The purpose of this brief commentary is to describe the growing effort to discover new drugs that can be used to treat disease caused by Mycobacterium tuberculosis. In this regard, it is estimated that over 8 million people contract tuberculosis each year, and approximately 2 to 3 million people die of this disease (6). In addition, it is thought that as many as 2 billion people have been exposed to the tuberculosis bacillus and are therefore at risk of developing active disease. In certain areas of the world, such as countries within the former Soviet Union, the case rates are climbing alarmingly. This problem is further compounded by a dramatic increase in multidrug-resistant strains of M. tuberculosis (10). An additional factor is human immunodeficiency virus, which has significantly increased the incidence of tuberculosis in sub-Saharan Africa and elsewhere (5).
While the long-term solution is a better vaccine (14), for the next several decades there will have to be continued reliance on chemotherapy. In this regard, however, there have been few additions to the existing “main-line” drugs for a considerable time. While there are some promising new agents, such as the longer-acting rifamycins, fluoroquinolones, oxazolidinones, and nitroimidazopyrans (1, 4, 7, 8, 11–13, 15, 17–19), there are as yet no extensive clinical trial data to determine the efficacy of these compounds.
Finding new drugs represents a challenge. Therapy currently takes a considerable time, and it is hoped that new compounds can be found that will reduce the total duration of treatment regimens. Drugs are also needed that will be effective against the growing number of drug-resistant strains and against bacilli that may be in a state of latency.
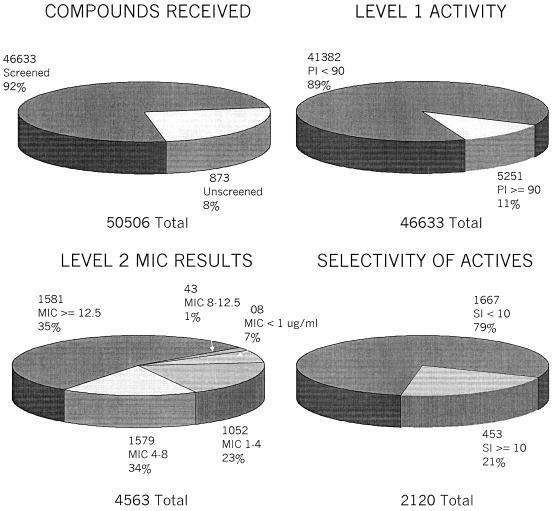
As part of the overall research effort at the National Institute of Allergy and Infectious Diseases, the National Institutes of Health has established a screening program at several institutions to efficiently screen large numbers of compounds for possible activity against M. tuberculosis. An acquisitions program receives from both academic and commercial sources compounds which are initially screened for in vitro activity. To date, over 50,000 compounds have been provided to the program (Fig. 1).
FIG. 1.
Progress of the in vitro tuberculosis drug screening program. Level 1 testing determines the percent inhibition (PI) for compounds; drugs having a PI of >90 advance to level 2. An initial cutoff of 12.5 μg/ml was amended to 6.25 μg/ml to reduce the number of compounds that could be screened further. Level 2 compounds are then further selected on the basis of the SI (SI equals IC50/MIC) for further testing in the macrophage infection and mouse therapy assays.
Each compound is first screened for activity at 6.25 μg/ml (or the molar equivalent of the highest-molecular-weight compounds in a series of congeners) against cultures of M. tuberculosis H37Rv in BACTEC 12B medium using a microplate alamar blue assay (2). Compounds that are active in this assay are reconfirmed using a BACTEC 460 radiometric system.
In the next stage of the procedure, the MIC of the compound is determined. In addition, the compound evaluated for cytotoxicity in cultures of Vero cells to determine a 50% inhibitory concentration (IC50). A selectivity index (SI) can then be calculated by dividing the IC50 by the MIC; if the SI is >10, the compound is then evaluated further.
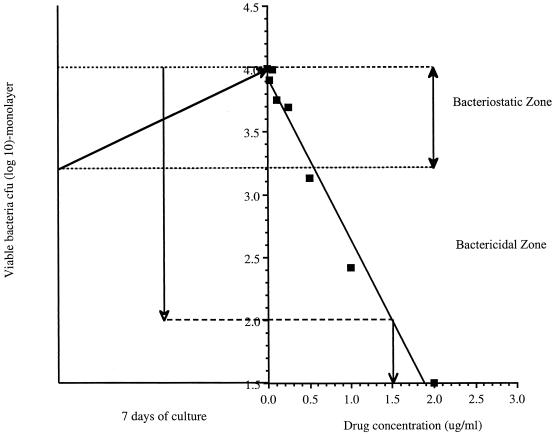
The next stage of screening uses the bone marrow macrophage infection assay (16) to determine if the compound can inhibit the growth of M. tuberculosis in its intracellular environment. Cultures of bone marrow-derived macrophages are infected with M. tuberculosis and then cultured for 7 to 8 days in the presence of a range of concentrations of the test compound. The monolayers are then lysed and plated on 7H11 agar to determine the numbers of surviving bacteria. By plotting the bacterial load in each culture against the drug concentration, one can determine whether a compound is bacteriostatic or bactericidal under these relevant physiological conditions. The concentration of drug reducing the bacterial load by 1 log unit (90% reduction [EC90]) or by 2 log units (99% reduction [EC99]) is recorded as a numerical readout (Fig. 2).
