Abstract
Objective:
To identify post-discharge outcome phenotypes and risk factors for poor outcomes using insurance claims data.
Design:
Retrospective cohort study
Setting:
Single quaternary center
Patients:
Children without pre-existing tracheostomy who required ≥ 3 days of invasive mechanical ventilation, survived the hospitalization, and had post-discharge insurance eligibility in Colorado’s All Payer Claims Database.
Interventions:
None
Measurements and Main Results:
We used unsupervised machine learning to identify functional outcome phenotypes based on claims data representative of post-discharge morbidities. We assessed health trajectory by comparing change in number of insurance claims between quarters 1 and 4 of the post-discharge year. Regression analyses identified variables associated with unfavorable outcomes. The 381 subjects had median age 3.3 years (interquartile range 0.9, 12) and 147 (39%) had a complex chronic condition. Primary diagnoses were respiratory (41%), injury (23%), and neurologic (11%). We identified three phenotypes: lower morbidity (n=300), higher morbidity (n=62), and 1-year non-survivors (n=19). Complex chronic conditions most strongly predicted the non-survivor phenotype. Longer PICU stays and tracheostomy placement most strongly predicted the higher morbidity phenotype. Patients with high but improving post-discharge resource use were differentiated by high illness severity and long PICU stays. Patients with persistently high or increasing resource use were differentiated by complex chronic conditions and tracheostomy placement.
Conclusions:
New morbidities are common after prolonged mechanical ventilation. Identifying phenotypes at high risk of post-discharge morbidity may facilitate prognostic enrichment in clinical trials.
Keywords: critical care outcome; intensive care units, pediatric; unsupervised machine learning; child; administrative claims, healthcare; respiratory failure
Introduction
Each year, nearly 97,000 children are cared for in U.S. Pediatric Intensive Care Units (PICUs) (1). PICU mortality rates approximate 2%, leading researchers, clinicians, and families of PICU survivors to call for increased attention on long-term morbidities following critical illness, termed Post-Intensive Care Syndrome-pediatrics (PICS-p) (2–10). Improved ability to identify patients at high risk for post-discharge sequelae could allow for targeting of interventions within the PICU or immediately post-discharge and inform provider and family expectations of post-discharge morbidities.
A systematic understanding of post-discharge functional outcomes and healthcare needs is challenged by fragmented healthcare databases and delivery systems, incomplete study enrollment and losses to follow-up, and high resource requirements for prospective longitudinal studies. To overcome these challenges, we identified a cohort of children who survived a hospitalization for acute respiratory failure and linked their hospitalization records to post-discharge insurance claims. Our primary aim was to identify novel claims-based outcome phenotypes reflective of post-discharge functional status using an unsupervised machine learning approach. Our secondary aim was to evaluate recovery trajectory based on frequency of claims during the post-discharge year. Lastly, we identified patient and hospitalization factors predictive of outcome phenotype and health trajectory. We hypothesized that patients could be grouped into functional outcome phenotypes identifiable by patient and hospitalization characteristics.
Materials and Methods
The study received a waiver of consent from Colorado’s Multiple Institutional Review Board (Protocol #18–0488).
We conducted this study at an urban, quaternary children’s hospital that serves as a 7-state referral center with 32–48 PICU beds (summer-winter) and a separate cardiac ICU. Using our Virtual Pediatric Systems database (11), we identified children without a tracheostomy who survived a PICU hospitalization between January 1st, 2013 and December 31st, 2016 during which they required invasive mechanical ventilation for at least 3 days. Next, we obtained insurance claims and death data from the University of Colorado’s electronic data warehouse, which maintains a linkage for our health system’s patients with the Colorado All Payer Claims Database (APCD) and the state death registry. We collected claims data for patients with insurance eligibility in the APCD for any duration within the 12 months post-discharge. We also collected pre-admission claims within the 13 months to 1 month prior to hospital admission. We categorized claims using previously established methods (12, 13) (Table E1).
