Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) primarily targets lipid-producing cells for viral tropism. In this review, we connect systemic lipid couriers, particularly high-density lipoproteins (HDLs) and exosomes, with the neurological facets of SARS-CoV-2 infection. We discuss how SARS-CoV-2 preferentially targets lipid-secreting cells and usurps host cell lipid metabolism for efficient replication and systemic spreading. Besides providing natural veils for viral materials against host immunity, the inherent properties of some of these endogenous lipid particles to traverse the blood–brain barrier (BBB) also offer alternative routes for SARS-CoV-2 neurotropism. Importantly, virus-driven neurological aberrations mediated by HDLs and exosomes are fueled by lipid rafts, which are implicated in the production and transmigration of these lipid particles across the BBB. Finally, we discuss how repurposing existing drugs targeting lipid rafts and cholesterol homeostasis may be beneficial toward alleviating the global coronavirus disease 2019 (COVID-19) disease burden.
Keywords: SARS-CoV-2, lipids, neurotropism, HDLs, exosomes, COVID-19
Epidemiology and clinical manifestations of neurological symptoms in COVID-19
Growing evidence document neurological complications associated with COVID-19 infection and vaccinations [1., 2., 3.], and the latest studies suggest that coronavirus attacks on the brain are multipronged [4]. Following a first reported case of meningitis associated with SARS-CoV-2 [5], a series of systematic cohort studies further confirmed the neurological impacts of COVID-19. In a nationwide study conducted in the UK, approximately 62% of patients with COVID-19 followed on an online portal exhibited cerebrovascular aftermaths, represented by ischemic stroke, altered mental status, and encephalopathy [1]. A separate study reported that a cluster of infected patients with encephalopathy escalated to a severe form that resembled multiple sclerosis, characterized by deleterious inflammation and loss of myelination in both the brain and spinal cord [6]. Neurological consequences of COVID-19 infection were supported by similar studies conducted in Italy, China, and the USA, which reported symptoms including depression, anxiety, and sleep difficulties in COVID-19 survivors [7., 8., 9.]. Furthermore, the transcriptome profiles in neurons and astrocytes of patients with COVID-19 resemble those of neurological disorders, including schizophrenia and depression [10]. Longitudinal examination of pre- and postinfection individuals revealed reduction in global brain size and a larger cognitive decline compared with noninfected control subjects [11]. In addition to the infection per se, viral containment measures such as physical distancing and lockdown also contribute to neuropsychiatric symptoms associated with COVID-19, principally moderated by social isolation and the feeling of loneliness [12]. Given the scale of the COVID-19 pandemic, it is foreseeable that a staggering number of COVID-19 survivors may be left grappling with debilitating, lifelong neuropsychiatric sequelae from the infection. It is therefore important that research delves into molecular mechanisms governing the neurological impact of SARS-CoV-2 infection. In this review, we sought to provide an overview of how SARS-CoV-2 infection may affect the brain via either direct viral neurotropism or indirect propagation of systemic inflammation – with a central focus on how the virus may exploit host systemic lipid metabolism to achieve neuroinvasion and trigger neurological assaults.
Routes of SARS-CoV-2 neurotropism
Viral entry into the brain generally takes either a transneuronal route initiating from the olfactory mucosa or a hematogenous route implicating the BBB (Box 1). The olfactory mucosa lines the nasal cavity and borders the brain. As the loss of smell is a common clinical presentation of COVID-19 [13], olfactory transmucosal invasion denotes a possible means of SARS-CoV-2 entry into the central nervous system (CNS) [14]. Postmortem examination and autopsy reports of patients with COVID-19 [15,16], however, were discordant with an olfactory route of viral entry. Instead, widespread endothelial dysfunction [15] and the presence of viral particles in the endothelium of frontal lobe tissues was noted [16], suggesting that SARS-CoV-2 might principally undertake a hematogenous route of neurological entry.
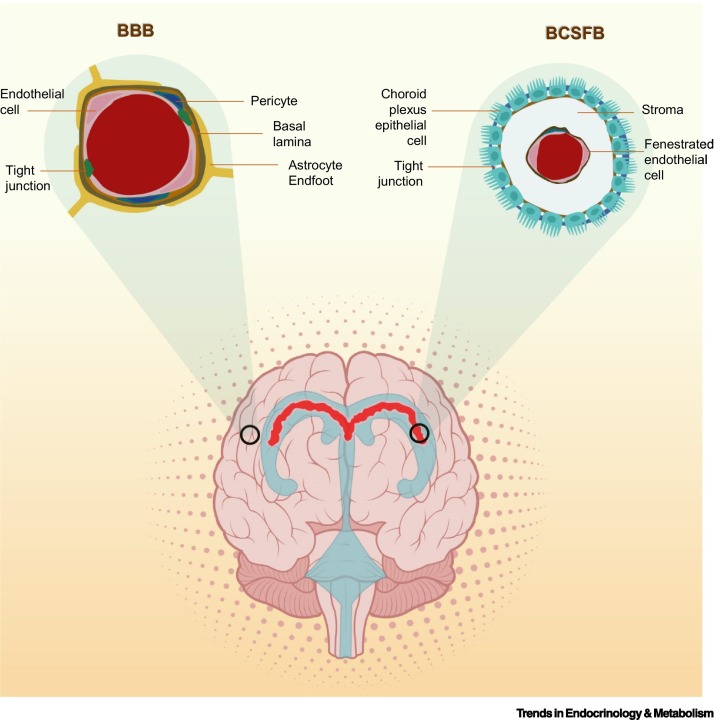
Box 1. Cell types constituting the natural barriers to the brain.
The BBB and BCSFB are two natural barriers that modulate access to the brain. The BBB is a modulated interface separating the CNS from the peripheral circulation. The NVU comprises cells of both vascular and neural origin, including cerebral microvascular endothelial cells and their associated astrocytes, pericytes, neurons, and extracellular matrix [133], which function synergistically to bring about neurovascular coupling. The cross-sectional circumference of a cerebral capillary lumen is enclosed by a single endothelial cell joined by tight junctions, which restricts the paracellular transport of water-soluble substances from the blood into the brain. Pericytes are anatomically attached at irregular intervals to the abluminal side of the cerebral endothelium and confer mechanical support to the endothelial cells. Extracellular matrix constituents, including collagen, heparin sulfate proteoglycans, laminin, fibronectin, and other matrix proteins, form the basal lamina that ensheaths the cerebral vascular endothelium. Astrocytes form specialized extensions (i.e., astrocyte endfoot) that are in contact with and border the basal lamina. The ChP constitutes the interface between the blood and the CSF, which consists of a tight cuboidal epithelium with plentiful villi surrounding a connective stroma. Cerebral blood vessels that comprise fenestrated capillary loops penetrate the stroma centrally, forming the BCSFB. The tight epithelium made up of ChP epithelial cells modulates the fluxes of water-soluble molecules and regulates the molecular composition of the CSF [134].
Alt-text: Box 1
Transcellular and paracellular branches of the hematogenous route
Despite accumulating evidence on the neurotropism of SARS-CoV-2, precisely how the virus navigates across different cell types of the brain, particularly cells of the neurovascular units (NVUs) (Box 1) that constitute the physical barriers delineating the brain parenchyma from the systemic circulation (Figure 1 ), has remained contradictory. A series of recent studies have illuminated the brain cell tropism of SARS-CoV-2 [17., 18., 19.], despite somewhat conflicting results. Lancaster and colleagues demonstrated a predominant expression of angiotensin-converting enzyme 2 ( ACE2 ) (see Glossary) in mature choroid plexus (ChP) cells constituting the blood–cerebrospinal fluid barrier (BCSFB) (Box 1 and Figure 1) relative to other neuron clusters and progenitors [17]. Using SARS-CoV-2 spike pseudovirions expressing GFP and live virus to infect brain organoids, the authors showed prevailing SARS-CoV-2 neurotropism for ChP epithelial cells, particularly subpopulations expressing abundant lipoproteins, and minimal susceptibility of both neurons and glia to SARS-CoV-2 infection. Importantly, SARS-CoV-2 infection triggers apolipoprotein A1 (APOA1) expression and copious formation of lipid vesicles within infected cells [17], indicating that viral entry induces enhanced lipid production and lipoprotein secretion. SARS-CoV-2 infection of the ChP epithelium also damages intercellular tight junctions and overall barrier integrity. Thus, infection of the ChPs promotes both transcellular and paracellular modes of viral neurotropism. Gaining access to the cerebrospinal fluid (CSF) may unlock several other neurological tissues and cell types to viral entry. Reports on the presence of SARS-CoV-2 in the CSF have been conflicting [20., 21., 22.], but, generally, viral materials are detected in the CSF only occasionally. Using brain slices of healthy human brain exposed to SARS-CoV-2, Crunfli et al. showed that a majority of the SARS-CoV-2 spike protein-positive cells (~58%) were also positive for the astrocyte-specific marker glial fibrillary acidic protein, and they concluded that astrocytes denote the major permissive cell type for SARS-CoV-2 infection of the CNS [18]. In another separate study based on pericyte-like cell (PLC)-containing cortical organoids (PCCOs), Gleeson and colleagues observed productive SARS-CoV-2 infection of PCCOs in both PLCs and the neighboring astrocytes that together constitute the NVUs [19]. The discrepancies in neural cell-type tropism across these studies may be associated with different viral quantities and exposure duration, as well as inherent differences in the infection models. Indeed, Lancaster and colleagues observed sparse but specific neuronal and glial infection in sliced organoids challenged with higher viral loads and longer exposure time [17]. Nonetheless, autopsy reports and infection models cumulatively underscore the susceptibility of cells constituting the neurological barriers (i.e., endothelial cells, astrocytes, and pericytes of the BBB and ChP cells of the BCSFB) to SARS-CoV-2 infection and point to a predominantly hematogenous route of SARS-CoV-2 neurotropism.
