Abstract
Current therapeutic approaches for glaucoma aim to reduce intraocular pressure (IOP), which is the only available and reliable strategy proven to control the risk of disease development and progression. Omidenepag isopropyl (OMDI) is a novel topical ocular hypotensive agent that was launched onto the market for the treatment of glaucoma and ocular hypertension (OHT). After topical instillation and during corneal penetration, OMDI is converted into the active metabolite omidenepag (OMD), which behaves as a non-prostaglandin, selective E-prostanoid subtype 2 (EP2) receptor agonist. The topical administration of 0.002% OMDI once-daily (QD) possesses a 20–35% IOP-lowering effect, comparable to that of prostaglandin analogs targeting F-prostanoid (FP) receptor QD, which are the current first-line for pharmaceutical reduction of IOP. However, the mechanism of action and adverse events (AEs) of OMDI are different from those of FP receptor agonists. OMDI reduces IOP by enhancing both conventional trabecular and uveoscleral outflow facilities without complications of prostaglandin-associated periorbitopathy (PAP) seen with FP receptor agonists. Moreover, OMDI was also effective and well-tolerated in non-/poor responders to latanoprost and showed a stable IOP-lowering effect for one year, and its concomitant use with timolol enhanced the IOP-lowering effect. OMDI demonstrated acceptable safety and tolerability with good adherence and can be used in almost every patient. However, OMDI has some AEs such as conjunctival hyperemia, corneal thickening, macular edema/cystoid macular edema and ocular inflammation. Moreover, OMDI is contraindicated in patients who are allergic to the product, in aphakic or pseudophakic eyes, and in combination with tafluprost eye drops. If used appropriately in the right patients, OMDI could be an effective treatment option for glaucoma and OHT as a first-line alternative to FP agonists. Here, we summarize the results of clinical studies of OMDI and discuss its efficacy and patient tolerability in glaucoma and OHT in this review.
Keywords: omidenepag, EP2 receptor, intraocular pressure, adverse event, prostaglandin, glaucoma
Introduction
The number of blind and visually impaired people due to glaucoma increased markedly, and this upward trend will continue with the growth and aging of populations, making glaucoma an important target for prevention and treatment.1–4 Current therapeutic approaches for glaucoma aim to reduce intraocular pressure (IOP), which is the only available and reliable strategy shown to control the risk of disease development and progression.5–8 In 1977, the low-concentration topical administration of prostaglandin F2α (PGF2α) or prostaglandin E2 (PGE2) was demonstrated to decrease intraocular pressure (IOP) by stimulating the respective F-prostanoid (FP) receptor or E-prostanoid (EP) receptor.9 Then, prostaglandin analogs targeting FP receptors to lower IOP were developed to have a better penetration into the anterior chamber and fewer side effects.10,11 Today, four prostaglandin derivatives (latanoprost, tafluprost, travoprost, and bimatoprost) targeting the FP receptor, or FP receptor agonists, have been released. They are most commonly used as once-daily eye drops across the world, being the first-line pharmaceutical treatment for glaucoma because of their greatest IOP-lowering effect of all glaucoma eye drops, efficacy in all forms of glaucoma, suppression of diurnal IOP variation, additive effects in combination with other types of glaucoma eye drops, few systemic side effects, and good patient adherence.12,13 FP receptor agonists decrease IOP by primarily increasing aqueous humor drainage via the uveoscleral outflow pathway by stimulating Gq-protein-mediated increases in intracellular calcium concentrations and various signaling cascades in the ciliary body and trabecular meshwork.12–14
However, despite the high efficacy and tolerability of FP receptor agonists administered once a day, there are some unmet clinical needs. First, in addition to common ocular side effects such as conjunctival hyperemia and eye irritation, the long-term or unilateral usage of FP receptor agonists frequently induces distinctive local side effects, named prostaglandin-associated periorbitopathy (PAP), including hyperpigmentation in the iris and around the eyelids, eyelash growth, blepharochalasis involution, periorbital fat loss, enophthalmos, deepening of the upper eyelid sulcus (DUES), and hardening of eyelids and ptosis.13,15 Periorbital tissue changes could hinder the long-term management of glaucoma by making IOP measurements difficult, increasing ophthalmic surgical failures of trabeculectomy, and reducing treatment adherence due to cosmetic problems.15–17 Therefore, PAP is a well-known clinical and cosmetic concern in patients receiving FP receptor agonists.15,16 Second, a small number of patients do not respond to FP receptor agonists and have less than 10% or 15% IOP reduction (non-/poor responders to FP agonists). It was found that 28% of Japanese patients in a retrospective study and 52% of predominantly Caucasian patients in a prospective randomized study were non-/poor responders to latanoprost.18,19 Therefore, an effective, well-tolerated, alternative monotherapy with a novel mechanism of action would be clinically beneficial to treat glaucoma patients with PAP or non-/poor responders to latanoprost or other FP agonists. In other words, the current developmental goal for glaucoma ophthalmic solutions would be to provide a therapy with IOP-lowering effects equivalent to those of latanoprost throughout the day with a once-daily drop, the possibility of use in combination with other eye drops, presenting fewer side effects, and with less inter-individual variability in efficacy.
Besides the FP receptor for PGF2α, the EP subtype 1–4 receptors for PGE2 also play an important role in IOP regulation.20 In recent years, several attempts have been made to find compounds affecting IOP by modulating prostaglandin signaling on multiple receptor subtypes. Of particular interest in the present context is the E-prostanoid subtype 2 (EP2) receptor.20–22 Evolving research suggests that selective EP2 receptor agonists, including DE-117 (omidenepag isopropyl, OMDI), PF-04217329 (taprenepag isopropyl), AGN-210961 (aganepag isopropyl) and JV-GL1 (PGN-9856 isopropyl), have an IOP-lowering potential in glaucoma and OHT, and some of these compounds are in clinical development.23–33 Against this backdrop, several Phase I to III clinical trials of OMDI were conducted.25–27,34,35 The phase I trial aimed to investigate the pharmacokinetic properties, safety, and IOP-lowering effect in a small number of healthy people.34 The next Phase II trials were designed to assess the efficacy and safety in a larger group of patients.25,35 Then, the Phase III clinical trials compared the efficacy and safety against the current standard treatment in a large number of patients, at least several hundred.26,27 Finally, a 0.002% OMDI ophthalmic solution (Eybelis, Santen Pharmaceutical Co., Ltd., Osaka, Japan), the world’s first commercially available EP2 receptor agonist, was approved in Japan in September 2018 as a new class of ocular hypotensive agents for the treatment of glaucoma and OHT.23–28,33 Furthermore, OMDI was the first once-daily ophthalmic drug with a novel mechanism of action to demonstrate non-inferiority in lowering IOP with minimal side effects in a direct comparison study with latanoprost.26 OMDI is currently also approved in Singapore, South Korea, Taiwan, and Thailand. Moreover, India, Malaysia, the Philippines, and the United States have pending reviews by their local regulatory agencies. Various clinical trials are revealing OMDI’s potential as an alternative first-line drug for glaucoma and OHT treatment, as well as precautions for its use. Here, we summarize the results of relevant studies13,15–20,23–29,34,36–45 and discuss its efficacy23–28,35,44–49 and patient tolerability13,15,25–28,34–36,50–65 in glaucoma and OHT in this review.
Pharmacological Profile of OMDI
OMDI, a selective EP2 receptor agonist with a non-prostaglandin structure, was co-developed by Ube Industries Ltd. (Tokyo, Japan) and Santen Pharmaceutical Co. Ltd. (Osaka, Japan). FP receptor agonists, the current first-line and most prescribed glaucoma eye drops, have inherent problems with non-/poor responders and ocular local side effects.15–19 Moreover, beta-blockers, another first-line ocular hypotensive agents, are not effective as FP receptor agonists in lowering IOP, and have a problem with systemic side effects.36,37 Therefore, there was a requirement for the development of a new first-line ophthalmic agent for glaucoma and OHT with a novel mechanism of action and efficacy and safety comparable to or higher than those of a FP receptor agonist.
