Abstract
Background
Remimazolam is a new medication with sedative and hypnotic effects. It has been demonstrated non-inferior to propofol in general anesthesia with regard to efficacy and safety. However, whether general anesthesia with remimazolam is better than propofol in terms of patients’ recovery quality remains unknown.
Patients and Methods
Patients enrolled in this study were randomized to remimazolam or propofol group. In remimazolam group, general anesthesia was induced with remimazolam and sufentanil and maintained with remimazolam and remifentanil. In propofol group, general anesthesia was induced with propofol and sufentanil and maintained with propofol and remifentanil. Neuromuscular blocking agent cisatracurium was also injected during anesthesia. Sedation level was monitored by bispectral index (BIS). Our primary outcome was the quality of patients’ postoperative recovery, using the Quality of Recovery-15 (QoR-15) scale. Secondary outcomes included SpO2, HR, MBP and frequency of application of vasoactive drugs during anesthesia, as well as incidences of adverse events in the post anesthesia care unit (PACU).
Results
The global scores of QoR-15 scale were lower in remimazolam group at postoperative day 1 and day 3 compared to propofol group, but differences between the two groups only had clinical significance at postoperative day 1. Among the five dimensions of QoR-15 scale, scores for physical comfort and emotional state were lower in remimazolam group than propofol group. MBP and HR were higher in remimazolam group than propofol group after anesthesia induction. SpO2 was similar in the two groups. The frequency of application of vasoactive drugs during anesthesia was higher in propofol group than remimazolam group. There was no statistical difference in the incidences of adverse events between the two groups.
Conclusion
General anesthesia with remimazolam can provide more stable hemodynamics but also cause temporary reduction in the quality of recovery in patients undergoing urologic surgery, compared to propofol.
Keywords: remimazolam, propofol, quality of recovery, urologic surgery
Introduction
Although we have all kinds of sedatives and hypnotics now, the selection of an ideal medication for general anesthesia is still challenging. Even the widely used, classic anesthetic-propofol, has a lot of defects, such as injection pain, cardio-respiratory depression, metabolic acidosis, hyperlipidemia and hepatomegaly.1 An ideal medication for anesthesia should contain some major characteristics, like short onset and offset, constant context-sensitive half time, few hemodynamic side effects, minimal nephrotoxicity and hepatotoxicity, and metabolites without pharmacodynamic activity.
Recently, a new benzodiazepine-remimazolam is approved for clinical practice.2,3 Remimazolam is an ester-based gamma-aminobutyric acid type A (GABAA) receptor agonist, with sedative and hypnotic effects. Its metabolism is mainly induced by tissue esterase, independent of liver and kidney function, and the metabolites are inactive.4–6 Moreover, like other benzodiazepine, remimazolam can be antagonized by flumazenil.7,8 These nearly perfect characteristics of remimazolam have greatly inspired scientists. Many researches have demonstrated its efficacy and safety in general anesthesia.9–11 However, few studies pay attention to patients’ postoperative recovery so far, which is also crucial for patients’ prognosis.
Thus, this study was designed to investigate the quality of recovery after general anesthesia with remimazolam in patients’ undergoing urologic surgery. Propofol was used in the control group. The patient self-reported Quality of Recovery-15 scale (QoR-15) was applied to assess the quality of patients’ postoperative recovery.12
Patients and Methods
Design and Patients
This was a prospective, double-blinded, randomized controlled clinical trial and approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (KY-2021-0085). It was registered at the Chinese Clinical Trials Registry (ChiCTR2100041986). Written informed consent was obtained from all participants. This study complied with the 1964 Helsinki declaration and its later amendments. Patients aged 18–84 years old, with American Society of Anesthesiologists (ASA) physical status I to III and body mass index (BMI) 18–28 kg/m2, scheduled for urologic surgery under general anesthesia were screened for this study. Exclusion criteria included pregnant or breastfeeding women and those who had a birth plan within 3 months, obvious respiratory or circulatory dysfunction before surgery, significantly abnormal blood routine tests and blood biochemical index, severe neuropsychiatric disorders, use of benzodiazepines or opioids within 1 month, those with contraindications or allergy to benzodiazepines, opioids, propofol and their components, and those estimated as difficult airway. An investigator neither involved in clinical care nor the follow-up, enrolled participants and later generated the random allocation sequence. All participants were randomly assigned 1:1 to either the remimazolam group or the propofol group based on the random number generated from the web (https://www.random.org/). The group arrangement was concealed with an envelope, which was opened by the anesthesiologist after the patient entered the operation room. Thus, anesthesiologists were not blind of the group assignment as they need to administer the medication accordingly. But the follow-up investigators and the patients were not informed of group allocation.
