Abstract
Purpose
To study the efficacy of Ba Zhen Tang in delaying skin photoaging and its potential mechanism based on network pharmacology and molecular docking.
Methods
First, we screened the active components and targets of Ba Zhen Tang by Traditional Chinese Medicine Database and Analysis Platform (TCMSP) and The Universal Protein Resource (UniProt). The target genes of skin photoaging were obtained from GeneCards and GeneMap database. Then, we analyzed the protein–protein interaction (PPI) by STRING database. The network map was constructed by Cytoscape. Finally, we performed Gene Ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis by Metascape database. The molecular docking via Autodock Vina and Pymol. Furthermore, skin photoaging cellular models were established, and the effects of Ba Zhen Tang on ameliorating skin photoaging were investigated.
Results
A total of 160 active ingredients in Ba Zhen Tang and 60 targets of Ba Zhen Tang for delaying skin photoaging were identified. By GO enrichment analysis, 1153 biological process entries, 45 cellular component entries and 89 molecular functional entries were obtained. A total of 155 signal pathways were obtained by KEGG analysis. Ba Zhen Tang is related to MAPK signaling pathway, TNF signaling pathway and AGE-RAGE signaling pathway in diabetic complications, etc., which directly affect the key nodes of photoaging. The molecular docking results showed that there was a certain affinity between the main compounds (kaempferol, quercetin, β-sitosterol, naringenin) and core target genes (PTGS2, CASP3, MAPK1, MAPK3, TP53). Ba Zhen Tang-treated mouse serum inhibited the senescence and p16INK4a expression of human immortalized keratinocyte (HaCaT) cells irradiated by ultraviolet-B (UVB).
Conclusion
Our study elucidated the potential pharmacological mechanism of Ba Zhen Tang in the treatment of photoaging through multiple targets and pathways. The therapeutic effects of Ba Zhen Tang on skin photoaging were validated in cellular models.
Keywords: Ba Zhen Tang, skin photoaging, network pharmacology, molecular docking, mechanism
Introduction
Skin Photoaging (Photoaging) is premature aging of the skin caused by repeated exposure to ultraviolet radiation, which will lead to a series of clinical and histological changes. Photoaged skin loses elasticity and appears dry with coarse wrinkles, irregular pigmentation, telangiectasis and even an increased risk of tumors. The histologic features are the accumulation of degraded elastotic material in the dermis coupled with reduced collagen, atypical keratinocytes and epidermal thickening.1,2 Many mechanisms are linked to photoaging: DNA damage, oxidative stress, shortening of the telomere, chronic inflammation, cell apoptosis, the role of microRNA and accumulation of advanced glycation end products (AGEs).3–6 Skin photoaging is mainly caused by ultraviolet radiation (UV), which leads to the production of excess reactive oxygen species (ROS). On the one hand, ROS directly causes oxidative damage to cells, on the other hand, ROS activates nuclear factor kappa-B, thereby inducing the expression of cell surface cytokines that promote photoaging, such as interleukin 1 (IL1), epidermal growth factor (EGF) and tumor necrosis factor alpha (TNF-α) in keratinocytes and dermal cells. Then the activation of the cell surface cytokines leads to the upregulation of downstream signal transduction pathways, such as MAPK signal transduction pathway. Accordingly, transcription factors c-Fos and c-Jun undergo heterodimerization and the formation of Activator protein-1 (AP-1) manifests, thus matrix metalloproteinases (MMPs) are overexpressed. UV radiation also can damage deoxyribonucleic acid (DNA) directly or indirectly, resulting in shortening of telomeres.7 Sunscreen, topical retinoids, 5-fluorouracil cream, topical or oral antioxidants and face rejuvenation therapy such as laser have been widely and successfully performed in the prevention and treatment of photoaging.7 Moreover, traditional Chinese medicine has been shown to be a potentially effective technique due to its safer and more convenient characteristic.
Ba Zhen Tang originated from the ancient prescription “Zheng Ti Lei Yao”, which is a combination of Sijunzi decoction and Siwu decoction. Sijunzi Decoction strengthens the spleen and Qi, Siwu Decoction nourishes blood, and Bazhen Decoction combines the powers of two sides. Ba Zhen Tang is composed of Ginseng (Renshen: RS), Atractylodes macrocephala (Baizhu: BZ), Poria (Fuling: FL), Angelica (Danggui: DG), Chuanxiong (CX), White peony (Baishao: BS), Radix rehmanniae preparata (Shudihuang: SDH) and Licorice (Gancao: GC). Clinical studies have also demonstrated that Ba Zhen Tang can be used to treat lots of symptoms, such as anemia, fatigue, dull complexion, pale complexion, pale tongue, thin-white coating, fine-faint pulse and large-forceless-empty pulse.8 According to Traditional Chinese Medicine, there is a correlation between the skin aging and the deficiency of Kidney Essence, stagnation of Liver-Qi, deficiency of Heart-Blood and deficiency of Qi-Blood. Ba Zhen Tang could protect skin from lack of Qi and Blood. When the body’s Qi and Blood is sufficient, people will get ruddy complexion, vigor and vitality. It has been reported that Ba Zhen Tang has the effect of inhibiting oxidative stress, inflammation and apoptosis in mice.8 There is increasing evidence that anti-inflammatory, antioxidant, and other mechanisms can delay skin aging.9,10 Ba Zhen Tang can dramatically activate the mouse X-linked inhibitor of apoptosis protein (XIAP), to prevent apoptosis of oocytes and granulosa cells, thus treating premature ovarian failure.11 To sum up, the active ingredients of Ba Zhen Tang are involved in the process of inhibiting aging. Whereas the mechanism of defer skin photoaging has not been reported.
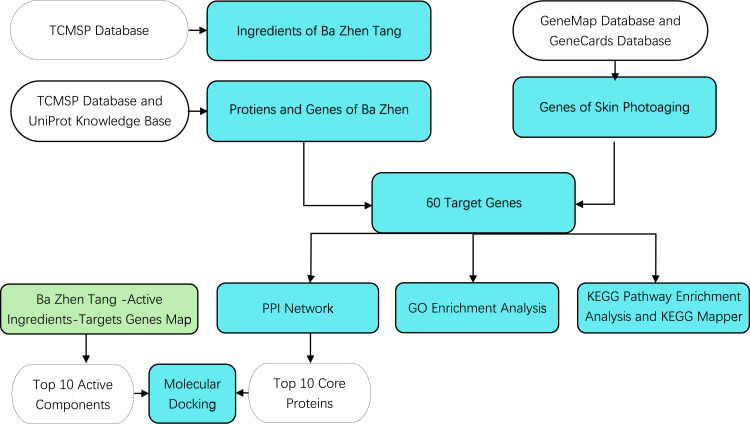
Network pharmacology measures the regulatory effect of drugs on the biomolecular network from a systematic and holistic perspective, which is characterized by its systematic nature, relevance and predictability. The word “network” composed of many elements such as TCM herbs, targets, diseases, and syndromes. By establishing and analyzing the biological network, we can carry out systematic research on the mechanism of herbal formulae and biological basis of diseases.12 Molecular docking is an established in silico structure-based method widely used in drug discovery. Docking enables the identification of novel compounds of therapeutic interest, predicting ligand–target interactions at a molecular level, or delineating structure-activity relationships (SAR), without knowing a priori the chemical structure of other target modulators. Emerging uses and applications of molecular docking, including adverse reaction prediction, polypharmacology, drug repurposing, and target fishing and profiling.13 In this study, we will elucidate the mechanism of Ba Zhen Tang in delaying skin photoaging at the molecular level based on network pharmacology and molecular docking. Furthermore, the vitro studies have been done to validate its anti-photoaging effects. A whole process flowchart is shown in Figure 1.
Figure 1.
Flowchart showing the network pharmacology and molecular docking for the investigation of the anti-photoaging effect of Ba Zhen Tang. Cyan box refers to main process, transparent color box refers to database or source of data for the main process, green box refers to the origin of top 10 active components.
Materials and Methods
Screening of the Main Active Ingredients of Ba Zhen Tang
The ingredients of Ba Zhen Tang were obtained from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform database (TCMSP, https://tcmsp-e.com). TCMSP is a unique systems pharmacology platform of Chinese herbal medicines that captures the relationships between drugs, targets and diseases, as well as pharmacokinetic properties for natural compounds involving oral bioavailability (OB), drug-likeness (DL), intestinal epithelial permeability, blood-brain-barrier, aqueous solubility, etc.14 Enter “Renshen”, “Baizhu”, “Fuling”, “Danggui”, “Chuanxiong”, “Baishao”, “Shudihuang” and “Gancao” to find the corresponding active ingredients. DL ≥0.18 and OB ≥30% were used as the criteria for primary screening.15
Potential Target of Ba Zhen Tang
The target protein of Ba Zhen Tang was predicted by TCMSP database according to the main active components. Select “Verified” and “Homo sapiens” option in the UniProt Knowledge Base (https://www.uniprot.org/),16 finally, the potential target gene corresponding to the eight position herbs in Ba Zhen Tang was obtained.
