Abstract
Background
The effect of the sequential combination of chemotherapy and immune checkpoint inhibitors (ICIs) remains unclear. Here, we evaluated the efficacy of different chemotherapy regimens administered after ICIs in advanced non‐small cell lung cancer (NSCLC), compared to the same regimens administered without previous ICIs.
Methods
We retrospectively included all patients treated between 2015 and 2019 for an advanced NSCLC, receiving a salvage chemotherapy just after ICI (CAI group) comparing them to ICI naive patients (CWPI group) undergoing the same chemotherapy at Bordeaux University Hospital. The primary outcome was the time to treatment discontinuation (TTD), and secondary endpoints were overall survival (OS) and overall response rate (ORR).
Results
A total of 152 patients were included, with 34/23 (CAI/CWPI) receiving paclitaxel/bevacizumab (PB), 24/11 paclitaxel (P), 27/12 gemcitabine (G) and 6/15 pemetrexed (PE). Characteristics were comparable, except for CAI treated with PB (more patients with an ECOG PS ≤1 [p <0.001]). Median number of lines received was higher in CAI for all groups. There was no difference between CAI and CWPI for TTD, OS and ORR. However, PB was associated with a nonsignificant increase in OS in the CAI group (HR = 0.65; 95% CI: 0.38–1.2, p = 0.17].
Conclusion
Our data showed no difference in TTD, OS and ORR regardless of chemotherapy, but a trend towards an increased OS with PB when given after an ICI, while patients received chemotherapy later in the CAI group. This suggests that a sequential combination of ICI followed by chemotherapy could be an interesting strategy in advanced NSCLC for selected patients.
Keywords: angiogenesis inhibitors, immune checkpoint inhibitor, non‐small cell lung cancer, salvage chemotherapy, sequential treatment

INTRODUCTION
Immune checkpoint inhibitors (ICIs) have revolutionized the management of non‐small cell lung cancer (NSCLC). 1 Nevertheless, chemotherapy remains essential. Through its immunogenic properties, chemotherapy is able to potentialize the effect of immunotherapy. 2 Indeed, chemotherapy induces immunogenic death and modulates the immunosuppressive landscape of the tumor microenvironment. Therefore, ICIs have been successfully developed combined with chemotherapy as a first‐line treatment with almost half of the patients alive at 2 years. 3 The synergistic effect of both molecules is well established. 4 However, the potential effect of their sequential combination remains unclear. Sequential combination could be an interesting alternative, to challenge different immune populations in a complementary way.
Previous data suggest that some patients experienced an unexpected prolonged overall survival (OS) under salvage chemotherapy after nivolumab exposure. 5 Studies frequently reported an increased antitumor effect especially for overall response rate (ORR). 6 , 7 , 8 However, impact on treatment duration and survival exhibits controversial results from previous studies. Furthermore, most studies are retrospective, with a small number of patients and historical comparisons. The largest one including Japanese patients, showed a significant improvement in ORR (ratio 1.71; p = 0.004) after adjustment for patient characteristics when chemotherapy was administered after ICIs. However, there was no difference in progression‐free survival (PFS) or OS. 9
Herein, we aimed to evaluate the efficacy of different chemotherapy regimens, administered after an ICI in advanced NSCLC, compared to the same regimens administered without previous ICIs.
METHODS
Patients
We retrospectively included all consecutive patients with stage III/IV histologically proven NSCLC diagnosed between January 2015, and December 2019, inaccessible to a local therapy, treated at the Bordeaux University Hospital (France) who received at least one infusion of chemotherapy of interest. We studied four chemotherapy regimens: pemetrexed (PE), gemcitabine (G), weekly paclitaxel (P) and paclitaxel plus bevacizumab (PB). 10 , 11 , 12 , 13 We did not study docetaxel because this chemotherapy is not commonly used in our institution due to its worse toxicity profile compared to paclitaxel. 14
Eligible patients could be treated after a relapse. Patients treated with more than one studied chemotherapy regimen were only included in the group of the first salvage chemotherapy. We excluded patients treated with a combination of immunotherapy and chemotherapy as first‐line treatment, with pemetrexed as maintenance therapy, or treated for another cancer.
The following data were collected at inclusion: demographic and anthropometric characteristics, smoking status, histology, TNM (eighth classification), PD‐L1 status, mutational status and presence of brain/liver metastasis.
Clinical endpoints
The primary outcome was the time to treatment discontinuation (TTD) (from the first day of chemotherapy to the treatment discontinuation or death). Secondary endpoints were OS (from the first day of chemotherapy of interest to death or lost to follow‐up), PFS (from the first day of chemotherapy of interest until progression or death), and ORR (corresponding to a complete or partial response [RECIST 1.1 criteria]). Disease control rate (DCR) (percentage of patients achieving a complete, partial or stable disease) was also investigated.
