Abstract
Many patients with systemic autoimmune rheumatic diseases (SARDs) require immunosuppression to reduce disease activity, but this also has important possible detrimental impacts on immune responses following vaccination. The phase III clinical trials for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines did not include those who are immunosuppressed. Fortunately, we now have a clearer idea of how immune responses following SARS-CoV-2 vaccination has for the immunosuppressed, with much of the data being within a year of its introduction. Here, we summarize what is known in this rapidly evolving field about the impact immunosuppression has on humoral immunogenicity including waning immunity and additional doses, breakthrough infection rates and severity, disease flare rates, along with additional considerations and remaining unanswered questions.
Introduction
Immunosuppressed individuals with systemic autoimmune rheumatic diseases (SARDs) may be vulnerable to both severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and severe coronavirus disease 2019 (COVID-19) outcomes [1, 2, 3, 4]. Therefore, SARS-CoV-2 vaccination may be especially crucial for individuals with SARDs. However, the SARS-CoV-2 vaccine clinical trials did not include SARDs or other immunosuppressive conditions. Immunosuppressive medications and underlying altered immunity may impact vaccine immunogenicity and result in lower effectiveness, resulting in breakthrough infection after vaccination. Specific immunosuppressive medications may particularly impact SARS-CoV-2 vaccine response and also affect the immune response to SARS-CoV-2 infection [5]. Temporary discontinuation around SARS-CoV-2 vaccine doses has been proposed to optimize immune response [6]. However, immunosuppressive medication disruption and immune activation after vaccination could also lead to underlying SARD flares and increased disease activity. Also, it is possible that the immune response may wane more quickly in immunosuppressed individuals than the general population, necessitating the need for additional vaccine doses. Finally, immunosuppressed patients may have prolonged viral shedding that may introduce new variants [7,8]. Thus, there are a myriad of considerations patients and providers with SARDs must balance related to SARS-CoV-2 vaccination (see Table 1 ).
Table 1.
Selected studies evaluating breakthrough infection after COVID-19 vaccination among patients with systemic autoimmune rheumatic diseases.
| First authorRef | Study design | Location Calendar time n of COVID-19 after vaccination |
Key findings/immunosuppressive medications implicated | Comments |
|---|---|---|---|---|
| Cook et al. [30] | Case series | Boston, MA, USA 30-Jan-2021 to 31-Jul-2021 n = 16 breakthrough cases |
|
|
| Lawson-Tovey et al. [31] | Case series | Europe 19-Jan-2021 to 27-Jul-2021 n = 38 after any vaccine dose; n = 10 breakthrough cases |
|
|
| Liew et al. [32] | Case series | International 5-Jan-2021 to 30-Sep-2021 n = 197 after any vaccine dose; n = 87 breakthrough cases |
|
|
| Sun et al. [33] | Retrospective cohort study | United States 10-Dec-2020 to 16-Sep-2021 n = 664,722 after any does with either RA (n = 13,445), other immune dysfunction condition, or healthy comparator; n = 22,917 breakthrough cases (n = 511 among RA) |
|
|
| Papagoras et al. [34] | Case series | Greece 1-Mar-2020 to 31-Aug-2021 n = 48 after any vaccine dose; n = 29 breakthrough cases |
|
-Cases identified from the Greek Rheumatology Society voluntary physician registry |
| Ahmed et al. [35] | Prospective cohort study | India Mar-2021 to Oct-2021 n = 630 who provided blood 4–6 weeks after second vaccine dose (n = 47 breakthrough cases |
|
|
| Vanni et al. [36] | Case series | Boston, MA, USA 13-Aug-2021 to 25-Oct-2021 n = 2 |
|
|
| Calabrese et al. [37] | Case series | Cleveland, OH, USA Dec-2020 to 15-Dec-2021 n = 74 after any vaccine dose on B cell depleting therapy for immune-mediated inflammatory diseases; n = 68 breakthrough cases |
|
|
CI, confidence interval; EHR, electronic health record; EULAR, European Alliance of Associations for Rheumatology; HR, hazard ratio; ILD, interstitial lung disease; RA, rheumatoid arthritis; RBD, receptor binding domain; SARD, systemic autoimmune rheumatic disease; TNF, tumor necrosis factor.
In this narrative review, we will provide an overview of the current evidence related to humoral immunogenicity from SARS-CoV-2 vaccination, focusing on specific immunosuppressive drugs, waning immunity, medication discontinuation around vaccination, and additional doses. We will also discuss the studies investigating clinical cases of breakthrough infection after vaccination. Finally, we will detail studies investigating disease flare or increased disease activity after SARS-CoV-2 vaccination.