FIG. 2.
Growth of M. tuberculosis in macrophages treated with test compounds in the macrophage infection assay. Cells are infected and cultured in the presence of a range of doses of the test compound. One week later, the monolayers are lysed and the numbers of surviving bacteria are determined by plating (squares). This data are then plotted against the drug concentration to determine whether the compound is bactericidal or bacteriostatic. In this example, EC99 values were determined as the control CFU value (4.0) minus 2 log units (2.0), plotted against a simple curve of inhibition, which provides the information that 1.5 μg of drug per ml clears 99% of the bacterial load.
Compounds that perform well in the macrophage infection assay are then tested in a mouse model if sufficient amounts of the compounds are available. After the maximum tolerated dose for a compound is determined, mice are exposed to a low-dose aerosol infection with M. tuberculosis (9). Twenty days later, as the bacterial load reaches its peak in control animals, the test compound is administered daily at appropriate dosages for 45 days. The route of administration is usually gavage, although subcutaneous or intraperitoneal injection can sometimes be given for a limited time.
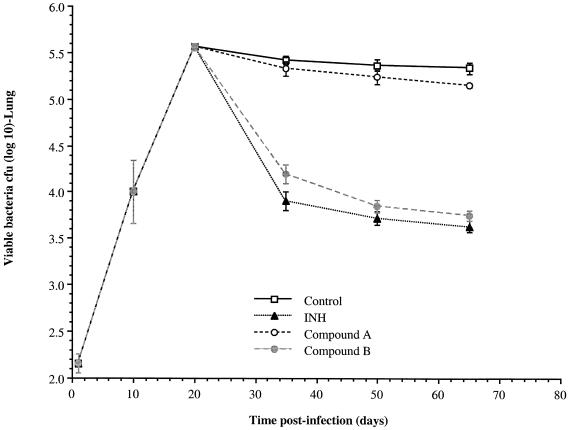
At 15, 30, and 45 days of therapy, groups of five mice are removed and euthanatized. The lungs and spleen are homogenized and plated on agar to determine the bacterial load. A positive control group of mice is given isoniazid (25 mg/kg of body weight/day) for comparison. The bacterial load for each test group is then plotted against time and compared to the values for untreated control mice (Fig. 3). Under these conditions, a reduction of 0.7 log unit or greater is considered statistically significant, as determined by analysis of variance. Compounds that provide this level of reduction are defined as active in this in vivo model.
FIG. 3.
Mouse aerosol infection assay. After about 3 weeks, the infection in the lungs is strongly contained by host immunity and a chronic disease state ensues. Therapy is begun at day 20, and the effects on the bacterial load are determined at 15-day intervals. In the results shown here (shown as mean and standard deviation), compound B had activity comparable to that of the isoniazid (INH) positive control. Compounds that reduce the bacterial load by 0.7 log unit or greater by day 65 are considered active.
Additional in vivo models are under development in this program. These include use of gamma interferon gene knockout mice, in which the bacterial load in the lungs cannot be prevented from reaching very high levels (3). In current evaluation studies, these mice are given a short course of treatment for 10 days to determine if such treatment is sufficient to detect positive drug activity and thus can be used as a new model with a much shorter duration than the standard assay.
To date, 11% of the compounds tested (5,251 compounds), have been highly active in vitro and 453 have an SI of >10. Of these, 340 have undergone evaluations in the macrophage infection model, and 53 have been tested in vivo. Of these, nine, from various compound classes, have been found to significantly reduce the bacterial load in the lungs of infected mice. While these results are encouraging, it is clear that much more work needs to be done, including the acquisition and testing of many more test compounds if the ultimate goal is to be achieved. Institutions or organizations possessing compounds that may have antimycobacterial properties are encouraged to visit www.taacf.org.
ACKNOWLEDGMENTS
This work was supported by NIH programs AI-95384, AI-95385, and AI-95364 and interagency agreement AI-5106-04 (supervised by Barbara Laughon, NIAID).