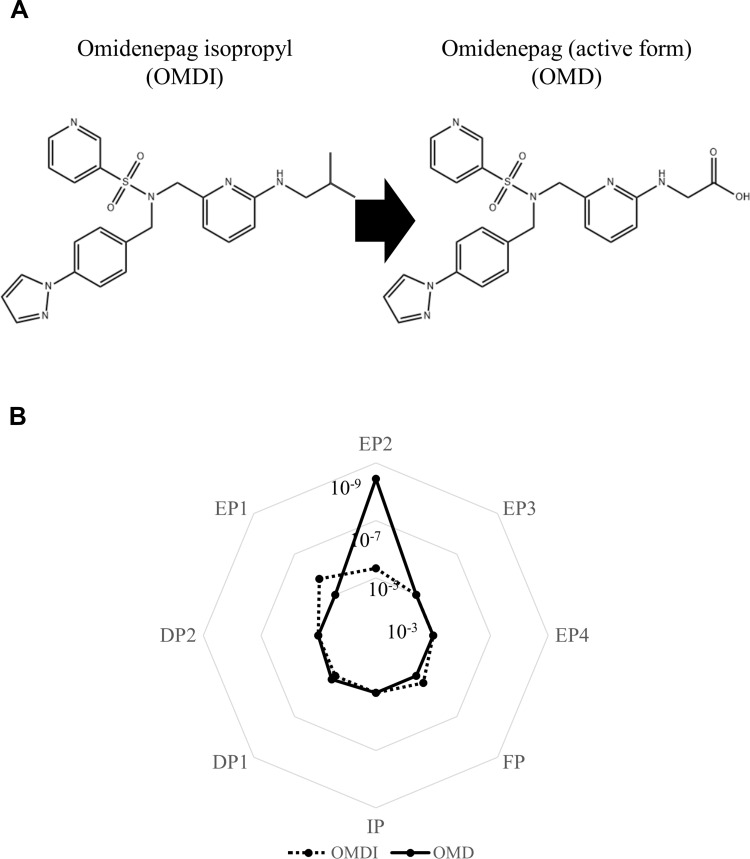
The pharmacodynamic properties of OMDI have been clarified by various recent studies.13,15,20,23–29,38–45 OMDI is an isopropyl ester prodrug that undergoes hydrolysis to its active metabolite (omidenepag, OMD) during corneal penetration (Figure 1A), which behaves a non-prostaglandin, selective EP2 receptor agonist.23–28,38 In in vitro studies using human recombinant prostanoid receptors, OMD was shown to selectively bind to the EP2 receptor (Ki = 3.6 nM) and possess high agonistic activity (EC50 = 8.3 nM) toward the EP2 receptor, with no detectable effects on other prostanoid receptors (Figure 1B). OMDI showed weak or no binding affinity to the prostanoid receptors tested, including EP1, EP2, and FP (Figure 1B).23 Following the topical administration of OMDI in rabbits, OMDI was below the limit of detection and OMD was the only detectable form in the aqueous humor.23 Therefore, the unique clinical side effects due to nonspecific binding to the FP receptor, or PAP, do not occur.15 Recent in vivo and in vitro studies showed that OMD has no or little effect on adipocyte differentiation, adipogenesis, and eye lash growth, which are PAP-related organizational changes seen with FP agonist use.39–41 Moreover, OMD has different effects on 3D human orbital fibroblast organoids as compared to bimatoprost acid, which indicate that it may not induce deepening of upper eyelid sulcus (DUES).42 Antiglaucoma drugs such as OMD and bimatoprost acid modulate the structures and physical properties of Grave’s orbitopathy-related human orbital fibroblast 3D organoids in different manners by modifying the gene expressions of ECM, ECM regulatory factors, and inflammatory cytokines.43
Figure 1.
Chemical structures of OMDI and its active form, OMD (A), and their binding affinity to prostanoid receptors evaluated with Ki value (mol/L) (B). OMDI shows no or little binding to the prostanoid receptors, while OMD has strong selective binding affinity to the EP2 receptor.
Note: Data from Kirihara et al.23
Abbreviations: OMDI, omidenepag isopropyl; OMD, omidenepag.
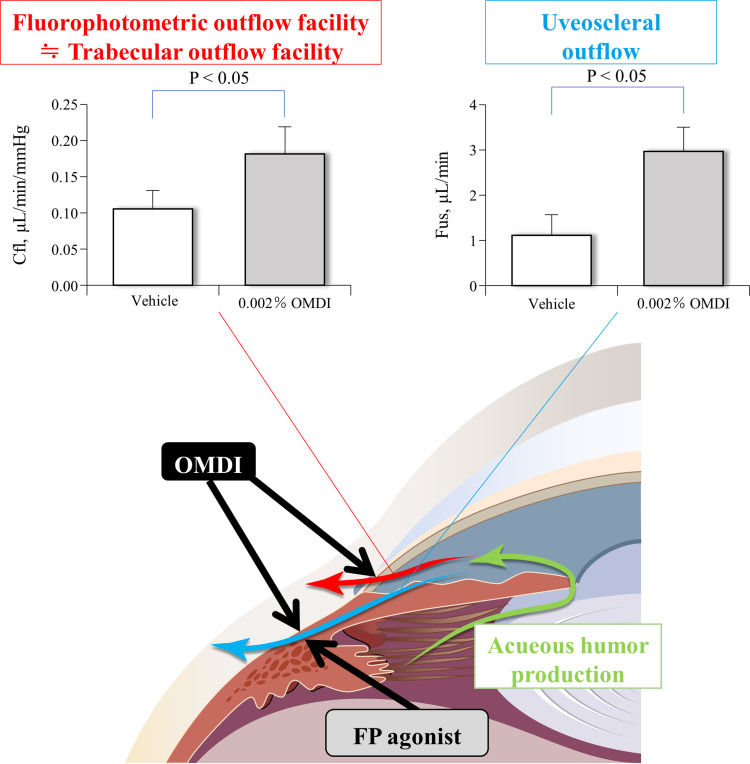
The EP2 receptor is a Gs-coupled transmembrane receptor found in the ciliary body and trabecular meshwork. EP2 receptor agonists decrease IOP by increasing both trabecular and uveoscleral outflow by stimulating a Gs-protein-mediated elevations in intracellular adenosine 3’,5-cyclic monophosphate (cAMP) levels and various signaling cascades in the ciliary body and trabecular meshwork.13,20,29 In monkeys with laser-induced OHT, treatment with 0.002% OMDI ophthalmic solution for 1 week resulted in a significant 71% increase in the aqueous outflow facility (thought to be the conventional, pressure-dependent trabecular outflow facility) and a significant 176% increase in the uveoscleral outflow compared with the control vehicle treatment (p < 0.05, respectively) (Figure 2).24 No significant difference was seen in aqueous humor production for 0.002% OMDI ophthalmic solution compared with vehicle.24 Therefore, OMDI is thought to lower IOP by promoting aqueous humor outflow via a dual mechanism of action, increasing both the trabecular outflow facility and uveoscleral outflow as a result of EP2 receptor stimulation.23,24 Another recent study with human trabecular meshwork (TM) cells, monkey Schlemm’s canal endothelial (SCE) cells, and porcine ciliary muscle showed that the IOP-lowering effect of OMD through the conventional outflow pathway is exerted by increasing outflow facilities with the modulation of TM cell fibrosis and SCE cell permeability.44
Figure 2.
Mechanism of action for IOP reduction with topical administration of OMDI in OHT monkey eyes. OMDI reduces IOP by enhancing both conventional trabecular and uveoscleral outflow facilities, while FP agonists do it mainly via uveoscleral outflow.
Notes: Aqueous humor outflow parameters following treatment with vehicle or 0.002% OMDI are represented as mean±SEM. Statistical significance was analyzed with a paired t-test. Data from Fuwa et al.24
Abbreviations: Cfl, fluorophotometric outflow facility; Fus, uveoscleral outflow; OMDI, omidenepag isopropyl; FP agonist, F-prostanoid receptor agonist.
In monkeys without OHT, a single topical administration of 0.0006% OMDI ophthalmic solution in combination with 0.5% timolol maleate (timolol), 1% brinzolamide, 0.01% netarsudil mesylate, 0.4% ripasudil hydrochloride hydrate, or 0.15% brimonidine tartrate resulted in an IOP reduction that was significantly lower than that seen with monotherapy with any of these drugs alone (p < 0.05, respectively).45 Namely, OMDI has additive IOP-lowering effects when administered in combination with other classes of antiglaucoma eye drops such as β-adrenergic antagonists, carbonic anhydrase inhibitors, Rho-associated coiled-coil containing protein kinases inhibitors, and α2-adrenergic agonists, because of its unique mechanisms of action.45
The pharmacokinetic properties of OMDI ophthalmic solution 0.002% in humans have been investigated in a phase I trial of seven Japanese and seven Caucasian healthy volunteers.34 Following treatment with 0.002% OMDI ophthalmic solution QD, OMD was rapidly absorbed in both subjects and the maximum plasma concentration (Cmax) of the active metabolite (OMD) was achieved (almost 30–40 pg/mL) after 10–15 min. The half-life (t½) of OMD was approximately 30 min, and the plasma concentrations were below the limit of quantification 4 hours after eye drop administration.34 Moreover, OMD pharmacokinetic parameters were similar between Japanese and Caucasian subjects, and there was no accumulation of OMD after 7 days of dosing.34
Efficacy – IOP-Lowering Effect of OMDI
Table 1 summarizes the twelve clinical trials registered in the US National Library of Medicine for DE-117, the OMDI ophthalmic solution (searched on 2022/1/11). These are being or were conducted in the US, Japan, India, South Korea, Singapore, and Taiwan. Currently, nine have been completed and the results of seven have been described in clinical articles. Seven are phase III clinical trials for OMDI ophthalmic solution 0.002%. Two of these, SPECTRUM 3 (NCT03691649) and SPECTRUM 4 (NCT03691662), are in patients with glaucoma or OHT; the other two, RENGE (NCT02822729) and PEONY (NCT02981446), are in patients with open-angle glaucoma (OAG) or OHT; the other one, AYAME (NCT02623738), is in patients with POAG or OHT; and the others, FUJI (NCT02822742) and SPECTRUM 5 (NCT03697811), are in patients with POAG or OHT who are non-/poor responders to latanoprost.
Table 1.
