Abstract
Purpose
To characterize the clinical outcomes of a novel ab interno minimally invasive procedure with the STREAMLINE® Surgical System for creation of incisional goniotomies and canal of Schlemm viscodilation in eyes with mild to severe primary open-angle glaucoma (POAG).
Methods
In a prospective, single-arm, first-in-human case series, 20 eyes of 20 subjects with mild to severe POAG underwent creation of incisional goniotomies and canal of Schlemm viscodilation following phacoemulsification cataract extraction after washout of all intraocular pressure (IOP)-lowering medications. The angle surgery portion was performed with a single-use handpiece tipped with a microcannula that creates precise goniotomies through the trabecular meshwork into the canal of Schlemm and delivers a small volume of ophthalmic viscosurgical device directly into the canal via precise catheterization. Outcomes in this interim analysis included mean reduction in IOP and medications through 6 months of follow-up, as well as the proportion of eyes achieving IOP reduction ≥20% from baseline.
Results
At month 6, mean IOP reduction of ≥20% from baseline was achieved in 89.5% of eyes (17/19). Mean (standard deviation) medicated IOP at screening was 16.3 (3.6) mmHg and unmedicated baseline IOP (after washout) was 23.5 (2.5) mmHg. Mean IOP was significantly reduced from baseline through 6 months of follow-up to 14.7 (2.4) mmHg (p<0.001), representing an IOP reduction of 8.8 mmHg (36.9%). Overall, 57.9% (11/19) of eyes decreased dependence on IOP-lowering medications by at least one medication, and 42.1% (8/19) were medication free. Mean medication use was reduced from 2.0 (0.8) at screening to 1.1 (1.1) at 6 months (p<0.001). Three eyes had transient IOP spikes treated with topical medications.
Conclusion
The creation of incisional goniotomies and canal of Schlemm viscodilation safely and effectively reduced IOP and the need for IOP-lowering medications by both clinically and statistically significant magnitudes in eyes with mild to severe POAG undergoing concomitant phacoemulsification cataract extraction through the first 6 months of follow-up.
Keywords: trabecular meshwork, canal of Schlemm, viscodilation, MIGS, glaucoma, goniotomy
Introduction
Surgical glaucoma treatments have evolved significantly over the past decade, motivated in large part by the unmet need for effective options to treat mild to moderate glaucoma without the risks associated with more traditional filtration procedures.1,2 In general, these procedures seek to enhance aqueous egress through the conventional aqueous humor outflow system by one of three means: catheterizing and injecting viscoelastic into the outflow system to enhance its function (canaloplasty); incising or excising the tissue (variations of goniotomy and trabeculotomy); or bypassing the trabecular meshwork (TM) with an implantable stenting device (of which several are available).1–4
On October 8, 2021, the STREAMLINE® Surgical System (New World Medical, Rancho Cucamonga, CA) was cleared via the 510(k) pathway by the US Food and Drug Administration (FDA) for use during ocular surgery to deliver small amounts of viscoelastic fluid. The single-use, disposable instrument consists of a surgical grade stainless-steel cannula and a polymer handset. The stainless-steel cannula is comprised of a long thin neck that allows for access to the TM through a clear corneal incision. The handset features an actuator button that, when fully depressed, retracts a polymer outer sleeve, allowing a stainless-steel inner cutting cannula tip to incise the TM, creating an incisional goniotomy 150 µm in diameter into the canal of Schlemm while simultaneously delivering a small volume of ophthalmic viscosurgical device (OVD) (approximately 7 µL per application) via focal incisional catheterization to viscodilate the canal, stretch the TM, and flush the distal collector channels. Before use, the device is loaded with an adequate volume of OVD to perform up to eight incisional goniotomies along several clock hours of the TM. The procedure can be performed either in combination with cataract surgery or as a standalone procedure, broadening its applicability to both phakic and pseudophakic eyes.
Herein, we report a preliminary analysis of an ongoing first-in-human study of incisional goniotomies and canal of Schlemm viscodilation in eyes with mild to severe primary open-angle glaucoma (POAG).