Humoral immunogenicity
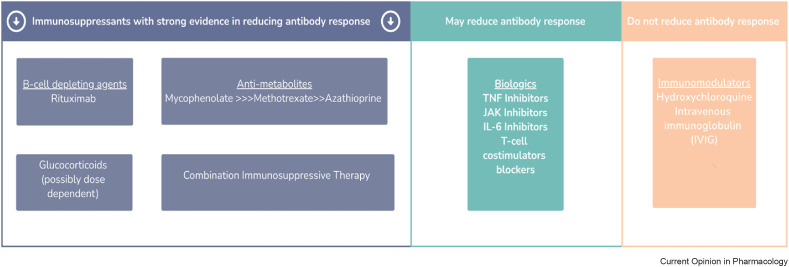
Humoral response in immunosuppressed patients with rheumatic and musculoskeletal diseases have been reported to be diminished [9, 10∗∗, 11, 12∗∗]. It is now well appreciated that depending on the type of immunosuppressive therapy, such as B cell depleting agents and mycophenolate mofetil, the antibody response can be dampened significantly. It is not surprising then that rheumatic patients treated with such agents would be more vulnerable to severe COVID-19 infection despite vaccination. This section will review the body of evidence that supports how different immunosuppressive therapies can blunt the humoral response (see Figure 1 ).
Figure 1.
Key immunosuppressants associated with reducing antibody response after SARS-CoV-2 vaccination. Those with the strongest evidence are highlighted in the left panel (purple), while those that may reduce antibody response are in the middle panel (green), and those that do not reduce antibody response are in the right panel (orange).
B-cell depleting agents
B cell-depleting agents, such as rituximab, have been reported to be the most significant offender in reducing humoral response after SARS-CoV-2 vaccination [12∗∗, 13, 14, 15, 16∗∗]. In fact, some studies have demonstrated that patients who receive rituximab do not mount any antibody response which in particular can be concerning for those on maintenance therapy. Duration since rituximab exposure may be an important contributor to improved humoral response [17]. In a retrospective study of 56 patients treated with rituximab, the longer the period between the patient's last exposure of rituximab and SARS-CoV-2 vaccination was associated with a positive serologic response, with those who had exposure > 12 months had the best response [18]. Additionally, B cell reconstitution has been reported to be a reliable marker of seroresponse in those with vasculitis or rheumatoid arthritis [14,17,18].
Antimetabolites
Mycophenolate mofetil and mycophenolic acid have been reported in multiple studies to blunt the humoral response [12,19]. In the rheumatic arm of the national vaccine response study at Johns Hopkins, patients taking mycophenolate reported rates of seroconversion as low as 27% after the first dose of the SARS-CoV-2 mRNA vaccine, with improvement to 73% after the second dose [9,12]. Even more reduced response has been reported in patients on anti-metabolite regimens containing MMF in single organ transplant recipients [11,19]. Interestingly, azathioprine has not been reported to have a significant impact on the humoral response [12].
Methotrexate has been reported to have lower rates of seroconversion, but reports have not been consistent [9,10,16,19]. For example, in the Johns Hopkins cohort, there was no significant impact of methotrexate on humoral response. However, in other cohort studies that included a US based cohort at New York University Langone with a European validation cohort, humoral immune response was diminished. Only 62.2% of patients on background methotrexate demonstrated an adequate response after two-dose vaccination [20].
Glucocorticoids
The dose of glucocorticoids and its direct effects on humoral response after SARS-CoV-2 vaccination still needs further study. However, since most people with SARDs are on combination therapy with other immunosuppressants, it may be difficult to isolate the sole effects of steroids alone. Nonetheless, a well characterized US cohort study, the COVARiPAD study, demonstrated that antibody titers were lower in patients who receive low dose prednisone (< 7.5mg/daily) [10].
Other Biologics/DMARDs
In one of the largest observational studies of 686 patients with rheumatic diseases, seroconversion was lower for those on immunosuppression, such as abatacept (71% seroconverted), JAK inhibitors (90% seroconverted), while other immunosuppressants (leflunomide, hydroxychloroquine, TNF inhibitors, IL-6 inhibitors, and IL-17 inhibitors) did not significantly impact seroconversion [21]. In contrast, in a prospective cohort of 133 patients, JAK inhibitors and other biologics such as TNF inhibitors, IL-12/IL-23 had a modest impact on antibody formation [10].
Combination therapy
Combination therapy with multiple agents, in particular with B-cell depleting therapies, can contribute to a poor humoral response. Connolly and colleagues reported on 20 patients who did not have detectable anti-RBD antibodies at a median of 30 days after the second dose of SARS-CoV-2 mRNA vaccine and found that 90% of the patients were on multiple immunosuppressive agents, and maintenance corticosteroids were a part of 16 (80%) participant regimens [13].
Waning immunity
While antibody responses are detectable for a minimum of 6 months following the initial series of SARS-CoV-2 mRNA vaccination in immunocompetent individuals [22,23], titers do tend to wane during this period potentially leading to loss of protective responses. Due to attenuated humoral responses associated with immunosuppression, the impact immunosuppression has on antibody decay kinetics is critical to inform providers about the need for additional boosting or administration of pre-exposure prophylaxis.