Figure 1.
Natural barriers of the brain.
Two portals exist for accessing the brain – the BBB and the BCSFB. While endothelial cells comprise the primary cell type for both barriers, transmigration across the latter also involves transit across the ChP cells (see Box 1). Passage of soluble substances and ions across the BBB is modulated by adjoining endothelial cells connected by tight junctions. As the endothelial cells constituting the mature BSCFB are fenestrated, passage of soluble substances is regulated by a tight epithelium comprising ChP cells connected by tight junctions. Abbreviations: BBB, blood–brain barrier; BCSFB, blood–cerebrospinal fluid barrier; ChP, choroid plexus.
SARS-CoV-2 targets lipid metabolism and secretory pathway of host cell
An important prerequisite for viral entry through a hematogenous route is the presence of the virus in blood. SARS-CoV-2 RNA has been detected in serological samples, particularly from severe cases in acute stages [23,24]. To achieve neurotropism, blood-borne viral particles need to evade the host’s surveillance system in addition to thwarting the BBB. Indeed, it is noteworthy that the preferential cell types for viral tropism across different tissues, whether in the peripheral system or the CNS, are predominantly lipid-secreting cells. Outside the CNS, the type II alveolar epithelial cells that denote the primary infection sites in the lungs are responsible for the secretion of pulmonary surfactant composed largely of lipids [25]. In the gut, differentiated enterocytes identified by APOA1, which exhibit highest expression of ACE2, are readily infected by both SARS-CoV and SARS-CoV-2 [26]. Within the CNS, astrocytes and ChP cells, both identified as permissive cells for viral entry in separate studies [17,18], represent the major cellular sources of lipoproteins in the CSF [27]. The accumulation of abundant lipid droplets (LDs) denotes a key cytopathogenic feature of SARS-CoV-2-infected cells [28]. Positive-sense RNA viruses such as SARS-CoV-2 exploit host cell lipid membranes to construct viral replication organelles [29]. A prominent feature of CoV infection is the recruitment of host cell membranes to form replication organelles, which can take varying forms ranging from double-membrane organelles to vesicle pockets, and serve to shield viral RNAs from cellular sensors of the host’s innate immune system [29,30]. Viruses usurp the host’s lipid metabolism to maximize their replication process (Figure 2). SARS-CoV-2 targets LDs, the major intracellular neutral lipid depots, to sustain their replication [31,32]. Pharmacological inhibition of acyl-CoA:diacylglycerol acyltransferase-1 (DGAT-1) that mediates the final step of triacylglycerol (TAG) neutral lipid synthesis curtails SAR-CoV-2 replication and prevents cell death in infected monocytes [31]. Pharmacological inhibition of fatty acid synthesis inhibits the replication of numerous variants of SARS-CoV-2 in vitro [33].
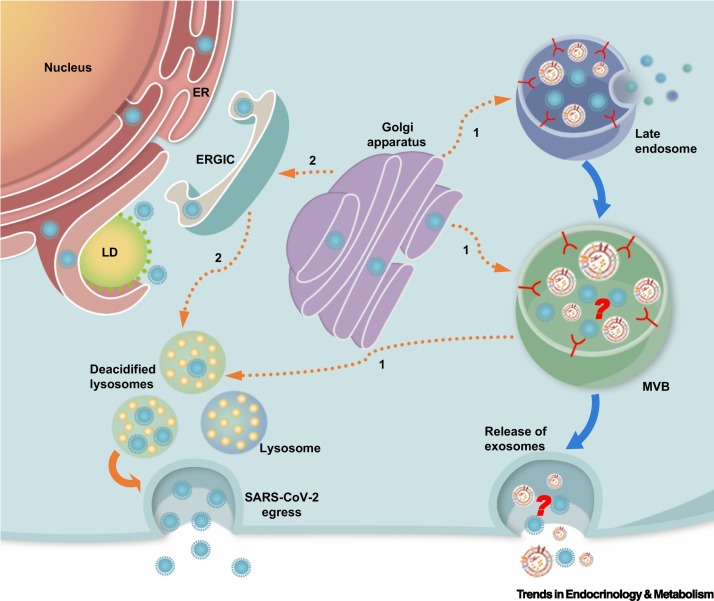
Figure 2.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) targets lipid depot and secretory machinery of host cell.
SARS-CoV-2 usurps the host’s lipid metabolism to maximize the replication process by targeting LDs of host cells, the major cellular neutral lipid depot, to obtain energy substrates for sustaining their replication cycles. The virus also uses the host cell’s secretory machinery for egress. Newly synthesized coronavirus proteins in the ER are packaged into mature virions in Golgi compartments. After reaching the Golgi apparatus, SARS-CoV-2 virions traffic to lysosomes and use exocytic lysosomes to egress. These exosomes are deacidified compared with normal lysosomes that do not contain the virions. The virus may undertake (1) a direct route from the Golgi apparatus to lysosomes via the late endosomes or MVBs (orange broken lines) or may adopt (2) a more roundabout path that involves retrograde transport back to the ER/ERGIC to finally reach the lysosomes (orange broken lines). If SARS-CoV-2 enters lysosomes through the late endosomes or MVBs, it is likely that viral contents and exosomes come into physical contact before the extracellular release of exosomes. Orange unbroken lines indicate the path of SARS-CoV-2 egress; broken lines represent possible paths. Blue unbroken lines represent the path of exosome formation leading to their final extracellular release. Abbreviations: ER, endoplasmic reticulum; ERGIC, ER-Golgi intermediate compartment; LD, lipid droplet; MVB, multivesicular body.
Coronaviruses also exploit the host’s secretory machinery to ensure effective biogenesis, assembly, and export of mature virions to achieve systemic propagation. Indeed, an examination of the SARS-CoV-2 interactome for host-derived virus-interacting proteins uncovered several proteins along the host’s secretory pathway, particularly proteins implicated in the maintenance of endoplasmic reticulum (ER)-Golgi homeostasis [34]. Based on observations using transmission electron microscopy, it was previously assumed that coronaviruses leverage vesicles of the biosynthetic secretory pathway to reach the plasma membrane for extracellular release [35]. Recent work by Altan-Bonnet and colleagues revealed that instead of the normal biosynthetic secretory pathway, β-coronaviruses, including SARS-CoV-2, rely on deacidified lysosomes for unconventional egress [36] (Figure 2). Competitive inhibition of Rab7 GTPase that regulates the maturation of multivesicular bodies (MVBs) into lysosomes effectively blocks viral egress, suggesting that β-coronaviruses may enter lysosomes via late endosomes or MVBs [36,37]. Given the role of MVBs as precursors to exosomes, it is plausible that viral contents contact exosomes before extracellular release.
The cellular tropism of SARS-CoV-2 for lipid-secreting cells indicates that the virus may exploit endogenous lipid materials of different forms, such as lipoproteins and exosomes, as Trojan horses to achieve immune evasion in their systemic spreading. In the following sections, we summarize current literature and discuss the possible involvement of two major forms of endogenous, systemic lipid couriers, namely, lipoproteins and extracellular vesicles (EVs), particularly exosomes, in the neurotropism of SARS-CoV-2.