Interventions
All enrolled patients received no premedication. Routine monitoring was conducted upon the arrival of the operation room, including electrocardiogram, pulse oxygen saturation (SpO2), noninvasive blood pressure (NIBP) and the bispectral index (BIS). In remimazolam group, general anesthesia was induced with intravenous injection of remimazolam (0.2–0.3) mg/kg and sufentanil (0.3–0.5) μg/kg. Anesthesia was maintained with remimazolam (1–2) mg/kg/h and remifentanil (0.2–0.3) μg/kg/min. In propofol group, general anesthesia was induced with intravenous injection of propofol (2–3) mg/kg and sufentanil (0.3–0.5) μg/kg. Anesthesia was maintained with propofol (4–10) mg/kg/h and remifentanil (0.2–0.3) μg/kg/min. Besides, neuromuscular blocking agent cisatracurium was applied with the dose of (0.1–0.2) mg/kg during anesthesia induction and (0.06–0.12) mg/kg/h during anesthesia maintenance. Patients were intubated after anesthesia induction and mechanical ventilation was provided with a tidal volume of (6–8) mL/kg. The ventilatory frequency was adjusted to maintain the end-tidal carbon dioxide concentration at 35–45 mmHg. Anesthetic depth was monitored with BIS and medications were titrated to keep BIS range between 40 and 60. Vasoactive agents were administered accordingly to maintain the blood pressure and heart rate within 20% of pre-induction values. When the operation procedure was completed, patients were transferred to the post anesthesia care unit (PACU) for recovery.
Outcome Measures
The primary outcomes of our study were the scores of QoR-15 scale. We used the Chinese version of QoR-15 questionnaire, which was validated as efficient and reliable as the original English version.13 It reflected the quality of postoperative functional recovery from five dimensions: physical comfort, emotional state, physical independence, psychological support and pain.12 The QoR-15 scores were recorded at 1 day before surgery, postoperative day 1 (POD1) and postoperative day 3 (POD3).
The secondary outcomes included SpO2, heart rate (HR), mean blood pressure (MBP) and the frequency of application of vasoactive drugs during anesthesia, as well as incidences of adverse events during anesthesia recovery. The detection time points of SpO2, HR and MBP included 5 min before anesthesia induction, 1min after induction, 1 min after intubation and the beginning of surgery. Among them, data recorded at 5 min before anesthesia induction was regarded as baseline. Adverse events during anesthesia recovery were recorded in PACU, which contained nausea and vomiting, hypoxemia, fluctuation of blood pressure, intraoperative awareness, somnolence and emergency delirium. Hypoxemia was defined as SpO2 less than 90%. When changes of NIBP exceeded 20% of the baseline value, it was considered as the occurrence of blood pressure fluctuation. Intraoperative awareness was assessed with modified Brice interview.14,15 Somnolence was regarded as a state of sleepiness and falling asleep without stimulation. The Nursing Delirium Screening Scale (Nu-DESC) was used to screen patients with emergency delirium.16
Patient demographic and clinical parameters including age, sex, BMI, education level, ASA physical status, duration of anesthesia and duration of operation were also collected. Besides, we used the age-adjusted Charlson Comorbidity Index (CCI) and surgical Apgar score to estimate patients’ preoperative physical condition and surgical outcomes. CCI was a useful tool to identify patients with multiple chronic diseases.17,18 The surgical Apgar score was a 10-point surgical outcomes score, based on the lowest HR, lowest MBP and estimated blood loss, which can well discriminate patients at high or low risk of major complications and death of operation.19
Statistical Analysis
The sample size was calculated according to QoR-15 scores at POD3. The minimal clinically important difference was 8 according to previous research.20 When this trial was registered on January 11th 2021, we calculated the sample size referring to previous studies, which indicated the standard deviation (SD) of QoR-15 scale was 14.12,21 Considering a dropout rate of 20%, a sample size of 166 could achieve a power of 90% with a type 1 error of 0.05. After the trial was approved by Ethics Committee on February 23rd 2021, we conducted a pilot study. Based on our pilot study, the SD of global QoR-15 score was 12 at postoperative day 3. Thus, we recalculated it and a total sample of 98 subjects was required to achieve a power of 90% with a type 1 error of 0.05. We finally included 128 subjects for analysis in this study.
All data were presented as mean (SD), median (interquartile range, IQR), or number (percentage) as appropriate. Quantitative variables were tested for normality with Shapiro–Wilk test. Comparison of normally distributed quantitative variables between groups were performed with student t test, and asymmetric distributed quantitative data was analyzed with Mann–Whitney U-test. Qualitative variables between groups were compared with Chi-square test or Fisher exact test as needed. Data for QoR-15 scores were also presented with median difference and its 95% confidence interval (CI). The median difference is the median of all pairwise differences between observations in the two groups and the 95% CI was estimated by Hodges–Lehmann. Data for the frequency of adverse events were reported with proportion difference and its 95% CI. The 95% CI for the proportion difference was calculated using the Wilson procedure without a correction for continuity. In other parameters, like patient demographic parameters, SpO2, HR, MBP and the frequency of vasoactive drugs application, the median or proportion differences and their 95% CI were calculated with the above methods. All analysis was assessed with a two tailed test and P value less than 0.05 was considered to be statistically significant.