Footnotes
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
The members of the Tuberculosis Drug Screening Program are J. Secrist, S. Anathan, C. Kwong, J. Maddry, and R. Reynolds (all at Southern Research Institute, Birmingham, Ala.); A. Poffenberger, M. Michael, and L. Miller (all at Southern Research Institute, Frederick, Md.); J. Krahenbuhl, L. Adams, and A. Biswas (all at National Hansen's Disease Laboratory Research Branch, Baton Rouge, La.), S. Franzblau (at Institute for Tuberculosis Research, University of Illinois, ••••)., D. Rouse and D. Winfield (at Research Triangle Institute, Research Triangle Park, N.C.; and J. Brooks and I. Orme (at Colorado State University, ••••).
REFERENCES
- 1.Brooks J V, Orme I M. Evaluation of once-weekly therapy for tuberculosis using isoniazid plus rifamycins in the mouse aerosol infection model. Antimicrob Agents Chemother. 1998;42:3047–3048. doi: 10.1128/aac.42.11.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins L, Franzblau S G. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother. 1997;41:1004–1009. doi: 10.1128/aac.41.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cynamon M H, Klemens S P, Sharpe C A, Chase S. Activities of several novel oxazolidinones against Mycobacterium tuberculosis in a murine model. Antimicrob Agents Chemother. 1999;43:1189–1191. doi: 10.1128/aac.43.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Cock K M, Chaisson R E. Will DOTS do it? A reappraisal of tuberculosis control in countries with high rates of HIV infection. Int J Tuberc Lung Dis. 1999;3:457–465. [PubMed] [Google Scholar]
- 6.Dye C, Scheele S, Dolin P, Pathania V, Raviglione M C. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 7.Ji B, Lounis N, Maslo C, Truffot-Pernot C, Bonnafous P, Grosset J. In vitro and in vivo activities of moxifloxacin and clinafloxacin against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1998;42:2066–2069. doi: 10.1128/aac.42.8.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji B, Lounis N, Truffot-Pernot C, Grosset J. In vitro and in vivo activities of levofloxacin against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1995;39:1341–1344. doi: 10.1128/aac.39.6.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly B P, Furney S K, Jessen M T, Orme I M. Low-dose aerosol infection model for testing drugs for efficacy against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1996;40:2809–2812. doi: 10.1128/aac.40.12.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keshavjee S, Becerra M C. Disintegrating health services and resurgent tuberculosis in post-Soviet Tajikistan: an example of structural violence. JAMA. 2000;283:1201. [PubMed] [Google Scholar]
- 11.Lenaerts A M, Chase S E, Chmielewski A J, Cynamon M H. Evaluation of rifapentine in long-term treatment regimens for tuberculosis in mice. Antimicrob Agents Chemother. 1999;43:2356–2360. doi: 10.1128/aac.43.10.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenaerts A M, Chase S E, Cynamon M H. Evaluation of rifalazil in a combination treatment regimen as an alternative to isoniazid-rifampin therapy in a mouse tuberculosis model. Antimicrob Agents Chemother. 2000;44:3167–3168. doi: 10.1128/aac.44.11.3167-3168.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyazaki E, Miyazaki M, Chen J M, Chaisson R E, Bishai W R. Moxifloxacin (BAY12–8039), a new 8-methoxyquinolone, is active in a mouse model of tuberculosis. Antimicrob Agents Chemother. 1999;43:85–89. doi: 10.1128/aac.43.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orme I M. Vaccination against tuberculosis: recent progress. Adv Vet Med. 1999;41:135–143. doi: 10.1016/s0065-3519(99)80013-5. [DOI] [PubMed] [Google Scholar]
- 15.Shoen C M, DeStefano M S, Cynamon M H. Durable cure for tuberculosis: rifalazil in combination with isoniazid in a murine model of Mycobacterium tuberculosis infection. Clin Infect Dis. 2000;30(Suppl. 3):S288–S290. doi: 10.1086/313876. [DOI] [PubMed] [Google Scholar]
- 16.Skinner P S, Furney S K, Jacobs M R, Klopman G, Ellner J J, Orme I M. A bone marrow-derived murine macrophage model for evaluating efficacy of antimycobacterial drugs under relevant physiological conditions. Antimicrob Agents Chemother. 1994;38:2557–2563. doi: 10.1128/aac.38.11.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skinner P S, Furney S K, Kleinert D A, Orme I M. Comparison of activities of fluoroquinolones in murine macrophages infected with Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1995;39:750–753. doi: 10.1128/AAC.39.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stover C K, Warrener P, VanDevanter D R, Sherman D R, Arain T M, Langhorne M H, Anderson S W, Towell J A, Yuan Y, McMurray D N, Kreiswirth B N, Barry C E, Baker W R. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature. 2000;405:962–966. doi: 10.1038/35016103. [DOI] [PubMed] [Google Scholar]
- 19.Traore I, Ji B, Lienhardt C, Bobin P, Grosset J. Determination of the minimal effective dosages of ofloxacin and sparfloxacin against M. leprae in the mouse foot pad system. Int J Lepr Other Mycobact Dis. 1996;64:142–145. [PubMed] [Google Scholar]