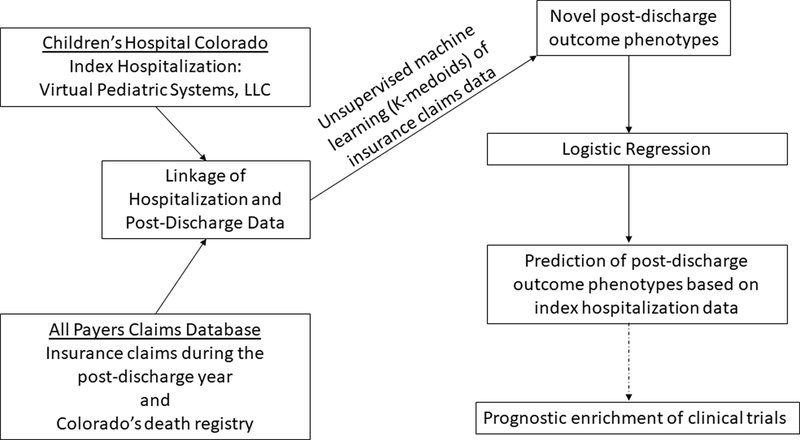
We collected patient and hospitalization characteristics from the electronic health record (EHR) and our site’s Virtual Pediatric Systems database (Figure 1). We identified comorbidities by chart review and categorized patients based on the Pediatric Medical Complexity Algorithm as having a pre-existing complex chronic condition (CCC), non-complex chronic condition, or no chronic condition (14). Acute Respiratory Distress Syndrome (ARDS) was defined using the Berlin definition and identified by a detection system embedded within our site’s EHR (15–17) (Table E2). A complicated PICU stay was defined as receipt of continuous renal replacement therapy, extracorporeal membrane oxygenation, in-hospital cardiopulmonary resuscitation, high-frequency oscillatory ventilation, or a tracheostomy. Additional methodological details are provided in the supplement.
Figure 1.
Linkage of hospitalization characteristics to post-discharge insurance claims representative of morbidity phenotypes to inform prognostic enrichment of future clinical trials aimed at decreasing post-discharge morbidities.
Statistical Analysis
We used partitioning around medoids, an unsupervised machine learning approach, to classify patients into distinct, non-overlapping clusters (phenotypes) with similar patterns of healthcare utilization based on 32 feature variables (e.g., hospital readmissions, outpatient claims, death) (Table E3) (13, 18). We determined cluster membership by minimizing the distance that defines dissimilarities (Gower dissimilarity matrix) between individuals assigned to each cluster (19, 20). In a separate analysis, we categorized health trajectory groups based on frequency of claims in each quarter of the post-discharge year as: low (fewer than 20 claims in any quarter), decreasing (quarter 4 [Q4]: quarter 1 [Q1] > 20% decrease), increasing (Q4:Q1 > 20% increase) or high static (Q4:Q1 < 20% change and more than 20 claims in a quarter).
We evaluated predictors of phenotype and health trajectory group using elastic net regularized logistic regression with 10-fold cross-validation to select the model with the minimum cross-validation error (Table E4). Models identified characteristics that distinguished each group in reference to the remaining cohort. Using the variables selected by the elastic net procedure, we used logistic regression and the Firth adjustment to make inferences and calculate the area under the receiver operating curve (AUROC) (21, 22). Analysis details are provided in the supplement.
Results
Of the 633 patients meeting inclusion criteria, 381 (60%) linked to records in the APCD. APCD-matched patients were more likely to have non-complex chronic conditions (14% versus 6%) than APCD-unmatched patients, although the proportion of patients with CCCs was the same (39%) (Table E5). More of the APCD-matched patients had complicated PICU stays (17% versus 11%, p=0.03) and died during the post-discharge year (19 [5%] vs 3 [1%], p=0.02).
