Abstract
Background:
Pancreatic acinar cell carcinoma (PACC) is rare, and its appropriate treatment remains unknown. We aim to explore the characteristics and optimal treatment of it.
Methods:
The data on clinicopathologic characteristics, molecular alteration, treatment, and survival of patients diagnosed with PACC at the Sun Yat-sen University Cancer Center from 2005 to 2020 were collected. The optimal treatment was explored by co-analyzing our results and published literatures.
Results:
Twenty-two PACC patients were enrolled. Eight of 17 non-metastatic patients received adjuvant chemotherapy. The patients receiving fluoropyrimidine-based regimen (n = 3) had a better median disease-free survival (mDFS) than those with gemcitabine-based regimen (n = 5) (unreached vs 27 months). Eight metastatic patients received first-line chemotherapy. Four patients received second-line chemotherapy. The objective response rate (ORR) of the fluoropyrimidine-based regimen was 85.7% (6/7), much better than that of the gemcitabine-based regimen (0/5). One patient who had responded to the first-line FOLFIRINOX (5-fluorouracil + oxaliplatin + leucovorin + irinotecan) regimen received olaparib as maintenance treatment for 5 months with good tolerance. Thirty-one published literatures, with a total of 86 cases, were included in the co-analysis. The ORR of the first-line fluoropyrimidine-based regimen (n = 47) was higher than that of gemcitabine-based regimen (n = 39) (59.6% vs 15.3%, P < .001). Eight of 11 patients treated with the FOLFIRINOX regimen achieved partial response (PR).
Conclusions:
For patients with metastasis, a fluorouracil-based regimen such as FOLFIRINOX may be preferred, and maintenance treatment of poly ADP-ribose polymerase (PARP) inhibitors after effective platinum-containing treatment for breast cancer susceptibility gene (BRCA) mutation patients must be assessed.
Keywords: Pancreatic acinar cell carcinoma, chemotherapy, fluorouracil, gemcitabine, olaparib
Introduction
Acinar cells are present in more than 80% of the pancreas, yet pancreatic acinar cell carcinoma (PACC) is rare, accounting for approximately 1% of all primary pancreatic neoplasms. 1 Recent studies have demonstrated that PACC was quite different from pancreatic ductal cell carcinoma (PDCC) in clinical, pathological, and molecular features and was associated with a significantly better survival.2 -4 The therapeutic strategy of PACC should be distinguished from that of PDCC.1,5,6
Radical surgery is recommended for non-metastatic PACC with a 5-year survival rate of 36.6% to 3.9%.7,8 Pancreatic acinar cell carcinoma was considered aggressive with a high recurrence rate and frequent metastasis after surgery.1,9,10 Systemic chemotherapy may play an essential role in improving prognosis. However, no prospective studies or meta-analyses documenting this fact have been reported. Retrospective studies comprising small case series or case reports resulted in the lack of high-quality evidence. Limited and selection-biased data make treatment decisions difficult. The authors collected data for 22 PACC patients in Sun Yat-sen University Cancer Center, based on clinicopathologic characteristics, molecular alteration, treatment, and survival. The role of chemotherapy and targeted therapy was explored. The study further compares fluoropyrimidine-based regimen with gemcitabine-based regimen by co-analyzing the results obtained herein and published literature. The authors aim to understand rare diseases better and optimize their treatment.
Material and Methods
Informed consent was obtained from all the patients in a written format. The institutional ethics committees approved this study to be conducted at Sun Yat-sen University Cancer Center, in accordance with the Declaration of Helsinki. The medical database at the center was scrutinized for patients treated between January 2005 and April 2020. Patients diagnosed with PACC who had all treatment and follow-up records were included in the study. If the patients have liver metastasis, liver biopsy was used to obtain a specimen. If it is difficult to obtain specimens from metastatic sites, the ultrasound-guided puncture was used to obtain specimens from primary lesions. At least 2 pathologists reviewed all the tumor specimens. Immunohistochemical staining was used to confirm acinar differentiation and to distinguish between acinar cell carcinomas and ductal adenocarcinomas. The standard for morphology is abound with acinar cell pathological differentiation (unlike rich ductal adenocarcinoma). The expression of SYN and CgA (for the identification of neuroendocrine tumor), and partial response (PR) and CD10 (for the identification of solid pseudopapilloma) was negative. Meanwhile, the specific expression of trypsin is detected in these cases, and the cases with controversial diagnosis of atypical morphology and histochemistry were excluded. Data on the clinicopathologic characteristics, molecular alteration, treatment, and survival were collected. The authors also searched the literature, including case reports or meeting abstracts describing therapeutic approaches for PACC in PubMed, Embase, and Cochrane library until August 25th, 2020. All literature involving systemic therapy containing gemcitabine or fluorouracil was included. An analysis based on the searched data and published data was conducted to improve the understanding of this rare disease and seek effective treatment. Cases receiving gemcitabine plus fluorouracil were categorized into a fluoropyrimidine-based regimen group.
