Abstract
Naringin and naringin’s aglycone naringenin belong to a subclass of flavonoids called flavanones. While many studies of pure naringenin and naringin and their food sources have shown beneficial health effects, including improved lipid metabolism, in animals and humans, the mechanisms underlying the lipid-lowering effects are not completely understood. In recent years, multiple studies using various in vitro and rodent models have revealed new mechanisms underlying the hypolipidemic effects of naringin and naringenin, including regulation of lipid digestion, reverse cholesterol transport, and LDL receptor expression. In addition, naringin and naringenin show diverse effects in populations with different health conditions, such as obesity and diabetes. Furthermore, a novel naringin and naringenin enriched food source citrus bergamia (bergamot) and other citrus fruits have recently been studied for lipid-lowering effects in animal models and human clinical trials. In this review, we provide an update on recent advances in naringin and naringenin and their enriched food sources on lipid metabolism and underlying mechanisms. Because absorption, distribution, metabolism, and excretion, particularly in the presence of food matrix, impact the bioavailability, which in turn affects the bioactivities of these flavonoids in vivo, we also summarize new findings from the pharmacokinetics studies and the interplays between the flavanones and gut microbiota.
Keywords: naringenin, naringin, lipid metabolism, bioavailability, pharmacokinetics
Introduction
Naringin and naringin’s aglycone naringenin belong to a subclass of flavonoids called flavanones, which are structurally different from other flavonoids due to the presence of a chiral carbon at the C2 position and no substitution at the C3 position [1]. Citrus fruits and juices such as oranges, grapefruits, and bergamots are the main sources of naringin and naringenin [1–3].
In general, pure naringin and naringenin were commonly used in animal and in vitro studies, while citrus fruit extracts, citrus peel extracts, whole citrus fruit, or citrus fruit juices were commonly used in human clinical trials. Several recent reviews have summarized many beneficial effects, including hypocholesterolemic effects, of naringin and naringenin in animal models and humans [4–7]. However, the mechanisms underlying the lipid-lowering effects are not completely understood. In recent years, new mechanisms underlying the hypolipidemic effects of naringin and naringenin have been reported. In addition, naringin and naringenin showed diverse effects in populations with different health conditions, such as obesity and diabetes. As a novel naringin and naringenin enriched food source, citrus bergamia (bergamot) has been studied for its lipid-lowering effects in animal models and human clinical trials [8–10]. Moreover, other citrus fruits, such as Citrus sinensis (orange), have also been investigated regarding lipid metabolism [11].
Therefore, in this review, we will summarize recent advances on citrus flavonoids, including naringin and naringenin, and other enriched food sources, on lipid metabolism and underlying mechanisms (Table 1). The bioavailability of citrus flavonoids can be impacted by many factors, including the food matrix. Emerging evidence also suggests that age and gender affect the pharmacokinetics, which in turn affect the bioactivities of these flavonoids in vivo. Therefore, recent advances on absorption, distribution, metabolism, and excretion (ADME) for naringin and naringenin and relationships with gut microbiota will also be discussed in this review.
Table 1.
Lipids lowering effects of naringin, naringenin, and its enriched food sources
| Model | Flavonoid | Key Findings | Mechanism | Reference | |
|---|---|---|---|---|---|
| In vitro study | Pancreatic lipase activity assay | Naringin (0.6 mM) | Synergistically inhibited PL activity with 0.6 mM emodin | PL inhibition | [45] |
|
| |||||
| Hela cells | Naringenin (0.01–100 μM for 24 h) | Decreased ABCA1 mRNA expression | Antagonist of LXRs | [48] | |
|
| |||||
| Human THP-1 macrophages | Naringenin (100 μM for 18 h) | Enhanced cholesterol efflux; Increased RCT | Increased NR1H3, ABCA1, and ABCG1 mRNA and protein expression | [50] | |
|
| |||||
| Murine RAW 264.7 macrophages | Naringenin (10–50 μM for 24 h) | Enhanced cholesterol efflux; Increased RCT | Increased Nr1h3, Abca1, and Abcg1 mRNA and protein expression; Decreased ATF6, GRP78, XBP-1 protein levels | [49] | |
|
| |||||
| Human HepG2 cells | Naringin (12.5 μM-50 μM for 24 h) | Dose dependently increased LDLR and CYP7A1 mRNA expression and protein level | SREBP-2 and PPARγ activation | [52] | |
|
| |||||
| Obesity-induced dyslipidemia | In vivo - mice Diet-induced obese male C57BL/6 mice; (n=10 per group) | Naringin 25–100 mg/kg for 8 weeks | Decreased body weight, liver weight, plasma LDL-C level, and hepatic TG and TC levels | Up-regulated hepatic Ldlr mRNA expression; Up-regulated hepatic p-AMPK α level; Reduced hepatic Srebp-1, Srebp-2, Pcsk9 mRNA expression | [53] |
|
| |||||
| In vivo - mice Ovariectomized obese C57BL/6 female mice (n=4–10 per group) | Naringenin 3% (WT/WT) for 11 weeks | No changes in serum and hepatic TG and TC levels; Decreased body weight, intraabdominal adiposity, and blood glucose levels; Decreased DAG levels in muscle | Decreased Fasn and Scd1 mRNA expression in muscle | [55] | |
|
| |||||
| In vivo - humans Class 1 obese patients with hypercholesterole mia (n=28) | Naringin 450 mg/d for 90 days | Decreased body weight, plasma TG, TC, and LDL-C levels | Undetermined | [56] | |
|
| |||||
| Diabetic dyslipidemia | In vivo - rats high fat-low streptozocin model of T2DM rats (n=5 per group) | Naringin 50–200 mg/kg bw/d for 9 weeks | Decreased plasma TC and TG, and hepatic TC levels; Increased hepatic HDL-C and HDL3-C levels; No changes in liver TG levels | Increased hepatic CPT activity; Increased paraoxonase activities in HDL; Increased hepatic Scarb1, Ahr, Lipc, and Lcat mRNA expression | [60] |
|
| |||||
| Simple dyslipidemia without other metabolic diseases | In vivo - mice Lean male Ldlr −/− C57BL/6 mice; (n=12 per group) | Naringenin 3% (WT/WT) for 8 weeks | Decreased body weight, epididymal white fat weight, plasma TG, LDL-C, VLDL-C, and liver TG levels; Increased plasma betahydroxybutyrate level | Enhanced hepatic Pnpla2, Cpt1a mRNA expression | [63] |
|
| |||||
| In vivo - humans Moderately hypercholesterole mic men and women (n=194) | Naringin 500 mg/d for 4 weeks | No changes in serum TG, HDL-C, and LDL-C level | Undetermined | [64] | |
|
| |||||
| Bergamot extracts with other nutraceuticals | In vivo - humans Patients with moderate dyslipidemia (n=11) | F105 (Bergamot fruit extract (BFP, 500 mg/d) with other 9 phytoextracts (220 mg/d) for 12 weeks | Decreased plasma TC, LDL-C, non-HDL-C, and C/HDL; Decreased TG, oxLDL, LDL/HDL, TG/HDL, oxLDL/HDL, and PAI levels in a subgroup of subjects with abnormal HbA1C, HOMA-IR score, or TG levels | Undetermined | [70] |
|
| |||||
| In vivo - humans Participants (n=50) with higher serum cholesterol (<500 mg/dl) | 1 tablet/d (whole bergamot extracts (200 mg), omega-3 (400 mg), trivalent chromium (10 μg), and red yeast rice (100 mg)) for 6 weeks | Decreased plasma TG, TC, LDL-C levels; Increased plasma HDL level | Undetermined | [71] | |
|
| |||||
| In vivo - humans Older adults with moderate dyslipidemia (n=98) | 1 tablet contains whole bergamot extract (250 mg), plant sterol esters (410 mg), orange oil, vitamin C (25 mg), Vitamin B6 (10 mg), Vitamin B12 (1000 μg), and folic acid (400 μg). 2 tablets/day for 12 weeks | Decreased body weight, circumstances, BMI, plasma TG, TC, and LDL-C levels | Undetermined | [72] | |
|
| |||||
| Citrus Sinensis (Orange) | In vivo - rats Diet-induced hyperlipidemic Male Wistar rats (n=10 per group) | Orange juice (2–8 ml/kg bw/day) | Decreased serum TG, TC, and LDL-C; Increased HDL-C level | Undetermined | [75] |
|
| |||||
| In vivo - humans Overweight/obese participants (n=100) | Orange juice with normal (299 mg) or high (745 mg) content of polyphenols (500 ml/d) for 12 weeks | Reduced body weight, BMI, waist circumference, plasma TG, ApoB levels by both juices; Increased plasma ApoA-1 level only by the juice with high polyphenols | Undetermined | [76] | |
|
| |||||
| In vivo - humans Obese participants (n=78) | Orange juice 500 ml/d combined with a reduced-calorie diet for 12 weeks | Reduced plasma TC and LDL-C levels in addition to the benefits by only a reduced-calorie diet | Undetermined | [77] | |
|
| |||||
| In vivo - humans Overweight or obese men (n=36) | Orange juice 250 ml/d compared to an energy and sugar content matched control drink for 12 weeks | Decreased plasma TG in the subjects with elevated TG at base line | Undetermined | [78] | |
|
| |||||
| In vivo - humans Healthy women (n=10) | Orange juice 300 ml/d for 60 days | Decreased serum TG, TC, and LDL-C levels; Increased Lactobacillus and Bifidobacterium in fecal samples; Increased acetic acid and decreased NH4+ level in fecal samples. The relative abundance of Akkermansia is negatively correlated with serum TG and LDL-C, but positively correlated with HDL-C | Improved gut microbiota | [39], [79] | |
|
| |||||
| In vivo - humans Healthy participants (n=15) | Orange juice 500 ml/d for 2 weeks | No changes in plasma glucose, TG, TC, LDL-C, and HDL-C levels; Decreased medium and long-chain acylcarnitine level; Increased short-chain acylcarnitine level | Undetermined | [80] | |
|
| |||||
| In vivo - humans Normal weight (n=17); or overweight/obese subjects (n=12/6) | Red orange juice 750 ml/d for 8 weeks | Decreased TC and LDL-C levels but no changes of TG in both normal weight and overweight/obese group; Decreased HDL-C and ApoA1 only in the normal weight group | Undetermined | [82] | |
|
| |||||
| In vivo - humans Obese women (n=11) | Red orange juice 500 ml/d for 12 weeks | Decreased plasma TC and LDL-C levels; No changes in body weight and TG level | Undetermined | [83] | |
|
| |||||
| In vivo - humans Obese participants (n=41) | Red orange juice 500 ml/d for 4 weeks | No changes in plasma TC, TG, LDL-C, and HDL-C levels | Undetermined | [84] | |
|
| |||||
| In vivo - mice Healthy lean male OF1 mice (n=8 per group) | Orange beverage for 12 weeks (fermentedpasteurized) 1:10 diluted in tap water, equal to 250 ml/d in human | Decreased TC, TG, LDL-C, and oxidized LDL levels; Increased HDL, total and reduced glutathione level | Anti-oxidation; | [87] | |
|
| |||||