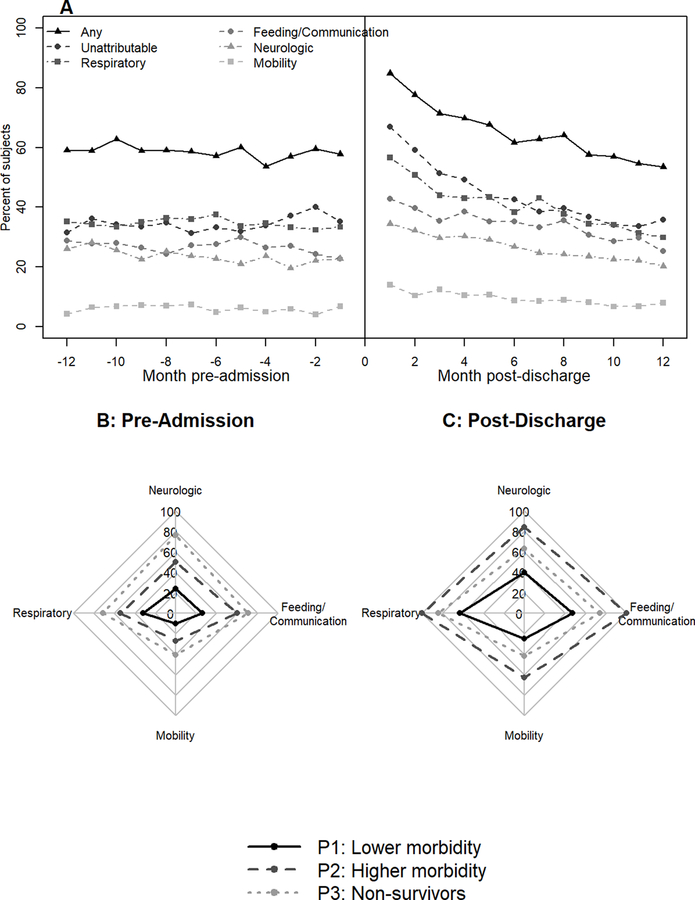
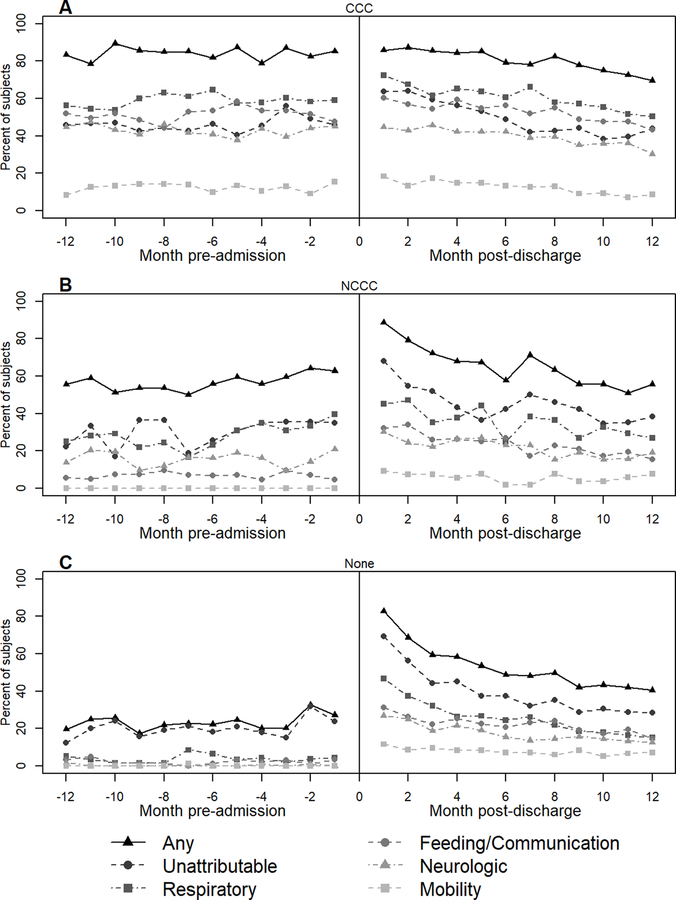
In the matched cohort of 381 patients, median age was 3.3 years (IQR 0.9, 12.3), 152 (40%) were female, and 147 (39%) had a pre-existing CCC (Table E5). The most common primary diagnoses were respiratory (41%), injury (23%) and neurologic (11%). More than half of the patients had moderate (n=106, 28%) or severe (n=135, 35%) ARDS. Patients required a median of 6.7 days (IQR 4.6, 9.9) of invasive mechanical ventilation. Durations of PICU and hospital stays were 10 (IQR 7, 15) and 22 (13, 43) days, respectively. Immediately following the hospitalization, 167 (44%) patients required durable medical equipment, most frequently in the feeding (30%) and respiratory (25%) domains (Table E6). During the year after discharge, 19 (5%) patients died, 126 (33%) were readmitted, 202 (53%) had an Emergency Department visit, and 272 (71%) had an outpatient pulmonary visit (Table E7). Respiratory (70%) domain was most frequently affected followed by feeding/communication (57%), neurologic (49%), and mobility (32%). Overall, health resource use was above pre-admission baseline across all domains during the months after discharge and decreased during the post-discharge year (Figure 2a, Figure E1). Claims immediately following discharge were similarly high (90%) for patients with and without pre-existing chronic conditions (Figure 3). However, in patients with a pre-existing CCC, this high rate of claims was similar to pre-admission baseline. In previously healthy patients, the recovery trajectory was characterized by a decline in health resource use during the post-discharge months with a plateau above their pre-admission baseline. A similar path was demonstrated by patients with a pre-existing non-complex chronic condition, although with greater variability throughout the post-discharge year.
Figure 2.
Health resource use during the year before admission and after discharge. A) Percent of subjects with at least 1 claim in a domain category during each 1-month period before admission and after hospital discharge; B) Percent of subjects in each phenotype with at least one claim in the domain category during the year before hospital admission (13 months to 1 month prior to admission). C) Percent of subjects in each phenotype with at least one claim in the domain category during the year after hospital discharge.
Figure 3.
Percent of subjects with at least 1 claim in a domain category during each 1-month period before admission and after hospital discharge, stratified by pre-existing Pediatric Medical Complexity Algorithm category: A) CCC: complex chronic condition, B) NCCC: non-complex chronic condition, C) No pre-existing chronic conditions.