Statistical analysis and ethical considerations
All statistical tests were performed with a type I error rate of 5%. Qualitative variables were described with numbers and percentages, and quantitative variables with numbers of nonmissing data, median with first and third quartiles or range. Survival variables were described with survival probabilities and curves using Kaplan–Meier method. The qualitative variables were compared with a Chi2, corrected Chi2 or nonparametric Fisher's exact test. The quantitative variables were compared with a Student t‐test (parametric) or a Wilcoxon test (nonparametric). The survival endpoints were compared using a log‐rank test. Cox regression models were used to adjust on stratification factor.
All statistical analyses were performed using R Statistical Software (version 4.1.0; R Foundation for Statistical Computing). The study was conducted in accordance with French legislation and ethical codes. This work complies to the protection of personal health data and the protection of privacy with the framework of application provided for by article 65‐2 of the amended Data Protection Act and the general data protection regulations and was registered with the following number CHUBX2020RE0270. The study was designed according to the STROBE guidelines (b).
RESULTS
Patient characteristics in the whole cohort
We screened 280 patients of which 152 were included (Figure 1), with 91 (59.9%) receiving chemotherapies of interest after ICI (CAI) and 61 (40.1%) the same chemotherapy regimen without previous ICI (CWPI). The most frequent chemotherapies were paclitaxel/bevacizumab (n = 57, 37.4%), gemcitabine (n = 39, 29.6%), paclitaxel (n = 35, 26.4%) and pemetrexed (n = 21, 6.6%) (Figure 1). The median number line of treatment received before the different studied chemotherapies was 2. 1 , 2 At chemotherapy initiation, 38 (25.0%) and 67 (44.1%) patients had liver and cerebral metastasis, respectively. The median OS (mOS) of the cohort was 6.5 (2.7–14.6) months. The comparison of data from the different chemotherapy subgroups, without stratifying them in CAI or CWPI, showed that PB was associated with higher TTD, PFS and OS than other chemotherapy subgroups (p <0.001).
FIGURE 1.
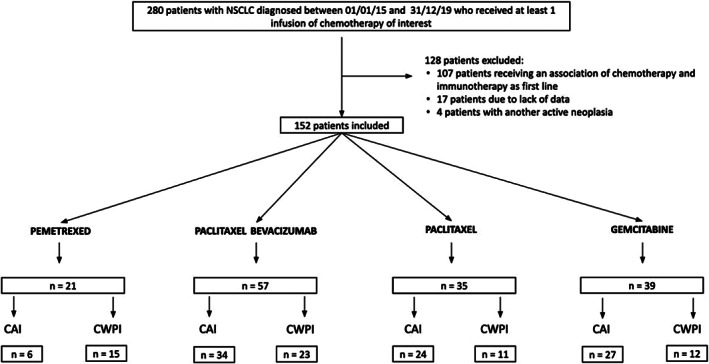
Flowchart of patient enrollment in chemotherapy after ICI and chemotherapy regimen without previous ICI. NSCLC, non‐small cell lung cancer; CAI, chemotherapy of interest after ICI; CWPI, chemotherapy regimen without previous ICI
Characteristics of CAI versus CWPI
In the CAI group, nivolumab was the most widely used ICI (n = 44, 48.9%), followed by pembrolizumab (n = 24, 26.7%) and atezolizumab (n = 22, 24.4%). ICIs were given in the majority as second‐ (72.9%) or third‐line (17.7%) and 82.5% of patients had an ECOG PS 0/1 before starting immunotherapy. Median TTD (mTTD) under ICI treatment was 2.7 months (1.9–6.3). There was no correlation between response duration under ICI and PD‐L1 status (p = 0.71).
After failure of studied chemotherapies, 38 (42%) patients in the CAI group received subsequent therapy, with 17 (19%) ICI rechallenges with a mPFS of 3.2 (1.7–4.6) months. In the CWPI group, 24 (39%) patients received another line after, including 20 (33%) patients receiving an ICI, with a mPFS of 3.9 (1–8.1) months.
The median follow‐up duration from chemotherapy initiation was 6.5 (2.7–14.6) months. At the date of the last follow‐up, 35 (23%) patients were still alive: 26 (74.3%) patients (CAI) and nine (25.7%) patients (CWPI). Among them, 19 (12.5%) patients remained under treatment, with six (10.5%) patients who were still receiving the studied chemotherapy in the PB subgroup: four in the CAI and two in the CWPI groups.
Considering all patients in both groups regardless of the type of chemotherapy, there was no difference for TTD (3.0 vs 1.9 months respectively; HR = 0.88, 95% CI: 0.63–1.20; p = 0.44), PFS (3.7 vs 3.1 months; HR = 0.91, 95% CI: 0.65–1.30; p = 0.58) and ORR (23% versus 10%; p = 0.24).
ICI administration was correlated with a better outcome, with a mOS from diagnosis of 26.1 months in the cohort of patients who received ICI, compared to 13.0 months in the cohort unexposed to ICI (HR = 0.52, 95% CI: 0.35–0.75; p < 0.001).