Summary of Clinical Trials Performed with Omidenepag Isopropyl (DE-117) Ophthalmic Solution
| NCT Number (Trial Feature) | Study Design (Phase, Allocation, Masking, Intervention Model) | Enrollment, n | Status | Condition | Arms | Treatment Duration | Primary Outcome Measure | Reference |
|---|---|---|---|---|---|---|---|---|
| NCT01654484 | I/II, randomized, single-masked, parallel group | 60 | Completed | POAG or OHT | DE-117 (0.003%, 0.01% and 0.03%) QD | 28 days | IOP change from baseline at each time point (08:00, 10:00, 12:00, and 16:00) | NA |
| DE-117 (0.003%, 0.01% and 0.03%) QD and tafluprost 0.0015% QD | ||||||||
| Tafluprost 0.0015% QD | ||||||||
| Placebo QD | ||||||||
| NCT01868126 | II, randomized, single-masked, parallel group | 91 | Completed | POAG or OHT | DE-117 (0.0003%, 0.001%, 0.002%, or 0.003%) QD | 4 weeks | IOP at each time point (08:00, 10:00, 12:00, and 16:00) and mean diurnal IOP | Aihara et al. J Glaucoma, 2019 |
| Latanoprost 0.005% QD | Number of subjects with adverse events | |||||||
| Placebo QD | ||||||||
| NCT02179008 (SEE-1) | II, randomized, double-masked, parallel group | 184 | Completed | POAG or OHT | DE-117 (0.0012%, 0.0016%, 0.002%, 0.0025%, or 0.003%) QD | 3 months | IOP at each time point (08:00, 10:00, 12:00, 16:00 and 18:00) and mean diurnal IOP | Aihara et al. J Glaucoma, 2019 |
| Latanoprost 0.005% QD | Number of subjects with adverse events | |||||||
| NCT02650063 | I, open-label, single group | 14 | Completed | Healthy Male Adults | DE-117 0.0025% QD | 1 week | Area under concentration-time curve (AUC) | Aihara et al, J Ocul Pharmacol, 2019 |
| Maximum plasma concentration (Cmax) | ||||||||
| Time to maximum plasma concentration (Tmax) | ||||||||
| Elimination half-life (T1/2) | ||||||||
| NCT02623738 (AYAME) | II/III, randomized, single-/double-masked, parallel group | 253 | Completed | POAG or OHT | DE-117 (0.002%, or 0.0025%) QD | 4 weeks | IOP at each time point (09:00, 13:00, and 17:00) and mean diurnal IOP change from baseline | Aihara et al. J Glaucoma, 2019, Aihara et al. Am J Ophthalmol, 2020 |
| Latanoprost 0.005% QD | Number of subjects with adverse events | |||||||
| Placebo QD | ||||||||
| NCT02822729 (RENGE) | III, randomized, open-label, parallel group | 125 | Completed | OAG or OHT | DE-117 0.002% QD | 52 weeks | IOP at each time point (09:00, 13:00, and 17:00) and mean diurnal IOP | Aihara et al, Jpn J Ophthalmol, 2021 |
| DE-117 0.002% QD and Timolol 0.5% BID | Number of subjects with adverse events | |||||||
| NCT02822742 (FUJI) | III, open-label, single group | 26 | Completed | POAG or OHT who are non-/poor responders to Latanoprost | Switching to DE-117 0.002% QD from Latanoprost 0.005% QD | 4 weeks | Mean diurnal IOP change from baseline | Aihara et al, Jpn J Ophthalmol, 2020 |
| NCT02981446 (PEONY) | III, randomized, single-masked, parallel group | 370 | Completed | OAG or OHT | DE-117 0.002% QD | 3 months | Mean diurnal IOP | NA |
| Latanoprost 0.005% QD | ||||||||
| NCT03691649 (SPECTRUM 3) | III, randomized, double-masked, parallel group | 427 | Unknown status | Glaucoma or OHT | Vehicle QD and DE-117 QD for 3 months, followed by DE-117 QD for 9 additional months | 12 months | IOP change from baseline | NA |
| Timolol Maleate 0.5% BID for 3 months, followed by DE-117 QD for 9 additional months | ||||||||
| NCT03691662 (SPECTRUM 4) | III, randomized, double-masked, parallel group | 409 | Unknown status | Glaucoma or OHT | Vehicle QD and DE-117 QD | 3 months | IOP change from baseline | NA |
| Timolol Maleate 0.5% BID | ||||||||
| NCT03697811 (SPECTRUM 5) | III, open-label, single group | 150 | Unknown status | POAG or OHT who are non-/poor responders to Latanoprost | Switching to DE-117 0.002% QD from Latanoprost 0.005% QD | 3 months | IOP change from baseline in Latanoprost low/non-responder subjects | NA |
| NCT03858894 (SPECTRUM 6) | II, randomized, double-masked, parallel group | 98 | Completed | POAG or OHT | Vehicle QD and DE-117 0.002% QD | 6 weeks | IOP at each time point (08:00, 12:00, and 16:00) | Olander et al. J Glaucoma, 2021 |
| DE-117 0.002% BID | Number of subjects with adverse events |
Notes: Data were obtained from the ClinicalTrials.gov website (https://clinicaltrials.gov/ct2/results?cond=&term=DE-117&cntry=&state=&city=&dist=&Search=Search) on January 11, 2022.
Abbreviations: NCT, national clinical trial; n, number of subjects; POAG, primary open-angle glaucoma; OHT, ocular hypertension; IOP, intraocular pressure; QD, quaque die; NA, not applicable; OAG, open-angle glaucoma, BID, bis die sumendum.
Table 2 summarizes the IOP-lowering effect of OMDI ophthalmic solutions in published articles related to clinical trials. For further investigation in a phase III clinical trial program, 3 randomized, masked, controlled, parallel group, multicenter, dose-finding studies (NCT01868126, NCT02179008, and NCT02623738) were consecutively conducted to determine the optimal dose concentrations of OMDI ophthalmic solution in patients with POAG or OHT. As a result, the 0.002% OMDI ophthalmic solution QD was found to have the optimal dose, which demonstrated stable IOP-lowering effects with good tolerance.25 Moreover, a recent phase II clinical trial (SPECTRUM 6) supported that the 0.002% OMDI ophthalmic solution was more favorable when administered QD than it was when administered twice-daily (BID), considering the benefit-risk profile because of the 3 times higher incidence of local tolerability issues (BID: 41.7%; QD: 14.0%) without significant difference in IOP reduction in the BID arm.35
Table 2.
Summary of Efficacy and Patient Tolerability of Omidenepag Isopropyl (DE-117) Ophthalmic Solution in Clinical Trials
| Reference | NCT Number (Trial Feature) | Enrollment Number | Condition | Primary Outcome Measure | Arms (n) | Baseline Mean Diurnal IOP (mmHg) | Results | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Efficacy | Patient Tolerability | ||||||||||
| Mean Diurnal IOP Change from Baseline (mmHg) | Mean Diurnal IOP Reduction Rate from Baseline (%) | AEs, n (%) | Serious AEs, n (%) | AEs Leading to Discontinuation, n (%) | |||||||
| Aihara et al. J Glaucoma, 2019 | NCT01868126 | 91 | POAG or OHT | IOP at each time point and mean diurnal IOP at week 4 | DE-117 0.0003% QD (17) | 24.6 | −3.12 | 12.7 | 0 | 0 | 0 |
| Number of subjects with adverse events | DE-117 0.001% QD (15) | 24.8 | −4.59 | 18.5 | 1 (6.7) | 0 | 0 | ||||
| DE-117 0.002% QD (14) | 24.8 | −7.39 | 29.8 | 9 (64.3) | 0 | 0 | |||||
| DE-117 0.003% QD (15) | 25.8 | −5.99 | 23.2 | 7 (46.7) | 0 | 0 | |||||
| Latanoprost 0.005% QD (15) | 25.5 | −7.77 | 30.5 | 1 (6.7) | 0 | 0 | |||||
| Placebo (15) | 25.5 | −1.94 | 7.6 | 2 (13.3) | 0 | 0 | |||||
| Aihara et al. J Glaucoma, 2019 | NCT02179008 (SEE-1) | 184 | POAG or OHT | IOP at each time point and mean diurnal IOP at month 3 | DE-117 0.0012% QD (32) | 25.1 | −4.17 | 16.6 | 19 (59.4) | 0 | 2 (6.3) |
| Number of subjects with adverse events | DE-117 0.0016% QD (29) | 25.5 | −4.69 | 18.4 | 15 (51.7) | 0 | 0 | ||||
| DE-117 0.002% QD (29) | 25.4 | −5.52 | 21.7 | 15 (51.7) | 1 (3.4) | 1 (3.4) | |||||
| DE-117 0.0025% QD (30) | 25.6 | −5.98 | 23.4 | 19 (63.3) | 1 (3.3) | 1 (3.3) | |||||
| DE-117 0.003% QD (31) | 24.8 | −5.28 | 21.3 | 19 (61.3) | 1 (3.2) | 2 (6.5) | |||||
| Latanoprost 0.005% QD (32) | 25.1 | −6.55 | 26.1 | 17 (53.1) | 0 | 0 | |||||
| Aihara et al. J Glaucoma, 2019 | NCT02623738 (AYAME) | 63 | POAG or OHT | IOP at each time point and mean diurnal IOP change from baseline at week 4 | DE-117 0.002% QD (22) | 23.8 | −5.11 | 21.5 | 9 (40.9) | 0 | 0 |
| Number of subjects with adverse events | DE-117 0.0025% QD (22) | 23.7 | −4.83 | 20.4 | 10 (45.5) | 0 | 1 (4.5) | ||||
| Placebo (19) | 23.4 | −2.18 | 9.3 | 2 (10.5) | 0 | 0 | |||||
| Aihara et al. Am J Ophthalmol, 2020 | NCT02623738 (AYAME) | 190 | POAG or OHT | IOP at each time point and mean diurnal IOP change from baseline at week 4 | DE-117 0.002% QD (94) | 23.78 | −5.93 | 24.9 | 46 (48.9) | 0 | 2 (2.1) |
| Number of subjects with adverse events | Latanoprost 0.005% QD (95) | 23.4 | −6.56 | 28.0 | 26 (27.1) | 0 | 2 (2.1) | ||||
| Aihara et al, Jpn J Ophthalmol, 2020 | NCT02822742 (FUJI) | 26 | POAG or OHT who were non-/poor responders to Latanoprost | Mean diurnal IOP change from baseline at week 4 | Switching to DE-117 0.002% QD from Latanoprost 0.005% QD (26) | 25.0, end of washout; 23.1, end of latanoprost run in | −4.87 from end of washout; −2.99 from end of latanoprost run in | 19.5 from end of washout; 12.9 from end of latanoprost run in | 5 (19.2) | 0 | 0 |
| Aihara et al, Jpn J Ophthalmol, 2021 | NCT02822729 (RENGE) | 125 | OAG or OHT | IOP at each time point and mean diurnal IOP at week 52 | Cohort 1: baseline diurnal IOP of ≥ 16– < 22 mmHg and treated with DE-117 0.002% QD (48) | 18.71 | −3.7 | 19.8 | 38 (79.2) | 3 (6.3) | 8 (16.7) |
| Number of subjects with adverse events | Cohort 2: baseline diurnal IOP of ≥ 22– ≤ 34 mmHg and treated with DE-117 0.002% QD (37) | 24.06 | −5.6 | 23.3 | 27 (73.0) | 1 (2.7) | 1 (2.7) | ||||
| Cohort 3: baseline diurnal IOP of ≥ 22– ≤ 34 mmHg and treated with DE-117 0.002% QD and Timolol 0.5% BID (40) | 23.14 | −8.4 | 36.3 | 37 (92.5) | 1 (2.5) | 7 (17.5) | |||||
| Olander et al. J Glaucoma, 2021 | NCT03858894 (SPECTRUM 6) | 98 | POAG or OHT | IOP at each time point at week 6 | Vehicle QD and DE-117 0.002% QD (50) | 24.55 | −6.18 | 25.2 | 7 (14.0) | 1 (2.0) | 0 |
| Number of subjects with adverse events | DE-117 0.002% BID (48) | 25.39 | −7.62 | 17.8 | 20 (41.7) | 0 | 4 (8.3) | ||||
Abbreviations: NCT, national clinical trial; IOP, intraocular pressure; AE, adverse event; n, number of subjects; POAG, primary open-angle glaucoma; OHT, ocular hypertension; QD, quaque die; OAG, open-angle glaucoma, BID, bis die sumendum.
