Abstract
Objective
There is limited literature on patients with a history of COVID-19 pneumonia who underwent anatomical lung resection for non-small cell lung cancer (NSCLC). This study was aimed to share the early postoperative outcomes in patients who underwent lung resection after COVID-19 pneumonia.
Materials and methods
We retrospectively evaluated 30 patients who underwent lobectomy with thoracotomy and systematic mediastinal lymph node dissection due to NSCLC in a single center between November 2018 and September 2021. The patients were divided into two groups regarding COVID-19 pneumonia history; the COVID-19 group consisted of 14 patients (46.7%) and the non-COVID-19 group 16 (53.3%) patients. The patients’ age, gender, comorbidity, Charlson Comorbidity Index (CCI) score, forced expiratory volume in 1 s (FEV1) value, tumor type and size, resection type, postoperative air leak duration, total drainage volume, drain removal time, postoperative complications, and length of stay (LOS) were recorded.
Results
9 (30%) patients were female, and 21 (70%) were male. The mean age was 62.1 ± 8.91 years. Our comparison of postoperative air leak duration, total drainage volume, time to drain removal, postoperative complications, and LOS between the COVID-19 and non-COVID-19 groups revealed no statistically significant difference.
Conclusion
Anatomical lung resection can be performed safely in NSCLC patients with a history of COVID-19 pneumonia without significant difference in early postoperative morbidity and mortality.
Keywords: COVID-19, Lung cancer, Thoracic surgery, Lobectomy
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has entailed a global crisis in the health system since the first half of 2020.1 , 2 The pandemic not only impacted daily life dramatically but also led to the death of millions of people.3 As of October 2021, approximately 239 million confirmed cases of COVID-19 and 4.87 million related deaths had been reported worldwide. According to World Health Organisation (WHO) data, about 234.1 million people have recovered from the disease so far.1 The SARS-CoV-2 can result in a broad spectrum of outcomes in its primary target, the lung, ranging from complete recovery to widespread sequelae.4 The early phase of SARS-CoV-2 infection generally manifests with diffuse pulmonary infiltrates, mucosal damage as a result of viremia in the bronchi, development of alveolitis and vasculitis, and distal parenchymal injury, whereas the later phase involves injury due to the weakening of mucosal and distal parenchymal connective tissue as a result of long-term corticosteroids use and pulmonary fibrosis.5
A history of COVID-19 pneumonia is increasingly common in patients scheduled for elective thoracic surgery. Early diagnosis and treatment are crucial in managing conditions that necessitate surgery, such as lung cancer, chest wall, and mediastinal diseases. Besides, it is clinically vital to provide the appropriate medical environment for patients who require thoracic surgery during the COVID-19 pandemic.6 , 7 There is limited published literature on the rates of complication, morbidity, and mortality rates in patients with a history of COVID-19 pneumonia who underwent anatomical lung resection for NSCLC. In this study, we share the early postoperative outcomes in patients with these features operated in our center and compare them with anatomical lung resection outcomes before the pandemic.
2. Materials and methods
2.1. Study setting and population
Our study has a retrospective, descriptive and cross-sectional design and was approved by the local ethics committee (Approval No:2021/206). The study population consisted of 30 patients who underwent lobectomy with thoracotomy and systematic mediastinal lymph node dissection due to NSCLC in our center between November 2018 and September 2021. Informed consent was obtained from all patients. All authors affirmed compliance with the World Medical Association (WMA) Declaration of Helsinki principles for medical research involving human subjects.
The patients were divided into two groups regarding COVID-19 pneumonia history. The COVID-19 group consisted of 14 patients (46.7%) with a history of the disease between April 2020 and September 2021. The non-COVID-19 group included 16 (53.3%) patients randomized by age, gender and comorbidity, who underwent anatomical lung resection and systematic mediastinal lymph node dissection between November 2018 and January 2020. All patients in the COVID-19 group had SARS-CoV-2 RT-PCR test positivity along with clinical and radiological compliance. All patients with a history of COVID-19 pneumonia had extensive pulmonary involvement on thorax computed tomography (Fig. 1 ). In all patients in the COVID-19 group, a lung nodule or mass was detected following SARS-CoV-2 infection. Two patients of the COVID-19 group were treated in the intensive care unit, and 12 patients COVID-19 ward. Patients in the COVID-19 group were divided into two groups according to the detection time of the lung nodule. The COVID-19 screening group consisted of those discovered during COVID-19 imaging, while the Subsequent screening group consisted of those detected during subsequent imaging. The cohort did not include patients who underwent lobectomy for benign etiologies, segmentectomy, or pneumonectomy for NSCLC and those without hilar and systematic mediastinal lymph node dissection. Data were obtained from physical and digital hospital archives. The patients’ age, gender, comorbidity, COVID-19 history, histopathological tumor type, resection type, duration of air leak from the chest tube, time to drain removal, postoperative complications, length of stay (LOS), and pathological TNM stages were noted. An air leak lasting longer than five days was considered a persistent air leakage. Postoperative complications were classified according to the Clavien-Dindo system.