Data is starting to emerge suggesting that most individuals with SARDs on immunosuppression likely maintain antibody titers that are protective. Data from the Johns Hopkins cohort revealed that while anti-RBD titers reduced by 2.8-fold between the 1-month to 6-month post-vaccination period, 96% of the study participants remained seropositive (96%) and 80% continued to maintain predicted protective neutralizing antibody titers [24,25]. Those on monotherapy had the high rates of high antibody titers compared to participants on combination therapy, and similar to antibody responses immediately post-vaccination those on therapies that B cell modulating therapies and T cell co-stimulation blockade were seronegative at the 6-month timepoint [24]. These early findings should be encouraging for immunosuppressed patients with SARDs, as initial antibody responses appear to be predictive of durable responses.
Additional vaccine doses and peri-vaccination hold
An additional 3rd SARS-CoV-2 vaccine dose in those who had a poor antibody response have found that up to 80% seroconvert in a case series of 18 patients [26]. Similarly, a fourth dose has also been reported to improve humoral response in a case report of a patient with rheumatoid arthritis even without pausing immunosuppression [27]. Interestingly, another case series of 18 patients from the Johns Hopkins cohort also demonstrated that a 4th additional dose augmented humoral response [28]. However, there was a subset of patients who did not hold immunosuppression who remained persistently negative.
Beyond additional vaccine doses, peri-vaccination hold of immunosuppressants can be a complementary strategy to improve humoral response in rheumatic patients on immunosuppression. Peri-vaccination hold of mycophenolate mofetil has been reported to augment humoral response in rheumatic patients [29]. In a case series of 24 patients who withheld mycophenolate, 22/24 (92%) had detectable antibodies compared with 112/171 (65%) who continued therapy. There were two participants who reported flares of their underlying disease requiring treatment in the peri-vaccination period, therefore careful assessment of disease activity should be done when optimizing vaccination strategies.
Breakthrough infection/severity
Several studies have investigated clinical outcomes among SARDs from COVID-19 breakthrough infection, defined by the US Centers for Disease Control and Prevention as a positive COVID-19 test 14 days or longer after completion of the initial vaccine series. Infections occurring between initial vaccination and that time point are considered partially vaccinated. The initial case series reporting breakthrough infections among SARDs was from Mass General Brigham in Boston, MA, USA [30]. This study identified 16 breakthrough infections among SARD patients [30]. Many of the medications also implicated with impaired humoral immunity after COVID-19 were over-represented (e.g., rituximab, mycophenolate mofetil, methotrexate) [30]. Some of the patients had severe outcomes and there were 2 deaths (both in patients with interstitial lung disease treated with rituximab) [30]. Other investigators analyzed European Alliance of Associations for Rheumatology to identify 38 COVID-19 cases after vaccination (10 breakthrough and 26 partially vaccinated infections)31. That study also reported two deaths among those with breakthrough infection (one with RA and Sjogren's syndrome treated with rituximab, 1 with RA treated with glucocorticoids) [31]. Finally, the Global Rheumatology Alliance reported a large case series of 197 COVID-19 cases occurring after vaccination, 87 of which qualified as breakthrough infections [32]. Among those with breakthrough infection, 22 were hospitalized (9 on rituximab, 3 on mycophenolate mofetil; 8 had pre-existing lung disease) and 5 died [32]. Overall, these case series show that some SARD patients are susceptible to severe clinical outcomes even after vaccination, particularly those with pre-existing lung disease and those treated with B cell depleting therapy or mycophenolate mofetil. However, firm conclusions are limited due to lack of denominator and small size of studies.
A large retrospective cohort study investigated breakthrough infection in conditions characterized by immune dysfunction using the National COVID Cohort Collaborative (N3C). This study compared COVID-19 risk after vaccination among patients with HIV infection, multiple sclerosis, rheumatoid arthritis, solid organ transplant, or bone marrow transplant to the general population (n = 664,722) [33]. The overall rate of COVID-19 after vaccination was 5.0 per 1000 person-months. Full vaccination was associated with 28% lower risk of COVID-19 compared to partial vaccination [33]. Compared to the general population, solid organ transplant (HR 2.16, 95% CI 1.96–2.38), HIV infection (HR 1.33, 95% CI 1.18–1.49), and rheumatoid arthritis (HR 1.20, 95% CI 1.09–1.32) were each associated with higher COVID-19 risk [33]. While some patients were hospitalized for COVID-19 after vaccination, the proportion was much lower compared to pre-vaccination cases [33]. A small study analyzing the Greek Rheumatology registry (n = 195) also reported improvement in the proportion of COVID-19 cases requiring hospitalization after vaccination among SARDs [34].
A large prospective cohort study in India linked humoral vaccine response to clinical outcomes for breakthrough infections among SARDs [35]. This prospective cohort study enrolled 630 patients with SARDs and measured anti-RBD antibodies 4–6 weeks after the 2nd vaccine dose [35]. They categorized patients based on the anti-RBD level as having a good response, inadequate response, or no response [35]. They contacted each patient every two months to identify COVID-19 cases. Their study coincided with the Delta wave in India [35]. Overall, there were 47 breakthrough infections (7.4% of patients) [35]. Those with no response to vaccination had nearly 4-fold increased risk of breakthrough infection (HR 3.6, 95%CI 1.6–8.0) compared to those with good response [35]. Thus, the humoral vaccine response may be a marker for risk of COVID-19.