A series of three clinical trials conducted in Japan have confirmed the IOP-lowering effects of OMDI: (1) the non-inferiority of OMDI over latanoprost (AYAME), (2) the long-term efficacy of OMDI and its additive effect in combination with timolol (RENGE), and (3) the efficacy of OMDI in non-/poor responders to latanoprost (FUJI). A Phase III, randomized, single-masked, active-controlled, parallel group, multicenter, non-inferiority study (AYAME) was conducted to compare the efficacies of treatment with 0.002% OMDI and 0.005% latanoprost ophthalmic solutions QD in patients with POAG and OHT for 4 weeks after a washout period (n = 190).26 At week 4, the mean diurnal IOP significantly decreased by 5.93 mmHg from baseline of 23.78 mmHg (24.9% reduction) in the OMDI group, and by 6.56 mmHg from baseline of 23.40 mmHg (28.0% reduction) in the latanoprost group (Table 2). However, the difference in mean diurnal IOP reduction from baseline to week 4 between two arms was 0.63 (95% CI: 0.01–1.26) mmHg in favor of latanoprost. The IOP reduction achieved with OMDI was found to be non-inferior to that achieved with latanoprost because the difference is within the standard level for non-inferiority (1.5 mmHg). Therefore, OMDI was shown to be the first ophthalmic solution in more than 20 years to show non-inferiority to latanoprost.46 The phase III, 3-month PEONY study, with a larger sample size (n = 370) and a study design similar to that of the AYAME study, has been completed in India, South Korea, Singapore, and Taiwan, and its results await publication. The phase III RENGE study (n = 125) also investigated the long-term, 12-month efficacy of 0.002% OMDI ophthalmic solution QD in patients with OAG and OHT divided into three cohorts.28 OMDI significantly decreased IOP over 52 weeks in a high-baseline IOP group with a baseline diurnal IOP of 22–34 mmHg, a low-baseline IOP group with a baseline diurnal IOP of 16–22 mmHg, and a concomitant-use-of-timolol group with a baseline diurnal IOP of 22 to 34 mmHg (p < 0.001%, respectively). Moreover, the combination therapy of OMDI and timolol resulted in additional IOP-lowering effects, confirming recent basic research results.45 In the phase III FUJI study (n = 24), 0.002% OMDI QD was also effective for patients who had failed to respond to latanoprost. The 4-week treatment with OMDI was well-tolerated and significantly reduced the mean diurnal IOP from baseline in patients who exhibited under 15% IOP reduction following 8-week treatment with latanoprost (non-/poor responders to latanoprost).27 Therefore, OMDI showed good IOP results both in patients with newly introduced or switching treatment. Further phase III studies of non-/poor responders to latanoprost (SPECTRUM 5) with a larger sample size over longer time periods are ongoing in the US.
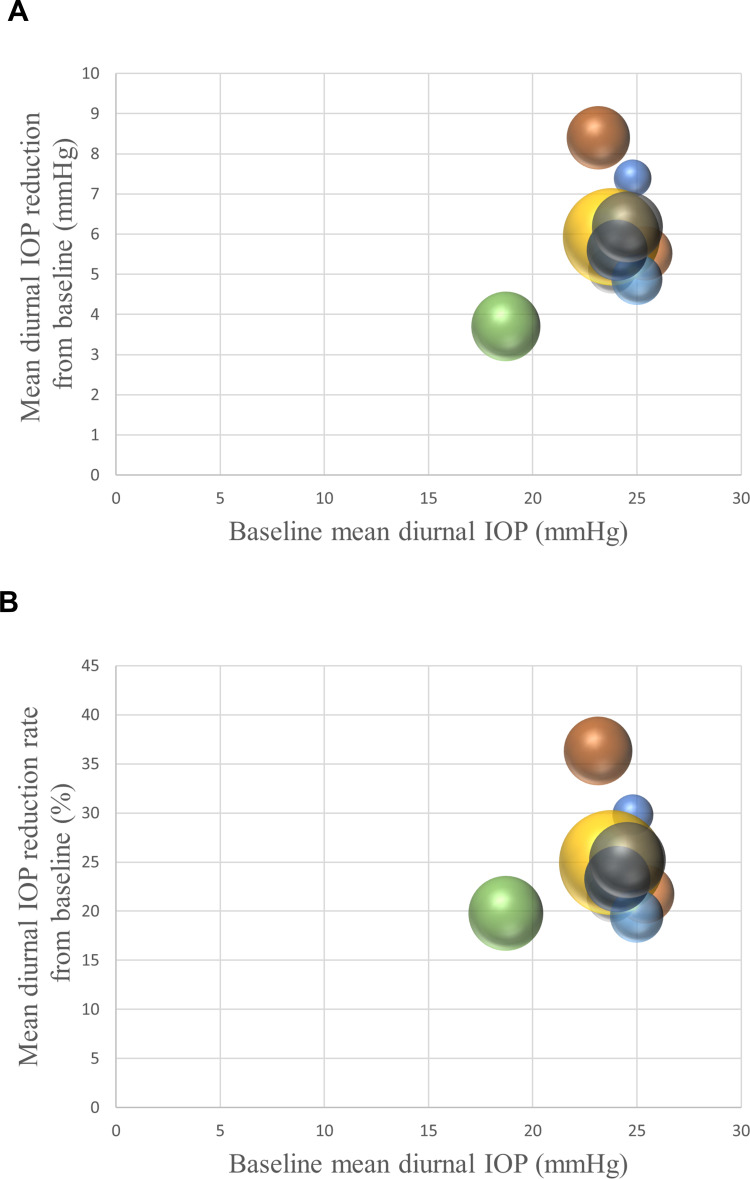
Figure 3 demonstrated the relationships between baseline mean diurnal IOP and mean diurnal IOP reduction or reduction rate from baseline with 0.002% OMDI QD treatment in published articles related to clinical trials using the bubble chart. OMDI was shown to lower mean diurnal IOP by 3.7–7.4 mmHg (20–30%) from baseline. Since OMDI is thought to lower IOP by increasing both trabecular and uveoscleral outflows through EP2 receptor stimulation, the IOP-lowering effects may be influenced by baseline IOP.23,24,44 There appears to be a positive correlation between baseline IOP and IOP reduction or reduction rate in Figure 3. One retrospective study supported this idea, as NTG patients with a mean baseline IOP of 15.7 mmHg showed a significant reduction in IOP with the topical administration of OMDI, but the range of IOP reduction was only 12.0%–12.8%.47 However, this is uncertain due to the insufficiency of randomized clinical trials in eyes with baseline IOP less than 21 mmHg, such as those with NTG. Therefore, further studies are warranted.
Figure 3.
Relationship between baseline mean diurnal IOP and mean diurnal IOP reduction (A) or reduction rate (B) with 0.002% OMDI QD treatment in the published articles related to the clinical trials.
Notes: The bubble size indicates the sample size for each study. Data from Aihara et al25–28 and Olander et al35.
Abbreviations: IOP, intraocular pressure; OMDI, omidenepag isopropyl; QD, quaque die.
Moreover, a prospective, open-label, experimental study demonstrated that 0.002% OMDI ophthalmic solution QD provided stable 24-hour IOP reduction throughout the day and night in OAGs or OHTs, which suggested that OMDI could be useful as a first-line treatment for new patients.48 Another clinically important aspect of the IOP-lowering effect of OMDI is the presence of non-/poor responders, defined as patients with IOP reductions of less than 10% at both 1–2 months and 3–4 months after initiation of administration.47,49 The frequency of non-responder patients with NTG for QD OMDI treatment in a retrospective study was 22.4%, which could be a higher percentage than those for FP receptor agonist treatment.47,49 Additional validation is needed to clarify this; however, careful follow-up is recommended after starting ophthalmic treatment.