Lipoproteins
Viewed from an evolutionary perspective, lipoproteins constitute an important aspect of innate immunity on top of its metabolic role in lipid transport [38]. Circulating apolipoproteins serve as the first line of defense against pathogenic invasion in many invertebrate species with an open circulatory system [39]. It is thus not surprising that viruses have evolved ways to evade or even exploit lipoprotein-mediated pathways in infecting their animal hosts. In a comprehensive study examining human proteins that physically interact with each of the SARS-CoV-2 proteins using affinity purification mass spectrometry, lipoprotein metabolism emerged as the top enriched pathway from proteins interacting with SARS-CoV-2-S [40]. The hepatitis C virus (HCV), for example, is a positive-strand RNA virus that circulates in a form bound to TAG-rich lipoproteins termed ‘lipoviroparticles’ [41,42]. HCVs seize the very low-density lipoprotein (VLDL) assembly and export pathway to leave hepatocytes and achieve systemic propagation within mammalian hosts [43], although the precise mechanistic details remain to be settled. Apart from tampering with VLDL machinery, HCVs also exploit HDL uptake via the scavenger receptor class B type I (SR-BI) to enhance cellular entry [44]. In a similar light, Wei et al. showed that SR-BI facilitates ACE-2-dependent entry of SARS-CoV-2 [45]. While SR-BI does not directly bind to SARS-CoV-2 spike protein (SARS-CoV-2-S), the S1 subunit of SARS-CoV-2-S binds to cholesterol and possibly HDL components [45]. SR-BI overexpression in Vero R6 cells increases SARS-CoV-2 infection, and HDLs enhance viral infection in a dose-dependent manner. Therefore, SARS-CoV-2 might exploit the physiological function of SR-BI in promoting cellular cholesterol uptake during its cellular entry and fusion. These findings confer a molecular link between COVID-19 pathogenesis and lipoprotein metabolism and corroborate the reported viral tropism of SARS-CoV-2, considering that the type II alveolar epithelial cells also express SR-BI to mediate a preferential uptake of vitamin E from HDLs over other lipoproteins [46]. Of the lipoproteins in the systemic circulation, only HDLs are able to traverse the BBB [27]. Indeed, the presence of caveolae-localized SR-BI on the apical surfaces of brain capillary endothelial cells facilitates the apical-to-basal transcytosis of HDLs across in vitro models of BBB [47,48] (Figure 3 ). HCVs infect endothelial cells of the BBB, and antibodies against SR-BI abrogated the infection [49]. With regard to the BCSFB, fluorescently tagged human apolipoprotein AI (Apo-AI) intravenously injected into mice was found localized to the ChP and subsequently detected in the CSF, indicating that circulating HDLs may gain access to the CNS predominantly via transmigration through the BCSFB [50]. Thus, the ability of HDLs to transverse the endothelial cells of the BBB and epithelial cells of the BSCFB may render these natural barriers susceptible to SARS-CoV-2 infection in the presence of circulating HDLs, given that HDLs are capable of associating with SARS-CoV-2 viral particles by the interaction between HDL cholesterol (HDL-C) and SARS-CoV-2-S [45].
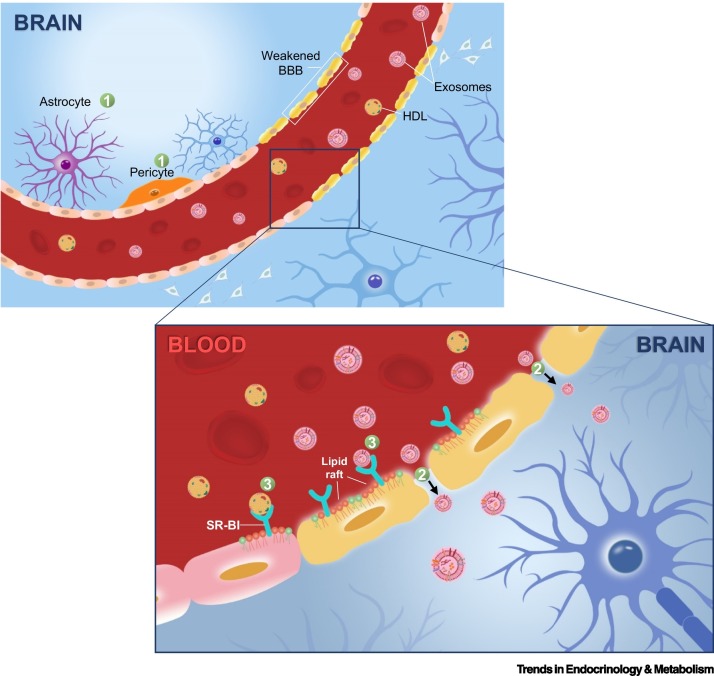
Figure 3.
Hematogenous route of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) neurotropism.
The hematogenous route of viral neurotropism comprises transcellular, paracellular, and Trojan horse modes of entry [116]. Transcellular mode (1) of entry requires viral binding and cellular uptake by specific cell types constituting the natural barriers of the brain, which requires these cells to possess plasma membrane receptors (e.g., ACE2) capable of recognizing and binding to the spike proteins of SARS-CoV-2. Some of these cell types enabling SARS-CoV-2 neurotropism transcellularly, as revealed by viral binding assays based on organoids and sectioned brain slices, include ChP epithelial cells of the BSCFB, as well as pericytes and astrocytes surrounding endothelial cells of the BBB [19., 20., 21.]. The paracellular mode (2) of entry entails a weakening of intercellular tight junctions between the cerebral vascular endothelial cells to facilitate an enhanced diffusion of substances across the BBB. The Trojan horse (3) mode of viral neurotropism typically involves leveraging host cells (e.g., blood cells) or endogenous lipid particles/vesicles, such as HDLs and exosomes that are central to this review, to achieve immune evasion from host’s surveillance in the process of traversing the BBB. The SR-BI receptor localized on lipid raft microdomains mediates the transmigration of HDLs and exosomes across the BBB [95]. Abbreviations: ACE2, angiotensin-converting enzyme 2; BBB, blood–brain barrier; BSCFB, blood–cerebrospinal fluid barrier; ChP, choroid plexus; HDL, high-density lipoprotein; SR-BI, scavenger receptor class B type I.
Mounting evidence indicates a massive remodeling of endogenous lipoprotein profiles upon SARS-CoV-2 infection [51., 52., 53., 54.]. The consensual lipoprotein signatures associated with SARS-CoV-2 infection are reductions in lipoprotein-associated cholesterols, particularly HDLs [55], and general increases in TAGs associated with low-density lipoproteins (LDLs) and VLDLs [51]. A serum profile of low HDL and high TAG before or during hospitalization strongly predicts the transition to severe COVID-19 [56]. Reductions in APOA1 were also predictive of the transition from mild to severe COVID-19 [57], concordant with the progressive reductions in APOA1 and HDL-C in patients with COVID-19 as disease severity increases [53,58]. In fact, a majority of published data on lipoprotein interactions with bacterial and viral infections specifically implicate HDLs [59]. For example, low APOA1 is associated with 30-day mortality in human sepsis patients, likely moderated by the degree of monocyte and platelet activation [60]. Apolipoprotein E (APOE) denotes the major constituent of HDL-like particles in the CNS [61] and is the most abundant apolipoprotein constituent of CSF lipoproteins [27]. Shi and colleagues reported an elevated rate of SARS-CoV-2 infection of neurons and astrocytes with APOE4/4 genotype relative to cells expressing APOE3 [62]. Lipoprotein particles from APOE4 astrocytes tended to be spherical and are often larger in diameter compared with those from APOE3 cultures [63]. Relative to APOE3, APOE4s preferentially bind to plasma lipoproteins with higher TAG content [64]. Thus, APOE4 astrocytes may possess a higher propensity to export TAG-rich lipoproteins, and it remains to be determined if such differences are relevant to their differential susceptibility to SARS-CoV-2 infection. Of note, APOE isoforms also differ in their binding affinities to complex with soluble β-amyloid peptides (Aβ) [65], and this disparity in APOE isoform-specific association has been a focus of considerable research in sporadic late-onset Alzheimer’s disease (AD) [61]. Related to SARS-CoV-2 neurotropism, the LDL scavenger receptor LDLRAD3, which displays neuron-enriched expression and modulates amyloid precursor protein (APP) trafficking in neurons [66], was found to mediate SARS-CoV-2 entry and infection independent of ACE2 [67].
