Abstract
Spatial navigation and event memory (termed episodic memory) are thought to be heavily intertwined, both in terms of their cognitive processes and underlying neural systems. Some theoretical models posit that both memory for places during navigation and episodic memory depend on highly overlapping brain systems. Here, we assessed this relationship by testing navigation in an individual with severe retrograde and anterograde amnesia; the amnesia stemmed from bilateral lesions in the medial temporal lobes from two separate strokes. The individual with amnesia and age-matched controls were tested on their memories for the locations of previously seen objects relative to distal mountain cues in an immersive virtual environment involving free ambulation. All participants were tested from both repeated and novel start locations and when a single distal mountain cue was unknowingly moved to determine if they relied on a single (beacon) cue to a greater extent than the collection of all distal cues. Compared to age-matched controls, the individual with amnesia showed no significant deficits in navigation from either the repeated or novel start points, although both the individual with amnesia and controls performed well above chance at placing objects near their correct locations. The individual with amnesia also relied on a combination of distal cues in a manner comparable to age-matched controls. Despite largely intact memory for locations using distal cues, the individual with amnesia walked longer paths, rotated more, and took longer to complete trials. Our findings suggest that memory for places during navigation and episodic memory may involve partially dissociable brain circuits and that other brain regions outside of the medial temporal lobe partially support some aspects of navigation. At the same time, the fact that the individual with amnesia walked more circuitous paths and had dense amnesia for autobiographic events supports the idea that the hippocampus may be important for binding information as part of a larger role in memory.
Keywords: Episodic Memory, Hippocampus, Spatial Navigation, Lesion, Allocentric, Amnesia, Medial Temporal Lobe Function
1). Introduction
Memory and navigation share some important links, which in turn suggests commonalities in their underlying cognitive processes and brain regions involved. For example, when we think of how to find a place such as our favorite restaurant, we may recall our last visit and use our memory for that event (termed an “episodic memory”) to help us retrieve the route to get there. Past studies also suggest similarities in the neural machinery involved in retrieving memories and locations. For example, place cells located in the hippocampus not only fire at specific spatial locations during navigation (Ekstrom et al., 2005; O’Keefe & Dostrovsky, 1971; Wilson & McNaughton, 1993), but also are active during the recall of memories about the navigational experience (Miller et al., 2013). Consistent with such observations, some models of hippocampal function have suggested that the same neural machinery important for processing space in the medial temporal lobes also plays a critical and fundamental role in non-spatial elements important to episodic memory (Bellmund et al., 2018; Buzsaki & Moser, 2013).
At the same time, there is increasing evidence that elements of navigation and memory are at least partially dissociable, both in terms of their underlying cognitive processes and brain networks. One large sample study found that individual ratings of autobiographical memory and mental imagery skills showed a low correlation with those of navigational and geometric/spatial skills (Fan et al., 2021). In addition, functional magnetic resonance imaging (fMRI) studies that investigated the patterns of activation and connectivity patterns showed only partially overlapping brain networks when participants retrieved temporal order (often linked to episodic memory) compared to spatial distances (Ekstrom et al., 2011; Petrican et al., 2020; Schedlbauer et al., 2014; Schedlbauer & Ekstrom, 2019). Studies of patients with impaired autobiographical memory suggest partially preserved spatial memory in some instances (Corkin, 2002; Herdman et al., 2015; Rosenbaum et al., 2000). Thus, some studies have suggested that the brain networks involved in episodic memory are partially independent from those involved in some of the components of involved in navigation skills (Eichenbaum, 2017; Ekstrom et al., 2017).
One brain area in which there remains uncertainty regarding the degree of overlap between memory and navigation is the human hippocampus. In support of a central role for the human hippocampus and medial temporal lobes in episodic memory, decades of work suggest that lesions to the human medial temporal lobe significantly affect the level of detail and precision with which individuals retrieve details about events in their past (Gilboa et al., 2006; Milner et al., 1968; Rosenbaum et al., 2009; Scoville & Milner, 1957). Regarding navigation, on the one hand, studies in rodents have revealed profound memory loss for hidden locations in novel environments such as the Morris Water Maze following hippocampal lesions (Morris et al., 1982). On the other hand, studies from humans with hippocampal lesions often show at least partially intact navigational skills. For example, one study found that when provided with a map, individuals with bilateral hippocampal lesions could readily navigate as long as there were no memory components involved (Urgolites et al., 2016). Other studies performed with individuals with hippocampal lesions further suggest largely accurate map drawings of familiar environments, although these maps lack some detail (Herdman et al., 2015; Maguire et al., 2006; Rosenbaum et al., 2015; Rosenbaum et al., 2000; Teng & Squire, 1999). These studies, however, do not address how individuals with hippocampal lesions remember locations during active navigation, and exactly how impairments in memory might affect active navigation.
In two previous studies, we found that a group of individuals with bilateral and unilateral hippocampal lesions could remember places using distal landmarks, often taken as a hallmark of “allocentric” navigation, in a virtual environment from both novel and repeated start points at levels well above chance. Individuals with hippocampal lesions, however, showed decrements in the precision of their search compared to age-matched controls (Kolarik et al., 2016, 2018). However, there are three important limitations with those studies. One was that all testing occurred in desktop virtual reality, which lacks vestibular, somatosensory, and proprioceptive cues (collectively termed “body-based” cues). Given that the medial temporal lobes have been theorized as important to multimodal integration of memories, particularly path integration (McNaughton et al., 2006), perhaps individuals with hippocampal lesions would show a greater deficit in memory for location when tested with body-based cues. This could also be relevant to determining any selective role for the human medial temporal lobes in allocentric (viewpoint independent and based on distal cues) compared to egocentric (viewpoint dependent) navigation as body-based cues provide richer encoding of both types of spatial knowledge (O’Keefe & Nadel, 1978). Additionally, the previous studies by Kolarik et al. (2016, 2018) did not involve detailed testing of memory and while all the individuals with medial temporal lobe lesions that were tested showed some memory impairments, the severity of each individual’s amnesia varied somewhat. Finally, not all individuals had bilateral hippocampal lesions, and an intact unilateral hippocampus may be able to support some aspects of spatial memory (Lee et al., 2002).
To address these limitations, we tested an individual, referred to as HML040, who experienced a bilateral lesion encompassing most of his medial temporal lobes due to multiple strokes. Based on a detailed neuropsychological work-up, which is described by Wank and colleagues (Wank et al., in press), HML040 scored in the severely impaired range on a battery of episodic memory tasks, including near floor performance on standardized episodic learning and memory tests after a long delay. A thorough work-up of his autobiographical memory (see Wank et al., in press) also revealed a profound episodic autobiographical memory impairment, with little access to memories from before his strokes and no access, to our knowledge, for episodic memories occurring after his strokes. To test HML040’s navigation skills, particularly his memory for places during navigation, we developed a task similar to that used in Kolarik et al. (2016, 2018) but tested HML040 (and age-matched controls) in a 5 × 5 meter room while wearing a wireless head-mounted display. Unlike Kolarik et al. (2016, 2018), we rendered the distal cues at “infinite” distance to ensure that the participants could not use these cues as possible egocentric or beaconing cues when the start locations differed from what was learned during acquisition. In addition, we included a condition in which one of the mountains moved to determine whether the participants used the collection of three mountains to remember a hidden location, the single mountain, or some combination of both.
If memory for places during navigation and episodic memory are heavily intertwined cognitive processes, we would expect HML040, who has dense amnesia, to show: 1) reduced memory for locations compared to the age-matched controls, 2) chance performance at finding the hidden targets, and 3) greater deficits from novel compared to repeated start locations. To briefly preface our results, HML040 showed comparable navigation performance to age-matched controls. Yet, HML040 did show longer search times and paths, suggesting some effect of his profoundly impaired memory on his navigation.
2). Methods
2.1). HML040 case history
HML040 obtained a law degree (J.D.: 20 total years of education) and worked as a tax attorney in a demanding office position for his career. He was 80 years old when he participated. HML040 experienced bilateral posterior cerebral artery strokes, the second of which resulted in the onset of his memory impairment a few years before our evaluation. HML040 had a history of hypertension but no other remarkable cardiovascular history prior to his strokes. According to HML040’s wife, prior to his second stroke, HML040 had no cognitive difficulties, was working full time, and was independent in activities of daily living. HML040’s wife reported that his memory impairment has been stable since his second stroke, neither worsening nor improving. HML040 was living in a memory care home when he participated in the current study. This limited our knowledge of his day-to-day navigational ability; however, his wife reported no concerns about his ability to move around his living space and the common areas of his memory care home.
2.1.1). Neuroimaging Data
HML040 had a pacemaker placed after his second stroke and we therefore relied on available clinical MRI and CT scans because 3T scans could not be safely conducted. Review of the CT and clinical scans revealed that his strokes affected the entire length of the hippocampus and surrounding MTL structures bilaterally. The damage is extensive, although there appears to be partial sparing of the anterior hippocampus. The lesions also extended into inferior temporo-occipital regions bilaterally, but more so on the left. There was evidence of left posterior thalamic involvement, but the lesion did not encompass the anteroventral or medial dorsal thalamic nuclei implicated in memory (Van Der Werf et al., 2003). Figure 1 shows eight axial images from the most recent CT scan, which was conducted shortly before we evaluated HML040, and provided better spatial resolution and MTL definition than an older MRI scan taken acutely after HML040’s second stroke. Our review of all scans revealed no evidence of age disproportionate cortical atrophy, or changes between the acute stage MRI and the CT obtained one and a half years later, suggesting no intervening clinical incident or new pathology. We also note that the earlier MRI showed moderate chronic deep and periventricular white matter ischemic changes, consistent with HML040’s history of hypertension. Due to the resolution of his scan and the fact that follow-ups are not possible due to his pacemaker, we are unable to accurately estimate volume loss within the medial temporal lobes. The CTs below, however, demonstrate clear and bilateral damage to the medial temporal lobes, which are likely to be the primary contributor to his amnesia.
Fig 1. CT imaging of HML040’s lesions in the axial view.
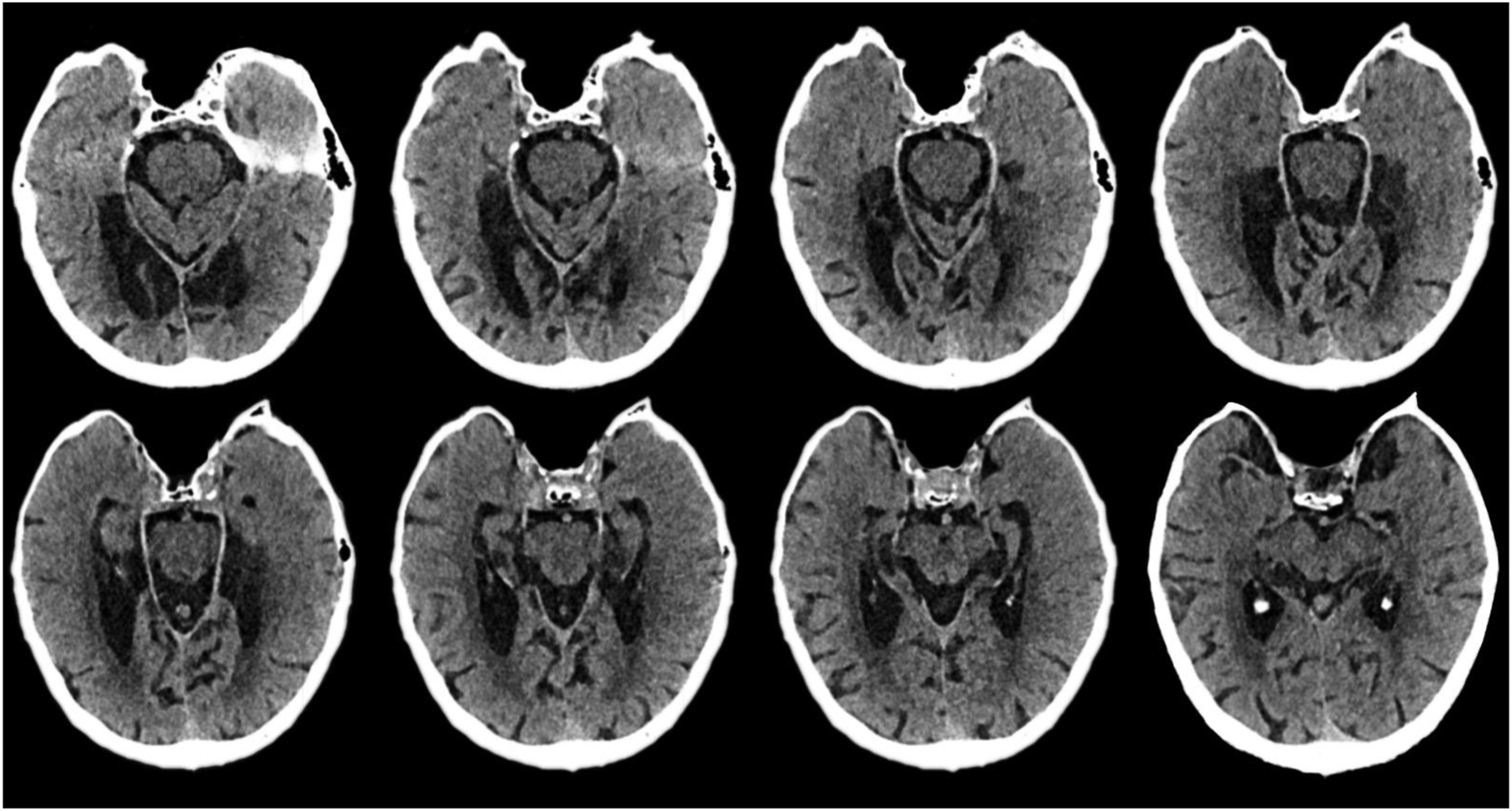
The images track the lesions from ventral (top left) regions to those more dorsal (bottom right).
