Abstract
Objective:
Comorbidities and risk factors have a major implication on incidence, complications, mortality, and management of community-acquired pneumonia complications and treatment outcomes. This study attempts to identify the same in the Indian population through a systematic review and meta-analysis.
Methods:
We screened observational studies (between January 1990 and February 2021) that reported potential comorbidities and other factors associated with increased risk of community-acquired pneumonia in the Indian population (⩾12 years) using PubMed, Google Scholar, and manual search. The risk of bias was identified using the Joanna Briggs Institute checklist for prevalence studies. Meta-analysis was conducted by using the random intercept logistic regression model.
Results:
Twenty-three studies were included in this analysis. The most prevalent comorbidities were chronic obstructive pulmonary disease (24.2%; 95% confidence interval: 16.4%–34.2%), hypertension (23.7%; 95% confidence interval: 13.6%–38.1%), and diabetes mellitus (16%; 95% confidence interval: 9.9%–24.7%). The prevalence of community-acquired pneumonia was high in patients with a current or previous history of smoking (51.4%; 95% confidence interval: 42.3%–61%) and advanced age ⩾50 years: (55.8%; 95% confidence interval: 48.4%–62%).
Conclusions:
Comorbid conditions like chronic obstructive pulmonary disease, hypertension, and diabetes mellitus and factors like advanced age and smoking history were common risk factors for community-acquired pneumonia in the Indian population.
Keywords: Community-acquired pneumonia, comorbidity, risk factor, India
Introduction
Community-acquired pneumonia (CAP) is a common infectious disease worldwide. Developing countries, including India, bear a significant brunt of the disease, impacting healthcare. India alone accounts for about one-fourth of the global pneumonia burden, with a case fatality rate (CFR) of 14%–30%. 1
Comorbidities and risk factors have major implications on the incidence, complications, mortality, and management of CAP, 2 posing significant challenges for clinicians. A study in Europe revealed that comorbid pathologies (chronic respiratory and cardiovascular diseases, dementia, cerebrovascular disease, human immunodeficiency virus (HIV), and chronic renal and liver disease) increase the risk of CAP by 2- to 4-fold. The same study also identified smoking and alcohol abuse as common risk factors associated with the disease. 2 Similar studies in the United States also reported advancing age as a common risk factor for increased incidence and related mortality.3,4
In India, there is limited information about the comorbid conditions and risk factors associated with increased risk of CAP. Although a few studies have reported such data, the evidence is scattered, and no comprehensive analysis is available to date.
Our study is the first systematic review and meta-analysis conducted to identify the comorbid conditions and risk factors that increase the risk of CAP in the Indian population. The results of this study would help clinicians implement targeted risk-reduction measures to reduce the disease burden of CAP in the country.
Methods
We conducted this study as per the Preferred Recording Items for Systematic Reviews and Meta-Analysis (PRISMA) statements (S1 PRISMA checklist). 5 Our study did not require ethical board approval because it did not contain human or animal trials.
Eligibility criteria
We included observational studies (cross-sectional studies and prospective or retrospective cohort studies) on patients with CAP, published in the last 31 years (January 1990–December 2020), that reported comorbidities and/or risk factors associated with CAP in Indian patients (>12 years of age). We excluded studies conducted outside India, in pediatric populations (<12 years of age), or in a language other than English. We also excluded case series, case reports, guidelines, and studies conducted outside the search period specified above.
Information sources
We systematically searched the following databases: PubMed; Google Scholar; National Institute of Science Communication, and Information Resources (NISCAIR); and Annotated Bibliography of Indian Medicine (ABIM), using a set of keywords such as “Community-Acquired Pneumonia,” “Incidence,” “comorbidities,” “risk factors,” and “bacterial etiology.” The reference list of retrieved studies was also screened to identify additional studies.
Data extraction
After removing the duplicates, we further screened the articles for eligibility and extracted the relevant information from eligible studies. Eligibility assessment and data extraction were done by two reviewers, and discrepancy, if any, was resolved by consensus.
Measurements
The two primary outcomes were the proportion of CAP patients with (a) comorbidity (clinical condition(s) simultaneously present in a patient) and (b) associated risk factors (factor(s) increasing an individual’s chances of developing a disease). Secondary outcomes were mortality and duration of hospital stay. Sensitivity analysis was carried out when data were arbitrary or unclear to determine the robustness of the outcomes to the assumptions made in performing the analysis. Sensitivity analysis was performed where required by omitting data, one at a time, to explore the effect of individual data on the overall pooled proportion.
Risk of bias
We identified the risk of bias in the included studies using the Joanna Briggs Institute Prevalence Critical Appraisal Tool. 6 This checklist had nine questions to which the reviewer responded as “Yes,” “No,” “unclear,” or “not applicable.” Each question to which the reviewer marked “Yes” was given one point. These scores were summed up and converted into a percentage. The risk of bias was performed by one reviewer and cross-checked by the second reviewer. Any discrepancy was resolved by consensus. The studies which obtained more than 60% as per the reviewer’s judgment were included in the analysis 7 (Supplemental Table 1).
Statistical analysis
Meta-analysis was carried out by using R Studio version 1.4.1106© 2009–2021. Entire data computations and results were done in R Studio. The proportion of CAP patients with comorbid conditions and potential risk factors was estimated with a 95% confidence interval (CI). The forest plot diagram was used to visualize heterogeneity among the studies. Degree of heterogeneity (I2 and Cochrane Q statistics, p value < 0.1) was used to quantify the observed variations with values of 25%, 50%, and 75% representing low, moderate, and high levels of heterogeneity, respectively.
