Abstract
Background:
Patterns of medication use and efficacy in aspirin-exacerbated respiratory disease (AERD) have not been well characterized, especially since the advent of respiratory biologics. Aspirin-therapy after desensitization (ATAD) is efficacious for upper and lower respiratory symptoms for AERD patients, though aspirin-related side effects can limit therapy. The optimal coordination of ATAD and respiratory biologics for treatment of AERD remains unclear.
Objective:
We aimed to characterize patterns of medication use and treatment experience with biologics and ATAD in AERD.
Methods:
We surveyed 98 patients with AERD recruited from the Brigham and Women’s Hospital AERD registry. Patients completed an online questionnaire describing their medication history and treatment experience.
Results:
Fifty-two (53.0%) patients reported a history of biologic use (omalizumab, mepolizumab, reslizumab, benralizumab, and/or dupilumab), and 84 (85.7%) reported undergoing aspirin desensitization. Twenty-four patients (24.4%) reported concurrent use of a biologic and ATAD. Compared to those taking ATAD alone, patients taking a biologic and ATAD concurrently were less likely to report that aspirin was effective for their AERD symptoms (OR 0.161 [95% CI 0.03–0.76], p=.02). While patients reported varying efficacy with biologics, dupilumab had the highest odds of patients reporting it worked “Very Well” (OR 17.58 [95% CI: 5.68–54.35], p <0.0001).
Conclusion:
Biologics are emerging as a treatment option for AERD, and are generally well tolerated. Biologic efficacy in AERD is variable by agent, though the majority of patients taking dupilumab found it to be effective. Patients on a biologic in conjunction with ATAD may represent a more severe subset AERD for which ATAD alone is insufficient.
Keywords: aspirin-exacerbated respiratory disease, respiratory biologic, aspirin desensitization, omalizumab, mepolizumab, reslizumab, benralizumab, dupilumab
Introduction
Aspirin-exacerbated respiratory disease (AERD), also known as NSAID-exacerbated respiratory disease, is a chronic inflammatory disease characterized by asthma, chronic rhinosinusitis with nasal polyps (CRSwNP), and respiratory reactions to cyclcooxygenase-1 inhibitors. Asthma and CRSwNP are a substantial burden for AERD patients,1,2 who frequently require trials of multiple medications to obtain adequate symptom control.3
Patterns of medication use in AERD have not been well characterized, especially since the advent of respiratory biologics, now approved for severe asthma and CRSwNP.4–10 Respiratory biologics are monoclonal antibodies targeting specific mediators in type 2 inflammatory pathways, which are dysregulated in AERD.11 Omalizumab (anti-IgE), mepolizumab (anti-IL-5), and dupilumab (anti-IL-4Rα) all have at least some efficacy in treatment of CRSwNP or asthma in patients with AERD.6,12–15 Notably, there have been no head to head trials comparing the efficacy of different biologics in the treatment of AERD.
Aspirin therapy after desensitization (ATAD) is a unique treatment modality for AERD, which has long been a mainstay of clinical management16. During desensitization, patients with AERD are given sequentially higher doses of aspirin until a respiratory reaction is provoked, after which a state of tolerance is reached, allowing for higher doses of aspirin to be administered; patients then take high-dose aspirin daily as a continuing medication. ATAD has been shown to improve lung function and quality of life, and also reduce sinonasal symptoms and medication use17–21. However, there is an increased risk of adverse events associated with ATAD compared to placebo18.
At this time, the optimal use and coordination of ATAD, respiratory biologics, and other medications in the treatment of AERD remains unclear. Additionally, there are minimal data regarding patient-reported experiences with these medications. Understanding patient perceptions of medication efficacy and tolerability, in addition to impacts on quality of life, is of foundational importance in understanding how to treat this complex disease. To this end, we sought to characterize patterns of medication use and therapeutic experience among patients with AERD.
Methods
We conducted a single-center, cross-sectional survey-based study at the Brigham and Women’s Hospital (BWH) in September 2020. The study was approved by the Mass General Brigham Institutional Review Board. Subjects provided informed consent and were compensated a nominal fee for their participation. Eligible patients were 18 years old or greater with a physician-confirmed diagnosis of AERD, followed for their AERD care at a Mass General Brigham facility, and enrolled in BWH AERD Registry (an IRB approved database of patients with AERD). An initial recruitment email and one reminder email was sent to the 303 eligible patients in the BWH AERD registry. The first 100 patients to respond were enrolled in the study. Patients completed an online questionnaire describing their medication history and AERD treatment experience. Additionally, patients also completed the 22 Item Sinonasal Outcome Test (SNOT-22) for assessment of sinonasal symptoms, the Asthma Control Test (ACT) for assessment of asthma symptoms, and the Healthy Days Core Module (HRQOL-4), a CDC-designed validated questionnaire assessing quality of life in the preceding 30 days. After enrollment, two patients did not complete the survey. Data from the electronic medical record was accessible to the study team, but only used to clarify major discrepancies in patient responses. Data were collected and managed using Research Electronic Data Capture (REDCap®). Data were exported and analyzed using Microsoft Excel (Redmond, WA), GraphPad Prism 9.2 (La Jolla, CA), and SAS v9.4 (Cary, NC). Student’s t test was used to compare means. The chi-square test, logistic regression, and multinomial logistic regression were used to assess relationships between categorical variables. The Generalized Estimating Equation was used to analyze patient-reported efficacy of biologic agents.
Results
Study Population and Demographics
Patient demographics are summarized in Table 1. Survey respondents included 60 (61.2%) women and 38 (38.8%) men. At the time of survey, mean age of participants was 51.8 ± 12.2 years. Mean age at asthma diagnosis was 33.2 ± 14.2 years, and mean age at nasal polyp diagnosis was 37.1 ± 12.4 years. Patients reported a mean of 3.0 ± 2.7 lifetime sinus surgeries. Patients reported using a median of 4 different classes of medications (range 1–7), including inhalers, intranasal corticosteroids, anti-leukotriene agents, antihistamines, high-dose aspirin, and respiratory biologics at the time of the survey (Figure 1). A greater percentage of women were on daily antihistamines, compared to men (60.0% vs 31.6%, p=0.006, Chi-square test). Otherwise, there were no age- or gender-associated differences in medication use.
