Introduction
Several weeks into the COVID-19 pandemic a new syndrome causing sudden critical illness in children was identified (1–4). Multisystem inflammatory syndrome in children (MIS-C), also called Pediatric Inflammatory Multisystem Syndrome Temporally associated with SARS-CoV-2 (PIMS-TS) (5), is a clinical syndrome of systemic inflammation, strongly associated with SARS-CoV-2 virus, that has some similarities to, but is clinically and biologically distinct from, Kawasaki disease shock (KDS), macrophage activation syndrome (MAS), and toxic shock syndrome (TSS) (6–8). MIS-C typically afflicts infected (and often asymptomatic) children 4–6 weeks after exposure to SARS-CoV-2 and can cause multi-organ involvement affecting the heart, lungs, kidneys, brain, skin, and gastrointestinal system (1). These patients often present with cardiogenic or distributive shock and/or with severe abdominal pathology requiring admission to the pediatric intensive care unit (PICU) (9). Feldstein et al. studied a cohort of 539 MIS-C patients throughout the United States of America and demonstrated a nearly 75% ICU admission rate, 45% vasopressor requirement with less than 2% mortality (1), with the majority of patients achieving a complete recovery. Although MIS-C is a rare new clinical entity, formative studies are shaping the basic immunopathologic mechanisms behind the syndrome (2). The focus of this review is to synthesize the currently published immunologic profiles of patients with MIS-C into a robust mechanistic schema, which will identify future areas of study to complete the identification of the immunologic pathophysiology of MIS-C, and to create a template that can be applied to several other auto inflammatory and post-infectious processes.
A pathologic immune response, direct SARS-CoV-2 effect, or Post-Infectious Syndrome?
The prevailing (non-mutually exclusive) hypotheses for the pathogenic etiology of MIS-C include 1) a post-infectious autoimmune mediated inflammatory process, 2) a cytokine storm instigated by a superantigen response, and 3) a dysregulated immune response to chronic exposure to SARS-CoV-2 viral antigens. There are data to support all three theories in the literature and the underlying etiology is likely complex and multifactorial.
Post Infectious Syndrome
The MIS-C inflammatory process begins within 4–6 weeks following an exposure or primary infection in a child who had previously been asymptomatic or only mildly symptomatic (10), with approximately half of patients testing positive for SARS-CoV-2 virus via nasopharyngeal swab RT-qPCR test (1). A post-infectious etiology for MIS-C is supported by several factors. Children with MIS-C have been found to express high levels of circulating IgG and IgA antibodies against SARS-CoV-2 and levels of IgM similar to convalesced patients, suggesting a post-infectious etiology (1). Additionally, a lower viral load (quantified by higher PCR cycle thresholds) has been reported in 10 patients with MIS-C compared with acute infections (11), with the absence of SARS-CoV-2 viremia demonstrated in a cohort of 14 MIS-C patients (12). When examining the immune phosphoproteome in 9 MIS-C patients, Gruber et al. showed that STAT-1 phosphorylation was not elevated, which would have been expected in an acute viral infection of the airway and lung mucosa (13). Finally, patients with MIS-C express elevated levels of SARS-CoV-2 anti-Spike protein and anti-receptor binding domain (RBD) IgG without significantly elevated IgM titers (11, 14, 15).
Superantigen Theory
MIS-C shares many clinical features with TSS, which is triggered by bacterial superantigens (SAg) such as Staphylococcal enterotoxin B (SEnB). Using structure-based computational models, investigators identified a superantigen-like motif in the SARS-CoV-2 spike protein S1/2 cleavage site that interacts with T-cell receptors (TCR) and MHC class II molecules, prompting a nonspecific T-cell activation and ‘cytokine storm’ (14). Using next-generation immunosequencing analysis of T cell repertoires, investigators have also reported a skewed (Vβ-skewing) T cell receptor repertoire in adult COVID-19 patients with severe hyperinflammation, as well as in children with MIS-C (15, 16), consistent with SAg triggered immune responses. These data support the hypothesis that MIS-C, as well as the cytokine storm observed in adult patients with severe COVID-19, may be mediated by SAg-like activity of the SARS-CoV-2 spike protein. The late timing of such a superantigen response in the course of infection and the propensity for only a minority of SARS-CoV-2-exposed individuals to manifest such a response maybe related to a second trigger in predisposed individuals that has not yet been identified. One explanation as to why the hyperinflammatory response to viral SAg would occur as a delayed response is the phenomenon associated with repeated exposure to bacterial or viral superantigens potentially causing the development of auto-immunity (14).