Methods
This is an ongoing prospective, nonrandomized, open-label, interventional first-in-human case series to characterize the safety and IOP-lowering effectiveness of incisional goniotomies and canal of Schlemm viscodilation in patients with mild to severe POAG undergoing cataract surgery at two centers in Mexico. Participating investigators are surgeons with extensive experience performing anterior chamber angle-based glaucoma surgery. The protocol and consent form were reviewed and approved by the Hospital Angeles Puebla ethics committee on November 10, 2020, and all study subjects signed the informed consent. The trial was registered (www.ClinicalTrials.gov NCT04700189) on January 7, 2021. This study followed all regulations laid down by the Declaration of Helsinki. The analysis presented in this report represents interim data from the first 20 subjects enrolled and followed through the first 6 months postoperatively.
Eligible subjects were adults at least 22 years of age with mild to severe POAG in at least one eye currently treated with one to three topical IOP-lowering medications who were scheduled for elective cataract surgery, whose preoperative IOP after washout of all IOP-lowering medications was between 21 and 36 mmHg inclusive. Women of childbearing potential, and patients with narrow or closed angles, advanced glaucoma (one or more of: visual field mean deviation worse than −12 dB; sensitivity ≤10 dB in two or more of the four central test points; cup–disc ratio >0.8; inability to safely undergo washout of IOP-lowering medications), any intraocular surgery in the prior 6 months or laser surgery in the prior 3 months (or any prior history of laser iridotomy), best-corrected visual acuity (BCVA) worse than 20/80 in either eye, or recent (prior 6 months) ocular inflammation or infection, were excluded.
Eligible subjects underwent a comprehensive screening assessment that included ascertainment of prior medical and ocular history, BCVA, anterior and posterior segment examination, IOP, gonioscopy, and automated perimetry. Subjects meeting eligibility criteria then initiated washout of IOP-lowering medications (28 days for prostaglandin analogues and beta-blockers, 14 days for alpha-agonists, and 5 days for miotics and carbonic anhydrase inhibitors; combination products based on the longest washout of components) and then attended a final eligibility visit at which the baseline unmedicated IOP was established. If both eyes qualified, the eye with higher baseline unmedicated IOP became the study eye (or the right eye if equal). Surgery was then performed as described below. Following surgery, assessments were conducted 1 day, 1 week, and 1, 3, and 6 months postoperatively. Medication use, BCVA, IOP, and anterior segment examination were performed at all visits; gonioscopy and posterior segment examination were performed at month 3. IOP was measured by experienced study personnel using Goldmann tonometry. Two measurements were taken at each assessment and averaged; a third was included if the first two differed by more than 3 mmHg.
The procedure was performed according to the manufacturer’s instructions for use5 following phacoemulsification cataract surgery and intraocular lens implantation. Before use, the device was loaded with OVD. The patient’s head was angled 45° away from the surgeon and the microscope angled 45° toward the surgeon. Under intraoperative direct gonioscopic visualization, the instrument was advanced through the cataract surgery incision across the anterior chamber to the nasal angle until the outer sleeve rested against the TM and slightly indented the tissue. The actuator button was then fully depressed, leading to retraction of the outer sleeve and allowing the inner cutting cannula to perform an incisional goniotomy. The actuator button was then held for 2 seconds to deliver the OVD into the canal of Schlemm. The tip was then withdrawn from the TM, the button released, and the procedure repeated over several clock hours of the drainage angle for a total of five to eight applications per eye. The postoperative medical regimen consisted of a fixed combination of prednisolone and gatifloxacin or tobramycin dosed every 2 hours initially and tapered off over 35 days.
The primary outcome measure of this ongoing study is the proportion of eyes with IOP reduction ≥20% from baseline at month 12. As this is a first-in-human trial, all analyses are considered exploratory and no specific hypotheses were tested; accordingly, the sample size was selected arbitrarily to represent an adequate data set with which to determine an estimate of the primary outcome. For this interim analysis, the effectiveness outcomes assessed included mean change from baseline in IOP and the mean change from screening (before washout) in the number of IOP-lowering medications; these were assessed using paired t-tests. The proportion of eyes achieving a ≥20% IOP reduction from baseline, as well as those achieving target IOP of <18 mmHg and <15 mmHg and those using fewer medications and no medications, all at month 6, were calculated. Safety outcomes included the nature and frequency of intraoperative and postoperative adverse events.
Results
Twenty eyes of 20 subjects were analyzed. One patient did not complete postoperative follow-up visits; the remaining 19 were analyzed through 6 months of follow-up. Demographic and baseline glaucoma data are given in Table 1. Based on the International Classification of Diseases 10th revision (ICD-10), eight eyes (40%) had mild glaucoma, eight eyes (40%) had moderate glaucoma and four eyes (20%) had severe glaucoma. Because our exclusion criteria for advanced glaucoma were more specific than the ICD-10 classification, eyes could have ICD-10 advanced glaucoma and still qualify for this study.
