Two things about COVID-19 might need attention. Preprints. 2020020315. (2020).
- 42.Vaarala M.H., Porvari K., Kyllonen A., Lukkarinen O., Vihko P. The TMPRSS2 gene encoding transmembrane serine protease is overexpressed in a majority of prostate cancer patients: detection of mutated TMPRSS2 form in a case of aggressive disease. Int. J. Cancer. 2001;94:705–710. doi: 10.1002/ijc.1526. [DOI] [PubMed] [Google Scholar]
- 43.Jazieh A.R., Akbulut H., Curigliano G., Rogado A., Alsharm A.A., Razis E.D., Mula-Hussain L., Errihani H., Khattak A., De Guzman R.B., Mathias C., Alkaiyat M.O.F., Jradi H., Rolfo C. C.-I.o.C.C. international research network on, impact of the COVID-19 pandemic on cancer care: a global collaborative study. JCO Glob. Oncol. 2020;6:1428–1438. doi: 10.1200/GO.20.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patt D., Gordan L., Diaz M., Okon T., Grady L., Harmison M., Markward N., Sullivan M., Peng J., Zhou A. Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for american seniors. JCO Clin. Cancer Inform. 2020;4:1059–1071. doi: 10.1200/CCI.20.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.