Abstract
Background:
Magnetic resonance elastography (MRE) is an accurate biomarker of liver fibrosis; however, limited data characterize its association with outcomes. We aimed to evaluate the association between liver stiffness (LS) on MRE and liver-related outcomes.
Methods:
This is a longitudinal, retrospective analysis of subjects at risk of NAFLD who had MRE assessment. LS was estimated using MRE, and liver fat was assessed using magnetic resonance imaging proton density fat fraction. Univariable and multivariable survival and regression analyses were used to assess the association between LS on MRE and liver-related outcomes including a cumulative primary outcome of hepatic decompensation, hepatocellular carcinoma (HCC), or death.
Results:
In all, 265 patients (68% women) with a mean age of 50 (±18) years and 44% Hispanic ethnicity and 45.3% with NAFLD were included. A total of 76 liver-related events or death occurred, and there was 453 person-years of follow-up time in 97 patients with available follow-up. Each 1-kPa increase in LS was associated with 2.20-fold (95% CI: 1.70–2.84, p < 0.001) increased odds of prevalent hepatic decompensation or HCC. A positive MEFIB index, a combination of MRE ⩾ 3.3 kPa and FIB-4 ⩾ 1.6, had a strong association with the primary outcome compared with those without, HR = 21.8 (95% CI: 4.28–111.4, p < 0.001). The MEFIB index had a sensitivity of 75% and specificity of 90%, and a negative score was associated with 98% negative predictive value for incident liver-related events or death.
Conclusion:
LS assessed by MRE is associated with hepatic decompensation and death, and the MEFIB combination of MRE with FIB-4 may have high negative predictive value for liver-related events.
Keywords: ascites, biomarker, cirrhosis, nonalcoholic fatty liver disease, noninvasive, portal hypertension, varices
Introduction
Nonalcoholic fatty liver disease (NAFLD) is emerging as a leading cause of chronic liver disease globally, and given the affected population, great emphasis has been placed on noninvasive assessment of disease.1,2 Despite the high prevalence of NAFLD, only a subset of patients develop decompensated cirrhosis and hepatocellular carcinoma (HCC). 3 Fibrosis stage on liver biopsy is strongly associated with outcomes; 4 however, liver biopsy is impractical to scale to the affected population, leaving a great need for noninvasive biomarkers associated with liver-related outcomes.
Magnetic resonance elastography (MRE) is a reproducible, 5 continuous measure of liver stiffness, the most accurate noninvasive test (NIT) for fibrosis stage on liver biopsy,6,7 and examines a much larger volume of the liver than a biopsy. An increase in liver stiffness on MRE is associated with fibrosis progression, 8 and MRE is increasingly being used as an end point in early stage NAFLD treatment trials.9,10 Emerging data from recent studies have suggested that liver stiffness on MRE may be associated with liver-related outcomes.11–14 Other NITs including the FIB-4 score (fibrosis index based on the 4 factor) and NAFLD Fibrosis Score (NFS) have demonstrated associations with liver-related events and death;15–17 however, when used alone, they have only modest discriminatory ability. Similarly, vibration-controlled transient elastography (VCTE) is associated with liver-related events among patients with chronic advanced liver disease, 18 but has limited discriminatory ability for liver-related events in patients with less advanced disease.
Recently, the combination of NITs has demonstrated improved accuracy for diagnosing significant fibrosis and NAFLD activity.19,20 Furthermore, the combination of liver stiffness on VCTE and platelet count have been used to identify patients with chronic liver disease with clinically significant portal hypertension. 21 A prospective study demonstrated that the combination of MRE ⩾ 3.3 kPa and FIB-4 ⩾ 1.6 (MEFIB index) provided excellent positive predictive value (PPV) of 97.1% for significant fibrosis and was validated in an independent cohort 20 to identify candidates for pharmacologic treatment for nonalcoholic (NASH) who have NASH with stage 2 fibrosis or higher. To date, studies evaluating the association between MRE and liver-related outcomes have not evaluated a combination of NITs to optimize diagnostic performance in clinical practice. Therefore, we hypothesized that liver stiffness on MRE would be associated with liver-related events and death and that the combination of MRE and FIB-4 would improve the ability to identify low- and high-risk groups. Using a well-characterized cohort of patients at risk of NAFLD, we evaluated the association between the liver stiffness on MRE and the use of the MEFIB index to predict hepatic decompensation or death.
Materials and methods
Study design
This study includes a cross-sectional and longitudinal analysis derived from two cohorts of patients who had a research MRE assessment and were at risk of NAFLD, with other causes of chronic liver disease excluded, with a retrospective evaluation for liver-related events and death. This study included 265 patients who underwent a standardized research visit including history, physical exam, and MRE assessment between 2011 and 2020 at the UCSD NAFLD Research Center.22–26 All patients provided written informed consent prior to enrolling in the study, and the study was approved by the UCSD Institutional Review Board. The reporting of this study conforms to the STROBE statement 27 with a completed checklist available in supplemental material.
Inclusion and exclusion criteria
The study population was derived from two cohorts at risk of NAFLD, defined as having other causes of chronic liver disease ruled out through history and laboratory testing. The Twin and Family Study (N = 141) 24 recruited participants who were twins, siblings, or parent–offspring pairs at least 18 years old residing in Southern California. Participants were excluded from the study if they met any of the following criteria: significant alcohol intake (defined as ⩾14 drinks/week for men or ⩾7 drinks/week for women) within the previous 2-year period or biochemical evidence of liver disease other than NAFLD.
Participants were also derived from the Familial Cirrhosis Study (N = 124), 28 which included probands with NAFLD cirrhosis defined by American Association for the Study of Liver Diseases (AASLD) and their first-degree family members (sibling, child, or parent). Exclusion criteria included significant alcohol intake (defined as ⩾14 drinks/week for men or ⩾7 drinks/week for women) within the previous 2-year period or biochemical evidence of liver disease other than NAFLD.