Acquisition of Skin Photoaging-Related Genes
By inputting “Skin photoaging” into the GeneMap database (https://omim.org/search/advanced/geneMap) and GeneCards database (The Human Gene Database, https://www.genecards.org/) to retrieve gene information,17,18 and collecting the two sets, the target gene data of skin photoaging can be gained.
Construction of Protein–Protein Interaction (PPI) Network
Firstly, take the intersection of the drug target gene and the disease target gene to get the putative targets, and the online Venn mapping software (http://www.bioinformatics.com.cn/static/others/jvenn/example.html) was used for visualization mapping. Then, the putative targets PPI network was constructed by STRING online database (https://www.string-db.org). STRING is a database of known and predicted protein–protein interactions. The interactions include physical and functional associations.19 Finally, the PPI network map obtained in STRING was stored in.tsv format, then imported it into Cytoscape 3.7.2 for network topology analysis and mapping.20
Gene Ontology (GO) Enrichment and Pathway Enrichment Analysis
Metascape database (https://metascape.org/) is a plethora of databases and tools that exist for gene annotation and gene list enrichment analysis.21 The target of Ba Zhen Tang delaying skin photoaging was inputted into the Metascape database with the organism limited to “Homo sapiens”, and other basic settings were the default value. Sorting the results according to the number of targets involved and graphing the top 20 items.
Molecular Docking
Molecular docking of the active components and core proteins in the first 10 degrees. The MOL2 format of 10 active compounds was gained from PubChem database (https://pubchem.ncbi.nlm.nih.gov) and 10 core protein PDB structures were downloaded from PDB database (PDB code: 1UNP, 3KJF, 1ALU, 5T01, 4FV5, 4QTB, 5I4Z, 5F1A, 5UUI, 5O1C) (http://www.rcsb.org).22,23 Proteins and compounds were introduced into the Autodock Tools-1.5.6 software to remove water and add hydrogen atoms, finally saved in PDBQT format. The main pharmacodynamic components of Ba Zhen Tang were docked with the core target gene by AutoDock vina. PyMOL was used to display the interaction diagram.
Experimental Animals
The experimental animals were specific pathogen free (SPF) healthy 6 to 8 months old male Sprague Dawley (SD) rats with body masses of 120–150 g provided by the Hubei Experimental Animal Research Center [License No. SCXK (E) 2010–0009]. The rats were raised at the Animal Experiment Center of the Hubei University of Chinese Medicine at a room temperature of 25°C±1°C and 40–60% relative humidity with a standard feed schedule. The study was approved by the Institutional Review Board of the Ethics Committee of Hubei University of Traditional Chinese Medicine. All animals were treated in compliance with the National Research Council’s Guide for the Care and Use of Laboratory Animals (1996).
Experimental Drugs
Ba Zhen Tang is mainly composed of Ginseng, Atractylodes macrocephala, Poria, Angelica, Chuanxiong, White peony, Radix rehmanniae preparata and Licorice. It has been identified as valid by the Hubei University of Chinese medicine. Through the traditional Chinese medicine preparation room of the Hospital of Traditional Chinese and Western Medicine affiliated with the Hubei University of Chinese Medicine, the medicine was combined at the mass ratio of 1:1:1:1:1:1:1:1 and formed into a liquid cream. Based on the recommended amount of regenerative medicine per unit of human body, the amount of regenerative medicine per unit rate was obtained and doubled. The normal method was used to concentrate the Ba Zhen Tang to a crude drug at 1.3 g/mL, which was stored at 4°C for reserve.
Preparation of the Ba Zhen Tang Serum
The randomized digital table method was used to divide the Ba Zhen Tang serum group and the control serum group, with 10 rats in each group. After 1 week of adaptive feeding of the SD rats, the Ba Zhen Tang serum group was given 2.5g/kg Ba Zhen Tang (according to the equivalent clinical dose),24 and the control serum group was given an equal volume of warm water once per day for seven consecutive days. After the last gavage, the rats were fasted for 12 h. The rats were then anesthetized with 10% chloral hydrate at 0.35 mL/100 g, after which blood was taken from the abdominal aorta. The blood was left at room temperature for 4 h, and then the serum was centrifuged for 10 min at 3000 r/min, and stored at −70°C.
Cell Culture
Human immortalized epidermal cells HaCaT were purchased from iCell company (Shanghai, China). The cells were cultured in DMEM medium that contained 10% fetal bovine serum (GIBICO, USA) and 1% penicillin-streptomycin double antibody. They were maintained in a cell incubator with 5% CO2 at 37°C.
UV Radiation and Treatment
HaCaT cells were divided into two groups: the control group and the Ba Zhen Tang group. The control group was continuously cultured for 24 h in a DMEM medium containing control serum. Ba Zhen Tang group was continuously cultured for 24 h in a DMEM medium containing 10% final serum concentration of Ba Zhen Tang. Then, HaCaT cells were irradiated with UVB (20 mJ/cm2) to induce cell senescence, UVB irradiation lasted for 5 days, once a day.
Senescence-Associated β-Galactosidase (SA-β-Gal) Staining
HaCaT cells were detected by SA-β-gal staining kit (C0602; Beyotime, Shanghai, China). 1 mL SA-β-gal staining fixative was added to make it fully cover the surface of the sample, which was placed in a wet box for 15 min at room temperature. Then, the sample was rinsed 3 times with PBS for 5 min each. The filter paper was used to absorb water, and an appropriate amount of staining working solution was added to make the working solution completely cover the edge of the sample. The slides were put in a wet box in a 37°C thermostat overnight. The collected images were observed under a microscope (Olympus, Japan). Senescent cells were stained dark blue.
Western Blot
Western blot was used to examine the expression of p16INK4a proteins in HaCaT cells. After the cells were lysed and centrifuged, the total protein was extracted. According to the measured protein molecular weight, 12% separating gel and 5% concentrated gel were configured separately. The sample was loaded at an amount of 50μg/porin. Electrophoresis was performed, and then the sample was transferred onto the membrane. After being removed from the blocking solution, the membrane was incubated with primary antibody against p16INK4a (1:1000; 10883-1-AP; Proteintech, USA). After washing the membrane, the secondary antibody (1:1000; ab7090; Abcam, USA) was incubated at room temperature for 1 h. The protein was developed by ECL kit and the grey value was quantified by ImageJ software (version 1.48).
Statistical Analysis
The data were analyzed by GraphPad 7.0. All quantitative data were displayed as the mean ±standard deviation. One-way analysis of variance was used for multiple comparisons. P < 0.05 was considered statistically significant.
Results
Main Active Ingredients in Ba Zhen Tang
According to the TCSMP database, 150 main active components were retrieved. Among them, the BS has 13 ingredients, RS has 22 ingredients, BZ has 7 ingredients, FL has 15 ingredients, DG has 2 ingredients, CX has 7 ingredients, SDH has 2 ingredients, and GC has 92 ingredients. There are 5 ingredients in common, and the main active ingredients of DG and SDH are common ingredients, the results are shown in Additional file 1.
Target Genes of Ba Zhen Tang
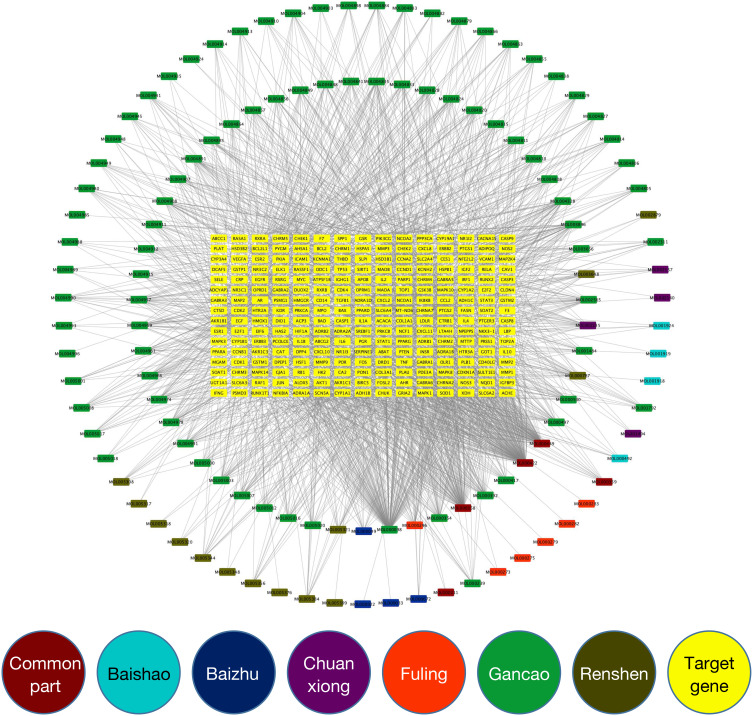
After removing ineffective active ingredients,122 active ingredients can be queried for corresponding effective target proteins. Then the 122 target proteins were standardized by UniProt database. Finally, 240 target genes of Ba Zhen Tang were obtained (Additional file 2). Cytoscape 3.7.2 was used to make the Ba Zhen Tang-active ingredients-targets gene map (Figure 2) The main active components of Ba Zhen Tang in the first 10 degrees are listed in Table 1.
