Abstract
Background
A standard treatment regimen for advanced non‐small cell lung cancer (NSCLC) patients with interstitial lung disease (ILD) has not been established since most clinical trials exclude such patients because of the high risk of acute exacerbation of ILD. This study aimed to prospectively investigate the efficacy and safety of carboplatin and nab‐paclitaxel as a first‐line regimen for NSCLC patients with ILD.
Methods
The enrolled patients had treatment‐naïve advanced NSCLC with ILD. The patients received 4–6 cycles of carboplatin (area under the curve = 5) on day 1 and nab‐paclitaxel 100 mg/m2 on days 1, 8, and 15 every 4 weeks. The primary endpoint was the completion rate of four or more cycles. Secondary endpoints included toxicity, overall response rate (ORR), disease control rate (DCR), progression‐free survival (PFS), and overall survival (OS).
Results
Twenty‐five patients were enrolled in this study. Nine patients had adenocarcinoma, 11 had squamous cell carcinoma, one had large cell carcinoma, and four had NSCLC, not otherwise specified. The completion rate of ≥4 cycles was 76% (95% confidence interval: 56.2%–88.8%), which met the primary endpoint. The ORR and DCR were 44% and 88%, respectively. The median PFS and OS were 5.8 months and 15.8 months, respectively. Three patients experienced grade ≥2 pneumonitis, and one patient met the acute exacerbation criteria.
Conclusion
The 4‐week modified regimen of carboplatin and nab‐paclitaxel showed tolerable toxicity with favorable efficacy in NSCLC patients with ILD. This regimen may be an effective treatment option for patients in real clinical settings.
Keywords: carboplatin, interstitial lung disease, nab‐paclitaxel, non‐small‐cell lung cancer
We performed a phase II feasibility study of carboplatin and nab‐paclitaxel for advanced non‐small cell lung cancer patients with interstitial lung disease. The patients received 4–6 cycles of carboplatin (AUC 5) on day 1 and nab‐paclitaxel 100 mg/m2 on days 1, 8 and 15 every 4 weeks. The 4‐week modified regimen of carboplatin and nab‐paclitaxel showed tolerable toxicity with favorable efficacy in NSCLC patients with ILD.

INTRODUCTION
Lung cancer is the leading cause of cancer‐related deaths worldwide. Patients with interstitial lung disease (ILD) are also at risk of developing lung cancer. The prevalence of ILD among patients with lung cancer is reported to be approximately 10%–20%. 1 The prevalence of ILD in our institution was 21.2%, and lung cancer is often comorbid with not only idiopathic pulmonary fibrosis (IPF) but also chronic hypersensitivity pneumonitis or ILD related to collagen diseases, such as rheumatoid arthritis (RA)‐associated lung disease. 2 Chemotherapy for lung cancer with ILD is known to cause treatment‐related acute exacerbation (AE) of existing ILD (AE‐ILD), and these complications are serious and often fatal. Therefore, lung cancer patients with ILD have been excluded from most clinical trials of chemotherapy, and there remains no definitive standard treatment for such patients.
On the basis of a small number of prospective and retrospective clinical trials for non‐small cell lung cancer (NSCLC) patients with ILD, carboplatin and paclitaxel are deemed the standard regimen in Japan. Minegishi et al. reported the safety and efficacy of carboplatin and weekly paclitaxel in patients with NSCLC and ILD. Their study showed an overall response rate (ORR) of 61% and median progression‐free survival (mPFS) and median overall survival (mOS) periods of 5.3 and 10.6 months, respectively. 3 The rate of AE‐ILD in their study was 5.6%. The combination of carboplatin and paclitaxel was also the most frequently used regimen in a surveillance study conducted by the Diffuse Lung Disease Research Group in Japan. 4
For lung cancer patients with driver gene abnormalities, such as EGFR mutation, ALK rearrangement, and ROS1 rearrangement, molecular target drugs are the first choice. However, few NSCLC patients with ILD show driver gene abnormalities, 5 and the molecular target drugs themselves also present a risk for AE‐ILD. Therefore, a new candidate cytotoxic cancer drug for NSCLC patients with ILD is warranted.
Nab‐paclitaxel is a 130‐nm albumin‐bound formulation of paclitaxel that can reach the tumor tissue more efficiently through binding of albumin receptors on endothelial cells and may be preferentially taken up by cancer cells. Carboplatin and nab‐paclitaxel demonstrated a significantly higher ORR in comparison with carboplatin and paclitaxel (33% vs. 25%) in a large multicenter international phase III study (CA031 study). 6 Moreover, nab‐paclitaxel and paclitaxel showed almost the same mPFS (6.3 vs. 5.8 months) and mOS (12.1 vs. 11.2 months). In terms of safety, significantly fewer incidences of grade ≥3 neutropenia, sensory neuropathy, myalgia, and arthralgia occurred in the carboplatin and nab‐paclitaxel arms. Thus, this regimen is a promising alternative for carboplatin and paclitaxel. However, information regarding the efficacy and safety of carboplatin and nab‐paclitaxel combination therapy for patients with NSCLC and ILD is currently limited. Among Japanese subsets of the CA031 study, approximately two‐thirds of the patients postponed treatment. 7 As a result, many treatments were performed every 4 weeks. One level of dose reduction was adopted in 33.8% of the patients, and two‐level dose reductions were performed in 31.1% of the patients. On the basis of this background and the fragile status of NSCLC patients with ILD, we conducted a multicenter phase II feasibility study to evaluate a regimen of carboplatin (area under the curve [AUC] = 5) and nab‐paclitaxel every 4 weeks for patients in a real clinical setting.