Table 1:
Patient Demographic and Clinical Characteristics
| Demographic and Clinical Characteristics (N = 98) | |
|---|---|
| Age (mean ± SD) | 51.8 ± 12.2 years |
| Age at Asthma Diagnosis (mean ± SD) | 33.2 ± 14.2 years |
| Age at Nasal Polyp Diagnosis (mean ± SD) | 37.1 ± 12.4 years |
| Number of Lifetime Endoscopic Sinus Surgeries (mean ± SD) | 3.0 ± 2.7 |
| ACT (mean ± SD) | 22.4 ± 3.4 |
| SNOT-22 (mean ± SD) | 22.9 ± 18.3 |
| Gender | n (%) |
| Male | 38 (38.8%) |
| Female | 60 (61.2%) |
| Race | n (%) |
| White | 89 (90.8) |
| Black | 3 (3.1) |
| More than one race | 3 (3.1) |
| Asian | 1 (1.0) |
| Other | 1 (1.0) |
| Prefer Not to Say | 1 (1.0) |
| Ethnicity | n (%) |
| Hispanic/Latino | 2 (2.0) |
| Total Annual Household Income | n (%) |
| $0–$35,000 | 5 (5.1) |
| $35,001–$70,000 | 15 (15.3) |
| $70,001–105,000 | 19 (19.3) |
| >$105,001 | 50 (51.0) |
| No response provided | 9 (9.1%) |
Figure 1: Current Medications.
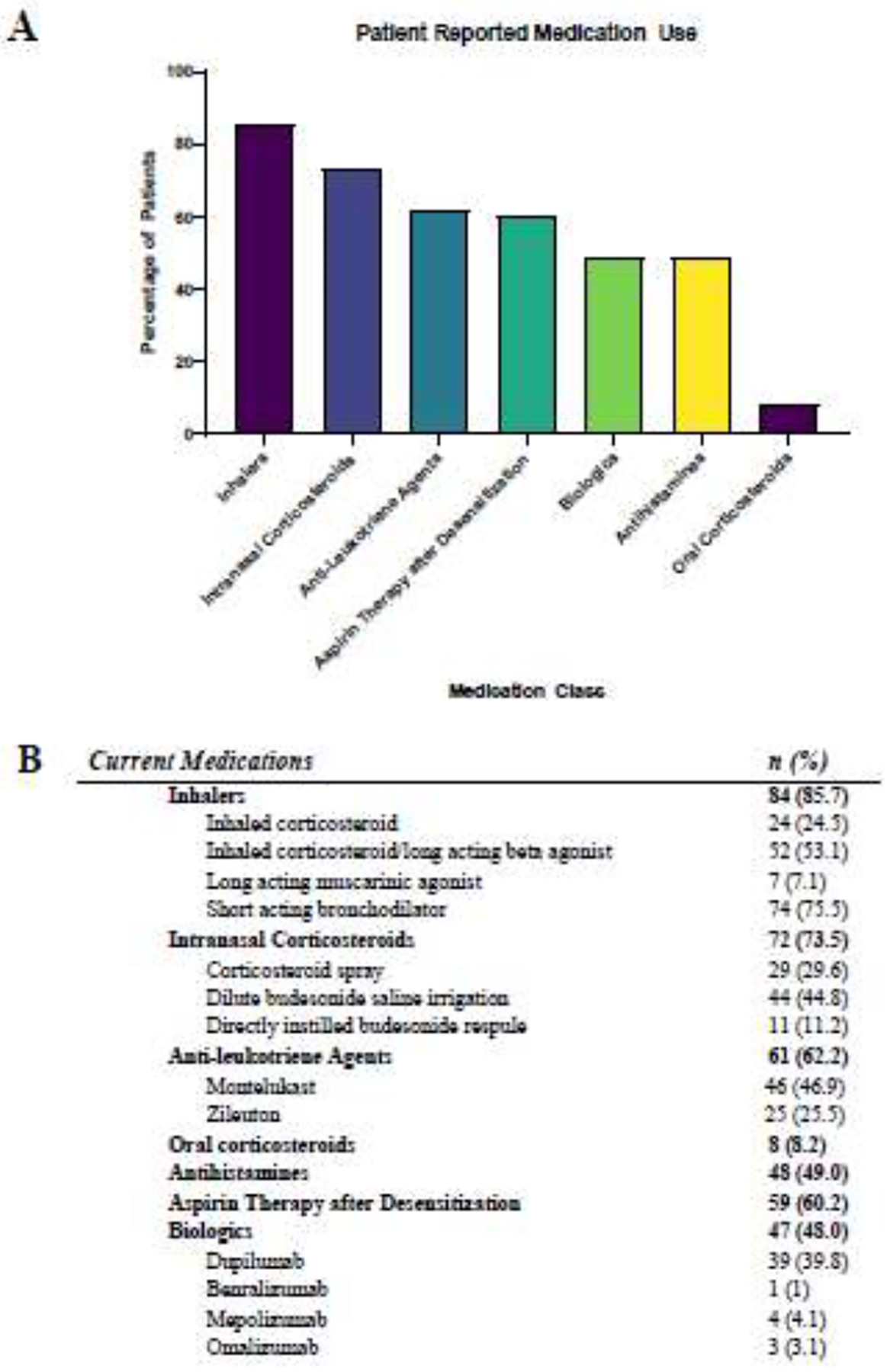
Patients self-reported the medications they were currently taking for the treatment of AERD through an online questionnaire. Specific numeric data and details of medication categories depicted in the bar graph (A) and noted in the table (B).
Aspirin therapy after desensitization
Eighty-four patients (85.7%) reported having undergone aspirin desensitization (Figure 2), with the majority (n=78) continuing daily aspirin (650mg – 1300mg daily) afterward. Unsuccessful desensitization (never reaching a state of tolerance) was the most common reason for not taking aspirin post-desensitization (n=4). Patients who continued aspirin therapy after desensitization (ATAD) reported a taking aspirin for a mean of 46.2 ± 40.5 months (range 1–240 months). Of these patients, 75.6% responded “Yes” when asked: “Was daily aspirin effective?” with response choices “Yes”, “No”, and “Not sure”. Among patients who found ATAD effective, most patients reported improvements in sinus symptoms and asthma, with fewer reporting improvements in otologic symptoms and general well-being (Figure 3). The most common reason for ATAD discontinuation was aspirin related side effects (n=10).