Patient Tolerability – OMDI Adverse Events and Patient Adherence
OMDI ophthalmic solution demonstrated acceptable safety and tolerability in 4 to 52 weeks treatment in patients with glaucoma or OHT according to clinical trials.25–28,35 Table 2 summarizes the adverse events (AEs) of OMDI in published articles related to the clinical trials.25–28,35 The frequency of AEs of 0.002% OMDI ophthalmic solution QD varied from 14.0% to 92.5% depending on the report. Similarly, the frequency of serious AEs varied from 0% to 6.3%, and AEs leading to discontinuation varied from 0% to 17.5%. Most ocular AEs were considered to be causally related to the study drug, and less frequently reported non-ocular AEs were considered unrelated to the study medication.25–28,35 Additionally, the serious AEs reported were all considered to be unrelated to the treatment.25–28,35 The ocular AEs leading to study withdrawal were of mild or moderate severity, including eye pain/foreign body sensation, photophobia, blurred vision, conjunctival hyperemia/conjunctivitis, macular edema/cystoid macular edema (ME/CME), and iritis with anterior chamber cells.25–28,35 All the cases resolved or were resolving during the study or following study withdrawal.25–28,35
Table 3 summarizes major AEs with a frequency of 1% or more associated with the administration of 0.002% OMDI ophthalmic solution QD in the published articles related to clinical trials.25–28,35 Overall, in patients receiving 0.002% OMDI ophthalmic solution QD (n = 360), AEs were observed in 53.6% (n = 193). The most frequently reported ocular AE was conjunctival hyperemia, which occurred in 19.7% (n = 71) of patients, followed by corneal thickening, which occurred in 5.0% (n = 18). The frequency of ME/CME which was the most common reason for study discontinuation in the RENGE study, being 4.4% (n = 16).28 The frequency of subjective symptoms was eye pain in 3.6% (n = 13), photophobia in 3.3% (n = 12), and blurred vision in 1.7% (n = 6). Anterior chamber cells were identified in 1.4% (n = 5) of subjects, while iritis with anterior chamber cells was only observed in 0.8% (n = 3). It is possible that these frequencies are underestimated, as not all clinical trials reported the presence or absence of all AEs. However, it should be useful to have an overview of the relatively frequent AEs that can occur with once-daily topical administration of 0.002% OMDI. Clinically, OMDI-related conjunctival hyperemia has a relatively high frequency, and concomitant OMDI and timolol treatment results in an increased incidence of conjunctival hyperemia.28 However, it should not be of large concern, as long as an adequate explanation is provided before prescribing, because it is always mild and weaker than that induced by 0.4% ripasudil and it usually settles down with time, as it does with FP agonists.50,51 Moreover, the average increase in corneal thickness with OMDI is mild, less than 20 µm, which is within the limits of the diurnal variation in corneal thickness, and has no clinical significance related to visual acuity or IOP measurements in most cases.28 However, corneal thickening could be a problem for those with a thinner cornea, for example due to a history of LASIK, because some may complain of decreased naked eye visual acuity with OMDI treatment. In addition, the drug should be discontinued or changed if subjective symptoms are severe, or if there are complications of ME/CME or iritis that may lead to vision loss. As a special note, in the RENGE study, all cases of OMDI-related ME/CME occurred in eyes with intraocular lens (IOL) implants, and the incidence of ME/CME in pseudophakia was almost half.28 Therefore, OMDI has been contraindicated in patients with pseudophakia or aphakia.28,52 Moreover, it should also be administered cautiously to patients with iritis or uveitis, which could show increased risk of CME.52,53 It should be noted that the use of OMDI is contraindicated in patients who are allergic to any of its components or who are receiving tafluprost, a FP agonist due to the possibility of it causing ocular inflammation.52,53 Therefore, it is advisable to avoid the concomitant use of OMDI and other FP agonist eye drops.52 Moreover, since there are no clinical reports on the concomitant use of OMDI with glaucoma eye drops other than timolol, it is recommended that OMDI should be used with caution until its safety is confirmed.52 Future clinical studies will be needed to determine the efficacies and AEs of OMDI in combination with other glaucoma ophthalmic solutions. Additional information should be provided by the post-marketing surveillance of OMDI.
Table 3.
Summary of Major Adverse Events Associated with the Topical Administration of 0.002% Omidenepag Isopropyl Ophthalmic Solution Once-Daily in Clinical Trials
| Reference | NCT Number (Trial Feature) | Subjects Analyzed, n | Major AEs Occurring at a Frequency of 1% or More | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Conjunctival Hyperemia, n (%) | Corneal Thickening, n (%) | Macular Edema/Cystoid Macular Edema, n (%) | Eye Pain, n (%) | Photophobia, n (%) | Blurred Vision, n (%) | Cells in the Anterior Chamber, n (%) | |||
| Total | 360 | 71 (19.7) | 18 (5.0) | 16 (4.4) | 13 (3.6) | 12 (3.3) | 6 (1.7) | 5 (1.4) | |
| Aihara et al. J Glaucoma, 2019 | NCT01868126 | 14 | 2 (14.3) | NA | NA | 2 (14.3) | 2 (14.3) | NA | NA |
| Aihara et al. J Glaucoma, 2019 | NCT02179008 (SEE-1) | 29 | 5 (17.2) | NA | NA | 2 (6.9) | 4 (13.8) | NA | NA |
| Aihara et al. J Glaucoma, 2019 | NCT02623738 (AYAME) | 22 | 5 (22.7) | 2 (9.1) | NA | NA | NA | NA | NA |
| Aihara et al. Am J Ophthalmol, 2020 | NCT02623738 (AYAME) | 94 | 23 (24.5) | 11 (11.7) | NA | 4 (4.3) | 4 (4.3) | 2 (2.1) | NA |
| Aihara et al, Jpn J Ophthalmol, 2020 | NCT02822742 (FUJI) | 26 | 2 (7.7) | NA | NA | NA | NA | NA | 2 (7.7) |
| Aihara et al, Jpn J Ophthalmol, 2021 | NCT02822729 (RENGE, Cohort 1) | 48 | 8 (16.7) | 0 (0.0) | 8 (16.7) | 2 (4.2) | NA | 1 (2.1) | 2 (4.2) |
| Aihara et al, Jpn J Ophthalmol, 2021 | NCT02822729 (RENGE, Cohort 2) | 37 | 8 (21.6) | 2 (5.4) | 2 (5.4) | 0 (0.0) | NA | 1 (2.7) | 0 (0.0) |
| Aihara et al, Jpn J Ophthalmol, 2021 | NCT02822729 (RENGE, Cohort 3) | 40 | 18 (45.0) | 3 (7.5) | 6 (15.0) | 3 (7.5) | NA | 2 (5.0) | 1 (2.5) |
| Olander et al. J Glaucoma, 2021 | NCT03858894 (SPECTRUM 6) | 50 | 0 (0.0) | NA | NA | NA | 2 (4.0) | NA | NA |
Abbreviations: NCT, national clinical trial; AE, adverse event; n, number of subjects; NA, not applicable.
As above, OMDI is usually well-tolerated and should have good treatment adherence because of the QD dosing regimen, as is the case with FP agonists.25,34,36,54 In fact, OMDI treatment compliance rates and dosing adherence determinations for 1 or 3 months were reported to be high (≥75%) in a clinical trial.25 A post-marketing retrospective study of 0.002% OMDI ophthalmic solution also demonstrated that the early persistence of OMDI was good, and initial OMDI monotherapy does not largely differ from initial latanoprost monotherapy.55 Additionally, the OMDI discontinuation rate until 3 months was 22% in total, because of an insufficient IOP-lowering efficiency (11%), followed by conjunctival hyperemia (4%) and visual acuity disturbance (2%).55 Therefore, clinicians should also be cautious of early failure in OMDI treatment, and patients should be informed of the risks and benefits of treatment with OMDI to increase the likelihood of long-term adherence to self-medication with eye drops.
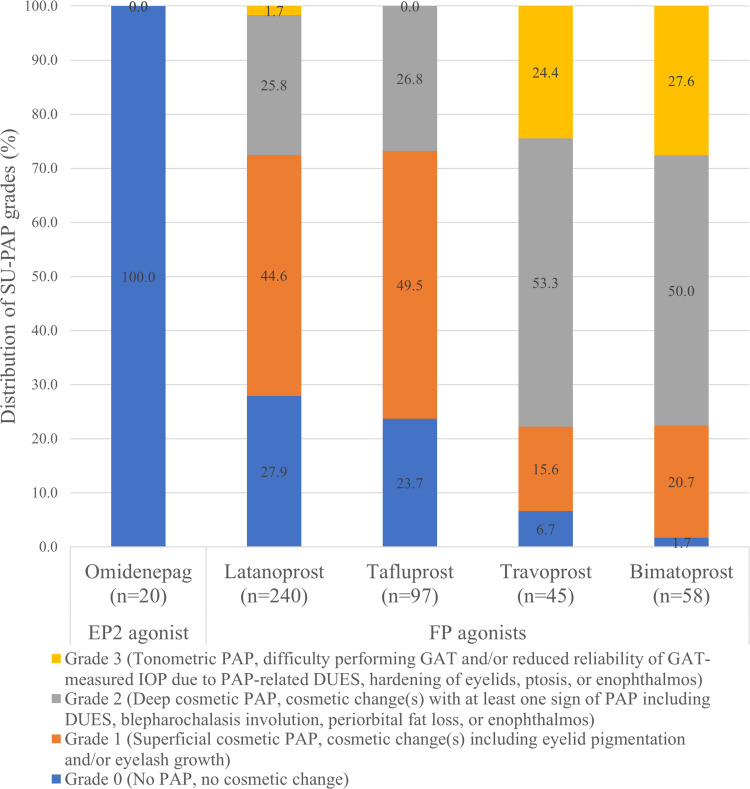
Additionally, it is clinically important to note that OMDI has no or little ocular PAP side effects, including eyelid pigmentation, eyelash growth, and DUES, because of the no binding affinity to the FP receptor.15,56 Previously, a grading or subjective measurement of PAP had been attempted for each PAP component such as conjunctival hyperemia, eyelash changes, and DUES. However, these measurements seem to be too complex to use in real-world situations and do not take into account the underlying mechanisms and difficulty of measuring IOP.15,57–59 Therefore, we recently constructed an in-house PAP grading system (Shimane University PAP Grading System, SU-PAP) which considers the mechanisms involved in the development of cosmetic PAPs (ie, superficial and deep), and the effect of PAP in glaucoma management (ie, difficult IOP measurements).15 SU-PAP can classify PAP into four grades, allowing for the assessment of its progression. (Figure 4). Moreover, in previous clinical trials, none of the eyes treated with OMDI showed PAP,25–28,35 and patients who were switched from conventional PGF2α analogs (FP agonists) to OMDI showed significant improvements in PAP signs including DUES over time without significant IOP changes.60–62 Currently, the results of long-term adherence of OMDI treatment are unknown. However, since OMDI does not induce PAP, it may not only be useful in the treatment of patients with PAP or those concerned by it, but also achieve a better long-term adherence than that of FP agonists.