Figure 2.
Ba Zhen Tang-active ingredient-target genes map. The 240 target genes of Ba Zhen Tang were arranged in yellow rectangle in the center. The 122 active components of Ba Zhen Tang were arranged in circle. Color classification of active ingredients: red belongs to 5 common ingredients, including DG and SDH, cyan belongs to BS. Blue belongs to BZ, purplish belongs to CX, Orange belongs to FL, green belongs to GC, brown belongs to RS.
Table 1.
Top 10 Active Ingredients in the Network of Ba Zhen Tang-Active Ingredients-Targets Genes Map
| MOL ID | Compound | Degree | OB% | DL | Medicine |
|---|---|---|---|---|---|
| MOL000422 | Kaempferol | 171 | 41.88 | 0.24 | BS, GC, RS |
| MOL000098 | Quercetin | 145 | 46.43 | 0.28 | GC |
| MOL000358 | Beta-Sitosterol | 105 | 36.91 | 0.75 | BS, DG, RS |
| MOL000449 | Stigmasterol | 90 | 43.83 | 0.76 | DG, RS, SDH |
| MOL003896 | 7-Methoxy-2-Methyl Isoflavone | 38 | 42.56 | 0.2 | GC |
| MOL004328 | Naringenin | 35 | 59.29 | 0.21 | GC |
| MOL000392 | Formononetin | 34 | 69.67 | 0.21 | GC |
| MOL002565 | Medicarpin | 30 | 49.22 | 0.34 | GC |
| MOL000354 | Isorhamnetin | 30 | 49.6 | 0.31 | GC |
| MOL000497 | Licochalcone A | 28 | 40.79 | 0.29 | GC |
Target Genes Related to Skin Photoaging
Using GeneMap and GeneCards database to screen “Skin photoaging” and “Homo sapiens”, we get 234 and 36 skin photoaging-related genes, respectively. Taken the two together, remove 5 duplicate values. Finally, 265 genes related to skin photoaging were received.
The Key Targets of Ba Zhen Tang for Anti-Skin Photoaging
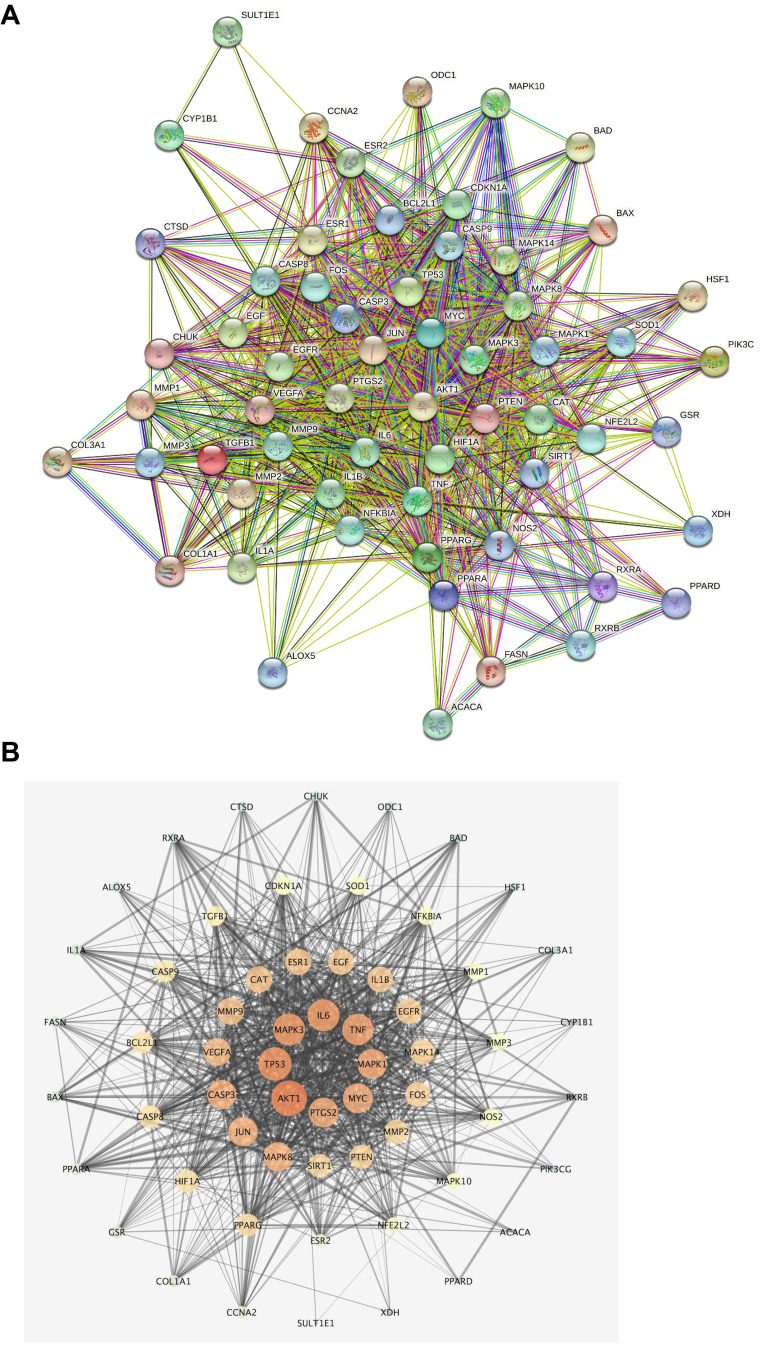
Among the 240 targets of Ba Zhen Tang and 265 genes related to skin photoaging, 60 of them are overlapped, suggesting that these 60 genes may be the key targets that mediate the anti-skin photoaging effects of Ba Zhen Tang, the 60 core target genes, the proteins they code, and their degrees are listed in Table 2. The potential key targets were analyzed by PPI analysis, and the results were saved as a file in TSV format and imported into Cytoscape to draw PPI network (Figure 3A and B).
Table 2.
60 Key Targets of Ba Zhen Tang for Delaying Skin Photoaging
| Gene Names | Protein Names | Degree |
|---|---|---|
| AKT1 | RAC-alpha serine/threonine-protein kinase | 53 |
| TP53 | Cellular tumor antigen p53 | 50 |
| MAPK3 | Mitogen-activated protein kinase 3 | 48 |
| IL6 | Interleukin-6 | 48 |
| TNF | Tumor necrosis factor | 47 |
| MAPK1 | Mitogen-activated protein kinase 1 | 45 |
| PTGS2 | Prostaglandin G/H synthase 2 | 45 |
| MYC | Myc proto-oncogene protein | 45 |
| CASP3 | Caspase-3 | 44 |
| JUN | Transcription factor AP-1 | 44 |
| MAPK8 | Mitogen-activated protein kinase 8 | 44 |
| VEGFA | Vascular endothelial growth factor A | 42 |
| MMP9 | Matrix metalloproteinase-9 | 41 |
| CAT | Catalase | 40 |
| ESR1 | Estrogen receptor | 40 |
| IL1B | Interleukin-1 beta | 39 |
| EGF | Pro-epidermal growth factor | 39 |
| EGFR | Epidermal growth factor receptor | 39 |
| MAPK14 | Mitogen-activated protein kinase 14 | 38 |
| FOS | Proto-oncogene c-Fos | 38 |
| MMP2 | 72 kDa type IV collagenase | 37 |
| PTEN | Phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase | 37 |
| SIRT1 | NAD-dependent protein deacetylase sirtuin-1 | 36 |
| PPARG | Peroxisome proliferator-activated receptor gamma | 35 |
| CASP8 | Caspase-8 | 35 |
| HIF1A | Hypoxia-inducible factor 1-alpha | 35 |
| BCL2L1 | Bcl-2-like protein 1 | 34 |
| CASP9 | Caspase-9 | 33 |
| TGFB1 | Transforming growth factor beta-1 proprotein | 31 |
| SOD1 | Superoxide dismutase | 29 |
| CDKN1A | Cyclin-dependent kinase inhibitor 1 | 29 |
| NFKBIA | NF-kappa-B inhibitor alpha | 28 |
| MMP3 | Stromelysin-1 | 27 |
| MMP1 | Interstitial collagenase | 27 |
| NOS2 | Nitric oxide synthase | 26 |
| MAPK10 | Mitogen-activated protein kinase 10 | 25 |
| NFE2L2 | Nuclear factor erythroid 2-related factor 2 | 24 |
| ESR2 | Estrogen receptor beta | 20 |
| CCNA2 | Cyclin-A2 | 19 |
| PPARA | Peroxisome proliferator-activated receptor alpha | 18 |
| GSR | Glutathione reductase | 18 |
| COL1A1 | Collagen alpha-1(I) chain | 18 |
| BAX | Apoptosis regulator | 17 |
| FASN | Fatty acid synthase | 16 |
| IL1A | Interleukin-1 alpha | 16 |
| ALOX5 | Polyunsaturated fatty acid 5-lipoxygenase | 15 |
| RXRA | Retinoic acid receptor RXR-alpha | 14 |
| CTSD | Cathepsin D | 13 |
| CHUK | Inhibitor of nuclear factor kappa-B kinase subunit alpha | 13 |
| ODC1 | Ornithine decarboxylase | 12 |
| BAD | Bcl2-associated agonist of cell death | 12 |
| HSF1 | Heat shock factor protein 1 | 11 |
| COL3A1 | Collagen alpha-1(III) chain | 9 |
| PIK3CG | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform | 7 |
| RXRB | Retinoic acid receptor RXR-beta | 7 |
| CYP1B1 | Cytochrome P450 1B1 | 7 |
| ACACA | Acetyl-CoA carboxylase 1 | 5 |
| XDH | Xanthine dehydrogenase/oxidase | 5 |
| PPARD | Peroxisome proliferator-activated receptor delta | 5 |
| SULT1E1 | Sulfotransferase 1E1 | 2 |
Figure 3.