Figure 2: Outcomes After Aspirin Desensitization.
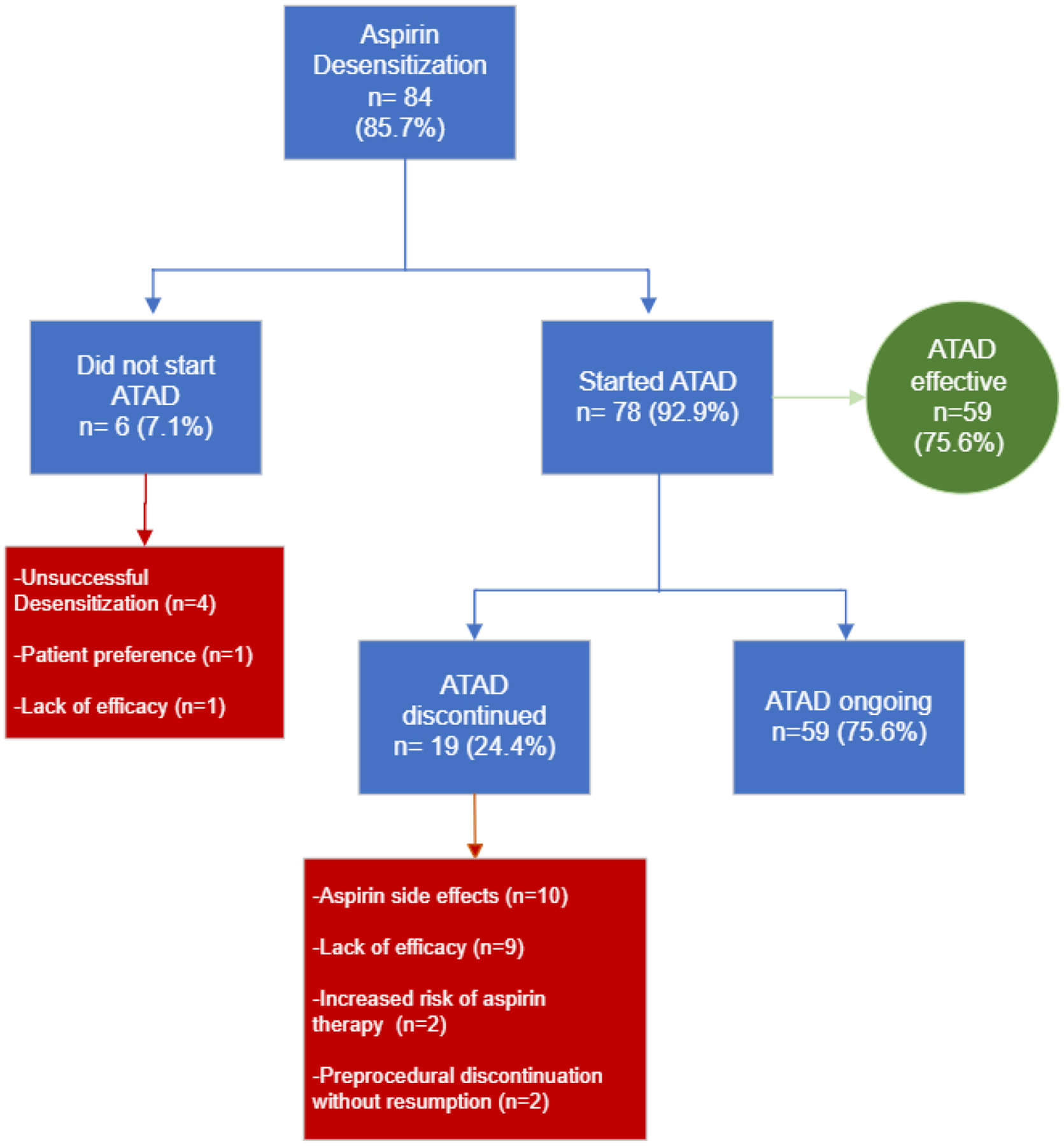
Patients self-reported reasons for not taking aspirin post-desensitization and discontinuing aspirin after daily aspirin therapy; categories of response were determined by review of patient comments and medical records. The green-outlined box lists the percentages of patients who found aspirin therapy after desensitization effective for a given AERD symptom.
Figure 3: Symptom Improvement after Aspirin Desensitization.