The neurological significance of plasma HDL-C reductions, particularly during the acute phase of COVID-19, deserves further attention. Low plasma HDL-Cs are associated with cognitive decline [61] in both elderly patients with AD [68] and middle-aged, cognitively normal individuals [69]. Conversely, raised HDL-C reduces the risk of late-onset AD [70]. Apart from cognitive performance, anomalies in HDL-C were also reported in patients with psychiatric disorders [71]. Decreases in CSF and serum APOA1 were observed in patients with schizophrenia [72], and lowered HDL-C was also recorded in major depression [73]. Mechanistic details underlying the neurologically beneficial effects of HDLs have yet to be fully understood, but they might be mediated by the inherent anti-inflammatory and antioxidant properties of lipid and/or protein cargoes of HDLs [74,75]. For example, massive remodeling of the HDL proteome upon SARS-CoV-2 infection, such as reduction in paraoxonase 1, renders HDLs from patients with COVID-19 less protective toward endothelial cells stimulated by proinflammatory cytokines compared with controls [76]. Lower circulating levels of HDLs were associated with greater deposition of cerebral β-amyloid (Aβ) in cognitively normal subjects and cognitively impaired elderly subjects independent of APOE4 [50]. HDL-mediated reverse cholesterol transport also serves to alleviate atherosclerotic burden in brain capillaries [47], which promotes the maintenance of normal cerebrovascular function particularly important to regular brain function, given that the brain alone comprises more than 25% of the total vascular network in the body [77]. Despite the associations between HDL-C fluctuations with cognitive health, a clear relationship between brain disorders and genetic defects in HDL assembly has been largely lacking, apart from Tangier disease [61], which implies that the aberrant neural phenotype, in several instances, may be instigated by metabolic perturbations in lipid trafficking instead [61]. Therefore, in addition to the potential role of HDLs in mediating SARS-CoV-2 neurotropism, the extended duration of diminished circulating HDLs, particularly in patients with acute COVID-19, warrants further investigation with respect to both its temporal effects on brain physiology and long-term aftermath in neurological function. Meta-analysis of published literature indicates that dyslipidemia (elevated LDLs and diminished HDLs) is associated with an increased risk of severe COVID-19 [78]. It follows that dyslipidemia may result in exacerbated neurological sequelae, principally mediated by lowered circulating HDLs, which translates to abated scavenging of cholesterol and reduced protection of the endothelium that are detrimental to the normal functioning of the brain.
Exosomes
Emerging evidence has underscored the role of exosomes in intercellular communication, especially pertaining to viral transmission [53,79., 80., 81.]. Intriguingly, exosomes and viruses share common characteristics in terms of their sizes, structures, and mechanisms of cellular production and uptake into recipient cells [82]. Importantly, lipid membrane composition of exosomes bears evident similarities to that of viral particles, especially in the enrichment of raft lipid constituents comprising cholesterol and sphingomyelin (SM) [83]. Preceding studies demonstrated compositional changes in exosomal protein and nucleic acid cargoes, as well as in the membrane lipidomes of circulating exosomes, both along the longitudinal trajectory [81] and across different severity stages of COVID-19 [53,84]. Proteins implicated in immune response and inflammation, as well as that in the activation of coagulation and complement pathways, were elevated in the plasma exosomes of patients with COVID-19 [81,84], which contribute to the pathological basis of the cytokine storm characteristic of severe SARS-CoV-2 infection.
By conferring an endogenous mask to conceal viral cargoes from host immune surveillance, exosomes contribute to immune evasion and facilitate viral propagation in the systemic circulation. For example, GD3 gangliosides on the surface of exosomes are causally linked to the functional arrest of T cells, giving rise to an immunosuppressive microenvironment that favors tumor progression. Functional inhibition of T cells was abrogated by the removal of sialic motifs from GD3-coated exosomes, demonstrating that immunosuppressive properties were conferred specifically by sialylation [85]. In a similar light, Song et al. discovered another type of sialylated lipids, monosialodihexosyl gangliosides (GM3s), as the only class of plasma lipids negatively associated with the counts of T cells and CD4+ T cells in patients with COVID-19 and measured increasing levels of GM3s on plasma exosomes isolated from patients of increasing disease severity [53]. In vitro treatment of alveolar epithelial cell lines (A549) with plasma exosomes from the hyperinflammatory phase of COVID-19 triggers an upregulation of sialic acid metabolism [81]. Enhanced sialylation on nanoparticles was shown to promote BBB permeation [86] by virtue of the electrostatic interactions between negative charges on sialic acids with N-acetylglucosamines enriched in human brain microvascular endothelial cells (HBMECs) constituting the BBB [87]. Moreover, sialic acid modifications allow prolonged residence of sialylated nanoparticles within the brain, possibly attributed to sialic acid receptors in the brain parenchyma [88]. It remains to be determined, however, if the enhanced sialylation observed in COVID-19 exosomes is virus or host driven. Apart from sialic acids, sterolomics identified significantly elevated level of 6-keto-5α-hydroxycholesterol on plasma exosomes from the viral incubation (presymptomatic) phase [81]. 6-Keto-5α-hydroxycholesterol contributes to suppression of immune response by inhibiting natural killer (NK) cell-mediated cytotoxicity [89] and impeding cytolytic action of CD8+ T lymphocytes [90], which may contribute to immune evasion of viral components couriered in exosomes. Indeed, SARS-CoV-2 RNA was detected in exosomes from patients with COVID-19, but not healthy subjects [84]. SARS-CoV-2-S antigen S2 and nucleocapsid proteins are present in the plasma exosomes from symptomatic patients with COVID-19, and immunization of mice with these human-derived exosomes induced the production of antibodies to SARS-CoV-2-S [91].
Sialylation facilitates the systemic spreading of numerous viruses via the hematogenous route, either in masking cellular antigenic sites to suppress immunoreactivity of the host or by promoting viral binding to host surfaces. The N-terminal domain of the S1 subunit of SARS-CoV-2-S contains a flat sialic acid-binding domain capable of interacting with sialoproteins and/or sialylated lipids [92]. Sialic acids, in particular gangliosides on lipid rafts, were proposed as auxiliary attachment coreceptors that act in concert with raft-localized ACE2 to promote the cellular entry of SARS-CoV-2 [93,94]. Besides sialic acids, other lipids on exosomal membranes may also facilitate SARS-CoV-2 entry into host cells. For example, Bohan et al. found that phosphatidylserine (PS) receptors facilitate the internalization of SARS-CoV-2 into the endosomal compartment of host cells via apoptotic mimicry, particularly in situations with low concentrations of the cognate ACE2 receptor [95]. PS was particularly enriched on exosomes from the hyperinflammatory phase of COVID-19 [81], suggesting that exosomal PS may be implicated in host cell entry in addition to the induction of systemic calcification and coagulation. In addition to lipids, proteins on exosomal membranes may also partake in SARS-CoV-2 recognition and binding. CD147 was shown to directly bind to the receptor binding domain of SARS-CoV-2-S with high affinity [96], rendering it a potential alternative receptor in SARS-CoV-2 entry independent of ACE2 [93]. Exosomal levels of CD147 increase in response to hypoxia [97] and hypoxia-induced malignant glioma [97]. Importantly, CD147 exhibited progressive increases in exosome-enriched EVs isolated from the serum of healthy controls to patients with mild and severe COVID-19 [98].
Exosomes also act in trans on distant sites to alter the metabolic status of recipient tissues and cells, thereby mediating intertissue crosstalk that can be either virus or host driven. Exosomes released from infected parent cells retain biological properties to infect and evoke metabolic responses in distant organs and tissues. Kwon et al. showed that exosome-enriched EVs conveying SARS-CoV-2 RNA provided an alternative route of viral entry into human cardiomyocyte cultures and induced an enhanced expression of inflammation-related genes in the recipient cells [99]. In a separate study, plasma exosomes from the hyperinflammatory phase, but not the resolution phase, triggered an enhanced translation of SARS-CoV structural proteins in A549 cells as revealed by RNA sequencing. Relative to the resolution phase, exosomes from the hyperinflammatory phase also induced elevated expression of genes controlling hemostasis in a hepatocyte cell line, in agreement with the disease pathology of exacerbated hypercoagulopathy under the hyperinflammatory phase [81]. Exosomal protein cargoes such as CD147 can mediate neurological insults in ischemic stroke by increasing BBB permeability via microvascular matrix metalloproteinase-9 and promoting microvascular thrombosis, the effects of which were attenuated by treatment with anti-CD147 function-blocking antibody [100]. CD147 is also a cell recognition molecule implicated in neuron–glia interactions [101]. Overexpression of CD147, an additional regulatory subunit of the gamma-secretase (GS) complex [102], increases cellular production of Aβ peptides under hypoxia [97]. Thus, it is evident that SARS-CoV-2 infection leads to drastic modifications in exosomal composition, which promote viral binding, facilitate immune evasion, and induce systemic inflammation and coagulation. Some of these modifications, such as increases in CD147 and presenilin-1 (PS-1) [81], can be potentially detrimental to brain metabolism and cognitive function (see following section). Virus-induced increases in exosomal CD147 and gangliosides bearing sialic acids can enhance exosomal transmigration across the BBB [88,100], thus promoting viral neurotropism. In fact, numerous studies demonstrated the inherent ability of exosomes to traverse the BBB by transcellular migration even in the absence of virus-induced modifications, and exosome transmigration into the brain is further enhanced when the integrity of neurological barriers is compromised under a proinflammatory milieu [103., 104., 105.]. Thus, exosomes carrying viral materials and proinflammatory proteins may cross the BBB thwarted by systemic inflammation with increasing ease, particularly during the hyperinflammatory phase of COVID-19, thereby constituting an alternative route of SARS-CoV-2 neurotropism (Figure 3).