Table 1.
Demographic and Baseline Glaucoma Status Data for the Study Sample (N=20)
| Parameter | Value |
|---|---|
| Age (years), mean (SD) | 64 |
| Gender, n (%) | |
| Male | 3 |
| Female | 17 |
| Ethnicity, n (%) | |
| Hispanic | 20 (100) |
| Operative eye, n (%) | |
| Right | 10 (50) |
| Left | 10 (50) |
| Number of IOP-lowering medications, n (%) | |
| 1 | 6 (30) |
| 2 | 8 (40) |
| 3 | 6 (30) |
| Glaucoma severity, n (%) | |
| Mild | 8 (40) |
| Moderate | 8 (40) |
| Severe | 4 (20) |
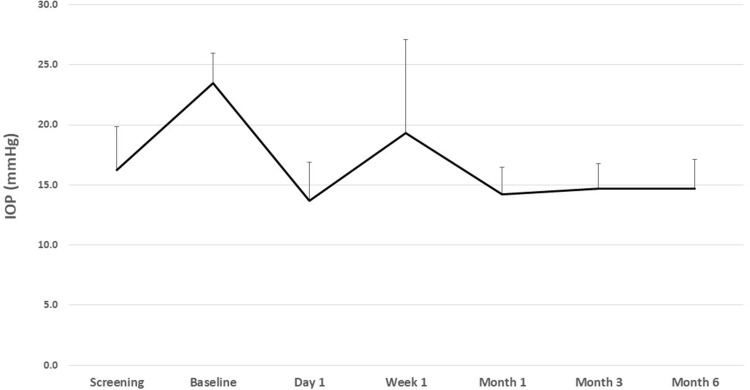
Mean IOP data at each time point are given in Table 2 and Figure 1. Mean (standard deviation) medicated IOP at screening was 16.3 (3.6) mmHg and unmedicated baseline IOP (after washout) was 23.5 (2.5) mmHg. Mean IOP was significantly reduced from baseline as soon as the first postoperative day, to 13.7 (2.5) mmHg (p<0.001) and, after stabilization, ranged from 14.2 to 14.7 mmHg at months 1–6 (p<0.001 at each time point). Mean IOP reduction after completion of postoperative steroid drops (at month 1 and beyond) ranged from 8.8 to 9.3 mmHg, representing percent reductions ranging from 36.8% to 39.2%. At month 6, a mean IOP reduction of ≥20% from baseline was achieved in 89.5% of eyes (17/19); 94.7% of eyes (18/19) achieved IOP <18 mmHg and 47.4% (9/19) achieved IOP <15 mmHg.
Table 2.
Mean IOP and Changes from Baseline at Each Visit (N=20)
| Screening | Baseline | Day 1 | Week 1 | Month 1 | Month 3 | Month 6 | |
|---|---|---|---|---|---|---|---|
| Number of eyes | 20 | 20 | 20 | 20 | 19 | 19 | 19 |
| IOP (mmHg) | |||||||
| Mean (SD) | 16.3 (3.6) | 23.5 (2.5) | 13.7 (3.2) | 19.3 (7.8) | 14.2 (2.3) | 14.7 (2.1) | 14.7 (2.4) |
| Mean (SD) change from baseline | – | – | 9.8 (3.9) | 4.2 (8.2) | 9.3 (2.9) | 8.8 (3.5) | 8.8 (3.2) |
| Mean (SD) % change from baseline | – | – | 41.1 (14.5) | 17.0 (33.8) | 39.2 (9.9) | 36.8 (11.4) | 36.9 (11.0) |
| Significance* | – | – | <0.001 | 0.035 | <0.0001 | <0.001 | <0.001 |
| Medications (n) | |||||||
| Mean (SD) | 2.0 (0.8) | 0 (0) | 0 (0) | 0.4 (0.8) | 1.0 (1.2) | 1.0 (1.1) | 1.1 (1.1) |
| Mean (SD) change from baseline | – | – | 2.0 (0.8) | 1.6 (1.0) | 1.0 (0.9) | 1.0 (0.9) | 0.9 (0.9) |
| Mean (SD) % change from baseline | – | – | 100 (0) | 81.7 (38.2) | 57.9 (48.2) | 56.1 (47.2) | 49.1 (47.6) |
| Significance* | – | – | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Notes: *The p-value is for comparison of mean IOP at each time point to baseline. This is an ongoing study where data will be presented later. Data to be collected include IOP, IOP-lowering medications, and safety; and will be deidentified. These pooled results will be analyzed and shared in a manuscript at the one-year mark. No other study documents will be made available. This study complies with the Declaration of Helsinki and all subjects signed informed consent. The research was approved by Hospital Angeles Puebla ethics committee because it is an external committee. Clínica Láser does not have its own IRB.