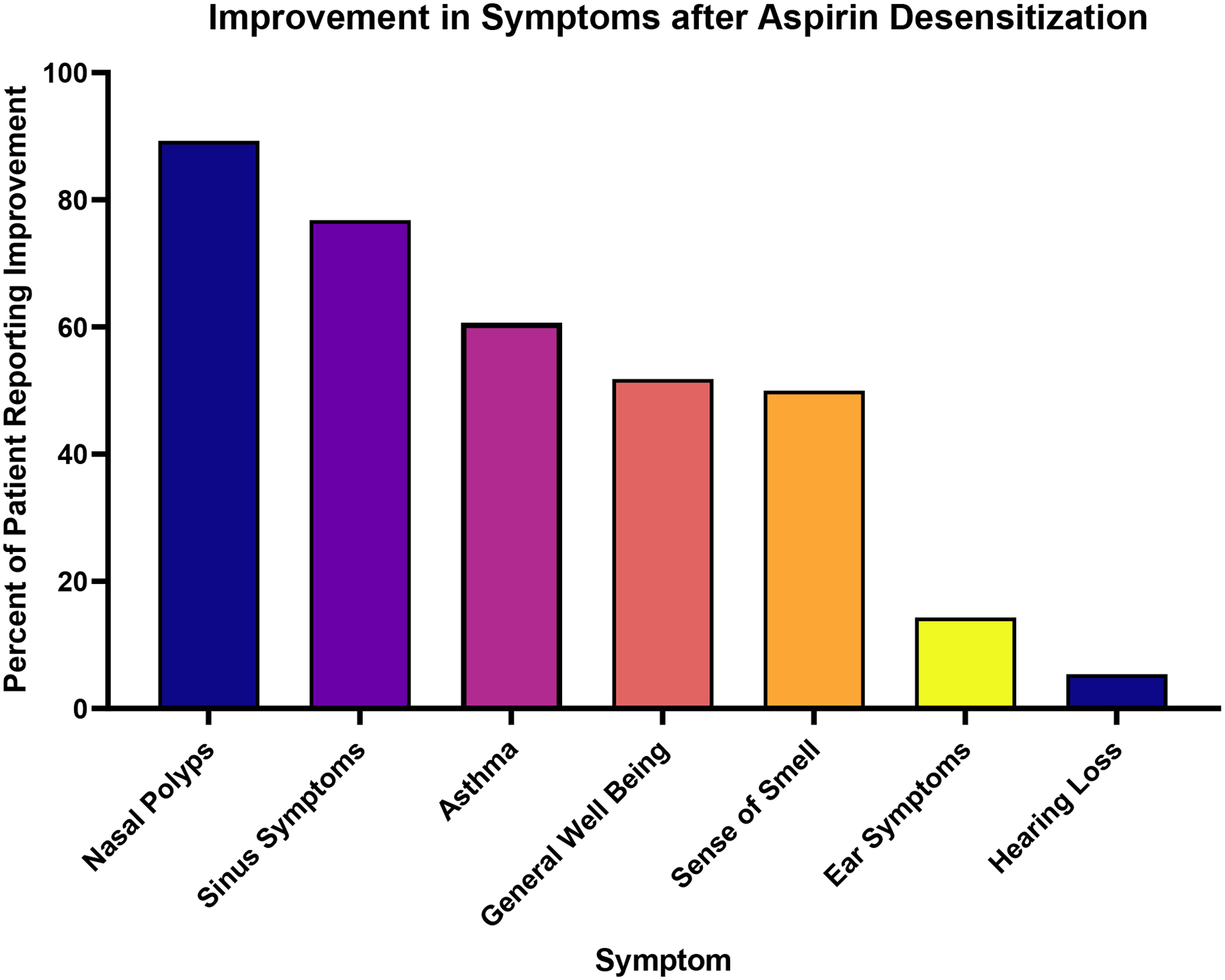
Patients who reported that aspirin-therapy after desensitization (ATAD) was effective (n=59) were then asked to report which symptoms were improved by ATAD. Patients were permitted to select multiple symptoms. The majority of patients reported ATAD as effective in treating nasal polyps and sinus symptoms.
Respiratory biologic treatment
Fifty-two (53.0%) patients reported history of respiratory biologic use (omalizumab, mepolizumab, reslizumab, benralizumab, and/or dupilumab); 20 patients had trialed multiple biologic agents (Figure 4). There were 30 patients who started but then discontinued a biologic, and among those, all but four were transitioned to an alternative biologic. The most common reason cited for biologic discontinuation or transition was lack of efficacy. There were only five instances of biologic side effects reported as the reason for discontinuation: hives and post-injection dizziness with omalizumab, body aches with mepolizumab, muscle aches with reslizumab, and gastric side effects with dupilumab. The majority of patients who transitioned biologic therapy ultimately started dupilumab; no patients had switched from dupilumab to another biologic. Patients were asked to rate how well a respiratory biologic worked for them, with response options “Very Well”, “OK”, “Not at all” and “Not Sure.” Compared to other biologics, dupilumab had the highest odds of participants reporting it worked “Very Well,” (OR 17.58 [95% CI: 5.68–54.35, p <0.0001], Generalized Estimating Equation). Specifically, 85.7% of patients on dupilumab reported it worked “Very Well,” compared to 40.0% of those who took omalizumab, 26.1% of patients who took mepolizumab/reslizumab, and none of the patients who took benralizumab (Figure 5).
Figure 4: Outcomes of biologic therapy among AERD patients.
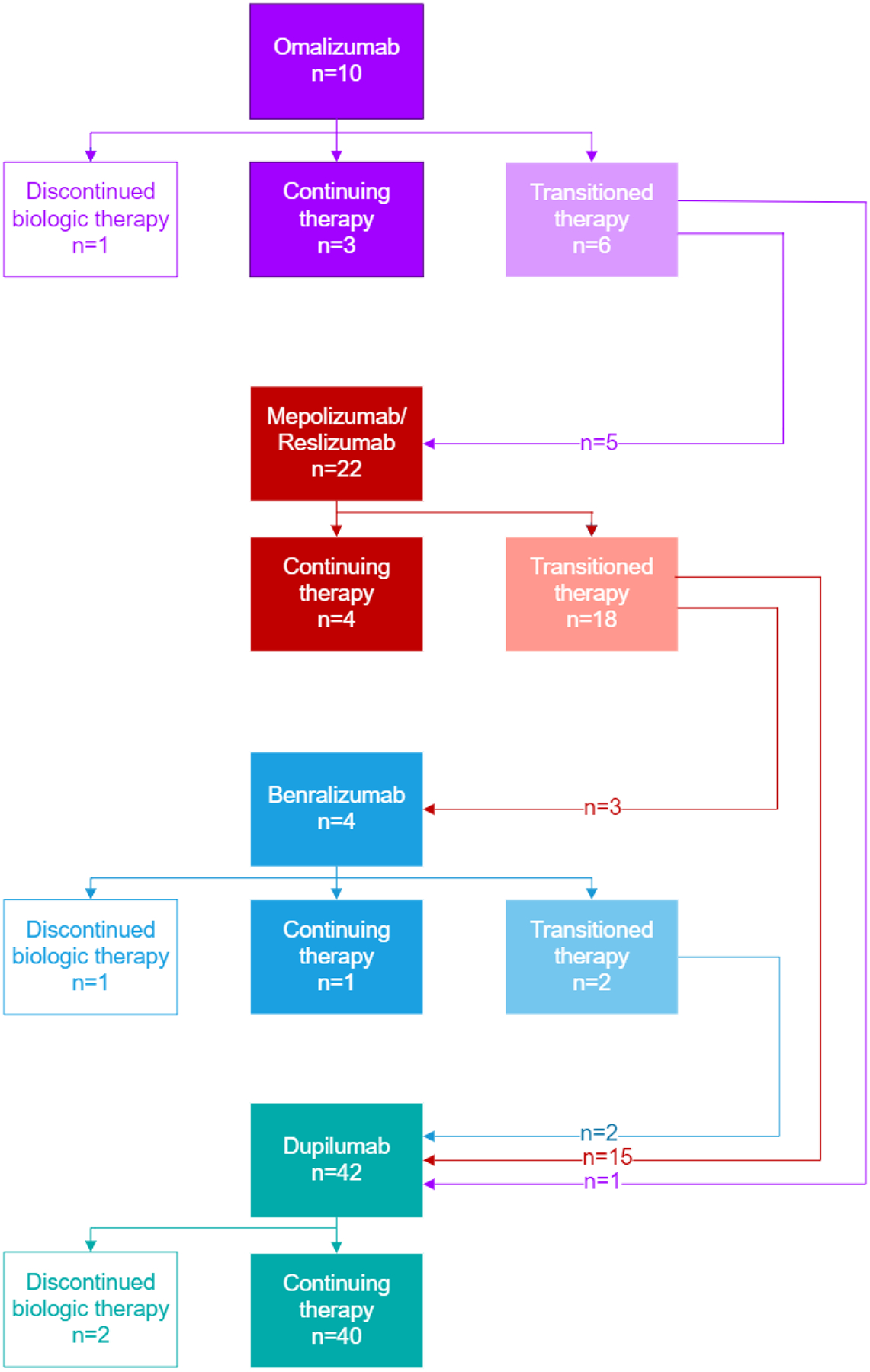
Most patients who discontinued to a biologic transitioned to a different biologic agent, with 19 patients ultimately being transitioned to dupilumab. Only four patients discontinued biologic therapy entirely.