All of these previous studies investigated breakthrough infection after 2 doses of mRNA vaccine (or one of J&J), but the US CDC now recommends an additional dose to complete the initial series for immunocompromised patients. The Mass General Brigham group reported their first 2 cases of breakthrough infection occurring in patients with rheumatoid arthritis on TNF inhibitor monotherapy [36]. A recent preprint investigated breakthrough infection after CD20 inhibitor use for immune-mediated inflammatory diseases at a single center and detailed 74 COVID-19 cases after vaccination (45 occurring after 2 vaccine doses and 23 occurring after 3 doses) [37]. More work is needed to investigate breakthrough infection after 3 or 4 vaccine doses and current variants.
Disease flares after SARS-CoV-2 vaccine series
In one of the largest physician-reported registries of inflammatory/autoimmune rheumatic musculoskeletal diseases (n = 5121 participants from 30 countries), it was found that flares were reported in 4.4% of cases, with 1.5% resulting in medication changes [38]. Results from the COVID-19 Global Rheumatology Alliance Vaccine Survey (n = 2860 participants) also found that flares that required medication changes occurred in only 4.6% of patients [39]. In a smaller study of the Johns Hopkins cohort (n = 1377 patients with RMD), 11% reported flares that required treatment [40]. There were no reports of severe flares in this group. The most common reactions were local reactions such as injection site pain and fatigue which did not interfere with daily activity. Of note, flares were associated with a flare in the 6 months preceding vaccination, prior COVID-19, and use of combination immunomodulatory therapy potentially highlighting patients who may have had more refractory disease at baseline.
Since SARDs represent a heterogeneous group of many autoimmune diseases, there have now also been reports on the risk of disease flare in specific autoimmune diseases such as systemic lupus erythematosus (SLE). In the VACOLUP study, 696 participants from 30 countries were included to determine the risk of flare after COVID vaccine administration. 21 (3%) of 696 patients reported a medically confirmed SLE flare [41]. 15 of 21 (71%) of the cases required a change in SLE treatment and 4 patients were admitted to the hospital [41]. Similar to the Johns Hopkins cohort [40], having a flare during the past year before vaccination was associated with an increased risk for SLE flare after vaccination [41]. A more recent study of 90 SLE patients in a multiethnic/multiracial cohort also demonstrated that postvaccination flares occurred in 11.4% of patients, and only 1.3% of these were severe [42]. Another small single-center prospective study of 71 RA patients receiving an additional dose of COVID-19 vaccine showed no pre/post vaccine differences in patient-reported disease activity or in cellular markers of immune activation in a subset [43]. A study performed in Hong Kong using administrative data among 5493 RA patients showed no association between vaccine receipt and RA flare as well as no difference in RA drug prescriptions after vaccination [44]. However, a randomized trial of holding vs. continuing methotrexate in RA patients after COVID-19 vaccine did show higher disease activity in those who held methotrexate [45]. This suggests that immunosuppressive medication changes around vaccination, rather than vaccine-specific effects, could explain at least some RMD flares after vaccination.
Overall, risk of severe flare after COVID vaccination was relatively uncommon in multiple studies and may be similar to the baseline rate of SARDs. These findings emphasize the safety of SARS-CoV-2 vaccines for immunosuppressed patients with SARDs.
Additional considerations
In the immunocompetent population, germinal center (GC) responses in draining lymph nodes last for a minimum of 6 months [46] which are critical for optimizing antibody diversity, affinity, and function. The duration of GC responses likely has major implications for the immunosuppressed: if immunosuppression is held peri-vaccination, restarting these medications likely are occurring early in the GC response. Blunting GC responses will theoretically attenuate cross-variant neutralization responses, as observed in the setting of MMF or TNFi. GCs are absent in patients who underwent kidney transplantation, partially due to the use of MMF [47]. In the setting of SARS-CoV-2 vaccination, TNFi monotherapy users possessed greatly reduced cross-variant neutralization titers to both B.1.351 (Beta) and B.1.617.2 (Delta) variants [48], possibly through the loss of GC structures as observed in the tonsils of patients with RA on TNFi [49]. Fortunately, a third dose restored these titers to predicted protective levels suggesting highlighting the critical importance of these additional doses [48].
The question about the sufficiency of T cell responses in vaccine-induced protective immunity continues to be debated. While a clear correlate of protection is neutralizing antibodies, SARS-CoV-2 vaccination in immunocompetent individuals generates robust and broadly reactive T cell responses, including the generation of memory T cells [50,51]. It has been suggested that neutralizing antibodies mediate protection from infection, while cellular responses influence severity of disease and infection resolution [52]. T cell responses may be particularly important in the setting of B cell depletion, which confers the greatest risk of poor or absent humoral responses as discussed above. Patients with multiple sclerosis on BCDT mounted modestly reduced follicular T cell responses in the blood [53], a cell necessary to generate effective GC responses. While CD8+ T cell frequencies are elevated, the increased incidence of breakthrough infections in these patients (as discussed above) likely suggests that T cells are not protective in this setting. This may be due to T cell dysfunction, as reductions in cytokine production by S-specific CD4+ T cells have been observed in patients on BCDT [15].