Outcome Phenotypes
Using post-discharge claims, we identified three distinct patient phenotypes (Table E8). Broadly, the lower morbidity phenotype included more than 75% (n=300) of the cohort and had the lowest rates of health resource use during the post-discharge year. The higher morbidity phenotype included the 62 (16%) patients with the most health resource use and the 1-year non-survivor phenotype included the 19 (5%) patients who died during the post-discharge year. The pattern of health resource use was particularly evident in rates of hospitalization (lower morbidity: 20%, higher morbidity: 90%, non-survivors: 47%) and Emergency Department visits (lower morbidity: 43%, higher morbidity: 97%, non-survivors: 63%). Across all phenotypes, most patients had an outpatient pulmonary visit after discharge (71%). However, the median number of pulmonary visits per patient for the higher morbidity phenotype far exceeded the other phenotypes (lower morbidity: 1 [IQR 0, 4], higher morbidity: 13.5 [IQR 8, 18], non-survivors: 3 [IQR 0, 12.5]). This was mirrored in the respiratory and tracheostomy durable medical equipment claims (Table E7). Medications filled after discharge showed a similar pattern with all higher morbidity phenotype patients having a medication claim versus 76% and 79% of the lower morbidity and non-survivor phenotypes, respectively. Additionally, the frequency of medication claims per patient was far greater in the higher morbidity phenotype (Table E7). Domain-specific health resource use (the composite of all domain-specific claims data) showed high morbidity rates, elevated above baseline, across all 3 phenotypes (Figure 2b and 2c). Feeding/communication and neurologic morbidities were prevalent in nearly all patients in the higher morbidity phenotype, more than half of the patients in the non-survivor phenotype, and more than a quarter of patients in the lower morbidity phenotype. In the lower morbidity phenotype, respiratory morbidities were present in 190 (63%) patients compared with 32% of patients with pre-admission data. This suggests a high rate of new respiratory morbidity even amongst the lower morbidity cohort. The mobility domain was less frequently affected but showed morbidity rates of 25–63% across the phenotypes.
We performed univariable analyses to test for characteristics associated with phenotype membership (Table E9). In multivariable analyses, patient, admission, and hospitalization characteristics contributed to model accuracy across all three models (Table E10). The models most accurately discriminated patients in the non-survivor phenotype (AUROC 0.87 [95% CI: 0.82, 0.93]) and the most strongly predictive characteristic was having a pre-existing CCC. The models also showed good discrimination for the lower morbidity phenotype (AUROC 0.80 [95% CI 0.75, 0.85]). The characteristics that most strongly predicted membership in the lower morbidity phenotype were patient (lack of a CCC and non-Hispanic ethnicity), admission (lower PIM2 score), and hospitalization (lack of tracheostomy placement) factors. The model showed lower discrimination for the higher morbidity phenotype (AUROC of 0.67 [95% CI: 0.59, 0.74]). Hospitalization factors (tracheostomy placement and longer PICU stays) most strongly predicted membership in this phenotype.
Health Trajectories
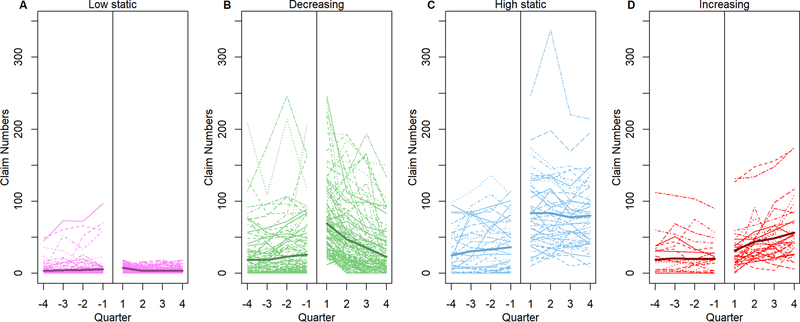
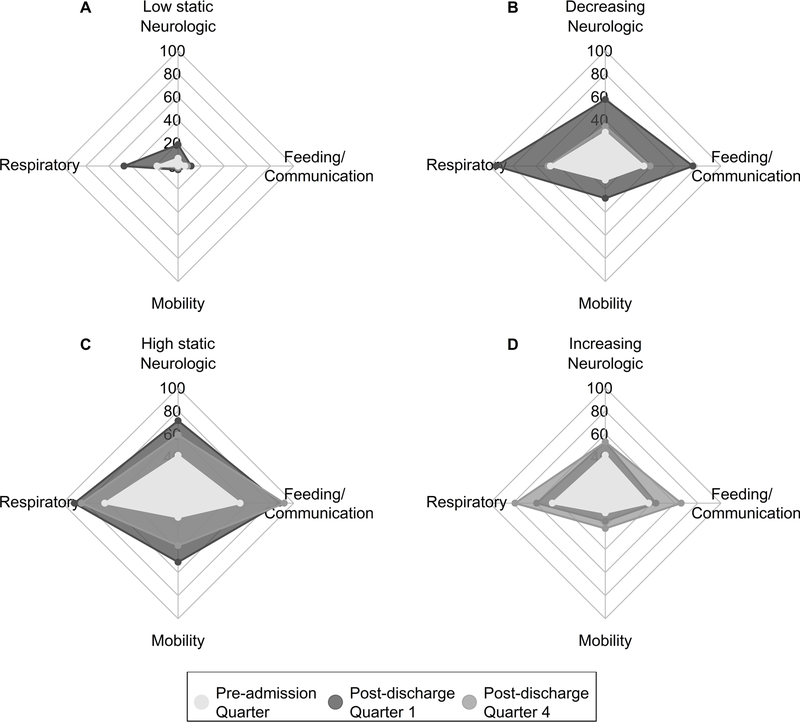