Figure 5: Patient Reported Efficacy of Biologic Agents in AERD.
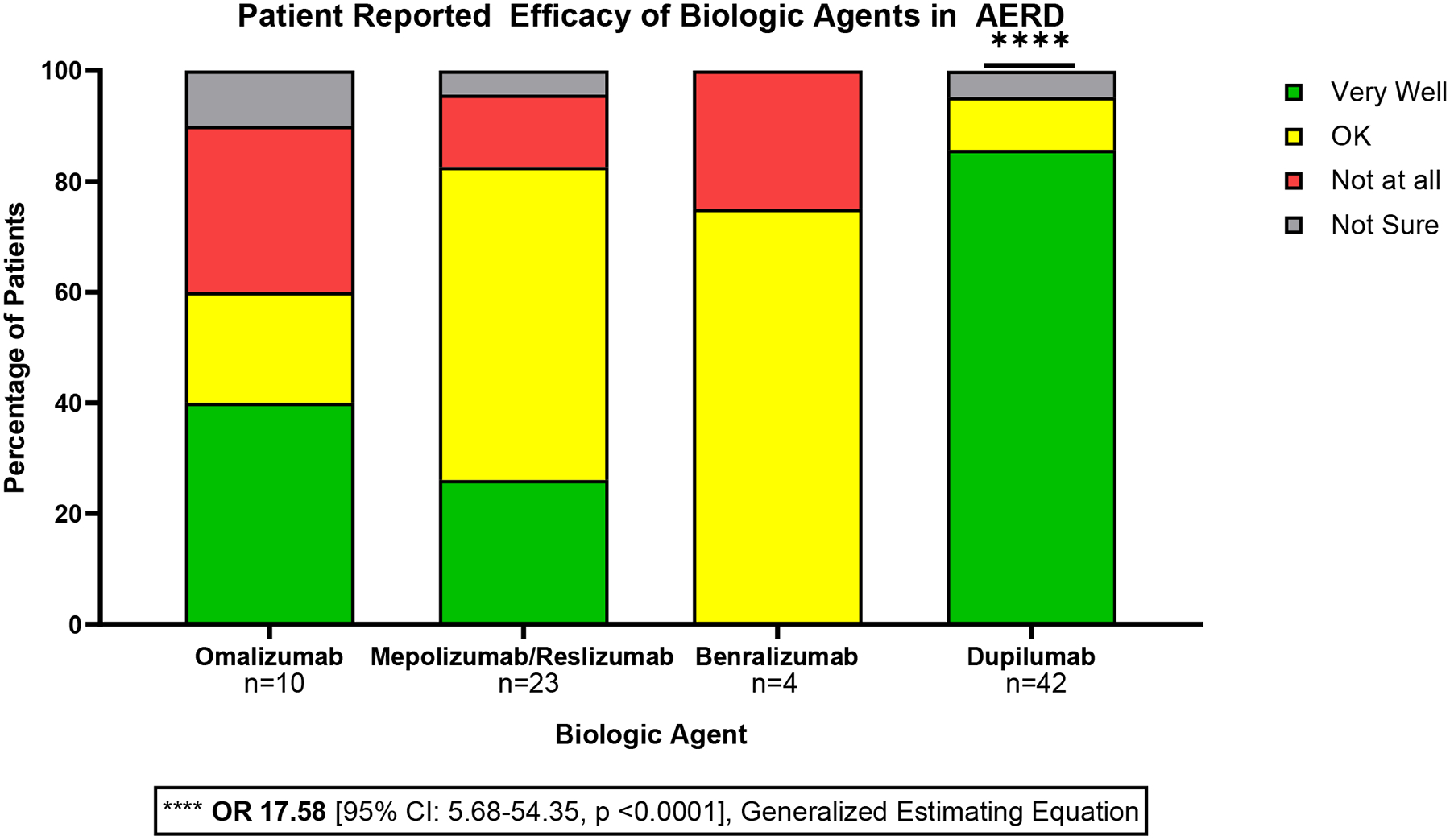
Patients who used a biologic agent were asked “How well did this medication work for you?” with response options “Very Well”, “OK”, “Not at all” and “Not Sure.” Patients were most likely to report that dupilumab worked “Very Well” (OR 17.58 [95% CI: 5.68–54.35, p <0.0001], Generalized Estimating Equation).