Clinical assessment and laboratory tests
All patients underwent a standardized clinical evaluation including a detailed history and a physical examination, which included vital signs, height, weight, and anthropometric measurements, performed by a trained clinical investigator. Body mass index (BMI) was defined as the body weight (in kilograms) divided by height (in meters) squared. Alcohol consumption was documented outside clinical visits and confirmed in the research clinic using the Alcohol Use Disorders Identifications Test (AUDIT) and the Skinner questionnaire. Other causes of liver disease were systematically ruled out based on history and laboratory tests. Patients underwent the following biochemical tests: glucose, albumin, hemoglobin A1c, alanine aminotransferase, aspartate aminotransferase, total bilirubin, alkaline phosphatase, fasting lipid panel, platelets, insulin, and international normalized ratio. FIB-429 and NFS 30 were calculated as described previously. Participants were instructed to fast for a minimum of 8 h prior to collection of laboratory tests.
Magnetic resonance imaging
Abdominal magnetic resonance imaging (MRI) was obtained on a single 3T MR scanner (GE Signa EXCITE HDxt, GE Healthcare, Waukesha, WI) at the UCSD MR3T Research Laboratory using previously described methods.31–35 Magnetic resonance imaging proton density fat fraction (MRI-PDFF) was used to measure hepatic steatosis, and NAFLD is defined as having an MRI-PDFF ⩾ 5% or a liver stiffness ⩾ 3.63 kPa plus a metabolic risk factor after exclusion of other causes of liver disease. Liver stiffness was estimated using 2-dimensional MRE, which is the most accurate biomarker for the quantitative assessment of liver stiffness as a surrogate for hepatic fibrosis.20,36,37 A passive driver was fitted around the body over the liver and connected to an acoustic active driver that delivered continuous vibrations at 60 Hz to produce shear waves in the liver, which were processed to generate elastograms depicting liver stiffness. Four slices were assessed, and co-localized regions of interest (ROIs) were manually specified as previously described. 38 Assessment of the mean MRE value was performed independent of knowledge of clinical outcomes with the exception of those apparent on imaging.
Justification for evaluating MRE cut points and MEFIB index
The cut point of ⩾3.63 kPa for advanced fibrosis is the most well-validated MRE cut point and has been evaluated in multiple studies6,39 and an individual patient meta-analysis 7 with excellent diagnostic accuracy (c = 0.93) for advanced fibrosis (stage 3–4). Furthermore, a liver stiffness measurement (LSM) cut point of 5 kPa on MRE has high specificity (90%) for the presence of cirrhosis and was included in the analysis for liver-related outcomes. 7
The MEFIB combination of MRE and FIB-4 has identified a cut point that leverages MRE to identify patients with stage 2 fibrosis or higher with a high PPV.20,40 The index includes patients who are at intermediate risk by FIB-4 and accurately identifies those who actually have stage 2 fibrosis or higher. The population identified by NITs, including the MEFIB index, may be used to select candidates for clinical trials. Assessing their long-term risk and documenting low risk for those excluded is an important clinical as well as validation question, which this study is designed to address.
Patient follow-up
Two modes of follow-up were employed to adjudicate patient outcomes. Patients were contacted by telephone, and an outcome questionnaire was administered. Three attempts were made to contact each patient prior to stating that the patient was lost to follow-up. In addition, the electronic health record was reviewed for each patient by two hepatologists (V.A. and N.T.) to assess for hepatic decompensation, HCC, or death. Patients with no follow-up time were only evaluated in cross-section for outcomes present at baseline.
Outcome measures
The primary outcome was a cumulative end point including ascites, hepatic encephalopathy, varices needing treatment, HCC, and death and for assessment of incident events was the time to the earliest event. Ascites was defined per AASLD guidance by imaging or physical examination. 41 Hepatic encephalopathy was defined as brain dysfunction caused by liver dysfunction and/or portosystemic shunting per practice guidelines. 42 Varices needing treatment were defined as medium/large varices, small varices with high-risk stigmata, decompensated patients with small varices, or variceal hemorrhage per AASLD guidance. 43 HCC was defined by histology or Liver Reporting and Data Systems (LI-RADS) for definite HCC.
Secondary outcomes included each individual component of the primary outcome and liver transplantation.
Statistical analysis
We hypothesized that there would be an association between liver stiffness on MRE and the cumulative outcome. Power analysis was performed assuming that 15% of the cohort had MRE ⩾ 3.63 kPa, consistent with advanced fibrosis, and that the presence of MRE ⩾ 3.63 kPa was associated with a 15% event rate over the follow-up period compared with an event rate of 2% for the cumulative outcome in those without advanced fibrosis. A sample size of 264 would provide 80% power with a two-tailed alpha of 0.05; therefore, we had adequate power to test and confirm our hypothesis. For patient characteristics, an ANOVA was performed on continuous variables presented as mean [standard deviation (SD)], and Kruskal–Wallis was performed on those presented as median [interquartile range (IQR)]. Chi-square or Fisher’s exact test was conducted as appropriate on all categorical variables. Kaplan–Meier curves were generated to evaluate for the cumulative incidence of liver-related events or death for the subset of patients with longitudinal follow-up. For the cumulative outcome, time was measured to the first event. Patients were censored at the last known follow-up in the clinical record. Logistic regression was used to assess for the prevalent association between liver-related events and liver stiffness at baseline. For patients with follow-up and without the event at baseline, Cox proportional hazard models were used to calculate hazard ratios (HRs). Dichotomous thresholds on MRE at cut points of 3.63 and 5 kPa were also evaluated. The combination of FIB-4 and MRE in the MEFIB index (MRE ⩾ 3.3 kPa and FIB-4 ⩾ 1.6) was evaluated for its association with outcomes. All statistical analyses were performed using SAS 9.4 (SAS Institute), and a p value less than 0.05 was considered statistically significant.