Finally, vaccine hesitancy remains an important issue among people with SARDs related to potential for disease flare, timing of vaccine receipt related to high disease activity and immunosuppressive medication use, and the novelty of SARS-CoV-2 [54, 55, 56] vaccines. More research is needed to show that SARS-CoV-2 vaccines are safe and effective and to develop specific interventions to increase vaccine update.
Future research
Within a year of the introduction of SARS-CoV-2 vaccines, we impressively have a solid idea of how various classes of immunosuppressive influence antibody titers. But these data have open additional questions regarding the relationship between immunogenicity, protection, and the complete impact of immunosuppression has on both. For example, all studies have found large variation in antibody titers among study participants for any given immunosuppressive class. Whether this is due to concomitant medication use, disease state or activity, sleep quality (as has been observed with hepatitis A vaccination [57]), or host genetics will require additional efforts to deconvolute.
Furthermore, these responses assessed immediately after SARS-CoV-2 vaccination do not fully represent the mature humoral response. As discussed above, germinal center responses linger for months after vaccination in the immunocompetent, which serves to improve antibody quality by diversifying antibody repertoire. We only have a limited idea of the impact of immunosuppression on antibody quality, which is limited to TNFi. This has implications on whether to hold medications, as they will be restarted when germinal center responses are clearly ongoing. The NIH/NIAID-sponsored ACV01 clinical trial, which seeks to examine the impact of additional doses of SARS-CoV-2 vaccine and holding either methotrexate or mycophenolate on antibody and cellular responses [58].
Finally, questions remain about the robustness and importance of T cell responses particularly for non-B cell depletion therapies. Also, the generation of mucosal immunity vis-a-vis S-specific IgA remains unclear, with little data published thus far [59]. As these and other questions are answered over the following months and years, this will provide the most comprehensive collective dataset that will certainly inform about how to improve the outcomes with other vaccines these patients require.
Funding/support
JJP is funded by NIH/NIAMS (K23 AR073927). JAS is funded by NIH/NIAMS (R01 AR077607, P30 AR070253, and P30 AR072577) and the R. Bruce and Joan M. Mickey Research Scholar Fund. AHJK is funded by NIH/NIAMS (P30 AR073752), NIAID (UL1 9AI110483), NCATS (UL1 TR002345), The Leona M. and Harry B. Helmsley Charitable Trust, Multiple Sclerosis Society, and Rheumatology Research Foundation.
Credit author statement
Paik and Alfred, Julie J. Paik: conceptualization, writing – original draft, and writing – review & editing. Jeffrey A. Sparks: conceptualization, writing – original draft, and writing – review & editing. Alfred H.J. Kim: conceptualization, writing – original draft, and writing – review & editing.
Conflict of interest statement
JJP reports research support from Pfizer and Kezar Inc, and consultancy fees from Pfizer, Alexion, Kezar, Roivant, EMD Serono, Argenx, and Guidepoint all unrelated to this work. JAS reports research support from Bristol Myers Squibb and consultancy fees from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer unrelated to this work. AHJK reports research support from GlaxoSmithKline and Foghorn Therapeutics, and consultancy fees from Alexion Pharmaceuticals, Aurinia Pharmaceuticals, Exagen Diagnostics, GlaxoSmithKline, and Pfizer unrelated to this work.
This review comes from a themed issue on Rheumatology (2023)
Edited by Larry Moreland and Kristine Kuhn
References
- 1.Conway R., Grimshaw A.A., Konig M.F., et al. SARS-CoV-2 infection and COVID-19 outcomes in rheumatic diseases: a systematic literature review and meta-analysis. Arthritis Rheumatol. 2021;74:766–775. doi: 10.1002/art.42030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D'Silva K.M., Serling-Boyd N., Wallwork R., et al. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US 'hot spot. Ann Rheum Dis. 2020;79:1156–1162. doi: 10.1136/annrheumdis-2020-217888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Silva K.M., Jorge A., Cohen A., et al. COVID-19 outcomes in patients with systemic autoimmune rheumatic diseases compared to the general population: a US multicenter, comparative cohort study. Arthritis Rheumatol. 2021;73:914–920. doi: 10.1002/art.41619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.England B.R., Roul P., Yang Y., et al. Risk of COVID-19 in rheumatoid arthritis: a national veterans affairs matched cohort study in at-risk individuals. Arthritis Rheumatol. 2021;73:2179–2188. doi: 10.1002/art.41800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sparks J.A., Wallace Z.S., Seet A.M., et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: results from the COVID-19 Global Rheumatology Alliance physician registry. Ann Rheum Dis. 2021;80:1137–1146. doi: 10.1136/annrheumdis-2021-220418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtis J.R., Johnson S.R., Anthony D.D., et al. American college of Rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 3. Arthritis Rheumatol. 2021;73:e60–e75. doi: 10.1002/art.41928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi B., Choudhary M.C., Regan J., et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med. 2020;383:2291–2293. doi: 10.1056/NEJMc2031364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choudhary M.C., Crain C.R., Qiu X., Hanage W., Li J.Z. Severe Acute respiratory syndrome coronavirus 2 (SARS-CoV-2) sequence characteristics of coronavirus disease 2019 (COVID-19) persistence and reinfection. Clin Infect Dis. 2022;74:237–245. doi: 10.1093/cid/ciab380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyarsky B.J., Ruddy J.A., Connolly C.M., et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021;80:1098–1099. doi: 10.1136/annrheumdis-2021-220289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepak P., Kim W., Paley M.A., et al. Effect of immunosuppression on the immunogenicity of mRNA vaccines to SARS-CoV-2 : a prospective cohort study. Ann Intern Med. 2021;174:1572–1585. doi: 10.7326/M21-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the first prospective studies demonstrating the importance of how different immunosuppressants affect immunogenicity of SARS-CoV-2 vaccines.