Results
Literature search and screening
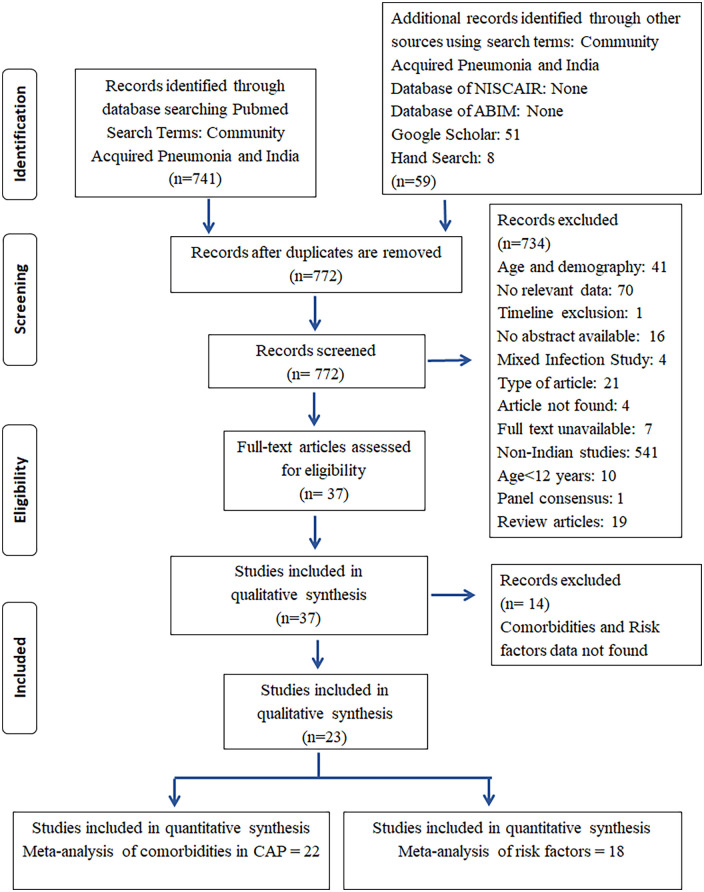
The PRISMA flowchart summarizing the entire search process is given in Figure 1. A total of 799 studies were retrieved from PubMed, Google Scholar, and hand search. No relevant study was obtained from ABIM and NISCAIR databases. Observational studies (cross-sectional studies and prospective or retrospective cohort studies) enrolling hospitalized as well as ambulatory patients with CAP were included. In all, 772 citations were identified after removing the duplicates. A total of 23 studies8–30 were included in the qualitative synthesis of which 22 studies reporting different comorbidities8–29 were considered for the quantitative synthesis of CAP patients with comorbidities and 18 studies8–10,12–17,20–22,25–30 reporting various risk factors were utilized for identifying the associated risk factors. Table 1 represents the characteristics of the studies included in the analysis.
Figure 1.
PRISMA flow diagram.
PRISMA: Preferred Recording Items for Systematic Reviews and Meta-Analysis; NISCAIR: National Institute of Science Communication and Information Resources; ABIM: Annotated Bibliography of Indian Medicine.
Table 1.
Characteristics of the study included in the analysis.
| First author | Year of publication | Study design | Setting | Age (or range) of enrolled patients (in years) | Number of patients with CAP |
|---|---|---|---|---|---|
| Dutt et al. 8 | 2014 | Retrospective observational study | Tertiary care center | 30–75 | 105 |
| Shah et al. 9 | 2010 | Prospective observational study | Tertiary care hospital | >65 | 150 |
| Dharmadhikari et al. 10 | 2013 | Prospective observational study | Tertiary care hospital | >15 | 65 |
| Sreekanth and Reddy 11 | 2015 | Prospective observational study | Tertiary care hospital | >18 | 50 |
| Dey et al. 12 | 1997 | Prospective observational study | Tertiary care hospital | ⩾50 | 72 |
| Bansal et al. 13 | 2004 | Prospective observational study | Tertiary care hospital | >15 | 70 |
| Shah et al. 14 | 2010 | Prospective observational study | Tertiary care hospital | 15–80 | 100 |
| Jain et al. 15 | 2014 | Prospective observational study | Tertiary care hospital | >15 | 120 |
| Kejriwal et al. 16 | 2015 | Prospective observational study | Tertiary care hospital | >14 | 60 |
| Shrikhande et al. 17 | 2015 | Prospective observational study | Tertiary care hospital | >12 | 50 |
| Acharya et al. 18 | 2014 | Prospective observational cross-sectional study | Tertiary care hospital | 14–70 | 100 |
| Kotwani et al. 19 | 2015 | Retrospective observational cross-sectional study | Tertiary care hospital | >18 | 261 |
| Ravindranath and Raju 20 | 2016 | Prospective observational study | Tertiary care hospital | 55.71 | 150 |
| Para et al. 21 | 2018 | Prospective observational study | Tertiary care cum referral facility | ⩾18 | 225 |
| Lamb and Patil 22 | 2018 | Observational prospective descriptive study | Tertiary care hospital | >12 | 50 |
| Ayyappa et al. 23 | 2018 | Retrospective study | Tertiary care hospital | 14–78 | 100 |
| Mane et al. 24 | 2018 | Prospective observational study | Urban hospital | >18 | 121 |
| Kallakatta and Kuruvilla 25 | 2017 | Prospective study | Tertiary care hospital | 19–90 | 102 |
| Roshni et al. 26 | 2018 | Observational study | Tertiary care hospital | >15 | 50 |
| Mahendra et al. 27 | 2018 | Prospective study | Tertiary care hospital | 54.03 | 100 |
| Aruna et al. 28 | 2019 | Prospective observational study | Tertiary care hospital | 19–88 | 60 |
| Vanjare et al. 29 | 2020 | Retrospective study | Tertiary care center | 70.4 ± 8.1 | 108 |
| Kanishan et al. 30 | 2020 | Descriptive study | Tertiary care center | >18 | 220 |
CAP: community-acquired pneumonia.
Primary outcomes
Comorbidities
Chronic obstructive pulmonary disease
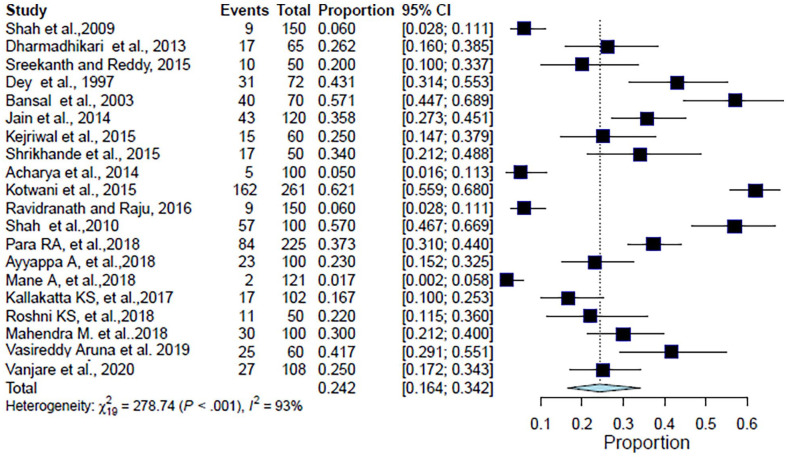
The analysis included 2114 patients from 20 studies. The pooled proportion of CAP patients with chronic obstructive pulmonary disease (COPD) as a comorbid condition was 24.2% (95% CI: 16.4%–34.2%, I2 = 93.2%, p < 0.001) (Table 2, Figure 2). The forest plot showed a significant degree of heterogeneity (Figure 2).9–21,23–29
Table 2.