Results
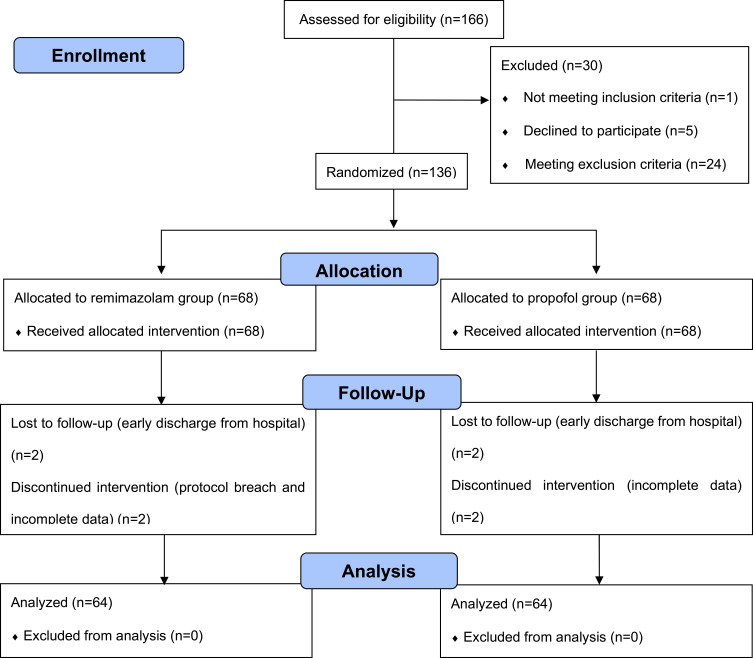
From February 2021 to August 2021, 166 patients were initially screened for eligibility, of whom 30 were excluded and 136 participants were randomly allocated to either the remimazolam group or the propofol group. Later, 8 patients discontinued the intervention. Thus, 128 participants were finally analyzed in this study. The detailed information was shown in the flow diagram (Figure 1). The demographic and clinical characteristics including preoperative QoR-15 scores of the two groups were comparable and detailed in Table 1.
Figure 1.
CONSORT 2010 flow diagram.
Table 1.
Patient Demographic and Clinical Parameters (n = 64 in Each Group)
| Remimazolam Group | Propofol Group | Difference (95% CI) | P value | |
|---|---|---|---|---|
| Sex, n (%) | 0.451 | |||
| Male | 41.0(64.1) | 45.0(70.3) | 6.3%(−9.9% to 21.9%) | |
| Female | 23.0(35.9) | 19.0(29.7) | 6.3%(−9.9% to 21.9%) | |
| Age, (yr) | 52.5(17.5) | 50.0(25.8) | −4.0(−10.0 to 2.0) | 0.163 |
| BMI, (kg.m−2) | 25.2(3.9) | 23.7(5.3) | −0.8(−2.0 to 0.1) | 0.077 |
| Education level, n (%) | 0.823 | |||
| Elementary school and below | 15.0(23.4) | 13.0(20.3) | 3.1%(−11.2% to 17.3%) | |
| Middle school | 31.0(48.4) | 30.0(46.9) | 1.6%(−15.3% to 18.3%) | |
| College and above | 18.0(28.1) | 21.0(32.8) | 4.7%(−11.1% to 20.2%) | |
| ASA physical status, n (%) | 0.203 | |||
| I | 11.0(17.2) | 19.0(29.7) | 12.5%(−2.2% to 26.6%) | |
| II | 45.0(70.3) | 36.0(56.3) | 14.1%(−2.6% to 29.7%) | |
| III | 8.0(12.5) | 9.0(14.1) | 1.6%(−10.6% to 13.7%) | |
| Duration of anesthesia, (min) | 108.0(82.0) | 80.0(85.3) | −17.0(−37.0 to 4.0) | 0.093 |
| Duration of operation, (min) | 78.5(76.0) | 63.0(80.8) | −14.0(−31.0 to 2.0) | 0.085 |
| CCI score | 2.0(2.0) | 3.0(5.0) | 0.0(−1.0 to 1.0) | 0.509 |
| Surgical Apgar score | 9.0(1.0) | 9.0(1.0) | 0.0(0.0 to 1.0) | 0.164 |
| Preoperative QoR-15 scores | 142.0(10.8) | 141.0(14.0) | 0.0(−3.0 to 2.0) | 0.869 |
Notes: Data were presented as median (interquartile range) or number (percentage). Sugical Apgar score, a 10-point surgical outcomes score. Difference (reported with 95% CI) were the median or proportion difference between the two groups. The median difference was the median of all pairwise differences between observations in the two groups, rather than the difference between the group medians. No statistically significant differences between groups were noted.
Abbreviations: BMI, body mass index; ASA, American Society of Anesthesiologists; CCI, Charlson comorbidity index; QoR-15, Quality of Recovery-15 scale; CI, confidence interval.
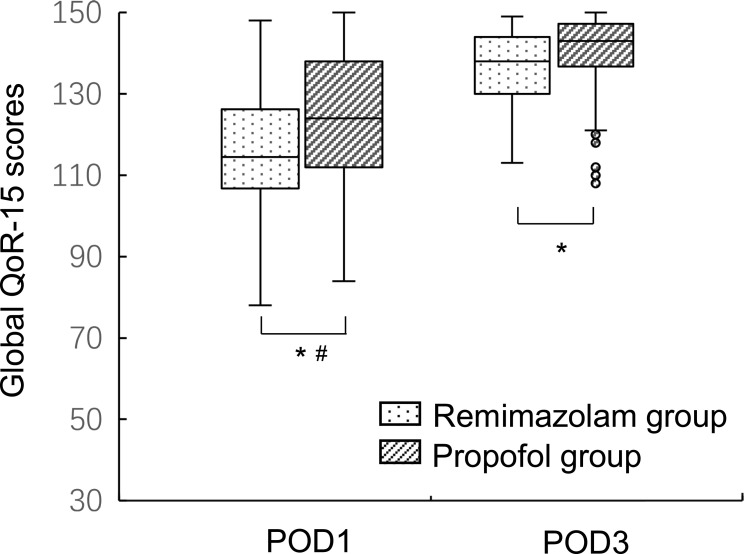
The postoperative QoR-15 scores were presented as median (interquartile range) in Table 2. The global QoR-15 scores at POD1 were 114.5 (20.5) in remimazolam group and 124.0 (26.0) in propofol group (P=0.004). The global QoR-15 scores at POD3 were 138.0 (14.0) in remimazolam group and 143.0 (11.5) in propofol group (P=0.007). Since the minimal clinically important significance for global QoR-15 score was 8, difference between the two groups only had a clinical significance at POD1 (Figure 2). Among the five dimensions of QoR-15 scale, scores for physical comfort and emotional state were lower in remimazolam group than propofol group at POD1 and POD3. There was no statistical difference in scores for pain, physical independence and psychological support between the two groups either at POD1 or POD3.