PPI network of Ba Zhen Tang against photoaging. (A) The core target PPI network constructed by STRING database. (B) The topology analysis of 60 core target protein networks by Cytoscape and arranged in a ring according to the degree value.
GO Enrichment Analysis
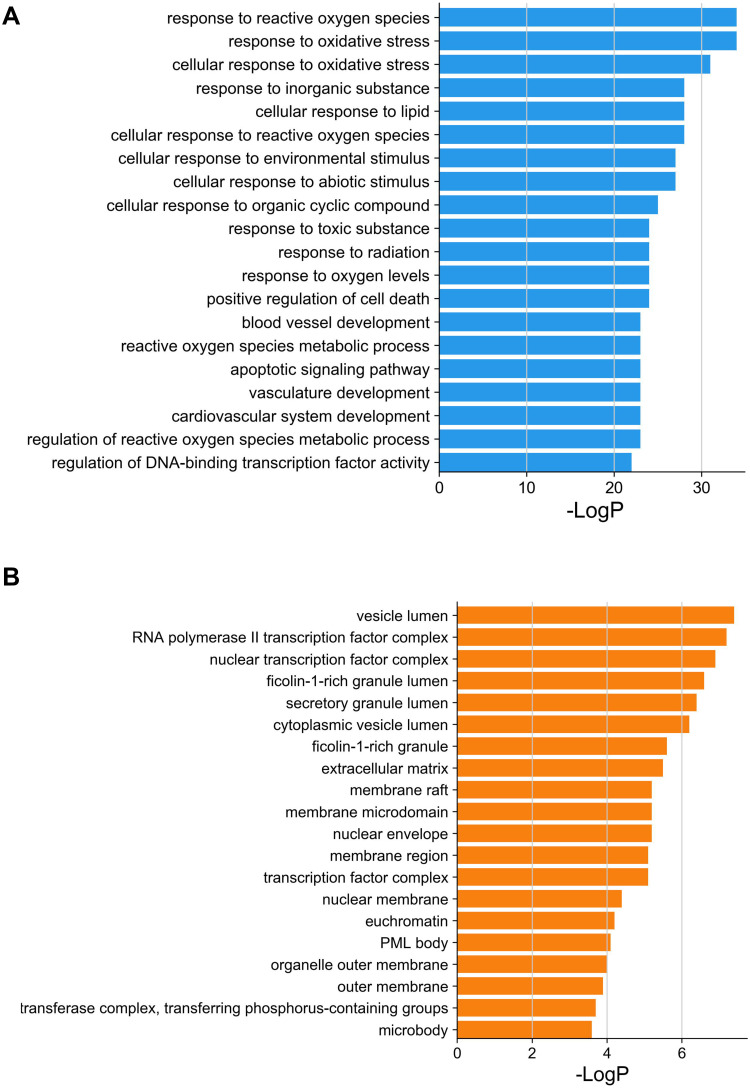
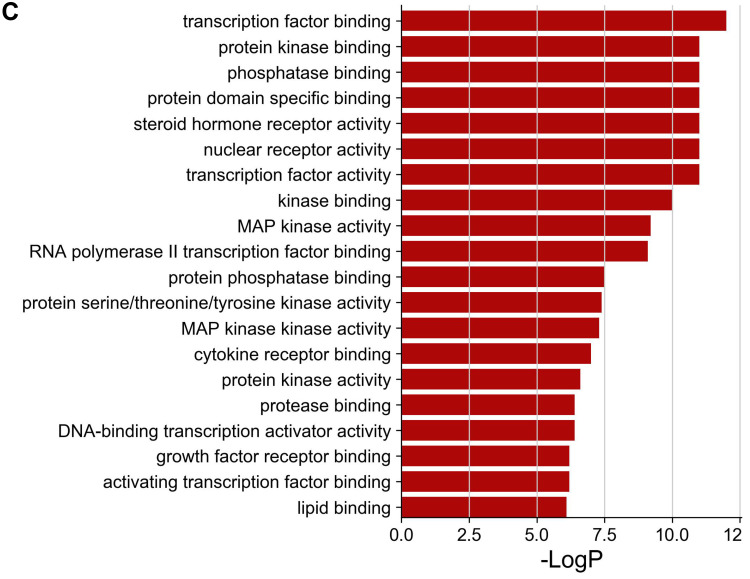
The Metascape database was used for GO enrichment analysis, the number of biological process (BP), cellular component (CC), and molecular function (MF) entries was 1153, 45 and 89, respectively. The top 20 of them were screened and represented as bar graph (Figure 4A–C).
Figure 4.
Continued.
Figure 4.
GO enrichment analysis of putative therapeutic targets. (A) Biological process. (B) Cellular component. (C) Molecular function.