METHODS
Patients
Eligible patients were aged 20–79 years at enrollment, with histologically or cytologically confirmed NSCLC with ILD. The eligibility criteria for lung cancer were stage IIIA/IIIB without the indication of curative operation or chemoradiotherapy, stage IV (TNM classification, seventh edition), or postoperative recurrence. The eligibility criteria for ILD included idiopathic interstitial pneumonitis (IIPs) and secondary ILD. Patients treated by pirfenidone or inhalation of N‐acetylcysteine (NAC) were eligible if comorbid ILD was stable. ILD was confirmed by high‐resolution chest computed tomography (HRCT) (≤2.5‐mm slices). The image criteria for eligibility were the presence at least two of the following radiological findings judged by central review by three pulmonologists: honeycombing, traction bronchiectasis, ground‐glass opacity, and interlobular septal thickening. We presumed that it was difficult to diagnose usual interstitial pneumonitis (UIP) accurately, and we wanted to exclude the cases that showed only slight fibrotic change. Moreover, our inclusion criteria included not only IPF but also other IIPs and secondary ILD. Therefore, we defined our own imaging criteria. Patients who received prior chemotherapy for lung cancer were ineligible, excluding those who experienced postoperative recurrence ≥6 months after the day of the final adjuvant chemotherapy administration. In addition, patients who received radiation therapy were ineligible, excluding patients who received palliative radiation therapy except for primary lesion and were judged to be stable for at least 2 weeks after the completion of radiation therapy. The following criteria were also required for eligibility: an Eastern Cooperative Oncology Group performance status (PS) of 0 or 1; adequate function of the bone marrow, liver, kidneys, and lungs (alveolar O2 pressure ≥70 Torr); life expectancy of at least 12 weeks; and less than grade 2 peripheral neuropathy. The key exclusion criteria were the experience of AE of ILD; administration of steroids or immunosuppressants for ILD within 3 months of enrollment; pleural effusion, pericardial effusion, and ascites requiring drainage; serious complications; symptomatic brain metastasis; a history of severe drug hypersensitivity reaction; and participation in the trial judged to be inappropriate by the investigators.
The study protocol was approved by the institutional review board of Tokyo Medical and Dental University (R2014‐014) and each participating institution. All patients provided written informed consent before enrollment. This study was registered at the University Medical Hospital Information Network (UMIN) Clinical Trials Registry (UMIN000015662).
Study design and treatment
This study was designed as a prospective, multicenter, single‐arm, phase II trial. The primary endpoint was the completion rate of ≥4 cycles of this combination therapy. Secondary endpoints included toxicity, ORR, disease control rate (DCR), PFS, and OS. We had intentions to set the incidence rate of AE‐ILD as the primary endpoint, but due to the difficulty in achieving the required number of enrollments and the difficulty in diagnosing AE‐ILD, we elected the completion rate of four or more cycles of treatment as the primary endpoint. Using this endpoint, we aimed to judge whether the study regimen could be comprehensively evaluated based on its tolerability and efficacy. Patients received carboplatin (AUC = 5) on day 1 and nab‐paclitaxel 100 mg/m 2 on days 1, 8, and 15 every 4 weeks for 4–6 cycles or until disease progression or unacceptable toxicity was observed. If a severe adverse event occurred during a given cycle, the doses of carboplatin and nab‐paclitaxel were reduced in the subsequent cycles. Such adverse events included grade 4 neutropenia, grade 3 thrombocytopenia, febrile neutropenia, or other grade ≥3 nonhematological toxicities. The first dose reduction consisted of carboplatin (AUC = 4) and nab‐paclitaxel 80 mg/m2, and the subsequent dose reductions consisted of carboplatin (AUC = 3) and nab‐paclitaxel 60 mg/m2. If patients required further reduction, the protocol treatment was terminated.