Table 2.
Postoperative QoR-15 Scores (n = 64 in Each Group)
| Remimazolam Group | Propofol Group | Median Difference (95% CI) | P value | |
|---|---|---|---|---|
| QoR-15 scores | ||||
| POD1 | 114.5(20.5) | 124.0(26.0) | 9.0(3.0 to 14.0) | 0.004* |
| POD3 | 138.0(14.0) | 143.0(11.5) | 4.0(1.0 to 7.0) | 0.007* |
| QoR-15 dimensions | ||||
| Pain | ||||
| POD1 | 18.5(5.0) | 20.0(3.8) | 0.0(0.0 to 1.0) | 0.293 |
| POD3 | 20.0(2.0) | 20.0(1.8) | 0.0(0.0 to 0.0) | 0.616 |
| Physical comfort | ||||
| POD1 | 37.5(10.5) | 41.5(10.8) | 3.0(1.0 to 6.0) | 0.018* |
| POD3 | 45.5(6.0) | 47.5(5.0) | 1.0(0.0 to 3.0) | 0.026* |
| Physical independence | ||||
| POD1 | 2.5(13.8) | 10.0(15.8) | 0.0(0.0 to 3.0) | 0.200 |
| POD3 | 17.5(5.8) | 18.0(4.8) | 1.0(0.0 to 2.0) | 0.124 |
| Psychological support | ||||
| POD1 | 20.0(0.0) | 20.0(1.0) | 0.0(0.0 to 0.0) | 0.182 |
| POD3 | 20.0(0.0) | 20.0(0.0) | 0.0(0.0 to 0.0) | 0.058 |
| Emotional state | ||||
| POD1 | 36.5(5.8) | 38.0(3.0) | 2.0(1.0 to 3.0) | 0.000* |
| POD3 | 39.0(3.0) | 39.0(2.0) | 1.0(0.0 to 1.0) | 0.019* |
Notes: Data were presented as median (interquartile range). The median difference (reported with 95% confidence intervals) is the median of all pairwise differences between observations in the two groups. It is not the difference between the group medians. *p < 0.05, compared with propofol group.
Abbreviations: QoR-15, Quality of Recovery-15; POD1, postoperative day 1; POD3, postoperative day 3; CI, confidence interval.
Figure 2.
The global QoR-15 scores of remimazolam group and propofol group at POD1 and POD3. Notes: Data were expressed as median (horizontal bar), interquartile range (box), the maximum and minimum values (upper and lower edges) and the outliers (circles). *Statistical significance, #Clinical significance.
Abbreviations: QoR-15, Quality of Recovery-15; POD1, postoperative day 1; POD3, postoperative day 3.
MBP, HR and SpO2 and application of vasoactive drugs during anesthesia were shown in Table 3. Baseline data were comparable between the two groups. After anesthesia induction, the MBP was higher in remimazolam group (94.4±12.9 mmHg) than propofol group (87.4±11.9 mmHg). But it became similar between the two groups after intubation and at the beginning of surgery. That might be attributed to the use of vasoactive drugs to maintain hemodynamic stabilization after anesthesia induction. The HR was significantly faster in remimazolam group (75.5±13.0 beats/min) than propofol group (65.7±11.5 beats/min) after anesthesia induction, but it went back to a similar state at the beginning of surgery. There was no difference in SpO2 between the two groups at these time points. The frequency of vasoactive drugs application during anesthesia was higher in propofol group than remimazolam group (56.3% for propofol group vs 28.1% for remimazolam group).
Table 3.