2.1.2). Neuropsychological Testing
HML040’s neuropsychological profile is reported in Table 1 and described in detail by Wank and colleagues (Wank et al., in press). Based on our neuropsychological testing and review of his background, we estimated his premorbid functioning as high average. Consistent with his advanced education and demanding occupational background, his Similarities score was high average, which is correlated with measures of premorbid function in healthy adults (Bright & van der Linde, 2020). On the Wechsler Adult Intelligence Scale (WAIS-IV edition; Wechsler, 2008), HML040 demonstrated average verbal comprehension and low average perceptual reasoning, suggesting a drop from our premorbid estimate for him. As detailed below, this likely reflects the fact that HML040 has several different cognitive impairments.
Table 1. HML040’s neuropsychological performance.
Note: Standardized scores from the subtests and indices of the WAIS-IV and WMS-IV are scaled scores and index scores, respectively, and derived from the published norms. Z-scores are reported for all general semantic (except Pyramids and Palm Trees), executive function, autobiographical memory tests. Both fluency tests, Boston Naming Test-II, and Trails z-scores were based on norms from Heaton et al., 2004. The z-score for Cambridge Naming Test was calculated using a different, unpublished control sample of healthy middle age and older adults (N = 32). For Pyramids and Palm Trees, percent correct is reported in the standardized score column and a score of >90% is considered within normal limits (WNL) per published norms. The normative sample for the Autobiographical Memory Interview (N = 34) comes from Kopelman et al., 1989.
| Raw Score | Standardized Score | Interpretation | |
|---|---|---|---|
| WAIS-IV | |||
| Similarities | 30 | 14 | High Average |
| Vocabulary | 45 | 12 | Average |
| Information | 11 | 8 | Average |
| Block Design | 20 | 7 | Low Average |
| Matrix Reasoning | 8 | 8 | Average |
| Visual Puzzles | 6 | 6 | Low Average |
| Digit Span | 21 | 8 | Average |
| Arithmetic | 11 | 8 | Average |
| Symbol Search | 4 | 2 | Impaired |
| Digit Symbol Coding | 15 | 3 | Impaired |
| Full Scale IQ | . | 83 | Low Average |
| Verbal Comprehension Index | . | 107 | Average |
| Perceptual Reasoning Index | . | 82 | Low Average |
| Working Memory Index | . | 89 | Low Average |
| Processing Speed Index | . | 59 | Impaired |
| WMS-IV | . | ||
| Logical Memory I | 11 | 4 | Impaired |
| Logical Memory II | 0 | 1 | Impaired |
| Verbal Paired Associates I | 3 | 3 | Impaired |
| Verbal Paired Associates II | 0 | 1 | Impaired |
| Visual Reproduction I | 14 | 4 | Impaired |
| Visual Reproduction II | 0 | 2 | Impaired |
| Symbol Span | 10 | 8 | Average |
| Auditory Memory Index | . | 51 | Impaired |
| Visual Memory Index | . | 58 | Impaired |
| Immediate Memory Index | . | 60 | Impaired |
| Delayed Memory Index | . | 43 | Impaired |
| General Semantics | |||
| Animal Fluency | 9 | −2.5 | Impaired |
| Boston Naming Test-II | 36 | −2.4 | Impaired |
| Cambridge Naming Test | 49 | −11.59 | Impaired |
| Pyramids and Palm Trees (Words) | 48 | 92.31% | WNL |
| Processing Speed/Executive Function | |||
| Trails A | 90.9” | −2.9 | Impaired |
| Trails B | >300” | <−3 | Impaired |
| F+A+S | 38 | −0.3 | Average |
| Autobiographical Memory Interview | |||
| Personal Semantic Childhood | 4.50 | −5.13 | Impaired |
| Personal Semantic Young Adult | 8.50 | −5.59 | Impaired |
| Personal Semantic Recent | 5.00 | −16.09 | Impaired |
| Autobiographical Incidents Childhood | 2.00 | −3.43 | Impaired |
| Autobiographical Incidents Young Adult | 0.00 | −4.78 | Impaired |
| Autobiographical Incidents Recent | 0.00 | −7.22 | Impaired |
WAIS-IV = Wechsler Adult Intelligence Scale, Fourth Edition, WMS-IV = Wechsler Memory Scale, Fourth Edition.
HML040’s deficits in learning and memory, however, were particularly severe. He consistently performed near the floor on the subtests of the Wechsler Memory Scale, Fourth edition (WMS-IV; Wechsler, 2009). As a result, his performance was profoundly impaired for verbal and non-verbal memory, at immediate and longer delays. Similarly, he exhibited profound impairment on multiple measures of autobiographical memory. While these results are detailed in Wank and colleagues (Wank et al., in press), we note here that HML040 has severely impaired retrieval of episodic memories for his personal life, as well as significantly impaired personal semantics, or knowledge about his life history (Renoult et al., 2012). As noted in Table 1, HML040’s autobiographical memory impairment for episodes and personal semantics extends back to the remote childhood period (Autobiographical Memory Interview, Kopelman et al., 1989). As reported in Wank and colleagues (Wank et al., in press), when given the Autobiographical Interview (Levine et al., 2002), HML040 was unable to generate any episodic memories from the anterograde domain, and his retrograde episodic memories lacked episodic detail. He also struggled to provide an elaborative narrative of his life story, instead relying on abstract facts. As discussed in Wank and colleagues (Wank et al., in press), HML040’s autobiographical memory impairment is so severe that we believe it has affected his ability to accurately and reliably know his personality traits.
In sum, HML040 presents as severely amnesic, with difficulty retrieving episodes and personal semantics. His personal semantic impairment is most significant for facts associated with spatiotemporal contexts (as detailed in Wank et al., in press). Our characterization of HML040 as amnesic is supported by his behavior in informal interactions as well. In both casual conversation and during breaks between cognitive tests, he frequently requires reorientation to time and place, repeats himself, and he occasionally reports feeling like he “just woke up.”
Beyond learning and memory, HML040’s neuropsychological profile is mixed, with areas of spared performance as well as additional cognitive impairments. HML040 has received extensive testing of semantic memory (Wank et al., in press), with overall mixed performance on tests of “general semantics” (Renoult et al., 2012). We have summarized his semantic memory performance as suggesting a particular difficulty for unique information from non-personal categories of knowledge (Wank et al., in press). His performance on a test of basic semantic knowledge comprehension was spared (Pyramids and Palm Trees; Howard & Patterson, 1992), and his Vocabulary and Information subtests on the WAIS-IV were in the average range. However, he showed mild to moderate object naming impairment (Boston Naming Test-II, Kaplan et al., 2001; Cambridge Naming Test, Adlam et al., 2010), and his verbal fluency was impaired on generating words for animals.
In other cognitive domains for executive functioning, HML040 showed normal working memory on the WAIS-IV, and his performance was low average on generating words that begin with a particular letter, namely “F”, “A”, and “S” (Benton et al., 1983). Whereas performance on Trails B was impaired, we believe he was likely hindered by slow mental and psychomotor processing speed as demonstrated by the WAIS-IV tasks and Trails A. Notably, Trails B was normatively similar to Trails A. We did not observe any clinical signs of frontal lobe impairment in our interactions with HML040. Performance on visuospatial processing tests, namely Block Design, Visual Puzzles, and Matrix Reasoning from the WAIS-IV, were low average to average, respectively. Factoring out the impact of processing speed on Block Design raises HML040’s performance to the average range as well (scaled score of 8).
2.2). Control Participants
We included data from nine cognitively healthy older adults (2 female) from the surrounding Tucson area whose ages ranged from 72 to 82 with a mean age of 77.67 and whose education level ranged from 16 to 20 years with a mean education level of 18.11 years. According to a single patient-to-group t-test approach (Crawford & Howell, 1998), HML040 did not significantly differ from the controls on age, education, or verbal intelligence (p’s ≥ .25), with the latter measured by the Verbal Comprehension Index from the Wechsler Adult Intelligence Scale (controls: M = 120, SD = 13.63).
The controls were screened for cognitive impairment using an established profile-based approach developed by Bondi and colleagues (Bondi et al., 2014; Grilli et al., 2018; McAvan et al., 2021). This approach covers four domains with a total of eight traditional neuropsychological tests (two for each domain). These domains are speed/executive function, language, memory, and visuospatial functioning. Individuals are considered cognitively unimpaired/clinically normal for age/education, and thus eligible, if both of the following conditions are met: 1) they do not perform more than one standard deviation below the age- and education-corrected normative mean on both scores in one domain, and 2) they do not perform more than one standard deviation below the age- and education-corrected normative mean on one test in three domains.
We used Trail Making Test A and B (Reitan & Wolfson, 1993) as our two measures of speed/executive function, and we used the Boston Naming Test (Goodglass et al., 2001) and animal fluency from the Controlled Oral Word Association Test (Benton, 1969) as our measures of language function. We used the California Verbal Learning Test long delay recall (Delis et al., 2000) and Rey-Osterrieth Complex Figure Test (RCFT) delay recall (Rey, 1941) scores as our measures of memory. Finally, for visuospatial functioning, we used Block Design from the Wechsler Adult Intelligence Scale (Wechsler, 2008) and RCFT Copy scores. These older adults were taken from a larger sample of older adults who were tested on the same paradigm in a recently published paper (McAvan et al. 2021). All participants received monetary compensation for their time. All participants had normal or corrected-to-normal color vision, normal or corrected-to-normal hearing, and reported no history of cardio-vascular problems or motion sickness. Written informed consent was obtained before the experiment, and the methods were approved by the Institutional Review Board (IRB) at the University of Arizona.
2.3). Experimental Design
Participants were tested on an immersive virtual reality analog of the Morris Water Maze created in Unity 3D (Unity Technologies ApS, San Francisco, CA) using the Landmarks virtual reality navigation package (Starrett et al., 2020). The task was the same task used by McAvan et al. (2021) which previously demonstrated that while older adults had reduced precision in navigation, they had preserved strategy use (i.e., no difference in finding the hidden target from novel vs. repeated start points) when compared to younger adults. The task had participants physically explore a virtual environment with the use of a wireless HMD. Participants were tested in a space approximately 5 × 5 meters in size with distal mountains serving as navigational stimuli to give a sense of a much greater space approximately 750 × 750 meters in size (see Figure 2A–D for the wireless HMD setup and environment). Four distal mountains were visible from within the testing space. The environment also contained a snow-covered floor and three unique objects (book, puzzle cube, and teapot) on pedestals which served as the hidden targets for navigation.
Fig 2. Overview of the task setup.