Characteristics of chemotherapy subgroups
Baseline characteristics of each group are summarized in Table 1. Patients were older in the CWPI group receiving P (p < 0.001). Regarding the PS, patients receiving PB had a better general condition in the CAI group compared to the CWPI group (p < 0.001). For the other chemotherapy groups, there were no statistically significant differences for PS (P: p = 1.0; G: p = 0.2; PE: p = 1.0).
TABLE 1.
Baseline characteristics of patients
| Type of chemotherapy | Paclitaxel‐bevacizumab | Paclitaxel | Pemetrexed | Gemcitabine | ||||
|---|---|---|---|---|---|---|---|---|
| n = 57 | n = 35 | n = 21 | n = 39 | |||||
| n = 152 | CAI | CWPI | CAI | CWPI | CAI | CWPI | CAI | CWPI |
| (n = 34) | (n = 23) | (n = 24) | (n = 11) | (n = 6) | (n = 15) | (n = 27) | (n = 12) | |
| Median age (years) (Q1–Q3) | 57.5 (53–62) | 59 (50–65) | 63 (53.5–67) | 70 (65.5–79) | 61 (68.5–70.2) | 66 (60.5–72.5) | 61 (58.5–66) | 64.5 (56.8–70.5) |
| Male Gender | 22 (64.7) | 16 (69.6) | 15 (62.5) | 7 (63.6) | 3 (50) | 7 (46.7) | 19 (70.4) | 5 (41.7) |
| Smoking status | ||||||||
| Never | 2 (5.9) | 1 (4.3) | 0 (0) | 1 (9.1) | 0 (0) | 4 (26.7) | 2 (7.4) | 2 (16.7) |
| Former | 28 (82.4) | 15 (65.2) | 19 (79.2) | 9 (81.8) | 6 (100) | 6 (40) | 20 (74.1) | 6 (50) |
| Current | 4 (11.8) | 7 (30.4) | 5 (20.8) | 1 (9.1) | 0 (0) | 5 (33.3) | 5 (18.5) | 4 (33.3) |
| Histologic subtype | ||||||||
| Adenocarcinoma | 33 (97.5) | 23 (100) | 15 (62.5) | 7 (63.6) | 6 (0) | 12 (80) | 14 (51.9) | 6 (50) |
| Squamous | 0 (0) | 0 (0) | 8 (33.3) | 3 (27.3) | 0 (0) | 1 (6.7) | 12 (44.4) | 6 (50) |
| Other | 1 (2.9) | 0 (0) | 1 (4.2) | 1 (9.1) | 0 (0) | 2 (13.3) | 1 (3.7) | 0 (0) |
| Line of chemotherapy of interest | ||||||||
| 1 | 0 (0) | 0 (0) | 0 (0) | 9 (81.8) | 0 (0) | 6 (40) | 0 (0) | 2 (16.7) |
| 2 | 2 (5.9) | 21 (91.3) | 2 (8.3) | 2 (18.2) | 0 (0) | 8 (53.3) | 1 (3.7) | 4 (33.3) |
| ≥3 | 32 (94.1) | 2 (8.7) | 22 (91.7) | 0 (0) | 6 (100) | 1 (6.7) | 26 (96.3) | 6 (50) |
| Median number of lines received (range) | 3.5 (3–7) | 2 (2–4) | 3 (2–6) | 2 (1–2) | 4 (3–5) | 2 (1–3) | 4 (2–6) | 3 (1–5) |
| PD‐L1 status a | ||||||||
| <1% | 14 (41.2) | 15 (65.2) | 9 (37.5) | 1 (9.1) | 1 (16.7) | 5 (33.3) | 9 (33.3) | 1 (8.3) |
| 1%–49% | 8 (23.5) | 3 (13.0) | 6 (25) | 3 (27.3) | 4 (66.7) | 5 (33.3) | 7 (25.9) | 1 (8.3) |
| >50% | 5 (14.7) | 0 (0) | 4 (16.7) | 2 (18.2) | 1 (16.7) | 0 (0) | 4 (14.8) | 2(16.6) |
| Unknown | 7 (20.6) | 5 (21.8) | 5 (20.8) | 5 (45.4) | 0 (0) | 5 (33.3) | 7 (25.9) | 8 (66.6) |
| EGFR (%) | 0 (0) | 1 (4.5) | 0 (0) | 0 (0) | 0 (0) | 1 (7.1) | 1(3.7) | 0 (0) |
| ALK (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| KRAS (%) | 14 (41.2) | 7 (30.4) | 5 (20.8) | 3 (27.2) | 3 (50) | 4 (26.7) | 8 (29.6) | 2 (16.7) |
| BRAF (%) | 2 (5.8) | 4 (17.4) | 1 (4.1) | 1 (9) | 0 (0) | 1 (6.7) | 1 (3.7) | 0 (0) |
| ROS (%) | 1 (2.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| STK11 (%) | 2 (5.8) | 1 (14.3) | 4 (16.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| ECOG PS b | ||||||||
| ≤1 | 27 (79.4) | 11 (47.8) | 8 (33.3) | 4 (36.4) | 4 (66.6) | 8 (53.3) | 15 (55.6) | 4 (33.3) |
| >1 | 7 (20.6) | 12 (52.1) | 16 (66.6) | 7 (63.6) | 2 (33.3) | 7 (46.7) | 12 (44.4) | 8 (66.7) |
| Type of ICI | ||||||||
| Nivolumab | 17 (50) | – | 8 (33.3) | – | 3 (50) | – | 16 (59.3) | – |
| Pembrolizumab | 11 (32.4) | 8 (33.3) | 2 (2) | 3 (11.1) | ||||
| Atezolizumab | 6 (17.6) | 7 (29.1) | 1 (16.7) | 8 (29.6) | ||||
| Other | 0 (0) | 1 (4.2) | 0 (0) | 0 (0) | ||||
| Presence of brain metastasis b | 23 (67.6) | 11 (47.8) | 10 (41.7) | 3 (27.3) | 1 (16.7) | 6 (40) | 8 (29.6) | 5 (41.7) |
| Presence of liver metastasis b | 9 (26.5) | 6 (26.1) | 10 (41.7) | 1 (9.1) | 2 (33.3) | 1 (6.7) | 5 (18.5) | 4 (33.3) |
| Interstitial lung disease at the diagnostics | 1 (2.9) | 0 (0.0) | 0 (0.0) | 1 (9.1) | 0 (0.0) | 0 (0.0) | 1 (3.7) | 0 (0.0) |
Note: Data are shown as n (%); medians are defined with their with first and third quartiles or range.