Baseline and treatment assessments
Patient assessments including PS, symptoms, and blood tests were conducted within 2 weeks before enrollment and on the day of protocol treatment. A pulmonary function test was conducted within 2 weeks before enrollment, and predicted forced vital capacity (FVC) was calculated based on the reference value published by the Japanese Respiratory Society in 2001. 8 CT scans of the chest and abdomen, CT or magnetic resonance imaging (MRI) of the brain, and bone scintigraphy or positron emission tomography (PET) were performed for baseline tumor assessment within 28 days before registration. Tumor response was assessed at baseline and every 8 weeks by using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. When a patient was documented as having a complete response (CR) or partial response (PR), confirmatory evaluation after 4 weeks was not mandatory. Confirmatory evaluation was usually performed 8 weeks after the initial response. Stable disease (SD) was considered when a response was confirmed at least once after enrollment at a minimum of an 8‐week interval. Unconfirmed ORR was defined by the proportion of patients with CR or PR on at least one visit. Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). AE‐ILD was diagnosed according to the following criteria: (1) worsening of dyspnea, (2) new ground‐glass opacities or consolidation on chest HRCT, (3) decreased partial pressure of oxygen in arterial blood (PaO2 ≥ 10 Torr under the same condition), and (4) no clinical evidence of infection, pulmonary embolism, congestive heart failure, or pneumothorax, within 1 month after administration of the last chemotherapy. This criterion was based on “Guideline for the diagnosis and treatment of idiopathic interstitial pneumonitis” published by the Japanese Respiratory Society in 2005. 9
Statistical analysis
The primary endpoint was the completion rate of four or more cycles of treatment. The secondary endpoints were ORR, DCR, PFS, OS, and safety (frequency of AE‐ILD and frequency of other adverse effects). In a previous CA031 phase III study, the completion rate of ≥4 cycles of carboplatin and nab‐paclitaxel among Japanese patients was 58% (Satouchi M, 2012, unpublished data). We expected that during the current study, patients would be able to safely complete ≥4 cycles to some extent, although the fragile condition of patients with advanced NSCLC and ILD generally imparts a worsening clinical course, including the occurrence of AE‐ILD. Due to the above reason, we set the expected completion rate of ≥4 cycles to 65%, while the threshold was set at 40% depending on the results of the clinical trial of CBDCA and nab‐paclitaxel for NSCLC without ILD. Using the One Arm Binomial program (Cancer Research and Biostatistics, https://stattools.crab.org/Calculators/oneArmBinomial.html), we estimated that 25 patients would be required to produce a statistical power of 80% with a one‐sided type I error of 5%. Therefore, the target number of patients was set at 30 to allow for potential dropouts. Efficacy and safety analyses were planned for patients who received at least some of the treatment protocols. PFS was calculated from the date of enrollment to the earlier date of judged progression or death from any cause. Data were censored on the last day of confirmation of clinical PFS. OS was defined as the interval between the date of enrollment and the date of death from any cause. Data were censored on the last date of confirmation of survival in the survival cases. PFS and OS were estimated using the Kaplan–Meier method. Statistical analysis of the completion rate, PFS, OS, ORR, and DCR were performed using R version 4.0.2 (https://www.r-project.org/). The difference between squamous cell carcinoma (Sq) and non‐Sq were compared with the use of Fisher's exact test and log‐rank test.
RESULTS
Patient characteristics
Between January 2015 and March 2019, 25 patients from seven institutions of Yushima Lung Cancer Oncology Group (YLOG), which was the minimum number of required participants based on the statistical plan, were enrolled in this study. All 25 patients received protocol treatment and were eligible for further analysis. Table 1 shows the baseline characteristics of the enrolled patients. The median age was 71 years (range, 63–76 years), and 23 patients were male. Fifteen patients had PS 0 (60%), and the rest were PS 1 (40%). Adenocarcinoma, Sq, and large cell carcinoma were observed in nine (36%), 11 (44%), and one (4%) patient, respectively, and the others were categorized as not otherwise specified. Nine patients (36%) were EGFR mutation negative, and the rest of patients were unknown. Seven patients (28%) were ALK rearrangement negative, and the rest of patients were unknown. Two patients (16%) were never smokers. Among the imaging findings for ILD on HRCT, honeycombing, traction bronchiectasis, interlobular septal thickening, and ground grass opacity were observed in 12 (48%), 23 (92%), 21 (84%), and 21 (84%) patients, respectively. The cases with honeycombing might be considered to have UIP pattern, while the cases without honeycombing and with bronchiectasis might be considered to have probable UIP pattern. The remaining case was diagnosed as nonspecific interstitial pneumonia (NSIP). Two patients were diagnosed with RA, manifesting as RA‐related ILD at enrollment. Two other patients showed anticyclic citrullinated peptide (CCP) antibody positivity without symptoms of RA at the time of enrollment, but one of them was later diagnosed with RA and RA lung. The other patients were considered idiopathic. The positive incidence rate and median serum level of KL‐6 were 84% and 747 U/ml, respectively. The median PaO2 and %FVC were 81.6 Torr and 80.6%, respectively. The number of modified gender, age and physiology (mGAP) index scores 10 of two, three, and four were 4 (16%), 16 (64%) and 5 (20%), respectively. Twenty patients were categorized as mGAP stage I, whereas 5 patients were categorized as mGAP stage II.
TABLE 1.