KEGG Pathway Enrichment Analysis and KEGG Mapper
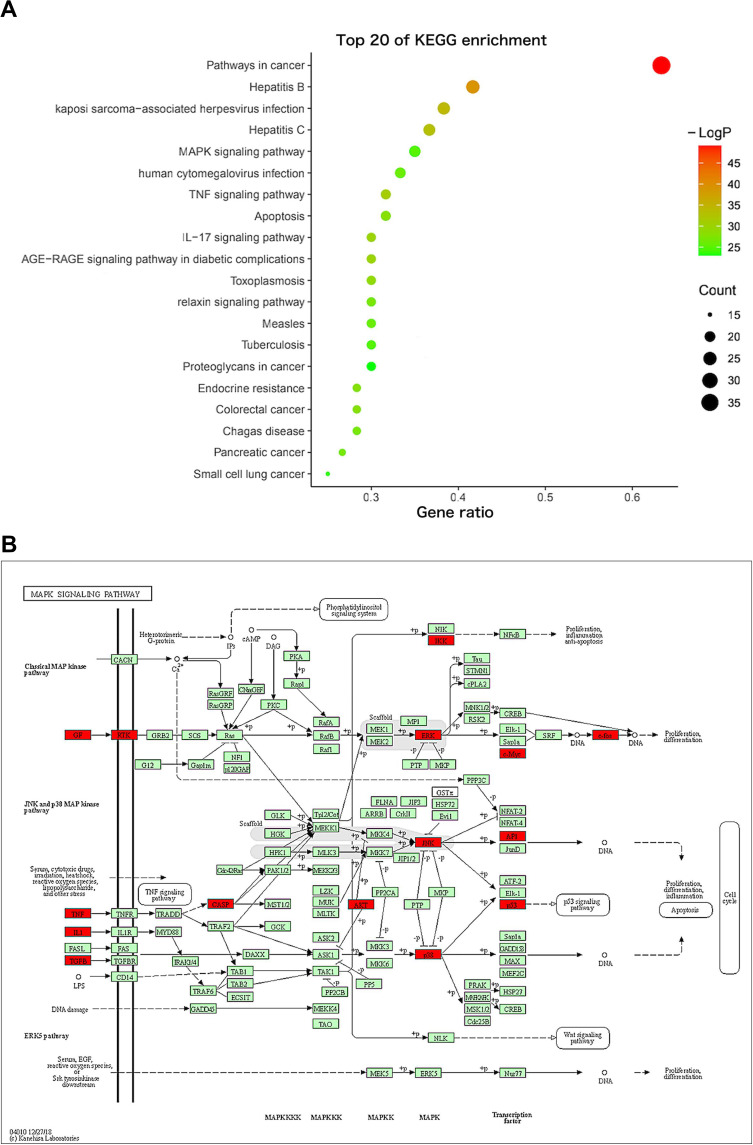
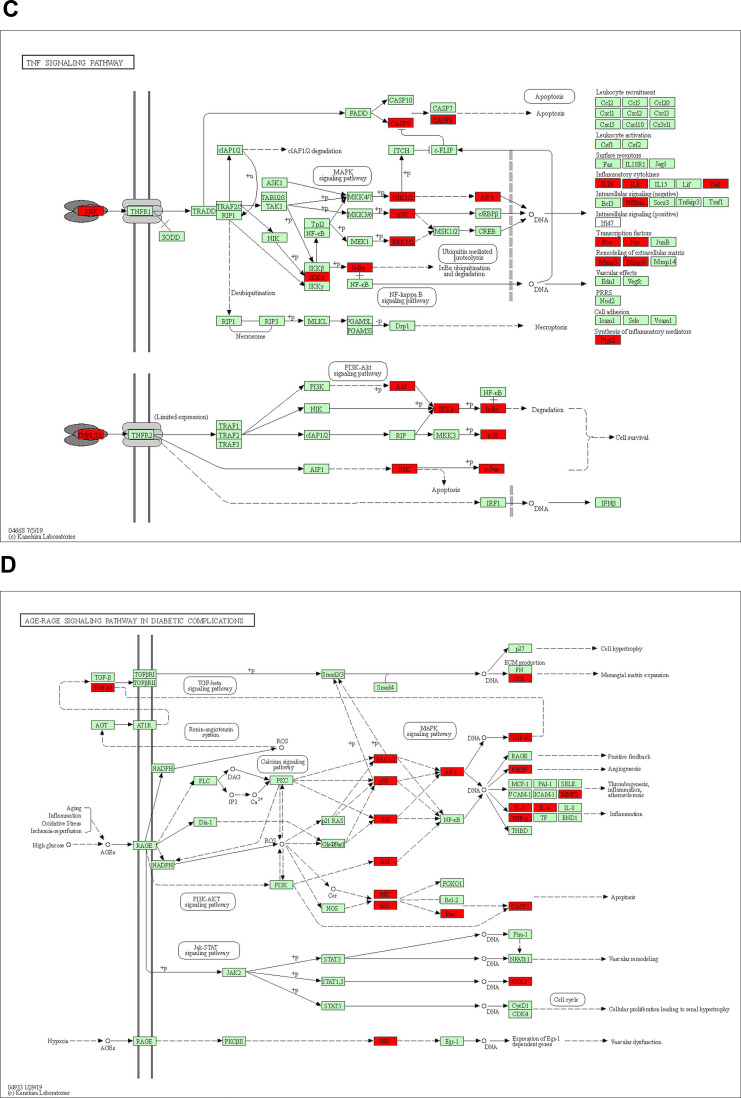
One hundred and fifty-five signaling pathways were identified, and the top 20 entries were selected and represented by a graphical bubble (Figure 5A). The targets of Ba Zhen Tang for anti-photoaging are mainly related to MAPK pathway, TNF pathway, AGE-RAGE signaling pathway in diabetic complications (Figure 5B–D), the IL-17 signal pathway, PI3K-AKT pathway and apoptosis also play an important role in it.
Figure 5.
Continued.
Figure 5.
KEGG pathway enrichment analysis. (A) The ordinate represents the names of the pathways; the abscissa represents the ratio of the number of enriched genes in each pathway to the core genes; the color of the dot corresponds to the P value; the size of the dots reflects the number of genes. (B) MAPK signaling pathway, enriched with 19 core target genes. (C) TNF signaling pathway, enriched with 18 core target genes. (D) AGE-RAGE signaling pathway in diabetic complications, enriched with 18 core target genes.
Molecular Docking
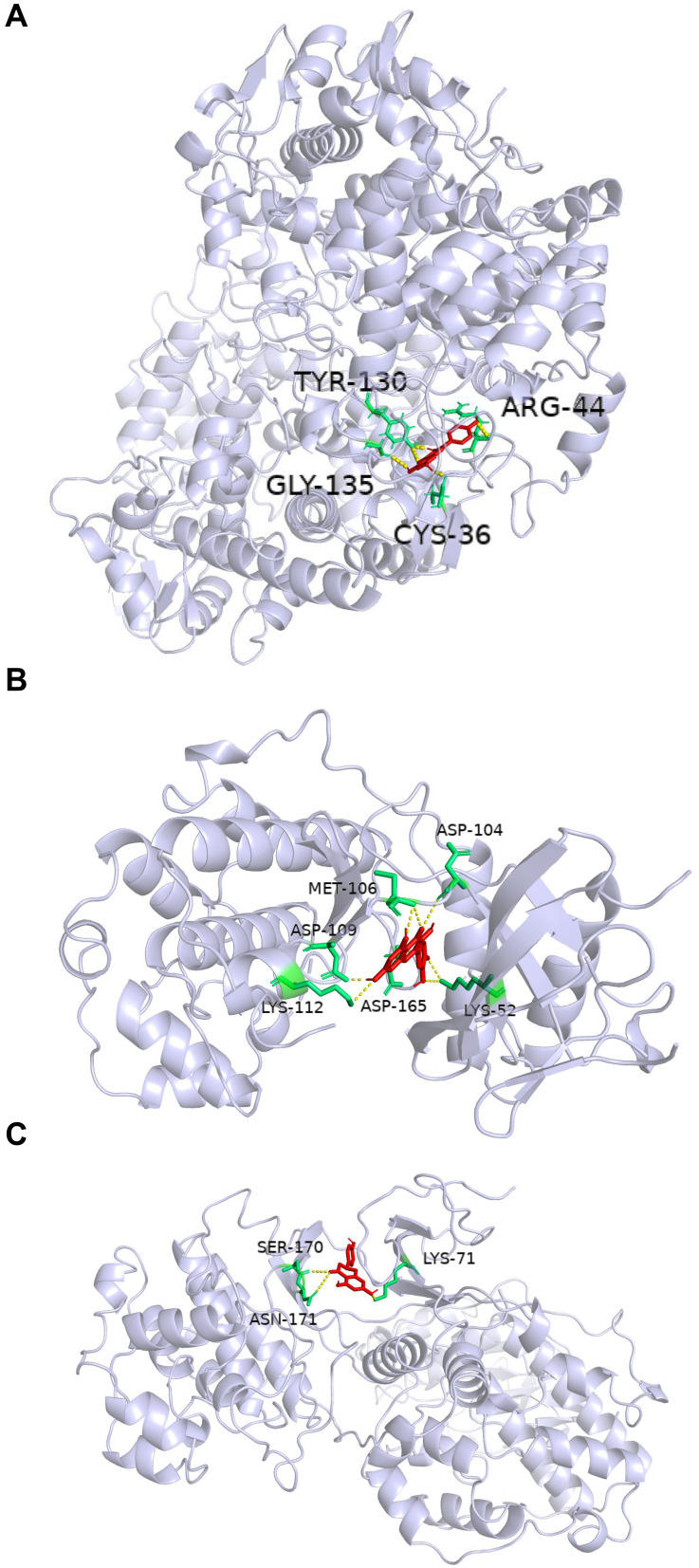
In order to verify the accuracy of prediction target of Ba Zhen Tang active components, the top 10 components of Ba Zhen Tang were docked with the top 10 core targets (Table 3). The binding score is less than −6kcal/mol, which indicates that it is prone to interaction. Receptor protein PTGS2 combines best with all compounds. The other conformations such as Kaempferol, β-sitosterol with CASP3, quercetin with TP53, naringenin with MAPK3, and quercetin, licorice chalcone a, naringenin with MAPK1 also had great contact. We select three representative groups for composition analysis. The conformations of receptor protein PTGS2 and the ligand kaempferol, receptor protein MAPK1 with the ligand quercetin, and receptor protein MAPK3 and the ligand naringenin are shown in Figure 6.
Table 3.