| In vivo - humans Participants with moderate hypercholesterolemia (n=18) | Orange beverage 500 ml/d for 2 weeks (fermentedpasteurized) | Decreased plasma TC, TG, LDL-C, and LDL/HDL ratio and other cardiovascular risk factors | Undetermined | [88] | |
|
| |||||
| In vivo - humans Healthy participants (n=26) | Orange juice (20% of energy requirement) with or in between three meals; for 2 weeks in a 4-week cross-over study | Decreased fat mass and serum gamma-glutamyl transferase when taking orange juice with meals; Increased fat mass and a trend of increased postprandial insulin sensitivity when taking between the meals; No changes in body weight and TG level | Undetermined | [89] | |
|
| |||||
| Other citrus fruits | In vivo - mice Diet-induced obese C57BL/6J mice (n=8 per group) | Citrus junos Tanaka peel extract 5% w/w for 10 weeks | Decreased plasma TC and LDL-C level | PPARα activation; Increased CPT1 level | [90] |
|
| |||||
| In vivo - mice Diet-induced obese C57BL/6J mice (n=8 per group) | Immature Citrus reticulata (mandarin) dry fruits extracts 1% w/w for 11 weeks |
Decreased body weight, epidydimal fat pad weight, serum TG and TC level; Decreased adipocyte size and hepatic steatosis; Improved cold tolerance | Increased mRNA expression of Ucp1 and thermogenic genes in inguinal WAT | [91] | |
|
| |||||
| In vivo - mice Diet-induced obese C57BL/6J mice (n=8 per group) | Citrus aurantium (bitter orange) blossoms extract 50 –200 mg/kg bw/d for 12 weeks | Decreased body weight, serum TG, TC, LDL-C, LPS, and leptin level; Improved liver oxidation damage and steatosis | Enhanced mitochondrial fatty acid beta-oxidation; Inhibited chronic low-grade inflammation; Reversed gut dysbiosis; | [92] | |
|
| |||||
| In vivo - mice Diet-induced obese C57BL/6J mice (n=7–8 per group) | Citrus tumida peel extract 5% w/w for 4 weeks | Decreased body weight, epidydimal, perirenal, and subcutaneous fat pad weight; Decreased serum TG and TC level | Undetermined | [93] | |
|
| |||||
| In vivo - hamsters Diet-induced hyperlipidemic Hamsters (n=80) | Citrus changshan-huyou peel extract 25–100 mg/kg bw/d for 4 weeks | Decreased serum TG, TC, and LDL-C level; Increased SOD activity and decreased MDA level; Decreased serum and hepatic TNF-a and IL-6 level | PPARα/γ activation; Increased hepatic CYP7A1 content; Decreased oxidative stress and inflammation | [94] | |
1. Advances in Absorption, Distribution, Metabolism, and Excretion (ADME) and Pharmacokinetics
1.1. Advances in ADME
The general ADME information for flavonoids has recently been reviewed elsewhere [12–16]; therefore, only pharmacokinetic aspects related to a better understanding of this review are discussed in this paper.
It is believed that once entering into the cells, naringin and naringenin undergo phase I (e.g., oxidation or demethylation by cytochrome P450 monooxygenases) and phase II (e.g., glucuronidation, sulfation, or methylation) metabolism, either in the intestinal cells or the liver. Moreover, it is increasingly recognized that gut microbiota can metabolize flavonoids into phenolic and aromatic ring-fission catabolites, further increasing their bioavailability [13].
Using deuterated naringin (D4-naringin), it was reported that 21 flavonoid metabolites and 11 phenolic catabolites were detected in rat urine and/or feces samples after a single dose of D4-naringin at 42 mg/kg [17]. Urine was the major route for excretion. While the unmetabolized D4-naringin was barely detectable in both urine and feces, D4-naringenin, D4-3-(4’-hydroxyphenyl)propionic acid (D4-HPPA), D4-p-coumaric acid (D4-p-CA), and D4-hippuric acid (D4-HA) were found to be the major metabolites in the urine, and only D4-HPPA was abundant in the feces. These four major metabolites corresponded to 56.9% of D4-naringin intake [17].
Conceivably, when the pure form of naringenin is orally ingested, it follows the same paths as the naringenin derived from naringin. Naringenin is known to be metabolized by phase II enzymes in the small intestine to generate flavanone glucuronides or sulfates [13, 16]. However, naringenin metabolism in the stomach and colon remains elusive. In a recent single-pass intestinal perfusion study of naringenin, which involved perfusing the naringenin solutions in anesthetized mice and collecting and measuring the metabolites from the outflow perfusates from segments of the gastrointestinal tract, new insights into naringenin metabolism in the regions of the stomach and colon, which previously were not well studied, were reported [18]. Higher concentrations of phase II metabolites naringenin glucuronides and naringenin sulfate were found in the colonic samples than from gastric samples, indicating higher glucuronosyltransferase and sulfotransferase activities in the colon and/or the contribution from the colonic microbiota (for glucuronides). In contrast, only a few phase I metabolites were identified, including apigenin found in both samples. Similar to naringin, naringenin that is not absorbed passes through the small intestinal lumen and reaches the colon, where it is metabolized by microbiota into gut microbial metabolites, such as HPPA and phloroglucinol [18]. Interestingly, HPPA and phloroglucinol were also found in the gastric samples, suggesting the presence of microorganisms capable of metabolizing naringenin in the stomach [18].
As citrus flavanones are beneficial for many chronic diseases that involve many organs, a new study reported the tissue distribution of naringin and its metabolites in the plasma and 14 tissues after a single oral dose (42 mg/kg) of purified naringin in rats [19]. In addition to the gastrointestinal (GI) tract, free unchanged naringin was found in the trachea, lung, kidney, fat, liver, muscle, heart, plasma, brain, and spleen in descending order of amount within 24 hr [19]. After absorption and first-pass metabolism in the GI tract, generated metabolites were transported to the liver for further metabolism. The major metabolites found in the liver, the central organ for lipid and cholesterol metabolism, include naringenin, naringenin-7-O-sulfate, free naringin, naringenin-4’-O glucuronide, and apiferol, flavone-derived alcohol (flavan-4-ol) [19]. It is interesting to note that different tissues have different dominant forms of the metabolites, with naringenin glucuronides as the dominant forms in the plasma and free naringenin and naringenin-7-O-sulfate as the dominant forms in the GI tract, liver, and other tissues. This is possibly due to differences in tissue distribution of phase II metabolizing enzymes [19].
The differences in ADME among pure naringenin and naringin and food sources containing naringin and naringenin have not been well studied. When a whole citrus fruit is consumed, the food matrix may interfere with digestion and absorption of naringin and naringenin; however, the specific impacts of the food matrix on the ADME have not been well studied [3]. Studies on other polyphenols, such as quercetin, caffeic acid, gallic acid, and p-coumaric acid in the food sources, have shown that the polyphenols’ absorption is affected by the food matrix [20, 21]. Bioaccessibility, defined as the fraction of polyphenols released from the food matrix during digestion, and becomes accessible for intestinal absorption, is determined by soluble and insoluble fiber or the presence of lipophilic compounds in the food matrix [20, 21]. Chemical structure, such as the presence of a sugar moiety on a compound [20], also affects the bioaccessibility and bioavailability of polyphenols.
Differences in flavanone bioaccessibility were reported in one study [22]. Although its content in orange juice was lower, hesperidin was more bioaccessible from orange juice than from the orange segments [22]. Similarly, significant differences in naringenin bioavailability from different food sources have been reported [23]. In a randomized cross-over trial, the subjects consumed either orange juice or the same batch of fresh oranges, which contained 2.4-fold more citrus flavanones (based on hesperidin and narirutin content); however, after the treatment of glucuronidase and sulfatase, there were no significant differences in hesperetin excretion in 24 hr urine samples and only an average of 1.7-fold more of naringenin when consuming the fresh oranges [23]. The differences in bioavailability of naringenin from these food sources could be due to the fiber matrix of oranges, as the orange juice is mechanically processed and is, therefore, more bioavailable [23]. Moreover, citrus flavanones are also located in the albedo (the inner part of the peel) and therefore are not usually consumed when eating oranges [24]. However, commercial juicing allows the albedo to contact the juice sacks of oranges, adding flavanones, such as naringin and naringenin, to the juice that would not normally be consumed from whole oranges [24].
1.2. Advances in pharmacokinetics
The pharmacokinetics of naringenin after a single dose of 135 mg in solid dispersion capsules in healthy human subjects were reported [25]. The average maximal plasma concentration achieved (Cmax) is ~ 7.39±2.83 μM, and it took ~3.67 hr (Tmax) to reach the Cmax [25]. Similarly, the pharmacokinetics of naringenin from single oral ingestion of the whole orange (Citrus sinensis) extract in healthy adults were recently reported [26]. The Cmax, when dosed with 150 mg of naringenin (NAR150) from the extract, is 15.76±7.88 μM and was achieved ~ 3 hr (Tmax) post-ingestion. The Cmax reached 48.45±7.88 μM in the 600 mg dose (NAR600) with no significant differences in Tmax compared to the NAR150. The calculated T1/2 for both NAR150 and NAR600 were similar, and ~ 3 hr [26]. Aging is known to affect all phases of pharmacokinetic processes, including absorption, distribution, metabolism, and excretion [27]. Specifically, aging delays gastric emptying and peristalsis, changes bowel surface area, decreases splanchnic blood flow, and alters cytochrome P450 enzymatic activity [27, 28]. Aged rats (20 months old) single-dosed with 42 mg/kg pure naringin had significantly delayed Tmax and prolonged half-washout time (t1/2) compared to adult rats [29]. The Cmax and area under the plasma concentration-time curve (AUC) were also about two times higher than in adult rats, which could be due to (1) the longer resident time of naringin in the GI tract, leading to complete absorption, and (2) weakened metabolic and excretive elimination [29]. Tissue distribution was also different between aged and adult rats, with aged rats having significantly higher levels of naringin and naringenin accumulation in the lung and trachea [29].