Computed tomographic scans or magnetic resonance imaging were used for tumor assessment. Revised Response Evaluation Criteria In Solid Tumours (RECIST) guideline (version 1.1) was adopted for tumor response evaluation.
Tumor specimens and matched blood samples were used for next-generation sequences (NGS). High-depth sequencing and 4 types of tumor variation (including point mutation, insertion loss of small fragments, copy number variation, and currently known fusion genes) were detected through the 1021 gene panel platform (including somatic mutation, germline mutation, and tumor mutational burden [TMB]).
All quantitative data were analyzed using the R version 3.6.2. Kaplan–Meier methods estimated survival. Log-rank tests were performed for the estimation of differences in survival. The objective response rate (ORR) was calculated using the chi-square test. A 2-tailed P value <.05 was considered statistically significant.
Results
Clinicopathological characteristics
A total of 27 PACC patients were identified from 4508 patients diagnosed with pancreatic neoplasms in the Sun Yat-sen University Cancer Center. Five patients without treatment were excluded, and 22 patients were enrolled in the study. There were 17 non-metastatic patients and 5 metastatic patients at initial diagnosis. All patients without metastasis had radical surgery and 9 of them developed metastasis. Table 1 shows the patients’ baseline characteristics. Most patients had bulky primary disease with a median size of 9.2 cm (range from 3 to 17 cm). Main metastatic sites were the liver (n = 10), distant lymph node (n = 6), and peritoneum (n = 5). One patient was diagnosed as mixed acinar-endocrine carcinoma and 2 patients as mixed acinar-ductal carcinoma. All of them had a predominantly acinar differentiation. Serum CA-199 level (43.46-123.32 U/ml) was found in 7 patients. Elevated alpha-fetoprotein (AFP) level (50.43-23778.56 ng/ml) was detected in 3 patients who developed liver metastasis. Three patients with elevated serum lipase levels suffered subcutaneous fat necrosis and developed distant metastasis.
Table 1.
Patient demographics and clinicopathological characteristics (n = 22).
| Characteristics | No. of patients |
|---|---|
| Sex | |
| Male | 15 (68.2%) |
| Female | 7 (31.8%) |
| Age, years | |
| Median (range) | 51 (7-79) |
| ECOG performance status | |
| 0 | 4 (18.2%) |
| 1 | 18 (81.8%) |
| Size of primary disease, cm | |
| Median (range) | 9.2 (3.0-17.0) |
| Location of primary lesions | |
| Head | 9 (40.9%) |
| Body/tail | 13 (59.1%) |
| Histology | |
| PACC | 19 (86.4%) |
| Mixed PACC | 3 (13.6%) |
| Common symptom | |
| Abdominal pain | 11 (50.0%) |
| Weight loss | 5 (28.6%) |
| Abdominal mass | 5 (28.6%) |
| Subcutaneous fat necrosis | 3 (13.6%) |
| Metastases at initial diagnosis | |
| Yes | 5 (22.7%) |
| No | 17 (77.3%) |
| Main metastatic site | |
| Liver | 10 (45.5%) |
| Distant lymph node | 6 (27.3%) |
| Peritoneum | 5 (22.7%) |
| Serum CA-199 | |
| Elevated | 7 (31.8%) |
| Normal | 15 (68.2%) |
| Serum AFP | |
| Elevated | 3 (13.6%) |
| Normal | 14 (63.6%) |
| Not available | 5 (22.7%) |
| Tumor family history | 4 (18.2%) |
| Non-metastases cases | n = 17 |
| Radical surgery | 17 (100%) |
| Adjuvant chemotherapy | 8 (47.1%) |
| Regional lymph node metastasis | 3 (17.6%) |
| High Ki-67 index (⩾30%) | 4 (23.5%) |
| Lymph-vascular invasion | 6 (35.3%) |
| Treatment for metastases cases | n = 14 |
| First palliative chemotherapy | 8 (57.1%) |
| Second palliative chemotherapy | 4 (28.6%) |
Abbreviations: AFP, alpha-fetoprotein; CA-199, carbohydrate antigen 199; ECOG, Eastern Cooperative Oncology Group; PACC, pancreatic acinar cell carcinoma.