There were no significant differences in average SNOT-22 score, ACT score, or number of lifetime sinus surgeries among patients on a biologic alone, ATAD and a biologic, ATAD alone, or neither ATAD nor a biologic. However, patients who had a history of biologic use were more likely to have had rapid post-operative polyp regrowth (polyp recurrence in less than 6 months vs. greater than 6 months) compared to those who had not used a biologic among those who had been on a biologic (OR 3.23 [95% CI 1.14–9.17] p=0.028, multinomial logistic regression). Compared to those not on a biologic, patients currently on a biologic reported more days in the past month where their physical health was not good, as per the HRQOL-4 (6.5 vs 2.5 days, p=0.006, Student’s t test).
Twenty-four patients (24.4%) reported concurrent use of ATAD and a biologic agent. Compared to all other participants, patients on ATAD and a biologic were on average older at study entry (57.1 vs 50.0 years, p=.01, Student’s t test) and at the time of nasal polyp diagnosis (42.8 vs 35.3 years, p=.01, Student’s t test), though there were not significant differences in mean duration of nasal polyposis. Compared to patients on ATAD alone, patients on ATAD and a biologic were less likely to respond “Yes” when asked if aspirin was effective for their AERD symptoms (OR 0.161 [95% CI 0.03–0.76], p=.02, Chi-square test) (Figure 6).
Figure 6: Patient reported efficacy of aspirin therapy after desensitization in AERD.
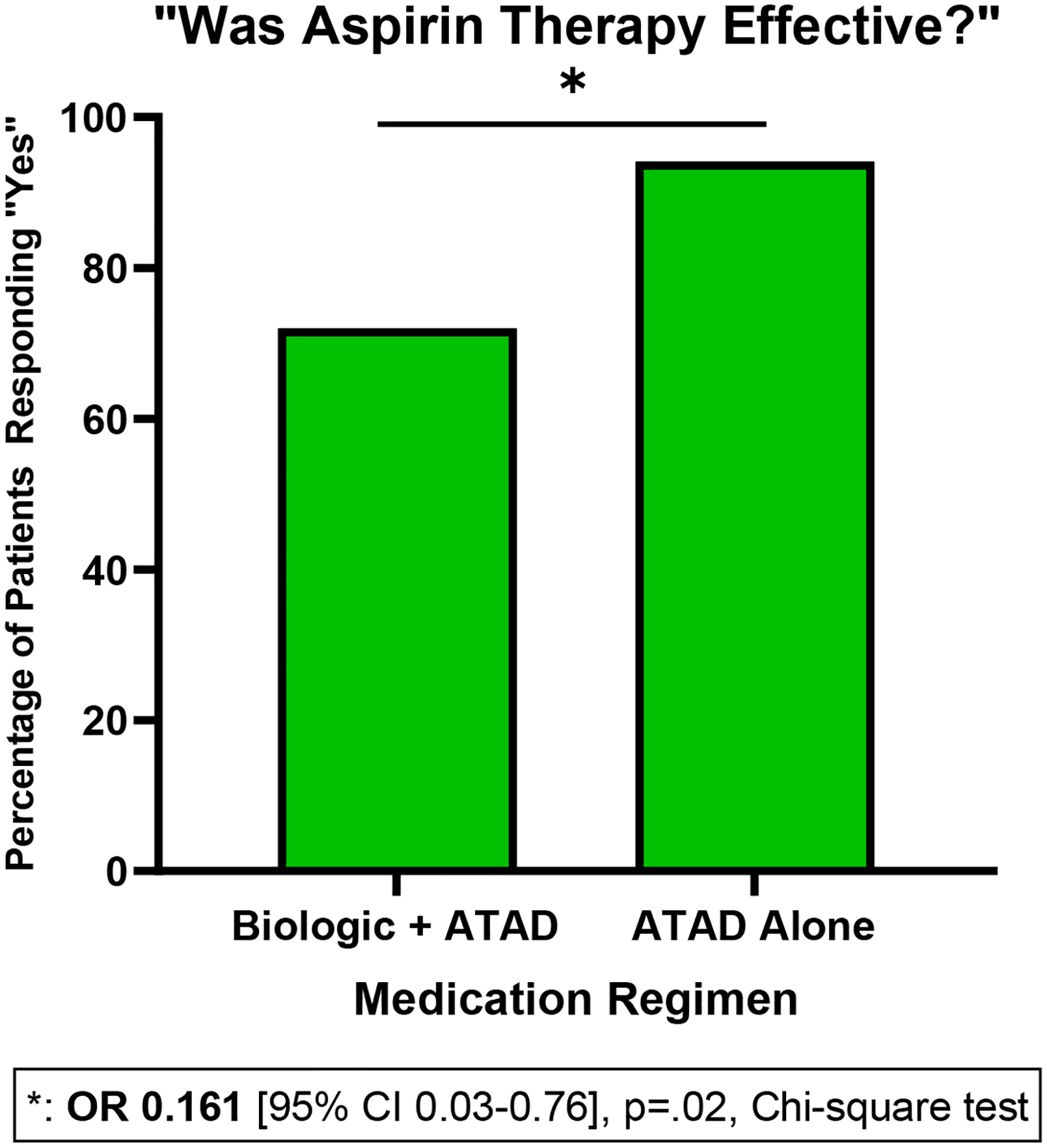
Patients who took aspirin therapy after desensitization (ATAD) were asked: “Was aspirin therapy effective?” with response options “Yes”, “No”, and “Not Sure.” Patients taking ATAD and a biologic were less likely to respond “Yes” compared to those who took ATAD alone (OR 0.161 [95% CI 0.03–0.76], p=.02, Chi-square test).
Twenty-three patients (23.4%) reported use of a biologic without aspirin. Sixteen of these patients reported taking ATAD in the past. Among this group, aspirin-related side effects (n=5) and lack of efficacy (n=7) were the most common reasons for discontinuation of ATAD.
Discussion
This study is among the first to characterize patterns of medication use and patient experience with respiratory biologics in AERD and provides pragmatic insights regarding current practices in the management of AERD at a large referral center. Our survey respondents were taking a median of four daily medications for the management of AERD, highlighting the substantial burden of this disease, as well as the potential challenges associated with cost, adherence, and quality of life. Clinicians caring for patients with AERD should anticipate prescribing multiple classes of medications to achieve adequate symptomatic control of asthma and CRSwNP.