There are sex-associated differences regarding naringin and naringenin absorption in rats and humans. The female aged rats had significantly higher AUC and t1/2, but no differences in Tmax and Cmax of naringenin compared to the male rats [29]. In addition, the female rats had a higher accumulation of naringin in the lung and trachea [29]. Significant sex-related differences in naringin and naringenin pharmacokinetics were also reported in adult rats and humans [30].
Interestingly, there are species differences regarding naringin and naringenin pharmacokinetics. For example, naringenin was shown to have a significant lag-time in human plasma after an oral dose of naringin compared to rats and dogs because of naringin metabolism in the human intestine, which also resulted in prolonging naringenin Tmax by 3.62±3.19 hr in humans compared to the rats and dogs [30]. These differences are thought to be caused by differences in blood flow rate, biochemical processes, and variable intestinal motility, which are related to body weight or body surface area of the animal species [30]. Species differences in naringin metabolism have also been reported. For example, while methylation, glucuronidation, and sulfation conjugation of metabolites were observed in rats (and dogs), only glucuronidation and sulfation conjugation occurred in humans [30]. Understanding species differences of naringenin and naringin pharmacokinetics is important because this can help translate data from studies performed on non-human species, such as rats and dogs, to human health [30].
2. Safety and Effective Dosage
There have not been many studies documenting adverse effects in humans of pure forms of naringin, naringenin, or the food sources of naringin and naringenin. Based on a previous single dose (135 mg) pharmacokinetic study [25], a randomized, placebo-controlled, single-ascending-dose clinical trial was carried out to analyze the adverse effects of naringenin and naringin. Ingestion of the doses ranged from 150 mg to 900 mg naringenin from the extract of whole oranges (Citrus sinensis), which contained 28% naringenin and 8.5% naringin, had no adverse events or changes in some liver and kidney function markers, such as blood urea nitrogen, creatinine, potassium, and albumin in the participants [26]. This study included only 18 participants; therefore, it may need to be repeated with larger sample sizes to confirm the safety of naringenin and naringin from the Citrus sinensis extract.
Since a previous in vitro study found that pure naringenin at 8 μM induced upregulation of thermogenic gene expression and increased energy expenditure in primary human adipocytes [31], a pharmacokinetics study was carried out to find the appropriate dosing to achieve 8 μM in the blood [26]. It was demonstrated that an intake of 300 mg of naringenin from Citrus sinensis extract twice daily was sufficient to produce 8 μM of naringin in the blood, leading to a beneficial effect in humans [26].
3. Naringenin and Its Food Source, Microbiota, and Gut Health
There is growing recognition that the bioavailability of dietary flavonoids, including citrus flavanones, is higher than previously thought when contributions of phenolic and aromatic ring-fission catabolites by gut microbiota are taken into consideration [12, 13]. Gut microbiota plays an important role in the absorption and metabolism of naringenin and naringin [19, 32, 33].
When incubated with the human fecal solution under anaerobic conditions, naringenin was degraded and metabolized to HPPA, 3-(phenyl)propionic acid, and low levels of 3-(4’-hydroxyphenyl)acetic acid over a 24 hr incubation [33]. Similarly, deuterated naringin (D4-naringin) was converted to D4-naringenin and D4-HPPA when incubated with the human fecal solution under anaerobic conditions, suggesting that deglycosylation (via glucosidase) and C-ring fission occurred during incubation [32]. An NADH-dependent reductase found in the human intestinal anaerobe Eubacterium ramulus was capable of cleaving the heterocyclic C-ring of naringenin [34]. Similarly, in addition to the plasma, many of the phenolic and aromatic ring fission catabolites were detected in the liver and kidney of rats treated with a single oral dose (42 mg/kg) of naringin, further supporting the roles of gut microbiota in naringenin metabolism [19].
Emerging evidence has begun to shed light on the biological activities for the phenolic and aromatic ring fission catabolites of naringenin and naringin. Similar to naringenin, its catabolite HPPA and 4-hydroxybenzoic acid also decreased total cholesterol (TC), triglyceride (TG) in the plasma and atherogenic index and increased plasma high density lipoprotein cholesterol (HDL-C) levels in 1% cholesterol diet-fed rats [35].
Due to gut microbes’ roles in naringenin and naringin metabolism, increasing certain microbiota populations may increase naringenin’s bioavailability and bioactivity. Probiotic bacteria Bifidobacterium longum and Lactobacillus rhamnosus were incubated with orange juice flavanone hesperetin-7-O-rutinoside, naringenin-7-O-rutinoside, hesperetin, and naringenin to determine if these probiotics could catabolize these flavanones. Both probiotics were able to induce ring fission, demethylation and/or dehydroxylation of hesperetin and naringenin, ultimately yielding 3-(phenyl)propionic acid [36]. Consistently, chronic administration of Bifidobacterium longum R0175 for 4 weeks increased the urinary excretion of metabolites and selected catabolites of orange juice flavanones, such as phenolic acids, indicating an increase in its bioavailability [37].
Interestingly, citrus flavanones have been found to affect gut bacterial growth and gene expression and increase the abundance of beneficial bacteria to produce short-chain fatty acids (SCFAs) [38, 39], which have been shown to reduce inflammation, improve intestinal barrier function, and exert chemopreventive effects in colonocytes [40]. When incubated with the bacteria Bifidobacterium catenulatum, naringenin significantly enhanced the growth and gene expression involved in cell metabolism, DNA repair, and molecular transport in the bacteria [38].
Recently, a clinical trial of 10 women consuming pasteurized orange juice for 2 months found that the juice stimulatedthe growth of Lactobacillus and Bifidobacterium species, which was accompanied by a significant increase in acetic acid in the fecal samples and significant reductions in blood TC, TG, and LDL cholesterol (LDL-C) [39]. Therefore, food sources of naringin and naringenin, such as pasteurized orange juice, may increase SCFA production from gut microbiota, resulting in beneficial effects, such as improved intestinal homeostasis and systemic lipid and cholesterol metabolism [41].
4. Modulation of Lipid Metabolism by Naringin and Naringenin
4.1. In vitro evidence
The lipid-lowering potentials of naringin and naringenin have been reviewed in the past [4–7]. In summary, the mechanisms of action include inhibition of cholesterol biosynthesis by targeting 3-hydroxy 3-methylglutaryl-CoA (HMG-CoA) reductase and acyl-CoA: cholesterol acyltransferase (ACAT) and increase in fatty acid beta-oxidation by activating peroxisome proliferator-activated receptor gamma (PPARγ) and alpha (PPARα) pathways, and upregulation of uncoupling protein 1 (UCP1) and 2 (UCP2), adipose triglycerides lipase (ATGL), and carnitine palmitoyl transferase 1A (CPT1A). However, using various cell models, recent studies have revealed some new mechanisms by which naringin and naringenin positively regulate lipid metabolism, which is reviewed here (Table 1).
4.1.1. Inhibition of pancreatic lipase
Dietary fats are mainly digested and absorbed in the small intestine. Efficient fat digestion relies on pancreatic lipase (PL) for subsequent absorption in the duodenum and jejunum. Therefore, inhibition of PL, especially by plant-derived inhibitors, is considered a promising strategy to reduce fat absorption and restore lipids homeostasis [42, 43]. It was reported that although naringin alone did not inhibit PL activity, naringin and emodin, a natural quinone compound mainly found in rhubarb [44], synergistically suppressed the PL activity derived from the porcine pancreas at the concentration of 0.6 mM [45].
4.1.2. Modulation of cholesterol efflux
Cholesterol efflux is a process by which free cholesterol is loaded to its primary acceptors, including high-density lipoprotein (HDL) and apolipoprotein A-I (ApoA-I), the initial and rate-limiting step in reverse cholesterol transport [46]. Liver X receptors (LXRs) regulate cholesterol efflux in peripheral tissues through transcriptional activation of genes of several intracellular cholesterol transporters, including apolipoprotein E (ApoE), ATP binding cassette transporter Abca1 and Abcg1, which facilitate the transfer of cholesterol to HDL and ApoA-I [47].
Fouache et al. examined the LXRs activation by several flavonoids, including naringenin, by transiently transfecting Hela cells with luciferase reporter driven by human LXRα gene promoter [48]. Naringenin (0.1–100 μM) did not stimulate LXRα promoter-driven luciferase activities in the absence of LXRα synthetic agonist T0901317. However, a significant decrease in luciferase activities occurred when transfected Hela cells were treated with naringenin and T0901317, suggesting the antagonist effects of naringenin on LXRα [48]. Decreased LXR target gene ABCA1 mRNA expression found in naringenin treated Hela cells further confirmed the effects. Docking simulation suggested that naringenin may have competed with the LXRα ligand from entering the LXRα ligand binding site [48].
In contrast, it has been reported that naringenin increased cholesterol efflux by enhancing mRNA and protein expression of LXRα (encoded by Nr1h3/NR1H3) and its target genes Abca1/ABCA1 and Abcg1/ABCG1 in cholesterol loaded murine RAW 264.7 macrophages and human THP-1 macrophages, respectively [49, 50]. The increase was further enhanced by ER stress inhibitor 4-phenylbutyric acid but was reduced by ER stress inducer tunicamycin [49]. Moreover, the protein level of activating transcription factor 6 (ATF6) and its downstream target genes glucose-regulated protein (Grp78) and X-box binding protein 1 (Xbp-1), both of which are associated with ER stress pathway and implicated in cholesterol metabolism, were significantly reduced by naringenin in a dose-dependent manner in RAW264.7 macrophages [49], suggesting that ER stress inhibition may be a potential mechanism underlying naringenin’s stimulatory effects on cholesterol efflux.