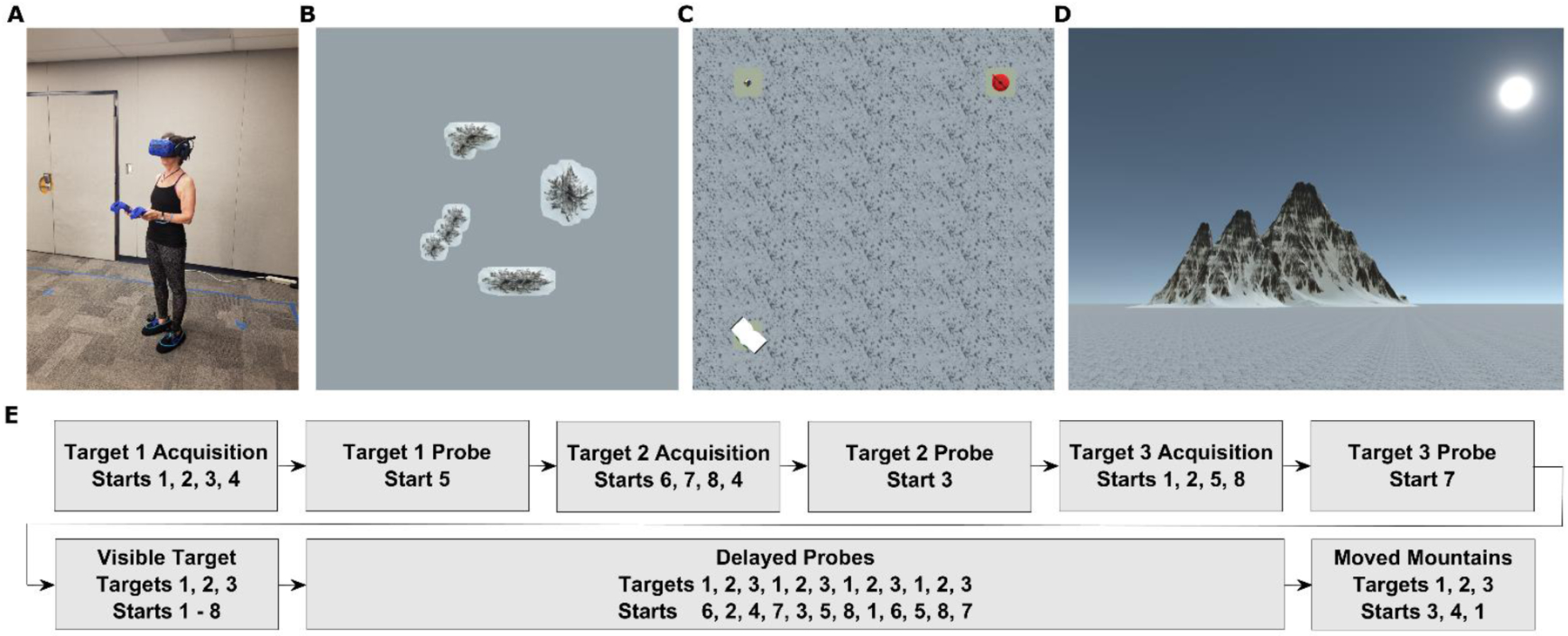
(A). Participant fitted with wireless HMD, battery pack, two controllers, and two foot covers with trackers (B). Bird’s-eye-view of entire virtual environment, including the 4 distal mountains (entire environment approximately 750×750 m in size) (C). Bird’s-eye-view of navigable virtual environment (approximately 5×5 m in size), with all three targets visible (D). Point-of-view from the participant within the virtual environment (E). Trial list with corresponding targets and starting locations.
After reading and signing the consent form, participants were blindfolded and led into the room where the experiment would take place. The purpose of the blindfold was to prevent participants from seeing the size and shape of the room in which the experiment would occur. Once they donned the wireless HMD, controllers, and battery pack, participants were then immersed in a practice virtual environment. Within this practice environment, participants were first allowed to freely wander around a small circular room for five trials. After freely exploring during these five trials, participants were then prompted to find and remember the location of a single target object for another five trials. Much like in the experimental portion, the practice target was not visible for the first 30 seconds of the trial. Once it became visible, participants then walked over to the target and interacted with it to move on. If they were confident that they had learned the location of the practice target, participants could then press a button on their controller to make the practice target appear before the 30 second period had passed. The practice session lasted approximately 10 minutes.
After the practice, participants then moved on to the experimental portion of the study. During this portion, participants were tasked with completing five blocks of navigation (Figure 1E), with breaks offered in-between each block. The five blocks that participants completed were three “acquisition blocks,” one “visible targets block,” and one “delayed probes block.”
During each of the acquisition blocks, participants experienced 16 trials wherein they were trained on a single target object across four different starting locations in sequence. For the first trial of every block, participants were instructed to simply explore the virtual environment. Within each block, the target object was invisible at the start of each trial and participants could either explore the space for 30 seconds or indicate the target object’s location with a button press before 30 seconds; when one of these events occurred, the target object would appear. After this, the participants walked over to and interacted with the target object to progress the experiment. Participants were disoriented at the end of each trial to ensure that they did not use their body orientation from trial to trial to find the hidden target. After the 16 training trials within each block, participants experienced a single “probe trial” wherein they had to walk to the remembered location of the target object and make a response to progress. Each acquisition probe trial involved a novel starting point compared to the rest of the acquisition block (see Figure 2E for starting locations).
After three acquisition blocks, participants then performed a single block of eight trials in which they cycled through the target objects (book, puzzle cube, teapot) and starting locations (1–8) in sequential order (e.g., start 1 to book, start 2 to puzzle cube, … start 8 to puzzle cube). The prompted target object was visible from the start and participants simply had to locate it, walk to it, and interact with it to progress. This block therefore involved “beaconing” navigation and served as a means of controlling for motor deficits, fatigue, and general ability to perform the task. If a participant significantly differed from others during the visible target block, then one could reasonably say that they were unable to perform the entire task effectively.
After the visible target block, participants then performed a single block of 15 delayed probe trials that were otherwise like the probe trial at the end of each acquisition block: participants were prompted to walk to the remembered location of a target object and make a response when they believed that they had found the location of the target. After an initial 12 probe trials, participants performed three more probe trials in which, unbeknownst to them, one of the distal mountains rotated 20 degrees around the target object. On each of the “moved mountain” trials, a different mountain was rotated around each target, and each mountain was one of two mountains visible when looking at the target from the center of the virtual environment. The purpose of testing participants on the moved mountain condition was to examine whether participants utilized a single mountain as a more egocentric beaconing cue, or a combination of the mountains to derive allocentric coordinates. To help mitigate feelings of fatigue or motion sickness, participants were offered breaks in-between every block. Throughout the experiment, participants’ locations within the virtual space were recorded at a rate of 10Hz.
2.4). Data Analysis
Data processing and analyses were completed in Excel 2019 (Microsoft Corporation, Redmond, WA) and MATLAB 2020a (The MathWorks Inc., Natick, MA). Individual paths for all participants were calculated by summing the distances between each sampled data point to derive various measures including the path from the start location to the response location, the path from the response location to the target location, and total path length. Distance to target (or “distance error”) was the shortest path between the participant’s response and the target. To understand some of the trends in the raw data more in depth, we compared HML040 with the control group using a modified t-test as described by Crawford and Howell (1998). This allowed us to compare HML040’s values with the control group’s sampled means (Crawford & Howell 1998). To accommodate for the disparity in comparative sample sizes, we used Hedges’ G to measure effect size. Due to a small number of responses made across trials during acquisition (unlike probe trials, responses were not required during acquisition), we used either the distance from the target when a response was made, or, when no response was given, we used the distance from the target after the 30 seconds. For acquisition probe trials, however, which occurred immediately after acquisition trials and were from a new start location, a response was required. Because a Euclidean distance error of 0 indicates an optimal response, we removed any outliers that were above 2.5 standard deviations from the mean (for a total of 2.74% of trials removed). For all other measures (e.g., response time, total path, etc.) we removed any outliers that were above or below 2.5 standard deviations from the mean (for a total of 3.75% of trials removed).
To determine if participants weighted the moved mountain more than the other three static mountains (or vice versa), we looked at response distance from where the target would be if it moved with the mountain (EMM) over that same distance added to their response distance from the actual static location of the target (EMM + E3M). A value of 1 would indicate completely weighting the three unmoved mountains over the single moved mountain. A value of 0 would indicate completely weighting the single moved mountain over the three unmoved mountains. Values in-between 0 and 1 would indicate partial weighting of both. A value less than 0.50 would indicate that participants weighted the moved mountain more than the static mountains, a value of greater than 0.50 would indicate that participants weighted the static mountains more than the moved mountain, and a value of 0.50 would indicate equal weighting of both.
To examine path complexity (or “tortuosity”), we looked at the Fractal Dimensionality of each path, more specifically we used the equation D = 1+ log(path length)/log(path measurements) to get a FD value between 1 and 2 with 1 being perfectly straight and 2 being a completely random path (Mandelbrot 1982).
To determine whether HML040 or controls’ memory for hidden targets exceeded chance, we employed a bootstrapping procedure consistent with earlier approaches (Kolarik et al. 2016). Briefly, the bootstrapping procedure involved calculating the Euclidean distance between the response on the current trial and the unprompted targets (two targets) on the other delayed probe trial (15 trials) for all participants. This resulted in a series of chance trajectories which were then shuffled randomly 10,000 times using the bootstrp function in MATLAB to generate a single “chance surrogate” participant. We then repeated this 100 times to create a group of 100 “chance surrogate” participants that we could then use as a comparison for HML040. We then compared HML040 and controls against these bootstrapped distributions to determine if memory for a single target exceeded that expected by chance.
3). Results
3.1). HML040’s memory for target locations during acquisition is statistically comparable, although numerically worse, to healthy age-matched controls
We first investigated the paths taken by HML040 and controls on specific trials to determine if there were any differences in performance during acquisition trials, which represented his attempts to learn the new environment. We also wished to determine how HML040 compared to the controls when switching from a repeated starting location to a novel starting location experienced for the first time during the acquisition probe trials. For these comparisons, we used three trials of repeated starting locations and three trials of novel starting locations per block. For example, in the first acquisition block in which participants were trained on target 1 (the book), the repeated start trials were trials 4, 8, and 12 and the novel start trials were trials 5, 9, and 13. We excluded trial 1 as a novel trial because participants were simply exploring the environment on this trial (i.e., first exposure) and we excluded trial 16 as a repeated trial to balance the comparisons. As seen in Figures 3 and 4, HML040 performed numerically worse, but statistically comparable, to the healthy controls on distance error for both repeated and novel starting locations. When averaging placement error across targets, HML040 performed numerically worse on both repeated (HML040: M = 3.64, SD = 0.80, Controls: M = 2.54, SD = 0.49; t[8] = 2.15, p = 0.06, MD = 1.10, 95% Control CI = [2.24, 2.84], Hedges’ g = 2.27) and novel (HML040: M = 3.48, SD = 1.17, Controls: M = 2.84, SD = 0.32; t[8] = 1.94, p = 0.09, MD = 0.65, 95% Control CI = [2.64, 3.03], Hedges’ g = 2.04) starting locations, but statistically comparable to healthy controls (although the analyses approached significance). This suggests that by the end of the acquisition block, HML040 had learned the locations of the hidden targets to comparable degrees as the control group, although he acquired the memories for locations less efficiently than the controls.
Fig 3. Example paths.
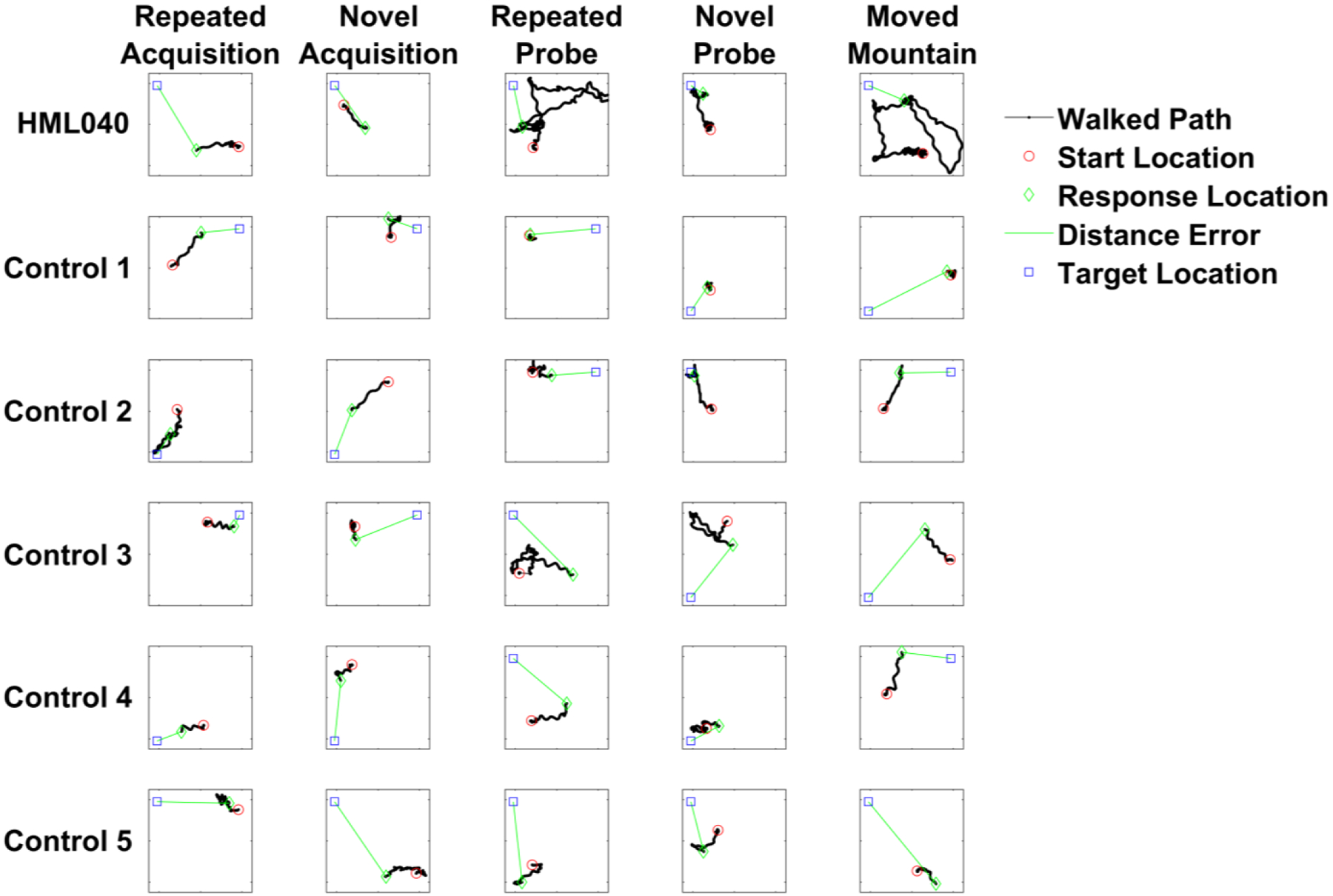
Some example paths walked through the virtual environment by HML040 and five selected controls on repeated acquisition, novel acquisition, repeated probes, novel probes, and moved mountain trials. Response location is where participants marked where they believed the target was and distance error was the Euclidean distance between the response location and actual target location.