Abbreviations: CAI, chemotherapy of interest after ICI; CWPI, chemotherapy regimen without previous ICI; ECOG PS, Eastern Cooperative Oncology Group performance‐status scores; ICI, immune checkpoint inhibitor. Q1–Q3, quartile 1‐quartile 3.
Determined by immunohistochemistry.
At the initiation of the studied chemotherapy.
Other clinical parameters were well balanced between subgroups. The median number of lines received was higher in all CAI groups.
In CAI groups, median duration between last IPI infusion and first following cycle of chemotherapy were 21 (14–28) (PB), 21 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 (P), 34 (32–46) (PE) and 16 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 (G) days.
Efficacy of studied chemotherapies
mTTD were not significantly different between CAI and CWPI in all the chemotherapy subgroups (PB: HR = 0.84, 95% CI: 0.48–1.50; p = 0.55; P: HR = 0.93, 95% CI: 0.44–1.90; p = 0.83; PE: HR = 0.91, 95% CI: 0.35–2.40; p = 0.84; G: HR = 0.74, 95% CI: 0.37–1.50; p = 0.42) (Table 2, Figure 2).
TABLE 2.
Survival and response in the different chemotherapy subgroups
| mTTD | mOS | mPFS | ORR | DCR | ||
|---|---|---|---|---|---|---|
| (months) | (months) | (months) | (%) | (%) | ||
| Paclitaxel‐ Bevacizumab | CAI (n = 34) | 5.5 | 18.3 | 6 | 43 | 90 |
| CWPI (n = 23) | 3.3 | 6.6 | 4.1 | 39 | 78 | |
| Paclitaxel | CAI (n = 24) | 1.45 | 4,5 | 3.25 | 24 | 59 |
| CWPI (n = 11) | 2.8 | 5.8 | 3.1 | 10 | 60 | |
| Pemetrexed | CAI (n = 6) | 2.25 | 8.6 | 2.95 | 33 | 50 |
| CWPI (n = 15) | 1.4 | 3.8 | 2.1 | 18 | 54 | |
| Gemcitabine | CAI (n = 27) | 1.6 | 5.7 | 2 | 18 | 59 |
| CWPI (n = 12) | 1.5 | 4.05 | 2.35 | 10 | 33 |
Abbreviations: CAI, chemotherapy of interest after ICI; CWPI, chemotherapy regimen without previous ICI; mTTD, median time to treatment discontinuation; mOS, median overall survival; mPFS, median progression‐free survival; ORR, objective response rate; DCR, disease control rate.
FIGURE 2.
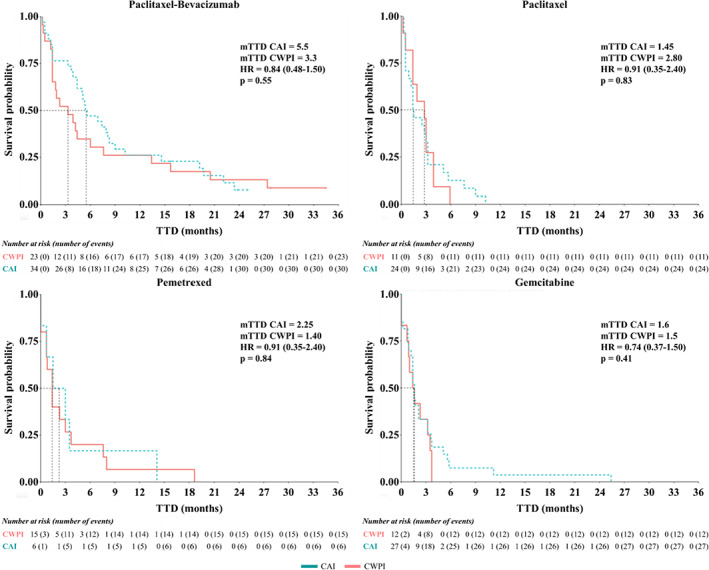
Kaplan–Meier estimates of time to discontinuation (TTD) according to the different types of chemotherapy regimen. HR, hazard ratio; CAI, chemotherapy of interest after ICI; CWPI, chemotherapy regimen without previous ICI; mTTD, median time to discontinuation
Evaluation of the response was available for 71 patients in the CAI and 49 patients in the CWPI groups. ORR was not statistically different between the CAI and CWPI groups for all chemotherapy regimens; however, there was a trend to higher response rates for the CAI groups especially for P, PE and G (Table 2). There was also no difference in DCR between the CAI and CWPI groups.