- 11.Boyarsky B.J., Werbel W.A., Avery R.K., et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325:1784–1786. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddy J.A., Connolly C.M., Boyarsky B.J., et al. High antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021;80:1351–1352. doi: 10.1136/annrheumdis-2021-220656. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the earliest observations demonstrating the negative impact of immunosuppression, especially mycophenolate and rituximab, on antibody reponse after SARS-CoV-2 vaccination.
- 13.Connolly C.M., Boyarsky B.J., Ruddy J.A., et al. Absence of humoral response after two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases: a case series. Ann Intern Med. 2021;174:1332–1334. doi: 10.7326/M21-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connolly C.M., Koenig D., Ravi S.N., et al. Correspondence on "SARS-CoV-2 vaccination in rituximab-treated patients: evidence for impaired humoral but inducible cellular immune response" by Bonelli et al. Ann Rheum Dis. 2021;80:e164. doi: 10.1136/annrheumdis-2021-220972. [DOI] [PubMed] [Google Scholar]
- 15.Moor M.B., Suter-Riniker F., Horn M.P., et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol. 2021;3:e789–e797. doi: 10.1016/S2665-9913(21)00251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekel L., Steenhuis M., Hooijberg F., et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in The Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol. 2021;3:e778–e788. doi: 10.1016/S2665-9913(21)00222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the largest observational studies of patients with son immunosuppression demonstrating the detrimental impact on humoral responses of BCDT and delaying the second dose of SARS-CoV-2 vaccination for those on MTX.
- 17.Stefanski A.L., Rincon-Arevalo H., Schrezenmeier E., et al. B cell numbers predict humoral and cellular response upon SARS-CoV-2 vaccination among patients treated with rituximab. Arthritis Rheumatol. 2021 doi: 10.1002/art.42060. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jinich S., Schultz K., Jannat-Khah D., Spiera R. B cell reconstitution is strongly associated with COVID-19 vaccine responsiveness in rheumatic disease patients who received treatment with rituximab. Arthritis Rheumatol. 2022;74:776–782. doi: 10.1002/art.42034. [DOI] [PubMed] [Google Scholar]
- 19.Boyarsky B.J., Werbel W.A., Avery R.K., et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haberman R.H., Herati R., Simon D., et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis. 2021;80:1339–1344. doi: 10.1136/annrheumdis-2021-220597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furer V., Eviatar T., Zisman D., et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]; One of the largest observational studies highlighting that seroconversion was lower in those taking abatacept while others did not significant impact seroconversion (such as leflunomide, IL-6 and IL-17 inhibitors).
- 22.Doria-Rose N., Suthar M.S., Makowski M., et al. Antibody persistence through 6 Months after the second dose of mRNA-1273 vaccine for covid-19. N Engl J Med. 2021;384:2259–2261. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pegu A., O'Connell S.E., Schmidt S.D., et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science. 2021;373:1372–1377. doi: 10.1126/science.abj4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S., Chiang T.P., Connolly C.M., et al. Antibody durability 6 months after two doses of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal disease. Lancet Rheumatol. 2022;4:e241–e243. doi: 10.1016/S2665-9913(21)00417-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the first reports examining the decay in seropositivity rates months after SARS-CoV-2 vaccination.