Study-wise details of comorbidities in community-acquired pneumonia in Indian studies.
| Comorbid condition | Number of comorbid subjects | Total number of subjects with CAP | Proportion | 95% CI | Reference |
|---|---|---|---|---|---|
| Diabetes mellitus | 96 | 105 | 0.914 | (0.844–0.960) | Dutt et al. 8 |
| 24 | 150 | 0.160 | (0.105–0.229) | Shah et al. 9 | |
| 13 | 65 | 0.200 | (0.111–0.318) | Dharmadhikari et al. 10 | |
| 8 | 50 | 0.160 | (0.072–0.291) | Sreekanth and Reddy 11 | |
| 6 | 72 | 0.083 | (0.031–0.173) | Dey et al. 12 | |
| 3 | 70 | 0.043 | (0.009–0.120) | Bansal et al. 13 | |
| 13 | 100 | 0.130 | (0.071–0.212) | Shah et al. 14 | |
| 08 | 120 | 0.067 | (0.029–0.127) | Jain et al. 15 | |
| 13 | 60 | 0.217 | (0.121–0.342) | Kejriwal et al. 16 | |
| 06 | 50 | 0.120 | (0.045–0.243) | Shrikhande et al. 17 | |
| 10 | 100 | 0.100 | (0.049–0.176) | Acharya et al. 18 | |
| 12 | 261 | 0.046 | (0.024–0.079) | Kotwani et al. 19 | |
| 24 | 150 | 0.160 | (0.105–0.229) | Ravindranath and Raju 20 | |
| 36 | 225 | 0.160 | (0.115–0.215) | Para et al. 21 | |
| 10 | 100 | 0.100 | (0.049–0.176) | Ayyappa et al. 23 | |
| 6 | 50 | 0.120 | (0.045–0.243) | Lamb and Patil 22 | |
| 2 | 121 | 0.017 | (0.002–0.058) | Mane et al. 24 | |
| 25 | 102 | 0.245 | (0.165–0.340) | Kallakatta and Kuruvilla 25 | |
| 6 | 50 | 0.120 | (0.045–0.243) | Roshni et al. 26 | |
| 25 | 100 | 0.250 | (0.169–0.347) | Mahendra et al. 27 | |
| 20 | 60 | 0.333 | (0.217–0.467) | Aruna et al. 28 | |
| 74 | 104 | 0.712 | (0.614–0.796) | Vanjare et al. 29 | |
| Total | 440 | 2265 | – | – | – |
| Hypertension | 80 | 105 | 0.762 | (0.669–0.840) | Dutt et al. 8 |
| 54 | 150 | 0.360 | (0.283–0.442) | Shah et al. 9 | |
| 3 | 50 | 0.060 | (0.013–0.166) | Sreekanth and Reddy 11 | |
| 8 | 72 | 0.111 | (0.049–0.207) | Dey et al. 12 | |
| 6 | 50 | 0.120 | (0.045–0.243) | Shrikhande et al. 17 | |
| 54 | 150 | 0.360 | (0.283–0.442) | Ravindranath and Raju 20 | |
| 92 | 225 | 0.409 | (0.344–0.476) | Para et al. 21 | |
| 8 | 100 | 0.080 | (0.035–0.152) | Ayyappa et al. 23 | |
| 6 | 50 | 0.120 | (0.045–0.243) | Lamb and Patil 22 | |
| 4 | 50 | 0.080 | (0.022–0.192) | Roshni et al. 26 | |
| 41 | 100 | 0.410 | (0.313–0.513) | Mahendra et al. 27 | |
| 54 | 108 | 0.500 | (0.402–0.598) | Vanjare et al. 29 | |
| Total | 410 | 1210 | – | – | – |
| Chronic obstructive pulmonary disease | 9 | 150 | 0.060 | (0.028–0.111) | Shah et al. 9 |
| 17 | 65 | 0.262 | (0.160–0.385) | Dharmadhikari et al. 10 | |
| 10 | 50 | 0.200 | (0.100–0.337) | Sreekanth and Reddy 11 | |
| 31 | 72 | 0.431 | (0.314–0.553) | Dey et al. 12 | |
| 40 | 70 | 0.571 | (0.447–0.689) | Bansal et al. 13 | |
| 57 | 100 | 0.570 | (0.467–0.669) | Shah et al. 14 | |
| 43 | 120 | 0.358 | (0.273–0.451) | Jain et al. 15 | |
| 15 | 60 | 0.250 | (0.147–0.379) | Kejriwal et al. 16 | |
| 17 | 50 | 0.340 | (0.212–0.488) | Shrikhande et al. 17 | |
| 5 | 100 | 0.050 | (0.016–0.113) | Acharya et al. 18 | |
| 162 | 261 | 0.621 | (0.559–0.680) | Kotwani et al. 19 | |
| 9 | 150 | 0.060 | (0.028–0.111) | Ravindranath and Raju 20 | |
| 84 | 225 | 0.373 | (0.310–0.440) | Para et al. 21 | |
| 23 | 100 | 0.230 | (0.152–0.325) | Ayyappa et al. 23 | |
| 2 | 121 | 0.017 | (0.002–0.058) | Mane et al. 24 | |
| 17 | 102 | 0.167 | (0.100–0.253) | Kallakatta and Kuruvilla 25 | |
| 11 | 50 | 0.220 | (0.115–0.360) | Roshni et al. 26 | |
| 30 | 100 | 0.300 | (0.212–0.400) | Mahendra et al. 27 | |
| 25 | 60 | 0.417 | (0.291–0.551) | Aruna et al. 28 | |
| 27 | 108 | 0.250 | (0.172–0.343) | Vanjare et al. 29 | |
| Total | 634 | 2114 | – | – | – |
| Asthma | 2 | 65 | 0.031 | (0.004–0.107) | Dharmadhikari et al. 10 |
| 9 | 72 | 0.125 | (0.059–0.224) | Dey et al. 12 | |
| 10 | 100 | 0.100 | (0.049–0.176) | Acharya et al. 18 | |
| 6 | 50 | 0.120 | (0.045–0.243) | Lamb and Patil 22 | |
| 1 | 121 | 0.008 | (0.000–0.045) | Mane et al. 24 | |
| 11 | 100 | 0.110 | (0.056–0.188) | Mahendra et al. 27 | |
| Total | 39 | 508 | – | – | – |
| Chronic kidney disease | 1 | 65 | 0.015 | (0.000–0.083) | Dharmadhikari et al. 10 |