Figure 4.
Distribution of PAP grade in each prostanoid receptor agonist using patient groups as assessed by SU-PAP, which considers mechanisms involved in the development of cosmetic PAP and effects of PAP in glaucoma management. SU-PAP can classify PAP into four levels, allowing for the assessment of PAP progression. OMDI did not cause PAP.15
Note: Data from Tanito et al.
Abbreviations: PAP, prostaglandin-associated periorbitopathy; SU-PAP, Shimane University PAP Grading System; OMDI, omidenepag isopropyl.
Case of OMDI-Related Macular Edema
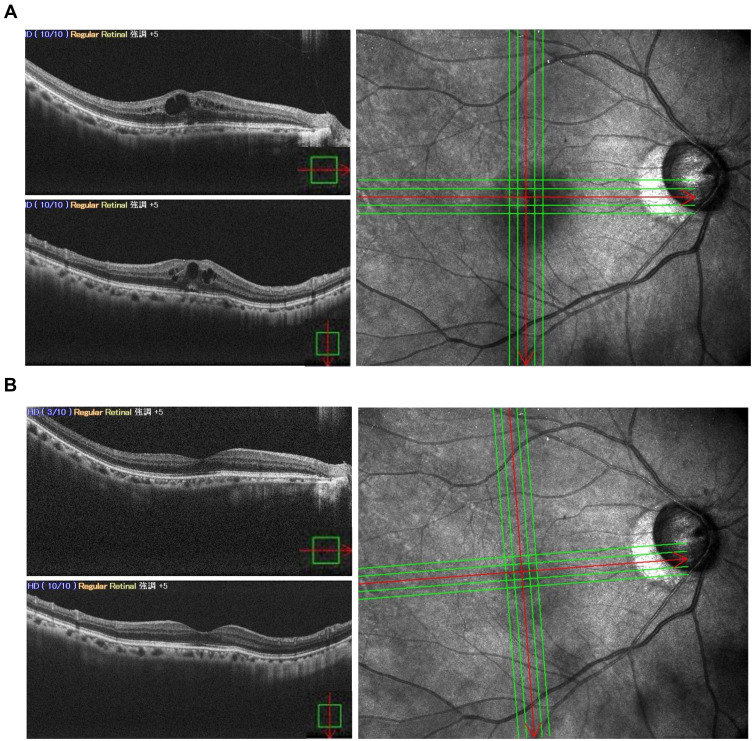
The mechanism of ME/CME and iritis caused by OMDI has not been elucidated to date. However, considering that PGE2 could cause ocular inflammatory reactions similar to those caused by PGF2α and all OMDI-related ME/CME cases occurred in IOL-implanted eyes in the clinical trial, EP2 stimulation may cause them when the blood-aqueous or blood-retinal barriers in the treated eye had been damaged.13,53,63–65 Thus, the effectiveness and safety of OMDI in secondary glaucoma subtypes, in which the eyes received ocular surgery or were inflicted with other retinal diseases, should be closely evaluated. We experienced a case of ME/CME associated with the accidental usage of OMDI in patients with glaucoma after uncomplicated cataract surgery and successfully treated with sub-Tenon triamcinolone injection (STTA) and sub-Tenon injection of corticosteroids. Consent to publish the case report was obtained. This report does not contain any personal information that could lead to the identification of the patient.
A 64-year-old Japanese female patient was referred to the Matsue Red Cross Hospital with a complaint of blurred vision in the right eye. Her general clinical history was unremarkable. She had been taking 2% carteolol, a nonselective β-adrenoceptor antagonist ophthalmic solution, QD for ocular hypertension and underwent uneventful cataract surgery with posterior chamber IOL implantation in her right eye 3 years before the onset of symptoms. She was prescribed and started using 0.002% OMDI ophthalmic solution QD additionally for 5 months before the onset. The best-corrected visual acuity (BCVA) was 20/25 and the IOP was 10 mmHg at initial examination. The anterior and posterior chambers were clear, and the anterior chamber flare was 17.9 pc/msec. Fundoscopy and spectral domain optical coherence tomography (SD-OCT) revealed a CME with serous retinal detachment (SRD) (Figure 5A). The patient wanted to improve her symptoms promptly, so OMDI was discontinued, and the patient was treated with STTA 20mg on the same day. After two months, an improvement in subjective symptoms was obtained, and the BCVA improved to 20/20. The IOP was 12 mmHg, and the flare was 10.7 pc/msec. SD-OCT showed resolution of the CME with SRD (Figure 5B). The case has been recurrence-free for 3 months, and the IOP has been controlled at 10–12 mmHg.
Figure 5.
(A) Five months after starting 0.002% OMDI QD treatment, CME with SRD in the right pseudophakic eye was shown on SD-OCT. There was no pathology that could cause CME other than the use of OMDI for IOL-implanted eyes. The discontinuation of OMDI and administration of STTA was decided. (B) Two months later, the recovery of CME with SRD was observed. The horizontal and vertical red arrows indicate the cross-sectional line scans in 5A and 5B.
Abbreviations: OMDI, omidenepag isopropyl; QD, quaque die; CME, cystoid macular edema; SRD, serous retinal detachment; SD-OCT, spectral domain optical coherence tomography; IOL, intraocular lens; STTA, sub-Tenon triamcinolone injection.
In the RENGE study, all cases of OMDI-related ME/CME were mild or moderate in severity, and after discontinuation of OMDI treatment, standard treatment with topical nonsteroidal anti-inflammatory drugs or steroids was successful, and the time from onset of ME/CME to recovery was reported to be 89.5 days.13,28 It is well-known that the topical administration of FP agonists after cataract surgery can lead to CME, which is usually treated with the discontinuation of eye drops, corticosteroid eye drops, and nonsteroidal anti-inflammatory drugs (NSAIDs), although the recovery can require 1 month or more.53,63–65 One case report demonstrated that STTA resulted in the early disappearance of CME associated with prostaglandin treatment after cataract surgery.63 Therefore, we selected STTA for OMDI-related ME/CME treatment, which resulted in a relatively early improvement of ME/CME and subjective symptoms. Moreover, since the CME decreased relatively early after sub-Tenon injection of corticosteroids and the flare value also decreased, some inflammation may have been involved in the development of OMDI-related ME/CME.
Conclusion
OMDI is the world’s first commercially available selective EP2 receptor agonist, which promotes aqueous humor outflow via the trabecular and uveoscleral outflow pathways. The once-daily topical administration of 0.002% OMDI possesses IOP-lowering effects non-inferior to those of latanoprost QD, without PAP complications. OMDI was also effective and well-tolerated in non-/poor responders to latanoprost in short term. Furthermore, it also showed a stable IOP-lowering effect for a long period, and its concomitant use with timolol enhanced the IOP-lowering effect. Moreover, OMDI demonstrated acceptable safety and tolerability with good adherence, and can be used in almost every patient. However, OMDI shows some AEs such as conjunctival hyperemia, corneal thickening, ME/CME, and ocular inflammation. Especially, since there are no clinical reports on the concomitant use of OMDI with glaucoma eye drops other than timolol, it is recommended that OMDI should be used with caution until safety is confirmed. Additionally, OMDI is contraindicated in patients who are allergic to the product, in aphakic or pseudophakic eyes, and in combination with tafluprost eye drops. If used appropriately in the right patients, OMDI could be an effective treatment option for glaucoma and OHT as a first-line alternative to FP agonists.
Funding Statement
There is no funding to report.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Santen Pharmaceutical Co., Ltd. provided information on the status of latest clinical trials of OMDI and assisted us in the preparation of Figures 1 and 2. No involvement was made in the writing or reviewing. The authors report no conflicts of interest in this work.