- 25.Khoury D.S., Cromer D., Reynaldi A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 26.Connolly C.M., Teles M., Frey S., et al. Booster-dose SARS-CoV-2 vaccination in patients with autoimmune disease: a case series. Ann Rheum Dis. 2022;81:291–293. doi: 10.1136/annrheumdis-2021-221206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albach F.N., Burmester G.R., Biesen R. Successful BNT162b2 booster vaccinations in a patient with rheumatoid arthritis and initially negative antibody response. Ann Rheum Dis. 2021;80:1361–1362. doi: 10.1136/annrheumdis-2021-220834. [DOI] [PubMed] [Google Scholar]
- 28.Teles M., Connolly C.M., Frey S., et al. Attenuated response to fourth dose SARS-CoV-2 vaccination in patients with autoimmune disease: a case series. Ann Rheum Dis. 2022;81:738–740. doi: 10.1136/annrheumdis-2021-221641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Connolly C.M., Chiang T.P., Boyarsky B.J., et al. Temporary hold of mycophenolate augments humoral response to SARS-CoV-2 vaccination in patients with rheumatic and musculoskeletal diseases: a case series. Ann Rheum Dis. 2022;81:293–295. doi: 10.1136/annrheumdis-2021-221252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C., Patel N.J., D'Silva K.M., et al. Clinical characteristics and outcomes of COVID-19 breakthrough infections among vaccinated patients with systemic autoimmune rheumatic diseases. Ann Rheum Dis. 2022;81:289–291. doi: 10.1136/annrheumdis-2021-221326. [DOI] [PMC free article] [PubMed] [Google Scholar]; This initial case series reported severe outcomes for breakthrough infection for patients with SARDs, particularly those on rituximab with interstitial lung disease.
- Lawson-Tovey S., Hyrich K.L., Gossec L., et al. SARS-CoV-2 infection after vaccination in patients with inflammatory rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2022;81:145. doi: 10.1136/annrheumdis-2021-221217. 50. [DOI] [PubMed] [Google Scholar]; This case series detailed the first partially vaccinated and breakthrough infections among SARDs in EULAR registries.
- Liew J., Gianfrancesco M., Harrison C., et al. SARS-CoV-2 breakthrough infections among vaccinated individuals with rheumatic disease: results from the COVID-19 Global Rheumatology Alliance provider registry. RMD Open. 2022;8 doi: 10.1136/rmdopen-2021-002187. [DOI] [PMC free article] [PubMed] [Google Scholar]; This large case series showed that many hospitalized breakthrough cases were on B cell depleting therapy or mycophenolate mofetil.
- Sun J., Zheng Q., Madhira V., et al. Association between immune dysfunction and COVID-19 breakthrough infection after SARS-CoV-2 vaccination in the US. JAMA Intern Med. 2022;182:153–162. doi: 10.1001/jamainternmed.2021.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]; Investigators performed a large US retrospective cohort study and found that patients with RA and other immune dysfunction conditions were associated with increased risk for breakthrough infection compared to healthy controls.
- 34.Papagoras C., Fragoulis G.E., Zioga N., et al. Better outcomes of COVID-19 in vaccinated compared to unvaccinated patients with systemic rheumatic diseases. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-221539. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Ahmed S., Mehta P., Paul A., et al. Postvaccination antibody titres predict protection against COVID-19 in patients with autoimmune diseases: survival analysis in a prospective cohort. Ann Rheum Dis. 2022;81:868–874. doi: 10.1136/annrheumdis-2021-221922. [DOI] [PubMed] [Google Scholar]; This prospective cohort study performed in India linked poor humoral vaccine response after vaccination with risk of breakthrough infection among SARDs.
- 36.Vanni K.M., Patel N.J., DiIorio M., et al. Breakthrough infection after three doses of COVID-19 mRNA vaccine in systemic autoimmune rheumatic diseases: two cases in patients on TNF inhibitor monotherapy. RMD Open. 2022;8 doi: 10.1136/rmdopen-2021-002082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calabrese C.M., Kirchner E., Husni E.M., Moss B.P., Fernandez A.J., Jin Y., Calabrese L.H. Breakthrough SARS-CoV-2 infections in immune mediated disease patients undergoing B cell depleting therapy. medRxiv. 2022:22271289. doi: 10.1101/2022.02.21.22271289. 0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado P.M., Lawson-Tovey S., Strangfeld A., et al. Safety of vaccination against SARS-CoV-2 in people with rheumatic and musculoskeletal diseases: results from the EULAR Coronavirus Vaccine (COVAX) physician-reported registry. Ann Rheum Dis. 2022;81:695–709. doi: 10.1136/annrheumdis-2021-221490. [DOI] [PubMed] [Google Scholar]; Largest physician reported registry of SARDs on the prevalence of flares after SARS-COV-2 vaccination. Flare rate was reported to be 4.4%.
- 39.Sattui S.E., Liew J.W., Kennedy K., et al. Early experience of COVID-19 vaccination in adults with systemic rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance Vaccine Survey. RMD Open. 2021;7:e001814. doi: 10.1136/rmdopen-2021-001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly C.M., Ruddy J.A., Boyarsky B.J., et al. Disease flare and reactogenicity in patients with rheumatic and musculoskeletal diseases following two-dose SARS-CoV-2 messenger RNA vaccination. Arthritis Rheumatol. 2022;74:28–32. doi: 10.1002/art.41924. [DOI] [PMC free article] [PubMed] [Google Scholar]; First study in SARDs to report that flare rate after primary series of SARS-CoV-2 vaccine was 11%.