Molecular Docking
| Components | Proteins | Docking Score (-kcal/mol) |
|---|---|---|
| Kaempferol | AKT1 | 6.7 |
| TNF | 6.5 | |
| PTGS2 | 9.3 | |
| CASP3 | 7.2 | |
| JUN | 5.8 | |
| Quercetin | AKT1 | 6.8 |
| TP53 | 7.9 | |
| IL6 | 7.1 | |
| TNF | 7.0 | |
| MAPK1 | 9.3 | |
| PTGS2 | 9.4 | |
| MYC | 6.1 | |
| CASP3 | 7.7 | |
| JUN | 6.0 | |
| Beta-Sitosterol | PTGS2 | 9.5 |
| CASP3 | 7.3 | |
| JUN | 6.5 | |
| Stigmasterol | PTGS2 | 8.1 |
| 7-Methoxy-2-Methyl Isoflavone | PTGS2 | 8.9 |
| Naringenin | AKT1 | 7.0 |
| MAPK3 | 9.1 | |
| MAPK1 | 8.7 | |
| PTGS2 | 9.5 | |
| CASP3 | 7.2 | |
| Formononetin | PTGS2 | 8.9 |
| JUN | 5.6 | |
| Medicarpin | PTGS2 | 8.0 |
| Isorhamnetin | PTGS2 | 8.5 |
| Licochalcone A | MAPK1 | 8.2 |
| PTGS2 | 8.3 |
Figure 6.
Molecular docking diagram. (A) Kaempferol and PTGS2 form hydrogen bonds at TYR-130, ARG-44, GLY-135, and CYS-36. (B) Quercetin and MAPK1 form hydrogen bonds at MET-106, ASP-104, ASP-109, ASP-165, LYS-52, and LYS-112. (C) Naringenin and MAPK3 form hydrogen bonds at LYS-71, ASN-171, and SER-170.
Ba Zhen Tang Treatment Reduces Cell Senescence and p16INK4a Expression in UV-Irradiated HaCaT Cells
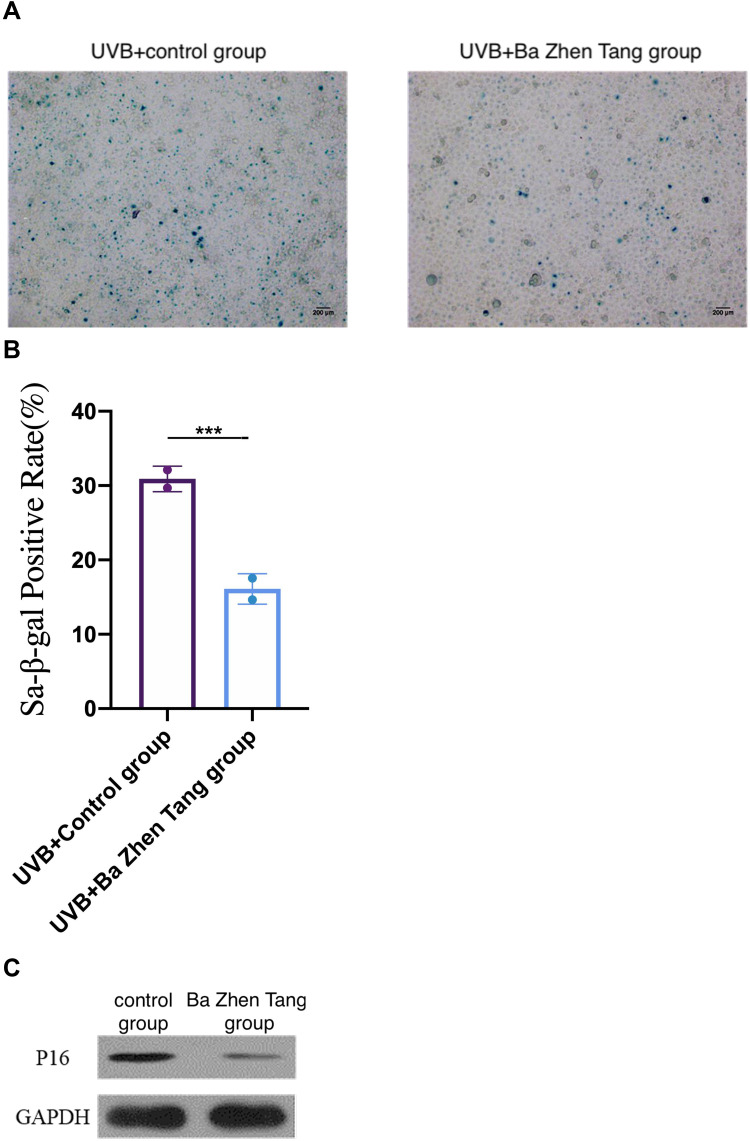
HaCaT cells were pretreated with Ba Zhen Tang serum for 24 h and irradiated by UVB (20 mJ/cm2). Control cells were cultured in the same condition with control serum pretreatment. After 5 days, SA-β-gal staining was utilized for detecting cell senescence. We found that SA-β-gal-positive rate was 30.9 ± 1.21% in the control group, and 16.1 ±1.45% in the Ba Zhen Tang group. SA-β-gal staining was distinctly decreased in Ba Zhen Tang serum treated HaCaT cells compared to control cells (Figure 7A and B).
Figure 7.
Ba Zhen Tang treatment inhibits cell senescence and p16INK4a expression. (A and B) The senescence of Hacat cells treated with Ba Zhen Tang serum was significantly lower than the control group. Scale bar: 200 μm. (C) Compared with the control group, the expression of p16INK4a was significantly decreased in the Ba Zhen Tang group. ***p < 0.001.
Western blot was performed to detect the expression of cell senescence marker p16INK4a. We found that Ba Zhen Tang serum treatment distinctly decreased the expression of p16INK4a in UV-irradiated HaCaT cells compared with control cells (Figure 7C, Table 4).
Table 4.
Expression of p16INK4a in HaCaT Cells of Each Experimental Group (± S)
| Group | p16INK4a/GAPDH Protein |
|---|---|
| Control group | 0.42±0.04* |
| Ba Zhen Tang group | 0.16±0.03 |
Note: *Compared with Ba Zhen Tang group, P < 0.05.
Discussion
In addition to the endogenous aging of the skin, since the skin is the outermost layer of the body, it is easier to suffer from UV, thus induces oxidative stress and photoaging.7 Ba Zhen Tang has the functions of invigorating Qi, nourishing Blood, beautifying skin, it was used to treat Chloasma in Chinese dermatology.25 In addition, Ba Zhen Tang has been proved to regulate the process of oxidative stress, inflammation and apoptosis, therefore, its effect in delaying photoaging and promoting facial rejuvenation is expected, but its mechanism of delaying skin photoaging is not clear. We elucidate the mechanism of Ba Zhen Tang in delaying skin photoaging based on network pharmacology, molecular docking and vitro experiment.
Through collecting the effective components of Ba Zhen Tang, we found that there are many anti-photoaging components, including kaempferol, quercetin, beta-sitosterol and stigmasterol, etc.
Kaempferol is a natural flavonoid, widely distributed in many edible plants, fruits and traditional medicines. It has been shown to have antioxidant, anti-inflammatory, anti-cancer and anti-bacterial effects.26,27 Kaempferol could protect human cells from oxidative damage and apoptosis induced by hydrogen peroxide through the signal pathways of BAX/Bcl-2 and Caspase-3.28 Kaempferol is the precursor of the COQ ring, and Coq is an important component of the mitochondrial electron transport chain, a significant antioxidant present in all cell membranes and associated with many aging diseases.29 Therefore, the addition of Kaempferol may be a potential treatment for photoaging.
Quercetin is a flavonol compound that is widely found in nature and has a variety of biological activities. It is a drug that selectively removes senescent cells.30 Quercetin can increase the activity of AMP-activated protein kinase (AMPK) and reduce the secretion of pro-inflammatory factors, such as IL-8, IFN-β, etc., to achieve the purpose of eliminating aging fibroblasts.31 Inflammation and oxidative stress amplify each other and accelerate the aging of skin tissue. Quercetin has been known to inhibit TNF-α, IL-1β, IL-6 and a large number of NO and ROS secreted by macrophages during aging. The main mechanism is the formation of semi-quinone free radicals between phenolic hydroxyl and radical, and terminates the free radical chain reaction, thus achieving the effect of delaying aging.32
It has been reported that beta-sitosterol plays a protective role in the chemical-induced injury of tissues, organs and apoptosis.33,34 It enters the cell membrane via a ESR1 mediated PI3K/GSK3 beta signal transduction pathway to enhance the resistance to oxidative stress and lipid peroxidation to slow the aging process.35
We found that AKT1, TP53, MAPK3, IL6, TNF, MAPK1, PTGS2, Myc, Casp3 and Jun occupied the central position in the core targets, and they may be the main regulators of Ba Zhen Tang in the treatment of photoaging. The results of molecular docking also proved that they had good binding activity with the main active components of Ba Zhen Tang.