Fig. 1.
Axial Thorax CT Images of patients in the COVID-19 group
Patient 1: (a–b) Subsegmental atelectasis and bilateral multifocal ground-glass areas are seen on thorax CT, (c) pre-operative thorax CT demonstrates a left lung lower lobe periferal nodule (the nodule marked with white arrow)
Patient 2: (d–f) Bilaterally ground-glass opacities are seen on thorax CT, (d) pre-operative thorax CT reveals a central left upper lobe mass. (the mass marked with white arrow).
Pulmonary function tests and transthoracic echocardiography were applied in all patients before surgery. Besides, all patients underwent positron emission tomography/computerized tomography and cranial magnetic resonance imaging for clinical staging and metastasis screening. The stair-climbing test was applied to all patients to evaluate lung capacity, gas exchange, muscle strength and cardiac output before lung resection. Patients with less than 4% desaturations during the stair-climbing test were considered suitable for lung resection. Informed consent was obtained from all patients participating in the study before the operation. Possible risks and uncertainties related to COVID-19 were also mentioned, and informed consent was obtained from the patients in COVID-19 group. Our surgical team omitted lobectomy for NSCLC in patients with distant metastases, mediastinal lymph node positivity, and features unfavorable for achieving R0 surgical margins with lobectomy. We performed all surgeries under general anesthesia with a double-lumen endotracheal tube followed by a posterolateral thoracotomy. The team used a thoraco-abdominal stapler (4.8 mm height) for bronchial divisions and a linear stapler (4.8 mm height) for pulmonary fissure divisions.
Patients with a history of COVID-19 underwent surgery at least six weeks after the disease, following extensive preoperative preparation. From March 11, 2020, when the first case of SARS-CoV-2 infection was officially reported, all patients submitted two combined oropharyngeal-nasal swabs taken 48 h apart for SARS-CoV-2 RT-PCR testing before surgery. Only patients with negative results qualify for surgery. We performed the surgeries with minimal staff, and the surgical team wore full personal protective equipment (PPE).
2.2. Statistical analysis
IBM SPSS for Windows Version 22.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Descriptive data were presented as frequency and percentage for categorical variables and mean and standard deviation (median + range) for quantitative variables. The conformity of data to normal distribution was checked with the Shapiro–Wilk test. In subgroups, univariate analyzes were performed using the chi-squared test and Mann–Whitney U-test. A p-value <0.05 was considered statistically significant.
3. Results
A total of 30 patients underwent lobectomy and systematic mediastinal lymph node dissection for NSCLC. 9 (30%) patients were female, and 21 (70%) were male. The mean age was 62.1 ± 8.91 years. The COVID-19 group consisted of 14 patients. 4 of them were female, and 10 were male. The mean age of the group was 60.7. Lung cancer was detected on COVID-19 imaging in 9 (64.2%) of the patients, while it was discovered in 5 (35.8%) of the patients during subsequent clinical evaluations. There were 16 patients in the non-COVID-19 group. 5 of them were female, and 11 were male. In the whole cohort, 11 (36.6%) patients had essential hypertension, 10 (30%) diabetes mellitus, 5 (16.7%) chronic obstructive pulmonary disease (COPD), 2 (6.6%) asthma, 2 (6.6%) heart valve disease, 1 (3.3%) atrial fibrillation and 1 (3.3%) chronic renal failure. 5 patients (16.7%) had no comorbidities. The Charlson Comorbidity indexes and pulmonary function test results by groups are presented in Table 1 .
Table-1.