| 2 | 50 | 0.040 | (0.005–0.137) | Sreekanth and Reddy 11 | |
| 1 | 72 | 0.014 | (0.000–0.075) | Dey et al. 12 | |
| 1 | 70 | 0.014 | (0.000–0.077) | Bansal et al. 13 | |
| 27 | 225 | 0.120 | (0.081–0.170) | Para et al. 21 | |
| 4 | 100 | 0.040 | (0.011–0.099) | Ayyappa et al. 23 | |
| 5 | 100 | 0.050 | (0.016–0.113) | Mahendra et al. 27 | |
| Total | 41 | 682 | – | – | – |
| Heart diseases | 4 | 65 | 0.062 | (0.017–0.150) | Dharmadhikari et al. 10 |
| 3 | 50 | 0.060 | (0.013–0.165) | Sreekanth and Reddy 11 | |
| 8 | 72 | 0.111 | (0.049–0.207) | Dey et al. 12 | |
| 5 | 70 | 0.071 | (0.024–0.159) | Bansal et al. 13 | |
| 2 | 261 | 0.008 | (0.001–0.027) | Kotwani et al. 19 | |
| 40 | 120 | 0.333 | (0.250–0.425) | Jain et al. 15 | |
| 24 | 225 | 0.107 | (0.070–0.155) | Para et al. 21 | |
| 7 | 60 | 0.117 | (0.048–0.226) | Aruna et al. 28 | |
| Total | 93 | 923 | – | – | |
| Tuberculosis | 2 | 72 | 0.028 | (0.003–0.097) | Dey et al. 12 |
| 11 | 100 | 0.110 | (0.056–0.188) | Acharya et al. 18 | |
| 5 | 50 | 0.100 | (0.033–0.218) | Lamb and Patil 22 | |
| 14 | 100 | 0.140 | (0.079–0.224) | Mahendra et al. 27 | |
| Total | 32 | 322 | – | ||
| Neoplastic diseases | 1 | 65 | 0.015 | (0.000–0.083) | Dharmadhikari et al. 10 |
| 7 | 72 | 0.097 | (0.040–0.190) | Dey et al. 12 | |
| 2 | 70 | 0.029 | (0.003–0.099) | Bansal et al. 13 | |
| 26 | 100 | 0.260 | (0.177–0.357) | Acharya et al. 18 | |
| 2 | 261 | 0.008 | (0.001–0.027) | Kotwani et al. 19 | |
| Total | 38 | 568 | – | – | – |
| 2 | 65 | 0.031 | (0.004–0.107) | Dharmadhikari et al. 10 | |
| 21 | 100 | 0.210 | (0.135–0.303) | Shah et al. 14 | |
| 2 | 72 | 0.028 | (0.003–0.097) | Dey et al. 12 | |
| 18 | 102 | 0.176 | (0.108–0.264) | Kallakatta and Kuruvilla 25 | |
| Total | 43 | 339 | – | – | – |
| Cerebrovascular accident | 2 | 65 | 0.031 | (0.004–0.107) | Dharmadhikari et al. 10 |
| 2 | 70 | 0.029 | (0.003–0.099) | Bansal et al. 13 | |
| 3 | 120 | 0.025 | (0.005–0.071) | Jain et al. 15 | |
| 3 | 60 | 0.050 | (0.010–0.139) | Aruna et al. 28 | |
| Total | 10 | 315 | – | – | – |
| Chronic liver diseases | 1 | 50 | 0.020 | (0.001–0.106) | Sreekanth and Reddy 11 |
| 2 | 70 | 0.029 | (0.003–0.099) | Bansal et al. 13 | |
| 3 | 120 | 0.025 | (0.005–0.071) | Jain et al. 15 | |
| 3 | 225 | 0.013 | (0.003–0.038) | Para et al. 21 | |
| 4 | 121 | 0.033 | (0.009–0.082) | Mane et al. 24 | |
| 4 | 60 | 0.067 | (0.018–0.162) | Aruna et al. 28 | |
| Total | 17 | 646 | – | – | – |
| Altered consciousness | 8 | 100 | 0.080 | (0.035–0.152) | Shah et al. 14 |
| 13 | 60 | 0.217 | (0.121–0.342) | Kejriwal et al. 16 | |
| 43 | 225 | 0.191 | (0.142–0.249) | Para et al. 21 | |
| 3 | 50 | 0.060 | (0.013–0.165) | Lamb and Patil 22 | |
| 5 | 102 | 0.050 | (0.016–0.111) | Kallakatta and Kuruvilla 25 | |
| 10 | 60 | 0.167 | (0.083–0.285) | Aruna et al. 28 | |
| Total | 82 | 597 | – | – | – |
| HIV | 2 | 50 | 0.040 | (0.004–0.137) | Sreekanth and Reddy 11 |
| 8 | 50 | 0.160 | (0.071–0.291) | Lamb and Patil 22 | |
| 3 | 100 | 0.030 | (0.006–0.085) | Mahendra et al. 27 | |
| Total | 13 | 200 | |||
| Bronchiectasis | 1 | 72 | 0.014 | (0.000–0.075) | Dharmadhikari et al. 10 |
| 4 | 65 | 0.062 | (0.017–0.150) | Dey et al. 12 | |
| 13 | 100 | 0.130 | (0.071–0.212) | Mahendra et al. 27 | |
| Total | 18 | 237 | – | – | – |
CAP: community-acquired pneumonia; CI: confidence interval; HIV: human immunodeficiency virus.
Figure 2.
Forest plot for meta-analysis of the proportion of CAP patients with COPD.
CAP: community-acquired pneumonia; COPD: chronic obstructive pulmonary disease; CI: confidence interval.
Hypertension
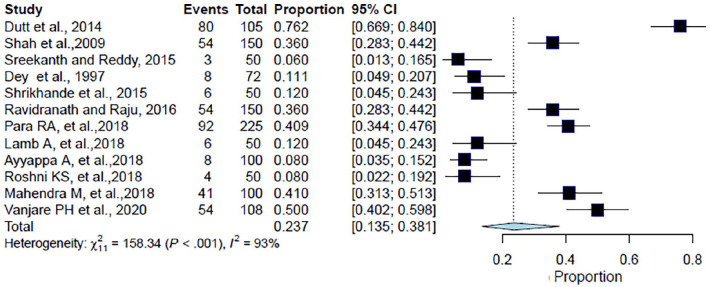
Of the 1210 patients included in the analysis from 12 studies, the pooled proportion of CAP patients with hypertension as a comorbid condition was 23.7% (95% CI: 13.5%–38.1%, I2 = 93.1%, p < 0.001) (Table 2, Figure 3). The forest plot showed a significant degree of heterogeneity (Figure 3).8,9,11,12,17,20–23,26,27,29
Figure 3.
Forest plot for meta-analysis of the proportion of CAP patients with hypertension.