Prostaglandin G/H synthase 2 (PTGS2) is a rate-limiting enzyme in prostaglandin synthesis. PGE2 is the predominant PTGS2-derived prostaglandin present in the oocyte microenvironment during the periconceptional period. Periconceptional PGE2 signaling durably impacts oocytes, conferring increased resistance to spontaneous apoptosis in blastocysts.36 The AKT/protein kinase B signaling channel can regulate cell proliferation and growth, and participate in the cellular process of cell apoptosis. An in vitro study found that when young human serum was used to treat an aging muscle cell model, Akt phosphorylation may increase compared to the older one.37 TP53 is a stress-inducible transcription factor for genes involved in metabolism, autophagy, senescence, apoptosis, cell cycle arrest, and DNA repair.38 Inflammation in the absence of infection is a hallmark of aging,39 After treating mice with Ba Zhen Tang, inflammatory cytokines such as IL6 and TNF reduced.8 Myc is a key transcription factor involved in cell cycle and cell senescence.40 Caspase-3 has been implicated in aging. It can trigger widespread damage and degeneration, playing a key role in causing cell death.41 And c-Jun can be used as an indicator for early diagnosis of photoaging skin.42
GO enrichment analysis comprises three independent ontologies: Biological process, Molecular function and Cellular component. Biological process refers to a biological objective to which the gene or gene product contributes. Molecular function is defined as the biochemical activity. Cellular component refers to the place in the cell where a gene product is active. The relationships between a gene product (or gene-product group) to biological process, molecular function and cellular component are one-to-many, reflecting the biological reality that a particular protein may function in several processes, contain domains that carry out diverse molecular functions, and participate in multiple alternative interactions with other proteins, organelles or locations in the cell.43 GO enrichment analysis showed that Ba Zhen Tang was involved in many biological processes, such as response to reactive oxygen species and oxidative stress, vascular system development and apoptosis, which were closely related to photoaging, the results are consistent with early studies. Ba Zhen Tang acts on cellular components such as vesicular cavity, transcription factor complex and nuclear membrane, with the participation of transcription factors, enzymes, receptors and lipids.
KEGG pathway enrichment analysis mainly involved MAPK signaling pathway, Tumor necrosis factors signaling pathway and AGE-RAGE signaling pathway in diabetic complications. Ba Zhen Tang also has effects on PI3K-AKT signaling pathway, apoptosis and IL-17 signaling pathway, etc., indicating that Ba Zhen Tang can act on skin photoaging through various ways.
One of the most significant metabolic pathways involved in aging is the mitogen-activated protein kinases (MAPK) signaling pathway.44 MAPK signaling pathway exists in most cells and plays a major role in cell proliferation, differentiation, transformation and apoptosis.45 In mammals, the MAPK signaling pathway is divided into four different groups. The c-Jun N-terminal kinase (JNK) pathway, one of the four groups, has been proven to regulate cell proliferation, differentiation, apoptosis and inflammation.46 Tumor necrosis factor α (TNF-α) is an inflammatory mediator overexpressed in the skin as a response to ultraviolet radiation.47 TNFα is known to stimulate JNK, p38 MAPK and NF-κB signal transduction pathways, resulting in IL-6 release.48 Advanced glycation end products (AGEs) are predominantly synthesized during chronic hyperglycemic conditions or aging. Apoptosis, oxidative stress, autophagy, and necroptosis that are considerably infuenced by the AGE-RAGE signaling.49 P38 MAPK pathway is associated with TNF signaling pathway, AGE-RAGE signal pathway in diabetic complications, PI3K-AKT signal pathway and apoptosis, it is suggested that these pathways are essential for delaying photoaging.
Senescence-associated β-galactosidase (SA-β-Gal) is a widely used marker of senescent cells in vitro and in vivo.50 The cell-cycle regulating gene, p16INK4A, encoding an inhibitor of cyclin-dependent kinases 4 and 6, is considered to play an important role in cellular aging and in premature senescence.51 Our study strongly suggests that SA-β-Gal and p16INK4a were decreased in the Ba Zhen Tang serum group compared with the control group. It shows that the serum of mice treated with Ba Zhen Tang can significantly reduce the aging degree of skin tissue in vitro.
Conclusion
The target of Ba Zhen Tang in treating photoaging, gene function analysis and molecular docking predicted by this study are consistent with the existing research reports, it shows the accuracy of target prediction in network pharmacology and molecular docking, and demonstrates the characteristics of multi-component, multi-target and multi-pathway of traditional Chinese medicine preparation. In addition, the location of the target sites and the mapping of the pathways suggest the importance of the MAPK pathway, TNF pathway and AGE-RAGE signal pathway in diabetic complications, it provides a direction for further research on the mechanism of delaying photoaging by Ba Zhen Tang. In the future, more data of Ba Zhen Tang anti-photoaging can be studied, including the vitro experiments to verify the regulation of Ba Zhen Tang on MAPK signaling pathways, TNF signaling pathways and AGE-RAGE signaling pathway in diabetic complications. In addition, through the drug development experiments of Ba Zhen Tang and its active ingredients, more evidence will be provided for the development of drugs to delay photoaging.
Funding Statement
National Natural Science Foundation of China (81674039, 81873347); Key Projects of Scientific Research Fund of Traditional Chinese Medicine of Hubei Health Commission (ZY2021Z011).Training Project Funding Plan of Young-aged Talent of Health System in Hubei Province (2020-2023); Research and Innovation Fund for Graduate Students of Jianghan University (211051001).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zhang S, Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27(5):729–738. doi: 10.1177/0963689717725755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Celia B, Hughes M, Williams GM, Pageon H, Fourtanier A, Green AC. Dietary antioxidant capacity and skin photoaging: a 15-year longitudinal study. J Invest Dermatol. 2021;141(4S):1111–1118. doi: 10.1016/j.jid.2020.06.026 [DOI] [PubMed] [Google Scholar]
- 3.Young AR, Claveau J, Rossi AB. Ultraviolet radiation and the skin: photobiology and sunscreen photoprotection. J Am Acad Dermatol. 2016;76(3S1):S100–S109. doi: 10.1016/j.jaad.2016.09.038 [DOI] [PubMed] [Google Scholar]
- 4.Panich U, Sititithumcharee G, Rathv Iboon N, Jirawatnotai S. Ultraviolet radiation-induced skin aging: the role of DNA damage and oxidative stress in epidermal stem cell damage mediated skin aging. Stem Cells Int. 2016;2016:7370642. doi: 10.1155/2016/7370642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerasymchuk M, Cherkasova V, Kovalchuk O, Kovalchuk I. The role of microRNAs in organismal and skin aging. Int J Mol Sci. 2020;21:15. doi: 10.3390/ijms21155281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xin C, Wang Y, Liu M, Zhang B, Yang S. Correlation analysis between advanced glycation end products detected noninvasively and skin aging factors. J Cosmet Dermatol. 2021;20(1):243–248. doi: 10.1111/JOCD.13452 [DOI] [PubMed] [Google Scholar]
- 7.Poon F, Kang S, Chien AL. Mechanisms and treatments of photoaging. Photodermatol Photoimmunol Photomed. 2015;31(2):65–74. doi: 10.1111/phpp.12145 [DOI] [PubMed] [Google Scholar]
- 8.Song E, Fu J, Xia X, Su C, Song Y. Bazhen decoction protects against Acetaminophen induced acute liver injury by inhibiting oxidative stress, inflammation and apoptosis in mice. PLoS One. 2014;9(9):e107405. doi: 10.1371/journal.pone.0107405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Y, Ge J, Huang C, Liu H, Jiang H. Application of mesenchymal stem cell therapy for aging frailty: from mechanisms to therapeutics. Theranostics. 2021;11(12):5675–5685. doi: 10.7150/THNO.46436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi HZ, Zeng JC, Shi SH, Giannakopoulos H, Zhang QZ, Le AD. Extracellular vesicles of GMSCs alleviate aging-related cell senescence. J Dent Res. 2021;100(3):283–292. doi: 10.1177/0022034520962463 [DOI] [PubMed] [Google Scholar]
- 11.Liu C, Li Q, Yang Y. Effects of the modified bazhen decoction in the treatment of premature ovarian failure in rats. Ann Clin Lab Sci. 2019;49(1):16–22. [PubMed] [Google Scholar]