Demographic characteristics and pathological findings of patients in COVID-19 and non-COVID-19 groups.
| Baseline characteristic | COVID-19 Group |
non-COVID-19 Group |
Total sample |
|||
|---|---|---|---|---|---|---|
| mean n | SD % | mean n | SD % | mean n | SD % | |
| Age | 60.7 | 8.39 | 63.4 | 9.41 | 62.1 | 8.91 |
| Gender | ||||||
| Female | 4 | 28.6 | 5 | 31.2 | 9 | 30.0 |
| Male | 10 | 71.4 | 11 | 68.8 | 21 | 70.0 |
| Comorbidity | ||||||
| DM | 6 | 42.9 | 4 | 25.0 | 10 | 30.0 |
| HT | 4 | 28.6 | 7 | 43.7 | 11 | 36.6 |
| COPD | 3 | 21.4 | 2 | 12.5 | 5 | 16.7 |
| Asthma | 2 | 14.3 | - | - | 2 | 6.6 |
| CKD | - | - | 1 | 6.2 | 1 | 3.3 |
| Valvular heart disease | 1 | 7.1 | 1 | 6.2 | 2 | 6.6 |
| Atrial fibrillation | 1 | 7.1 | - | - | 1 | 3.3 |
| None | 1 | 7.1 | 4 | 25.0 | 5 | 16.7 |
| CCI score | ||||||
| 0–1 | 2 | 14.3 | 3 | 18.7 | 5 | 16.7 |
| 2–3 | 8 | 57.1 | 10 | 62.5 | 18 | 60 |
| 4–5 | 4 | 28.6 | 3 | 18.7 | 7 | 23.3 |
| >6 | - | - | - | - | - | - |
| FEV1% | 78.4 | 6,6 | 79,6 | 7,5 | 79,1 | 7,0 |
| Tumour size (cm) | 3.17 | 1.51 | 3.23 | .96 | 3.2 | 1.22 |
| Tumour location | ||||||
| Right upper lobe | 3 | 21.4 | 1 | 6.2 | 4 | 13.3 |
| Right middle lobe | 2 | 14.3 | 3 | 18.7 | 5 | 16.7 |
| Right lower lobe | 3 | 21.4 | 2 | 12.5 | 5 | 16.7 |
| Left upper lobe | 3 | 21.4 | 5 | 31.3 | 8 | 26.7 |
| Left lower lobe | 3 | 21.4 | 5 | 31.3 | 8 | 26.7 |
| Lung cancer histologic type | ||||||
| Adenocarcinoma | 7 | 50.0 | 8 | 50.0 | 15 | 50.0 |
| Squamous cell carcinoma | 6 | 42.9 | 6 | 37.5 | 12 | 40.0 |
| Neuroendocrine tumor | 1 | 7.1 | 2 | 12.5 | 3 | 10.0 |
| Pathological TNM stage | ||||||
| Stage IA | 9 | 64.3 | 6 | 37.5 | 15 | 50.0 |
| Stage IB | 2 | 14.3 | 6 | 37.5 | 8 | 26.7 |
| Stage IIA | - | - | 1 | 6.2 | 1 | 3.3 |
| Stage IIB | 1 | 7.1 | 1 | 6.2 | 2 | 6.6 |
| Stage IIIA | 2 | 14.3 | 2 | 12.5 | 4 | 13.3 |
| Time to surgery after COVID-19 (weeks) | 25.79 | 15.0 | - | - | - | - |
Note: DM: Diabetes Mellitus, HT: Hypertension, COPD: Chronic Obstructive Pulmonary Disease, CKD: Chronic Kidney Disease, CCI: Charlson Co-morbidity Index.