Results
Characteristics of the study population
Two hundred sixty-five patients who underwent MRE were included. Participants had a mean age of 50 (±18) years, were predominantly female (68%), and were 46% White and 44% Hispanic. The prevalence of NAFLD was 45.3%. Advanced fibrosis (MRE ⩾ 3.63 kPa) on MRE was present in 18% of the cohort and 12% had MRE > 5 kPa. The mean BMI was 29 (±7) kg/m2 (Table 1). A total of 29 patients had 76 events [ascites (n = 17), hepatic encephalopathy (n = 17), varices needing treatment (n = 18), HCC (n = 10), and death n = 14)] and 453 person-years of follow-up among 97 patients with longitudinal follow-up data. Participants without follow-up data were only assessed for prevalent events at the time of MRE (Supplemental Figure 1). Four patients underwent subsequent liver transplantation. Higher liver stiffness was associated with older age, higher BMI, Hispanic ethnicity, the presence of diabetes, and hypertension (Table 1).
Table 1.
Clinical, demographic, and imaging characteristics by liver stiffness on MRE.
| MRE liver stiffness | Total N | <3.63 kPa N = 218 |
3.63–5 kPa N = 15 |
>5 kPa N = 32 |
p |
|---|---|---|---|---|---|
| Demographic | |||||
| Age in years, mean (SD) | 265 | 47.4 (17.8) | 60.9 (14.6) | 62.5 (10.1) | <0.0001 |
| Male, n (%) | 265 | 67 (30.7%) | 8 (53.3%) | 9 (28.1%) | 0.1716 |
| BMI (kg/m2), mean (SD) | 255 | 28.5 (7.3) | 32.3 (4.7) | 31.0 (6.1) | 0.0350 |
| White, n (%) | 256 | 107 (50.9%) | 3 (21.4%) | 8 (25.0%) | 0.0038 |
| Hispanic, n (%) | 256 | 84 (40.0%) | 8 (57.1%) | 21 (65.6%) | 0.0149 |
| Diabetes, n (%) | 265 | 22 (10.1%) | 6 (40.0%) | 22 (68.8%) | <0.0001 |
| Hypertension, n (%) | 265 | 50 (22.9%) | 8 (53.3%) | 16 (50.0%) | 0.0005 |
| Hyperlipidemia, n (%) | 265 | 41 (18.8%) | 4 (26.7%) | 7 (21.9%) | 0.7165 |
| Current smoker, n (%) | 236 | 17 (8.8%) | 1 (7.7%) | 3 (10.3%) | 0.8956 |
| Family history | |||||
| Liver disease, n (%) | 265 | 20 (9.2%) | 1 (6.7%) | 3 (9.4%) | 1.0000 |
| Biochemical profile | |||||
| HbA1c (%), median (IQR) | 259 | 5.6 (0.6) | 6.2 (1.2) | 6.2 (1.6) | 0.0001 |
| AST (U/l), median (IQR) | 260 | 22.0 (9.0) | 32.0 (17.0) | 53.0 (38.0) | <0.0001 |
| ALT (U/l), median (IQR) | 259 | 19.5 (13.0) | 31.0 (31.0) | 32.0 (33.0) | <0.0001 |
| Alkaline phosphatase (U/l), median (IQR) | 260 | 71.0 (25.0) | 102.0 (46.0) | 107.0 (49.5) | <0.0001 |
| Total bilirubin (mg/dl), median (IQR) | 260 | 0.4 (0.3) | 0.6 (0.4) | 0.8 (1.0) | <0.0001 |
| Albumin (g/dl), median (IQR) | 260 | 4.5 (0.4) | 4.3 (0.2) | 4.1 (0.6) | <0.0001 |
| HOMA-IR median (IQR) | 243 | 2.5 (2.6) | 7.3 (4.4) | 10.3 (13.4) | <0.0001 |
| Triglycerides (mg/dl), median (IQR) | 259 | 98.0 (71.0) | 138.0 (99.0) | 105.0 (81.0) | 0.1785 |
| HDL (mg/dl), median (IQR) | 257 | 56.0 (22.0) | 43.0 (10.0) | 41.0 (21.0) | <0.0001 |
| LDL (mg/dl), median (IQR) | 255 | 106.0 (48.0) | 85.0 (43.0) | 84.0 (35.0) | 0.0010 |
| Platelet count (109/l), median (IQR) | 259 | 257.0 (82.5) | 189.0 (86.0) | 119.0 (90.5) | <0.0001 |
| INR, median (IQR) | 259 | 1.0 (0.1) | 1.1 (0.2) | 1.2 (0.2) | <0.0001 |
| Clinical scores | |||||
| FIB-4, median (IQR) | 256 | 0.9 (0.7) | 1.7 (1.7) | 4.2 (3.6) | <0.0001 |
| NAFLD Fibrosis Score, median (IQR) | 246 | –2.5 (1.7) | 0.3 (2.5) | 1.2 (2.1) | <0.0001 |
| MELD score, median (IQR) | 256 | 6.0 (1.0) | 7.0 (1.0) | 8.0 (5.0) | <0.0001 |
| Imaging | |||||
| MRI-PDFF (%), mean (SD) | 265 | 6.0 (6.9) | 9.5 (6.5) | 5.9 (5.0) | 0.1426 |
| MRE (kPa), mean (SD) | 265 | 2.2 (0.4) | 4.3 (0.4) | 7.3 (2.1) | – |
| NAFLD diagnosis, N (%) | 265 | 77 (35.3%) | 14 (93.3%) | 29 (90.6%) | <0.0001 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; BMI, body mass index; FIB-4, fibrosis index based on the 4 factors; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment method for insulin resistance [calculated as (fasting insulin (μU/ml) × fasting glucose (mmol/l)) / 22.5]; IQR, interquartile range; INR, international normalized ratio; LDL, low-density lipoprotein; MRI-PDFF, MRI-based proton density fat fraction; MRE, magnetic resonance elastography; SD, standard deviation.
ANOVA performed on continuous variables presented as mean (SD), and Kruskal–Wallis was performed on all other continuous variables. Chi-square or Fisher’s exact test was conducted as appropriate on all categorical variables. Statistically significant p values are denoted in bold.