Baseline patient characteristics (n = 25)
| Number of patients | % | |
|---|---|---|
| Age, years | ||
| Median (range) | 71 (63–76) | |
| Gender | ||
| Male | 23 | 92 |
| Female | 2 | 8 |
| Performance status (ECOG) | ||
| 0 | 15 | 60 |
| 1 | 10 | 40 |
| Histology | ||
| Adenocarcinoma | 9 | 36 |
| Squamous cell carcinoma | 11 | 44 |
| Large cell carcinoma | 1 | 4 |
| Not otherwise specified (NOS) | 4 | 16 |
| Clinical stage | ||
| IIIA | 2 | 8 |
| IIIB | 8 | 32 |
| IV | 12 | 48 |
| Recurrence after surgical resection | 3 | 12 |
| Smoking status | ||
| Current | 23 | 92 |
| Former or never | 2 | 8 |
| ILD findings on HRCT | ||
| Honeycombing | 12 | 48 |
| Traction bronchiectasis | 23 | 92 |
| Interlobular septal thickening | 21 | 84 |
| Ground‐glass opacity | 21 | 84 |
| ILD etiology | ||
| Idiopathic | 21 | 84 |
| Collagen vascular disease‐associated (all rheumatoid arthritis) | 2 | 8 |
| Anti‐CCP antibody (+), later diagnosis of rheumatoid arthritis | 1 | 4 |
| Anti‐CCP antibody (+), but no definitive diagnosis of collagen vascular disease | 1 | 4 |
| KL‐6, U/ml | ||
| Median (range) | 747 (408–2439) | |
| 500≤ | 21 | 84 |
| 500> | 4 | 16 |
| PaO2 Torr | ||
| Median (range) | 81.6 (73–98.3) | |
| %FVC | ||
| Median (range) | 80.6 (54.4–110.5) | |
| Modified GAP index | ||
| 2 | 4 | 16 |
| 3 | 16 | 64 |
| 4 | 5 | 20 |
Abbreviations: CCP, cyclic citrullinated peptide; ECOG, Eastern Cooperative Oncology Group; FVC, forced volume capacity; GAP, gender, age and physiology; HRCT, high resolution computed tomography; ILD, interstitial lung disease.
Treatment delivery and efficacy
Table 2 presents the data for treatment delivery. Nineteen patients (76%, 95% confidence interval [CI]: 56.2–88.8%) received four or more cycles of the protocol treatment. Of the 25 patients enrolled in our study, one patient (4%) achieved CR, 10 patients (40%) showed PR, and 11 patients (44%) had SD. Only one patient showed progressive disease (PD), and two patients had a disease status that could not be evaluated. Thus, the ORR and DCR were 44% (95% CI: 26.7%–62.9%) and 88% (95% CI: 69.2%–96.7%), respectively. The unconfirmed ORR response rate was 64% (95% CI: 44.4%–79.8%). A waterfall plot of the maximum tumor reduction rate is shown in Figure 1. The median PFS and OS were 5.8 months (95% CI: 4.4–7.9 months) and 15.8 months (95% CI: 11.0–26.5 months), respectively (Figure 2).
TABLE 2.
Treatment delivery and response (n = 25)
| Number of patients | % | |
|---|---|---|
| Treatment delivery (cycles) | ||
| 1 | 2 | 8 |
| 2 | 2 | 8 |
| 3 | 2 | 8 |
| 4 | 10 | 40 |
| 5 | 2 | 8 |
| 6 | 7 | 28 |
| 4 or more than 4 | 19 | 76 |
| Objective response | ||
| CR | 1 | 4 |
| PR | 10 | 40 |
| SD | 11 | 44 |
| PD | 1 | 4 |
| NE | 2 | 8 |
| ORR | 11 | 44 |
| DCR | 22 | 88 |
| Unconfirmed ORR | 16 | 64 |
Abbreviations: CR, complete response; DCR, disease controll rate; NE, not evaluable; ORR, overall response rate; PD, progressive disease; PR partial response; SD, stable disease.
FIGURE 1.
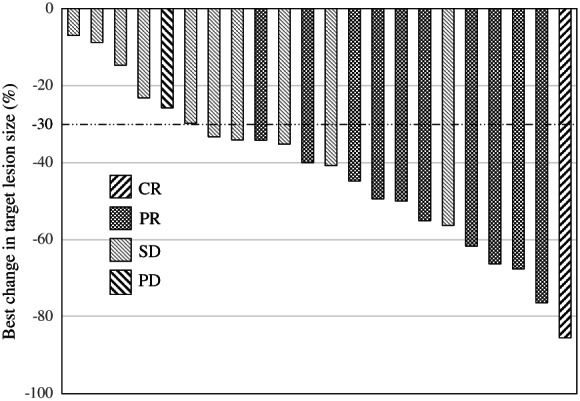
Waterfall plot showing the maximum percentage change from baseline in the size of tumors. CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease
FIGURE 2.
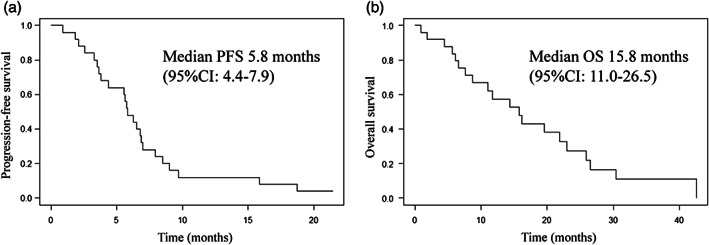
Kaplan–Meier curves of progression‐free survival (PFS) (a) and overall survival (OS) (b). CI, confidence interval
We compared the differences regarding efficacy between the Sq and non‐Sq groups despite each sub‐group having a small sample size. The ORR was 54.5% (95% CI: 28.0%–78.7%) in the Sq group (n = 11) and 35.7% (95% CI: 16.2%–61.4%) in the non‐Sq group (n = 14) (p = 0.43). The treatment efficacy in the Sq group tended to be higher than that in the non‐Sq group, although not significantly. The median PFS was 5.8 months (95% CI: 5.3–not available [NA]) in the Sq group and 6.1 months (95% CI: 3.3–15.9 months) in the non‐Sq group (hazard ratio [HR] 1.03, 95% CI: 0.46–2.32, p = 0.14). The median OS was 19.5 months (95% CI: 11.7–NA) in the Sq group and 11 months (95% CI: 5.6–NA) in the non‐Sq group (HR 0.47, 95% CI: 0.18–1.22, p = 0.69). The median PFS was almost the same between the Sq and non‐Sq groups. The median OS in the Sq group was longer than that in the non‐Sq group, although not significantly (Figure S1).