Similar effects were observed in human THP-1 macrophages [50]. Naringenin up-regulated ABCA1, ABCG1, and LXRα mRNA and protein expression at the dose of 100 μM and exerted a synergic effect with T0901317 [50]. It was found that small interference RNA (siRNA) of AMP-activated protein kinase (AMPK) alpha 1 and alpha 2 or AMPK chemical inhibitor compound C reversed naringenin’s effects [50], suggesting that the upregulation of ABCA1 and ABCG1 by naringenin in human macrophages were AMPK dependent.
In summary, the effects of naringenin in LXR and its downstream targets are inconsistent among in vitro studies. Such discrepancy may be due to the tissue specific effects since different cell models (i.e., Hela cells [48] and macrophages [49, 50]) were used in these studies. In addition, the study designs were different. The murine RAW 264.7 [49] and human THP-1 macrophages [50] were pre-treated with cholesterol to induce foam cells and then treated with naringenin, whereas the Hella cells were treated by naringenin without being pre-loaded with cholesterol [48]. The underlying mechanisms by which naringenin differentially regulated the LXR signaling warrant future investigation. Moreover, it is noted that up to 100 μM of naringenin were used in the in vitro studies, which was intended to compensate for short treatment duration and different microenvironment in cells models compared to that of animal models. However, higher doses used in vitro may not be translatable to human health since such concentrations are not physiologically achievable through diet.
4.1.3. Modulation of the LDL receptor
The clearance of LDL-C largely depends on the membrane LDL receptor (LDLR) expression, primarily the hepatic LDLR, as it accounts for the clearance of the majority of plasma LDL-C in the human body [51].
Naringin dose-dependently (12.5 μM-50 μM) enhanced both LDLR mRNA and protein expression in cholesterol-treated human HepG2 cells, which was accompanied by elevated sterol regulatory element-binding protein 2 (SREBP-2) mRNA and protein expression [52]. In addition, mRNA and protein expression of hepatic CYP7A1, an enzyme responsible for bile acid synthesis from cholesterol, was significantly increased by naringin through PPARγ activation, suggesting an increased cholesterol clearance by the liver [52].
4.2. Lipid-lowering effects in obesity-associated dyslipidemia
Naringin and naringenin have been shown to be beneficial for weight loss and obesity-induced dyslipidemia by reducing blood TG, TC, and LDL-C levels [5–7]. The underlying mechanisms include reduced TG accumulation and enhanced fatty acid oxidation in hepatocytes and adipocytes [4, 6]. Recent studies have provided new insights into the mechanisms underlying lipid-regulatory effects of naringin and naringenin in the obese state in mice models and in both sexes (Table 1).
It has been reported that eight weeks of naringin treatment by gavage (25, 50, 100 mg/kg/day) decreased body weight, liver weight, plasma LDL-C level, hepatic TG level, and hepatic TC levels in a dose-dependent manner in high-fat diet-induced obese male C57BL/6J mice [53]. It was found that hepatic phospho-AMPK alpha (p-AMPK α) protein levels and Ldlr mRNA expression were up-regulated, whereas proprotein convertase subtilisin/kexin type 9 (Pcsk9) mRNA expression was reduced in the liver. PCSK9, mainly produced by hepatocytes, decreases membrane LDL receptor level by binding LDL receptor to stimulate lysosomal degradation [54]. Therefore, the study suggests that naringin may reduce plasma LDL-C levels by increasing hepatic LDLR content through inhibiting Pcsk9 mRNA expression [53].
However, the lipid-lowering effects by naringenin seen in the male mice were not observed in obese ovariectomized female mice. It was reported that in the obese ovariectomized C57BL/6J mice fed with 3% naringenin, there were no changes in plasma TC and TG levels, although body weight, intraabdominal adiposity, and blood glucose levels were decreased [55]. Interestingly, naringenin significantly reduced diacylglycerol, but not TG levels, in the muscle. These findings were accompanied by decreased gene expression in fatty acid synthase (Fasn) and stearoyl-CoA desaturase 1 (Scd1), suggesting that lipid biosynthesis was inhibited by naringenin in the muscle of obese ovariectomized mice [55].
Naringin’s effects on obesity-associated dyslipidemia were confirmed in a recent human trial in class 1 obese patients (BMI 30–34.9 kg/m2) with hypercholesterolemia (total cholesterol from 200–400 mg/dL) [56]. Daily oral intake of 450 mg naringin for 90 days reduced body weight and plasma TG, TC, and LDL-C levels in class 1 obese patients [56].
4.3. Lipid-lowering effects in diabetic dyslipidemia
Naringin and naringenin are known to activate AMPK pathways to increase lipid oxidation and inhibit HMG-CoA reductase to decrease cholesterol biosynthesis in diabetic rodent models [5, 6, 57]. In addition, naringenin could reduce the secretion of hepatic ApoB through potentiating intracellular insulin signaling in diabetic mice models [58]. ApoB is a structural component of VLDL, and insulin promotes ApoB degradation and decreases its secretion from hepatocytes [59].
In a recent study, naringin was found to dose-dependently (50–200 mg/kg/d) decrease the plasma TC and TG and hepatic TC levels and increase HDL-C levels while having no effect on liver TG levels in a rat model of high fat/low streptozocin induced type 2 diabetes [60] (Table 1). Naringin increased paraoxonase activities in HDL and hepatic CPT activity, and mRNA expression of hepatic scavenger receptor class B, member 1 (Scarb1) gene and aryl hydrocarbon receptor (Ahr), but not HMG-CoA reductase (Hmgc), were significantly increased to the highest levels at 200 mg/kg of naringin whereas mRNA expression of hepatic lipase (Lipc) and lecithin-cholesterol acyltransferase (Lcat) were significantly increased to the highest at 50 mg/kg [60]. Ahr gene encodes the transcription factor that has been reported to attenuate cholesterol biosynthesis [61]. Scarb1 gene encodes a membrane protein that facilitates HDL recycling in hepatocytes. Lcat gene encodes the protein that increases HDL3-C formation by esterifying free cholesterol within HDL3-C particles [62]. The study suggests that naringin may reduce plasma and hepatic cholesterol and increase HDL3-C levels by increasing hepatic Ahr, Scarb1, and Lcat gene expression in type 2 diabetes. It is worth noting that plasma insulin and amylase levels were both significantly decreased by naringin in the study, indicating possible effects of naringin on the pancreas. Future studies are warranted to investigate the potential effects of naringin on pancreatic function in type 2 diabetes.
4.4. Lipid regulatory effects in models without obesity or other metabolic diseases
As previously reviewed, the studies of the lipid regulatory effects by naringin and naringenin mainly used the obesity or other metabolic disease models. Here, we summarize the recent studies by naringin and naringenin in the simple dyslipidemia condition without obesity or other metabolic diseases (Table 1).
Male Ldlr −/− C57BL/6 mice were pair-fed a chow diet with or without 3% (wt/wt) naringenin for 8 weeks [63]. Naringenin significantly reduced adiposity and decreased plasma TG, VLDL, and LDL-C levels in these lean mice [63]. Although hepatic cholesterol was not changed, hepatic TG was shown a trend ofdecrease by naringenin compared to the controls, which were accompanied by increased hepatic mRNA expression of peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (Pgc1a) and patatin like phospholipase domain containing 2 (Pnpla2, also known as adipose triglyceride lipase) [63]. The results suggest that the hypotriglyceridemic and hypocholesterolemic effects of naringenin in the lean Ldlr −/− mice are likely due to enhanced fatty acid oxidation, similar to the findings observed in Ldlr −/− mice under high-fat diet-induced obese conditions [63].
Human clinical trials in moderately hypercholesterolemic or healthy subjects are limited. Demonty et al.[64] examined the effects of naringin on decreasing serum LDL-C levels in moderately hypercholesterolemic men and women. Daily 500 mg of naringin for 4 weeks did not change serum TC, LDL-C, or HDL-C levels compared with the placebo group [64].
Collectively, the lipid-lowering effects of naringin and naringenin are more consistent in male mice models but not in obese female mice, suggesting sex differences in the beneficial effects of naringin and naringenin. In addition, the effects of naringin and naringenin on lipid metabolisms are divergent in human trials. Naringin reduced body weight and plasma lipids in obese participants [56], but not in non-obese subjects with hypercholesterolemia [64], which may be due to the differences in baseline health status and treatment durations (90 days vs. 28 days). Overall, current human clinical trials regarding the lipid-lowering effects of naringin and naringenin are limited in numbers and with relatively small sample sizes in some of the trials. More studies with large sample sizes and different concentrations of naringin/naringenin administration are warranted to further explore the lipid-lowering effects of naringin and naringenin in humans.
5. Modulation of Lipid Metabolism by Naringin- and Naringenin-Enriched Food Sources
5.1. Citrus bergamia (Bergamot)
Naringin is a flavonoid commonly found in citrus fruits, including citrus bergamia. Citrus bergamia, also known as bergamot, is traditionally used to produce essential oil but not fruit juice due to its bitter and sour taste [65]. Bergamot contains multiple flavonoids, including naringin, neohesperidin, neoeriocitrin, neodiosmin, and eriodictyol, whose concentrations are relatively higher than other citrus fruits [66–68]. In addition, diglycosidic conjugates of neohesperidin and naringin, brutieridin and melitidin, are found in higher content in bergamot but not in orange and lemon [69]. The unique flavonoid profile of bergamot is distinct from other citrus fruits, making bergamot a popular target to examine its health-promoting effects [10, 65] (Table 1).
Bergamot is known to reduce blood TC, LDL-C, and TG level by regulating several enzyme activities involved in lipid metabolism, including HMG-CoA reductase, pancreatic cholesterol ester hydrolase (pCEH), ACAT, LCAT, and CETP, possibly through MAPK, PPARγ, and AMPK activation [8, 9]. However, more investigations of upstream mechanisms leading to the lipid-lowering effects of bergamot are needed. As for human clinical trials, it was concluded that the changes of lipid profiles of bergamot are not consistent, especially the HDL-C levels [8]. This may be due to the heterogeneous designs, including different sample sizes, treatment duration, and bergamot forms and concentrations consumed.