Factors associated with prevalent liver-related outcomes
Twenty-one participants had 40 events at baseline. Higher LSM was associated with prevalent liver-related outcomes [odds ratio (OR) = 2.20 per 1 kPa increase; 95% confidence interval (CI): 1.70–2.84, p < 0.0001]. The association was amplified at higher LSM with MRE of 3.63–5.0 kPa and >5 kPa associated with an OR = 13.4 (95% CI: 2.7–66.6, p = 0.002) and OR = 41.6 (95% CI: 12.4–139.7, p < 0.001), respectively, for the cumulative outcome compared with MRE < 3.63 kPa and remained significant in multivariable analysis adjusted for age and sex: OR = 7.4 (95% CI: 1.3–41.5, p = 0.022) and OR = 26.2 (95% CI: 7.6–90.5, p < 0.001). In sensitivity analysis limited to subjects with NAFLD at baseline (n = 120), higher LSM with MRE of 3.63–5.0 kPa and >5 kPa associated with an OR = 12.7 (95% CI: 1.06–150.70, p = 0.045) and OR = 70.93 (95% CI: 8.66–580.87, p < 0.001), respectively, for the cumulative outcome compared with MRE < 3.63 kPa. Similarly, in the subset of patients who did not meet the study definition for NAFLD, the results remained consistent with MRE ⩾ 3.63 kPa associated with a higher risk of the primary outcome, OR = 15.33 (95% CI: 1.21–193.6, p = 0.035), compared with MRE < 3.63 kPa. A 1-unit increase in FIB-4 [OR = 1.94 (95% CI: 1.40–2.68, p < 0.001)] and NFS [OR = 1.73 (95% CI: 1.16–2.66, p = 0.01)] was associated with higher odds of liver-related outcome or death (Table 2).
Table 2.
Factors associated with prevalent liver-related outcomes.
| Liver-related outcomes or death OR (95% CI) |
p value | |
|---|---|---|
| MRE | ||
| <3.63 kPa (referent) | Ref | |
| 3.63–5 kPa | 13.37 (2.68–66.64) | 0.0015 |
| >5 kPa | 41.61 (12.40–139.66) | <0.0001 |
| Demographic and biochemical | ||
| BMI (kg/m2) | 1.04 (0.99–1.10) | 0.1397 |
| HbA1c (%) | 1.42 (1.03–1.95) | 0.0309 |
| AST (U/l) (per 5-unit increase) | 1.20 (1.10–1.30) | <0.0001 |
| ALT (U/l) (per 5-unit increase) | 1.04 (0.98–1.10) | 0.1881 |
| Alkaline phosphatase (U/l) (per 5-unit increase) | 1.25 (1.15–1.36) | <0.0001 |
| Total bilirubin (mg/dl) | 18.23 (6.55–50.77) | <0.0001 |
| Albumin (g/dl) | 0.007 (0.001–0.046) | <0.0001 |
| HOMA-IR | 1.07 (1.03–1.12) | 0.0026 |
| Triglycerides (mg/dl) (per 5-unit increase) | 1.02 (0.99–1.04) | 0.1409 |
| HDL (mg/dl) (per 5-unit increase) | 0.78 (0.65–0.94) | 0.0083 |
| LDL (mg/dl) (per 5-unit increase) | 0.88 (0.81–0.95) | 0.0017 |
| Platelet count (109/l) (per 10-unit increase) | 0.77 (0.71–0.84) | <0.0001 |
| Clinical score | ||
| FIB-4 | 2.39 (1.77–3.22) | <0.0001 |
| NAFLD Fibrosis Score | 3.07 (2.08–4.53) | <0.0001 |
| MELD score (per 1-unit increase) | 1.63 (1.36–1.96) | <0.0001 |
| Positive MEFIB | 42.89 (13.26–138.73) | <0.0001 |
| Imaging | ||
| MRI-PDFF | 0.90 (0.80–1.02) | 0.0959 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; FIB-4, fibrosis index based on the 4 factors; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment method for insulin resistance (calculated as (fasting insulin (μU/ml) × fasting glucose (mmol/l)) / 22.5); INR, international normalized ratio; LDL, low-density lipoprotein; MRI-PDFF, MRI-based proton density fat fraction. Statistically significant p values are denoted in bold.
Higher liver stiffness on MRE was associated with an increased risk for each liver-related event (Table 3). Liver-related events increased in frequency with higher categories of liver stiffness (<3.63 kPa, 3.63–5 kPa, and >5 kPa) for varices needing treatment (1.9%, 20%, and 34%), ascites (1.4%, 6.7%, and 41%), hepatic encephalopathy (1.4%, 13.3%, and 37.5%), and death (0.5%, 20%, and 18.8%). The risk of HCC was low in MRE < 3.63 (0.5%) but similar in 3.63–5 kPa (20.0%) and >5 kPa (18.8%). The results remained consistent on sensitivity analysis of a composite outcome of only hepatic decompensation (varices needing treatment or ascites or hepatic encephalopathy) [OR = 2.39 per 1 kPa increase (95% CI: 1.82–3.13, p ⩽ 0.0001)]. The ordinal categories 3.63–5.0 kPa and >5 kPa were associated with an OR = 13.19 (95% CI: 2.65–65.71, p = 0.0016) and OR = 52.75 (95% CI: 15.77–176.51, p ⩽ 0.0001), respectively, for the hepatic decompensation compared with MRE < 3.63 kPa. Similarly, in sensitivity analysis evaluating the association between MRE and risk of HCC, higher liver stiffness was associated with increased risk of HCC [OR = 1.55 per 1 kPa increase (95% CI: 1.26–1.91, p ⩽ 0.0001)]. The ordinal categories 3.63–5.0 kPa and >5 kPa were associated with an OR = 53.5 (95% CI: 5.17–553.55, p = 0.0008) and OR = 49.39 (95% CI: 5.72–426.42, p = 0.0004) for HCC compared with <3.63 kPa, respectively.