Safety
The major adverse events associated with the treatments are shown in Table 3. The most common hematological grade 3 or 4 adverse event was neutropenia (n = 12; 48%), while febrile neutropenia was not observed. The most common nonhematological adverse events were fatigue (n = 19; 76%), followed by appetite loss (n = 15; 60%). Peripheral neuropathy was observed in 10 patients (40%) without grade 3 or 4 neuropathy. One patient experienced sudden left internal carotid artery thrombosis due to Trousseau syndrome on day 18 after one administration of chemotherapy, resulting in death on day 20. Our group judged that this event was not treatment‐related.
TABLE 3.
Major treatment‐related adverse events
| Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | All grades (N) | All grades (%) | Grades 3,4 (N) | Grades 3,4 (%) |
|---|---|---|---|---|---|---|---|---|
| Hematological | ||||||||
| Leukocytosis | 2 | 10 | 9 | 0 | 21 | 84 | 9 | 36 |
| Neutropenia | 1 | 8 | 9 | 3 | 21 | 84 | 12 | 48 |
| Thrombocytopenia | 7 | 1 | 4 | 0 | 12 | 48 | 4 | 16 |
| Anemia | 8 | 11 | 6 | 0 | 25 | 100 | 6 | 24 |
| Febrile neutropenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nonhematological | ||||||||
| Appetite loss | 8 | 6 | 1 | 0 | 15 | 60 | 1 | 4 |
| Conspitation | 9 | 5 | 0 | 0 | 14 | 56 | 0 | 0 |
| Diarrhea | 1 | 0 | 0 | 0 | 1 | 4 | 0 | 0 |
| Fatigue | 10 | 5 | 4 | 0 | 19 | 76 | 4 | 16 |
| Nausea | 6 | 5 | 0 | 0 | 11 | 44 | 0 | 0 |
| Peripheral sensory neuropathy | 5 | 5 | 0 | 0 | 10 | 40 | 0 | 0 |
| Pneumonitis | 0 | 1 | 1 | 1 | 3 | 12 | 2 | 8 |
| ALT increased | 8 | 0 | 0 | 0 | 8 | 32 | 0 | 0 |
| AST increased | 10 | 0 | 0 | 0 | 10 | 40 | 0 | 0 |
| Creatinine increased | 8 | 0 | 0 | 0 | 8 | 32 | 0 | 0 |
Abbreviations:ALT, alanine aminotransferase; AST, asparatate aminotransferase.
During protocol treatment, four patients (16%) needed one level dose reduction, and two patients (8%) required two level dose reduction due to neutropenia, thrombopenia, prolonged thrombopenia and assessment by doctors. Seven patients (28%) required course delays due to prolonged myelosuppression, fatigue, infection, and suspicion of infection.
Pulmonary toxicities
During the planned study period, the predefined chemotherapy‐induced AE‐ILD was observed in one patient who showed G4 pneumonitis, recovered once, but eventually died due to lung cancer progression. The other two patients who had shown pneumonitis did not meet the predefined AE‐ILD criteria because they were able to maintain their PaO2. However, they were administered high‐dose intravenous steroids in accordance with the physician's decision. Thus, these cases may be included in AE‐ILD. At more than 28 days after completion of the protocol treatment, two patients showed AE‐ILD. One patient developed AE after two months of the final nab‐paclitaxel administration, and the other showed AE during second‐line chemotherapy with vinorelbine.
Subsequent chemotherapy
Among the 25 patients, 15 (60%) received subsequent chemotherapy as second‐line treatment. S‐1 (n = 11), docetaxel (n = 3), and carboplatin plus pemetrexed (n = 1) were used as second‐line regimens. Six patients received third‐line treatment. Nivolumab was used in two patients as third‐ and fourth‐line treatments.
DISCUSSION
Our findings showed the efficacy and safety of a modified 4‐week carboplatin and nab‐paclitaxel regimen for patients with advanced NSCLC with ILD. Since the completion rate for four or more cycles of treatment was 76%, the primary endpoint of our study was met. This result is consistent with the findings of two prospective studies 11 , 12 that reported completion rates of 78% and 63.9%, respectively.
To date, only a few published prospective trials have investigated the efficacy and safety of platinum doublet chemotherapy for patients with NSCLC and ILD because of the risk of AE‐ILD (Table 4). Fukuizumi et al. reported a phase II study of carboplatin and weekly paclitaxel, showing that the ORR, mPFS, and mOS were 69.7%, 6.3 months, and 19.8 months, respectively, 13 in addition to the study mentioned in the introduction. 3 In two recent prospective phase II studies of carboplatin and nab‐paclitaxel, 11 , 12 Kenmotsu et al. performed a clinical trial on 92 patients with mild or moderate ILD and reported that the ORR, mPFS, and mOS were 51%, 6.2 months, and 15.4 months, respectively, while Asahina et al. showed that the ORR, mPFS, and mOS were 55.6%, 5.3 months, and 15.4 months, respectively. Several retrospective studies of carboplatin and nab‐paclitaxel have demonstrated similar results (Table 4). 16 , 17 , 18 , 19 , 20 The median PFS and OS in our study were comparable to the values reported in these studies. Taken together, these findings confirmed that our 4‐week modified carboplatin and nab‐paclitaxel regimen showed similar efficacies. From the point of the difference of efficacy between the Sq and non‐Sq groups, the ORR was higher, and the mOS in the Sq group was longer than that in the non‐Sq group. These results are consistent with the previous studies, 6 , 12 , 21 but the reason remains unknown.