Chronic Response to Viral Antigen Exposure
Several studies support the theory that MIS-C may be secondary to chronic exposure to SARS-CoV-2 viral antigens. Cell surface markers on T lymphocytes were found to be consistent with antigenic activation and lymphocyte exhaustion in a cohort of 14 MIS-C patients compred with 16 matched pediatric COVID-19 cases, suggestive of prolonged stimulation (17). Moreover, 90% of MIS-C patients present with severe gastrointestinal (GI) symptoms, suggesting possible gastrointestinal involvement (1) and the possibility of distrupted gut permeability and microbial translocation (18, 19). Yonker et al. found that some children with MIS-C have a prolonged presence of SARS-CoV-2 in the GI tract, as detected by stool RT-PCR (7 of 12 patient samples processed detected viral RNA), and expressed elevation in systemic zonulin levels (mass cytometry), a marker of increased intestinal permeability, and may suggest that chronic low systemic exposure to SARS-CoV-2 by gastrointenstinal-vascular tranlocation may be associated with MIS-C (20). To support this hypothesis, a MIS-C patient treated with a Zonulin antagonist caused decreased plasma SARS-CoV-2 Spike antigen levels and improved clinical course compared to currently available therapies (20). Contributory to this argument are the high IgA titers found in MIS-C patients, indicative of elevated mucosal immunity (15). Some have hypothesized that less severe disease portends a persistent subacute infectious state or incomplete eradication causing prolonged exposure to viral material and triggering a systemic inflammatory process (17, 21).
Immunologic mechanisms of the systemic inflammatory process in MIS-C