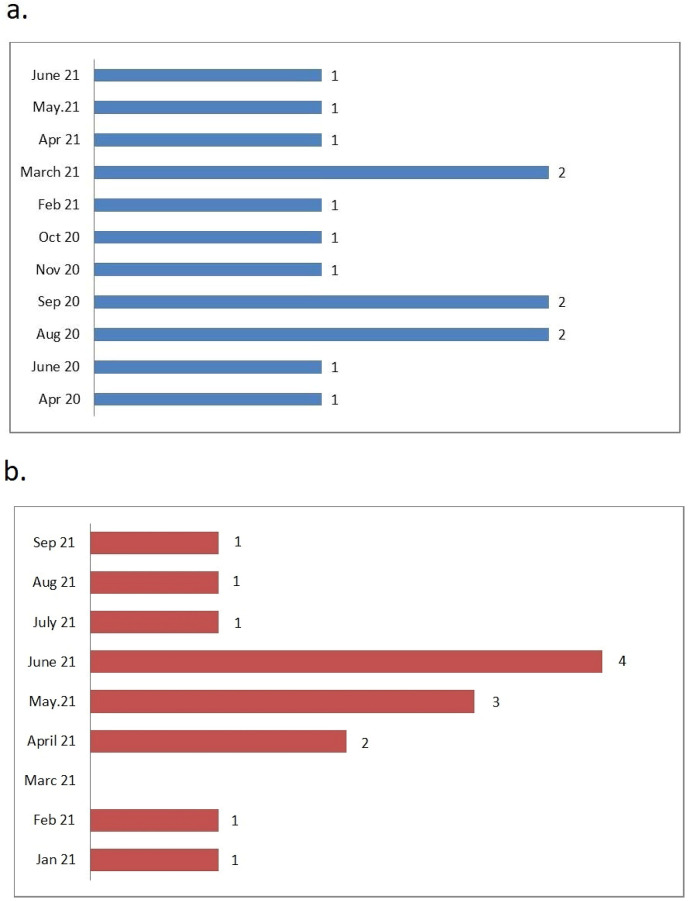
The SARS-CoV-2 infection and operation dates of the patients in the COVID-19 group are shown in Fig. 2 . In the COVID-19 group, the mean time to surgery after SARS-CoV-2 infection was 25.79 ± 15 weeks. Mean times from SARS-CoV-2 infection to lung cancer surgery were 16.33 ± 5.33 and 42.8 ± 10.7 weeks in the COVID-19 screening group and Subsequent screening group, respectively.
Fig. 2.
Distribution of SARS-CoV-2 infection and anatomic lung resection numbers by months in patients in the COVID-19 group
(a) Time of SARS-CoV-2 infection in patients in the COVID-19 group, (b) Time to perform anatomical lung resection in patients in the COVID-19 group.
Eight (%26.7) patients underwent left upper lobectomy, 8 (%26.7) left lower lobectomy, 5 (16.7%) right middle lobectomy, 5 (16.7%) right lower lobectomy, 4 (13.3%) right upper lobectomy. Table 1 shows the demographic characteristics and pathological findings of patients in the COVID-19 and non-COVID-19 groups.
The postoperative air leak duration was 3.36 ± 2.27 days in the COVID-19 group and 2.94 ± 1.52 days in the non-COVID-19 group. The difference was not statistically significant (p = 0.854). Besides, there were 2 cases of persistent air leakage in the COVID-19 group and none in the non-COVID-19 group, with no significant difference again (p = 0.209). Furthermore, the mean total drainage volume was 725 ± 619.4 ml in the COVID-19 group and 893.7 ± 565 ml in the non-COVID group (p = 0.355). Moreover, the mean time to drain removal was 6.36 ± 2.46 days in the COVID-19 group and 4.69 ± 1.49 days in the non-COVID-19 group. The difference was not significant between groups (p = 0.052) (Table 2 ). Finally, the mean LOS was 7.64 ± 2.49 days in the COVID-19 group and 6.56 ± 1.41 days in the non-COVID-19 group (p = 0.377).
Table-2.
Comparison of COVID-19 and non-COVID-19 groups by postoperative air leak.
| Variables | COVID-19 Group |
Non-COVID-19 Group |
p-valuea | ||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| Air leak duration (days) | 3.36 | 2.27 | 2.94 | 1.52 | .854 |
| Total drainage volume (ml) | 725 | 619.4 | 893.7 | 565.0 | .355 |
| Time to drain removal (days) | 6.36 | 2.46 | 4.69 | 1.49 | .052 |
| Length of hospital stay (days) | 7.64 | 2.49 | 6.56 | 1.41 | .377 |
Four patients had postoperative complications in each group (p = 1.00). There were three (21.4%) minor and one (6.2%) major complications Clavien-Dindo classification in the COVID-19 group, and four (25.5%) minor complications in the non-COVID-19 group. In addition, two (14.2%) patients had surgical complications in the COVID-19 group. One of these patients had a wound site infection, while the other had chylothorax and residual space. In the non-COVID-19 group, 2 (12.5%) patients had wound site infections (Table 3 ).
Table-3.