Next, we evaluated claim frequency during the post-discharge year in the 316 (83%) patients who had complete (≥ 12 months) post-discharge APCD eligibility (Table E11). Based on claims frequencies, we defined four health resource use groups: low static (n=124, 39%), decreasing (n=111, 35%), high static (n=49, 16%), and increasing (n=32, 10%) (Figure 4). Relative to the pre-admission year, all groups except the low static group had a higher average number of claims relative to their pre-admission baseline during the months after discharge. However, the claim trajectories differed. Patients in the decreasing group had claim frequencies immediately following discharge that were approximately double that of pre-admission baseline and the claim frequencies approximated baseline levels by the end of the post-discharge year. Patients in the high static and increasing groups had higher claim frequencies relative to their pre-admission baseline and this higher rate persisted during the post-discharge year. Among those with changing resource needs (decreasing and increasing groups), the change in resource use was displayed across domains (Figure 5, Table E12). Patients with increasing use had higher quarter 4 claim frequencies in the feeding/communication and respiratory domains with smaller increases in neurologic and mobility domains.
Figure 4.
Resource use trajectory based on claims during the year before admission (13 months to 1 month prior to admission) and after discharge. Dotted lines are individual trajectories, and solid line is the average number of claims across patients at each quarter.
Figure 5.
Percent of subjects in each health resource use group with at least one claim in the domain category during the quarter of the preadmission year proximal to the index admission (months −1 to −4) and quarters 1 and 4 of the post-discharge year.
We performed univariable analyses to evaluate characteristics associated with health trajectory group (Table E13). In multivariable analyses, patient, admission, and hospitalization factors contributed to model accuracy across all three groups (Table E10). The models most accurately predicted membership in the low static group (AUROC 0.83 [95% CI: 0.78, 0.87]). Characteristics that most accurately predicted low static resource use were patient (female sex, lack of pre-existing CCC) and hospitalization (lack of tracheostomy placement and shorter PICU stay) factors. The models had good accuracy for discrimination of the high static or increasing group (AUROC 0.72 [95% CI: 0.66, 0.79]). Characteristics that most accurately predicted high static or increasing group membership were patient (pre-existing CCC) and hospitalization (tracheostomy placement) factors. The models less accurately predicted patients with decreasing health resource use (AUROC 0.66 [95% CI: 0.60, 0.73]). Characteristics that most accurately predicted decreasing health resource use were admission (higher PRISM score, lack of a primary injury diagnosis) and hospitalization (longer PICU stay) factors.
Discussion
In this study of critically ill and injured children who required at least 3 days of invasive mechanical ventilation, insurance claims data indicate high rates of post-discharge healthcare needs. This includes high rates of hospital readmission, Emergency Department visits, and post-discharge morbidities. An unsupervised machine learning approach identified three distinct phenotypes: 1) lower morbidity, 2) higher morbidity, and 3) 1-year non-survivors. Membership in these phenotypes and health trajectory phenotypes were predicted by a combination of patient, admission, and hospitalization characteristics. Additionally, both resolution and acquisition of morbidities tracked across domains. These results suggest that an identifiable subset of critically ill children at high risk of post-discharge morbidities may facilitate prognostic enrichment in clinical trials aimed at decreasing long-term morbidities.