There are several distict immunologic profiles that render MIS-C a unique systemic inflammatory syndrome (Figure 1). Compared to other inflammatory syndromes, the distinct constellation of symptoms in MIS-C are due to the specific nature of the tissue targets and inflammatory cascade identified in this syndrome. For simplicity, humoral and cellular adaptive immunity and the innate response can be considered separately, although, these systems are deeply intertwined.
Figure 1.
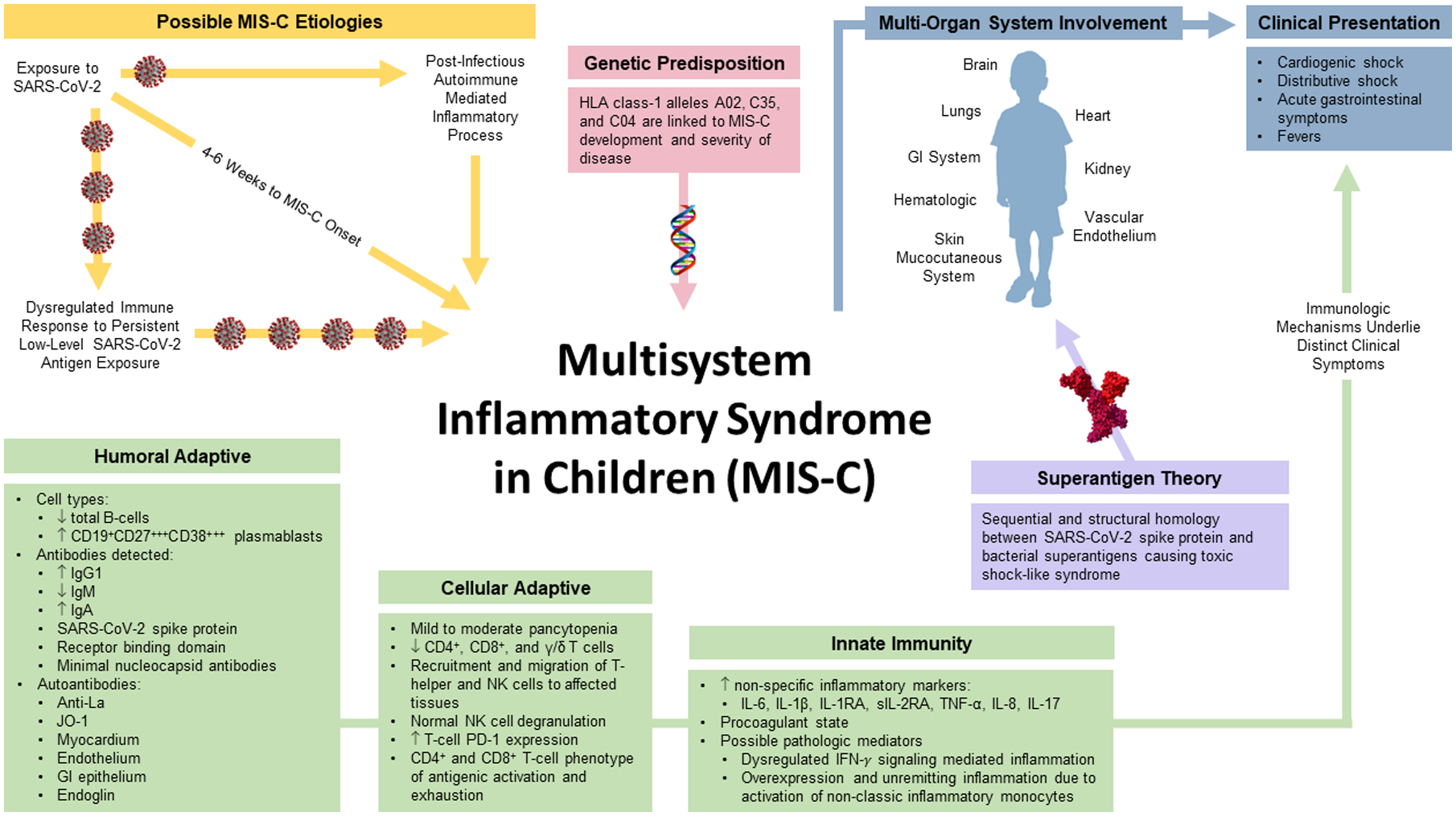
Humoral Adaptive Immunity
Microarray data in approximately 50 patients with MIS-C has found elevated levels of specific autoantibody targets, some of which are similar and others distinct from several well-characterized auto inflammatory disorders (13, 22). Elevated anti-La and anti-Jo-1 antibodies have been noted in MIS-C, which is common in Sjögren’s syndrome and idiopathic inflammatory myopathies, respectively. Additionally, autoantibodies targeting myocardial and endothelial tissue, as well as gastrointestinal epithelium and cellular immune mediator targets were also identified (13, 15). Autoantibodies against endoglin, which target the structural integrity of arteries are found in Kawasaki Disease and were demonstrated in a cohort of 23 patients with MIS-C. Furthermore, there is evidence that serum antibodies of severely ill (from 14 severe of 23 total cases) MIS-C bind human cardiac microvascular endothelial cells (15). While the absolute number of B-cells may decrease during the acute phase of MIS-C, there is an increased proportion of CD19+CD27+CD38+ plasmablasts (from 25 MIS-C patients) (23) and proliferating plasmablasts (15). In convalesced adult patients, plasmablast counts return to baseline approximately 2–4 weeks post infection, indicative of a dysregulated adaptive plasmablast response to SARS-CoV-2 (17) in MIS-C patients. The possibility of circulating peripheral blood immune complexes was explored, but none were discovered in blood samples from patients with MIS-C (12). However, the presence of circulating peripheral blood immune complexes in tissue biopsy samples has yet to be examined.