CAP: community-acquired pneumonia; CI: confidence interval.
Diabetes mellitus
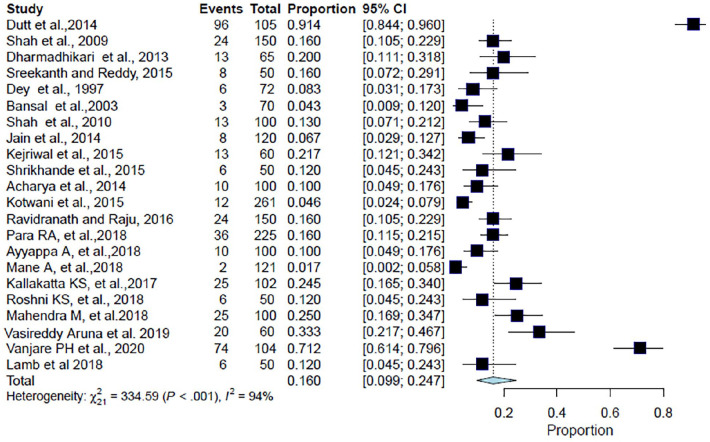
Overall, 2265 patients were included in the analysis from 22 studies. The pooled proportion of CAP patients with diabetes was found to be 16% (95% CI: 9.9%–24.7%; I2 = 93.7%; p < 0.001) (Table 2, Figure 4). The forest plot showed a significant degree of heterogeneity (Figure 4).8–29
Figure 4.
Forest plot for meta-analysis of the proportion of CAP patients with diabetes.
CAP: community-acquired pneumonia; CI: confidence interval.
Other comorbidities
The other comorbidities associated with CAP patients were chronic kidney disease: 3.7% (95% CI: 1.9%–7.4%; I2 = 67.4%);10–13,21,23,27 heart disease: 7.9% (95% CI: 3.9%–15.5%; I2 = 88.8%);10–13,15,19,21,28 asthma: 6.9% (95% CI: 3.6%–12.7%; I2 = 52.4%);10,12,18,22,24,27 bronchiectasis: 5.9% (95% CI: 2.1%–15%; I2 = 67.8%);10,12,27 neoplastic diseases: 4.1% (95% CI: 1.1%–4.1%; I2 = 90.5%);10,12,13,18,19 altered consciousness: 11.7% (95% CI: 7.2%–18.3%; I2 = 73.6%);14,16,21,22,25,28 structural lung disease: 8.6% (95% CI: 3.2%–21.3%; I2 = 80.4%);10,12,14,25 and HIV: 6.0% (95% CI: 2.3%–15.1%; I2 = 75.7%).11,22,27 Apart from these, comorbidities such as chronic liver disease (2.6%);11,13,15,21,24,28 cerebrovascular accident (3.2%),10,13,15,28 and tuberculosis (9.4%)12,18,22,27 were also reported in a few studies with no statistically significant association with CAP (Table 2).
Associated risk factors
Smoking
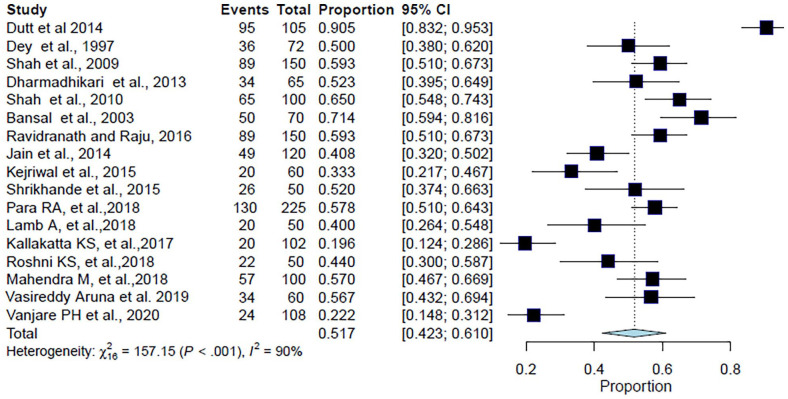
A total of 1637 patients were included in this analysis from 17 studies. The pooled population of CAP patients with a previous or current history of smoking was 51.7% (95% CI: 42.3%–61%; I2 = 89.8%; p < 0.001) (Table 3, Figure 5). The forest plot showed a significant degree of heterogeneity (Figure 5).8–10,12–17,20–22,25–29
Table 3.
Study-wise details of risk factors in CAP in Indian Studies.
| Risk factors | Number of risk factor subjects | Total number of subjects with CAP | Proportion | 95% CI | Reference |
|---|---|---|---|---|---|
| Smoking | 95 | 105 | 0.905 | (0.832–0.953) | Dutt et al. 8 |
| 89 | 150 | 0.593 | (0.510–0.673) | Shah et al. 9 | |
| 34 | 65 | 0.523 | (0.395–0.649) | Dharmadhikari et al. 10 | |
| 36 | 72 | 0.500 | (0.380–0.620) | Dey et al. 12 | |
| 50 | 70 | 0.714 | (0.594–0.816) | Bansal et al. 13 | |
| 65 | 100 | 0.650 | (0.548–0.743) | Shah et al. 14 | |
| 49 | 120 | 0.408 | (0.320–0.502) | Jain et al. 15 | |
| 20 | 60 | 0.333 | (0.217–0.467) | Kejriwal et al. 16 | |
| 26 | 50 | 0.520 | (0.374–0.663) | Shrikhande et al. 17 | |
| 89 | 150 | 0.593 | (0.510–0.673) | Ravindranath and Raju 20 | |
| 130 | 225 | 0.578 | (0.510–0.548) | Para et al. 21 | |
| 20 | 50 | 0.400 | (0.264–0.548) | Lamb and Patil 22 | |
| 20 | 102 | 0.196 | (0.124–0.286) | Kallakatta and Kuruvilla 25 | |
| 22 | 50 | 0.440 | (0.300–0.587) | Roshni et al. 26 | |
| 57 | 100 | 0.570 | (0.467–0.669) | Mahendra et al. 27 | |
| 34 | 60 | 0.567 | (0.432–0.694) | Aruna et al. 28 | |
| 24 | 108 | 0.222 | (0.418–0.312) | Vanjare et al. 29 | |
| Total | 860 | 1637 | – | – | – |
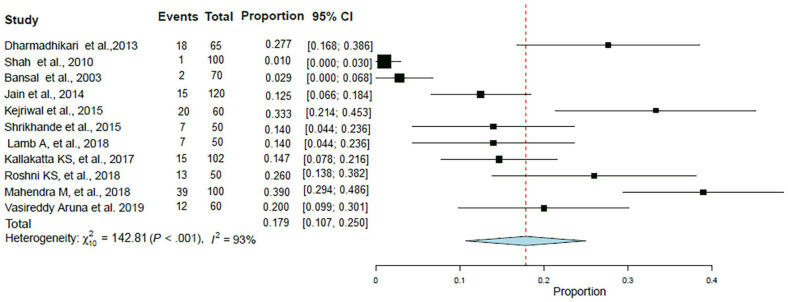
| Alcoholism | 18 | 65 | 0.277 | (0.168–0.386) | Dharmadhikari et al. 10 |
| 2 | 70 | 0.029 | (0.000–0.068) | Bansal et al. 13 | |
| 1 | 100 | 0.010 | (0.000–0.030) | Shah et al. 14 | |
| 15 | 120 | 0.125 | (0.066–0.184) | Jain et al. 15 | |
| 20 | 60 | 0.333 | (0.214–0.453) | Kejriwal et al. 16 | |
| 7 | 50 | 0.140 | (0.044–0.236) | Shrikhande et al. 17 | |
| 7 | 50 | 0.140 | (0.044–0.236) | Lamb and Patil 22 | |
| 15 | 102 | 0.147 | (0.078–0.216) | Kallakatta and Kuruvilla 25 | |
| 13 | 50 | 0.260 | (0.138–0.382) | Roshni et al. 26 | |
| 39 | 100 | 0.390 | (0.294–0.486) | Mahendra et al. 27 | |
| 12 | 60 | 0.200 | (0.099–0.301) | Aruna et al. 28 | |
| Total | 149 | 827 | – | – | – |
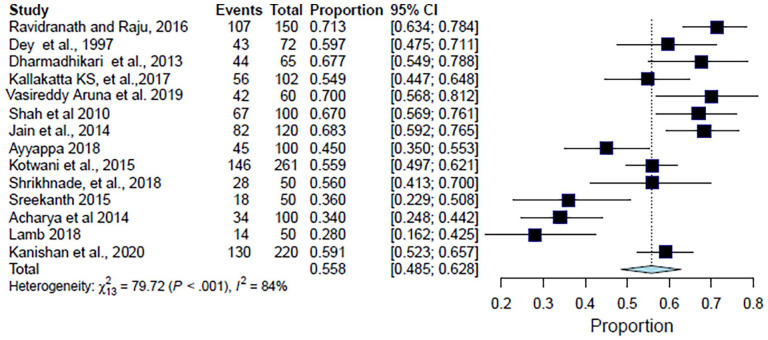