SpO2, HR, MBP and Application of Vasoactive Drugs During Anesthesia (n = 64 in Each Group)
| Remimazolam Group | Propofol Group | Difference (95% CI) | P value | |
|---|---|---|---|---|
| SpO2 (%) | ||||
| Baseline | 100.0(1.0) | 100.0(1.0) | 0.0(0.0 to 0.0) | 0.973 |
| 1 min after induction | 100.0(0.0) | 100.0(0.0) | 0.0(0.0 to 0.0) | 0.440 |
| 1 min after intubation | 100.0(0.0) | 100.0(0.0) | 0.0(0.0 to 0.0) | 0.156 |
| At the beginning of surgery | 100.0(0.0) | 100.0(0.0) | 0.0(0.0 to 0.0) | 0.081 |
| HR (beats/min) | ||||
| Baseline | 77.7±14.7 | 74.7±12.3 | 2.9(−1.8 to 7.7) | 0.225 |
| 1 min after induction | 75.5±13.0 | 65.7±11.5 | 9.8(5.5 to 14.1) | 0.000* |
| 1 min after intubation | 74.0(15.8) | 66.0(21.8) | −9.0(−14.0 to −4.0) | 0.001* |
| At the beginning of surgery | 61.0(19.3) | 58.5(11.8) | −2.0(−6.0 to 2.0) | 0.203 |
| MBP (mmHg) | ||||
| Baseline | 108.8±12.6 | 106.0±12.3 | 2.8(−1.6 to 7.1) | 0.210 |
| 1 min after induction | 94.4±12.9 | 87.4±11.9 | 7.0(2.7 to 11.4) | 0.002* |
| 1 min after intubation | 93.0(17.0) | 93.0(30.8) | −2.0(−7.0 to 5.0) | 0.642 |
| At the beginning of surgery | 86.5(17.0) | 86.0(24.0) | −1.0(−5.0 to 4.0) | 0.800 |
| Application of vasoactive drugs | 18.0(28.1%) | 36.0(56.3%) | 28.1%(11.0% to 43.0%) | 0.001* |
Notes: Data for HR and MBP at baseline and 1 min after induction were presented as mean ± standard deviation and the difference (95% CI) was the mean difference and its 95% CI. Data for application of vasoactive drugs were presented as number (percentage) and the difference (95% CI) was the proportion difference and its 95% CI. Other data were presented as median (interquartile range), and the difference (95% CI) was the median of all pairwise differences between observations in the two groups, rather than the difference between the group medians. *p < 0.05, compared with propofol group.
Abbreviations: SpO2, pulse oxygen saturation; HR, heart rate; MBP, mean blood pressure; CI, confidence interval.
During anesthesia recovery, the detailed adverse events were recorded and presented in Table 4. The incidence of total adverse events was 26.6% in remimazolam group and 21.9% in propofol group. Among them, incidences of nausea and vomiting, somnolence, and emergency delirium in remimazolam group was higher than propofol group, while the incidence of fluctuation of blood pressure was higher in propofol group than remimazolam group. Nevertheless, there was no statistical significance either in total adverse events or the single one between the two groups. No cases of hypoxia or intraoperative awareness occurred during anesthesia recovery in the two groups.
Table 4.
Adverse Events in Anesthesia Recovery Period (n = 64 in Each Group)
| Adverse Events | Remimazolam Group | Propofol Group | Difference of Proportions (95% Confidence Interval) | P value |
|---|---|---|---|---|
| Nausea and vomiting | 7.0(10.9%) | 3.0(4.7%) | 6.3%(−3.7% to 16.7%) | 0.188 |
| Hypoxemia | 0.0(0.0%) | 0.0(0.0%) | —— | —— |
| Fluctuation of blood pressure | 5.0(7.8%) | 10.0(15.6%) | 7.8%(−3.7% to 19.5%) | 0.169 |
| Intraoperative awareness | 0.0(0.0%) | 0.0(0.0%) | —— | —— |
| Somnolence | 4.0(6.3%) | 1.0(1.6%) | 4.7%(−3.1% to 13.5%) | 0.362 |
| Emergency delirium | 6.0(9.4%) | 2.0(3.1%) | 6.3%(−2.8% to 16.1%) | 0.283 |
| Total | 17.0(26.6%) | 14.0(21.9%) | 4.7%(−10.1% to 19.3%) | 0.536 |
Notes: Data were shown with the number (percentage). No statistically significant differences between groups were noted.
Discussion
In this study, we compared general anesthesia with remimazolam versus propofol in terms of postoperative recovery in patients undergoing urologic surgery. Our results revealed a lower QoR-15 score in remimazolam group than propofol group at POD1 and POD3. But differences between the two groups only had a clinical meaning at POD1. In other words, the quality of patients’ recovery in general anesthesia with remimazolam dropped temporarily one day after urologic surgery, but went back to rough parity with the level of propofol anesthesia three days after surgery. Further analysis revealed the reduction of postoperative recovery in remimazolam group focused on the aspects of physical comfort and emotional state. This study is very important, as it provided us a way to evaluate general anesthesia with remimazolam from the view of postoperative recovery.
Postoperative recovery is a complex process and affected mainly by factors from patients, surgery and anesthesia. In this study, we used CCI and surgical Apgar scores to assess patients’ preoperative comorbidities and surgical outcomes. CCI is an extensively studied comorbidity index for predicting perioperative mortality and has been proved to be valid and reliable to measure preoperative comorbidity in clinical study.22,23 The surgical Apgar score, unlike the obstetrical Apgar score, is calculated from the blood loss, lowest MBP, and lowest HR during the operation, which is strongly correlated with postoperative outcomes.24,25 With the surgical Apgar score, we can quickly and objectively assess how well the operation has been done for a patient. In this study, the CCI and surgical Apgar scores as well as other demographic data between the two groups were similar. That is, the characteristics of patients and surgeries were comparable in the two groups.