mPFS were not significantly different between both groups regardless of the type of chemotherapy (Table 2 and Figure S1). mOS was not significantly increased in the CAI group (Figure 3). However, in the PB cohort, there was a trend towards improvement in OS for the CAI group, with nearly a 3‐fold increase of the mOS: 18.3 months (CAI) versus 6.6 months (CWPI) (p = 0.17).
FIGURE 3.
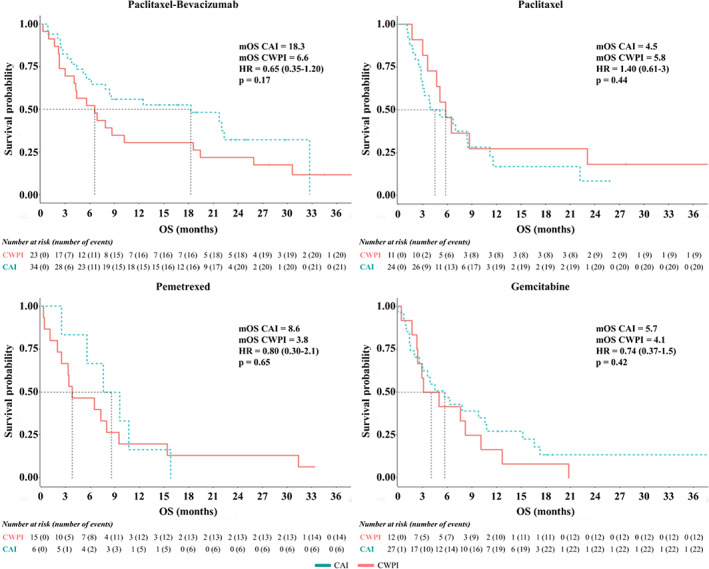
Kaplan–Meier estimates of overall survival (OS) according to the different types of chemotherapy regimen. HR, hazard ratio; CAI, chemotherapy of interest after ICI; CWPI, chemotherapy regimen without previous ICI; mOS, median overall survival
Swimmer plots representing immunotherapy and chemotherapy response durations in each subgroup are shown in Figure 4. We observed heterogeneous patterns of response to chemotherapy regardless of the protocol and the addition of prior immunotherapy. There was no evidence of a prolonged response to chemotherapy due to immunotherapy, but these swimmer plots showed that some patients could experience an unexpected prolonged response to chemotherapy even in unresponsive tumor for immunotherapy.
FIGURE 4.
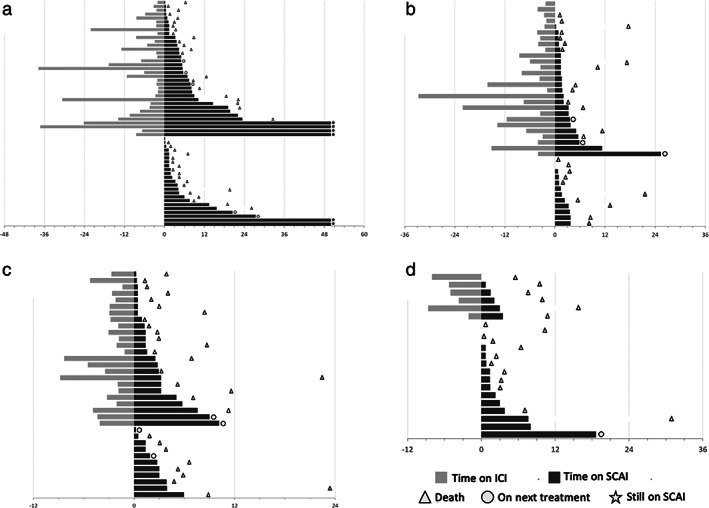
Swimmer plots showing treatment durations of immunotherapy (light gray) and chemotherapy (dark gray) in the different chemotherapy subgroups. (a) paclitaxel bevacizumab; (b) gemcitabine; (c) paclitaxel; (d) pemetrexed. Each bar represents one subject in the study. Time is written in weeks
DISCUSSION
The most important challenge in advanced NSCLC is the first‐line setting, to rapidly and sustainably control cancer disease. With combinations arising in first‐line, there is a crucial need to study efficient therapeutic strategies for subsequent lines, to improve survival while preserving quality of life. However, chemotherapies commonly used as next‐line treatment are historically associated with low response rates. Here, we investigated clinical reports of seemingly increased responses to chemotherapy in a post‐immunotherapy setting.