Cellular Adaptive Immunity
There seems to be a specific subset of activated, inflammatory T cells that are targeted for tissue and vascular endothelia recruitment. These changes are likely caused by either tissue-specific autoantibodies, direct viral invasion of tissues, and/or T cell activation by SAg. Mild to moderate lymphopenia is a hallmark of disease in MIS-C with decreased absolute numbers of B cells, α/β T cells (CD4+ and CD8+) and γ/δ T cells. Cellular migration, consumption, and recruitment may contribute to this leukopenia, mediated by interleukin (IL)-17, CCL20 and CCL28 (T helper cell mucosal recruitment) (10, 13), CCL19, CXCL10, and CDCP1 (T cell and NK cell recruitment to affected tissues) (22, 23), and upregulation of the surface αLβ2 integrin, CD11a/CD18 (leukocyte activation, differentiation and endothelial adhesion) (24, 25).
Deep cellular immune profiling has identified an adaptive immune phenotype suggestive of persistent CD4+ and CD8+ T-cell antigenic activation and exhaustion, represented by a significant elevation in programmed cell death protein (PD)-1 expression compared with acutely infected subjects. Both CD4+ and CD8+ T-cells have been found to be highly proliferative. In addition, “vascular patrolling” CD8+ T-cells expressing CX3CR1 were present in increased numbers, indicative of an activated vascular endothelium (17).
Innate Immunity
The innate immune system is essential for initial rapid detection and killing of viruses, production of interferons as well as proinflammatory cytokines and subsequent activation of adaptive immunity. Nonspecific inflammatory markers, including ESR, CRP, D-dimer, ferritin, and fibrinogen, are elevated in patients with MIS-C. In contrast to patients with Kawasaki disease, MIS-C patients typically present with mild thrombocytopenia and elevation of PT, PTT, and INR, with evidence of a procoagulant state (26). Troponin and brain natriuretic peptide (BNP) elevation are also distinguishing features of MIS-C in contrast to other childhood systemic auto inflammatory syndromes (4, 27).
Two main inflammatory pathways have emerged as the most likely pathologic mediators of MIS-C: 1) the IFN-ɣ mediated inflammatory pathway and 2) the non-classic inflammatory monocyte pathway. Increased circulating levels of IFN-ɣ, IL-18, CXCL10 (IP-10), CCL5 (RANTES), and GM-CSF suggest systemic inflammation due to a dysregulated IFN-ɣ signaling cascade (12). Additionally, the negative regulator of IFN-ɣ signaling, TWEAK, was not found to be elevated (22).
However, several factors suggest a response initiated by inflammatory monocytes. Elevated levels of CCL2 (MCP-1), IL-1α and IL-1RA (12), a decrease in the proportion of classic, CD14high monocytes, and an increase in the proportion of non-classical CD14low, CD16high monocytes with CD64high expression are indicative of an activated, cytokine-producing phenotype. High percentages of neutrophils also express high levels of CD64 and neutrophils and monocytes express high levels of S100A-family alarmins, suggestive of an active inflammatory process (15, 23, 24). In addition, cell surface expression of ICAM1 on both neutrophils and monocytes are indicative of leukocyte activation (13). Decreased expression of HLA-DR and CD86 was observed on monocytes and dendritic cells, consistent with reduced antigen-presenting capacity (15, 23). Finally, both pro- and anti-inflammatory changes have been seen in the neutrophils of a cohort of 21 children with MIS-C including the proinflammatory markers αMβ2 integrin CD11b/18, CD66b, and components of neutrophil extracellular traps, as well as the anti-inflammatory markers leukocyte-associated immunoglobulin-like receptor-1 (LAIR-1) and programmed death-ligand 1 (PD-L1) (28).