Figure 1 .
Mean IOP at each time point.
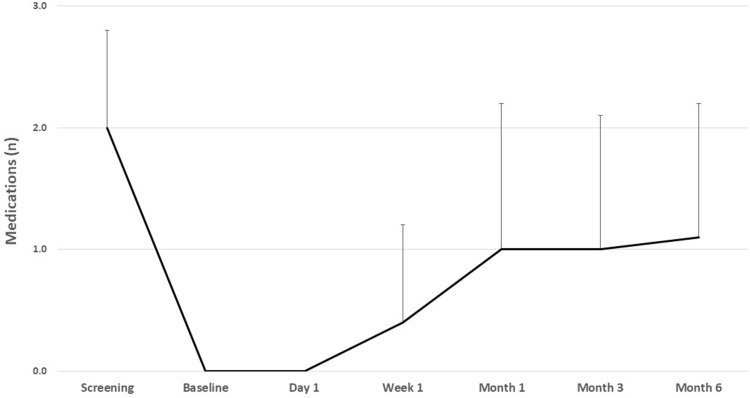
Mean medication use data at each time point are given in Table 2 and Figure 2. Subjects used a mean of 2.0 (0.8) medications at screening, and this was significantly reduced (p<0.001) at every postoperative time point. After postoperative stability (month 1 and beyond), the mean number of medications used ranged from 1.0 to 1.1, representing a mean reduction of 0.9–1.0 medications and percent reductions of 49.1–57.9%. At month 6, 57.9% (11/19) of subjects were using fewer medications than at screening and 42.1% (8/19) were medication free.
Figure 2 .
Mean medication use at each time point.
The procedure was well tolerated in all eyes, with no intraocular complications related to the glaucoma procedure. Three eyes experienced a steroid-related IOP elevation treated successfully with short-term topical medical therapy. One eye was exited from the study at week one owing to accidental chemical exposure to the face of the patient, unrelated to the study, that required care outside the study parameters.
Discussion
Preliminary analysis of this ongoing first-in-human study in eyes with mild to severe open-angle glaucoma demonstrates that incisional goniotomies and canal of Schlemm viscodilation using the STREAMLINE Surgical System provide safe and effective reduction of both IOP and the need for IOP-lowering medications through the first 6 postoperative months. At month 6, mean IOP was reduced by 37% and medications by nearly 50% compared to preoperative values.
Creating incisional goniotomies and canal of Schlemm viscodilation over several clock hours of the drainage angle lowers IOP through several potential mechanisms. The incisional goniotomy overcomes the well-documented resistance to aqueous outflow caused by diseased TM.6,7 OVD delivery dilates the canal, stretches the TM, and flushes the distal collector channels. The canal and collector channels are important post-TM sources of aqueous outflow resistance.8–12 Histology demonstrates a reduction of approximately 50% in both cross-sectional diameter and outflow facility of the canal in glaucomatous versus healthy eyes.10 The collector channels can also contribute to post-TM outflow resistance, as elevated IOP can lead to herniation of the canal’s inner wall into the collector channel ostia, causing obstruction.11 Thus, incisional goniotomies and canal of Schlemm viscodilation address both TM and post-TM resistance to aqueous outflow.