HDLs and exosomes evoke neurological effects dependent on lipid rafts
Lipid rafts are increasingly recognized as membrane portals to pathogens, stemming from observations that a variety of pathogens leverage lipid rafts to achieve host entry [106]. Lipid rafts are not only central to mechanisms underlying the endogenous biogenesis and neurotropism of both HDLs and exosomes but also can fine-tune the cargo composition and the biological properties of the latter, henceforth modulating the neurological effects of exosomes. Nascent HDLs share notable similarities in lipidome composition (i.e., enriched in cholesterol and SMs) with lipid rafts, suggesting that nascent HDLs may be derived from raft-like regions of the plasma membranes [107]. Likewise, exosomes originate from within MVBs that are enriched in cholesterol [108]. A growing body of literature points to the key involvement of lipid raft components in the reorganization of MVBs, which produces intraluminal vesicles that serve as progenitors to exosomes [109]. In terms of neurotropism, it is important to recognize that ACE2 and SR-BI are both localized on lipid rafts [110,111]. Moreover, both ACE2 and SR-BI are expressed on endothelial cells [112,113], and SR-BI facilitates the transcytosis of HDLs across a monolayer of HBMECs [114]. Similarly, transcellular migration of exosomes across HBMECs proceeds via lipid raft-mediated endocytosis [105]. Lipid raft content of exosomes modulates the preferential localization of raft-associated membrane proteins such as PS-1. An enhanced level of raft lipids elevates the anisotropy (or reduces the fluidity) of exosomal membranes, which enhances the localization of PS-1 onto plasma exosomes in the hyperinflammatory phase of COVID-19 [81]. GS cleaves NOTCH-1 and induces interleukin-6 production in a positive feedback loop, perpetuating systemic inflammation that underlies the hyperinflammatory phase of COVID-19 [115]. GS also cleaves APP to generate Aβ peptides [116] implicated in cognitive impairment [117]. An enhanced level of plasma Aβ40 peptides in the hyperinflammatory phase relative to the resolution phase was measured in a small set of patients with COVID-19 [81]. Thus, lipid raft content alters the biological properties of exosomes and may enhance their transmigration across the BBB to impinge on brain metabolism.
Spillover effects from systemic inflammation
It is also important to recognize that SARS-CoV-2 can trigger neurological assaults even without the viruses actually crossing the BBB and entering the brain. Increased pathological infiltration of circulating immune cells into the brain was observed in patients with COVID-19 [118]. Systemic inflammation in the peripheral circulation can inundate the CNS through proinflammatory relay mechanisms [10]. Neurological complications may result from the pathological leakage of peripheral proteins and lipids, particularly for metabolites that are inherently able to traverse the BBB. An illustrative example is plasma lysophosphatidylcholines (LPCs) that were progressively increased in patients with COVID-19 of increasing severity [53]. LPCs elicit proinflammatory effects on vascular tissues [119] and are able to traverse BBB via the Mfsd2a transporter [120]. Several proteins of the complement cascade were also significantly increased in the circulation of patients with COVID-19 [54,81]. While most complement proteins do not normally traverse the BBB, they can gain neural access when the BBB is compromised by systemic inflammation, inducing collateral damage to the brain and its associated vasculature [121].
Repurposing drugs targeting lipid metabolism for COVID-19
Drug repurposing offers an expedited track to alleviate the rapidly expanding disease burden imposed by COVID-19. Given the extensive involvement of lipids in COVID-19 pathogenesis, it may be useful to evaluate lipid-targeting drugs for treating COVID-19 and its neurological sequelae. While upstream pathways triggered by SARS-CoV-2 infection may differ from those of other viruses, downstream dysregulation of host metabolism leading to adverse outcomes may converge on common pathways [122]. Therefore, targeting host lipid metabolism represents a feasible long-term approach against COVID-19 and other viral infections. As SARS-CoV-2 targets host membrane lipids for cellular entry and replication, antivirals that interfere with membrane lipid homeostasis may deter viral infection. In this regard, snake venom phospholipase A2 (PLA2) exhibited potent virucidal effects attributed to their lipolytic activity on membrane phospholipids [123]. Indeed, enhanced LPC production in patients with COVID-19 may have resulted from induction of endogenous PLA2 activity against SARS-CoV-2 [53]. Bemcentinib, which is orally bioavailable and currently in Phase 2 trials for non-small-cell lung cancer, may deter SARS-CoV-2 cellular entry via inhibition of PS receptors [95]. Various pharmacological inhibitors targeting fatty acid and neutral lipid biogenesis, including orlistat, triacsin C [30], and A922500 [31], hold potential for curbing SARS-CoV-2 replication within infected host cells. Drugs targeting lipid rafts that fuel the Trojan horses, such as glycyrrhizic acid that induces cholesterol-dependent disruption to lipid rafts, may also be useful. Glycyrrhizic acid is a natural product currently used for treating viral hepatitis and cutaneous inflammation [124]. Some statins, such as rosuvastatin and simvastatin, capable of raising HDL-C [125] and minimizing the duration of diminished HDL-C in patients with COVID-19, may be favorable toward neurological outcome. Reports on the outcome of statin use in patients with COVID-19, however, have been conflicting [126,127]. As HDLs also mediate ACE2-dependent SARS-CoV-2 entry [45], the precise timing of drug application in consideration of disease course may be crucial to maximize the potentially beneficial aspects of statins.
Concluding remarks
With the rising toll of patients with COVID-19 encumbered with neurological manifestations, it is imperative to develop better diagnostic tools that can delineate high-risk patients, hopefully at an earlier time point from the inception of neurological symptoms, in order to leave a wider window for therapeutic intervention. On top of conventional clinical indices of systemic inflammation, omics-driven technologies provide a comprehensive array of additional, noninvasive metabolite markers in the peripheral circulation [128] that may better reflect the extent of damage specific to the CNS, particularly important during the hyperinflammatory phase of the disease. To this end, metabolites and lipids of predominantly neurological origin are promising candidates to convey brain-specific aberrations. 24S-Hydroxycholesterol (24S-OHC), for instance, is produced via CYP46A1 that is exclusively localized to neurons in the brain and retina [129] and provides an indirect correlate to the number of metabolically active neurons in the brain. Spillover of 24S-OHC into the peripheral circulation reflects BBB integrity. Given the neurological implications of diminished HDLs during the course of COVID-19, it is also worthy to examine if the extent of HDL reduction correlates to neurological outcome in survivors on a long-term basis. Nonetheless, the COVID-19 pandemic has largely suspended in-person visits and displaced several clinical follow-ups as the pandemic extends in scale. The pandemic may accelerate a changing mindset toward use of remote assessment and in-home study interventions in clinical research [130], which will be particularly important to resume translational research under the extended constraints imposed by COVID-19. Questions also remain pertaining to whether lipoproteins and exosomes are effective couriers of the full SARS-CoV-2 virus or viral materials in the peripheral circulation and if engineering the content of these lipid particles confers additional ways to combat against the virus-driven Trojan horses. For example, exosomes from mesenchymal stem cells are proposed as immunomodulatory agents for treating COVID-19 [131]. In addition, convalescent plasma therapy improves clinical symptoms in patients with severe COVID-19 [132]. Given the distinct compositional profiles and biological effects evoked by plasma exosomes isolated from successive phases of COVID-19 [81], exosomes from resolution stages may confer useful insights pertaining to exosome engineering against COVID-19 (see Outstanding questions).
Outstanding questions.
Are endogenous lipid couriers such as HDLs and exosomes effective carriers for SARS-CoV-2 and/or its viral materials? Are these lipid particles infectious by themselves? Because SARS-CoV-2-S binds to cholesterol, does SARS-CoV-2 associate with HDLs in the circulation to form lipoviral particles?
Can we engineer exosomes with specific cargoes and lipid membrane compositions to combat against virus-driven Trojan horses in the peripheral circulation?
Can conventional plasma lipid indices (e.g., HDL-C) and/or plasma levels of metabolites/lipids that originate primarily from the CNS (e.g., 24-OHC) serve as early prognostic indicators of neurological outcome in patients with COVID-19?
Alt-text: Outstanding questions
Acknowledgments
Acknowledgments
This work was financially supported by grants from the National Natural Science Foundation of China (NSFC92057202 to G.S., NSFC 91954207 to X.H.), the National Key R&D Program of China (2018YFA0506900, 2018YFA0800901 to G.S.), and the Agilent Applications and Core Technology – University Research Grant (to G.S.). The authors thank Xiaojie Liu from LipidALL Technologies for artistic illustrations.
Declaration of interests
S.M.L. is an employee of LipidALL Technologies. G.S. receives research funding from the Agilent Applications and Core Technology - University Research. The other author declares no competing interests.