Table 3.
Liver-related outcomes and death by liver stiffness on MRE.
| Total number of events | MRE liver stiffness | p value a | |||
|---|---|---|---|---|---|
| <3.63 kPa N = 218 |
3.63–5 kPa N = 15 |
>5 kPa N = 32 |
|||
| Varices needing treatment, N (%) | 18 | 4 (1.9%) | 3 (20.0%) | 11 (34.4%) | <0.0001 |
| Ascites, N (%) | 17 | 3 (1.4%) | 1 (6.7%) | 13 (40.6%) | <0.0001 |
| Hepatic encephalopathy, N (%) | 17 | 3 (1.4%) | 2 (13.3%) | 12 (37.5%) | <0.0001 |
| Hepatocellular carcinoma, N (%) | 10 | 1 (0.5%) | 3 (20.0%) | 6 (18.8%) | <0.0001 |
| Liver transplant, N (%) | 4 | 0 | 0 | 4 (12.5%) | 0.0003 |
| Death, N (%) | 14 | 5 (2.3%) | 1 (6.7%) | 8 (25.0%) | <0.0001 |
p value calculated using Fisher’s exact test. Statistically significant p values are denoted in bold.
MEFIB index and liver-related outcomes or death
A positive MEFIB index, defined as a combination of MRE ⩾ 3.3 kPa and FIB-4 ⩾ 1.6, was present in 39 patients and had a strong association with prevalent and future incident liver-related outcomes or death compared with those with a negative MEFIB (59.0% versus 2.7%). The OR for the primary outcome was OR = 42.9 (95% CI: 13.3–138.7, p < 0.001) for a positive MEFIB versus a negative MEFIB and remained significant in multivariable analysis adjusted for age and sex, OR = 24.1 (95% CI: 7.2–81.4, p < 0.001).
Factors associated with incident liver-related outcomes or death
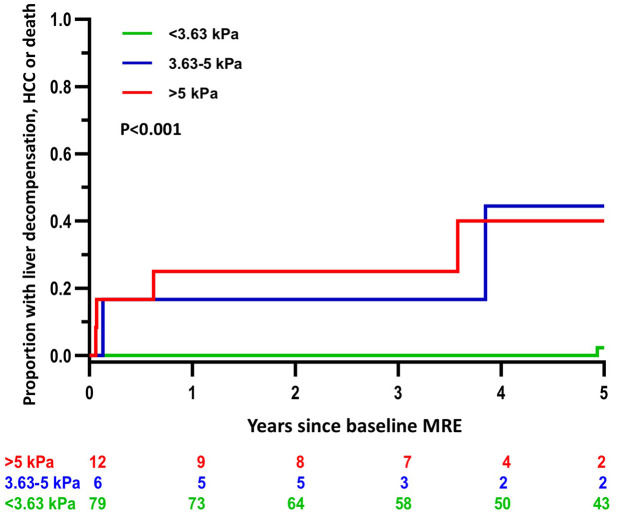
Among participants without liver-related events at baseline with longitudinal follow-up after MRE (n = 97 and 40.2% with NAFLD), 12 incident cumulative events occurred over 453 person-years of follow-up in eight patients. In addition, 24 incident events occurred in 21 patients with at least one event at baseline. The incidence of the cumulative outcome was 0.5%, 10.0%, and 10.3% per person-year in the <3.63 kPa, 3.63–5 kPa, and >5 kPa groups, respectively. The 3-year risk of incident decompensation, HCC, or death was 0% for MRE < 3.63 kPa, 16.7% for MRE = 3.63–5 kPa, and 25% for MRE > 5 kPa among those without a decompensating event at baseline. The incident risk of liver-related outcomes and death increased significantly across the three liver stiffness categories, p < 0.001 (Figure 1). A positive MEFIB index was also associated with a higher risk of incident events, HR = 21.84 (95% CI: 4.28–111.4, p < 0.001) (Table 4; Figure 2). Two incident events in patients with a negative MEFIB index occurred at 4.93 and 8.38 years after baseline. Importantly, the sensitivity and specificity of MEFIB for the cumulative outcome was 75% and 90%, and a negative score was associated with a high negative predictive value (NPV) of 98% for liver-related events or death.
Figure 1.
Five-year cumulative incidence of hepatic decompensation, hepatocellular carcinoma, or death by liver stiffness cut points on MRE < 3.63, 3.63–5 kPa, and >5 kPa.
Table 4.
Factors associated with incident liver-related outcomes or death.
| Liver-related outcomes or death Hazard ratios (95% CI) |
p value | |
|---|---|---|
| MRE | ||
| <3.63 kPa (referent) | ref | |
| 3.63–5 kPa | 17.09 (2.38–122.75) | 0.0048 |
| >5 kPa | 16.58 (2.90–94.79) | 0.0016 |
| Demographic and biochemical | ||
| BMI (kg/m2) | 1.02 (0.92–1.13) | 0.6660 |
| HbA1c (%) | 1.69 (0.91–3.14) | 0.0941 |
| AST (U/l) (per 5-unit increase) | 1.17 (1.07–1.28) | 0.0007 |
| ALT (U/l) (per 5-unit increase) | 1.13 (1.05–1.21) | 0.0008 |
| Alkaline phosphatase (U/l) (per 5-unit increase) | 1.11 (0.99–1.26) | 0.0823 |
| Total bilirubin (mg/dl) | 5.90 (1.25–27.82) | 0.0249 |
| Albumin (g/dl) | 0.16 (0.01–1.94) | 0.1512 |
| HOMA-IR | 1.03 (0.98–1.07) | 0.2637 |
| Triglycerides (mg/dl) (per 5-unit increase) | 1.03 (0.99–1.07) | 0.1790 |
| HDL (mg/dl) (per 5-unit increase) | 0.60 (0.40–0.89) | 0.0107 |
| LDL (mg/dl) (per 5-unit increase) | 0.96 (0.86–1.07) | 0.4486 |
| Platelet count (109/l) (per 10-unit increase) | 0.85 (0.76–0.96) | 0.0077 |
| Clinical score | ||
| FIB-4 | 1.94 (1.40–2.68) | <0.0001 |
| NAFLD Fibrosis Score | 1.73 (1.13–2.66) | 0.0115 |
| MELD score (per 1-unit increase) | 1.34 (1.07–1.69) | 0.0122 |
| Positive MEFIB | 21.84 (4.28–111.40) | 0.0002 |
| Imaging | ||
| MRI-PDFF | 1.03 (0.94–1.13) | 0.5187 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; FIB-4, fibrosis index based on the 4 factors; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment method for insulin resistance [calculated as (fasting insulin (μU/ml) × fasting glucose (mmol/l)) / 22.5]; INR, international normalized ratio; LDL, low-density lipoprotein; MRI-PDFF, MRI-based proton density fat fraction. Statistically significant p values are denoted in bold.