In addition to the studies on lipid regulatory effect by bergamot alone, some studies have examined the combined effects by formulating bergamot with other foods or phytochemicals to enhance the bioactive effects. In an earlier clinical study, the regulatory effects on the lipid profile of nutraceutical F105, which contained 250 mg bergamot fruit extract (BFE) with 9 other phytoextracts (110 mg) from other fruits or plants, were examined in 11 participants with moderate dyslipidemia [70]. After taking 2 capsules of F105 at dinner time for 12 weeks, the plasma TC, LDL-C, non-HDL-C, C/HDL, and ApoB levels were significantly reduced in all 11 subjects, along with improved antioxidative parameters and no changes in body weight [70]. In addition, the plasma TG, oxLDL, LDL/HDL, TG/HDL, oxLDL/HDL, and PAI levels were significantly decreased in a subgroup of 8 subjects who had pre-diabetes [70]. The study suggests that BFE combined with other antioxidative phytoextracts may be beneficial in people with dyslipidemia, particularly in individuals with pre-diabetes.
Similar hypolipidemic effects were reported using a combination of whole bergamot extracts (200 mg), omega-3 (400 mg), trivalent chromium (10 μg), and red yeast rice (100 mg) in a pilot randomized human trial conducted in 50 subjects with higher serum cholesterol levels [71]. After 6 weeks of intervention, serum TG, TC, and LDL-C levels were significantly decreased, while serum HDL levels were increased in all subjects [71].
In another randomized, double-blinded, placebo-controlled study, bergamot extract was formulated with plant sterol esters, orange oil, vitamin C, vitamin B6, vitamin B12, and folic acid as CitriCholess to treat 98 older dyslipidemic adults [72]. After 12 weeks of supplementation, the body weight, serum TG, TC, and LDL-C levels were significantly decreased [72].
5.2. Citrus sinensis
Citrus sinensis (orange) is a fruit consumed worldwide, known to contain various flavanones, vitamins, and other bioactive compounds [73]. Both peel and fleshy parts of orange and orange juice have a certain amount of naringenin and its glycoside form narirutin, which were bioavailable to humans [1, 73, 74]. However, not many reviews have specifically summarized studies on this topic. Therefore, we briefly review the lipid-lowering effects of orange and orange juice in animal models and human clinical trials (Table 1).
In male Wistar rats with diet-induced hyperlipidemia, blood TG, TC, and LDL-C levels were significantly reduced after 8 weeks of orange juice intervention at the dose of 2, 5, and 8 ml/kg/d, respectively [75]. The HDL-C levels were increased by orange juice treatment at a dose of 8 ml/kg/d [75].
In overweight or obese human subjects, a 12-week consumption of 500 ml/d orange juice containing 299 mg polyphenols significantly reduced the body weight, BMI waist circumference, plasma TG and ApoB levels [76]. The ApoA-I levels were significantly increased when orange albedo and pulp extracts were added into the orange juice and increased the polyphenols content to 745 mg [76]. In another study in obese participants consuming a reduced-calorie diet, additional 500 ml orange juice consumption for 12 weeks reduced the TC and LDL-C levels to a greater extent than the participants who did not consume orange juice [77]. Although a 12-week 250 ml/d orange juice consumption did not change lipid profile in overweight/obese men with normal TG levels, the plasma TG levels were significantly reduced in subjects with hypertriglyceridemia [78]. Interestingly, the TG levels were significantly increased after consuming energy- and sugars- matched control drinks, suggesting that elevated blood TG levels caused by regular consumption of sweet drinks are likely alleviated by the bioactive phytochemicals [78].
In the non-obese healthy population, serum TG, TC, and LDL-C levels were significantly decreased after consuming orange juice (300 ml/d) for 60 days in the women whose BMI were 24.1±3.3 kg/m2 on average [39]. Another similar trial in the healthy women further investigated the effects of orange juice consumption on gut microbiota and their correlation with metabolic biomarkers [79]. There were increases in Actinobacteria and decreases in Firmicutes and Bacteroidetes in the fecal samples after the orange juice consumption. The relative abundance of the Akkermansia family was increased by orange juice consumption, which was negatively correlated with blood TG and LDL-C but positively correlated with HDL-C levels [79]. Therefore, orange juice may have hypotriglyceridemic and hypocholesterolemic effects in healthy women with normal body weights. Whether these lipid-lowering effects of orange juice consumption are mediated through modulating gut microbiota warrants further investigation.
The efficacy of orange juice consumption for a short term in a healthy population has also been reported. Two weeks of orange juice consumption at 500 ml/d did not improve plasma glucose, TG, TC, LDL-C, and HDL-C levels in healthy participants [80]. However, analysis of dried blood spots indicated that while medium and long-chain acylcarnitine levels were significantly decreased, short-chain acylcarnitines were increased after orange juice intake [80], suggesting that relatively short-term consumption of orange juice may not change serum lipid profiles but may stimulate mitochondrial and peroxisomal fatty acid beta-oxidation [80].
Red (or blood) orange is another variety of orange containing lycopene and anthocyanin that are not usually found in other citrus fruits [81]. It has been reported that blood TC and LDL-C were significantly decreased after 8 weeks of red-orange juice consumption at 750 ml/d in both normal weight and overweight/obese participants, while the body weight and blood TG were not affected [82]. Similarly, 12 weeks of red orange juice consumption at 500 ml/d significantly decreased serum TC and LDL-C levels with no change of body weight and TG levels in obese women [83]. In contrast, 4 weeks of consumption of red orange juice at 500 ml/d did not change the blood TG, TC, LDL-C, or HDL-C levels in the obese participants [84]. In addition, there were no differences in these parameters between red-orange juice and blonde orange juice consumption, suggesting that, at the dose of 500 ml, there were no extra beneficial effects from red orange juice compared with regular blond orange juice [84].
As described above, although long-term (8–12 weeks) orange juice consumption reduced body weight, blood TG, TC, and LDL-C levels in humans, the extent of positive effects of orange juice consumption were not uniform among these trials. This discrepancy may be due to the differences in testing doses, duration of orange juice consumption, polyphenols content in orange juice, and the initial metabolic status of the individuals.
The industrial food process, such as alcoholic fermentation, has been used to improve the concentrations of the bioactive compounds, such as flavanones and carotenoids, in fruit juices [85]. In addition, the alcoholic-fermented orange juice (orange beverage) contains moderate alcohol, which may be associated with reduced risks of cardiovascular disease [85, 86]. Therefore, the fermentation process may increase the beneficial effects of regular orange juice.
The effects of fermentation were studied in animal models. Male OF1 mice under standard diet were randomized into either water, orange juice, orange beverage, or aqueous alcohol solution group for 12 weeks [87]. Blood TG, TC, and LDL levels were significantly reduced while HDL levels were increased in the mice fed with the orange beverage but not with the juice or aqueous alcohol [87]. The results demonstrated greater effects on lipid profiles by the orange beverage than the orange juice, which may suggest that synergistic effects may have occurred between orange juice and alcohol in orange beverage [87].
Daily consumption of 500 ml of low-alcohol orange beverage for 2 weeks significantly decreased plasma TC, LDL-C, and LDL-C/HDL-C ratio in the subjects with moderate hypercholesterolemia. However, blood TG levels were not affected [88]. Interestingly, the changes in these lipid parameters were not observed by orange juice consumption at the same dose and duration in the healthy population, as shown in a previous study [80].
The mice and human studies suggest that alcoholic fermentation of orange juice may improve the lipid-lowering effects of regular orange juice, which is likely associated with the synergistic effects of low-grade alcohol and bioactive components in the orange juice. However, the in vivo studies are still limited both in mice and humans. Lipid-lowering effects of alcoholic-fermented orange juice warrant future investigations.
Though orange juice may exert beneficial effects in humans, the sugar content as extra energy intake outside of 3 meals may be a potential metabolic risk. In a recent cross-over clinical trial, healthy participants aged between 20 to 45 years old consumed orange juice either within each meal or no less than 2 hours after each meal for 2 weeks [89]. It was found that though blood TG levels were not changed after both interventions, fat mass and gamma-glutamyl transferase were decreased by orange juice consumption within each meal [89]. In contrast, fat mass was significantly increased along with a trend of decrease in postprandial insulin sensitivity by orange juice consumption after each meal [89]. These results suggest that the timing of orange juice consumption may not have direct effects on lipid profiles, at least on blood TG, but drinking orange juice in-between meals may be associated with increased risks of obesity and diabetes compared with consuming orange juice together with meals in healthy people [89].
5.3. Other citrus fruits
In addition to bergamot and orange, several other citrus fruits enriched in naringin and naringenin were reported with lipid-lowering effects in various rodent models (Table 1).
It was reported that Citrus junos Tanaka peel extract intervention for 10 weeks significantly decreased plasma TC and LDL-C levels in C57BL/6J mice under high cholesterol diet [90]. In addition, increased body weight, serum TG and TC levels by high-fat diet with or without constant light exposure were significantly reduced by 11 weeks’ consumption of mandarin (Citrus reticulata) dry fruit extract (1% w/w) [91], 12 weeks’ consumption of the bitter orange (Citrus aurantium L. var. amara Engl.) blossoms extract (50–200 mg/kg/d) [92], or 4 weeks’ consumption of Citrus tumida peel extract (5% w/w), respectively [93].
In a recent study, Citrus changshan-huyou peel extract administration by gavage was reported to dose-dependently reduce serum TC, TG, and LDL-C in high-fat diet-induced hyperlipidemic hamsters, with the doses at 25, 50, 100 mg/kg/d for 4 weeks [94].
6. Summary and Conclusions
Citrus flavonoids naringin, naringenin, or their extracts from food sources, such as bergamot and orange juice, are promising bioactive compounds in disease prevention and/or treatment. Recent studies have revealed new information on their ADME and pharmacokinetics. Age, sex, and species all affect naringin and naringenin’s ADME. In addition, there is a close interplay between gut microbiota and naringin and naringenin metabolism in that gut microbiota makes naringin and naringenin more bioavailable, and naringenin and orange juice modulate the relative abundance of gut microbiota and improve gut health.
Recent studies have revealed new mechanisms, including regulation of lipid digestion, cholesterol efflux, and LDL receptor expression and stability (Figure 1). However, higher doses of naringin and naringenin (e.g., 100 μM) were used in some in vitro studies, raising concerns about whether the effects observed in those studies are relevant in vivo and to human health.