Preliminary outcomes of incisional goniotomies and canal of Schlemm viscodilation compare favorably to outcomes with TM bypass implants. In the pivotal trial evaluating the first-generation iStent combined with phacoemulsification, mean medicated IOP reduction from unmedicated baseline to 12 months (6-month data not reported) was 8.4 mmHg, IOP reduction of ≥20% was achieved in 66% of eyes, mean medication reduction was 1.4 medications, and 85% were medication free.13 In the second-generation iStent Inject pivotal trial, two devices were implanted at the time of cataract surgery.14 At 24 months (the only time point reported), mean unmedicated diurnal IOP reduction from baseline was 7.0 mmHg, IOP reduction of ≥20% from baseline was achieved in 75.8% of eyes, mean medication reduction was 1.2 medications, and 84% of eyes were medication free. In the Hydrus pivotal trial, 12-month results (6-month data not reported) of combined surgery with phacoemulsification demonstrated mean diurnal unmedicated IOP reduction of 8.5 mmHg, and 85.9% of eyes with ≥20% IOP reduction from baseline; 24-month medication reduction (the only time point reported) was 1.4 medications, and 78% of eyes were medication-free.15 The safety profile of these stents was similar to that of incisional goniotomies and canal of Schlemm viscodilation, in that no serious adverse events were reported in any of these approaches.
OVD delivery with this newly described approach produces viscodilation of the canal of Schlemm and the distal collector channels, similarly to canaloplasty procedures. Histologic analysis of human cadaver eyes injected with fluoresceinated OVD demonstrated that incisional goniotomies and viscodilation deliver OVD throughout the entire canal and into the distal collector channels after one application.16 The outcomes observed in this study compare favorably to commercialized forms of canaloplasty, none of which has been evaluated in prospective regulatory trials. VISCO360 (Sight Sciences, Menlo Park, CA) is an ab interno procedure requiring successive cannulation of each 180° half of the canal through a common goniotomy. An 18-month study of eyes with mild to moderate open-angle glaucoma undergoing VISCO360 demonstrated mean IOP reduction of 36% and mean medication reduction of 32%.17 In a pair of 12-month VISCO360 studies that evaluated outcomes in eyes with mild to moderate open-angle glaucoma and high (≥18 mmHg) and low (<18 mmHg) baseline IOP, mean IOP was reduced by 22–41% in high IOP eyes, while mean medication reduction was 45–89% in low IOP eyes; IOP reduction of ≥20% was achieved in 87% of eyes, and 32–86% of eyes were medication free.18,19 Among studies of 12–24 months’ duration of canaloplasty performed with the iTrack® microcatheter (Nova Eye Medical, Fremont, CA; previously reported as ab interno canaloplasty, or ABiC) in eyes with open-angle glaucoma, mean IOP reductions of 25–40% have been reported, with 78.4% achieving IOP reductions of ≥20% from baseline; mean medication reductions of 39–97% and medication-free rates of 25–80% have also been reported.20–25
Creating incisional goniotomies with concomitant canal of Schlemm viscodilation over several clock hours of the drainage angle represents a meaningful advancement in the ongoing development of minimally invasive ab interno procedures for patients with mild to severe glaucoma who seek reduction of IOP, IOP-lowering medications, or both. The procedure provides incisional goniotomy and catheterization, potentially minimizing or eliminating the risk of canal tissue damage related to difficult or false passage as seen with other cannulas currently in use, and leaves no permanently implanted device, which eliminates the risk of implant-related complications, including occlusion and migration. Implanted devices also pose a risk of corneal endothelial cell loss, most notably with glaucoma drainage devices,26 but also with minimally invasive glaucoma implants27 and sustained-release glaucoma drug delivery systems.28
Strengths of this study include its prospective design and use of an industry-standard primary outcome (≥20% reduction in IOP from baseline).29 In addition, subjects in this study underwent washout of IOP-lowering medications preoperatively to provide a more accurate estimate of surgical reduction of IOP. Limitations include both small sample size and short follow-up; a larger sample is being collected, and follow-up is planned through 12 months for the full data set, including assessment of pachymetry and specular microscopy, which will be collected at the final visit and compared to baseline values. Given that this device has been cleared by the FDA and the instrument is available for use, the need to disseminate preliminary outcomes data supports the reporting of this interim analysis. An additional limitation is the lack of a phacoemulsification-only control group, as phacoemulsification alone is known to reduce both IOP and the need for IOP-lowering medications in glaucomatous eyes.30 However, the magnitude of both IOP reduction and medication reduction in this study is significantly greater than would be expected from phacoemulsification alone, supporting the efficacy of the incisional goniotomy procedure. Also, the IOP outcomes presented in this interim analysis were not assessed after drug washout.
In summary, incisional goniotomy and catheterization with viscodilation of the canal of Schlemm over several clock hours at the time of cataract extraction safely and effectively reduces IOP and the need for IOP-lowering medications by both clinically and statistically significant magnitudes in eyes with medically controlled mild to severe POAG.