Figure 2.
Five-year cumulative incidence of hepatic decompensation, hepatocellular carcinoma, or death by MEFIB index, with positive defined as a combination of MRE ⩾ 3.3 kPa and FIB-4 ⩾ 1.6.
Discussion
Using a well-phenotyped cohort of subjects with MRE assessment and liver-related outcome assessment, we demonstrate that liver stiffness on MRE is strongly associated with liver-related outcomes or death. The risk of decompensation and death increased with liver stiffness greater than or equal to the established cut point of 3.63 kPa and was highest among those with liver stiffness greater than 5 kPa where there was a 25% risk of decompensation within 1 year. Importantly, MEFIB index, which is positive when MRE ⩾ 3.3 kPa and FIB-4 ⩾ 1.6, had high NPV, 98%, to rule out future liver-related events.
In context with published literature
MRE is the most accurate biomarker of liver fibrosis in NAFLD; 44 however, it has been evaluated primarily against liver biopsy as the reference standard. Liver biopsy interpretation suffers from only modest inter- and intra-observer agreement for the assessment of characteristics of histologic NAFLD including fibrosis stage.45,46 Instead, assessment of the direct association between MRE and liver-related outcomes and death is critical to determining its value as a biomarker of liver disease.
Matsui and colleagues performed a cross-sectional analysis evaluating the association between liver stiffness on MRE and gastroesophageal varices and demonstrated that a combination of MRE (<4.2 kPa) and platelet count (>180,000/µl) can rule out varices but did not report on other liver-related outcomes or conduct longitudinal analysis for incident events. 47 Han et al. 11 also evaluated the cross-sectional association between MRE and cirrhosis as well as decompensation and identified an optimal cut point of 6.48 kPa with moderate diagnostic accuracy, c = 0.71. Finally, Gidener et al. 12 performed a longitudinal study evaluating the ability to predict future cirrhosis development among noncirrhotic patients and the ability to predict hepatic decompensation among 194 patients with compensated NAFLD cirrhosis with longitudinal follow-up. While the authors report only a small increase in diagnostic accuracy in models combining FIB-4 and liver stiffness on MRE, the article did not evaluate the combination of the two tests to maximize sensitivity or specificity.
In this study, we evaluate prevalent liver-related outcomes and evaluate the incidence rate for future outcomes in a subset of patients with longitudinal follow-up without decompensation at baseline. Importantly, we explored the previously validated MEFIB, which had demonstrated high PPV for significant fibrosis and, in this study, demonstrated a high NPV for liver-related events in those with a negative score. The risk of incident decompensation was highest in those with MRE > 5 kPa with a stepwise increase for all hepatic decompensation except HCC, which had a similar risk in those with MRE = 3.63–5 kPa and those with MRE > 5 kPa. If validated, this finding may have implications for future thresholds for HCC screening guided by NITs. Importantly, two incident events did occur in the low-risk group approximately 5 and 8 years after MRE assessment supporting the need for ongoing follow-up in at-risk populations.
Strengths and limitations
Although the study evaluated a diverse, well-characterized cohort, certain limitations are acknowledged. Importantly, the cohort was single center and only a subset of patients had longitudinal follow-up with a limited number of events. However, the association between MRE and incident outcomes has been limited with only one other single center study published to date. 12 While our findings corroborate the prior study, we also evaluate a previously validated index: the MEFIB with practical implications for how a negative score could be used to effectively exclude liver-related events or death (NPV 98%). Furthermore, the study was retrospective; however, our study participants all had a standardized research visit at baseline to systematically evaluate for other causes of liver disease including alcohol, whereas previous studies have relied completely on chart review. Patients with NAFLD are at risk of extrahepatic events including major adverse cardiovascular events, which we did not capture. However, all-cause mortality among those without hepatic decompensation or HCC was low at 3 deaths and represented 21% of deaths in the study cohort. Finally, the study did not assess the impact of change in MRE value on liver-related outcomes, and patients may have participated in clinical trials during the study period affecting their risk of future outcomes. However, there is no US Food and Drug Administration (FDA)-approved treatment for NAFLD, and the impact of an effective treatment in a clinical trial would only attenuate the association between a single MRE and outcomes. Future, multicenter, prospective studies will be required to corroborate the findings and the association between liver stiffness on MRE and liver-related events and death.
Implications for future research
This study corroborates the importance a single LSM on MRE; however, future longitudinal studies of the implications of changes in liver stiffness over time are needed. In this study, no liver-related events happened within 4 years of a negative MEFIB index. Larger studies or meta-analysis may be required to better define the best interval for follow-up after a low liver stiffness value on MRE alone and in combination with other NITs. Future studies may consider evaluating other combinations of blood-based tests with MRE, including the Enhanced Liver Fibrosis (ELF) score, which has demonstrated an association with NAFLD progression. 48 Importantly, while the risk of portal hypertensive complications increased with higher liver stiffness, the risk of HCC appeared to plateau with LSM ⩾ 3.63 kPa, and future studies should evaluate how to incorporate liver stiffness on MRE into identifying high-risk NAFLD populations for HCC screening who do not have biopsy-proven cirrhosis. In conclusion, liver stiffness on MRE has a strong association with future liver-related events and death, and the combination of MRE and FIB-4 using the MEFIB threshold demonstrated high accuracy to exclude liver-related events or death.