Figure1. Potential mechanisms underlying the effects of naringin and naringenin on lipid metabolism.
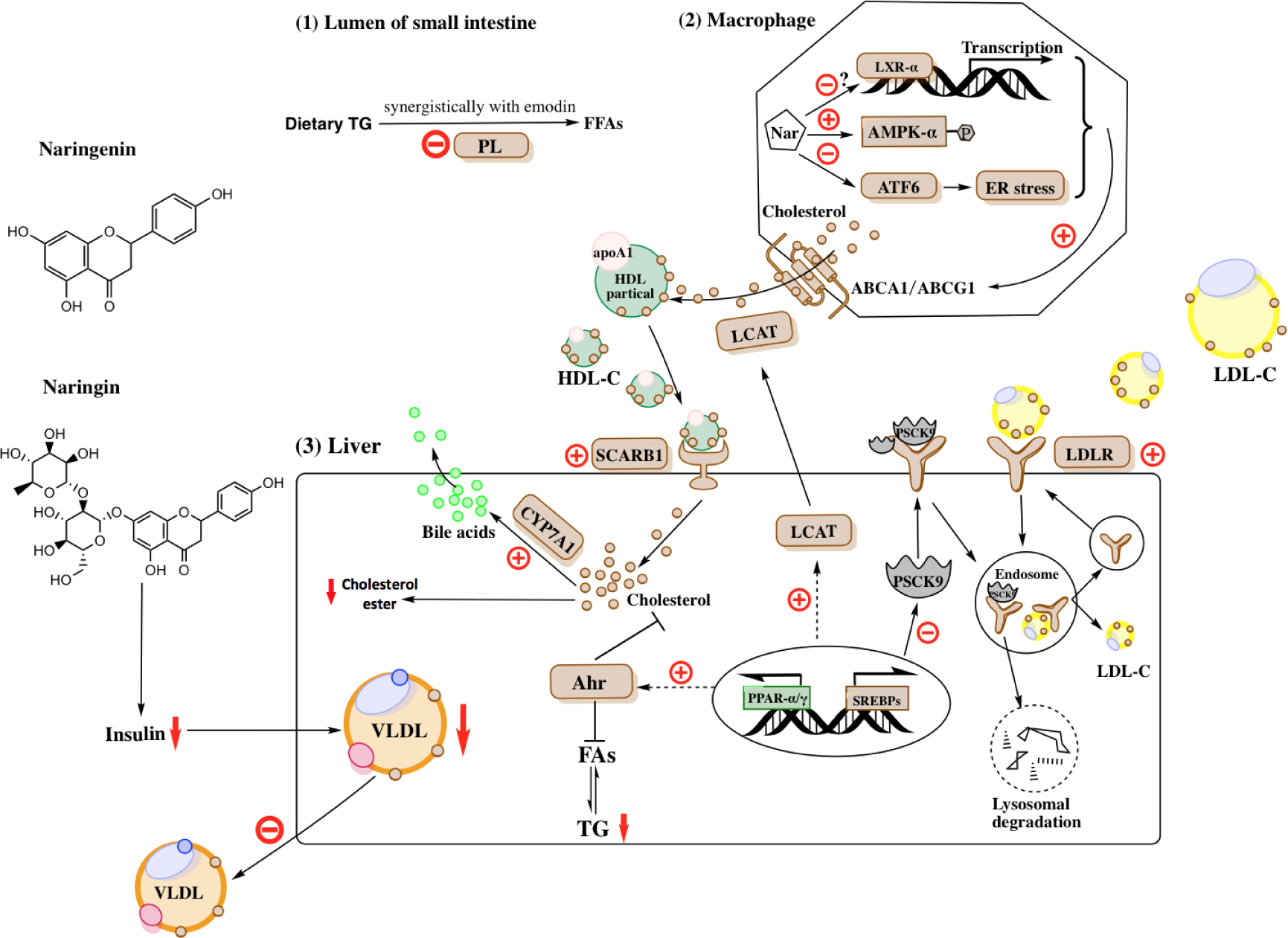
Naringin and naringenin have been shown lipid-lowering effects in various animal models and human trials. Recent studies have revealed novel mechanisms of naringin and naringenin on lipid metabolism (1) Naringin synergistically inhibits PL activity with emodin to decrease dietary fat absorption in the small intestine; (2) Naringin and naringenin increase reverse cholesterol transport by up-regulating ABCA1/ABCG1, SCARB1, and LCAT expression in macrophages and hepatocytes. The up-regulation of ABCA1/ABCG1 in macrophages is likely mediated through ER stress-ATF6 pathway, AMPK pathway, and LXR-α activation; (3) Naringin and naringenin up-regulate hepatic LDLR by suppressing PSCK9, which contributes to LDL-C clearance from circulation. In addition, naringin and naringenin decrease hepatic cholesterol ester content by inhibiting cholesterol biosynthesis through up-regulating Ahr expression and increasing cholesterol clearance through CYP7A1, which converts cholesterol to bile acids. Moreover, up-regulated Ahr expression contributes to decreased hepatic TG level by inhibiting FA synthesis. Further, naringenin and naringenin may decrease VLDL production through improving hepatic insulin sensitivity.
PL, pancreatic lipase; HDL, High-density lipoprotein; ApoA1, apolipoprotein A1; HDL-C, High-density lipoprotein cholesterol; Nar, naringin/naringenin; ABCA1, ATP-binding cassette subfamily A member1; ABCG1, ATP-binding cassette subfamily G member1; SCARB1, Scavenger receptor class B type 1; LCAT, lecithin-cholesterol acyltransferase; ER, endoplasmic reticulum; ATF6, activating transcription factor 6; AMPK, AMP-activated protein kinase; LXR, Liver X receptor; LDL-C, Low-density lipoprotein cholesterol; LDLR, Low-density lipoprotein receptor; PSCK9, Proprotein convertase subtilisin/kexin type 9; Ahr, aryl hydrocarbon receptor; CYP7A1, cytochrome P450 family 7 subfamily A member 1; FAs, fatty acids; TG, triglyceride; PPAR, Peroxisome proliferator-activated receptor; SREBPs, Sterol regulatory element-binding protein.
Recently, several studies have investigated the lipid regulatory effects of bergamot alone or with other phytoextracts or nutraceuticals in humans. The combinations have shown greater lipid-lowering effects than bergamot alone, which may be a promising strategy for dyslipidemia management in the future. In addition to bergamot, other citrus fruits such as Citrus junos Tanaka, Citrus changshan-huyou, mandarin, bitter orange, and Citrus tumida also have shown lipid-lowering effects in rodents, with more consistent TC reduction than other lipids.
Orange juice reduces body weight and fat mass and improves lipid profile in rodents and humans. Recent studies have suggested that alcoholic fermentation of orange juice may provide better lipid-lowering effects than regular orange juice due to the presence of alcohol and enhanced bioactive compounds after fermentation. Also, the timing of consuming orange juice may have different results. Drinking orange juice together with meals may be a better choice than consuming after meals. However, whether this timing has similar effects in obese or diabetic individuals needs to be investigated.
In summary, pure naringin and naringenin and their enriched citrus fruits appear to have lipid regulatory potentials, especially in the management of hypercholesterolemia in humans. However, the results were not consistent in all clinical trials due to differences in study design, participants’ baseline health condition, and the dose and duration of treatment. Further studies are needed to determine the optimized doses, intervention period, and timing to achieve the efficient lipid-lowering effects of naringin, naringenin, or other citrus fruits.
Highlights.
Naringin and naringin’s aglycone naringenin belong to a subclass of flavonoids called flavanones.
Many studies have shown beneficial health effects, including improved lipid metabolism, by naringenin, naringin, and their food sources in animals and humans; however, the mechanisms underlying the lipid-lowering effects are still not completely understood.
An update on recent advances in citrus flavanone naringin and naringenin and their enriched food sources on lipid metabolism and underlying mechanisms is provided.
Processes that impact bioavailability and interplays between naringin and naringenin and gut microbiota are also summarized.
Acknowledgment
The work was supported by NIH 1R15AT010395 and AHA 19AIREA34480011 to SW and NIH 1R15DK114790–01A1 to LZ.