Glossary
- Angiotensin-converting enzyme 2 (ACE2)
cognate receptor for SARS-CoV-2 spike protein that mediates SAR-CoV-2 entry into host cell.
- Exosomes
a subtype of extracellular vesicles (EVs), approximately 30–150 nm in diameter, released by the retrograde transport and fusion of multivesicular bodies with the plasma membrane [135]. In our discussion on the role of EVs in this work, we refer to exosomes (or exosome-enriched EV fractions) generated along the endocytic pathway and not ectosomes that directly bud from the plasma membrane.
- Lipid rafts
specialized, nanoscale dynamic domains on membranes with localized enrichment in cholesterol, sphingolipids, and glycosphingolipids, modulating a myriad of biological processes such as cell signaling and membrane trafficking by recruiting specific membrane-associated proteins [136].
- Presenilin-1 (PS-1)
one of the four core proteins and catalytic subunit of the gamma-secretase (GS) complex that mediates the production of Aβ peptides from amyloid precursor protein.
References
- 1.Varatharaj A., et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7:875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marshall M. How COVID-19 can damage the brain. Nature. 2020;585:342–343. doi: 10.1038/d41586-020-02599-5. [DOI] [PubMed] [Google Scholar]
- 3.Patone M., et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat. Med. 2021;27:2144–2153. doi: 10.1038/s41591-021-01556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall M. COVID and the brain: researchers zero in on how damage occurs. Nature. 2021;595:484–485. doi: 10.1038/d41586-021-01693-6. [DOI] [PubMed] [Google Scholar]
- 5.Moriguchi T., et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int. J. Infect. Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paterson R.W., et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazza M.G., et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav. Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taquet M., et al. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang A.C., et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature. 2021;595:565–571. doi: 10.1038/s41586-021-03710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douaud G., et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604:697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Numbers K., Brodaty H. The effects of the COVID-19 pandemic on people with dementia. Nat. Rev. Neurol. 2021;17:69–70. doi: 10.1038/s41582-020-00450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lechien J.R., et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur. Arch. Otorhinolaryngol. 2020;277:2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meinhardt J., et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021;24:168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 15.Bryce C., et al. Pathophysiology of SARS-CoV-2: the Mount Sinai COVID-19 autopsy experience. Mod. Pathol. 2021;34:1456–1467. doi: 10.1038/s41379-021-00793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paniz-Mondolfi A., et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J. Med. Virol. 2020;92:699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pellegrini L., et al. SARS-CoV-2 infects the brain choroid plexus and disrupts the blood-CSF barrier in human brain organoids. Cell Stem Cell. 2020;27:951–961.e5. doi: 10.1016/j.stem.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crunfli F., et al. Morphological, cellular and molecular basis of brain infection in COVID-19 patients. medRxiv. 2022 doi: 10.1101/2020.10.09.20207464. Published online January 20, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L., et al. A human three-dimensional neural-perivascular ‘assembloid’ promotes astrocytic development and enables modeling of SARS-CoV-2 neuropathology. Nat. Med. 2021;27:1600–1606. doi: 10.1038/s41591-021-01443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Y.H., et al. SARS-CoV-2 detected in cerebrospinal fluid by PCR in a case of COVID-19 encephalitis. Brain Behav. Immun. 2020;87:149. doi: 10.1016/j.bbi.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellon M., et al. Cerebrospinal fluid features in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reverse transcription polymerase chain reaction (RT-PCR) positive patients. Clin. Infect. Dis. 2021;73:e3102–e3105. doi: 10.1093/cid/ciaa1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Destras G., et al. Systematic SARS-CoV-2 screening in cerebrospinal fluid during the COVID-19 pandemic. Lancet Microbe. 2020;1 doi: 10.1016/S2666-5247(20)30066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng L., et al. SARS-CoV-2 can be detected in urine, blood, anal swabs, and oropharyngeal swabs specimens. J. Med. Virol. 2020;92:1676–1680. doi: 10.1002/jmv.25936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersson M.I., et al. SARS-CoV-2 RNA detected in blood products from patients with COVID-19 is not associated with infectious virus. Wellcome Open Res. 2020;5:181. doi: 10.12688/wellcomeopenres.16002.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goerke J. Pulmonary surfactant: functions and molecular composition. Biochim. Biophys. Acta. 1998;1408:79–89. doi: 10.1016/s0925-4439(98)00060-x. [DOI] [PubMed] [Google Scholar]
- 26.Lamers M.M., et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50–54. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H., Eckel R.H. What are lipoproteins doing in the brain? Trends Endocrinol. Metab. 2014;25:8–14. doi: 10.1016/j.tem.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nardacci R., et al. Evidences for lipid involvement in SARS-CoV-2 cytopathogenesis. Cell Death Dis. 2021;12:263. doi: 10.1038/s41419-021-03527-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolff G., et al. Double-membrane vesicles as platforms for viral replication. Trends Microbiol. 2020;28:1022–1033. doi: 10.1016/j.tim.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams C.G., et al. Inhibitors of VPS34 and fatty-acid metabolism suppress SARS-CoV-2 replication. Cell Rep. 2021;36 doi: 10.1016/j.celrep.2021.109479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dias S.S.G., et al. Lipid droplets fuel SARS-CoV-2 replication and production of inflammatory mediators. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1009127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bosch M., et al. Mammalian lipid droplets are innate immune hubs integrating cell metabolism and host defense. Science. 2020;370 doi: 10.1126/science.aay8085. [DOI] [PubMed] [Google Scholar]
- 33.Chu J., et al. Pharmacological inhibition of fatty acid synthesis blocks SARS-CoV-2 replication. Nat. Metab. 2021;3:1466–1475. doi: 10.1038/s42255-021-00479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sicari D., et al. Role of the early secretory pathway in SARS-CoV-2 infection. J. Cell Biol. 2020;219 doi: 10.1083/jcb.202006005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tooze J., et al. Sorting of progeny coronavirus from condensed secretory proteins at the exit from the trans-Golgi network of AtT20 cells. J. Cell Biol. 1987;105:1215–1226. doi: 10.1083/jcb.105.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghosh S., et al. β-Coronaviruses use lysosomes for egress instead of the biosynthetic secretory pathway. Cell. 2020;183:1520–1535 e14. doi: 10.1016/j.cell.2020.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X., et al. Actively or passively deacidified lysosomes push β-coronavirus egress. Cell Death Dis. 2021;12:235. doi: 10.1038/s41419-021-03501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meilhac O., et al. High-density lipoproteins are bug scavengers. Biomolecules. 2020;10:598. doi: 10.3390/biom10040598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niere M., et al. Insect immune activation by recombinant Galleria mellonella apolipophorin III(1) Biochim. Biophys. Acta. 1999;1433:16–26. doi: 10.1016/s0167-4838(99)00148-x. [DOI] [PubMed] [Google Scholar]
- 40.Gordon D.E., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Negro F. Abnormalities of lipid metabolism in hepatitis C virus infection. Gut. 2010;59:1279–1287. doi: 10.1136/gut.2009.192732. [DOI] [PubMed] [Google Scholar]
- 42.Aizawa Y., et al. Chronic hepatitis C virus infection and lipoprotein metabolism. World J. Gastroenterol. 2015;21:10299–10313. doi: 10.3748/wjg.v21.i36.10299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Icard V., et al. Secretion of hepatitis C virus envelope glycoproteins depends on assembly of apolipoprotein B positive lipoproteins. PLoS One. 2009;4 doi: 10.1371/journal.pone.0004233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dreux M., et al. Receptor complementation and mutagenesis reveal SR-BI as an essential HCV entry factor and functionally imply its intra- and extra-cellular domains. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei C., et al. HDL-scavenger receptor B type 1 facilitates SARS-CoV-2 entry. Nat. Metab. 2020;2:1391–1400. doi: 10.1038/s42255-020-00324-0. [DOI] [PubMed] [Google Scholar]
- 46.Kolleck I., et al. HDL is the major source of vitamin E for type II pneumocytes. Free Radic. Biol. Med. 1999;27:882–890. doi: 10.1016/s0891-5849(99)00139-2. [DOI] [PubMed] [Google Scholar]