Our “real life” study, evaluating all available ICIs in France, showed no significant differences in terms of TTD, PFS and OS whatever chemotherapy regimen was used. Nevertheless, PB chemotherapy administered after ICI tended to increase OS and TTD, although these results were not significant. Interestingly, OS were comparable between groups while CAI patients experienced this treatment in a more advanced line. This could be partly be explained by the 30% crossover rate to ICI in the CWPI group. Thereby, this emphasizes that every patient should receive immunotherapy in the absence of contraindications without compromising its outcome.
Similar results have been reported, specifically by Kato et al. in the largest cohort with Japanese patients, in which ORR for CAI was 18%, versus 11% for CWPI, without differences in PFS or OS. 9 In the literature, very few other studies have assessed the efficacy of chemotherapy after immunotherapy including a comparator arm in NSCLC. Constantini et al. also underlined similar PFS for treatments received after nivolumab compared to historical studies. 5 Most of these studies, compared to historical data or to a comparator arm, reported a gain in ORR. However, comparison between all these studies is difficult due to the heterogeneity between chemotherapy and ICI regimens. Furthermore, most of them were performed on Asian and/or small cohorts.
The question of a potential “post‐immunotherapy effect” has also been raised in head and neck carcinoma, where Saleh et al. have reported an ORR of 30%, three to five times higher than historical trials. 15 In metastatic urothelial carcinoma, Szabados et al. have found an ORR of 64% for patients experiencing chemotherapy after immunotherapy versus 21% for patients experiencing chemotherapy after failure of first‐line platinum‐based therapy. 16 However, all these studies were performed on small cohorts, with different chemotherapy regimens, and with probably an effect according to tumor type.
There is a strong rationale to study the combination between chemotherapy and ICI, due to the important interplay between these therapeutic modalities. Indeed, immunotherapy sensitizes tumors to chemotherapy by prior activation of the immune system, which will be able to better react to chemotherapy‐induced antigen release. 17 However, chemotherapy could also resensitize to further ICI therapy. Indeed, chemotherapy agents can modulate immune cells, increasing antitumor response while inhibiting some immunosuppressive cells. 2 The optimal timing of PD‐1/PD‐L1 blockade and chemotherapy, concomitant or sequential, remains unknown beyond the first‐line setting. Some preclinical data comparing both strategies in mouse models reported an increased antitumor activity for chemotherapy when given before ICIs, compared to concomitant chemoimmunotherapy. 18 More investigations have to be done to study such a strategy in a human setting and to determine which patient would benefit from a sequential treatment.
Our study showed that the association PB after immunotherapy tended to increase OS. VEGF, which mediates angiogenesis, inhibits antitumor immune cells while promoting immunosuppressive cells. 19 Some studies in small cohorts assessing combination of antiangiogenic drugs with docetaxel after ICI showed an ORR between 36% 20 and 50%. 21 There are few data on the specific efficacy of PB following ICI. In a cohort of 33 patients, Bilger et al found an ORR of 42%, a PFS of 6.2 months and a 1‐year OS not reached, consistent with our results. 22 In comparison, the phase III trial Ultimate evaluating the association between paclitaxel and bevacizumab showed an ORR of 22.5%, a PFS of 5.4 months and an OS of 9.9 months. 13 Our study, which is the first to compare PB administered after or without an ICI, confirms that this combination is a valuable strategy after ICI failure.
The “post‐immunotherapy effect” could also be explained by a transient concomitant effect due to the long‐term binding of antibodies to lymphocytes, 23 and extended half‐life of ICI (25–27 days according to molecule). 24 Immunotherapy antibodies are still circulating at the beginning of chemotherapy. Another strategy could be to continue immunotherapy beyond progression, associated to a new line of chemotherapy, to maintain an immunological pressure on tumor cells, chemotherapy being effective on tumor clones that are resistant to the immune response. Such strategies have been evaluated in Hodgkin's lymphoma (n = 30) showing an effect of continuing immunotherapy with chemotherapy. 25 This continuation strategy is currently being evaluated in NSCLC (NCT03656094, NCT03083808).
With the improvement of patient survival, some of them benefit from a rechallenge of immunotherapy alone. Therefore, knowing the efficacy of chemotherapy after ICI as monotherapy remains topical. Finding the better sequential strategy to prioritize one drug among others is challenging. In patients with melanoma, such strategies were studied, using ipilimumab combined to nab‐paclitaxel/bevacizumab 26 with a 1‐year improvement in mOS. However, the difference was not significant because of the small number of patients (n = 24). These sequential strategies are highly relevant, due to the reciprocal interactions between immunotherapy and chemotherapy mechanisms of action, highlighting the importance of considering multiple rechallenges. In this sequential strategy, determining specific resistances to ICI of each tumor could help to identify the most appropriate chemotherapy to be administered.