Here, we used QoR-15 scale to measure the quality of postoperative recovery. It is a patient-reported outcome measurement but more efficient and reliable than the Post-operative Quality Recovery Scale (PQRS) to evaluate patients’ recovery after anesthesia and surgery.13 Recently, it was recommended as an endpoint in comparative anesthesia studies.26 Lots of studies have applied QoR-15 scale as their outcomes but the time points they tested varied. The original study of QoR-15 scale administered the questionnaire before surgery as baseline. In this study, we also tested QoR-15 scale 1 day before surgery, which showed similar preoperative QoR-15 scores in the two groups. However, a recent study in day case surgery revealed that QoR-15 scores tested within 24h preoperatively may not represent a true baseline due to factors like fatigue, anxiety and others.27 Thus, baseline for QoR-15 scale is controversial. Actually, many studies only tested it postoperatively and the timepoint of 24h after surgery was most commonly applied.28,29 Chazapis M suggested a follow-up to 48h postoperatively, as most patients recovered 2 days after surgery.27 In this study, we checked QoR-15 scores at POD1 and POD3, as the first day after surgery was the most commonly used timepoint and the third day was when the majority had already recovered. Previous study revealed the minimal clinically important difference of global QoR-15 scores was 8, but the authors updated it to be 6 lately.30 The paper was formally published in November 2021, when data collection of our study was already finished. Even with this updated value, our sample size was enough to reach a power of 80% with a type 1 error of 0.05, and our data still revealed a statistical and clinical difference in total QoR-15 scores at POD1 between the two groups, while difference between the two groups at POD3 only had statistical but not clinical important significance. Among the five dimensions of QoR-15 scale, the decrease of QoR-15 scores in remimazolam group mainly reflected on physical comfort and emotional state.
Our study found a temporary decrease in the quality of recovery after general anesthesia with remimazolam. But the exact mechanism underlying this phenomenon is unclear. Considering the pharmacodynamics and pharmacokinetics of remimazolam, it seems unlikely to occur the effect of drug accumulation.31 However, a study evaluating the efficacy and safety of remimazolam versus propofol for general anesthesia showed a longer time to extubation in remimazolam group than propofol group.9 Liu also found remimazolam may worsen elderly patients’ postoperative cognitive functions in upper gastrointestinal endoscopy.32 Like remifentanil, remimazolam, when doses at the top end of the response curve, might appear undesirable desensitization effects and rebound phenomenon once stopped, such as anxiety.5 Cases about remimazolam tolerance have been reported in benzodiazepine long-term users.33 Our study showed the reduction in the quality of recovery in remimazolam group was in terms of physical comfort and emotional state, which reminded us to concern patients’ mental conditions.
Postoperative delirium is a mental problem puzzling anesthesiologist many years and use of benzodiazepines is associated with delirium.34 Here, we used Nu-DESC to assess emergency delirium in PACU. Although there was no statistical significance, the incidence of emergency delirium in remimazolam group was higher than propofol group. However, the average operation time in our study was less than 2 hours, other studies with longer time use of remimazolam might get a significant difference. Recently, a clinical trial to evaluate the effect of remimazolam compared with propofol on the incidence of delirium after cardiac surgery was registered.35 Its results may answer this question more precisely.
Other adverse events during anesthesia emergence, including nausea and vomiting, hypoxemia, fluctuation of blood pressure, intraoperative awareness and somnolence, were similar between the two groups. It has to be noted that we did not use the antagonist-flumazenil in this study. With this reversal agent, we can control the sedation level of remimazolam more precisely, while the incidence of adverse events may also vary. Meanwhile, we should know that these adverse events were secondary outcomes of this study. Since the sample size was calculated according to the primary outcomes, it may be not sufficient for these secondary outcomes. Thus, further studies focusing on the adverse events of remimazolam as primary outcomes are needed.
Besides, our study also showed a more moderate decrease of MBP and relatively higher HR after anesthesia induction in remimazolam group than propofol group. The MBP in propofol group elevated after intubation partly due to the use of vasoactive drugs. In total, fewer vasoactive drugs were needed in remimazolam group than propofol group during anesthesia. This is consistent with other researches, which all revealed a more stable hemodynamics in remimazolam group.9,36
Nevertheless, there are some limitations in this trial. To bring more subjects into the study, the inclusion criteria was relatively broad, as patients aged 18–84 years old, with ASA class I to III were included. However, it is worth noting that previous studies have proved the efficacy and safety of general anesthesia with remimazolam in ASA class III and elderly patients.37,38 Even though the effect of age and ASA class was small on time to extubation with remimazolam anesthesia, lower doses of remimazolam were recommended for some fragile elderly patients.10,39 Thus, further clinical data would help to understand the effect of general anesthesia with remimazolam on quality of recovery in fragile elderly patients. Second, the filling of QoR-15 scale questionnaire was partly by our investigators, as some of the patients were illiterate. The elaboration of investigators and the understanding of patients might have a difference. Future studies with patients’ self-completed QoR-15 scale would be more precise to evaluate the effect of general anesthesia with remimazolam. Third, to reduce the influence of surgery types as confounding factors, we only included urologic surgeries in this study. In other types of operations, the effect of general anesthesia with remimazolam on patients’ postoperative recovery need further validation.
Conclusion
In summary, remimazolam is a promising medication in general anesthesia due to its relatively stable hemodynamic feature. However, patients’ postoperative recovery should be concerned in general anesthesia with remimazolam before it is widely promoted.40 We demonstrated that general anesthesia with remimazolam temporarily impaired the quality of recovery in patients undergoing urologic surgery. Further clinical trials with other types of operation are needed to confirm the long-term impact of remimazolam on patients’ recovery.
Acknowledgments
We thank our anesthesiology and urologic surgery colleagues for their cooperation in facilitating this trial. We are also grateful to Siyue Chen and Siqi Tang for their kind assistance.