Fig 4. HML040 versus Control Group accuracy on novel and repeated viewpoints in acquisition trials.
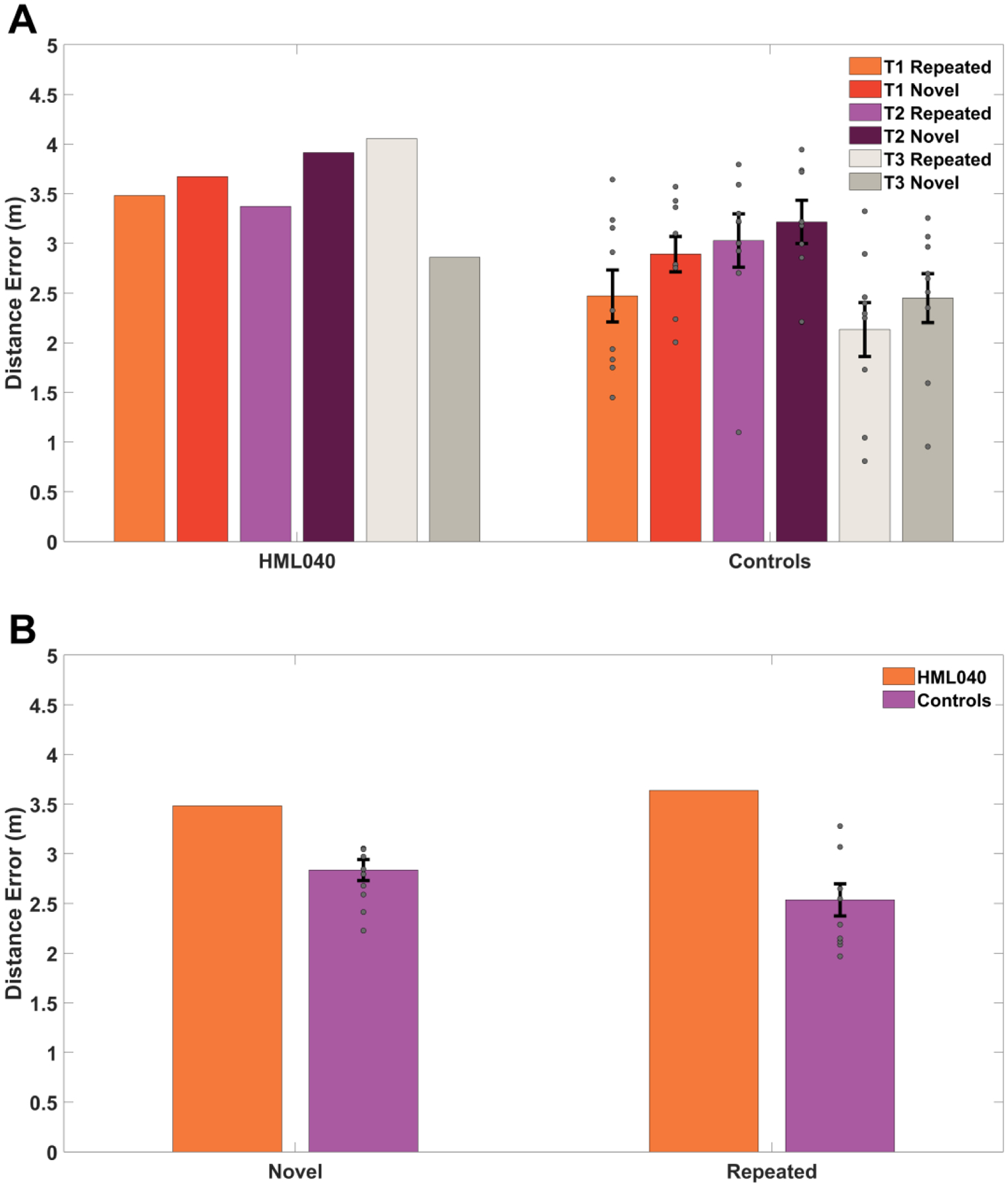
(A). Comparison of trials across targets 1, 2, and 3 and (B). trials collapsed across targets. Distance error is the Euclidean distance between where participants placed the targets and where they were located in the virtual environment. Novel locations were those in which a start location/target location pairing was new to the participants and Repeated trials were those in which a start location/target location pairing was previously seen. Grey dots represent the control group’s data (some points may overlap). All error bars are the standard error of the mean (SEM). There were no statistically significant differences between HML040 and controls.
3.2). HML040 remembered the target location comparably to healthy controls and better than chance on the delayed probe trials
Next, we tested whether there were any significant differences in distance error between HML040 and controls during the delayed probe trials. Note that these delayed probe trials occurred approximately 10–15 minutes after acquisition. Also please note that each data point involves the average of two different probe trials for novel start locations and two different probe trials for repeated start locations. We also included a comparison with chance performance to ensure that all groups were not simply guessing (see section 2.4 Data Analysis for bootstrapping procedures for determining chance). Unlike the acquisition trials in which the participants were given the choice of making a response if they felt confident that they were close to the target location, the delayed probe trials forced every participant to make a response. This was to directly test how well they were able to recall the location of each hidden target rather than potentially relying on HML040’s or the controls’ confidence in their memory.
If HML040 learned the location of the hidden targets, his distance error should be like that of the control group while still performing better than chance. Alternatively, if HML040 was unable to learn the location of each hidden target, we would reasonably expect him to perform worse than the healthy controls and show no difference from our calculated chance performance. Figure 5 shows the responses made for all targets during the experiment normalized to a center location for HML040 and controls. As can be seen from Figure 5, HML040 and control placements showed similar distance errors throughout the experiment. Averaging these distance errors for delayed probe trials only (trials 60–71, i.e., excluding when the mountain moved), as shown in Figure 6A, HML040 performed slightly numerically better (M = 2.70, SD = 1.57) across delayed probe trials when compared to the control group (M = 2.78, SD = 0.36), albeit not significantly (t[8] = −0.22, p = 0.83, MD = 0.08, 95% Control CI = [2.56, 3.01], Hedges’ g = 0.23). He also performed significantly better than chance (t[99] = −840.14, p < 0.01, MD = 0.59, 95% Chance Surrogate CI = [3.2705, 3.2707], Hedges’ g = 724.70) when compared to our chance surrogates (M = 3.29, SD < 0.001). Thus, HML040 was able to remember and recall the locations of the hidden targets at levels comparable to age-matched healthy controls and better than levels predicted by chance. We also compared our control group to our calculated chance performance and found that the control group (M = 2.78, SD = 0.36) performed significantly better than chance (M = 3.29, SD < 0.001) as well (t[8] = −4.03, p < 0.01, MD = 0.51, 95% Control CI = [−0.77, −0.21], Hedges’ g = 5.09).
Fig 5. All trial responses made by HML040 and controls.
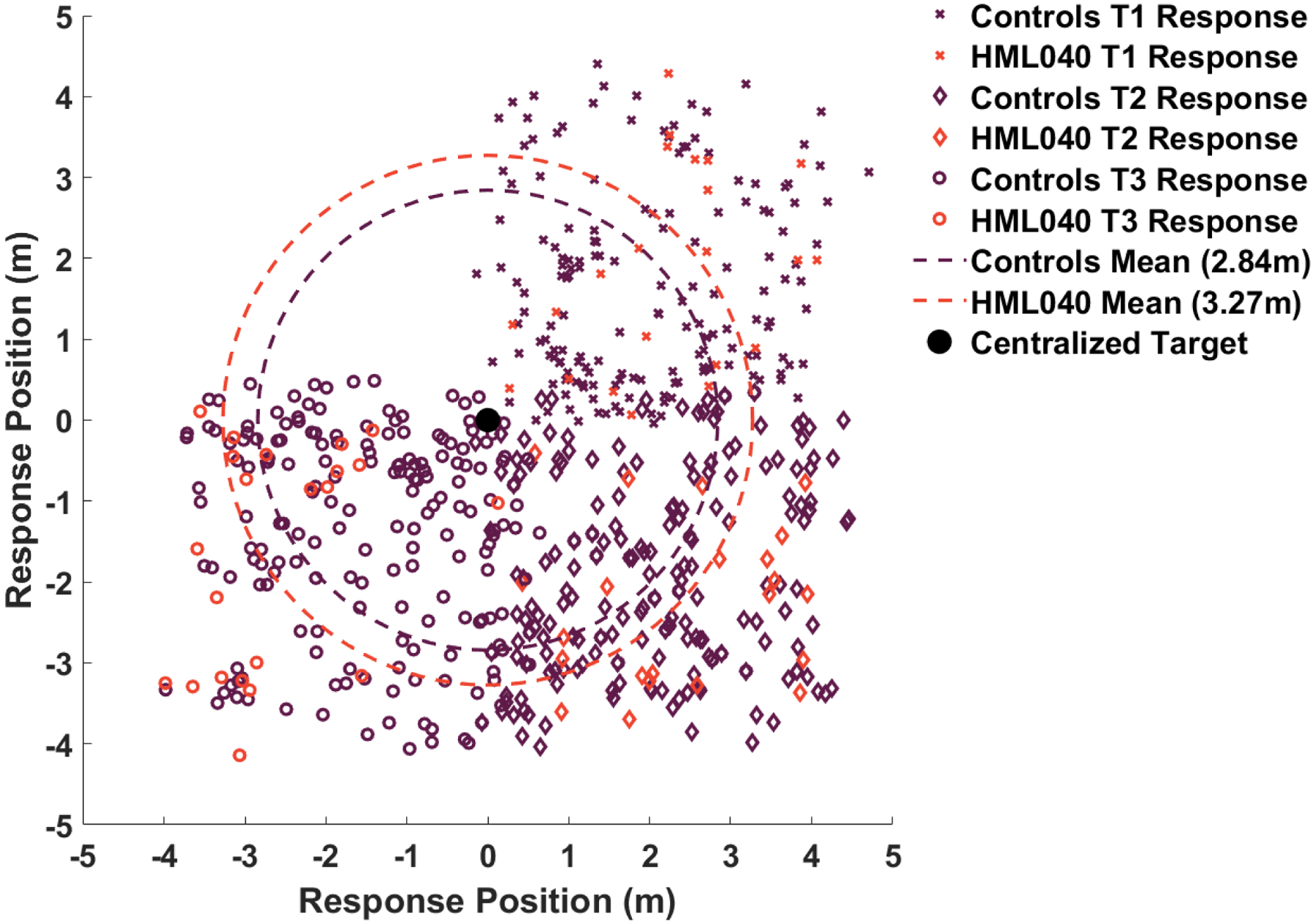
X’s correspond to responses in which participants were tasked with finding target 1 and marked where they thought it was, diamonds correspond to responses in which participants were tasked with finding target 2 and marked where they thought it was, and circles correspond to responses in which participants were tasked with finding target 3 and marked where they thought it was. All responses are centralized around (0,0).
Fig 6. Accuracy in delayed probe trials between HML040 and Control Group.