There are some limitations in our findings. First, it is a retrospective study, with small groups with a lack of power not allowing multivariate nor subgroup analysis. Moreover, given its “real‐life” nature, the first scanographic evaluation under chemotherapy was not performed at the same time. This could have affected the PFS, and we preferred TTD as the primary endpoint. Finally, even if both groups were statistically comparable for most of the clinical features, ECOG was significantly lower in the CAI groups treated with PB or G, which may lead to a bias in survival analysis.
In conclusion, this study, including all the approved ICIs and the main chemotherapy regimens for the management of NSCLC showed no difference in TTD, OS and ORR regardless of chemotherapy but a trend towards an increased OS with paclitaxel/bevacizumab when given after an ICI. This could be a valuable option in advanced NSCLC for selected patients. However, our data have to be confirmed in larger cohorts, specifically studying paclitaxel/bevacizumab regimens. Sequential combination strategies need further investigation, and the place of such therapeutic strategy has to be determined since combinations are validated as first‐line treatment.
CONFLICT OF INTEREST
None.
Supporting information
Appendix S1 Supplementary Information
ACKNOWLEDGMENTS
The authors thank the patients and their families, and all medical and paramedical staff from the Medical Oncology and the Pneumology Departments who contributed to the management of patients. We would also like to thank Professor Olivier Adotevi for his comprehensive review of the manuscript.
Heraudet L, Delon T, Veillon R, Vergnenègre C, Lepetit H, Daste A, et al. Effect of prior immunotherapy on the efficacy of chemotherapy in advanced non‐small cell lung cancer: A retrospective study. Thorac Cancer. 2022;13:1391–1400. 10.1111/1759-7714.14403
REFERENCES
- 1. Reck M, Rodríguez–Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Updated analysis of KEYNOTE‐024: Pembrolizumab versus platinum‐based chemotherapy for advanced non–small‐cell lung cancer with PD‐L1 tumor proportion score of 50% or greater. J Clin Oncol. 2019;37:537–46. 10.1200/JCO.18.00149 [DOI] [PubMed] [Google Scholar]
- 2. Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28:690–714. 10.1016/j.ccell.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 3. Gadgeel S, Rodríguez‐Abreu D, Speranza G, Esteban E, Felip E, Dómine M, et al. Updated analysis from KEYNOTE‐189: Pembrolizumab or placebo plus Pemetrexed and platinum for previously untreated metastatic nonsquamous non–small‐cell lung cancer. J Clin Oncol. 2020;38:1505–17. 10.1200/JCO.19.03136 [DOI] [PubMed] [Google Scholar]
- 4. Galluzzi L, Humeau J, Buqué A, Zitvogel L, Kroemer G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat Rev Clin Oncol. 2020;17:725–41. 10.1038/s41571-020-0413-z [DOI] [PubMed] [Google Scholar]
- 5. Costantini A, Corny J, Fallet V, Renet S, Friard S, Chouaid C, et al. Efficacy of next treatment received after nivolumab progression in patients with advanced nonsmall cell lung cancer. ERJ Open Res. 2018;4:00120–2017. 10.1183/23120541.00120-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park SE, Lee SH, Ahn JS, Ahn M‐J, Park K, Sun J‐M. Increased response rates to salvage chemotherapy administered after PD‐1/PD‐L1 inhibitors in patients with non–small cell lung cancer. J Thorac Oncol. 2018;13:106–11. 10.1016/j.jtho.2017.10.011 [DOI] [PubMed] [Google Scholar]
- 7. Ogawara D, Soda H, Iwasaki K, Suyama T, Taniguchi H, Fukuda Y, et al. Remarkable response of nivolumab‐refractory lung cancer to salvage chemotherapy, Thorac. Cancer. 2018;9:175–80. 10.1111/1759-7714.12543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bersanelli M, Buti S, Giannarelli D, Leonetti A, Cortellini A, Russo GL, et al. Chemotherapy in non‐small cell lung cancer patients after prior immunotherapy: the multicenter retrospective CLARITY study. Lung Cancer. 2020;150:123–31. 10.1016/j.lungcan.2020.10.008 [DOI] [PubMed] [Google Scholar]
- 9. Kato R, Hayashi H, Chiba Y, Miyawaki E, Shimizu J, Ozaki T, et al. Propensity score–weighted analysis of chemotherapy after PD‐1 inhibitors versus chemotherapy alone in patients with non–small cell lung cancer (WJOG10217L). J Immunother Cancer. 2020;8:e000350. 10.1136/jitc-2019-000350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of Pemetrexed versus docetaxel in patients with non–small‐cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97. 10.1200/JCO.2004.08.163 [DOI] [PubMed] [Google Scholar]