Unsupervised machine learning identified three distinct outcome phenotypes. The non-survivor phenotype likely represents patients for whom the index hospitalization was a step along a path of downward decline, ultimately resulting in death. This phenotype nearly universally had pre-existing CCCs and presented with high illness severities. Of note, the non-survivor phenotype had fewer patients with health resource use in each domain relative to the higher morbidity phenotype. This may be reflective of a shortened follow-up duration due to death. The higher morbidity phenotype represents an intermediate cohort, most with pre-existing CCCs and high illness severities, but with a higher-intensity PICU experience including more complicated and longer PICU stays. Although duration of PICU stay may be primarily driven by non-modifiable patient and illness characteristics (23–27), adult studies aimed at decreasing durations of mechanical ventilation have successfully lessened exposure to the potentially toxic environment of the ICU and pediatric studies aimed at similar objectives are underway (28–33). Lastly, the lower morbidity phenotype represents the cohort expected to have the best outcomes based on the lack of pre-existing chronic conditions and lower illness severity. Despite this, across all domains, 25–63% of these patients suffered morbidities. In summary, each outcome phenotype may benefit from a distinct approach to mitigate morbidities.
Studies evaluating PICS in adults identify age, severity of illness, and length of ICU stay as predictors of impairment severity while separating pre-existing chronic conditions as predictors of recovery trajectory (34). Our data suggest similar patterns exist in PICS-p, as pre-existing CCCs are key drivers of unfavorable health trajectories. Additionally, our results suggest that hospitalization factors (illness severity, PICU length of stay) identify patients with potentially recoverable morbidities as demonstrated by the decreasing resource use during the post-discharge year. Additionally, health trajectory was pervasive across domains. For example, in those with an improving trajectory, morbidity rates decreased by nearly 50% across all domains between the first and fourth quarters of the post-discharge year and a similar pattern but inverse direction occurred in the cohort with increasing resource use. The significant overlap of domain morbidities may be more pronounced in PICS-p compared to adult PICS because children experience ongoing growth and development with a morbidity in one domain affecting ongoing development across other domains. For example, a child with a respiratory morbidity may have less capacity to engage in play, limiting their physical, cognitive, and socioemotional development.
Leading researchers in adult critical care and PICS have highlighted the adult ICU survivor as a medically complex patient who requires multidisciplinary care with a focus on the transition between the ICU and the medical home to optimize recovery (35). Pediatric studies also support targeted interventions in these complex patients involving multidisciplinary outpatient care to decrease subsequent hospitalizations (36–39). In addition to medical care, efforts extending beyond the medical home to include individualized schooling, emotional and psychiatric support, and family support may further facilitate an optimal environment for recovery. Additionally, studies should assess whether duration of time spent in recovery permanently alters developmental trajectory.
This study is limited by its single center design and missing data as 40% of patients did not match in the APCD. While this introduces a potential for bias, our follow-up cohort is likely representative of the general PICU population, as it does not rely on patient or family engagement for enrollment or post-discharge data collection. Additionally, inpatient characteristics were similar between APCD-matched and -unmatched subjects except that the unmatched patients had fewer non-complex chronic conditions, complicated PICU stays, and post-discharge deaths. Post-discharge deaths may be underrepresented as reliable data collection was only available within Colorado. Patient characterization was limited to presence or absence of a CCC without notation of type or number of CCCs and we are unable to characterize social disadvantage, both of which may affect outcomes. The study hospital serves a broad population and, while this likely represents health resource use patterns across Colorado particularly Medicaid insured patients which account for 60% of pediatric hospitalizations, additional studies are needed to determine generalizability both graphically and by existence and type of insurance (40). While claims data are likely to identify most significant morbidities, some morbidities and their recovery/deterioration trajectory may be missed due to poor healthcare access or discontinuation of services not resulting in improvements. Importantly, our data do not include mental health services, limiting neurologic/psychiatric morbidities to medication data.
Conclusions
As identified using state all payer claims insurance data, reducing morbidities and improving health trajectory may be targetable outcomes for interventional trials of PICU or post-discharge therapies. Prognostic enrichment using clinical phenotypes at high risk of morbidity identified by high illness severity and longer, more complicated PICU stays may enhance trial design. Optimizing outcomes will require accounting for complex interactions between pre-existing patient factors such as chronic conditions that are linked to health trajectory and hospitalization characteristics associated with new morbidities.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number K23HD096018. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study was also supported by the Health Data Compass Data Warehouse project (healthdatacompass.org). Virtual Pediatric Systems data were provided by our site’s collection of data for the Virtual Pediatric Systems (VPS), LLC. No endorsement or editorial restriction of the interpretation of these data or opinions of the authors has been implied or stated.
Conflicts of Interest and Sources of Funding:
Research support was provided to Dr. Maddux by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K23HD096018) and the Francis Family Foundation. The funder did not participate in the work. For the remaining authors, no conflicts of interest were declared.