Postoperative complications by groups.
| Variables | COVID-19 Group |
non-COVID-19 Group |
||
|---|---|---|---|---|
| n | % | n | % | |
| Postoperative complicationsa | ||||
| Minor | 3 | 21.4 | 4 | 25.5 |
| Major | 1 | 6.2 | - | - |
| None | 10 | 71.4 | 12 | 75.0 |
| Surgical complications | ||||
| Residual space | 1 | 7.1 | - | - |
| Chylothorax | 1 | 7.1 | - | - |
| Wound infection | 1 | 7.1 | 2 | 12.5 |
| Pulmonary complications | ||||
| Atelectasis | 2 | 14.3 | 1 | 6.2 |
| Pneumonia | 1 | 7.1 | - | - |
| Cardiac complications | ||||
| Arrhythmia | 1 | 7.1 | 1 | 6.2 |
| Cerebrovascular complications | ||||
| Lacunar stroke | 1 | 7.1 | - | - |
Postoperative complications classified according to the Clavien-Dindo system.
There were also pulmonary, cardiac, and cerebrovascular complications in our cohort. 2 (14.3%) patients developed early atelectasis and 1 (7.1%) postoperative pneumonia in the COVID-19 group, whereas 1 (6.2%) patient had atelectasis in the non-COVID-19 group. We detected arrhythmia in one patient in each group. The only cerebrovascular complication was a lacunar stroke that developed on the postoperative 7th day in a patient with a history of tuberculosis in the COVID-19 group. The patient's echocardiography and bilateral carotid and lower extremity deep venous Doppler examinations did not reveal any etiological findings that could cause embolism. Following anticoagulant therapy, neurological symptoms regressed, and the patient was uneventfully discharged.
The examination of pathological specimens revealed a mean tumor size of 3.2 ± 1.2 cm. Histopathological diagnosis was adenocarcinoma in 15 (50%) patients, squamous cell carcinoma in 12 (24%), and neuroendocrine tumor in 3 (10%). The pathological staging of the patients was as follows: 15 patients (50%) were staged as IA, eight patients (26.7%) were staged as IB, one patient (3.3%) was staged as IIA, two patients (6.6%) were staged as IIB, and four patients (3.3%) were staged as IIIA. Nine patients (64.3%) were staged as IA, two (14.3%) as IB, one (7.1%) as IIB, and two (14.3%) as IIIA in the COVID-19 group. Table 4 shows the pathological stages of patients in the COVID-19 group according to the time of the lung cancer diagnosis.
Table 4.
Pathological stages of patients in the COVID-19 group according to the time of lung cancer diagnosis.
| Pathologic TNM Stage | COVID-19 Screening Group |
Subsequent Screening Group |
Total sample |
|||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Stage IA | 7 | 77.8 | 2 | 40 | 9 | 64.3 |
| Stage IB | 1 | 11.1 | 1 | 20 | 2 | 14.3 |
| Stage IIA | - | - | - | - | - | - |
| Stage IIB | - | - | 1 | 20 | 1 | 7.1 |
| Stage IIIA | 1 | 11.1 | 1 | 20 | 2 | 14.3 |
4. Discussion
The present study is one of the few in the literature to investigate early postoperative outcomes in patients with a history of COVID-19 pneumonia who underwent anatomical lung resection for NSCLC. Postoperative air leak duration, total drainage volume, time to drain removal, postoperative complications, and LOS between the COVID-19 and non-COVID-19 groups revealed no statistically significant difference.