- 41.Felten R., Kawka L., Dubois M., et al. Tolerance of COVID-19 vaccination in patients with systemic lupus erythematosus: the international VACOLUP study. Lancet Rheumatol. 2021;3:e613–e615. doi: 10.1016/S2665-9913(21)00221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Izmirly P.M., Kim M.Y., Samanovic M., et al. Evaluation of immune response and disease status in systemic lupus erythematosus patients following SARS-CoV-2 vaccination. Arthritis Rheumatol. 2022;74:284–294. doi: 10.1002/art.41937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tedeschi S.K., Stratton J., Ellrodt J.E., et al. Rheumatoid arthritis disease activity assessed by patient-reported outcomes and flow cytometry before and after an additional dose of COVID-19 vaccine. Ann Rheum Dis. 2022 doi: 10.1136/annrheumdis-2022-222232. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X., Tong X., Yeung W.W.Y., et al. Two-dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Ann Rheum Dis. 2022;81:564–568. doi: 10.1136/annrheumdis-2021-221571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Araujo C.S.R., Medeiros-Ribeiro A.C., Saad C.G.S., et al. Two-week methotrexate discontinuation in patients with rheumatoid arthritis vaccinated with inactivated SARS-CoV-2 vaccine: a randomised clinical trial. Ann Rheum Dis. 2022;81:889–897. doi: 10.1136/annrheumdis-2021-221916. [DOI] [PubMed] [Google Scholar]
- Kim W., Zhou J.Q., Horvath S.C., et al. Germinal centre-driven maturation of B cell response to mRNA vaccination. Nature. 2022;604:141–145. doi: 10.1038/s41586-022-04527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using paired blood and draining lymph node aspirates in immunocompetent SARS-CoV-2 vaccinees, this report identified the extraordinary length of GC responses that occur following the initial series of mRNA vaccination.
- Lederer K., Bettini E., Parvathaneni K., et al. Germinal center responses to SARS-CoV-2 mRNA vaccines in healthy and immunocompromised individuals. Cell. 2022;185:1008–1024 e15. doi: 10.1016/j.cell.2022.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]; Similar to ref 46, paired blood and draining lymph node specimens from kidney transplant recipients were examined to identify the absence of GCs and neutralizing antibody responses.
- Chen R.E., Gorman M.J., Zhu D.Y., et al. Reduced antibody activity against SARS-CoV-2 B.1.617.2 delta virus in serum of mRNA-vaccinated individuals receiving tumor necrosis factor-alpha inhibitors. Med (N Y) 2021;2:1327–13241 e4. doi: 10.1016/j.medj.2021.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the first reports to examine cross-variant neutralization responses using infection SARS-CoV-2 virus, identifying the lack of response to Beta and Delta variants in TNFi users.
- 49.Anolik J.H., Ravikumar R., Barnard J., et al. Cutting edge: anti-tumor necrosis factor therapy in rheumatoid arthritis inhibits memory B lymphocytes via effects on lymphoid germinal centers and follicular dendritic cell networks. J Immunol. 2008;180:688–692. doi: 10.4049/jimmunol.180.2.688. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Z., Mateus J., Coelho C.H., et al. Humoral and cellular immune memory to four COVID-19 vaccines. bioRxiv. 2022 doi: 10.1101/2022.03.18.484953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goel R.R., Painter M.M., Apostolidis S.A., et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. 2021;374:abm0829. doi: 10.1126/science.abm0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kedzierska K., Thomas P.G. Count on us: T cells in SARS-CoV-2 infection and vaccination. Cell Rep Med. 2022;3:100562. doi: 10.1016/j.xcrm.2022.100562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolidis S.A., Kakara M., Painter M.M., et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med. 2021;27:1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; A thorough examination of cellular compartments in BCDT recipients within patients with multiple sclerosis, identifying a reduction of Spike-specific memory B cells but preservation of follicular helper T cells in the periphery (blood).
- 54.Ledbetter S.S., Xie F., Stewart P., et al. COVID-19 vaccine uptake and vaccine hesitancy in Rheumatology patients receiving immunomodulatory therapies treated in community practice settings. Arthritis Rheumatol. 2022 doi: 10.1002/art.42067. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Putman M., Kennedy K., Sirotich E., et al. COVID-19 vaccine perceptions and uptake: results from the COVID-19 global Rheumatology alliance vaccine Survey. Lancet Rheumatol. 2022;4:e237–e240. doi: 10.1016/S2665-9913(22)00001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Felten R., Dubois M., Ugarte-Gil M.F., et al. Cluster analysis reveals three main patterns of beliefs and intention with respect to SARS-CoV-2 vaccination in patients with autoimmune and inflammatory diseases. Rheumatology (Oxford) 2021;60:SI68–SI76. doi: 10.1093/rheumatology/keab432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lange T., Perras B., Fehm H.L., Born J. Sleep enhances the human antibody response to hepatitis A vaccination. Psychosom Med. 2003;65:831–835. doi: 10.1097/01.psy.0000091382.61178.f1. [DOI] [PubMed] [Google Scholar]
- 58.https://clinicaltrials.gov/ct2/show/NCT05000216.
- 59.Geisen U.M., Berner D.K., Tran F., et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021;80:1306–1311. doi: 10.1136/annrheumdis-2021-220272. [DOI] [PMC free article] [PubMed] [Google Scholar]