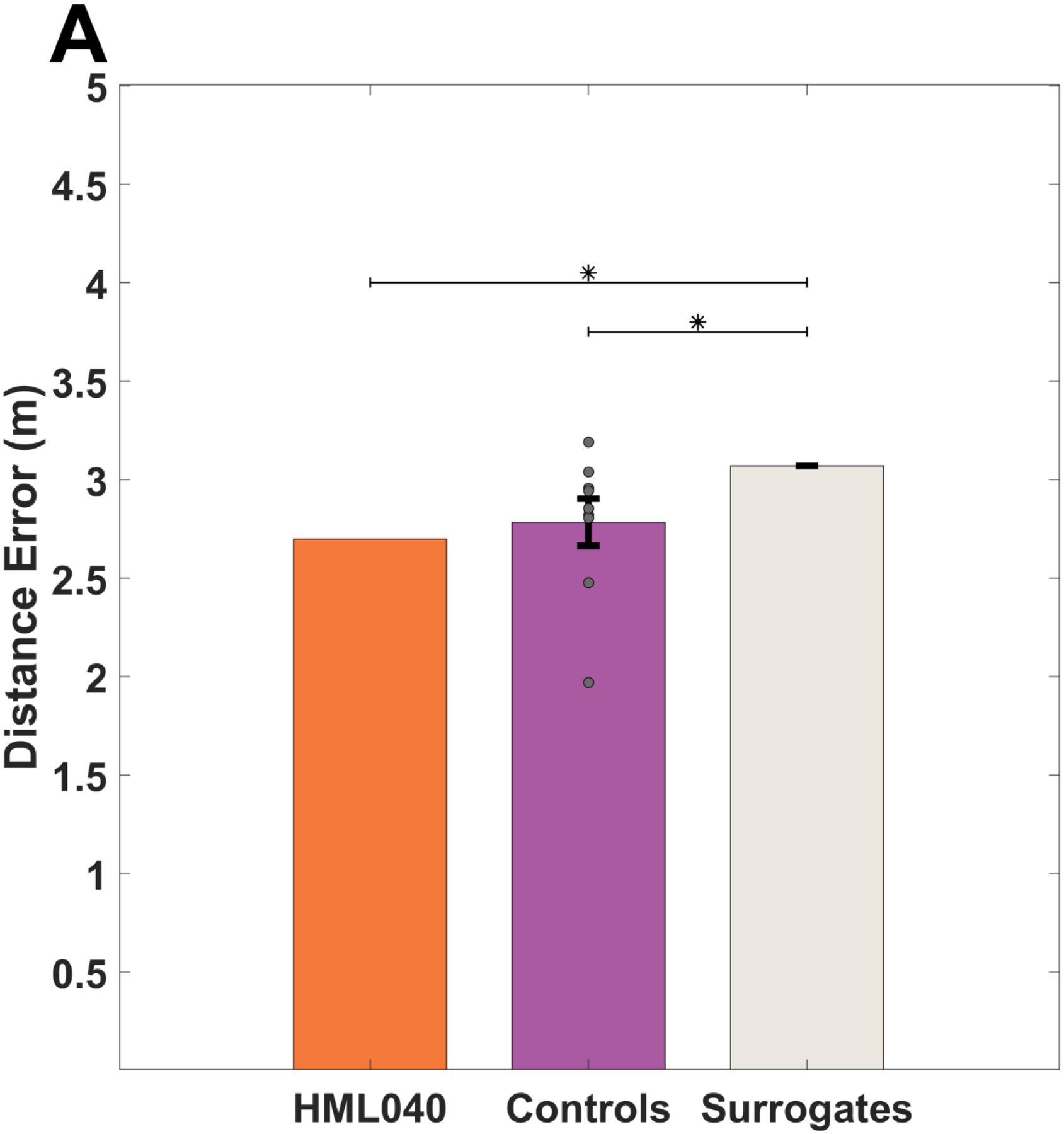
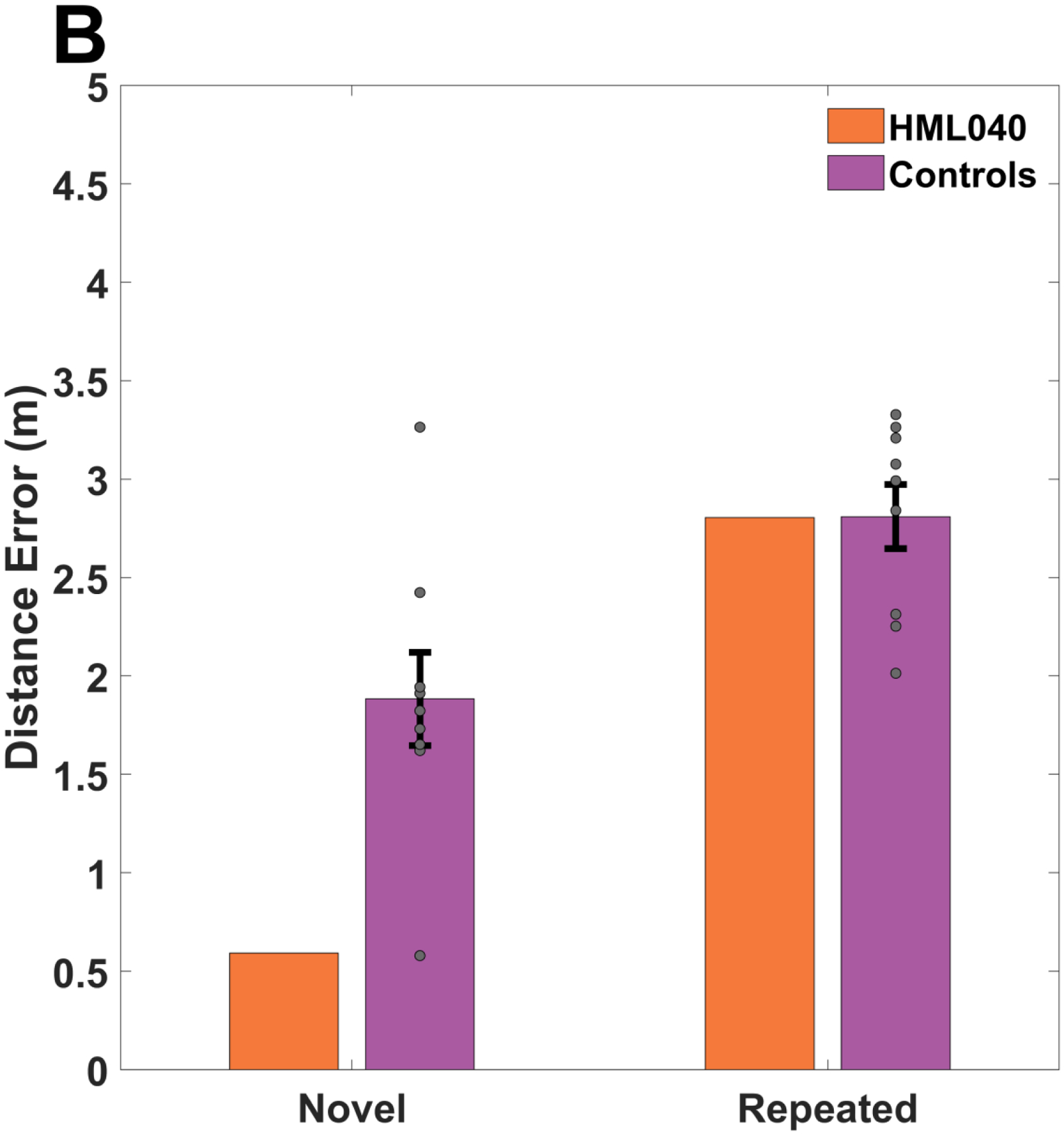
(A). HML040 versus Control Group and Chance Group accuracy averaged across all delayed probes. There was no statistically significant difference between HML and controls although HML040 performed significantly better than chance, as indicated by the mixed surrogate chance group. (B). HML040 versus Control Group accuracy for novel and repeated trials. Despite having a smaller distance from the target on novel trials compared to the control group, for both repeated and novel trials, HML040 did not perform statistically different from the controls. Distance error is the Euclidean distance between where participants placed the targets and where they were actually located in the virtual environment. Novel locations were those in which a start location-target location pairing was new to the participants and Repeated trials were those in which a start location-target location pairing was previously seen. Grey dots represent the control group’s data (some points may overlap). All error bars are the standard error of the mean (SEM).
To further examine the HML040’s memory for the hidden targets during the delayed probes, we also looked at a subset of trials in which each participant started from a location that was either directly repeated from within the acquisition block (trials 65 & 70) or a completely novel target-start pairing (trials 66 & 67). As demonstrated in Figure 6B, we saw no significant differences in distance error between HML040 (Repeated: M = 2.80, SD = 1.06; Novel: M = 0.59, SD = 0.17) and controls (Repeated: M = 2.81, SD = 0.49 ; Novel: M = 1.88, SD = 0.71) and for either the repeated or novel starting points (Repeated: t[8] = −0.01, p = 0.99, MD = 0.03, 95% Control CI = [2.51, 3.11], Hedges’ g = 0.01; Novel: t[8] = −1.72, p = 0.12, MD = 1.29, 95% Control CI = [1.44, 2.32], Hedges’ g = 1.81). The lack of a difference for repeated vs. novel start locations is consistent with what we observed for acquisition trials. These findings imply a similar ability to remember the previously learned hidden target locations, and the possibility to generalize from a previously unseen starting location.
3.3). HML040 and controls show a similar reliance on a combination of static distal cues and a single moved mountain cue
To examine possible differences in navigational strategies between HML040 and controls, we looked at a subset of trials in which we explicitly moved one of the distal mountain cues. Doing so would give us a relative weighting of a single moved mountain compared to three static mountains (see section 2.3 Experimental Design above). As shown in Figure 7, we found that both HML040 and controls showed a weighted value of less than 0.50, meaning that they weighted the single moved mountain slightly more than the static mountains. This comparison involved averaging three different trials (72, 73, 74) involving the moved mountain for each participant. To determine any differences between HML040 and controls, we compared HML040’s weight value (M = 0.47, SD = 0.003) to that of the controls (M = 0.47, SD = 0.02) using a Crawford’s modified t-test. We found no significant difference between HML040 and the control group (t[8] = 0.08, p = 0.94, MD < 0.001, 95% Control CI = [0.46, 0.48], Hedges’ g = 0.08). This suggests that both groups showed a similar reliance on weighting a single mountain slightly more than multiple cues.
Figure 7. HML040 versus Control Group on the moved mountain weight values.
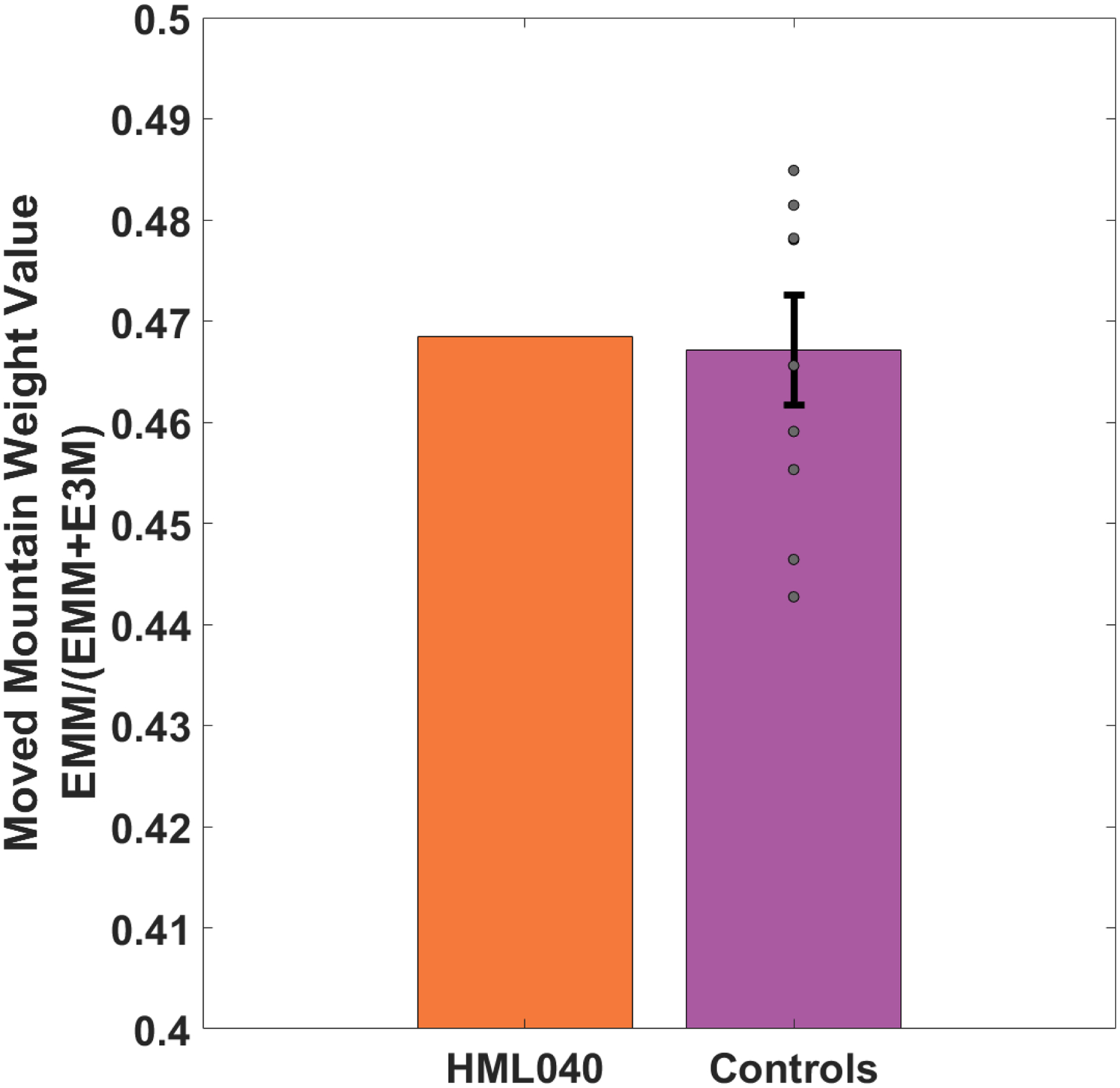
HML040 is not significantly different than the control group in terms of weighting beacon vs. allocentric cues based on the response distance from where the target would be if it moved with the mountain (EMM) over that same distance added to their response distance from the actual static location of the target (EMM + E3M). Grey dots represent the control group’s data (some points may overlap). All error bars are the standard error of the mean (SEM).