Supplemental Material
Supplemental material, sj-jpg-1-tag-10.1177_17562848221093869 for Prognostic utility of magnetic resonance elastography and MEFIB index in predicting liver-related outcomes and mortality in individuals at risk of and with nonalcoholic fatty liver disease by Veeral Ajmera, Khang Nguyen, Nobuharu Tamaki, Suzanne Sharpton, Ricki Bettencourt and Rohit Loomba in Therapeutic Advances in Gastroenterology
Footnotes
Author contributions: Veeral Ajmera: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Khang Nguyen: Data curation; Writing – review & editing.
Nobuharu Tamaki: Data curation; Writing – review & editing.
Suzanne Sharpton: Writing – review & editing.
Ricki Bettencourt: Data curation; Formal analysis; Writing – review & editing.
Rohit Loomba: Conceptualization; Data curation; Investigation; Methodology; Resources; Supervision; Writing – review & editing.
ORCID iDs: Suzanne Sharpton  https://orcid.org/0000-0001-7722-5716
https://orcid.org/0000-0001-7722-5716
Rohit Loomba  https://orcid.org/0000-0002-4845-9991
https://orcid.org/0000-0002-4845-9991
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: RL receives additional funding support from NIEHS (5P42ES010337), NCATS (5UL1TR001442), NIDDK (R01DK106419 and P30DK120515), and DOD PRCRP (CA170674P2). VA is supported by NIDDK (K23DK119460).
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Loomba serves as a consultant or advisory board member for Bird Rock Bio, Celgene, Enanta, GRI Bio, Madrigal, Metacrine, NGM, Sanofi, Arrowhead Research, Galmed, GNI, Novo Nordisk, Merck, Siemens, Pfizer, Gilead, and Glympse Bio. In addition, his institution has received grant support from Allergan, BMS, BI, Daiichi-Sankyo Inc., Eli-Lilly, Galectin, Galmed, GE, Genfit, Intercept, Janssen Inc, Madrigal, Merck, NGM, Pfizer, Prometheus, Siemens, and Sirius. He is also the co-founder of Liponexus Inc.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Veeral Ajmera, NAFLD Research Center, Division of Gastroenterology, University of California at San Diego, 9500 Gilman Drive, ACTRI Building, 1W507, La Jolla, CA 92093-0887, USA.
Khang Nguyen, NAFLD Research Center, Division of Gastroenterology, University of California at San Diego, La Jolla, CA, USA.
Nobuharu Tamaki, NAFLD Research Center, Division of Gastroenterology, University of California at San Diego, La Jolla, CA, USADepartment of Gastroenterology and Hepatology, Musashino Red Cross Hospital, Tokyo, Japan.
Suzanne Sharpton, NAFLD Research Center, Division of Gastroenterology, University of California at San Diego, La Jolla, CA, USA.
Ricki Bettencourt, NAFLD Research Center, Division of Gastroenterology, University of California at San Diego, La Jolla, CA, USA.
Rohit Loomba, NAFLD Research Center, Division of Gastroenterology, University of California at San Diego, 9500 Gilman Drive, ACTRI Building, 2W202, La Jolla, CA 92093-0887, USA.
References
- 1. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64: 73–84. [DOI] [PubMed] [Google Scholar]
- 2. Loomba R, Wong R, Fraysse J, et al. Nonalcoholic fatty liver disease progression rates to cirrhosis and progression of cirrhosis to decompensation and mortality: a real world analysis of Medicare data. Aliment Pharmacol Ther 2020; 51: 1149–1159. [DOI] [PubMed] [Google Scholar]
- 3. Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021; 184: 2537–2564. [DOI] [PubMed] [Google Scholar]
- 4. Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology 2017; 65: 1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Venkatesh SK, Wang G, Teo LL, et al. Magnetic resonance elastography of liver in healthy Asians: normal liver stiffness quantification and reproducibility assessment. J Magn Reson Imaging 2014; 39: 1–8. [DOI] [PubMed] [Google Scholar]
- 6. Park CC, Nguyen P, Hernandez C, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology 2017; 152: 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hsu C, Caussy C, Imajo K, et al. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol 2019; 17: 630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ajmera VH, Liu A, Singh S, et al. Clinical utility of an increase in magnetic resonance elastography in predicting fibrosis progression in nonalcoholic fatty liver disease. Hepatology 2020; 71: 849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakajima A, Eguchi Y, Yoneda M, et al. Randomised clinical trial: pemafibrate, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), versus placebo in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2021; 54: 1263–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Flint A, Andersen G, Hockings P, et al. Randomised clinical trial: semaglutide versus placebo reduced liver steatosis but not liver stiffness in subjects with non-alcoholic fatty liver disease assessed by magnetic resonance imaging. Aliment Pharmacol Ther 2021; 54: 1150–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Han MAT, Vipani A, Noureddin N, et al. MR elastography-based liver fibrosis correlates with liver events in nonalcoholic fatty liver patients: a multicenter study. Liver Int 2020; 40: 2242–2251. [DOI] [PubMed] [Google Scholar]
- 12. Gidener T, Ahmed OT, Larson JJ, et al. Liver stiffness by magnetic resonance elastography predicts future cirrhosis, decompensation and death in NAFLD. Clin Gastroenterol Hepatol 2021; 19: 1915–1924.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tamaki N, Higuchi M, Kurosaki M, et al. Risk difference of liver-related and cardiovascular events by liver fibrosis status in nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. Epub ahead of print 16 July 2021. DOI: 10.1016/j.cgh.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Higuchi M, Tamaki N, Kurosaki M, et al. Longitudinal association of magnetic resonance elastography-associated liver stiffness with complications and mortality. Aliment Pharmacol Ther 2022; 55: 292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hagström H, Nasr P, Ekstedt M, et al. Accuracy of noninvasive scoring systems in assessing risk of death and liver-related endpoints in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2019; 17: 1148–1156. [DOI] [PubMed] [Google Scholar]