TABLE 4.
Summary of the prospective trials for NSCLC patients with ILD, retrospective trials of CBDCA plus nab‐paclitaxel for NSCLC patients with ILD, and CA031 trial
| Author | Regimen | Number | Study design | ORR (%) | mPFS (months) | mOS (months) | AE‐ILD (%) | FN (%) | CBDCA AUC | 1‐cycle duration (weeks) |
|---|---|---|---|---|---|---|---|---|---|---|
| Minegishi et al. 3 | CBDCA+weekly PTX | 18 | P | 61.1 | 5.3 | 10.6 | 5.6 | 5.6 | 5 | 4w |
| Fukuizumi et al. 13 | CBDCA+weekly PTX | 33 | P | 69.7 | 6.3 | 19.8 | 12 | 15.2 | 5 | 4w |
| Sekine et al. 14 | CBDCA+S‐1 | 21 | P | 33.3 | 4.2 | 9.7 | 9.5 | 0 | 5 | 3w |
| Hanabishi et al. 15 | CBDCA+S‐1 | 33 | P | 33.3 | 4.8 | 12.8 | 6.1 | 0 | 5 | 4w |
| Yasuda et al. 16 | CBDCA+nab‐PTX | 12 | R | 66.7 | 5.1 | 14.9 | 8.3 | 8.3 | 6 | 3w |
| Fujita et al. 17 | CBDCA+nab‐PTX | 8 | R | 50 | 5.6 | 8.1 | 0 | 0 | 5 or 6 | 4w |
| Igawa et al. 18 | CBDCA+nab‐PTX | 34 | R | 38.2 | 5.8 | 12.7 | 5.7 | 20 | 5 or 6 | 4w |
| Niwa et al. 19 | CBDCA+nab‐PTX | 9 | R | 55.6 | 5.8 | 11.5 | 0 | 33.3 | 5 or 6 | 4‐6w |
| Araya et al. 20 | CBDCA+nab‐PTX | 9 | R | 77.8 | 5.8 | 8 | 0 | 0 | 5 or 6 | 4w |
| Kenmotsu et al. 11 | CBDCA+nab‐PTX | 94 | P | 51 | 6.2 | 15.4 | 4.3 | 8.7 | 6 | 3w |
| Asahina et al. 12 | CBDCA+nab‐PTX | 36 | P | 55.6 | 5.3 | 15.4 | 5.6 | 0 | 6 | 3w |
| this study | CBDCA+nab‐PTX | 25 | P | 44 | 5.8 | 15.8 | 4 | 0 | 5 | 4w |
| Satouchi et al. 21 (CA031 in Japanese) | CBDCA+nab‐PTX | 74 | P | 35 | 6.9 | 16.7 | – | – | 6 | 3w |
| Socinski et al. (CA031) 6 | CBDCA+nab‐PTX | 521 | P | 33 | 6.3 | 12.1 | – | <1 | 6 | 3w |
Abbreviations: AE‐ILD, acute exacebration‐interstitial lung disease; AUC, area under the curve; CBDCA, carboplatin; FN, febrile neutropenia; ILD, interstitial lung disease; mOS, median overall survival; mPFS, median progression‐free survival; NSCLC, non‐small cell lung cancer; ORR, overall response rate; P, prospective; PTX, paclitaxel; R, retrospective.
Hematological toxicities were mild compared to those reported in previous prospective studies 11 , 12 and Japanese subsets enrolled in the CA031 study. 21 The rates of G ≥3 neutropenia and anemia in our study were 48% and 24%, respectively, while they were 75% and 48%, 64% and 36%, 69% and 32%, respectively, in each previous study. Moreover, no instances of febrile neutropenia were observed. Our protocol was slightly different in dose and schedule from the previous studies, and the rates of the dose reduction and course delay were low compared to the previous studies. These modifications may have contributed to the improved toxicity profiles, which were easy to manage in our clinical settings.
Moreover, the AE‐ILD rate among patients in our study was 4%, similar to that found in two prospective studies. In a recent meta‐analysis, the AE‐ILD rate was 8.07%, and in a subgroup analysis of patients treated with carboplatin and nab‐paclitaxel, the AE‐ILD rate was 4.98%. 22 On including the case that did not meet our criteria for AE‐ILD, the rate of AEs was 12%, which was slightly higher than that reported previously. This rate may have been influenced by the small sample size. We concluded that the toxicity of the carboplatin and nab‐paclitaxel regimen was acceptable.