- 47.Panzenboeck U., et al. ABCA1 and scavenger receptor class B, type I, are modulators of reverse sterol transport at an in vitro blood-brain barrier constituted of porcine brain capillary endothelial cells. J. Biol. Chem. 2002;277:42781–42789. doi: 10.1074/jbc.M207601200. [DOI] [PubMed] [Google Scholar]
- 48.Balazs Z., et al. Uptake and transport of high-density lipoprotein (HDL) and HDL-associated α-tocopherol by an in vitro blood-brain barrier model. J. Neurochem. 2004;89:939–950. doi: 10.1111/j.1471-4159.2004.02373.x. [DOI] [PubMed] [Google Scholar]
- 49.Fletcher N.F., et al. Hepatitis C virus infects the endothelial cells of the blood-brain barrier. Gastroenterology. 2012;142:634–643.e6. doi: 10.1053/j.gastro.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stukas S., et al. Intravenously injected human apolipoprotein A-I rapidly enters the central nervous system via the choroid plexus. J. Am. Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bruzzone C., et al. SARS-CoV-2 infection dysregulates the metabolomic and lipidomic profiles of serum. iScience. 2020;23 doi: 10.1016/j.isci.2020.101645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu G., et al. Lipoprotein-associated phospholipase A2 activity and mass as independent risk factor of stroke: a meta-analysis. Biomed. Res. Int. 2019;2019 doi: 10.1155/2019/8642784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song J.W., et al. Omics-driven systems interrogation of metabolic dysregulation in COVID-19 pathogenesis. Cell Metab. 2020;32:188–202.e5. doi: 10.1016/j.cmet.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen B., et al. Proteomic and metabolomic characterization of COVID-19 patient sera. Cell. 2020;182:59–72.e15. doi: 10.1016/j.cell.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Casari I., et al. Dissecting lipid metabolism alterations in SARS-CoV-2. Prog. Lipid Res. 2021;82 doi: 10.1016/j.plipres.2021.101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masana L., et al. Low HDL and high triglycerides predict COVID-19 severity. Sci. Rep. 2021;11:7217. doi: 10.1038/s41598-021-86747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nie S., et al. Metabolic disturbances and inflammatory dysfunction predict severity of coronavirus disease 2019 (COVID-19): a retrospective study. medRxiv. 2020 doi: 10.1101/2020.03.24.20042283. Published online March 26, 2020. [DOI] [Google Scholar]
- 58.Hu X., et al. Declined serum high density lipoprotein cholesterol is associated with the severity of COVID-19 infection. Clin. Chim. Acta. 2020;510:105–110. doi: 10.1016/j.cca.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kocar E., et al. Cholesterol, lipoproteins, and COVID-19: basic concepts and clinical applications. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2021;1866 doi: 10.1016/j.bbalip.2020.158849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barlage S., et al. Changes in HDL-associated apolipoproteins relate to mortality in human sepsis and correlate to monocyte and platelet activation. Intensive Care Med. 2009;35:1877–1885. doi: 10.1007/s00134-009-1609-y. [DOI] [PubMed] [Google Scholar]
- 61.Vitali C., et al. HDL and cholesterol handling in the brain. Cardiovasc. Res. 2014;103:405–413. doi: 10.1093/cvr/cvu148. [DOI] [PubMed] [Google Scholar]
- 62.Wang C., et al. ApoE-isoform-dependent SARS-CoV-2 neurotropism and cellular response. Cell Stem Cell. 2021;28:331–342.e5. doi: 10.1016/j.stem.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fagan A.M., et al. Unique lipoproteins secreted by primary astrocytes from wild type, apoE−/−, and human apoE transgenic mice. J. Biol. Chem. 1999;274:30001–30007. doi: 10.1074/jbc.274.42.30001. [DOI] [PubMed] [Google Scholar]
- 64.Weisgraber K.H. Apolipoprotein E distribution among human plasma lipoproteins: role of the cysteine-arginine interchange at residue 112. J. Lipid Res. 1990;31:1503–1511. [PubMed] [Google Scholar]
- 65.Strittmatter W.J., et al. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A. 1993;90:8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ranganathan S., et al. LRAD3, a novel low-density lipoprotein receptor family member that modulates amyloid precursor protein trafficking. J. Neurosci. 2011;31:10836–10846. doi: 10.1523/JNEUROSCI.5065-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu S., et al. Genome-wide CRISPR activation screen identifies candidate receptors for SARS-CoV-2 entry. Sci. China Life Sci. 2022;65:701–717. doi: 10.1007/s11427-021-1990-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Merched A., et al. Decreased high-density lipoprotein cholesterol and serum apolipoprotein AI concentrations are highly correlated with the severity of Alzheimer’s disease. Neurobiol. Aging. 2000;21:27–30. doi: 10.1016/s0197-4580(99)00103-7. [DOI] [PubMed] [Google Scholar]
- 69.Singh-Manoux A., et al. Low HDL cholesterol is a risk factor for deficit and decline in memory in midlife: the Whitehall II study. Arterioscler. Thromb. Vasc. Biol. 2008;28:1556–1562. doi: 10.1161/ATVBAHA.108.163998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reitz C., et al. Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset Alzheimer disease. Arch. Neurol. 2010;67:1491–1497. doi: 10.1001/archneurol.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mitchell A.J., et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders – a systematic review and meta-analysis. Schizophr. Bull. 2013;39:306–318. doi: 10.1093/schbul/sbr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang J.T., et al. Independent protein-profiling studies show a decrease in apolipoprotein A1 levels in schizophrenia CSF, brain and peripheral tissues. Mol. Psychiatry. 2008;13:1118–1128. doi: 10.1038/sj.mp.4002108. [DOI] [PubMed] [Google Scholar]
- 73.Lehto S.M., et al. Low HDL cholesterol associates with major depression in a sample with a 7-year history of depressive symptoms. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2008;32:1557–1561. doi: 10.1016/j.pnpbp.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 74.Wang X., et al. Localized increases in CEPT1 and ATGL elevate plasmalogen phosphatidylcholines in HDLs contributing to atheroprotective lipid profiles in hyperglycemic GCK-MODY. Redox Biol. 2021;40 doi: 10.1016/j.redox.2021.101855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosenblat M., et al. Paraoxonase 1 (PON1) enhances HDL-mediated macrophage cholesterol efflux via the ABCA1 transporter in association with increased HDL binding to the cells: a possible role for lysophosphatidylcholine. Atherosclerosis. 2005;179:69–77. doi: 10.1016/j.atherosclerosis.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 76.Begue F., et al. Altered high-density lipoprotein composition and functions during severe COVID-19. Sci. Rep. 2021;11:2291. doi: 10.1038/s41598-021-81638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stukas S., et al. High-density lipoproteins and cerebrovascular integrity in Alzheimer’s disease. Cell Metab. 2014;19:574–591. doi: 10.1016/j.cmet.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 78.Hariyanto T.I., Kurniawan A. Dyslipidemia is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes Metab. Syndr. 2020;14:1463–1465. doi: 10.1016/j.dsx.2020.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Izquierdo-Useros N., et al. HIV and mature dendritic cells: Trojan exosomes riding the Trojan horse? PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cosset F.L., Dreux M. HCV transmission by hepatic exosomes establishes a productive infection. J. Hepatol. 2014;60:674–675. doi: 10.1016/j.jhep.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 81.Lam S.M., et al. A multi-omics investigation of the composition and function of extracellular vesicles along the temporal trajectory of COVID-19. Nat. Metab. 2021;3:909–922. doi: 10.1038/s42255-021-00425-4. [DOI] [PubMed] [Google Scholar]
- 82.Hassanpour M., et al. The role of extracellular vesicles in COVID-19 virus infection. Infect. Genet. Evol. 2020;85 doi: 10.1016/j.meegid.2020.104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Skotland T., et al. Lipids in exosomes: current knowledge and the way forward. Prog. Lipid Res. 2017;66:30–41. doi: 10.1016/j.plipres.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 84.Barberis E., et al. Circulating exosomes are strongly involved in SARS-CoV-2 infection. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.632290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shenoy G.N., et al. Sialic acid-dependent inhibition of T cells by exosomal ganglioside GD3 in ovarian tumor microenvironments. J. Immunol. 2018;201:3750–3758. doi: 10.4049/jimmunol.1801041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kuo Y.C., et al. Targeting human brain cancer stem cells by curcumin-loaded nanoparticles grafted with anti-aldehyde dehydrogenase and sialic acid: colocalization of ALDH and CD44. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;102:362–372. doi: 10.1016/j.msec.2019.04.065. [DOI] [PubMed] [Google Scholar]
- 87.Wielgat P., et al. Sialic acid-modified nanoparticles – new approaches in the glioma management-perspective review. Int. J. Mol. Sci. 2021;22:7494. doi: 10.3390/ijms22147494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tosi G., et al. Sialic acid and glycopeptides conjugated PLGA nanoparticles for central nervous system targeting: in vivo pharmacological evidence and biodistribution. J. Control. Release. 2010;145:49–57. doi: 10.1016/j.jconrel.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 89.Kucuk O., et al. Inhibition of NK cell-mediated cytotoxicity by oxysterols. Cell. Immunol. 1992;139:541–549. doi: 10.1016/0008-8749(92)90091-3. [DOI] [PubMed] [Google Scholar]