This study confirms that patients with AERD consider aspirin therapy to be effective and well-tolerated, consistent with previous studies.16,22 The most common reasons for ATAD discontinuation were related to side effects and changes in risk/benefit stratification, rather than lack of efficacy. For patients on ATAD, clinicians should monitor aspirin-related side effects, and remain cognizant of medical history changes that would modify the risk for aspirin-related adverse events.
This study also highlights the emerging role of biologics in AERD management, with 49% of patients in this cohort currently on a respiratory biologic, with the majority on dupilumab. Dupilumab is FDA-approved for treatment of both CRSwNP and eosinophilic asthma, making it well-suited for AERD patients. While patients reported varying efficacy of biologics, the vast majority of patients who took dupilumab reported this agent worked “Very Well,” including 13 of 18 patients on dupilumab who had previously trialed a different biologic. Among that group, only one patient reported the prior biologic worked “Very Well”; in that patient, omalizumab was ultimately discontinued due to waning efficacy. While data suggests efficacy of dupilumab for the treatment of asthma and CRSwNP in AERD13, there remains a paucity of objective data comparing the efficacy of specific biologic agents in AERD. Nonetheless, the pattern observed in this cohort is consistent with previous data, highlighting the efficacy of dupilumab among AERD patients both in a real world setting23, and in those with previous inadequate response to biologic agents targeting IL-5 or IL-5Rα.24
In this study, 24 patients reported concurrent use of ATAD and a biologic. Aspirin desensitization followed by ATAD is a mainstay of AERD treatment. However, there remains little data to guide the approach to implementing biologic therapy among patients with AERD on or eligible for ATAD. In our cohort, patients on a biologic were less likely to find ATAD effective for their symptoms compared to those on ATAD alone. This pattern suggests that biologic initiation may reflect an escalation of care for those already on ATAD who do not experience adequate symptomatic relief. Patients taking both ATAD and a biologic may represent a subgroup of “medium” responders to ATAD: ATAD was sufficiently efficacious so as to not warrant discontinuation, but not enough to preclude the need for further therapy in the form of biologics. Alternatively, patients may continue on ATAD to maintain cross-tolerance to other NSAIDs. Thus, properly assessing patient response to ATAD could be a crucial step in identifying patients who may require biologic therapy. This approach would be supported by a recently proposed algorithm for the integration of biologics into AERD care.11
Additionally, a proportion of patients in our study were on a respiratory biologic without ATAD; a majority of those patients had taken ATAD in the past, with most discontinuing for aspirin related side effects and lack of efficacy. Thus, biologic therapy alone may be an option for those who tolerate ATAD poorly, or are otherwise at elevated risk for aspirin-related adverse effects, including those with renal impairment, a history of peptic ulcers, or taking other anticoagulant medications. While patients with AERD are often diagnosed at younger ages, the need to consider risk and comorbidities in management becomes especially crucial as patients become older given increased risk bleeding in elderly patients on aspirin.
Patients with AERD taking a biologic reported more days of feeling physically unwell compared to those not on a biologic based on HRQOL-4 responses. However, interestingly, there were no significant differences in mean SNOT-22 and ACT scores. The lack of significant difference in the latter may reflect the efficacy of biologic therapy in managing asthma and sinonasal disease burden; nonetheless, number of days of physical health impairment suggests that patients who initiate biologic therapy continue to have a poorer perception of their health. This data suggests that the goal of biologics in AERD may be to reduce symptoms to a manageable level, rather than to induce a complete resolution of symptoms. However, this study did not collect data regarding SNOT-22 and ACT scores prior to the initiation of biologics, which limits characterization of symptom reduction associated with biologic use.
Additionally, patients who had ever been on a biologic were more likely to have polyp growth in less than 6 months post-surgery. Thus, patients who ultimately require biologic therapy for AERD likely represent a subset of AERD patients with more severe disease. While biologic therapy may be efficacious as measured by clinical outcome scores, symptom severity and medication burden may continue to negatively influence patient perceptions of health.
This study presents some limitations. As a descriptive, survey-based investigation, the patient-reported data collected are largely subjective, introducing the possibility of recall bias. Regardless, patient perception of efficacy and tolerability remain valuable and important considerations when designing an optimal medication regimen. Additionally, the patients in the BWH AERD registry, by virtue of seeking treatment at our tertiary care center, may reflect a population of AERD patients with more severe disease requiring intensive therapy. Even within the BWH AERD registry, our cohort may represent a subgroup of individuals more likely to be on biologics due to particularly severe disease, and who may have been more likely to enroll in this study by virtue of more frequent contact with the medical system and greater investment in their treatment. Indeed, patients in this cohort report a mean of three lifetime endoscopic sinus surgeries, indicating substantial sinonasal disease.
Moreover, as a cross-sectional study, these data present patient status at a moment in time; thus, it is it not possible to discern from these data specifically whether observed characteristics necessarily reflect the inherent nature of the disease as opposed to the effect of therapy. We anticipate that many patients in this cohort are likely to initiate and discontinue both biologic therapy and ATAD over the course of time. Therefore, future studies should further characterize clinical outcomes among AERD patients on ATAD and biologics to define the optimal use of these modalities with respect to disease management, cost-effectiveness, and quality of life.
Finally, more than 70% of the patients in this study reported an annual household income greater than $70,000, with the majority on private health insurance plans, likely enabling greater access to biologic therapy from a cost perspective. Thus, our cohort’s experience may not be generalizable to other populations, especially those facing structural barriers to accessing affordable healthcare.
This study highlights the emerging role and efficacy of biologic agents in AERD, especially dupilumab. This study also reiterates the breadth of therapeutic interventions required to maintain symptomatic control in this complex disease. The data emphasize the benefits of ATAD, and while highlighting a need to carefully consider adverse effects of this therapy. Future studies will characterize the role of multimodal medical therapy on quality of life and AERD disease progression, and will evaluate the role of biologics in reducing medication burden in AERD.
Funding sources:
This work was supported by Regeneron, the National Institutes of Health (NIH grants U19AI095219, K23AI139352, R01HL128241, T32 AI007306), and by generous contributions from the Vinik and Kaye Families.