In attempts to develop a new standardized treatment, the oral intracellular inhibitor nintedanib, a key drug, was shown to slow IPF disease progression in phase III trials. 23 This drug is an anti‐fibrotic agent and an antiangiogenesis inhibitor. Nintedanib is also known to improve OS for lung adenocarcinoma in LUME‐Lung 1 and has been approved for lung adenocarcinoma in Europe. 24 Based on the results of these trials, a randomized phase III study of the combination of carboplatin plus nab‐paclitaxel with and without nintedanib for advanced NSCLC patients with IPF (J‐SONIC) is ongoing, 25 and carboplatin plus nab‐paclitaxel with nintedanib is expected to be a more promising regimen based on the results of a clinical trial of carboplatin plus nab‐paclitaxel.
Our study had several limitations. First, the number of included patients was small, and it was difficult to state any generalizable conclusions from only this study. Second, the severity of ILD was mild because the inclusion criteria were PS of 0 or 1 and alveolar O2 pressure ≥70 Torr, and we included patients who were comparatively able to maintain %FVC. Therefore, we consider that the results from this study can be adapted to only mild to moderate cases of ILD that meet the inclusion criteria. The safety data cannot be generalized to patients with severe ILD. However, from the point of %FVC, the severities of ILD were considered to be nearly comparable to that reported in previous studies. 11 , 12 Third, the inclusion criteria permitted secondary ILD in addition to IPF. However, the efficacy of carboplatin plus nab‐paclitaxel in patients with secondary ILD also needs to be determined in a real clinical setting.
In conclusion, carboplatin and nab‐paclitaxel showed tolerable toxicity with favorable efficacy in NSCLC patients with ILD. Considering the results of two recently reported prospective trials and several retrospective trials together, this modified regimen may be an effective treatment options for NSCLC patients with ILD in real clinical settings. Considering the substantial prevalence of lung cancer with ILD, further development of new treatment regimens is warranted.
CONFLICT OF INTEREST
HS received honoraria from Taiho Pharmaceutical and Bristol Myers Squibb.
KU received honoraria from Bristol Myers Squibb. YM, TT, TH, TM, and HF received honoraria from Taiho Pharmaceutical. The rest of the authors declare no conflict of interest.
Supporting information
Supplementary Figure S1 Kaplan–Meier curves of progression‐free survival (PFS) (a) and overall survival (OS) according to histology (squamous cell carcinoma [Sq] vs non‐Sq)
ACKNOWLEDGMENTS
We thank all the patients, their families, and the investigators who participated in this study. We thank Makoto Tomita for advising us on the issue with the statistical analysis. This research did not receive any specific grant from funding agencies in the public or commercial sectors. We would like to thank Editage for English language editing.
Sakashita H, Uchibori K, Jin Y, Tsutsui T, Honda T, Sakakibara R, et al. A phase II feasibility study of carboplatin and nab‐paclitaxel for advanced non‐small cell lung cancer patients with interstitial lung disease (YLOG0114). Thorac Cancer. 2022;13:1267–1275. 10.1111/1759-7714.14376
REFERENCES
- 1. Ogura T, Takigawa N, Tomii K, et al. Summary of the Japanese respiratory society statement for the treatment of lung cancer with comorbid interstitial pneumonia. Respir Investig. 2019;57(6):512–33. [DOI] [PubMed] [Google Scholar]
- 2. Kawahara T, Sakashita H, Suzuki T, Tateishi T, Miyazaki Y. Real world data of combined lung cancer and interstitial lung disease. J Thorac Dis. 2019;11(10):4144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Minegishi Y, Sudoh J, Kuribayasi H, Mizutani H, Seike M, Azuma A, et al. The safety and efficacy of weekly paclitaxel in combination with carboplatin for advanced non‐small cell lung cancer with idiopathic interstitial pneumonias. Lung Cancer. 2011;71(1):70–4. [DOI] [PubMed] [Google Scholar]
- 4. Minegishi Y, Gemma A, Homma S, Kishi K, Azuma A, Ogura T, et al. Acute exacerbation of idiopathic interstitial pneumonias related to chemotherapy for lung cancer: nationwide surveillance in Japan. ERJ Open Res. 2020;6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Honda T, Sakashita H, Masai K, Totsuka H, Motoi N, Kobayashi M, et al. Deleterious pulmonary surfactant system gene mutations in lung adenocarcinomas associated with usual interstitial pneumonia. JCO Precis Oncol. 2018;2:1–24. [DOI] [PubMed] [Google Scholar]
- 6. Socinski MA, Bondarenko I, Karaseva NA, Makhson AM, Vynnychenko I, Okamoto I, et al. Weekly nab‐paclitaxel in combination with carboplatin versus solvent‐based paclitaxel plus carboplatin as first‐line therapy in patients with advanced non‐small‐cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30(17):2055–62. [DOI] [PubMed] [Google Scholar]
- 7. Nogami N. Practice of administration management of nab‐paclitaxel, appropriate dose modification for effective administration of nab‐paclitaxel. Consensus cancer treatment. 2014;13(2):111–2. [Google Scholar]