- 16. Younes R, Caviglia GP, Govaere O, et al. Long-term outcomes and predictive ability of non-invasive scoring systems in patients with non-alcoholic fatty liver disease. J Hepatol 2021; 75: 786–794. [DOI] [PubMed] [Google Scholar]
- 17. Tamaki N, Kurosaki M, Takahashi Y, et al. Liver fibrosis and fatty liver as independent risk factors for cardiovascular disease. J Gastroenterol Hepatol 2021; 36: 2960–2966. [DOI] [PubMed] [Google Scholar]
- 18. Petta S, Sebastiani G, Viganò M, et al. Monitoring occurrence of liver-related events and survival by transient elastography in patients with nonalcoholic fatty liver disease and compensated advanced chronic liver disease. Clin Gastroenterol Hepatol 2021; 19: 806–815.e5. [DOI] [PubMed] [Google Scholar]
- 19. Newsome PN, Sasso M, Deeks JJ, et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol 2020; 5: 362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jung J, Loomba RR, Imajo K, et al. MRE combined with FIB-4 (MEFIB) index in detection of candidates for pharmacological treatment of NASH-related fibrosis. Gut 2021; 70: 1946–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Augustin S, Pons M, Maurice JB, et al. Expanding the Baveno VI criteria for the screening of varices in patients with compensated advanced chronic liver disease. Hepatology 2017; 66: 1980–1988. [DOI] [PubMed] [Google Scholar]
- 22. Le TA, Chen J, Changchien C, et al. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology 2012; 56: 922–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Loomba R, Sirlin CB, Ang B, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology 2015; 61: 1239–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Loomba R, Schork N, Chen CH, et al. Heritability of Hepatic Fibrosis and Steatosis Based on a Prospective Twin Study. Gastroenterology 2015; 149: 1784–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Doycheva I, Cui J, Nguyen P, et al. Non-invasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE. Aliment Pharmacol Ther 2016; 43: 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Loomba R, Cui J, Wolfson T, et al. Novel 3D magnetic resonance elastography for the noninvasive diagnosis of advanced fibrosis in NAFLD: a prospective study. Am J Gastroenterol 2016; 111: 986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 28. Caussy C, Soni M, Cui J, et al. Nonalcoholic fatty liver disease with cirrhosis increases familial risk for advanced fibrosis. J Clin Invest 2017; 127: 2697–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43: 1317–1325. [DOI] [PubMed] [Google Scholar]
- 30. Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45: 846–854. [DOI] [PubMed] [Google Scholar]
- 31. Tang A, Desai A, Hamilton G, et al. Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology 2015; 274: 416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tang A, Tan J, Sun M, et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology 2013; 267: 422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reeder SB, Robson PM, Yu H, et al. Quantification of hepatic steatosis with MRI: the effects of accurate fat spectral modeling. J Magn Reson Imaging 2009; 29: 1332–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hines CD, Frydrychowicz A, Hamilton G, et al. T(1) independent, T(2) (*) corrected chemical shift based fat-water separation with multi-peak fat spectral modeling is an accurate and precise measure of hepatic steatosis. J Magn Reson Imaging 2011; 33: 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Permutt Z, Le TA, Peterson MR, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non–alcoholic fatty liver disease – MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther 2012; 36: 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Imajo K, Kessoku T, Honda Y, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology 2016; 150: 626–637. [DOI] [PubMed] [Google Scholar]
- 37. Hsu E, Feghali-Bostwick CA. Insulin-like growth factor-II is increased in systemic sclerosis-associated pulmonary fibrosis and contributes to the fibrotic process via Jun N-terminal kinase- and phosphatidylinositol-3 kinase-dependent pathways. Am J Pathol 2008; 172: 1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J Hepatol 2016; 65: 1006–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Loomba R, Wolfson T, Ang B, et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology 2014; 60: 1920–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tamaki N, Imajo K, Sharpton S, et al. MRE plus FIB-4 (MEFIB) versus FAST in detection of candidates for pharmacological treatment of NASH-related fibrosis. Hepatology 2021; 75: 661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2021; 74: 1014–1048. [DOI] [PubMed] [Google Scholar]
- 42. Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study Of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014; 60: 715–735. [DOI] [PubMed] [Google Scholar]
- 43. Garcia-Tsao G, Abraldes JG, Berzigotti A, et al. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017; 65: 310–335. [DOI] [PubMed] [Google Scholar]
- 44. Ajmera V, Loomba R. Imaging biomarkers of NAFLD, NASH, and fibrosis. Mol Metab 2021; 50: 101167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005; 128: 1898–1906. [DOI] [PubMed] [Google Scholar]
- 46. Davison BA, Harrison SA, Cotter G, et al. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J Hepatol 2020; 73: 1322–1332. [DOI] [PubMed] [Google Scholar]
- 47. Matsui N, Imajo K, Yoneda M, et al. Magnetic resonance elastography increases usefulness and safety of non-invasive screening for esophageal varices. J Gastroenterol Hepatol 2018; 33: 2022–2028. [DOI] [PubMed] [Google Scholar]
- 48. Sanyal AJ, Harrison SA, Ratziu V, et al. The natural history of advanced fibrosis due to nonalcoholic steatohepatitis: data from the simtuzumab trials. Hepatology 2019; 70: 1913–1927. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-jpg-1-tag-10.1177_17562848221093869 for Prognostic utility of magnetic resonance elastography and MEFIB index in predicting liver-related outcomes and mortality in individuals at risk of and with nonalcoholic fatty liver disease by Veeral Ajmera, Khang Nguyen, Nobuharu Tamaki, Suzanne Sharpton, Ricki Bettencourt and Rohit Loomba in Therapeutic Advances in Gastroenterology