Copyright Form Disclosure: Dr. Maddux’s institution received funding from the Francis Family Foundation. Drs. Maddux and Miller’s institutions received funding from the National Institute of Child Health and Human Development (NICHD) (K23HD096018). Drs. Maddux, Mourani, and Miller received support for article research from the National Institutes of Health (NIH). Dr. Mourani’s institution received funding from the NIH. Dr. Bennett’s institution received funding from the NICHD, the National Center for Advancing Translational Sciences, and the National Institute of Allergy and Infectious Diseases. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
No reprints will be ordered.
Site: University of Colorado School of Medicine, Children’s Hospital Colorado
References
- 1.Benneyworth BD, Bennett WE, Carroll AE. Cross-sectional comparison of critically ill pediatric patients across hospitals with various levels of pediatric care. BMC Res Notes. 2015. Nov 19;8:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manning JC, Pinto NP, Rennick JE, et al. Conceptualizing Post Intensive Care Syndrome in Children-The PICS-p Framework. Pediatr Crit Care Med. 2018. Apr;19(4):298–300. [DOI] [PubMed] [Google Scholar]
- 3.Fink EL, Maddux AB, Pinto N, et al. A Core Outcome Set for Pediatric Critical Care. Crit Care Med. 2020. Oct 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollack MM, Holubkov R, Funai T, et al. Simultaneous Prediction of New Morbidity, Mortality, and Survival Without New Morbidity From Pediatric Intensive Care: A New Paradigm for Outcomes Assessment. Crit Care Med. 2015. Aug;43(8):1699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maddux AB, Pinto N, Fink EL, et al. Postdischarge Outcome Domains in Pediatric Critical Care and the Instruments Used to Evaluate Them: A Scoping Review. Crit Care Med. 2020. Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinto NP, Rhinesmith EW, Kim TY, et al. Long-Term Function After Pediatric Critical Illness: Results From the Survivor Outcomes Study. Pediatr Crit Care Med. 2017. Mar;18(3):e122–e30. [DOI] [PubMed] [Google Scholar]
- 7.Pollack MM, Banks R, Holubkov R, et al. Long-Term Outcome of PICU Patients Discharged With New, Functional Status Morbidity. Pediatr Crit Care Med. 2020. Oct 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watson RS, Asaro LA, Hertzog JH, et al. Long-Term Outcomes after Protocolized Sedation versus Usual Care in Ventilated Pediatric Patients. Am J Respir Crit Care Med. 2018. Jun 1;197(11):1457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson RS, Asaro LA, Hutchins L, et al. Risk Factors for Functional Decline and Impaired Quality of Life after Pediatric Respiratory Failure. Am J Respir Crit Care Med. 2019. Oct 1;200(7):900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimmerman JJ, Banks R, Berg RA, et al. Critical Illness Factors Associated With Long-Term Mortality and Health-Related Quality of Life Morbidity Following Community-Acquired Pediatric Septic Shock. Crit Care Med. 2020. Mar;48(3):319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wetzel RC, Sachedeva R, Rice TB. Are all ICUs the same? Paediatr Anaesth. 2011. Jul;21(7):787–93. [DOI] [PubMed] [Google Scholar]
- 12.ATC: Structure and Principles. Oslo, Norway: WHO Collaborating Centre for Drug Statistics Methodology: Norwegian Institute of Public Health; [updated February 15, 2018; November 26, 2018]; Available from: https://www.whocc.no/atc/structure_and_principles/. [Google Scholar]
- 13.Maddux AB, Sevick C, Cox-Martin M, et al. Novel Claims-Based Outcome Phenotypes in Survivors of Pediatric Traumatic Brain Injury. J Head Trauma Rehabil. 2021. Feb 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon TD, Haaland W, Hawley K, et al. Development and Validation of the Pediatric Medical Complexity Algorithm (PMCA) Version 3.0. Acad Pediatr. 2018. Jul;18(5):577–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khemani RG, Thomas NJ, Venkatachalam V, et al. Comparison of SpO2 to PaO2 based markers of lung disease severity for children with acute lung injury. Crit Care Med. 2012. Apr;40(4):1309–16. [DOI] [PubMed] [Google Scholar]
- 16.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. Jama. 2012. Jun 20;307(23):2526–33. [DOI] [PubMed] [Google Scholar]
- 17.Rice TW, Wheeler AP, Bernard GR, et al. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007. Aug;132(2):410–7. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman L, Rousseeuw PJ. In: Library WO, editor. Finding Groups in Data: An Introduction to Cluster Analysis: John Wiley & Sons, Inc; 2008. p. 68–125. [Google Scholar]
- 19.Gower JC. A General Coefficient of Similarity and Some of Its Properties. Biometrics. 2020;27(4):857–71. [Google Scholar]
- 20.Kassambara A. Practical Guide to Cluster Analysis in R: Unsupervised machine learning. 1 ed. France: Statistical tools for high-throughput data analysis; 2017. p. 15–40. [Google Scholar]
- 21.Firth D. Bias Reduction of Maximum Likelihood Estimates. Biometrika. 1993. 11/21/2019;80(1):27–38. [Google Scholar]
- 22.Heinze GP, Dunkler M, Southworth D, logistf H: Firth’s Bias-Reduced Logistic Regression. 1.23 ed: R (> 3.0.0); 2018.