Funding Statement
This study was supported by New World Medical.
Disclosure
Dr Sumit J Garg is a consultant for New World Medical, during the conduct of the study. Dr Malik Y Kahook is a consultant to New World Medical and his university receives fees on his behalf for this consultancy. In addition, Dr Kahook has a patent (no. 10,729,584) issued related to STREAMLINE Surgical System technology. The authors report no other conflicts of interest in this work.
References
- 1.Lavia C, Dallorto L, Maule M, Ceccarelli M, Fea AM. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta-analysis. PLoS One. 2017;12(8):e0183142. doi: 10.1371/journal.pone.0183142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richter GM, Coleman AL. Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmol. 2016;10:189–206. doi: 10.2147/OPTH.S80490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ansari E. An update on implants for Minimally Invasive Glaucoma Surgery (MIGS). Ophthalmol Ther. 2017;6(2):233–241. doi: 10.1007/s40123-017-0098-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pillunat LE, Erb C, Junemann AG, Kimmich F. Micro-invasive glaucoma surgery (MIGS): a review of surgical procedures using stents. Clin Ophthalmol. 2017;11:1583–1600. doi: 10.2147/OPTH.S135316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Streamline Surgical System. Instructions for Use. Rancho Cucamonga, CA: New World Medical; 2021. [Google Scholar]
- 6.Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963;69(6):783–801. doi: 10.1001/archopht.1963.00960040789022 [DOI] [PubMed] [Google Scholar]
- 7.Maepea O, Bill A. Pressures in the juxtacanalicular tissue and Schlemm’s canal in monkeys. Exp Eye Res. 1992;54(6):879–883. doi: 10.1016/0014-4835(92)90151-H [DOI] [PubMed] [Google Scholar]
- 8.O’Callaghan J, Cassidy PS, Humphries P. Open-angle glaucoma: therapeutically targeting the extracellular matrix of the conventional outflow pathway. Expert Opin Ther Targets. 2017;21(11):1037–1050. doi: 10.1080/14728222.2017.1386174 [DOI] [PubMed] [Google Scholar]
- 9.Martinez Sanchez GJ, Escobar Del Pozo C, Rocha Medina JA. Numerical model of aqueous humor drainage: effects of collector channel position. Med Eng Phys. 2019;65:24–30. doi: 10.1016/j.medengphy.2018.12.022 [DOI] [PubMed] [Google Scholar]
- 10.Allingham RR, de Kater AW, Ethier CR. Schlemm’s canal and primary open angle glaucoma: correlation between Schlemm’s canal dimensions and outflow facility. Exp Eye Res. 1996;62(1):101–109. doi: 10.1006/exer.1996.0012 [DOI] [PubMed] [Google Scholar]
- 11.Battista SA, Lu Z, Hofmann S, Freddo T, Overby DR, Gong H. Reduction of the available area for aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes. Invest Ophthalmol Vis Sci. 2008;49(12):5346–5352. doi: 10.1167/iovs.08-1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smit BA, Johnstone MA. Effects of viscoelastic injection into Schlemm’s canal in primate and human eyes: potential relevance to viscocanalostomy. Ophthalmology. 2002;109(4):786–792. doi: 10.1016/S0161-6420(01)01006-5 [DOI] [PubMed] [Google Scholar]
- 13.Samuelson TW, Katz LJ, Wells JM, Duh YJ, Giamporcaro JE. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118(3):459–467. doi: 10.1016/j.ophtha.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 14.Samuelson TW, Sarkisian SR Jr., Lubeck DM, et al. Prospective, randomized, controlled pivotal trial of an Ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract: two-year results. Ophthalmology. 2019;126(6):811–821. doi: 10.1016/j.ophtha.2019.03.006 [DOI] [PubMed] [Google Scholar]
- 15.Samuelson TW, Chang DF, Marquis R, et al. A Schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: the HORIZON study. Ophthalmology. 2019;126(1):29–37. doi: 10.1016/j.ophtha.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 16.Garg S, Ammar DA, Goldberg D, Seibold LK, Porteous E, Kahook M Histological analysis of a novel dual-port microcatheter used to target the conventional aqueous outflow pathway via an Ab-interno approach. American Society of Cataract and Refractive Surgery Annual Meeting. Washington DC; 2022. [Google Scholar]