Conclusions:
Evidence suggests that MIS-C patients have an activation of the innate immune system with massive pro-inflammatory cytokine production and ongoing maladaptive immune responses with dysregulated T- and B-cell function. While the timing supports a post-infectious process, there may also be ongoing and sustained antigenic stimulation of the immune system. Superantigens may also play a role in the pathogenesis of MIS-C, as well as autoantibodies targeting the specific organ systems. While these data remain hypothesis-generating, it is informative as a framework and template for investigation of currently known and future autoinflammatory conditions. Ongoing investigations into the genetic influences predisposing young patients to this disease will undoubdedly shed additional light on the associated immune perturbations related to MIS-C. Recent work already suggests that specific HLA haplotypes are more frequently associated with this disease entity (16).
Trials continue to evaluate best practice immunomodulatory therapies as mono- or combination therapy regimens which include corticosteroids, intravenous immunoglobulins (IVIG) as well as several investigational biologic treatments. Whereas corticosteroids mainly target nonspecific systemic inflammation suppression, several in-use and investigational therapies aim to target the specific immunologic and inflammatory derangements orbserved in this patient population. As this appears to be a self-limiting inflammatory process, there remains evidence suggesting that supportive care without immunomodulatory therapy is non-inferior to current recommended immune therapy in terms of clinical outcomes (29, 30). Nevertheless, further investigation and additional clinical trials of targeted immunomodulatory therapies are warranted in combination with the development of a continued deeper understanding of the immunopathology of MIS-C. IVIG may interfere with autoantibody targets and antagonistic monoclonal antibodies targeting inflammatory cytokines such as IL-1, IL6 or IFN-γ could prove effective as well.
A high level mechanistic understanding of the specific immune perturbations in each autoinflammatory disease will provide more precise therapeutic targets for investigation. Additional mechanistic studies in children are necessary to further understand this rare syndrome and best treatment modalities.
Conflicts of Interest and Source Funding:
KER has received funding from the NIH, NIGMS GM129763 and from the National Center for Advancing Translational Sciences (NCATS) UL1TR0002345. The remaining authors declare that they have no conflicts of interest to report.
Copyright Form Disclosure:
Drs. Mazer and Remy received support for article research from the National Institutes of Health (NIH). Dr. Lam’s institution received funding from the NIH, the National Heart, Lung, and Blood Institute, the National Institute of General Medical Sciences (NIGMS), and the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Shein received funding from Hill Ward Henderson. Dr. Remy received funding from the NIH/NIGMS (K08 GM129763). The remaining authors have disclosed that they do not have any potential conflicts of interest.
References:
- 1.Feldstein LR, Tenforde MW, Friedman KG, et al. : Characteristics and Outcomes of US Children and Adolescents With Multisystem Inflammatory Syndrome in Children (MIS-C) Compared With Severe Acute COVID-19. Jama 2021; 325:1074–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC: Multisystem Inflammatory Syndrom (MIS-C). Available at: https://www.cdc.gov/mis-c/. Accessed 6/13/21, 2021
- 3.Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19. Available at: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19. Accessed 6/13/2021, 2021
- 4.Feldstein LR, Rose EB, Horwitz SM, et al. : Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. The New England journal of medicine 2020; 383:334–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viner RM and Whittaker E: Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet (London, England) 2020; 395:1741–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung EW, Zachariah P, Gorelik M, et al. : Multisystem Inflammatory Syndrome Related to COVID-19 in Previously Healthy Children and Adolescents in New York City. Jama 2020; 324:294–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noval Rivas M, Porritt RA, Cheng MH, et al. : COVID-19-associated multisystem inflammatory syndrome in children (MIS-C): A novel disease that mimics toxic shock syndrome-the superantigen hypothesis. J Allergy Clin Immunol 2021; 147:57–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowley AH, Shulman ST and Arditi M: Immune pathogenesis of COVID-19-related multisystem inflammatory syndrome in children. J Clin Invest 2020; 