| Age ⩾ 50 years | 44 | 65 | 0.677 | (0.563–0.791) | Dharmadhikari et al. 10 |
| 18 | 50 | 0.360 | (0.229–0.508) | Sreekanth and Reddy 11 | |
| 43 | 72 | 0.597 | (0.484–0.711) | Dey et al. 12 | |
| 67 | 100 | 0.670 | (0.568–0.760) | Shah et al. 14 | |
| 82 | 120 | 0.683 | (0.592–0.765) | Jain et al. 15 | |
| 28 | 50 | 0.560 | (0.412–0.700) | Shrikhande et al. 17 | |
| 34 | 100 | 0.340 | (0.248–0.441) | Acharya et al. 18 | |
| 146 | 261 | 0.559 | (0.496–0.620) | Kotwani et al. 19 | |
| 107 | 150 | 0.713 | (0.641–0.786) | Ravindranath and Raju 20 | |
| 45 | 100 | 0.450 | (0.350–0.552) | Ayyappa et al. 23 | |
| 14 | 50 | 0.280 | (0.162–0.424) | Lamb and Patil 22 | |
| 56 | 102 | 0.549 | (0.452–0.646) | Kallakatta and Kuruvilla 25 | |
| 42 | 60 | 0.700 | (0.584–0.816) | Aruna et al. 28 | |
| 130 | 220 | 0.591 | (0.522–0.656) | Kanishan et al. 30 | |
| Total | 856 | 1500 | – | – | – |
CAP: community-acquired pneumonia; CI: confidence interval.
Figure 5.
Forest plot for meta-analysis of the proportion of CAP patients with previous or current history of smoking.
CAP: community-acquired pneumonia; CI: confidence interval.
Alcoholism
A total of 827 patients were included in this analysis from 11 studies. The pooled proportion of CAP patients with history of alcoholism was in 17.9% (95% CI: 10.7%–25%; I2 = 93%; p < 0.001) patients (Table 3, Figure 6). The forest plot showed a significant degree of heterogeneity (Figure 6).10,13–17,22,25–28
Figure 6.
Forest plot for meta-analysis of the proportion of CAP patients with alcoholism.
CAP: community-acquired pneumonia; CI: confidence interval.
Age ⩾ 50 years
The analysis for ages ⩾ 50 years included 1500 patients from 14 studies. The analysis suggested that the proportion of CAP patients for age ⩾ 50 years as a risk factor was found to be 55.8% (95% CI: 48.4%–62.8%; I2 = 83.7%; p < 0.001) (Table 3, Figure 7). The forest plot showed a significant degree of heterogeneity (Figure 7).10–12,14,15,17–20,22,23,25,28,30
Figure 7.
Forest plot for meta-analysis of the proportion of CAP patients of age ⩾ 50 years.
CAP: community-acquired pneumonia; CI: confidence interval.
Secondary outcomes
Mortality
Mortality in CAP patients was reported in 16 studies and ranged between 2.0% and 38% of patients.8–10,12–14,16,17,20,21,23–26,28,29
Duration of hospital stay
The duration of hospital stay for CAP patients was observed in six studies and ranged from 4.8 to 9.8 days.8,12,13,16,19,21
Sensitivity analysis
After analyzing the studies, the age group ⩾50 years was found as a significant risk factor associated with CAP. However, due to the overlapping of this age group (age-range) in a few studies (e.g. 45–55 years), clear data could not be extracted. Hence, sensitivity analysis was carried out to determine if substituting alternative values (number of patients) from the overlapping age group significantly affected the outcome of the meta-analysis. To perform sensitivity analysis, we included alternative values for the number of patients in overlapping age groups (one study at a time) to calculate the overall proportion of CAP patients with advanced age as a risk factor. After substituting alternative values for overlapping age groups, no significant (~3% difference) change in the overall proportion of CAP patients with advanced age (⩾50 years) was observed Hence, the number of patients from overlapping age groups were excluded, and only values from a clearly defined age group were included in the meta-analysis.
Discussion
Adequate recognition of comorbidities and risk factors associated with CAP not only helps in better management of the disease but also reduces financial burden. 31 This is of paramount importance in developing countries like India. In this systematic review and meta-analysis, we found that the presence of comorbid conditions—COPD, hypertension, and/or diabetes—and factors like smoking and advanced age increases the risk of CAP in India.