- 8. Clinical pulmonary functions committee of the Japanese Respiratory Society Reference values of Spirograms and arterial blood gas partial pressure in Japanese. JJRS. 2001;39(5):S1–17. [Google Scholar]
- 9. Guideline for the diagnosis and treatment of idiopathic interstitial pneumonitis. JJRS. 2005;43(3):179–207. [Google Scholar]
- 10. Kobayashi H, Omori S, Nakashima K, Wakuda K, Ono A, Kenmotsu H, et al. Modified GAP index for prediction of acute exacerbation of idiopathic pulmonary fibrosis in non‐small cell lung cancer. Respirology. 2017;22(7):1379–85. [DOI] [PubMed] [Google Scholar]
- 11. Kenmotsu H, Yoh K, Mori K, Ono A, Baba T, Fujiwara Y, et al. Phase II study of nab‐paclitaxel + carboplatin for patients with non‐small‐cell lung cancer and interstitial lung disease. Cancer Sci. 2019;110(12):3738–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Asahina H, Oizumi S, Takamura K, Harada T, Harada M, Yokouchi H, et al. A prospective phase II study of carboplatin and nab‐paclitaxel in patients with advanced non‐small cell lung cancer and concomitant interstitial lung disease (HOT1302). Lung Cancer. 2019;138:65–71. [DOI] [PubMed] [Google Scholar]
- 13. Fukuizumi A, Minegishi Y, Omori M, Atsumi K, Takano N, Hisakane K, et al. Weekly paclitaxel in combination with carboplatin for advanced non‐small‐cell lung cancer complicated by idiopathic interstitial pneumonias: a single‐arm phase II study. Int J Clin Oncol. 2019;24(12):1543–8. [DOI] [PubMed] [Google Scholar]
- 14. Sekine A, Satoh H, Baba T, Ikeda S, Okuda R, Shinohara T, et al. Safety and efficacy of S‐1 in combination with carboplatin in non‐small cell lung cancer patients with interstitial lung disease: a pilot study. Cancer Chemother Pharmacol. 2016;77(6):1245–52. [DOI] [PubMed] [Google Scholar]
- 15. Hanibuchi M, Kakiuchi S, Atagi S, Ogushi F, Shimizu E, Haku T, et al. A multicenter, open‐label, phase II trial of S‐1 plus carboplatin in advanced non‐small cell lung cancer patients with interstitial lung disease. Lung Cancer. 2018;125:93–9. [DOI] [PubMed] [Google Scholar]
- 16. Yasuda Y, Hattori Y, Tohnai R, Ito S, Kawa Y, Kono Y, et al. The safety and efficacy of carboplatin plus nanoparticle albumin‐bound paclitaxel in the treatment of non‐small cell lung cancer patients with interstitial lung disease. Jpn J Clin Oncol. 2018;48(1):89–93. [DOI] [PubMed] [Google Scholar]
- 17. Fujita T, Hiroishi T, Shikano K, Yanagisawa A, Hayama N, Amano H, et al. The safety and efficacy of treatment with nab‐paclitaxel and Carboplatin for patients with advanced squamous non‐small cell lung cancer concurrent with idiopathic interstitial pneumonias. Intern Med. 2018;57(13):1827–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Igawa S, Nishinarita N, Takakura A, Ozawa T, Harada S, Kusuhara S, et al. Real‐world evaluation of carboplatin plus a weekly dose of nab‐paclitaxel for patients with advanced non‐small‐cell lung cancer with interstitial lung disease. Cancer Manag Res. 2018;10:7013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Niwa H, Nakahara Y, Yokoba M, Mitsufuji H, Sasaki J, Masuda N. Safety and efficacy of carboplatin plus nab‐paclitaxel for treating advanced non‐small‐cell lung cancer with interstitial lung disease. Mol Clin Oncol. 2017;7(4):604–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Araya T, Kita T, Ueda T, Terada N, Sakai T, Yamamura K, et al. Real‐world evidence of safety and efficacy of carboplatin plus nanoparticle albumin‐bound paclitaxel in patients with advanced non‐small‐cell lung cancer and preexisting interstitial lung disease: a retrospective study. Can Respir J. 2019;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Satouchi M, Okamoto I, Sakai H, Yamamoto N, Ichinose Y, Ohmatsu H, et al. Efficacy and safety of weekly nab‐paclitaxel plus carboplatin in patients with advanced non‐small cell lung cancer. Lung Cancer. 2013;81(1):97–101. [DOI] [PubMed] [Google Scholar]
- 22. Wang Y, Miao L, Hu Y, Zhou Y. The efficacy and safety of first‐line chemotherapy in patients with non‐small cell lung cancer and interstitial lung disease: a systematic review and meta‐analysis. Front Oncol. 2020;10:1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabe U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–82. [DOI] [PubMed] [Google Scholar]
- 24. Reck M, Kaiser R, Mellemgaard A, Douillard JY, Orlov S, Krzakowski M, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non‐small‐cell lung cancer (LUME‐lung 1): a phase 3, double‐blind, randomised controlled trial. Lancet Oncol. 2014;15(2):143–55. [DOI] [PubMed] [Google Scholar]
- 25. Otsubo K, Kishimoto J, Kenmotsu H, Minegishi Y, Ichihara E, Shiraki A, et al. Treatment rationale and design for J‐SONIC: a randomized study of carboplatin plus nab‐paclitaxel with or without Nintedanib for advanced non‐small‐cell lung cancer with idiopathic pulmonary fibrosis. Clin Lung Cancer. 2018;19(1):e5–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1 Kaplan–Meier curves of progression‐free survival (PFS) (a) and overall survival (OS) according to histology (squamous cell carcinoma [Sq] vs non‐Sq)