Footnotes
All authors have no conflict interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barreca D, et al. , Flavanones: Citrus phytochemical with health-promoting properties. BioFactors, 2017. 43(4): p. 495–506. [DOI] [PubMed] [Google Scholar]
- 2.Orrego-Lagarón N, et al. , Absorption and disposition of naringenin and quercetin after simultaneous administration via intestinal perfusion in mice. Food & Function, 2016. 7(9): p. 3880–3889. [DOI] [PubMed] [Google Scholar]
- 3.Stevens Y, et al. , The Intestinal Fate of Citrus Flavanones and Their Effects on Gastrointestinal Health. Nutrients, 2019. 11(7): p. 1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alam MA, et al. , Effect of Citrus Flavonoids, Naringin and Naringenin, on Metabolic Syndrome and Their Mechanisms of Action. Advances in Nutrition, 2014. 5(4): p. 404–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nyane NA, et al. , Metformin-like antidiabetic, cardio-protective and non-glycemic effects of naringenin: Molecular and pharmacological insights. European Journal of Pharmacology, 2017. 803(January): p. 103–111. [DOI] [PubMed] [Google Scholar]
- 6.Raja Kumar S, et al. , Preventive Effect of Naringin on Metabolic Syndrome and Its Mechanism of Action: A Systematic Review. Evidence-based Complementary and Alternative Medicine, 2019. 2019: p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen R, et al. , Therapeutic potential of naringin: an overview. Pharmaceutical Biology, 2016. 54(12): p. 3203–3210. [DOI] [PubMed] [Google Scholar]
- 8.Lamiquiz-Moneo I, et al. , Effect of bergamot on lipid profile in humans: A systematic review. Crit Rev Food Sci Nutr, 2019: p. 1–11. [DOI] [PubMed] [Google Scholar]
- 9.Nauman C, M. and Johnson JJ, Clinical application of bergamot (Citrus bergamia) for reducing high cholesterol and cardiovascular disease markers. Integrative Food, Nutrition and Metabolism, 2019. 6(2): p. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carresi C, et al. , The Effect of Natural Antioxidants in the Development of Metabolic Syndrome: Focus on Bergamot Polyphenolic Fraction. Nutrients, 2020. 12(5): p. 1504-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Neil CE, et al. , 100% orange juice consumption is associated with better diet quality, improved nutrient adequacy, decreased risk for obesity, and improved biomarkers of health in adults: National Health and Nutrition Examination Survey, 2003–2006. Nutr J, 2012. 11: p. 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cassidy A and Minihane AM, The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am J Clin Nutr, 2017. 105(1): p. 10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kay CD, et al. , Anthocyanins and Flavanones Are More Bioavailable than Previously Perceived: A Review of Recent Evidence. Annu Rev Food Sci Technol, 2017. 8: p. 155–180. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez-Aquino E and Muriel P, Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J Gastroenterol, 2018. 24(16): p. 1679–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshi R, Kulkarni YA, and Wairkar S, Pharmacokinetic, pharmacodynamic and formulations aspects of Naringenin: An update. Life Sciences, 2018. 215: p. 43–56. [DOI] [PubMed] [Google Scholar]
- 16.Najmanova I, et al. , The pharmacokinetics of flavanones. Crit Rev Food Sci Nutr, 2020. 60(18): p. 3155–3171. [DOI] [PubMed] [Google Scholar]
- 17.Zeng X, et al. , Metabolite Profiling of Naringin in Rat Urine and Feces Using Stable Isotope-Labeling-Based Liquid Chromatography-Mass Spectrometry. J Agric Food Chem, 2020. 68(1): p. 409–417. [DOI] [PubMed] [Google Scholar]
- 18.Orrego-Lagarón N, et al. , Metabolic profile of naringenin in the stomach and colon using liquid chromatography/electrospray ionization linear ion trap quadrupole-Orbitrap-mass spectrometry (LC-ESI-LTQ-Orbitrap-MS) and LC-ESI-MS/MS. J Pharm Biomed Anal, 2016. 120: p. 38–45. [DOI] [PubMed] [Google Scholar]
- 19.Zeng X, et al. , Tissue distribution of naringin and derived metabolites in rats after a single oral administration. J Chromatogr B Analyt Technol Biomed Life Sci, 2020. 1136: p. 121846. [DOI] [PubMed] [Google Scholar]
- 20.Ovando-Martínez M, et al. , Simulated Gastrointestinal Digestion, Bioaccessibility and Antioxidant Capacity of Polyphenols from Red Chiltepin (Capsicum annuum L. Var. glabriusculum) Grown in Northwest Mexico. Plant Foods for Human Nutrition, 2018. 73(2): p. 116–121. [DOI] [PubMed] [Google Scholar]
- 21.Monfoulet LE, et al. , Effects of the apple matrix on the postprandial bioavailability of flavan-3-ols and nutrigenomic response of apple polyphenols in minipigs challenged with a high fat meal. Food Funct, 2020. [DOI] [PubMed] [Google Scholar]
- 22.Aschoff JK, et al. , In vitro bioaccessibility of carotenoids, flavonoids, and vitamin C from differently processed oranges and orange juices [Citrus sinensis (L.) Osbeck]. J Agric Food Chem, 2015. 63(2): p. 578–87. [DOI] [PubMed] [Google Scholar]
- 23.Aschoff JK, et al. , Urinary excretion of Citrus flavanones and their major catabolites after consumption of fresh oranges and pasteurized orange juice: A randomized cross-over study. Mol Nutr Food Res, 2016. 60(12): p. 2602–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho KKHY, Ferruzzi MG, and Wightman JD, Potential health benefits of (poly)phenols derived from fruit and 100% fruit juice. Nutrition Reviews, 2019. 78(2): p. 145–174. [DOI] [PubMed] [Google Scholar]
- 25.Kanaze FI, et al. , Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur J Clin Nutr, 2007. 61(4): p. 472–7. [DOI] [PubMed] [Google Scholar]
- 26.Rebello CJ, et al. , Safety and pharmacokinetics of naringenin: A randomized, controlled, single-ascending-dose clinical trial. Diabetes Obes Metab, 2020. 22(1): p. 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corsonello A, Pedone C, and Incalzi RA, Age-related pharmacokinetic and pharmacodynamic changes and related risk of adverse drug reactions. Curr Med Chem, 2010. 17(6): p. 571–84. [DOI] [PubMed] [Google Scholar]
- 28.Zheng H, et al. , Age-related changes in hepatic expression and activity of drug metabolizing enzymes in male wild-type and breast cancer resistance protein knockout mice. Biopharm Drug Dispos, 2018. 39(7): p. 344–353. [DOI] [PubMed] [Google Scholar]
- 29.Zeng X, et al. , Pharmacokinetics, Tissue Distribution, Metabolism, and Excretion of Naringin in Aged Rats. Front Pharmacol, 2019. 10: p. 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bai Y, et al. , Pharmacokinetics and Metabolism of Naringin and Active Metabolite Naringenin in Rats, Dogs, Humans, and the Differences Between Species. Front Pharmacol, 2020. 11: p. 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rebello CJ, et al. , Naringenin Promotes Thermogenic Gene Expression in Human White Adipose Tissue. Obesity (Silver Spring), 2019. 27(1): p. 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen T, et al. , Simultaneously Quantitative Analysis of Naringin and Its Major Human Gut Microbial Metabolites Naringenin and 3-(4’-Hydroxyphenyl) Propanoic Acid via Stable Isotope Deuterium-Labeling Coupled with RRLC-MS/MS Method. Molecules, 2019. 24(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pereira-Caro G, et al. , In vitro colonic catabolism of orange juice (poly)phenols. Molecular Nutrition & Food Research, 2015. 59(3): p. 465–475. [DOI] [PubMed] [Google Scholar]
- 34.Braune A, Gutschow M, and Blaut M, An NADH-Dependent Reductase from Eubacterium ramulus Catalyzes the Stereospecific Heteroring Cleavage of Flavanones and Flavanonols. Appl Environ Microbiol, 2019. 85(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeon S-M, et al. , Hypocholesterolemic and antioxidative effects of naringenin and its two metabolites in high-cholesterol fed rats. Translational Research, 2007. 149(1): p. 15–21. [DOI] [PubMed] [Google Scholar]
- 36.Pereira-Caro G, et al. , Catabolism of citrus flavanones by the probiotics Bifidobacterium longum and Lactobacillus rhamnosus. Eur J Nutr, 2018. 57(1): p. 231–242. [DOI] [PubMed] [Google Scholar]
- 37.Pereira-Caro G, et al. , Chronic administration of a microencapsulated probiotic enhances the bioavailability of orange juice flavanones in humans. Free Radic Biol Med, 2015. 84: p. 206–214. [DOI] [PubMed] [Google Scholar]
- 38.Firrman J, et al. , Analysis of Temporal Changes in Growth and Gene Expression for Commensal Gut Microbes in Response to the Polyphenol Naringenin. Microbiol Insights, 2018. 11: p. 1178636118775100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lima ACD, et al. , Effect of Daily Consumption of Orange Juice on the Levels of Blood Glucose, Lipids, and Gut Microbiota Metabolites: Controlled Clinical Trials. Journal of Medicinal Food, 2019. 22(2): p. 202–210. [DOI] [PubMed] [Google Scholar]
- 40.McNabney SM and Henagan TM, Short Chain Fatty Acids in the Colon and Peripheral Tissues: A Focus on Butyrate, Colon Cancer, Obesity and Insulin Resistance. Nutrients, 2017. 9(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He J, et al. , Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int J Mol Sci, 2020. 21(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu TT, et al. , Lipase Inhibitors for Obesity: A Review. Biomedicine and Pharmacotherapy, 2020. 128(November 2019). [DOI] [PubMed] [Google Scholar]
- 43.Rajan L, Palaniswamy D, and Mohankumar SK, Targeting obesity with plant-derived pancreatic lipase inhibitors: A comprehensive review. Pharmacological Research, 2020. 155(December 2019): p. 104681–104681. [DOI] [PubMed] [Google Scholar]
- 44.Ji X, et al. , Bioactive compounds from herbal medicines to manage dyslipidemia. Biomed Pharmacother, 2019. 118: p. 109338. [DOI] [PubMed] [Google Scholar]
- 45.Guo XX, et al. , Synergistic interactions of apigenin, naringin, quercetin and emodin on inhibition of 3T3-L1 preadipocyte differentiation and pancreas lipase activity. Obesity Research and Clinical Practice, 2016. 10(3): p. 327–339. [DOI] [PubMed] [Google Scholar]
- 46.Phillips MC, Molecular mechanisms of cellular cholesterol efflux. Journal of Biological Chemistry, 2014. 289(35): p. 24020–24029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao C and Dahlman-Wright K, Liver X receptor in cholesterol metabolism. Journal of Endocrinology, 2010. 204(3): p. 233–240. [DOI] [PubMed] [Google Scholar]