- 11. Crinò L, Mosconi AM, Scagliotti G, Selvaggi G, Novello S, Rinaldi M, et al. Gemcitabine as second‐line treatment for advanced non–small‐cell lung cancer: a phase II trial. J Clin Oncol. 1999;17:2081–1. 10.1200/JCO.1999.17.7.2081 [DOI] [PubMed] [Google Scholar]
- 12. Buccheri G, Ferrigno D. Second‐line weekly paclitaxel in patients with inoperable non‐small cell lung cancer who fail combination chemotherapy with cisplatin. Lung Cancer. 2004;45:227–36. 10.1016/j.lungcan.2004.01.011 [DOI] [PubMed] [Google Scholar]
- 13. Cortot AB, Audigier‐Valette C, Molinier O, Le Moulec S, Barlesi F, Zalcman G, et al. Weekly paclitaxel plus bevacizumab versus docetaxel as second‐ or third‐line treatment in advanced non‐squamous non–small‐cell lung cancer: results of the IFCT‐1103 ULTIMATE study. Eur J Cancer. 2020;131:27–36. 10.1016/j.ejca.2020.02.022 [DOI] [PubMed] [Google Scholar]
- 14. Esteban E, González de Sande L, Fernández Y, Corral N, Fra J, Muñiz I, et al. Prospective randomised phase II study of docetaxel versus paclitaxel administered weekly in patients with non‐small‐cell lung cancer previously treated with platinum‐based chemotherapy. Ann Oncol. 2003;14:1640–7. 10.1093/annonc/mdg456 [DOI] [PubMed] [Google Scholar]
- 15. Saleh K, Daste A, Martin N, Pons‐Tostivint E, Auperin A, Herrera‐Gomez RG, et al. Response to salvage chemotherapy after progression on immune checkpoint inhibitors in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Eur J Cancer. 2019;121:123–9. 10.1016/j.ejca.2019.08.026 [DOI] [PubMed] [Google Scholar]
- 16. Szabados B, van Dijk N, Tang YZ, van der Heijden MS, Wimalasingham A, Gomez de Liano A, et al. Response rate to chemotherapy after immune checkpoint inhibition in metastatic urothelial cancer. Eur Urol. 2018;73:149–52. 10.1016/j.eururo.2017.08.022 [DOI] [PubMed] [Google Scholar]
- 17. Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies, cancer. Immunol Res. 2015;3:436–43. 10.1158/2326-6066.CIR-15-0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nowak AK, Robinson BWS, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. n.d.;8. [PubMed] [Google Scholar]
- 19. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325–40. 10.1038/nrclinonc.2018.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brueckl WM, Reck M, Rittmeyer A, Kollmeier J, Wesseler C, Wiest GH, et al. Efficacy of docetaxel plus Ramucirumab as palliative third‐line therapy following second‐line immune‐checkpoint‐inhibitor treatment in patients with non‐small‐cell lung cancer stage IV. Clin Med Insights Oncol. 2020;14:117955492095135. 10.1177/1179554920951358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grohé C. Nintedanib + docetaxel in lung adenocarcinoma patients (pts) following treatment with immune checkpoint inhibitors (ICIs): updated efficacy and safety results of the ongoing non‐interventional study (NIS) VARGADO (NCT02392455). J Clin Oncol. 2020;38:9604–9604. 10.1200/JCO.2020.38.15_suppl.9604 [DOI] [Google Scholar]
- 22. Bilger G, Boré P, Valery S, Pinsolle J, Descourt R, Geier M, et al. Efficacy of weekly paclitaxel‐bevacizumab combination in advanced non squamous non‐small cell lung cancer (NSCLC): a retrospective multicentric study. Ann Oncol. 2019;30:v645. 10.1093/annonc/mdz260.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single‐agent anti–programmed Death‐1 (MDX‐1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. 10.1200/JCO.2009.26.7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Sousa Linhares A, Battin C, Jutz S, Leitner J, Hafner C, Tobias J, et al. Therapeutic PD‐L1 antibodies are more effective than PD‐1 antibodies in blocking PD‐1/PD‐L1 signaling. Sci Rep. 2019;9:11472. 10.1038/s41598-019-47910-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rossi C, Gilhodes J, Maerevoet M, Herbaux C, Morschhauser F, Brice P, et al. Efficacy of chemotherapy or chemo‐anti‐PD‐1 combination after failed anti‐PD‐1 therapy for relapsed and refractory hodgkin lymphoma: a series from lysa centers. Am J Hematol. 2018;93:1042–9. 10.1002/ajh.25154 [DOI] [PubMed] [Google Scholar]
- 26. Markovic SN, Suman VJ, Javed A, Reid JM, Wall DJ, Erickson LA, et al. Sequencing Ipilimumab immunotherapy before or after chemotherapy (nab‐paclitaxel and bevacizumab) for the treatment of BRAFwt (BRAF wild‐type) metastatic malignant melanoma: results of a study of academic and community cancer research united (ACCRU) RU261206I. Am J Clin Oncol. 2020;43:115–21. 10.1097/COC.0000000000000644 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supplementary Information