COVID-19 infection during the perioperative period is well known to be a risk factor for increased length of stay, morbidity, and mortality.8 SARS-CoV-2 infection shows a highly variable clinical course. The majority of infected people survive the disease asymptomatically or with mild symptoms. COVID-19 pneumonia is causing significant short and medium-term morbidity and mortality, with persisting symptoms and radiological alterations up to 6 months after symptoms onset.9 Surgery for carcinoid tumors, 2 cm nodules, and ground-glass opacity was recommended to be postponed in the early phases of the pandemic due to the unpredictable risk of SARS-CoV-2 infection.10
There isn't enough evidence in the literature about the outcomes of lung resection in patients with a COVID-19 pneumonia history. Elective thoracic surgery is recommended at least 4–6 weeks after SARS-CoV-2 RT-PCR negativity in patients with a history of COVID-19.11 In our study, the mean duration of SARS-CoV-2 infection to lung resection was 25.79 (range: 9–56) weeks. We believe that multiple factors affect the long period from SARS-CoV-2 infection to lung surgery. First of all, 5 of the patients with COVID-19 pneumonia could not be diagnosed with a lung mass during SARS-CoV-2 screening. Detection of the lung mass in these patients in the subsequent thorax imaging caused a prolonged period until lung resection. The average time from infection to lung surgery in 5 patients with delayed diagnosis of a lung mass was 42.8 ± 10.7 weeks. We believe two reasons why a lung nodule or mass was not discovered in these patients during infection screening. Malignant lesions, particularly ground-glass lung nodules, were shadowed by dense pulmonary infiltrates caused by COVID-19 pneumonia. The nodules went undiscovered because thick-section thorax CT was used for lung imaging while the number of individuals affected by the pandemic was large. It is noteworthy that the lung resection operations of patients with COVID-19 pneumonia were carried out from the first months of 2021, following mass vaccination activities. Patients in the COVID-19 group may have hesitated for lung surgery during the pre-vaccination period.
Studies report the rate of postoperative persistent air leakage between 2.38 and 13.3% in patients who underwent lobectomy for NSCLC.12, 13, 14, 15 In the present study, persistent air leakage was observed in two (14.2%) patients in the COVID-19 group, which was in line with the literature.
Regarding the time to drain removal, Suzuki et al reported a mean of four days (1–29) and Long et al also four days.3 , 4 , 6 , 7 However, in a systematic review of 39 studies, including seven with data on lobectomy with thoracotomy, Whitson et al indicated a mean of 5.7 days.12 , 13 , 16 In our study, the mean time to drain removal was 6.36 ± 2.46 days in the COVID-19 group and 4.69 ± 1.49 days in the non-COVID-19 group. The difference of approximately 2 days more in the COVID-19 group was not statistically significant (p = 0.052).
Another parameter in our investigation was LOS. In the literature, Erdogu et al cited a mean LOS of 5.5 days, Al-Ameri et al 6 days, Whitson et al. 13.3 days, and Geller et al 6 days.14, 15, 16, 17 In the present study, the mean LOS was 7.64 ± 2.49 days in the COVID-19 group and 6.56 ± 1.41 days in the non-COVID-19 group (p = 0.377). Besides, there was no prolonged hospital stay in the COVID-19 group.
Concerning postoperative complications, Long et al noted minor and major conditions in 23 (10.95%) patients, Erdoğu et al in 58 (32.2%), Whitson et al in 31.2%, and Geller et al in 169 (34%).13, 14, 15, 16 In our cohort, 4 patients had postoperative complications in each group, corresponding to 28.5% in the COVID-19 group and 25% in the non-COVID-19 group, without statistical significance (p = 1.00).
Testori et al reported a successful lung resection case for primary lung adenocarcinoma in a 46-year-old male patient with a history of COVID-19, with no complications as of early postoperative outcomes.18 The complication rates in the present study also attest to the viability of anatomical lung resection in patients with similar characteristics.
Three of the patients in the COVID-19 group underwent surgery in the pathologically advanced stage. The lung mass could not be diagnosed on COVID-19 imaging in two of these patients. Despite the limited number of patients in our study, it is noteworthy that the pathological stages of patients with a history of COVID-19 pneumonia diagnosed with lung cancer in the later period were more advanced. Delaying surgery for stage 1 NSCLC by 8 weeks was linked to pathological upstaging of the tumor, higher 30-day mortality, and shorter median survival, according to a large cohort study of 40,000 patients.19 Although the results of our study report that lung resection can be performed safely in patients with COVID-19 pneumonia, clinical, radiological, or laboratory evaluation methods are needed to determine the optimum time for early surgery in these patients.
4.1. Limitations
Since the long-term effects of COVID-19 infection are still unclear, our investigation of early postoperative outcomes has been based on a relatively small cohort. As time proceeds, there will be many more cases of lung resection in patients with a history of COVID-19 infection, allowing future studies with larger study populations.
5. Conclusion
COVID-19 infection primarily affects the lungs. However, the present study has shown that lung resection can be performed safely in NSCLC patients with a history of COVID-19 pneumonia without significant difference in early postoperative morbidity and mortality. Nevertheless, randomized controlled trials with a higher number of patients are necessary to draw firmer conclusions.
Funding of the article
This article was not financially supported by any company.
Ethics
The study was approved by the local ethics committee (Approval No:2021/206).