References
- 1.Bourne RR, Taylor HR, Flaxman SR, et al. Number of people blind or visually impaired by glaucoma worldwide and in world regions 1990–2010: a meta-analysis. PLoS One. 2016;11(10):e0162229. doi: 10.1371/journal.pone.0162229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Global Health. 2017;5(12):e1221–e34. doi: 10.1016/S2214-109X(17)30393-5 [DOI] [PubMed] [Google Scholar]
- 3.Steinmetz JD, Bourne RRA, Briant PS, Causes of blindness and vision impairment in. 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the right to sight: an analysis for the global burden of disease study. Lancet Global Health. 2021;9(2):e144–e60. doi: 10.1016/S2214-109X(20)30489-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuo M, Fukuda H, Omura T, Tanito M. Agreement in glaucoma type diagnosis between referring and referred ophthalmologists. Graefe’s Arch Clin Exp Ophthalmol. 2021;4:1–2. [DOI] [PubMed] [Google Scholar]
- 5.Ernest PJ, Schouten JS, Beckers HJ, Hendrikse F, Prins MH, Webers CA. An evidence-based review of prognostic factors for glaucomatous visual field progression. Ophthalmology. 2013;120(3):512–519. doi: 10.1016/j.ophtha.2012.09.005 [DOI] [PubMed] [Google Scholar]
- 6.Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268–1279. doi: 10.1001/archopht.120.10.1268 [DOI] [PubMed] [Google Scholar]
- 7.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–13; discussion 829–30. doi: 10.1001/archopht.120.6.829 [DOI] [PubMed] [Google Scholar]
- 8.Garway-Heath DF, Crabb DP, Bunce C, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;385(9975):1295–1304. doi: 10.1016/S0140-6736(14)62111-5 [DOI] [PubMed] [Google Scholar]
- 9.Camras CB, Bito LZ, Eakins KE. Reduction of intraocular pressure by prostaglandins applied topically to the eyes of conscious rabbits. Invest Ophthalmol Vis Sci. 1977;16(12):1125–1134. [PubMed] [Google Scholar]
- 10.Camras CB, Siebold EC, Lustgarten JS, et al. Maintained reduction of intraocular pressure by prostaglandin F2 alpha-1-isopropyl ester applied in multiple doses in ocular hypertensive and glaucoma patients. Ophthalmology. 1989;96(9):1329–36; discussion 36–7. doi: 10.1016/S0161-6420(89)32717-5 [DOI] [PubMed] [Google Scholar]
- 11.Camras CB, Schumer RA, Marsk A, et al. Intraocular pressure reduction with PhXA34, a new prostaglandin analogue, in patients with ocular hypertension. Arch Ophthalmol. 1992;110(12):1733–1738. doi: 10.1001/archopht.1992.01080240073034 [DOI] [PubMed] [Google Scholar]
- 12.Klimko PG, Sharif NA. Discovery, characterization and clinical utility of prostaglandin agonists for the treatment of glaucoma. Br J Pharmacol. 2019;176(8):1051–1058. doi: 10.1111/bph.14327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aihara M. Prostanoid receptor agonists for glaucoma treatment. Jpn J Ophthalmol. 2021;65(5):581–590. doi: 10.1007/s10384-021-00844-6 [DOI] [PubMed] [Google Scholar]
- 14.Winkler NS, Fautsch MP. Effects of prostaglandin analogues on aqueous humor outflow pathways. J Ocular Pharmacol Ther. 2014;30(2–3):102–109. doi: 10.1089/jop.2013.0179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanito M, Ishida A, Ichioka S, et al. Proposal of a simple grading system integrating cosmetic and tonometric aspects of prostaglandin-associated periorbitopathy. Medicine. 2021;100(34):e26874. doi: 10.1097/MD.0000000000026874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakata R, Chang PY, Sung KR, et al. Prostaglandin-associated periorbitopathy syndrome (PAPS): addressing an unmet clinical need. Semin Ophthalmol;2021. 1–8. doi: 10.1080/08820538.2021.2003824 [DOI] [PubMed] [Google Scholar]
- 17.Miki T, Naito T, Fujiwara M, et al. Effects of pre-surgical administration of prostaglandin analogs on the outcome of trabeculectomy. PLoS One. 2017;12(7):e0181550. doi: 10.1371/journal.pone.0181550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choplin N, Bernstein P, Batoosingh AL, Whitcup SM. A randomized, investigator-masked comparison of diurnal responder rates with bimatoprost and latanoprost in the lowering of intraocular pressure. Surv Ophthalmol. 2004;49(Suppl 1:):S19–25. doi: 10.1016/j.survophthal.2003.12.016 [DOI] [PubMed] [Google Scholar]
- 19.Ikeda Y, Mori K, Ishibashi T, Naruse S, Nakajima N, Kinoshita S. Latanoprost nonresponders with open-angle glaucoma in the Japanese population. Jpn J Ophthalmol. 2006;50(2):153–157. doi: 10.1007/s10384-005-0293-x [DOI] [PubMed] [Google Scholar]
- 20.Saeki T, Ota T, Aihara M, Araie M. Effects of prostanoid EP agonists on mouse intraocular pressure. Invest Ophthalmol Vis Sci. 2009;50(5):2201–2208. doi: 10.1167/iovs.08-2800 [DOI] [PubMed] [Google Scholar]
- 21.Woodward DF, Bogardus AM, Donello JE, et al. Molecular characterization and ocular hypotensive properties of the prostanoid EP2 receptor. J Ocular Pharmacol Ther. 1995;11(3):447–454. doi: 10.1089/jop.1995.11.447 [DOI] [PubMed] [Google Scholar]
- 22.Nilsson SF, Drecoll E, Lütjen-Drecoll E, et al. The prostanoid EP2 receptor agonist butaprost increases uveoscleral outflow in the cynomolgus monkey. Invest Ophthalmol Vis Sci. 2006;47(9):4042–4049. doi: 10.1167/iovs.05-1627 [DOI] [PubMed] [Google Scholar]
- 23.Kirihara T, Taniguchi T, Yamamura K, et al. Pharmacologic characterization of omidenepag isopropyl, a novel selective EP2 receptor agonist, as an ocular hypotensive agent. Invest Ophthalmol Vis Sci. 2018;59(1):145–153. doi: 10.1167/iovs.17-22745 [DOI] [PubMed] [Google Scholar]
- 24.Fuwa M, Toris CB, Fan S, et al. Effects of a novel selective EP2 receptor agonist, omidenepag isopropyl, on aqueous humor dynamics in laser-induced ocular hypertensive monkeys. J Ocular Pharmacol Ther. 2018;34(7):531–537. doi: 10.1089/jop.2017.0146 [DOI] [PubMed] [Google Scholar]
- 25.Aihara M, Lu F, Kawata H, et al. Phase 2, randomized, dose-finding studies of omidenepag isopropyl, a selective EP2 agonist, in patients with primary open-angle glaucoma or ocular hypertension. J Glaucoma. 2019;28(5):375–385. doi: 10.1097/IJG.0000000000001221 [DOI] [PubMed] [Google Scholar]
- 26.Aihara M, Lu F, Kawata H, Iwata A, Odani-Kawabata N, Shams NK. Omidenepag isopropyl versus latanoprost in primary open-angle glaucoma and ocular hypertension: the Phase 3 AYAME Study. Am J Ophthalmol. 2020;220:53–63. doi: 10.1016/j.ajo.2020.06.003 [DOI] [PubMed] [Google Scholar]
- 27.Aihara M, Ropo A, Lu F, et al. Intraocular pressure-lowering effect of omidenepag isopropyl in latanoprost non-/low-responder patients with primary open-angle glaucoma or ocular hypertension: the FUJI study. Jpn J Ophthalmol. 2020;64(4):398–406. doi: 10.1007/s10384-020-00748-x [DOI] [PubMed] [Google Scholar]
- 28.Aihara M, Lu F, Kawata H, Iwata A, Odani-Kawabata N. Twelve-month efficacy and safety of omidenepag isopropyl, a selective EP2 agonist, in open-angle glaucoma and ocular hypertension: the RENGE study. Jpn J Ophthalmol. 2021;65:810–819. doi: 10.1007/s10384-021-00868-y [DOI] [PubMed] [Google Scholar]
- 29.Prasanna G, Carreiro S, Anderson S, et al. Effect of PF-04217329 a prodrug of a selective prostaglandin EP(2) agonist on intraocular pressure in preclinical models of glaucoma. Exp Eye Res. 2011;93(3):256–264. doi: 10.1016/j.exer.2011.02.015 [DOI] [PubMed] [Google Scholar]
- 30.Schachar RA, Raber S, Courtney R, Zhang M. A phase 2, randomized, dose-response trial of taprenepag isopropyl (PF-04217329) versus latanoprost 0.005% in open-angle glaucoma and ocular hypertension. Curr Eye Res. 2011;36(9):809–817. doi: 10.3109/02713683.2011.593725 [DOI] [PubMed] [Google Scholar]
- 31.Yanochko GM, Affolter T, Eighmy JJ, et al. Investigation of ocular events associated with taprenepag isopropyl, a topical EP2 agonist in development for treatment of glaucoma. J Ocular Pharmacol Ther. 2014;30(5):429–439. doi: 10.1089/jop.2013.0222 [DOI] [PubMed] [Google Scholar]
- 32.Bertrand JA, Woodward DF, Sherwood JM, Wang JW, Overby DR. The role of EP(2) receptors in mediating the ultra-long-lasting intraocular pressure reduction by JV-GL1. Br J Ophthalmol. 2021;105(11):1610–1616. doi: 10.1136/bjophthalmol-2020-317762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwamura R, Tanaka M, Okanari E, et al. Identification of a selective, non-prostanoid EP2 receptor agonist for the treatment of glaucoma: omidenepag and its prodrug omidenepag isopropyl. J Med Chem. 2018;61(15):6869–6891. doi: 10.1021/acs.jmedchem.8b00808 [DOI] [PubMed] [Google Scholar]