Finally, we also compared HML040 with the control group on various dependent measures related to the movements and paths that he took to find the locations of the targets (see Table 2). During the visible target trials, which served to control for fatigue and motor deficits, we found no significant differences in any of our measures. This indicated that there were no differences in how HML040 and controls navigated to the visible objects. This in turn suggests that sensorimotor and/or fatigue, as far as we could measure them in this study with the visible trials, did not differ between HML040 and controls.
Table 2. Comparing HML040 to healthy controls on various dependent measures.
We found no difference in dependent measures related to walked distances, angular rotations, or total time on visible target trials between HML040 and controls. For probe trials and moved mountain trials, all measures for HML040 are significantly greater than controls except fractal dimensionality (which approached significance for moved mountain trials).
| HML040 | Controls | Mean Difference | 95% Confidence Interval (CI) | Crawford’s | Hedges’ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | (MD) | t | df | p | g | |||
| Visible Target Trials | Total Path (m) | 2.88 | 0.77 | 3.08 | 0.95 | 0.20 | [2.49, 3.67] | − 0.20 | 8 | 0.84 | 0.21 |
| Total Rotation (deg) | 902.04 | 971.49 | 677.10 | 398.24 | 224.94 | [430.19, 924.01] | 0.54 | 8 | 0.61 | 0.56 | |
| Excess Path (m) | 0.49 | 0.37 | 0.94 | 0.75 | 0.45 | [0.47, 1.41] | − 0.56 | 8 | 0.59 | 0.60 | |
| Excess Rotation (deg) | 833.42 | 981.83 | 594.93 | 398.69 | 238.49 | [347.74, 842.12] | 0.57 | 8 | 0.59 | 0.60 | |
| Total Time (ms) | 17920.50 | 5685.87 | 11386.50 | 5432.60 | 6534 | [8018.29, 14754.71] | 1.14 | 8 | 0.29 | 1.20 | |
| Fractal Dimensionality | 1.20 | 0.05 | 1.24 | 0.05 | 0.04 | [1.21, 1.28] | − 0.78 | 8 | 0.46 | 0.80 | |
| Delayed Probe Trials | Total Path (m) | 13.82 | 8.05 | 6.36 | 2.31 | 7.46 | [4.93, 7.79] | 3.10 | 8 | 0.02 | 3.23 |
| Total Rotation (deg) | 3848.85 | 2547.20 | 1699.33 | 470.734 | 2149.52 | [1407.47, 1991.18] | 4.33 | 8 | <0.01 | 4.57 | |
| (Excluding Moved | Excess Path (m) | 10.94 | 7.83 | 3.33 | 2.19 | 7.61 | [1.97, 4.69] | 3.29 | 8 | 0.01 | 3.47 |
| Excess Rotation (deg) | 3777.56 | 2554.31 | 1624.53 | 469.66 | 2153.03 | [1333.35, 1915.72] | 4.35 | 8 | <0.01 | 4.58 | |
| Total Time (ms) | 97202.00 | 55325.09 | 24818.81 | 11659.29 | 72383.19 | [17590.05, 32047.56] | 5.89 | 8 | <0.01 | 6.21 | |
| Response Time (ms) | 60190.83 | 31270.26 | 17081.77 | 10370.08 | 43109.06 | [10652.32, 23511.22] | 3.94 | 8 | <0.01 | 4.16 | |
| Fractal Dimensionality | 1.33 | 0.12 | 1.26 | 0.07 | 0.07 | [1.22, 1.31] | 0.88 | 8 | 0.41 | 1.00 | |
| Moved Mountain | Total Path (m) | 34.52 | 4.86 | 5.69 | 0.63 | 28.83 | [5.30, 6.08] | 43.62 | 8 | <0.01 | 45.76 |
| Trials | Total Rotation (deg) | 12281.21 | 3492.85 | 1434.19 | 354.44 | 10846.81 | [1214.44, 1653.94] | 29.03 | 8 | <0.01 | 30.60 |
| Excess Path (m) | 30.53 | 5.30 | 1.69 | 0.64 | 28.84 | [1.29, 2.08] | 42.57 | 8 | <0.01 | 45.06 | |
| Excess Rotation (deg) | 12251.43 | 3490.47 | 1384.22 | 351.96 | 10867.21 | [1166.01, 1602.43] | 29.29 | 8 | <0.01 | 30.88 | |
| Total Time (ms) | 198967.33 | 49292.79 | 19967.09 | 4715.95 | 179000.24 | [17043.20, 22890.98] | 36.01 | 8 | <0.01 | 37.96 | |
| Response Time (ms) | 184031.67 | 40873.07 | 12136.35 | 4969.91 | 171895.32 | [9619.90, 15747.33] | 32.90 | 8 | <0.01 | 34.59 | |
| Fractal Dimensionality | 1.47 | 0.01 | 1.24 | 0.10 | 0.23 | [1.18, 1.30] | 2.25 | 8 | 0.055 | 2.30 | |
Significant*.
Approaches~.
Not Significant.
We did find, however, that HML040 took longer and ambulated using more complex paths than controls in some situations. During the delayed probe trials, we found that HML040 walked more, rotated more, and took significantly longer in the task than the control group (Table 2). However, he did not have a significantly more tortuous paths (as measured using fractal dimensionality), suggesting that while he walked longer paths, they were not more complex in terms of turning patterns or tortuosity. During the moved mountain trials, we found that HML040 again walked greater distances, rotated more, and spent more time walking through the environment. The fractal dimensionality of his path for this comparison was more complex on the moved mountain trials, suggesting possible confusion. These findings suggest that although HML040 chose a location that was comparable in distance to the actual location of the target compared to the controls, his trajectories to get there differed from the controls in many ways. This, in turn, suggests some possible compensatory strategies related to his impaired episodic memory, issues we consider in depth later in the Discussion.
To ensure that there was no differing navigational ability within our sample including males and females, we compared MHL040 to males only. The overall results did not change except for one difference: during acquisition, the difference between the patient and controls for target 3 was previously trending significant and now became significant when excluding females. We also compared male to female controls and found no difference on any of our measures.
4). Discussion
In this study, we tested HML040, an individual who developed profound amnesia as a result of two separate strokes involving the medial temporal lobes. Critically, HML040 has severely impaired episodic learning and memory, based on prior assessment of his performance on standard neuropsychological tests of memory, and his retrograde and anterograde “real world” autobiographical memory (Table 1; Wank et al., in press). By assessing HML040 on a human analog of the rodent Morris Water Maze in immersive virtual reality, we had the unique opportunity to address the degree of possible overlap between memory for places during navigation and episodic memory. Intriguingly, despite his severe episodic memory impairments, HML040 performed similarly to age-matched control participants and above chance on tests of his memory for places using distal cues.
In our task, based in part on the commonly used spatial memory assay known as the Virtual Morris Water Maze, participants wore a wireless head-mounted display and freely walked around a 5 × 5 m environment in which four distal mountains were rendered at infinite distance. For the three trained targets in the acquisition phase, HML040 performed comparably on both novel and previously seen repeated viewpoints. This trend was the same in the delayed probe trials, in which HML040 performed numerically better, but statistically indistinguishably at finding the hidden targets from novel and repeated start locations compared to controls. In addition, when we moved one of the mountains on a subset of probe trials, HML040 showed no greater reliance on the single mountain than controls. Despite his apparent intact navigational memory and strategy use, HML040 showed longer paths (involving greater distances and rotations) and took more time to complete all trials. Longer exposure time at encoding has been shown to improve later recognition memory in individuals with amnesia (Hirst et al., 1986). However, it is not clear why at retrieval, when using a task with no pressure to respond quickly, we would expect longer exposure time to have a significant benefit on memory. It is also noteworthy that: 1) giving amnesic participants more exposure time at encoding does not seem to bring them to the level of controls, and 2) the memory benefits of longer exposure in amnesia can be sensitive to relatively modest procedural differences, such as using blocked versus inter-mixed study trial lengths (Verfaellie et al., 2010). Taken together, while HML040’s longer paths may have assisted his performance, it is unlikely that mere exposure time at encoding or retrieval had a meaningful role in HML040’s place memory.
While there is little debate that lesions to the medial temporal lobe significantly impact episodic memory, past studies have shown mixed results regarding the effect of such lesions on memory for places during navigation (Banta-Lavenex et al., 2014; Bartsch et al., 2010; Bohbot & Corkin, 2007; Herdman et al., 2015; Holdstock et al., 2000; Maguire et al., 2006; Parslow et al., 2005; Rosenbaum et al., 2000; Teng & Squire, 1999). We note that past studies have shown that individuals with hippocampal lesions, in many cases, show largely intact spatial memory for familiar environments learned in the past (Bohbot & Corkin, 2007; Herdman et al., 2015; Maguire et al., 2006; Rosenbaum et al., 2000; Teng & Squire, 1999). Because our study involved participants learning a new environment with objects they had never experienced before, we focused our considerations on navigation of novel spatial environments. In one study using novel environments, individuals with unilateral medial temporal lobe lesions learned the locations of a hidden sensor from either a novel or repeated start location in a small-sized room. Individuals with lesions primarily to the hippocampus showed no impairments at remembering the hidden location although individuals with lesions including right parahippocampal cortex did show impairments at remembering targets from novel start locations (Bohbot et al., 1998). Although our results are similar in terms of the effects of hippocampal lesions on memory for places, one important difference between our study and the Bohbot et al. study is that the Bohbot et al. study did not involve distal cues but rather navigation within a small room from different start-points.
In two studies using a desktop virtual reality version of a Morris Water Maze (i.e., no body-movements other than keyboard/joystick movements), individuals with both unilateral and bilateral hippocampal lesions showed well-above chance performance at remembering hidden locations from repeated and novel start points. Individuals with hippocampal lesions, however, showed some decrements in the precision of their memory compared to age-matched controls (Kolarik et al., 2016, 2018). Notably, the individuals in the Kolarik et al. studies were all young, while HML040 was older (80 years), with older adults reported to show deficits in navigation compared to younger adults (McAvan et al., 2021; Moffat et al., 2001, 2006). This in turn may explain why HML040 showed no deficit here compared to age-matched controls while those in the Kolarik studies did show a difference.
Somewhat in contrast to the studies mentioned above with individuals with hippocampal lesions navigating novel environments, rats consistently show profound impairments in the traditional Morris Water Maze when remembering the hidden platform during probe trials (Morris et al., 1982, 1986). However, there are important task-related differences between the species: rats have relatively poor vision compared to humans and it seems likely that visual-based place memory plays a more significant role for humans than rodents, with rodents likely using path integration strategies to a greater extent (Ekstrom et al., 2018; Lindner et al., 1997; Wolbers & Wiener, 2014). In addition, there are some differences in the brains of rats and humans as related to spatial navigation, particularly in the prefrontal and retrosplenial cortices, which also play relevant roles in memory for places (Ekstrom et al., 2014, 2018; Patai & Spiers, 2021). Therefore, it is plausible that differences in rat vs. human memory for places following hippocampal lesions could relate to possible differences in how the different species learn the task (i.e., reliance on vision) and brain structures that may be engaged during navigational place memory.
Our findings also suggested no selective impairment in memory from a start location repeated from acquisition (egocentric) compared to a novel start point (allocentric), sometimes taken as a measure for allocentric vs. egocentric navigation in the Morris Water Maze (Morris et al., 1986). Our finding stands somewhat in contrast to previous studies in humans with hippocampal lesions that have shown impairments in allocentric, but not egocentric, navigation (Banta-Lavenex et al., 2014; Bartsch et al., 2010; Holdstock et al., 2000; Parslow et al., 2005). The distinction between allocentric and egocentric navigation, however, can be difficult to pinpoint in small-scale (“vista”) environments like the traditional Morris Water Maze or other room-sized environments compared to the larger “environmental” space used here (Wolbers & Wiener, 2014). In addition, in some of the previously mentioned studies, the “egocentric” condition referred to what we employed here as the visible control and there was no explicit manipulation of a repeated vs. novel start point. In this way, the deficit in what appeared to be “allocentric” navigation may in fact have been in navigation from both a repeated and novel start point. In our study, we therefore compared navigation from the repeated and novel start points to allow more detailed assessment of different types of navigational impairments. We also disoriented participants before each trial to ensure that they could not readily track their bearing, which otherwise might have helped them better remember repeated start points. Finally, we included a condition in which we moved one of the distal landmarks, which demonstrated that neither HML040 nor controls relied completely on the allocentric cues (static distal landmarks) nor the single mountain cue (beacon cue). Together, these findings suggest that the HML040’s hippocampal lesion did not selectively impair his putative allocentric (or egocentric) navigation.