- 12.Wang X, Wang ZY, Zheng JH, Li S. TCM network pharmacology: a new trend towards combining computational, experimental and clinical approaches. Chin J Nat Med. 2021;19(1):1–11. doi: 10.1016/S1875-5364(21)60001-8 [DOI] [PubMed] [Google Scholar]
- 13.Pinzi L, Rastelli G. Molecular docking: shifting paradigms in drug discovery. Int J Mol Sci. 2019;20(8):1422–1467. doi: 10.3390/ijms20184331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6:13. doi: 10.1186/1758-2946-6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Xing J, Xu Y, et al. In silico ADME/T modelling for rational drug design. Q Rev Biophys. 2015;48(4):488–515. doi: 10.1017/s0033583515000190 [DOI] [PubMed] [Google Scholar]
- 16.Bateman A, Martin M-J, Orchard S. The UniProt consortium. UniProt: the universal protein knowledgebase in 2021.. Nucleic Acids Res. 2021;49(D1):D480–D489. doi: 10.1093/NAR/GKAA1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richards JE, Scott Hawley R.Chapter 11 - The gene hunt: how genetic maps are built and used. In: Richards JE, Scott Hawley R, editors. The Human Genome. 3rd ed. Academic Press; 2011:369–403. [Google Scholar]
- 18.Stelzer G, Rosen N, Plaschkes I, et al. The genecards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinformatics. 2016;54:1.30.1–1.30.33. doi: 10.1002/cpbi.5 [DOI] [PubMed] [Google Scholar]
- 19.Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–D613. doi: 10.1093/nar/gky1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y, Zhou B, Pache L, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10(1):1523. doi: 10.1038/s41467-019-09234-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S, Chen J, Cheng T, et al. PubChem in 2021: new data content and improved web interfaces. Nucleic Acids Res. 2021;49(D1):D1388–D1395. doi: 10.1093/nar/gkaa971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burley SK, Bhikadiya C, Bi C, et al. RCSB Protein Data Bank: powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 2021;49(D1):D437–D451. doi: 10.1093/nar/gkaa1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bunin DI, Chang PY, Doppalapudi RS, et al. Dose-dependent efficacy and safety toxicology of hydroxypyridinonate actinide decorporation agents in rodents: towards a safe and effective human dosing regimen. Radiat Res. 2013;179(2):171–182. doi: 10.1667/RR3115.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang ZW, Lian JW, Jian-wei effective treatment of melasma cases introduced. J Tradit Chin Med. 2011;10:3–4. [Google Scholar]
- 26.Ilk S, Sağlam N, Özgen M, Korkusuz F. Chitosan nanoparticles enhances the anti-quorum sensing activity of kaempferol. Int J Biol Macromol. 2017;94:(Pt A):653–662. doi: 10.1016/j.ijbiomac.2016.10.068 [DOI] [PubMed] [Google Scholar]
- 27.Kashyap D, Sharma A, Tuli HS, Sak K, Punia S, Mukherjee TK. Kaempferol - A dietary anticancer molecule with multiple mechanisms of action: recent trends and advancements. J Funct Foods. 2017;30:203–219. doi: 10.1016/j.jff.2017.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du W, An Y, He X, Zhang D, He W. Protection of kaempferol on oxidative stress-induced retinal pigment epithelial cell damage. Oxid Med Cell Longev. 2018;2018:1610751. doi: 10.1155/2018/1610751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernández-Del-Río L, Soubeyrand E, Basset GJ, Clarke CF. Metabolism of the flavonol kaempferol in kidney cells liberates the B-ring to enter coenzyme Q biosynthesis. Molecules. 2020;25(13):2955. doi: 10.3390/molecules25132955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirkland JL, Tchkonia T. Senolytic drugs: from discovery to translation. J Intern Med. 2020;288(5):518–536. doi: 10.1111/JOIM.13141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewinska A, Adamczyk-Grochala J, Bloniarz D, et al. AMPK-mediated senolytic and senostatic activity of quercetin surface functionalized Fe 3 O 4 nanoparticles during oxidant-induced senescence in human fibroblasts. Redox Biol. 2020;28:101337. doi: 10.1016/j.redox.2019.101337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang J, Diao P, Shu X, Li L, Xiong L. Quercetin and quercitrin attenuates the inflammatory response and oxidative stress in LPS-Induced RAW264.7 cells: in vitro assessment and a theoretical model. Biomed Res Int. 2019;2019:7039802. doi: 10.1155/2019/7039802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koc K, Geyikoglu F, Cakmak O, et al. The targets of β-sitosterol as a novel therapeutic against cardio-renal complications in acute renal ischemia/reperfusion damage. Naunyn-Schmiedeb Arch Phar. 2021;394(3):469–479. doi: 10.1007/S00210-020-01984-1 [DOI] [PubMed] [Google Scholar]
- 34.Parvez MK, Al-Dosari MS, Arbab AH, et al. Hepatoprotective effect of Solanum surattense leaf extract against chemical- induced oxidative and apoptotic injury in rats. BioMed Central. 2019;19(1):154. doi: 10.1186/s12906-019-2553-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi C, Wu F, Zhu XC, Xu J. Incorporation of beta-sitosterol into the membrane increases resistance to oxidative stress and lipid peroxidation via estrogen receptor-mediated PI3K/GSK3beta signaling. Biochim Biophys Acta. 2013;1830(3):2538–2544. doi: 10.1016/j.bbagen.2012.12.012 [DOI] [PubMed] [Google Scholar]
- 36.Nuttinck F, Jouneau A, Charpigny G, et al. Prosurvival effect of cumulus prostaglandin G/H synthase 2/prostaglandin2 signaling on bovine blastocyst: impact on in vivo posthatching development. Biol Reprod. 2017;96(3):531–541. doi: 10.1095/biolreprod.116.145367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen SL, Marshall RN, Edwards SJ, Lord JM, Lavery GG, Breen L. The effect of young and old ex vivo human serum on cellular protein synthesis and growth in an in vitro model of ageing. Am J Physiol Cell Physiol. 2021;321(1):C26–C37. doi: 10.1152/ajpcell.00093.2021 [DOI] [PubMed] [Google Scholar]
- 38.Sica V, Kroemer G. A bidirectional crosstalk between autophagy and TP53 determines the pace of aging. Mol Cell Oncol. 2020;7(5):1769434. doi: 10.1080/23723556.2020.1769434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14(10):576–590. doi: 10.1038/s41574-018-0059-4 [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Li Y, Peng Y, et al. ΔNp63α down-regulates c-Myc modulator MM1 via E3 ligase HERC3 in the regulation of cell senescence. Cell Death Differ. 2018;25(12):2118–2129. doi: 10.1038/s41418-018-0132-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tong Q, Zhang M, Cao X, Xu S, Wang D, Zhao Y. Expression and activation of Daphnia pulex Caspase-3 are involved in regulation of aging. Gene. 2017;634:37–46. doi: 10.1016/j.gene.2017.08.035 [DOI] [PubMed] [Google Scholar]
- 42.Shi LQ, Ruan CL. Expression and significance of MMP-7 c-Jun and c-Fos in rats skin photoaging. Asian Pac J Trop Med. 2013;6(10):768–770. doi: 10.1016/S1995-7645(13)60135-2 [DOI] [PubMed] [Google Scholar]
- 43.Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. Nat Genet. 2000;25(1):25–29. doi: 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moaddel R, Ubaida-Mohien C, Tanaka T, et al. Proteomics in aging research: a roadmap to clinical, translational research. Aging Cell. 2021;20(4):e13325. doi: 10.1111/ACEL.13325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu ZH, Cai M, Xiang J, et al. PI3K/Akt pathway contributes to neuroprotective effect of Tongxinluo against focal cerebral ischemia and reperfusion injury in rats. J Ethnopharmacol. 2016;181:8–19. doi: 10.1016/j.jep.2016.01.028 [DOI] [PubMed] [Google Scholar]
- 46.Kyosseva SV. Targeting MAPK signaling in age-related macular degeneration. Ophthalmol Eye Dis. 2016;8:23–30. doi: 10.4137/OED.S32200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mavrogonatou E, Konstantinou A, Kletsas D. Long-term exposure to TNF-α leads human skin fibroblasts to a p38 MAPK- and ROS-mediated premature senescence. Biogerontology. 2018;19(3–4):237–249. doi: 10.1007/s10522-018-9753-9 [DOI] [PubMed] [Google Scholar]
- 48.Rutting S, Xenaki D, Malouf M, et al. Short chain fatty acids increase TNFα-induced inflammation in primary human lung mesenchymal cells through the activation of p38 MAP kinase. Am J Physiol Lung Cell Mol Physiol. 2019;316(1):L157–L174. doi: 10.1152/ajplung.00306.2018 [DOI] [PubMed] [Google Scholar]
- 49.Waghela BN, Vaidya FU, Ranjan K, Chhipa AS, Tiwari BS, Pathak C. AGE-RAGE synergy influences programmed cell death signaling to promote cancer. Mol Cell Biochem. 2021;476(2):585–598. doi: 10.1007/s11010-020-03928-y [DOI] [PubMed] [Google Scholar]
- 50.Tokmakov AA, Sato KI. Activity and intracellular localization of senescence-associated β-galactosidase in aging Xenopus oocytes and eggs. Exp Gerontol. 2019;119(157–167):1873–6815. doi: 10.1016/j.exger.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 51.Ressler S, Bartkova J, Niederegger H, et al. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell. 2006;5(5):379–389. doi: 10.1111/j.1474-9726.2006.00231.x [DOI] [PubMed] [Google Scholar]