- 48.Fouache A, et al. , Flavonoids differentially modulate liver X receptors activity—Structure-function relationship analysis. Journal of Steroid Biochemistry and Molecular Biology, 2019. 190(February): p. 173–182. [DOI] [PubMed] [Google Scholar]
- 49.Xu X, et al. , Enhanced cellular cholesterol efflux by naringenin is mediated through inhibiting endoplasmic reticulum stress - ATF6 activity in macrophages. Biochim Biophys Acta Mol Cell Biol Lipids, 2019. 1864(10): p. 1472–1482. [DOI] [PubMed] [Google Scholar]
- 50.Saenz J, et al. , Grapefruit Flavonoid Naringenin Regulates the Expression of LXRα in THP-1 Macrophages by Modulating AMP-Activated Protein Kinase. Molecular Pharmaceutics, 2018. 15(5): p. 1735–1745. [DOI] [PubMed] [Google Scholar]
- 51.Khosravi M, Hosseini-Fard R, and Najafi M, Circulating low density lipoprotein (LDL). Horm Mol Biol Clin Investig, 2018. 35(2). [DOI] [PubMed] [Google Scholar]
- 52.Liang J, et al. , Naringin regulates cholesterol homeostasis and inhibits inflammation via modulating NF-κB and ERK signaling pathways in vitro. Pharmazie, 2016. 71(2): p. 101–108. [PubMed] [Google Scholar]
- 53.Sui GG, et al. , Naringin Activates AMPK Resulting in Altered Expression of SREBPs, PCSK9, and LDLR To Reduce Body Weight in Obese C57BL/6J Mice. J Agric Food Chem, 2018. 66(34): p. 8983–8990. [DOI] [PubMed] [Google Scholar]
- 54.Vekic J, et al. , Obesity and dyslipidemia. Metabolism: Clinical and Experimental, 2019. 92: p. 71–81. [DOI] [PubMed] [Google Scholar]
- 55.Ke JY, et al. , Citrus flavonoid, naringenin, increases locomotor activity and reduces diacylglycerol accumulation in skeletal muscle of obese ovariectomized mice. Molecular Nutrition and Food Research, 2016. 60(2): p. 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barajas-Vega JL, et al. , Naringin reduces body weight, plasma lipids and increases adiponectin levels in patients with dyslipidemia. Int J Vitam Nutr Res, 2020: p. 1–7. [DOI] [PubMed] [Google Scholar]
- 57.Hartogh DJD and Tsiani E, Antidiabetic properties of naringenin: A citrus fruit Polyphenol. Biomolecules, 2019. 9(3): p. 4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allister EM, et al. , Inhibition of apoB secretion from HepG2 cells by insulin is amplified by naringenin, independent of the insulin receptor. J Lipid Res, 2008. 49(10): p. 2218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haas ME, Attie AD, and Biddinger SB, The regulation of ApoB metabolism by insulin. Trends Endocrinol Metab, 2013. 24(8): p. 391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rotimi SO, et al. , Naringin enhances reverse cholesterol transport in high fat/low streptozocin induced diabetic rats. Biomedicine and Pharmacotherapy, 2018. 101(December 2017): p. 430–437. [DOI] [PubMed] [Google Scholar]
- 61.Tanos R, et al. , Aryl hydrocarbon receptor regulates the cholesterol biosynthetic pathway in a dioxin response element-independent manner. Hepatology, 2012. 55(6): p. 1994–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fotakis P, et al. , The Effect of Natural LCAT Mutations on the Biogenesis of HDL. Biochemistry, 2015. 54(21): p. 3348–59. [DOI] [PubMed] [Google Scholar]
- 63.Burke AC, et al. , Naringenin Supplementation to a Chow Diet Enhances Energy Expenditure and Fatty Acid Oxidation, and Reduces Adiposity in Lean, Pair-Fed Ldlr −/− Mice. Molecular Nutrition and Food Research, 2019. 63(6): p. 1–9. [DOI] [PubMed] [Google Scholar]
- 64.Demonty I, et al. , The citrus flavonoids hesperidin and naringin do not affect serum cholesterol in moderately hypercholesterolemic men and women. J Nutr, 2010. 140(9): p. 1615–20. [DOI] [PubMed] [Google Scholar]
- 65.Mannucci C, et al. , Clinical Pharmacology of Citrus bergamia: A Systematic Review. Phytother Res, 2017. 31(1): p. 27–39. [DOI] [PubMed] [Google Scholar]
- 66.Gattuso G, et al. , Distribution of flavonoids and furocoumarins in juices from cultivars of Citrus bergamia Risso. J Agric Food Chem, 2007. 55(24): p. 9921–7. [DOI] [PubMed] [Google Scholar]
- 67.Cautela D, Vella FM, and Laratta B, The Effect of Processing Methods on Phytochemical Composition in Bergamot Juice. Foods, 2019. 8(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gattuso G, et al. , Flavonoid composition of Citrus juices. Molecules, 2007. 12(8): p. 1641–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Di Donna L, et al. , Statin-like principles of bergamot fruit (Citrus bergamia): isolation of 3-hydroxymethylglutaryl flavonoid glycosides. J Nat Prod, 2009. 72(7): p. 1352–4. [DOI] [PubMed] [Google Scholar]
- 70.Babish JG, et al. , Synergistic in vitro antioxidant activity and observational clinical trial of F105, a phytochemical formulation including Citrus bergamia, in subjects with moderate cardiometabolic risk factors. Canadian Journal of Physiology and Pharmacology, 2016. 94(12): p. 1257–1266. [DOI] [PubMed] [Google Scholar]
- 71.Landi F, et al. , Effects of a New Combination of Medical Food on Endothelial Function and Lipid Profile in Dyslipidemic Subjects: A Pilot Randomized Trial. BioMed Research International, 2019. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cai Y, et al. , Effects of 12-week supplementation of Citrus bergamia extracts-based formulation CitriCholess on cholesterol and body weight in older adults with dyslipidemia: A randomized, double-blind, placebo-controlled trial. Lipids in Health and Disease, 2017. 16(1): p. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Favela-Hernandez JM, et al. , Chemistry and Pharmacology of Citrus sinensis. Molecules, 2016. 21(2): p. 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chanet A, et al. , Citrus flavanones: what is their role in cardiovascular protection? J Agric Food Chem, 2012. 60(36): p. 8809–22. [DOI] [PubMed] [Google Scholar]
- 75.Mallick N and Khan RA, Antihyperlipidemic effects of Citrus sinensis, Citrus paradisi, and their combinations. J Pharm Bioallied Sci, 2016. 8(2): p. 112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rangel-Huerta OD, et al. , Normal or High Polyphenol Concentration in Orange Juice Affects Antioxidant Activity, Blood Pressure, and Body Weight in Obese or Overweight Adults. The Journal of Nutrition, 2015. 145(8): p. 1808–1816. [DOI] [PubMed] [Google Scholar]
- 77.Ribeiro C, Dourado G, and Cesar T, Orange juice allied to a reduced-calorie diet results in weight loss and ameliorates obesity-related biomarkers: A randomized controlled trial. Nutrition, 2017. 38: p. 13–19. [DOI] [PubMed] [Google Scholar]
- 78.Simpson EJ, Mendis B, and Macdonald IA, Orange juice consumption and its effect on blood lipid profile and indices of the metabolic syndrome; A randomised, controlled trial in an at-risk population. Food and Function, 2016. 7(4): p. 1884–1891. [DOI] [PubMed] [Google Scholar]
- 79.Fidélix M, et al. , Microbiota modulation and effects on metabolic biomarkers by orange juice: a controlled clinical trial. Food and Function, 2020. 11(2): p. 1599–1610. [DOI] [PubMed] [Google Scholar]
- 80.Moreira V, et al. , Orange juice affects acylcarnitine metabolism in healthy volunteers as revealed by a mass-spectrometry based metabolomics approach. Food Research International, 2018. 107(November 2017): p. 346–352. [DOI] [PubMed] [Google Scholar]
- 81.Grosso G, et al. , Red orange: experimental models and epidemiological evidence of its benefits on human health. Oxid Med Cell Longev, 2013. 2013: p. 157240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Silveira JQ, Dourado GKZS, and Cesar TB, Red-fleshed sweet orange juice improves the risk factors for metabolic syndrome. International Journal of Food Sciences and Nutrition, 2015. 66(7): p. 830–836. [DOI] [PubMed] [Google Scholar]
- 83.Azzini E, et al. , Effect of Red Orange Juice Consumption on Body Composition and Nutritional Status in Overweight/Obese Female: A Pilot Study. Oxidative Medicine and Cellular Longevity, 2017. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hollands WJ, et al. , 4-Week consumption of anthocyanin-rich blood orange juice does not affect LDL-cholesterol or other biomarkers of CVD risk and glycaemia compared with standard orange juice: A randomised controlled trial. British Journal of Nutrition, 2018. 119(4): p. 415–421. [DOI] [PubMed] [Google Scholar]
- 85.Escudero-López B, et al. , Fermented orange juice: source of higher carotenoid and flavanone contents. J Agric Food Chem, 2013. 61(37): p. 8773–82. [DOI] [PubMed] [Google Scholar]
- 86.Golan R, Gepner Y, and Shai I, Wine and Health–New Evidence. European Journal of Clinical Nutrition, 2019. 72: p. 55–59. [DOI] [PubMed] [Google Scholar]
- 87.Escudero-López B, et al. , Consumption of orange fermented beverage reduces cardiovascular risk factors in healthy mice. Food and Chemical Toxicology, 2015. 78: p. 78–85. [DOI] [PubMed] [Google Scholar]
- 88.Cerrillo I, et al. , Effect of daily intake of a low-alcohol orange beverage on cardiovascular risk factors in hypercholesterolemic humans. Food Research International, 2019. 116(December 2017): p. 168–174. [DOI] [PubMed] [Google Scholar]
- 89.Hägele FA, et al. , High orange juice consumption with or in-between three meals a day differently affects energy balance in healthy subjects. Nutrition and Diabetes, 2018. 8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shin EJ, et al. , Citrus junos Tanaka peel ameliorates hepatic lipid accumulation in HepG2 cells and in mice fed a high-cholesterol diet. BMC Complementary and Alternative Medicine, 2016. 16(1): p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chou YC, Ho CT, and Pan MH, Immature citrus reticulata extract promotes browning of beige adipocytes in high-fat diet-induced C57BL/6 mice. Journal of Agricultural and Food Chemistry, 2018. 66(37): p. 9697–9703. [DOI] [PubMed] [Google Scholar]
- 92.Shen CY, et al. , Citrus aurantium L. var. amara Engl. inhibited lipid accumulation in 3T3-L1 cells and Caenorhabditis elegans and prevented obesity in high-fat diet-fed mice. Pharmacological Research, 2019. 147(June): p. 104347-104347. [DOI] [PubMed] [Google Scholar]
- 93.Sato M, et al. , Dietary Intake of Immature Citrus tumida Hort. ex Tanaka Peels Suppressed Body Weight Gain and Fat Accumulation in a Mouse Model of Acute Obesity. Journal of Nutritional Science and Vitaminology, 2019. 65(1): p. 19–23. [DOI] [PubMed] [Google Scholar]
- 94.Ling Y, et al. , Hypolipidemic effect of pure total flavonoids from peel of Citrus (PTFC) on hamsters of hyperlipidemia and its potential mechanism. Experimental Gerontology, 2020. 130(November 2019): p. 110786–110786. [DOI] [PubMed] [Google Scholar]