Abbreviations/Acronyms:
- AERD
Aspirin-exacerbated respiratory disease
- CRSwNP
Chronic rhinosinusitis with nasal polyps
- ATAD
Aspirin therapy after desensitization
- ACT
Asthma Control Test
- SNOT-22
22 Item Sinonasal Outcome Test
- HRQOL-4
Healthy Days Core Module
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: K.B. has served on scientific advisory boards for AstraZeneca, Regeneron, Sanofi and GlaxoSmithKline. T.L has served on scientific advisory boards for Regeneron, Sanofi, and GlaxoSmithKline. J.B. has served on scientific advisory boards for GlaxoSmithKline. J.M., D.G., R.M., and C.S. have no conflicts.
References
- 1.Bergmark RW, Palumbo M, Rahman S, et al. Aspirin-Exacerbated Respiratory Disease: Association Between Patient-Reported Sinus and Asthma Morbidity. The journal of allergy and clinical immunology In practice 2020. [DOI] [PubMed] [Google Scholar]
- 2.Alanin MC, Laidlaw T, Society TS, Hopkins C. The Burden of Non-steroidal anti-inflammatory exacerbated respiratory disease from the patient’s perspective - a qualitative analysis of posts from the Samter’s Society. Rhinology 2020;58:333–40. [DOI] [PubMed] [Google Scholar]
- 3.Ta V, White AA. Survey-Defined Patient Experiences With Aspirin-Exacerbated Respiratory Disease. J Allergy Clin Immunol Pract 2015;3:711–8. [DOI] [PubMed] [Google Scholar]
- 4.Bachert C, Han JK, Desrosiers M, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019;394:1638–50. [DOI] [PubMed] [Google Scholar]
- 5.Gevaert P, Omachi TA, Corren J, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol 2020;146:595–605. [DOI] [PubMed] [Google Scholar]
- 6.Tuttle KL, Buchheit KM, Laidlaw TM, Cahill KN. A retrospective analysis of mepolizumab in subjects with aspirin-exacerbated respiratory disease. The journal of allergy and clinical immunology In practice 2018;6:1045–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han JK, Bachert C, Fokkens W, et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 2021;9:1141–53. [DOI] [PubMed] [Google Scholar]
- 8.Castro M, Corren J, Pavord ID, et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N Engl J Med 2018;378:2486–96. [DOI] [PubMed] [Google Scholar]
- 9.Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014;371:1198–207. [DOI] [PubMed] [Google Scholar]
- 10.Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest 2004;125:1378–86. [DOI] [PubMed] [Google Scholar]
- 11.Buchheit KM, Laidlaw TM, Levy JM. Immunology-based recommendations for available and upcoming biologics in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2021;148:348–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi H, Fukutomi Y, Mitsui C, et al. Omalizumab for Aspirin Hypersensitivity and Leukotriene Overproduction in Aspirin-exacerbated Respiratory Disease. A Randomized Controlled Trial. Am J Respir Crit Care Med 2020;201:1488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laidlaw TM, Mullol J, Fan C, et al. Dupilumab improves nasal polyp burden and asthma control in patients with CRSwNP and AERD. J Allergy Clin Immunol Pract 2019;7:2462–5 e1. [DOI] [PubMed] [Google Scholar]
- 14.Buchheit KM, Lewis E, Gakpo D, et al. Mepolizumab targets multiple immune cells in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2021;148:574–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bachert C, Han JK, Desrosiers MY, et al. Efficacy and safety of benralizumab in chronic rhinosinusitis with nasal polyps: A randomized, placebo-controlled trial. J Allergy Clin Immunol 2021. [DOI] [PubMed] [Google Scholar]
- 16.Stevens WW, Jerschow E, Baptist AP, et al. The role of aspirin desensitization followed by oral aspirin therapy in managing patients with aspirin-exacerbated respiratory disease: A Work Group Report from the Rhinitis, Rhinosinusitis and Ocular Allergy Committee of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol 2021;147:827–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fruth K, Pogorzelski B, Schmidtmann I, et al. Low-dose aspirin desensitization in individuals with aspirin-exacerbated respiratory disease. Allergy 2013;68:659–65. [DOI] [PubMed] [Google Scholar]
- 18.Chu DK, Lee DJ, Lee KM, Schünemann HJ, Szczeklik W, Lee JM. Benefits and harms of aspirin desensitization for aspirin-exacerbated respiratory disease: a systematic review and meta-analysis. Int Forum Allergy Rhinol 2019;9:1409–19. [DOI] [PubMed] [Google Scholar]
- 19.Esmaeilzadeh H, Nabavi M, Aryan Z, et al. Aspirin desensitization for patients with aspirin-exacerbated respiratory disease: A randomized double-blind placebo-controlled trial. Clin Immunol 2015;160:349–57. [DOI] [PubMed] [Google Scholar]
- 20.Mortazavi N, Esmaeilzadeh H, Abbasinazari M, et al. Clinical and Immunological Efficacy of Aspirin Desensitization in Nasal Polyp Patients with Aspirin-Exacerbated Respiratory Disease. Iran J Pharm Res 2017;16:1639–47. [PMC free article] [PubMed] [Google Scholar]
- 21.Stevenson DD, Pleskow WW, Simon RA, et al. Aspirin-sensitive rhinosinusitis asthma: a double-blind crossover study of treatment with aspirin. J Allergy Clin Immunol 1984;73:500–7. [DOI] [PubMed] [Google Scholar]
- 22.Walters KM, Waldram JD, Woessner KM, White AA. Long-term Clinical Outcomes of Aspirin Desensitization With Continuous Daily Aspirin Therapy in Aspirin-exacerbated Respiratory Disease. American Journal of Rhinology & Allergy 2018;32:280–6. [DOI] [PubMed] [Google Scholar]
- 23.Wangberg H, Spierling Bagsic SR, Osuna L, White AA. Appraisal of the Real-World Effectiveness of Biologic Therapies in Aspirin-Exacerbated Respiratory Disease. J Allergy Clin Immunol Pract 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bavaro N, Gakpo D, Mittal A, Bensko J, Laidlaw TM, Buchheit KM. Efficacy of dupilumab in patients with aspirin-exacerbated respiratory disease and previous inadequate response to anti-IL-5 or anti-IL-5Rα in a real-world setting. The journal of allergy and clinical immunology In practice 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]