In this study, COPD, hypertension, and diabetes were present as comorbid conditions in 24.2%, 23.7%, and 16% of CAP patients, respectively. These findings are in line with a previous study conducted in Europe that identified COPD as a major comorbid condition increasing the risk of CAP. 2 Another observational study in the United Kingdom showed that 13% of people with COPD had more than one episode of CAP, of which 18.8% suffered from recurrent (⩾2 episodes) CAP. 32 Another prospective study in Serbia showed that 61.1% of CAP patients had hypertension as a comorbid condition. 33 This proportion is, however, considerably greater than the results obtained in our study. Similarly, McLaughlin et al. 34 in 2015 showed that patients with a history of diabetes mellitus were 3–6 times more likely to develop CAP as compared to patients without any comorbidity. Another study also reported diabetes (7.6%–28.5%), heart disease (6.9%–25.8%), and COPD (3.8%–15.4%) as common comorbid conditions associated with CAP in developed countries. 35 Our study identified other pathologic conditions—chronic kidney disease, neoplastic disease, asthma, bronchiectasis, structural lung disease, and altered consciousness—present in CAP patients but the proportion was relatively low to suggest a potential association with the risk of CAP.
Regarding risk factors, our study showed that more than half of the patients with CAP were ⩾50 years of age or reported a current or previous history of smoking. Similar findings were observed in other studies which identified advanced age and smoking as common risk factors associated with CAP and related fatality.2,36 Many studies showed that the risk of CAP increases with age. A study in the United States showed a significant increase in the overall incidence of CAP with ages ranging from 18.2 per 1000 person-years in the age group 65–69 years to as high as 52.3 per 1000 person-years in the age group above 85 years. 3 More than 25,000 pneumococcal-related deaths were estimated in the United States among adults aged ⩾50 years. 37 Another study in Germany showed a direct relationship between age and fatality rate in hospitalized patients with CAP; case fatality increased from 3.6% in patients <50 years old to 25.5% in those ⩾90 years. 38 Baik et al. 39 in 2000 also showed an increased risk of CAP among men aged 40–75 years. Our study also identified alcohol abuse as a risk factor. However, the proportion of CAP patients reporting alcohol intake was relatively smaller as compared to other risk factors suggesting a weaker association. Previous studies reported contrasting observations for alcohol abuse as a risk factor for CAP. While some studies reported alcohol intake as a risk factor,2,36 a recent global study showed that the link between alcohol intake and CAP was inconclusive. 40
Our study had a few limitations. First, the review did not include controls, such as case-control studies, to substantiate the association of risk factors with CAP. Second, we included studies with subjects presenting overlapping comorbidities. Third, publication bias was not assessed. Fourth, the present review did not evaluate the association of comorbidities and risk factors with incidence or case fatality, or factors predictive of CAP. Fifth, the meta-analysis showed significant heterogeneity which is commonly observed in epidemiological studies and could be attributed to several factors such as population characteristics; study design; differences in defining, measuring, and analyzing outcomes; criteria for patient selection; study objectives; period (i.e. the year when studies included were published); and statistical analysis. Therefore, despite the differences, the pooling of these studies was considered to be plausible, reasonable, and logical. Finally, the weighted mean of studies was not computed to determine the effect size of individual studies as this is not a common practice when high heterogeneity is observed.
Conclusion
This systematic review and meta-analysis identified diabetes, hypertension, and COPD as common comorbid pathologies and advanced age (age > 50 years), smoking, and alcohol abuse as risk factors associated with increased incidence of CAP in India.
Supplemental Material
Supplemental material, sj-docx-1-smo-10.1177_20503121221095485 for Systematic review and meta-analysis of comorbidities and associated risk factors in Indian patients of community-acquired pneumonia by Canna Jagdish Ghia and Gautam Sudhakar Rambhad in SAGE Open Medicine
Footnotes
Author contributions: Conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, writing—original draft preparation, writing—review and editing were contributed by Canna Ghia and Gautam Rambhad.
Data availability statement: The data that support the findings of this study are available from the corresponding author [Canna Ghia, canna.ghia@pfizer.com] upon reasonable request.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Not applicable (our study did not require ethical board approval because it did not contain human or animal trials).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This literature review was funded by Pfizer Limited.
Informed consent: Not applicable due to the nature of the study (systematic review from the published literature).
ORCID iD: Canna Jagdish Ghia  https://orcid.org/0000-0001-9839-3209
https://orcid.org/0000-0001-9839-3209
Supplemental material: Supplemental material for this article is available online.
References
- 1. Eshwara VK, Mukhopadhyay C, Rello J. Community-acquired bacterial pneumonia in adults: an update. Indian J Med Res 2020; 151(4): 287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Torres A, Peetermans WE, Viegi G, et al. Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax 2013; 68(11): 1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jackson ML, Neuzil KM, Thompson WW, et al. The burden of community-acquired pneumonia in seniors: results of a population-based study. Clin Infect Dis 2004; 39(11): 1642–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buzzo AR, Roberts C, Mollinedo LG, et al. Morbidity and mortality of pneumonia in adults in six Latin American countries. Int J Infect Dis 2013; 17(9): e673–e677. [DOI] [PubMed] [Google Scholar]
- 5. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Munn Z, Moola S, Lisy K, et al. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc 2015; 13(3): 147–153. [DOI] [PubMed] [Google Scholar]
- 7. Andargie A, Molla A, Tadese F, et al. Lost to follow-up and associated factors among patients with drug-resistant tuberculosis in Ethiopia: a systematic review and meta-analysis. PLoS ONE 2021; 16(3): e0248687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dutt TS, Tousheed SZ, Mohan BV. Community acquired pneumonia and cardiac diseases: a fatal association. Indian J Chest Dis Allied Sci 2014; 56(3): 153–156. [PubMed] [Google Scholar]
- 9. Shah BA, Ahmed W, Dhobi GN, et al. Validity of pneumonia severity index and CURB-65 severity scoring systems in community acquired pneumonia in an Indian setting. Indian J Chest Dis Allied Sci 2010; 52(1): 9–17. [PubMed] [Google Scholar]