Data Sharing Statement
All data generated or analyzed during this study were included in the published article. Further inquiries about the datasets can be directed to the corresponding author on reasonable request.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lundström S, Twycross R, Mihalyo M, et al. Propofol. J Pain Symptom Manage. 2010;40(3):466–470. doi: 10.1016/j.jpainsymman.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 2.Sneyd JR, Rigby-Jones AE. Remimazolam for anaesthesia or sedation. Curr Opin Anaesthesiol. 2020;33(4):506–511. doi: 10.1097/ACO.0000000000000877 [DOI] [PubMed] [Google Scholar]
- 3.Keam SJ. Remimazolam: first approval. Drugs. 2020;80(6):625–633. doi: 10.1007/s40265-020-01299-8 [DOI] [PubMed] [Google Scholar]
- 4.Stöhr T, Colin PJ, Ossig J, et al. Pharmacokinetic properties of remimazolam in subjects with hepatic or renal impairment. Br J Anaesth. 2021;127(3):415–423. doi: 10.1016/j.bja.2021.05.027 [DOI] [PubMed] [Google Scholar]
- 5.Wesolowski AM, Zaccagnino MP, Malapero RJ, et al. Remimazolam: pharmacologic considerations and clinical role in anesthesiology. Pharmacotherapy. 2016;36(9):1021–1027. doi: 10.1002/phar.1806 [DOI] [PubMed] [Google Scholar]
- 6.Kilpatrick GJ. Remimazolam: non-clinical and clinical profile of a new sedative/anesthetic agent. Front Pharmacol. 2021;12:690875. doi: 10.3389/fphar.2021.690875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aya D, Hoshi T, Yamaguchi H. Predicting the amount of flumazenil needed to antagonize remimazolam. Eur J Gastroenterol Hepatol. 2021;33(10):1335–1336. doi: 10.1097/MEG.0000000000002201 [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Sang N, Song K, et al. Psychomotor recovery following remimazolam-induced sedation and the effectiveness of flumazenil as an antidote. Clin Ther. 2020;42(4):614–624. doi: 10.1016/j.clinthera.2020.02.006 [DOI] [PubMed] [Google Scholar]
- 9.Doi M, Morita K, Takeda J, et al. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020;34(4):543–553. doi: 10.1007/s00540-020-02788-6 [DOI] [PubMed] [Google Scholar]
- 10.Zhou J, Leonowens C, Ivaturi VD, et al. Population pharmacokinetic/pharmacodynamic modeling for remimazolam in the induction and maintenance of general anesthesia in healthy subjects and in surgical subjects. J Clin Anesth. 2020;66:109899. doi: 10.1016/j.jclinane.2020.109899 [DOI] [PubMed] [Google Scholar]
- 11.Sheng XY, Liang Y, Yang XY, et al. Safety, pharmacokinetic and pharmacodynamic properties of single ascending dose and continuous infusion of remimazolam besylate in healthy Chinese volunteers. Eur J Clin Pharmacol. 2020;76(3):383–391. doi: 10.1007/s00228-019-02800-3 [DOI] [PubMed] [Google Scholar]
- 12.Stark PA, Myles PS, Burke JA. Development and psychometric evaluation of a postoperative quality of recovery score: the QoR-15. Anesthesiology. 2013;118(6):1332–1340. doi: 10.1097/ALN.0b013e318289b84b [DOI] [PubMed] [Google Scholar]
- 13.Bu XS, Zhang J, Zuo YX. Validation of the Chinese version of the quality of recovery-15 score and its comparison with the post-operative quality recovery scale. Patient. 2016;9(3):251–259. doi: 10.1007/s40271-015-0148-6 [DOI] [PubMed] [Google Scholar]
- 14.Brice DD, Hetherington RR, Utting JE. A simple study of awareness and dreaming during anaesthesia. Br J Anaesth. 1970;42(6):535–542. doi: 10.1093/bja/42.6.535 [DOI] [PubMed] [Google Scholar]
- 15.Mashour GA, Kent C, Picton P, et al. Assessment of intraoperative awareness with explicit recall: a comparison of 2 methods. Anesth Analg. 2013;116(4):889–891. doi: 10.1213/ANE.0b013e318281e9ad [DOI] [PubMed] [Google Scholar]
- 16.Radtke FM, Franck M, Schneider M, et al. Comparison of three scores to screen for delirium in the recovery room. Br J Anaesth. 2008;101(3):338–343. doi: 10.1093/bja/aen193 [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 18.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5 [DOI] [PubMed] [Google Scholar]
- 19.Regenbogen SE, Lancaster RT, Lipsitz SR, et al. Does the surgical apgar score measure intraoperative performance? Ann Surg. 2008;248(2):320–328. doi: 10.1097/SLA.0b013e318181c6b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myles PS, Myles DB, Galagher W, et al. Minimal clinically important difference for three quality of recovery scales. Anesthesiology. 2016;125(1):39–45. doi: 10.1097/ALN.0000000000001158 [DOI] [PubMed] [Google Scholar]
- 21.Barrington MJ, Seah GJ, Gotmaker R, et al. Quality of recovery after breast surgery: a multicenter randomized clinical trial comparing pectoral nerves interfascial plane (Pectoral Nerves II) block with surgical infiltration. Anesth Analg. 2020;130(6):1559–1567. doi: 10.1213/ANE.0000000000004371 [DOI] [PubMed] [Google Scholar]