Past studies with lesion cases provide some tentative support for the idea of a partial dissociation between memory for places during navigation and episodic memory. In one study by Robin et al. (2019) that compared an individual with a lesion to their parietal-occipital area, but little damage to their medial temporal lobes, to a group of individuals with bilateral medial temporal lobe lesions, both groups showed some impairments in episodic memory, particularly for scenes. However, the individual with the parietal occipital lesion showed mild impairments in episodic memory, but profound difficulties with real-world navigation. In contrast, the individuals with medial temporal lobe lesions exhibited more profound loss of episodic memory and less evidence for spatial navigation deficits (Robin et al., 2019). Due to the limited battery of navigation tests in this study, however, the comparison is largely speculative.
Consistent with some of these findings, taxi-drivers with lesions to the retrosplenial cortex show profound disorientation (Takahashi et al., 1997), although detailed reports of their episodic memory are not available. In contrast, the one reported case of a taxi-driver with medial temporal lobe lesions showed only mild, if any, navigation deficits despite dense amnesia (Maguire et al. 2006). Finally, the famous individual H.M. —who had dense amnesia— had largely intact place memory, both in an analogue of the Morris Water Maze and for drawing maps and navigating the city he lived in (Bohbot & Corkin 2007; Corkin, 2002). Such findings are consistent with proposals that posterior brain areas, such as retrosplenial cortex, play a more significant role in navigation compared to episodic memory, with episodic memory likely relying on the medial temporal lobes and anterior brain regions to a greater extent (Ekstrom et al., 2017). Notably, however, all of the aforementioned studies assayed memory for familiar environments while our study examined learning in a novel environment instead.
An inherent limitation of our study is that it involves comparing a rare individual with extremely dense amnesia to a group of controls. It is possible that other individuals with hippocampal lesions could show more profound deficits in navigation. Nonetheless, as has also been argued in theoretical papers on the topic (Medina & Fischer-Baum, 2017; Rosenbaum et al., 2014; Streese & Tranel, 2021), single case studies provide an important test of hypotheses in cognitive neuroscience. Particularly, if episodic memory and memory for places during navigation are heavily intertwined cognitive processes, any individual with severely impaired episodic memory should also show severely impaired memory for places during navigation. Therefore, the fact that HML040 showed deficits in autobiographical memory rather than memory for places during navigation is one important counterexample against this principle. The present study therefore represents a critical single dissociation supporting a basic assertation raised by earlier studies, namely that the human hippocampus plays less of a role in aspects of place memory during navigation than episodic memory.
Another potential limitation with our study is that the exact nature of the damage to HML040’s medial temporal lobes is difficult to characterize due to his pacemaker, which precluded detailed MRIs. However, high-resolution CT scans suggest extensive damage to the hippocampus and parahippocampal gyrus with some potential sparing of his anterior hippocampus. Given the extent of the damage to his medial temporal lobes, however, it is difficult to determine whether the anterior hippocampus would remain functional. Relatedly, HML040’s cognitive deficits go beyond episodic learning and memory and involve semantic memory as well. To a lesser extent, his mental processing speed also is affected. However, these cognitive deficits are not as severe as HML040’s profound deficits on tests of episodic memory. HML040’s episodic memory impairment, therefore, cannot be attributed to these other cognitive deficits. A detailed work-up further suggests that his semantic memory impairment may reflect a role for the MTL in personal and general knowledge (Wank et al., in press; Grilli & Verfaellie, 2016). It is also noteworthy that HML040’s dense amnesia is evident on tasks that are not timed. Taken together, we think it is unlikely that HML040’s contrasting performance on tests of episodic memory and navigation can be accounted for by a potential difference in the need for semantic knowledge or rapid mental processing speed on tests of these two cognitive domains.
The Morris Water Maze is one of the most widely tested and validated experimental paradigms that assays cognitive deficits in rodents following experimentally induced brain alterations (D’Hooge & De Deyn, 2001). Experimental work has validated the task in that rodents employ the distal cues to remember a hidden platform, as evidenced by their altered search pattern when the distal cues are blocked by a curtain and improved performance when a single local cue marks the hidden platform (Morris, 1984). As discussed above, while several groups have developed versions of the Morris Water Maze involving desktop virtual reality and room-sized environments, these assays have varied somewhat in their implementation and shown somewhat mixed results of the effects of hippocampal lesions on human navigation. Nonetheless, it is clear from all of these studies that performance improves dramatically (in both healthy controls and lesion patients) in the presence of a local cue marking the hidden location; in the few experiments that have directly tested this, altering the distal cues affects memory for the hidden target (Newman & Kaszniak, 2000; van Gerven et al, 2012), similar to the manipulations of local cues and landmark position in our task. Nonetheless, for our specific implementation of the Morris Water Maze, its sensitivity to spatial memory impairments in humans is unknown. We note that in a previous study comparing older and younger adults, older adults were impaired at remembering the hidden target location compared to younger adults and this impairment could not be attributed to issues with cybersickness or other aspects of the interface (McAvan et al., 2021). This suggests that the task is sufficiently difficult to detect age-related decline and would presumably detect more severe spatial memory difficulties. In other words, given HML040’s profound episodic memory impairment, he should have shown some impairments in our task if memory for places and episodic memory depended on the same brain circuits. Additional studies would be helpful in establishing whether our task is sensitive to spatial memory impairments, especially in individuals with lesions to areas like parietal cortex.
In conclusion, despite HML040’s dense amnesia for episodic memory, his memory for places during navigation remained at least partially intact. This suggests that these two cognitive processes depend on at least partially independent brain networks and that brain regions outside of the medial temporal lobes may be able to support aspects of navigation following medial temporal lobe damage. Thus, our findings provide additional support for the idea that navigation and episodic memory are partially dissociable.
We tested an individual with severely impaired episodic memory in a spatial task involving free ambulation and memory for hidden objects using distal landmarks.
This individual had a bilateral lesion to the medial temporal lobes due to two separate strokes.
Despite amnesia, this individual performed comparably to age-matched controls and significantly above chance when using distal cues to remember the hidden objects.
Our findings support models suggesting that episodic memory and memory for places experienced during navigation involve partially dissociable brain circuits.
Acknowledgements
This work was partially supported by the National Institutes of Health grant R01 NS114913 awarded to Arne Ekstrom and Matthew Grilli with other support from the University of Arizona, Tucson, AZ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT (Contributor Roles Taxonomy)
Andrew McAvan – Conceptualization, Data Curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing, Editing.
Aubrey Wank – Investigation, Supervision, Writing, Editing.
Steven Rapcsak – Resources, Writing, Editing.
Matthew Grilli – Funding Acquisition, Project Administration, Resources, Writing, Editing.
Arne Ekstrom – Conceptualization, Formal Analysis, Funding Acquisition, Methodology, Project Administration, Resources, Writing, Editing.
References
- Adlam ALR, Patterson K, Bozeat S, & Hodges JR (2010). The Cambridge semantic memory test battery: Detection of semantic deficits in semantic dementia and Alzheimer’s disease. Neurocase, 16(3), 193–207. doi: 10.1080/13554790903405693 [DOI] [PubMed] [Google Scholar]
- Banta-Lavenex P, Colombo F, Ribordy-Lambert F, & Lavenex P (2014). The human hippocampus beyond the cognitive map: Evidence from a densely amnesic patient. Frontiers in Human Neuroscience, 8(711), 1–18. doi: 10.3389/fnhum.2014.00711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch T, Schonfeld R, Muller FJ, Alfke K, Leplow B, Aldenhoff J, et al. (2010). Focal lesions of human hippocampal CA1 neurons in transient global amnesia impair place memory. Science, 328(5984), 1412–1415. doi: 10.1126/science.1188160 [DOI] [PubMed] [Google Scholar]
- Bellmund JLS, Gardenfors P, Moser EI, & Doeller CF (2018). Navigating cognition: Spatial codes for human thinking. Science, 362(6415). doi: 10.1126/science.aat6766 [DOI] [PubMed] [Google Scholar]
- Benton AL, Sivan AB, Hamsher K, Varney NR, & Spreen O (1983). Contributions to neuropsychological assessment. Oxford University Press, New York. [Google Scholar]
- Benton AL (1969). Development of a multilingual aphasia battery: Progress and problems. Journal of the Neurological Sciences, 9(1), 39–48. doi: 10.1016/0022-510X(69)90057-4 [DOI] [PubMed] [Google Scholar]
- Bohbot VD, & Corkin S (2007). Posterior parahippocampal place learning in H.M. Hippocampus, 17(9), 863–872. doi: 10.1002/hipo.20313 [DOI] [PubMed] [Google Scholar]
- Bohbot VD, Kalina M, Stepankova K, Spackova N, Petrides M, & Nadel L (1998). Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex. Neuropsychologia, 36(11), 1217–1238. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, et al. (2014). Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. Journal of Alzheimers Disease, 42(1), 275–289. doi: 10.3233/JAD-140276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright P, & van der Linde I (2020). Comparison of methods for estimating premorbid intelligence. Neuropsychol Rehabil, 30(1) 1–14. doi: 10.1080/09602011.2018.1445650 [DOI] [PubMed] [Google Scholar]
- Buzsaki G, & Moser EI (2013). Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nature Neuroscience, 16(2), 130–138. doi: 10.1038/nn.3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkin S (2002). What’s new with the amnesic patient H.M.?. Nat Rev Neurosci, 3, 153–160. doi: 10.1038/nrn726 [DOI] [PubMed] [Google Scholar]
- Crawford JR, & Howell DC (1998). Comparing and individual’s test score against norms derived from small samples. Clinical Neuropsychology, 12(4), 482–486. doi: 10.1076/clin.12.4.482.7241 [DOI] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, & Ober BA (2000). The California verbal learning test (CLVT-II). The Psychological Corporation. New York, NY. [Google Scholar]
- D’Hooge R, & De Deyn PP (2001). Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev, 36(1), 60–90. doi: 10.1016/s0165-0173(01)00067-4 [DOI] [PubMed] [Google Scholar]
- Eichenbaum H (2017). The role of the hippocampus in navigation is memory. Journal of Neurophysiology, 117(4), 1785–1796. doi: 10.1152/jn.00005.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Arnold AE, & Iaria G (2014). A critical review of the allocentric spatial representation and its neural underpinnings: toward a network-based perspective. Front Hum Neurosci, 8, 803. doi: 10.3389/fnhum.2014.00803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Caplan JB, Ho E, Shattuck K, Fried I, & Kahana MJ (2005). Human hippocampal theta activity during virtual navigation. Hippocampus, 15(7), 881–889. doi: 10.1002/hipo.20109 [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Copara MS, Isham EA, Wang WC, & Yonelinas AP (2011). Dissociable networks involved in spatial and temporal order source retrieval. Neuroimage, 56(3), 1803–1813. doi: 10.1016/j.neuroimage.2011.02.033 [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Huffman DJ, & Starrett M (2017). Interacting networks of brain regions underlie human spatial navigation: A review and novel synthesis of the literature. Journal of Neurophysiology, 118(6), 3328–3344. doi: 10.1152/jn.00531.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, & Ranganath C (2018). Space, Time and Episodic Memory: the Hippocampus is all over the Cognitive Map. Hippocampus, 28(9), 680–687. doi: 10.1002/hipo.22750 [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Spiers HJ, Bohbot VD, & Rosenbaum RS (2018). Human Spatial Navigation: Princeton University Press. [Google Scholar]
- Ekstrom AD, & Yonelinas AP (2020). Precision, binding, and the hippocampus: Precisely what are we talking about? Neuropsychologia, 138, 107341. doi: 10.1016/j.neuropsychologia.2020.107341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elward RL, Rugg MD, & Vargha-Khadem F (2021). When the brain, but not the person, remembers: Cortical reinstatement is modulated by retrieval goal in developmental amnesia. Neuropsychologia, 154. doi: 10.1016/j.neuropsychologia.2021.107788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan CL, Abdi H, & Levine B (2021). On the relationship between trait autobiographical episodic memory and spatial navigation. Memory & Cognition, 49(2), 265–275. doi: 10.3758/s13421-020-01093-7 [DOI] [PubMed] [Google Scholar]