- 23.Dosa NP, Boeing NM, Ms N, et al. Excess risk of severe acute illness in children with chronic health conditions. Pediatrics. 2001. Mar;107(3):499–504. [DOI] [PubMed] [Google Scholar]
- 24.Edwards JD, Houtrow AJ, Vasilevskis EE, et al. Chronic conditions among children admitted to U.S. pediatric intensive care units: their prevalence and impact on risk for mortality and prolonged length of stay*. Crit Care Med. 2012. Jul;40(7):2196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwards JD, Lucas AR, Stone PW, et al. Frequency, risk factors, and outcomes of early unplanned readmissions to PICUs. Crit Care Med. 2013. Dec;41(12):2773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odetola FO, Gebremariam A, Davis MM. Comorbid illnesses among critically ill hospitalized children: Impact on hospital resource use and mortality, 1997–2006. Pediatr Crit Care Med. 2010. Jul;11(4):457–63. [DOI] [PubMed] [Google Scholar]
- 27.Typpo KV, Petersen NJ, Petersen LA, et al. Children with chronic illness return to their baseline functional status after organ dysfunction on the first day of admission in the pediatric intensive care unit. J Pediatr. 2010. Jul;157(1):108–13.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choong K, Awladthani S, Khawaji A, et al. Early Exercise in Critically Ill Youth and Children, a Preliminary Evaluation: The wEECYCLE Pilot Trial. Pediatr Crit Care Med. 2017. Nov;18(11):e546–e54. [DOI] [PubMed] [Google Scholar]
- 29.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008. Jan 12;371(9607):126–34. [DOI] [PubMed] [Google Scholar]
- 30.Morris PE, Goad A, Thompson C, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008. Aug;36(8):2238–43. [DOI] [PubMed] [Google Scholar]
- 31.Schaller SJ, Anstey M, Blobner M, et al. Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet. 2016. Oct 1;388(10052):1377–88. [DOI] [PubMed] [Google Scholar]
- 32.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009. May 30;373(9678):1874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wieczorek B, Ascenzi J, Kim Y, et al. PICU Up!: Impact of a Quality Improvement Intervention to Promote Early Mobilization in Critically Ill Children. Pediatr Crit Care Med. 2016. Dec;17(12):e559–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azoulay E, Vincent JL, Angus DC, et al. Recovery after critical illness: putting the puzzle together-a consensus of 29. Crit Care. 2017. Dec 5;21(1):296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herridge MS. Fifty Years of Research in ARDS. Long-Term Follow-up after Acute Respiratory Distress Syndrome. Insights for Managing Medical Complexity after Critical Illness. Am J Respir Crit Care Med. 2017. Dec 1;196(11):1380–4. [DOI] [PubMed] [Google Scholar]
- 36.Ducharme-Crevier L, La KA, Francois T, et al. PICU Follow-Up Clinic: Patient and Family Outcomes 2 Months After Discharge. Pediatr Crit Care Med. 2021. Nov 1;22(11):935–43. [DOI] [PubMed] [Google Scholar]
- 37.Gordon JB, Colby HH, Bartelt T, et al. A tertiary care-primary care partnership model for medically complex and fragile children and youth with special health care needs. Arch Pediatr Adolesc Med. 2007. Oct;161(10):937–44. [DOI] [PubMed] [Google Scholar]
- 38.Graham RJ, McManus ML, Rodday AM, et al. Pediatric Specialty Care Model for Management of Chronic Respiratory Failure: Cost and Savings Implications and Misalignment With Payment Models. Pediatr Crit Care Med. 2018. May;19(5):412–20. [DOI] [PubMed] [Google Scholar]
- 39.Fitzgerald JC, Kelly NA, Hickey C, et al. Implementation of a Follow-Up System for Pediatric Sepsis Survivors in a Large Academic Pediatric Intensive Care Unit. Front Pediatr. 2021;9:691692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bucholz EM, Schuster MA, Toomey SL. Trends in 30-Day Readmission for Medicaid and Privately Insured Pediatric Patients: 2010–2017. Pediatrics. 2020. Aug;146(2). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.