Declaration of competing interest
The authors declare that they have no conflict of interest.
References
- 1.WHO, WHO Coronavirus Disease (COVID-19) dashboard. https://covid19.who.int/. (Accessed on 14/10/2021).
- 2.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verity R., Okell L.C., Dorigatti I., et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aesif S.W., Bribriesco A.C., Yadav R., et al. Pulmonary pathology of COVID-19 following 8 Weeks to 4 Months of severe disease: a report of three cases, including one with bilateral lung transplantation. Am J Clin Pathol. 2021;155:506–514. doi: 10.1093/ajcp/aqaa264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calabrese F., Pezzuto F., Fortarezza F., et al. Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European Pulmonary Pathologists. Virchows Arch. 2020;477:359–372. doi: 10.1007/s00428-020-02886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jheon S., Ahmed A.D., Fang V.W., et al. General thoracic surgery services across Asia during the 2020 COVID-19 pandemic. Asian Cardiovasc. Thorac. 2020;28:243–249. doi: 10.1177/0218492320926886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calabrò L., Peters S., Soria J.C., et al. Challenges in lung cancer therapy during the COVID-19 pandemic. Lancet Respir Med. 2020;8:542–544. doi: 10.1016/S2213-2600(20)30170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser S., Baranowski R., Patrini D., et al. Maintaining safe lung cancer surgery during the COVID-19 pandemic in a global city. EClinical. Med. 2021;39 doi: 10.1016/j.eclinm.2021.101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marando M., Fusi-Schmidhauser T., Tamburello A., et al. 1-year radiological, functional and quality-of-life outcomes in patients with SARS-CoV-2 pneumonia - a prospective observational study. NPJ Prim Care Respir Med. 2022;32(1):8. doi: 10.1038/s41533-022-00273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh A.P., Berman A.T., Marmarelis M.E., et al. Management of lung cancer during the COVID-19 pandemic. JCO. Oncol. Pract. 2020;16(9):579–586. doi: 10.1200/OP.20.00286. [DOI] [PubMed] [Google Scholar]
- 11.Lei S., Jiang F., Su W., et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. Eclinical. Med. 2020;21 doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki K., Saji H., Aokage K., et al. Comparison of pulmonary segmentectomy and lobectomy: safety results of a randomized trial. J Thorac Cardiovasc Surg. 2019;158:895–907. doi: 10.1016/j.jtcvs.2019.03.090. [DOI] [PubMed] [Google Scholar]
- 13.Long H., Tan Q., Luo Q., et al. Thoracoscopic surgery versus thoracotomy for lung cancer: short-term outcomes of a randomized trial. Ann Thorac Surg. 2018;105:386–392. doi: 10.1016/j.athoracsur.2017.08.045. [DOI] [PubMed] [Google Scholar]
- 14.Erdogu V., Akin H., Sonmezoglu Y., et al. Comparison of the video-assisted thoracoscopic lobectomy versus open thoracotomy for primary non-small cell lung cancer: single cohort study with 269 cases. Sisli Etfal Hastan. Tip. Bul. 2020;54:291–296. doi: 10.14744/SEMB.2020.60963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geller A.D., Zheng H., Mathisen D.J., Wright C.D., Lanuti M. Relative incremental costs of complications of lobectomy for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2018;155:1804–1811. doi: 10.1016/j.jtcvs.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 16.Whitson B.A., Groth S.S., Duval S.J., Swanson S.J., Maddaus M.A. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg. 2008;86:2008–2016. doi: 10.1016/j.athoracsur.2008.07.009. ; discussion 2016-8. [DOI] [PubMed] [Google Scholar]
- 17.Al-Ameri M., Bergman P., Franco-Cereceda A., Sartipy U. Video-assisted thoracoscopic versus open thoracotomy lobectomy: a Swedish nationwide cohort study. J Thorac Dis. 2020;10:3499–3506. doi: 10.21037/jtd.2018.05.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Testori A., Perroni G., Voulaz E., Crepaldi A., Alloisio M. Pulmonary lobectomy after COVID-19. Ann Thorac Surg. 2020;111:e181–e182. doi: 10.1016/j.athoracsur.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samson P., Patel A., Garrett T., et al. Effects of delayed surgical resection on short-term and long-term outcomes in clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2015;99:1906–1913. doi: 10.1016/j.athoracsur.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]