- 22.Kang HW, Kim SM, Kim WT, et al.; KORCC (Korean Renal Cell Carcinoma) Group. The age-adjusted Charlson comorbidity index as a predictor of overall survival of surgically treated non-metastatic clear cell renal cell carcinoma. J Cancer Res Clin Oncol. 2020;146(1):187–196. doi: 10.1007/s00432-019-03042-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koppie TM, Serio AM, Vickers AJ, et al. Age-adjusted Charlson comorbidity score is associated with treatment decisions and clinical outcomes for patients undergoing radical cystectomy for bladder cancer. Cancer. 2008;112(11):2384–2392. doi: 10.1002/cncr.23462 [DOI] [PubMed] [Google Scholar]
- 24.Lin YC, Chen YC, Yang CH, et al. Surgical apgar score is strongly associated with postoperative ICU admission. Sci Rep. 2021;11(1):115. doi: 10.1038/s41598-020-80393-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito T, Abbosh PH, Mehrazin R, et al. Surgical apgar score predicts an increased risk of major complications and death after renal mass excision. J Urol. 2015;193(6):1918–1922. doi: 10.1016/j.juro.2014.11.085 [DOI] [PubMed] [Google Scholar]
- 26.Léger M, Campfort M, Cayla C, et al. Postoperative quality of recovery measurements as endpoints in comparative anaesthesia studies: a systematic review. Br J Anaesth. 2021;126(6):e210–e212. doi: 10.1016/j.bja.2021.03.008 [DOI] [PubMed] [Google Scholar]
- 27.Chazapis M, Walker EM, Rooms MA, et al. Measuring quality of recovery-15 after day case surgery. Br J Anaesth. 2016;116(2):241–248. doi: 10.1093/bja/aev413 [DOI] [PubMed] [Google Scholar]
- 28.Ma J, Martin R, Chan B, et al. Using activity trackers to quantify postpartum ambulation: a prospective observational study of ambulation after regional anesthesia and analgesia interventions. Anesthesiology. 2018;128(3):598–608. doi: 10.1097/ALN.0000000000001979 [DOI] [PubMed] [Google Scholar]
- 29.Lu J, Wang JF, Guo CL, et al. Intravenously injected lidocaine or magnesium improves the quality of early recovery after laparoscopic cholecystectomy: a randomised controlled trial. Eur J Anaesthesiol. 2021;38(1):S1–S8. doi: 10.1097/EJA.0000000000001348 [DOI] [PubMed] [Google Scholar]
- 30.Myles PS, Myles DB. An updated minimal clinically important difference for the QoR-15 scale. Anesthesiology. 2021;135(5):934–935. doi: 10.1097/ALN.0000000000003977 [DOI] [PubMed] [Google Scholar]
- 31.Schüttler J, Eisenried A, Lerch M, et al. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part I. Pharmacokinetics and clinical pharmacodynamics. Anesthesiology. 2020;132(4):636–651. doi: 10.1097/ALN.0000000000003103 [DOI] [PubMed] [Google Scholar]
- 32.Tan Y, Ouyang W, Tang Y, et al. Effect of remimazolam tosilate on early cognitive function in elderly patients undergoing upper gastrointestinal endoscopy. J Gastroenterol Hepatol. 2021. doi: 10.1111/jgh.15761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshikawa H, Hosokawa M, Kashima Y, et al. Remimazolam tolerance in long-term benzodiazepine users: a case report of 2 cases. A a Pract. 2021;15(5):e01460. doi: 10.1213/XAA.0000000000001460 [DOI] [PubMed] [Google Scholar]
- 34.Kok L, Slooter AJ, Hillegers MH, et al. Benzodiazepine use and neuropsychiatric outcomes in the ICU: a systematic review. Crit Care Med. 2018;46(10):1673–1680. doi: 10.1097/CCM.0000000000003300 [DOI] [PubMed] [Google Scholar]
- 35.Yang M, Liu X, Yang D, et al. Effect of remimazolam besylate compared with propofol on the incidence of delirium after cardiac surgery: study protocol for a randomized trial. Trials. 2021;22(1):717. doi: 10.1186/s13063-021-05691-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai G, Pei L, Duan F, et al. Safety and efficacy of remimazolam compared with propofol in induction of general anesthesia. Minerva Anestesiol. 2021;87(10):1073–1079. doi: 10.23736/S0375-9393.21.15517-8 [DOI] [PubMed] [Google Scholar]
- 37.Doi M, Hirata N, Suzuki T, et al. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA Class III): results of a multicenter, randomized, double-blind, parallel-group comparative trial. J Anesth. 2020;34(4):491–501. doi: 10.1007/s00540-020-02776-w [DOI] [PubMed] [Google Scholar]
- 38.Nakanishi T, Sento Y, Kamimura Y, et al. Remimazolam for induction of anesthesia in elderly patients with severe aortic stenosis: a prospective, observational pilot study. BMC Anesthesiol. 2021;21(1):306. doi: 10.1186/s12871-021-01530-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lohmer LL, Schippers F, Petersen KU, Stoehr T, Schmith VD. Time-to-event modeling for remimazolam for the indication of induction and maintenance of general anesthesia. J Clin Pharmacol. 2020;60(4):505–514. doi: 10.1002/jcph.1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sneyd JR, Gambus PL, Rigby-Jones AE. Current status of perioperative hypnotics, role of benzodiazepines, and the case for remimazolam: a narrative review. Br J Anaesth. 2021;127(1):41–55. doi: 10.1016/j.bja.2021.03.028 [DOI] [PubMed] [Google Scholar]