- 90.Kucuk O., et al. Inhibition of cytolytic T lymphocyte activity by oxysterols. Lipids. 1994;29:657–660. doi: 10.1007/BF02536101. [DOI] [PubMed] [Google Scholar]
- 91.Bansal S., et al. SARS-CoV-2 infection in lung transplant recipients induces circulating exosomes with SARS-CoV-2 spike protein S2. Clin. Transl. Med. 2021;11 doi: 10.1002/ctm2.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fantini J., et al. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peng R., et al. Cell entry by SARS-CoV-2. Trends Biochem. Sci. 2021;46:848–860. doi: 10.1016/j.tibs.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Seyran M., et al. The structural basis of accelerated host cell entry by SARS-CoV-2dagger. FEBS J. 2021;288:5010–5020. doi: 10.1111/febs.15651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bohan D., et al. Phosphatidylserine receptors enhance SARS-CoV-2 infection. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang K., et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target. Ther. 2020;5:283. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xie J., et al. Resveratrol abrogates hypoxia-induced up-regulation of exosomal amyloid-β partially by inhibiting CD147. Neurochem. Res. 2019;44:1113–1126. doi: 10.1007/s11064-019-02742-3. [DOI] [PubMed] [Google Scholar]
- 98.Fujita Y., et al. Early prediction of COVID-19 severity using extracellular vesicle COPB2. J. Extracell. Vesicles. 2021;10 doi: 10.1002/jev2.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kwon Y., et al. Detection of viral RNA fragments in human iPSC cardiomyocytes following treatment with extracellular vesicles from SARS-CoV-2 coding sequence overexpressing lung epithelial cells. Stem Cell Res. Ther. 2020;11:514. doi: 10.1186/s13287-020-02033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jin R., et al. Inhibition of CD147 (cluster of differentiation 147) ameliorates acute ischemic stroke in mice by reducing thromboinflammation. Stroke. 2017;48:3356–3365. doi: 10.1161/STROKEAHA.117.018839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fadool J.M., Linser P.J. 5A11 antigen is a cell recognition molecule which is involved in neuronal-glial interactions in avian neural retina. Dev. Dyn. 1993;196:252–262. doi: 10.1002/aja.1001960406. [DOI] [PubMed] [Google Scholar]
- 102.Zhou S., et al. CD147 is a regulatory subunit of the γ-secretase complex in Alzheimer’s disease amyloid β-peptide production. Proc. Natl. Acad. Sci. U. S. A. 2005;102:7499–7504. doi: 10.1073/pnas.0502768102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yuan D., et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials. 2017;142:1–12. doi: 10.1016/j.biomaterials.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Betzer O., et al. In vivo neuroimaging of exosomes using gold nanoparticles. ACS Nano. 2017;11:10883–10893. doi: 10.1021/acsnano.7b04495. [DOI] [PubMed] [Google Scholar]
- 105.Chen C.C., et al. Elucidation of exosome migration across the blood-brain barrier model in vitro. Cell. Mol. Bioeng. 2016;9:509–529. doi: 10.1007/s12195-016-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rosenberger C.M., et al. Microbial pathogenesis: lipid rafts as pathogen portals. Curr. Biol. 2000;10:R823–R825. doi: 10.1016/s0960-9822(00)00788-0. [DOI] [PubMed] [Google Scholar]
- 107.Sorci-Thomas M.G., et al. Nascent high density lipoproteins formed by ABCA1 resemble lipid rafts and are structurally organized by three apoA-I monomers. J. Lipid Res. 2012;53:1890–1909. doi: 10.1194/jlr.M026674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mobius W., et al. Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic. 2003;4:222–231. doi: 10.1034/j.1600-0854.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 109.Skryabin G.O., et al. Lipid rafts in exosome biogenesis. Biochemistry (Mosc) 2020;85:177–191. doi: 10.1134/S0006297920020054. [DOI] [PubMed] [Google Scholar]
- 110.Lu Y., et al. Lipid rafts are involved in SARS-CoV entry into Vero E6 cells. Biochem. Biophys. Res. Commun. 2008;369:344–349. doi: 10.1016/j.bbrc.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rhainds D., et al. Localization and regulation of SR-BI in membrane rafts of HepG2 cells. J. Cell Sci. 2004;117:3095–3105. doi: 10.1242/jcs.01182. [DOI] [PubMed] [Google Scholar]
- 112.Ganesan L.P., et al. Scavenger receptor B1, the HDL receptor, is expressed abundantly in liver sinusoidal endothelial cells. Sci. Rep. 2016;6:20646. doi: 10.1038/srep20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hamming I., et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fung K.Y., et al. SR-BI mediated transcytosis of HDL in brain microvascular endothelial cells is independent of caveolin, clathrin, and PDZK1. Front. Physiol. 2017;8:841. doi: 10.3389/fphys.2017.00841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rizzo P., et al. COVID-19 in the heart and the lungs: could we ‘Notch’ the inflammatory storm? Basic Res. Cardiol. 2020;115:31. doi: 10.1007/s00395-020-0791-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Holmes O., et al. Effects of membrane lipids on the activity and processivity of purified gamma-secretase. Biochemistry. 2012;51:3565–3575. doi: 10.1021/bi300303g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Raven F., et al. Soluble gamma-secretase modulators attenuate Alzheimer’s β-amyloid pathology and induce conformational changes in presenilin 1. eBioMedicine. 2017;24:93–101. doi: 10.1016/j.ebiom.2017.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alquisiras-Burgos I., et al. Neurological complications associated with the blood-brain barrier damage induced by the inflammatory response during SARS-CoV-2 infection. Mol. Neurobiol. 2021;58:520–535. doi: 10.1007/s12035-020-02134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Goncalves I., et al. Evidence supporting a key role of Lp-PLA2-generated lysophosphatidylcholine in human atherosclerotic plaque inflammation. Arterioscler. Thromb. Vasc. Biol. 2012;32:1505–1512. doi: 10.1161/ATVBAHA.112.249854. [DOI] [PubMed] [Google Scholar]
- 120.Nguyen L.N., et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509:503–506. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- 121.Alexander J.J. Blood-brain barrier (BBB) and the complement landscape. Mol. Immunol. 2018;102:26–31. doi: 10.1016/j.molimm.2018.06.267. [DOI] [PubMed] [Google Scholar]
- 122.Ayres J.S. A metabolic handbook for the COVID-19 pandemic. Nat. Metab. 2020;2:572–585. doi: 10.1038/s42255-020-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Siniavin A.E., et al. Snake venom phospholipase A2s exhibit strong virucidal activity against SARS-CoV-2 and inhibit the viral spike glycoprotein interaction with ACE2. Cell. Mol. Life Sci. 2021;78:7777–7794. doi: 10.1007/s00018-021-03985-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bailly C., Vergoten G. Glycyrrhizin: an alternative drug for the treatment of COVID-19 infection and the associated respiratory syndrome? Pharmacol. Ther. 2020;214 doi: 10.1016/j.pharmthera.2020.107618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Barter P.J., et al. Effect of statins on HDL-C: a complex process unrelated to changes in LDL-C: analysis of the VOYAGER database. J. Lipid Res. 2010;51:1546–1553. doi: 10.1194/jlr.P002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shui G., et al. Comparative plasma lipidome between human and cynomolgus monkey: are plasma polar lipids good biomarkers for diabetic monkeys? PLoS One. 2011;6 doi: 10.1371/journal.pone.0019731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang X.J., et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab. 2020;32:176–187 e4. doi: 10.1016/j.cmet.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lam S.M., et al. High-coverage lipidomics for functional lipid and pathway analyses. Anal. Chim. Acta. 2021;1147:199–210. doi: 10.1016/j.aca.2020.11.024. [DOI] [PubMed] [Google Scholar]
- 129.Liao W.L., et al. Quantification of cholesterol-metabolizing P450s CYP27A1 and CYP46A1 in neural tissues reveals a lack of enzyme-product correlations in human retina but not human brain. J. Proteome Res. 2011;10:241–248. doi: 10.1021/pr1008898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Snider B.J., Holtzman D.M. Effects of COVID-19 on preclinical and clinical research in neurology: examples from research on neurodegeneration and Alzheimer’s disease. Neuron. 2021;109:3199–3202. doi: 10.1016/j.neuron.2021.08.026. [DOI] [PubMed] [Google Scholar]
- 131.Alzahrani F.A., et al. The potential use of mesenchymal stem cells and their derived exosomes as immunomodulatory agents for COVID-19 patients. Stem Cells Int. 2020;2020 doi: 10.1155/2020/8835986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Duan K., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. U. S. A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hawkins B.T., Davis T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 134.Ghersi-Egea J.F., et al. Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta Neuropathol. 2018;135:337–361. doi: 10.1007/s00401-018-1807-1. [DOI] [PubMed] [Google Scholar]
- 135.Mathieu M., et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 136.Lingwood D., Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]