- 34.Aihara M, Lu F, Kawata H, et al. Pharmacokinetics, safety, and intraocular pressure-lowering profile of omidenepag isopropyl, a selective, nonprostaglandin, prostanoid EP2 receptor agonist, in healthy Japanese and caucasian volunteers (Phase I Study). J Ocular Pharmacol Ther. 2019;35(10):542–550. doi: 10.1089/jop.2019.0044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olander KW, Sato MA, Abrams MA, et al. A randomized phase 2 trial comparing omidenepag isopropyl 0.002% once and twice daily in subjects with primary open-angle glaucoma or ocular hypertension (SPECTRUM-6). J Glaucoma. 2021;30(6):473–480. doi: 10.1097/IJG.0000000000001836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prum BE Jr, Rosenberg LF, Gedde SJ, et al. Primary open-angle glaucoma preferred practice pattern((R)) guidelines. Ophthalmology. 2016;123(1):P41–p111. doi: 10.1016/j.ophtha.2015.10.053 [DOI] [PubMed] [Google Scholar]
- 37.Müskens RP, Wolfs RC, Witteman JC, et al. Topical beta-blockers and mortality. Ophthalmology. 2008;115(11):2037–2043. doi: 10.1016/j.ophtha.2008.07.004 [DOI] [PubMed] [Google Scholar]
- 38.Impagnatiello F, Bastia E, Almirante N, et al. Prostaglandin analogues and nitric oxide contribution in the treatment of ocular hypertension and glaucoma. Br J Pharmacol. 2019;176(8):1079–1089. doi: 10.1111/bph.14328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto Y, Taniguchi T, Inazumi T, et al. Effects of the selective EP2 receptor agonist omidenepag on adipocyte differentiation in 3T3-L1 cells. J Ocular Pharmacol Ther. 2020;36(3):162–169. doi: 10.1089/jop.2019.0079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ida Y, Hikage F, Umetsu A, Ida H, Ohguro H. Omidenepag, a non-prostanoid EP2 receptor agonist, induces enlargement of the 3D organoid of 3T3-L1 cells. Sci Rep. 2020;10(1):16018. doi: 10.1038/s41598-020-72538-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esaki Y, Katsuta O, Kamio H, et al. The antiglaucoma agent and EP2 receptor agonist omidenepag does not affect eyelash growth in mice. J Ocular Pharmacol Ther. 2020;36(7):529–533. doi: 10.1089/jop.2020.0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hikage F, Ida Y, Ouchi Y, Watanabe M, Ohguro H. Omidenepag, a selective, prostanoid EP2 agonist, does not suppress adipogenesis in 3D organoids of human orbital fibroblasts. Transl Vis Sci Technol. 2021;10(4):6. doi: 10.1167/tvst.10.4.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ichioka H, Ida Y, Watanabe M, Ohguro H, Hikage F. Prostaglandin F2α and EP2 agonists, and a ROCK inhibitor modulate the formation of 3D organoids of Grave’s orbitopathy related human orbital fibroblasts. Exp Eye Res. 2021;205:108489. doi: 10.1016/j.exer.2021.108489 [DOI] [PubMed] [Google Scholar]
- 44.Nakamura N, Honjo M, Yamagishi R, Igarashi N, Sakata R, Aihara M. Effects of selective EP2 receptor agonist, omidenepag, on trabecular meshwork cells, Schlemm’s canal endothelial cells and ciliary muscle contraction. Sci Rep. 2021;11(1):16257. doi: 10.1038/s41598-021-95768-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuwa M, Shimazaki A, Odani-Kawabata N, et al. Additive intraocular pressure-lowering effects of a novel selective EP2 receptor agonist, omidenepag isopropyl, combined with existing antiglaucoma agents in conscious ocular normotensive monkeys. J Ocular Pharmacol Ther. 2021;37(4):223–229. doi: 10.1089/jop.2020.0071 [DOI] [PubMed] [Google Scholar]
- 46.Woodward DF, Wang JW, Stamer WD, Lütjen-Drecoll E, Krauss AH, Toris CB. Antiglaucoma EP(2) agonists: a long road that led somewhere. J Ocular Pharmacol Ther. 2019;35(9):469–474. doi: 10.1089/jop.2019.0041 [DOI] [PubMed] [Google Scholar]
- 47.Inoue K, Inoue J, Kunimatsu-Sanuki S, et al. Short-term efficacy and safety of omidenepag isopropyl in patients with normal-tension glaucoma. Clin Ophthalmol. 2020;14:2943–2949. doi: 10.2147/OPTH.S271789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shiratori N, Nishio Y, Takeda A, et al. Twenty-four-hour intraocular pressure control with omidenepag isopropyl 0.002% in patients with glaucoma and ocular hypertension. Clin Ophthalmol. 2021;15:3997–4003. doi: 10.2147/OPTH.S333042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inoue K, Setogawa A, Tomita G. Nonresponders to prostaglandin analogs among normal-tension glaucoma patients. J Ocular Pharmacol Ther. 2016;32(2):90–96. doi: 10.1089/jop.2015.0086 [DOI] [PubMed] [Google Scholar]
- 50.Terao E, Nakakura S, Fujisawa Y, et al. Time course of conjunctival hyperemia induced by omidenepag isopropyl ophthalmic solution 0.002%: a pilot, comparative study versus ripasudil 0.4. BMJ Open Ophthalmol. 2020;5(1):e000538. doi: 10.1136/bmjophth-2020-000538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Honrubia F, García-Sánchez J, Polo V, de la Casa JM, Soto J. Conjunctival hyperaemia with the use of latanoprost versus other prostaglandin analogues in patients with ocular hypertension or glaucoma: a meta-analysis of randomised clinical trials. Br J Ophthalmol. 2009;93(3):316–321. doi: 10.1136/bjo.2007.135111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santen Pharmaceutical. EYBELIS ophthalmic solution 0.002%: prescribing information [Japanese]; 2018. Available from: https://www.santen.co.jp/medical-channel/di/tenpu/DD067_eybelis.pdf. Accessed January 9, 2022.
- 53.Holló G, Aung T, Cantor LB, Aihara M. Cystoid macular edema related to cataract surgery and topical prostaglandin analogs: mechanism, diagnosis, and management. Surv Ophthalmol. 2020;65(5):496–512. doi: 10.1016/j.survophthal.2020.02.004 [DOI] [PubMed] [Google Scholar]
- 54.European Glaucoma Society Terminology and Guidelines for Glaucoma. 4th edition Chapter 3: treatment principles and options supported by the EGS foundation: part 1: foreword; introduction; glossary; Chapter 3 treatment principles and options. Br J Ophthalmol. 2017;101(6):130–195. doi: 10.1136/bjophthalmol-2016-EGSguideline.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakakura S, Kanamori A, Fukuma Y, Wakabayashi S, Nagata Y, Adachi M. Evaluation of early medication persistence with omidenepag isopropyl, a topical selective prostaglandin EP2 agonist, in patients with glaucoma: a retrospective two-institute study. BMJ open. 2021;11(1):e040301. doi: 10.1136/bmjopen-2020-040301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inoue K, Shiokawa M, Katakura S, et al. Periocular adverse reactions to omidenepag isopropyl: periocular adverse reactions to omidenepag isopropyl. Am J Ophthalmol. 2021;237:114–121. doi: 10.1016/j.ajo.2021.12.011 [DOI] [PubMed] [Google Scholar]
- 57.Alm A, Stjernschantz J. Effects on intraocular pressure and side effects of 0.005% latanoprost applied once daily, evening or morning. A comparison with timolol. Scandinavian latanoprost study group. Ophthalmology. 1995;102(12):1743–1752. doi: 10.1016/S0161-6420(95)30798-1 [DOI] [PubMed] [Google Scholar]
- 58.Johnstone MA. Hypertrichosis and increased pigmentation of eyelashes and adjacent hair in the region of the ipsilateral eyelids of patients treated with unilateral topical latanoprost. Am J Ophthalmol. 1997;124(4):544–547. doi: 10.1016/S0002-9394(14)70870-0 [DOI] [PubMed] [Google Scholar]
- 59.Kim HW, Choi YJ, Lee KW, Lee MJ. Periorbital changes associated with prostaglandin analogs in Korean patients. BMC Ophthalmol. 2017;17(1):126. doi: 10.1186/s12886-017-0521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakakura S, Terao E, Fujisawa Y, Tabuchi H, Kiuchi Y. Changes in prostaglandin-associated periorbital syndrome after switch from conventional prostaglandin F2α treatment to omidenepag isopropyl in 11 consecutive patients. J Glaucoma. 2020;29(4):326–328. doi: 10.1097/IJG.0000000000001442 [DOI] [PubMed] [Google Scholar]
- 61.Oogi S, Nakakura S, Terao E, Fujisawa Y, Tabuchi H, Kiuchi Y. One-year follow-up study of changes in prostaglandin-associated periorbital syndrome after switch from conventional prostaglandin f2alfa to omidenepag isopropyl. Cureus. 2020;12(8):e10064. doi: 10.7759/cureus.10064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sakata R, Fujishiro T, Saito H, et al. Recovery of deepening of the upper eyelid sulcus after switching from prostaglandin FP receptor agonists to EP2 receptor agonist: a 3-month prospective analysis. Jpn J Ophthalmol. 2021;65(5):591–597. doi: 10.1007/s10384-021-00855-3 [DOI] [PubMed] [Google Scholar]
- 63.Matsuura K, Sasaki S, Uotani R. Successful treatment of prostaglandin-induced cystoid macular edema with subtenon triamcinolone. Clin Ophthalmol. 2012;6:2105–2108. doi: 10.2147/OPTH.S39542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sacchi M, Villani E, Gilardoni F, Nucci P. Efficacy of intravitreal dexamethasone implant for prostaglandin-induced refractory pseudophakic cystoid macular edema: case report and review of the literature. Clin Ophthalmol. 2014;8:1253–1257. doi: 10.2147/OPTH.S63829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Makri OE, Tsapardoni FN, Plotas P, Ifantis N, Xanthopoulou PT, Georgakopoulos CD. Cystoid macular edema associated with preservative-free latanoprost after uncomplicated cataract surgery: case report and review of the literature. BMC Res Notes. 2017;10(1):127. doi: 10.1186/s13104-017-2448-5 [DOI] [PMC free article] [PubMed] [Google Scholar]