- 10. Dharmadhikari V, Joseph T, Kulkarni A. Bacteriological and clinical profile of community acquired pneumonia in hospitalized patients. Int J Pharm Bio Sci 2013; 4: 695–702. [Google Scholar]
- 11. Sreekanth A, Reddy S. Study of clinical presentations and treatment outcome of severe community acquired pneumonia in the department of pulmonology of a tertiary care hospital. IOSR J Dent Med Sci 2015; 14: 125–128. [Google Scholar]
- 12. Dey AB, Nagarkar KM, Kumar V. Clinical presentation and predictors of outcome in adult patients with community-acquired pneumonia. Natl Med J India 1997; 10(4): 169–172. [PubMed] [Google Scholar]
- 13. Bansal S, Kashyap S, Pal LS, et al. Clinical and bacteriological profile of community acquired pneumonia in Shimla, Himachal Pradesh. Indian J Chest Dis Allied Sci 2004; 46(1): 17–22. [PubMed] [Google Scholar]
- 14. Shah BA, Singh G, Naik MA, et al. Bacteriological and clinical profile of Community acquired pneumonia in hospitalized patients. Lung India 2010; 27(2): 54–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jain SK, Jain S, Trikha S. Study of clinical, radiological, and bacteriological profile of community-acquired pneumonia in hospitalized patients of Gajra Raja Medical College, Gwalior, Central India. Int J Sci Stud 2014; 2(6): 96–100. [Google Scholar]
- 16. Kejriwal A, Shenoi AS, Pusukuru R, et al. A clinical, bacteriological and radiological profile of community acquired pneumonia in Navi Mumbai, India. IOSR J Dent Med Sci 2015; 14(9): 58–61. [Google Scholar]
- 17. Shrikhande A, Khangarot s, Saxena A, et al. Clinical, the bacteriological and radiological study of community acquired pneumonia. J Evol Med Dent Sci 2015; 4(13): 2112–2119. [Google Scholar]
- 18. Acharya VK, Padyana M, Unnikrishnan B, et al. Microbiological profile and drug sensitivity pattern among community acquired pneumonia patients in tertiary care centre in Mangalore, coastal Karnataka, India. J Clin Diagn Res 2014; 8(6): MC04–MC06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kotwani A, Kumar S, Swain PK, et al. Antimicrobial drug prescribing patterns for community-acquired pneumonia in hospitalized patients: a retrospective pilot study from New Delhi, India. Indian J Pharmacol 2015; 47(4): 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ravindranath M, Raju CH. Validity of pneumonia severity index/pneumonia outcome research trial and Curb-65 severity scoring systems in community acquired pneumonia in Indian setting. Int J Adv Med 2016; 3: 338–344. [Google Scholar]
- 21. Para RA, Fomda BA, Jan RA, et al. Microbial etiology in hospitalized North Indian adults with community-acquired pneumonia. Lung India 2018; 35(2): 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lamb A, Patil AH. Epidemiology and clinical features of community acquired pneumonia: hospital based study. Int J Res Med Sci 2018; 6(7): 2260–2263. [Google Scholar]
- 23. Ayyappa A, Sambasiva Rao G, Patrudu BMS, et al. Clinico-radiological and bacteriological profile of community acquired pneumonia. Indian J Appl Res 2018; 8(8): 23–24. [Google Scholar]
- 24. Mane A, Gujar P, Gaikwad S, et al. Aetiological spectrum of severe community-acquired pneumonia in HIV-positive patients from Pune, India. Indian J Med Res 2018; 147(2): 202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kallakatta K, Kuruvilla TS. Bacteriological and clinical profile of community acquired pneumonia in hospitalized patients. Indian J Res 2017; 6(8): 13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roshni KS, Mishra PC, Mohapatra SC, et al. Clinical, microbiological and radiological study of community acquired pneumonia. IOSR J Dent Med Sci 2018; 17(2): 45–63. [Google Scholar]
- 27. Mahendra M, Jayaraj BS, Limaye S, et al. Factors influencing severity of community-acquired pneumonia. Lung India 2018; 35(4): 284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aruna V, Devalkar A, Lal SB, et al. A comparative study of community acquired pneumonia between adults and elderly patients. Indian J Appl Res 2019; 9: 33–37. [Google Scholar]
- 29. Vanjare PH, Kangogopal G, Wilson BP, et al. Clinical profile and predictors of mortality in the elderly with community-acquired pneumonia at a tertiary care hospital in South India. J Health Res 2020; 7: 20–23. [Google Scholar]
- 30. Kanishan C, Shetty VA, Hampana S, et al. Bacterial aetiology of community acquired pneumonia in a tertiary care hospital of Southern India. J Clin Diagn Res 2020; 14(2): DC05–DC09. [Google Scholar]
- 31. Polsky D, Bonafede M, Suaya JA. Comorbidities as a driver of the excess costs of community-acquired pneumonia in U.S. commercially-insured working age adults. BMC Health Serv Res 2012; 12(1): 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Williams NP, Coombs NA, Johnson MJ, et al. Seasonality, risk factors and burden of community-acquired pneumonia in COPD patients: a population database study using linked health care records. Int J Chron Obstruct Pulmon Dis 2017; 12: 313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cupurdija V, Lazic Z, Petrovic M, et al. Community-acquired pneumonia: economics of inpatient medical care vis-à-vis clinical severity. J Bras Pneumol 2015; 41(1): 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McLaughlin JM, Johnson MH, Kagan SA, et al. Clinical and economic burden of community-acquired pneumonia in the Veterans Health Administration, 2011: a retrospective cohort study. Infection 2015; 43(6): 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Curcio D, Cané A, Isturiz R. Redefining risk categories for pneumococcal disease in adults: critical analysis of the evidence. Int J Infect Dis 2015; 37: 30–35. [DOI] [PubMed] [Google Scholar]
- 36. Luna CM, Famiglietti A, Absi R, et al. Community-acquired pneumonia: etiology, epidemiology, and outcome at a teaching hospital in Argentina. Chest 2000; 118(5): 1344–1354. [DOI] [PubMed] [Google Scholar]
- 37. Weycker D, Strutton D, Edelsberg J, et al. Clinical and economic burden of pneumococcal disease in older US adults. Vaccine 2010; 28(31): 4955–4960. [DOI] [PubMed] [Google Scholar]
- 38. Ewig S, Birkner N, Strauss R, et al. New perspectives on community-acquired pneumonia in 388 406 patients. Thorax 2009; 64(12): 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baik I, Curhan GC, Rimm EB, et al. A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in US men and women. Arch Intern Med 2000; 160(20): 3082–3088. [DOI] [PubMed] [Google Scholar]
- 40. Almirall J, Serra-Prat M, Bolíbar I, et al. Risk factors for community-acquired pneumonia in adults: a systematic review of observational studies. Respiration 2017; 94(3): 299–311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-smo-10.1177_20503121221095485 for Systematic review and meta-analysis of comorbidities and associated risk factors in Indian patients of community-acquired pneumonia by Canna Jagdish Ghia and Gautam Sudhakar Rambhad in SAGE Open Medicine