- Gilboa A, Winocur G, Rosenbaum RS, Poreh A, Gao F, Black SE, et al. (2006). Hippocampal contributions to recollection in retrograde and anterograde amnesia. Hippocampus, 16(11), 966–980. doi: 10.1002/hipo.20226 [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, & Barresi B (2001). Boston diagnostic aphasia examination (3rd ed.). Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- Grilli MD, & Verfaellie M (2016). Experience-near but not experience-far autobiographical facts depend on the medial temporal lobe for retrieval: Evidence from amnesia. Neuropsychologia, 81, 180–185. doi: 10.1016/j.neuropsychologia.2015.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli MD, Wank AA, Bercel JJ, & Ryan L (2018). Evidence for reduced autobiographical memory episodic specificity in cognitively normal middle-aged and older individuals at increased risk for Alzheimer’s Disease dementia. Journal of the International Neuropsychological Society, 24(10), 1073–1083. doi: 10.1017/S1355617718000577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, & Grant I (2004). Revised comprehensive norms for expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Herdman KA, Calarco N, Moscovitch M, Hirshhorn M, & Rosenbaum RS (2015). Impoverished descriptions of familiar routes in three cases of hippocampal/medial temporal lobe amnesia. Cortex, 71, 248–263. doi: 10.1016/j.cortex.2015.06.008 [DOI] [PubMed] [Google Scholar]
- Hirshhorn M, Grady C, Rosenbaum RS, Winocur G, & Moscovitch M (2012). Brain regions involved in the retrieval of spatial and episodic details associated with a familiar environment: An fMRI study. Neuropsychologia, 50, 3094–3106. [DOI] [PubMed] [Google Scholar]
- Hirst W, Johnson MK, Kim JK, Phelps EA, Risse G, & Volpe BT (1986). Recognition and recall in amnesics. J Exp Psychol Learn Mem Cogn, 12(3), 445–451. [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Cezayirli E, Isaac CL, Aggleton JP, & Roberts N (2000). A comparison of egocentric and allocentric spatial memory in a patient with selective hippocampal damage. Neuropsychologia, 38(4), 410–425. doi: 10.1016/s00283932(99)00099-8 [DOI] [PubMed] [Google Scholar]
- Howard K & Patterson K (1992). Pyramids and palm trees: A test of semantic access from pictures and words. Thames Valley: Bury St Edmunds. [Google Scholar]
- Kaplan E, Goodglass H, & Weintraub S (2001). Boston Naming Test-2nd Edition. Pro-Ed: Austin, Texas. [Google Scholar]
- Kolarik BS, Baer T, Shahlaie K, Yonelinas AP, & Ekstrom AD (2018). Close but no cigar: Spatial precision deficits following medial temporal lobe lesions provide novel insight into theoretical models of navigation and memory. Hippocampus, 28(1), 31–41. doi: 10.1002/hipo.22801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolarik BS, Shahlaie K, Hassan B, Borders AA, Kaufman K, Gurkoff G, et al. (2016). Impairments in precision, rather than spatial strategy, characterize performance on the virtual Morris Water Maze: A case study. Neuropsychologia, 80, 90–101. doi: 10.1016/j.neuropsychologia.2015.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman MD, Wilson BA, & Baddeley AD (1989). The autobiographical memory interview: A new assessment of autobiographical and personal semantic memory in amnesic patients. Journal of Clinical and Experimental Neuropsychology, 11(5), 724–744. doi: 10.1080/01688638908400928 [DOI] [PubMed] [Google Scholar]
- Lee TM, Yip JT, & Jones-Gotman M (2002). Memory deficits after resection from left or right anterior temporal lobe in humans: a meta-analytic review. Epilepsia, 43(3), 283–291. doi: 10.1046/j.1528-1157.2002.09901.x [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G & Moscovitch M (2002). Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychol. Aging, 17(4), 677–689. doi: 10.1037/0882-7974.17.4.677 [DOI] [PubMed] [Google Scholar]
- Lindner MD, Plone MA, Schallert T, & Emerich DF (1997). Blind rats are not profoundly impaired in the reference memory Morris water maze and cannot be clearly discriminated from rats with cognitive deficits in the cued platform task. Brain research. Cognitive Brain Research, 5(4), 329–333. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Nannery R, & Spiers HJ (2006). Navigation around London by a taxi driver with bilateral hippocampal lesions. Brain, 129(11), 2894–2907. doi: 10.1093/brain/awl286 [DOI] [PubMed] [Google Scholar]
- Mandelbrot BB (1982). The Fractal Geometry of Nature. Freeman, New York. [Google Scholar]
- McAvan AS, Du YK, Oyao A, Doner S, Grilli MD, & Ekstrom AD (2021). Older adults show reduced spatial precision but preserved strategy-use during spatial navigation involving body-based cues. Frontiers in Aging Neuroscience, 13, 129. doi: 10.3389/fnagi.2021.640188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, & Moser MB (2006). Path integration and the neural basis of the ‘cognitive map’. Nat Rev Neurosci, 7(8), 663–678. doi: 10.1038/nrn1932 [DOI] [PubMed] [Google Scholar]
- Medina J, & Fischer-Baum S (2017). Single-case cognitive neuropsychology in the age of big data. Cognitive Neuropsychology, 34(7–8), 440–448. doi: 10.1080/02643294.2017.1321537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JF, Neufang M, Solway A, Brandt A, Trippel M, Mader I, et al. (2013). Neural activity in human hippocampal formation reveals the spatial context of retrieved memories. Science, 342(6162), 1111–1114. doi: 10.1126/science.1244056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B, Corkin S, & Teuber HL (1968). Further analysis of the hippocampal amnesic syndrome: 14-year follow-up study of HM. Neuropsychologia, 6(3), 215–234. doi: 10.1016/0028-3932(68)90021-3 [DOI] [Google Scholar]
- Moffat SD, Elkins W, & Resnick SM (2006). Age differences in the neural systems supporting human allocentric spatial navigation. Neurobiology of Aging, 27(7), 965–972. doi: 10.1016/j.neurobiolaging.2005.05.011 [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, & Resnick SM (2001). Age differences in spatial memory in a virtual environment navigation task. Neurobiology of Aging, 22(5), 787–796. [DOI] [PubMed] [Google Scholar]
- Morris R (1984). Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods, 11(1), 47–60. doi: 10.1016/0165-0270(84)90007-4 [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, & O’Keefe J (1982). Place navigation impaired in rats with hippocampal lesions. Nature, 297, 681–683. doi: 10.1038/297681a0 [DOI] [PubMed] [Google Scholar]
- Morris RGM, Hagan JJ, & Rawlins JNP (1986). Allocentric spatial learning by hippocampectomised rats: a further test of the “spatial mapping” and “working memory” theories of hippocampal function. The Quarterly Journal of Experimental Psychology, 38(4), 365–395. doi: 10.1080/14640748608402242 [DOI] [PubMed] [Google Scholar]
- Newman MC, & Kaszniak AW (2000). Spatial memory and aging: Performance on a human analog of the Morris water maze. Aging, Neuropsychology, and Cognition, 7(2), 86–93. doi: 10.1076/1382-5585(200006)7:2;1-U;FT086 [DOI] [Google Scholar]
- O’Keefe J, & Dostrovsky J (1971). The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res, 34(1), 171–175. doi: 10.1016/0006-8993(71)90358-1 [DOI] [PubMed] [Google Scholar]
- O’Keefe J, & Nadel L (1978). The Hippocampus as a Cognitive Map. Oxford: Clarendon Press. [Google Scholar]
- Parslow DM, Morris RG, Fleminger S, Rahman Q, Abrahams S, & Recce M (2005). Allocentric spatial memory in humans with hippocampal lesions. Acta Psycholgica, 118(1–2), 123–147. doi: 10.1016/j.actpsy.2004.10.006 [DOI] [PubMed] [Google Scholar]
- Patai EZ, & Spiers HJ (2021). The versatile wayfinder: Prefrontal contributions to spatial navigation. Trends in Cognitive Sciences. [DOI] [PubMed] [Google Scholar]
- Petrican R, Palombo DJ, Sheldon S, & Levine B (2020). The neural dynamics of individual differences in episodic autobiographical memory. eNeuro, 7(2). doi: 10.1523/ENEURO.0531-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM, & Wolfson D (1993). The Haldstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. Tucson: Neuropsychology Press. [Google Scholar]
- Renoult L, Davidson PSR, Palombo DJ, Moscovitch M, & Levine B (2012). Personal semantics: at the crossroads of semantic and episodic memory. Trends in Cognitive Sciences, 16(11) 550–558. doi: 10.1016/j.tics.2021.09.003 [DOI] [PubMed] [Google Scholar]
- Rey A (1941). L’examen psychologique dans les cas d’encephalopathie traumatique. Archives de Psychologie, 28, 286–340. [Google Scholar]
- Robin J, Hirshhorn M, Rosenbaum RS, Winocur G, Moscovitch M, & Grady CL (2015). Functional connectivity of hippocampal and prefrontal networks during episodic and spatial memory based on real‐world environments. Hippocampus, 25, 81–93 [DOI] [PubMed] [Google Scholar]
- Robin J, Rivest J, Rosenbaum RS, & Moscovitch M (2019). Remote spatial and autobiographical memory in cases of episodic amnesia and topographical disorientation. Cortex, 119, 237–257. doi: 10.1016/j.cortex.2019.04.013 [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Cassidy BN, & Herdman KA (2015). Patterns of preserved and impaired spatial memory in a case of developmental amnesia. Frontiers in Human Neuroscience, 9, 196. doi: 10.3389/fnhum.2015.00196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum RS, Gilboa A, Levine B, Winocur G, & Moscovitch M (2009). Amnesia as an impairment of detail generation and binding: evidence from personal, fictional, and semantic narratives in K.C. Neuropsychologia, 47(11), 2181–2187. doi: 10.1016/j.neuropsychologia.2008.11.028 [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Gilboa A, & Moscovitch M (2014). Case studies continue to illuminate the cognitive neuroscience of memory. Ann. N.Y. Acad. Sci, 1316(2014), 105–133. doi: 10.1111/nyas.12467 [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Priselac S, Kohler S, Black SE, Gao F, Nadel L, et al. (2000). Remote spatial memory in an amnesic person with extensive bilateral hippocampal lesions. Nature Neuroscience, 3(10), 1044–1048. doi: 10.1038/79867 [DOI] [PubMed] [Google Scholar]
- Schedlbauer AM, Copara MS, Watrous AJ, & Ekstrom AD (2014). Multiple interacting brain areas underlie successful spatiotemporal memory retrieval in humans. Sci Rep, 4, 6431. doi: 10.1038/srep06431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedlbauer AM, & Ekstrom AD (2019). Flexible network community organization during the encoding and retrieval of spatiotemporal episodic memories. Network Neuroscience, 3(4), 1070–1093. doi: 10.1162/netn_a_00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville WB, & Milner B (1957). Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry, 20(1), 11–21. doi: 10.1136/jnnp.20.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starrett MJ, McAvan AS, Huffman DJ, Stokes JD, Kyle CT, Smuda DN, et al. (2020). Landmarks: A solution for spatial navigation and memory experiments in virtual reality. Behavior Research Methods. doi: 10.3758/s13428-020-01481-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streese CD, & Tranel D (2021). Combined lesion-deficit and fMRI approaches in single-case studies: unique contributions to cognitive neuroscience. Current Opinion in Behavioral Sciences, 40, 58–63. doi: 10.1016/j.cobeha.2021.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Kawamura M, Shiota J, Kasahata N, & Hirayama K (1997). Pure topographic disorientation due to right retrosplenial lesion. Neurology, 49(2), 464–469. doi: 10.1212/wnl.49.2.464 [DOI] [PubMed] [Google Scholar]
- Teng E, & Squire LR (1999). Memory for places learned long ago is intact after hippocampal damage. Nature, 400(6745), 675–677. doi: 10.1038/23276 [DOI] [PubMed] [Google Scholar]
- Urgolites ZJ, Kim S, Hopkins RO, & Squire LR (2016). Map reading, navigating from maps, and the medial temporal lobe. Proceedings of the National Academy of Sciences of the United States of America, 113(50), 14289–14293. doi: 10.1073/pnas.1617786113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf YD, Jolles J, Witter MP, & Uylings HBM (2003). Contributions of thalamic nuclei to declarative memory functioning. Cortex, 39(4–5), 1047–1062. doi: 10.1016/S0010-9452(08)70877-3 [DOI] [PubMed] [Google Scholar]
- van Gerven DJH, Schneider AN, Wuitchik DM, & Skelton RW (2012). Direct measurement of spontaneous strategy selection in a virtual morris water maze shows females choose an allocentric strategy at least as often as males do. Behavioral Neuroscience, 126(3), 465–478. doi: 10.1037/a0027992 [DOI] [PubMed] [Google Scholar]
- Verfaellie M, LaRocque KF, & Rajaram S (2010). Benefits of immediate repetition versus long study presentation on memory in amnesia. Neuropsychology, 24(4), 457–464. doi: 10.1037/a0018625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wank AA, Robertson A, Thayer SC, Verfaellie M, Rapcsak SZ, & Grilli MD (2021). Autobiographical memory unknown: Pervasive autobiographical memory loss encompassing personality trait knowledge in an individual with medial temporal lobe amnesia. PsyArXiv. doi: 10.31234/osf.io/dva23 [DOI] [PubMed] [Google Scholar]
- Wechsler D (2008). WAIS-IV Manual. New York, NY. [Google Scholar]
- Wechsler D (2009). Wechsler Memory Scale-Fourth Edition. Pearson; San Antonio, TX. [Google Scholar]
- Wilson MA, & McNaughton BL (1993). Dynamics of the hippocampal ensemble code for space. Science, 261(5124), 1055–1058. doi: 10.1126/science.8351520 [DOI] [PubMed] [Google Scholar]
- Wolbers T, & Wiener JM (2014). Challenges in identifying the neural mechanisms that support spatial navigation: the impact of spatial scale. Frontiers in Human Neuroscience, 8(571), 1–12. doi: 10.3389/fnhum.2014.00571 [DOI] [PMC free article] [PubMed] [Google Scholar]