130:5619–5621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shekerdemian LS, Mahmood NR, Wolfe KK, et al. : Characteristics and Outcomes of Children With Coronavirus Disease 2019 (COVID-19) Infection Admitted to US and Canadian Pediatric Intensive Care Units. JAMA Pediatr 2020; 174:868–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kabeerdoss J, Pilania RK, Karkhele R, et al. : Severe COVID-19, multisystem inflammatory syndrome in children, and Kawasaki disease: immunological mechanisms, clinical manifestations and management. Rheumatol Int 2021; 41:19–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson EM, Diorio C, Goodwin EC, et al. : SARS-CoV-2 antibody responses in children with MIS-C and mild and severe COVID-19. J Pediatric Infect Dis Soc 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esteve-Sole A, Anton J, Pino-Ramirez RM, et al. : Similarities and differences between the immunopathogenesis of COVID-19-related pediatric multisystem inflammatory syndrome and Kawasaki disease. J Clin Invest 2021; 131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gruber CN, Patel RS, Trachtman R, et al. : Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C). Cell 2020; 183:982–995 e914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng MH, Zhang S, Porritt RA, et al. : Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation. Proc Natl Acad Sci U S A 2020; 117:25254–25262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramaswamy A, Brodsky NN, Sumida TS, et al. : Immune dysregulation and autoreactivity correlate with disease severity in SARS-CoV-2-associated multisystem inflammatory syndrome in children. Immunity 2021; 54:1083–1095 e1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porritt RA, Paschold L, Noval Rivas M, et al. : HLA class I-associated expansion of TRBV11–2 T cells in Multisystem Inflammatory Syndrome in Children. J Clin Invest 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vella LA, Giles JR, Baxter AE, et al. : Deep immune profiling of MIS-C demonstrates marked but transient immune activation compared to adult and pediatric COVID-19. Sci Immunol 2021; 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giron LB, Dweep H, Yin X, et al. : Plasma Markers of Disrupted Gut Permeability in Severe COVID-19 Patients. Front Immunol 2021; 12:686240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliva A, Miele MC, Di Timoteo F, et al. : Persistent Systemic Microbial Translocation and Intestinal Damage During Coronavirus Disease-19. Front Immunol 2021; 12:708149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yonker LM, Gilboa T, Ogata AF, et al. : Multisystem inflammatory syndrome in children is driven by zonulin-dependent loss of gut mucosal barrier. J Clin Invest 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weisberg SP, Connors TJ, Zhu Y, et al. : Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat Immunol 2021; 22:25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Consiglio CR, Cotugno N, Sardh F, et al. : The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell 2020; 183:968–981 e967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carter MJ, Fish M, Jennings A, et al. : Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med 2020; 26:1701–1707 [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Salido A, Cuenca-Carcelen S and Castillo-Robleda A: CD64, CD11a and CD18 leukocytes expression in children with SARS-CoV-2 multisystem inflammatory syndrome versus children with Kawasaki disease: Similar but not the same. Med Clin (Barc) 2021; 156:89–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walling BL and Kim M: LFA-1 in T Cell Migration and Differentiation. Front Immunol 2018; 9:952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pouletty M, Borocco C, Ouldali N, et al. : Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis 2020; 79:999–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee PY, Day-Lewis M, Henderson LA, et al. : Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflammatory syndrome in children. J Clin Invest 2020; 130:5942–5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seery V, Raiden SC, Algieri SC, et al. : Blood neutrophils from children with COVID-19 exhibit both inflammatory and anti-inflammatory markers. EBioMedicine 2021; 67:103357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McArdle AJ, Vito O, Patel H, et al. : Treatment of Multisystem Inflammatory Syndrome in Children. The New England journal of medicine 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davies P, Lillie J, Prayle A, et al. : Association Between Treatments and Short-Term Biochemical Improvements and Clinical Outcomes in Post-Severe Acute Respiratory Syndrome Coronavirus-2 Inflammatory Syndrome. Pediatr Crit Care Med 2021; 22:e285–e293 [DOI] [PMC free article] [PubMed] [Google Scholar]