- 17.Hughes T, Traynor M. Clinical results of Ab interno canaloplasty in patients with open-angle glaucoma. Clin Ophthalmol. 2020;14:3641–3650. doi: 10.2147/OPTH.S275087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ondrejka S, Korber N. 360 degrees Ab-interno Schlemm’s canal viscodilation in primary open-angle glaucoma. Clin Ophthalmol. 2019;13:1235–1246. doi: 10.2147/OPTH.S203917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tracer N, Dickerson JE Jr., Radcliffe NM. Circumferential viscodilation Ab interno combined with phacoemulsification for treatment of open-angle glaucoma: 12-month outcomes. Clin Ophthalmol. 2020;14:1357–1364. doi: 10.2147/OPTH.S252965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillmann K, Aref A, Niegowski LJ, Baumgartner JM. Combined Ab interno viscocanaloplasty (ABiC) in open-angle glaucoma: 12-month outcomes. Int Ophthalmol. 2021;41(10):3295–3301. doi: 10.1007/s10792-021-01891-1 [DOI] [PubMed] [Google Scholar]
- 21.Kazerounian S, Zimbelmann M, Lortscher M, Hommayda S, Tsirkinidou I, Muller M. Canaloplasty Ab interno (AbiC) - 2-Year-results of a novel Minimally Invasive Glaucoma Surgery (MIGS) Technique. Klin Monbl Augenheilkd. 2020;238(10):1113–1119. doi: 10.1055/a-1250-8431 [DOI] [PubMed] [Google Scholar]
- 22.Davids AM, Pahlitzsch M, Boeker A, Winterhalter S, Maier-Wenzel AK, Klamann M. Ab interno canaloplasty (ABiC)-12-month results of a new minimally invasive glaucoma surgery (MIGS). Graefes Arch Clin Exp Ophthalmol. 2019;257(9):1947–1953. doi: 10.1007/s00417-019-04366-3 [DOI] [PubMed] [Google Scholar]
- 23.Gallardo MJ, Supnet RA, Ahmed IIK. Circumferential viscodilation of Schlemm’s canal for open-angle glaucoma: Ab-interno vs Ab-externo canaloplasty with tensioning suture. Clin Ophthalmol. 2018;12:2493–2498. doi: 10.2147/OPTH.S178962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallardo MJ, Supnet RA, Ahmed IIK. Viscodilation of Schlemm’s canal for the reduction of IOP via an Ab-interno approach. Clin Ophthalmol. 2018;12:2149–2155. doi: 10.2147/OPTH.S177597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallardo MJ. 24-month efficacy of viscodilation of Schlemm’s canal and the distal outflow system with iTrack Ab-interno canaloplasty for the treatment of primary open-angle glaucoma. Clin Ophthalmol. 2021;15:1591–1599. doi: 10.2147/OPTH.S272506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Realini T, Gupta PK, Radcliffe NM, et al. The effects of glaucoma and glaucoma therapies on corneal endothelial cell density. J Glaucoma. 2021;30(3):209–218. doi: 10.1097/IJG.0000000000001722 [DOI] [PubMed] [Google Scholar]
- 27.Lass JH, Benetz BA, He J, et al. Corneal endothelial cell loss and morphometric changes 5 years after phacoemulsification with or without cypass micro-stent. Am J Ophthalmol. 2019;208:211–218. doi: 10.1016/j.ajo.2019.07.016 [DOI] [PubMed] [Google Scholar]
- 28.Medeiros FA, Walters TR, Kolko M, et al. Phase 3, randomized, 20-month study of bimatoprost implant in open-angle glaucoma and ocular hypertension (ARTEMIS 1). Ophthalmology. 2020;127(12):1627–1641. doi: 10.1016/j.ophtha.2020.06.018 [DOI] [PubMed] [Google Scholar]
- 29.Premarket studies of implantable Minimally Invasive Glaucoma Surgical (MIGS) devices: guidance for industry and food and drug administration staff; 2015. Available from: https://www.fda.gov/media/90950/download. Accessed March 17, 2021.
- 30.Armstrong JJ, Wasiuta T, Kiatos E, Malvankar-Mehta M, Hutnik CM. The effects of phacoemulsification on intraocular pressure and topical medication use in patients with glaucoma: a systematic review and meta-analysis of 3-year data. J Glaucoma. 2017;26(6):511–522. doi: 10.1097/IJG.0000000000000643 [DOI] [PubMed] [Google Scholar]