Gene alteration
Next-generation sequences on tumor samples were performed in 4 PACC patients. All patients showed microsatellite stability (MSS) and wild-type rat sarcoma (RAS)/v-raf murine sarcoma viral oncogene homolog B1 (BRAF) and low TMB (ranging from 6.72 to 8.16 mutations/mb). Two patients with breast cancer susceptibility gene 2 (BRCA2) germline mutation were identified, including 1 male patient with somatic mutation of IGF1R, FAT3, SMAD4, APC, SPENA, and MLH1 whose mother and sister were diagnosed with breast cancer. One female patient had a somatic mutation of TP53, CRTC1, SMAD4, ROS1, NTRK1, ATM, and KIF5B, whose grandmother was suspected of pancreatic tumor. One male patient showed mutation of TP53, PML, ATM, ENDRA, and ZNF703, whose father and grandfather were diagnosed with rectal cancer. No gene mutation was observed in the last patient.
Treatment for non-metastatic PACC
Seventeen non-metastatic patients underwent radical surgery with an median disease-free survival (mDFS) of 57 months. Eight of 17 (47.1%) patients received adjuvant chemotherapy. Nine of 17 (52.9%) patients developed metastasis after radical surgery. All 6 patients with pathological lymph-vascular invasion developed metastasis, and 3 of 11 patients without lymph-vascular invasion also developed metastasis. Ki-67 expression on the tumor was positive in 9 patients. By a cutoff of 30%, 4 patients with a high index of Ki-67 (⩾30%) developed metastasis, while 5 patients with a low index of Ki-67 (<30%) remained disease-free. Five of 8 (62.5%) patients in the adjuvant therapy group had lymph-vascular invasion or Ki-67 high index, while 2 of 9 (22.2%) patients in the non-adjuvant therapy group had these risk factors.
Most patients received adjuvant therapy of 2 to 4 cycles, and only 2 patients received more than 6 cycles with disease-free survival (DFS) over 4 years. Table 2 lists the specific information on adjuvant chemotherapy. In all, 5 of 8 patients with adjuvant therapy and 4 of 9 patients without adjuvant therapy developed metastasis. Median disease-free survival of 9 patients without adjuvant chemotherapy seemed numerically better than that of the 8 patients with adjuvant chemotherapy (69 vs 42 months). Median disease-free survival of 5 patients receiving gemcitabine-based adjuvant chemotherapy was 27 months, while that of 3 patients receiving fluoropyrimidine-based adjuvant chemotherapy was not reached.
Table 2.
Adjuvant chemotherapy for non-metastatic patients and outcome.
| Patient number, age | Regimes | Cycles | DFS (months) | LV invasion | Ki-67 index | Metastasis |
|---|---|---|---|---|---|---|
| 1 60-69 years | GEM | 4 | 5 | Yes | 70% | Yes |
| 1 50-59 years | GEM | 4 | 5 | Yes | Unknown | Yes |
| 1 50-59 years | S1 | 2 | 26 | Yes | Unknown | Yes |
| 2 50-59 years | GEMOX | 2 | 27 | Yes | 30% | Yes |
| 1 50-59 years | GEM | 10 | 57 | No | 30% | Yes |
| 2 40-49 years | S1 | 12 | 51 | No | 15% | No |
| 1 50-59 years | SOX | 5 | 62 | No | 20% | No |
| 1 10-19 years | GEM | 4 | 141 | No | 10% | No |
Abbreviations: DFS, disease-free survival; GEM, gemcitabine; GEMOX, gemcitabine + oxaliplatin; LV, lymph-vascular; S1, tegafur/gimeracil/potassium; SOX, S1 + oxaliplatin.
1/2 in Column 1 refers to sex (male or female).
Treatment for metastatic PACC patients
Eight metastatic PACC patients received first-line chemotherapy, including 5 patients at initial diagnosis and 3 patients after radical surgery. Four patients received second-line chemotherapy after failing to first-line chemotherapy. The chemotherapy regimens and results have been shown in Table 3. The ORR of the fluoropyrimidine-based regimen was 85.7% (6/7), much better than the gemcitabine-based regimen (0/5, 4 patients got progressive disease [PD]). Two patients who received the second-line FOLFIRINOX (5-fluorouracil + oxaliplatin + leucovorin + irinotecan) regimen achieved PR after failing to first-line AG (albumin-bound paclitaxel + gemcitabine) regimen. One patient developed PD with the second-line AG regimen, and 1 patient with RAS wide-type achieved stable disease (SD) to second-line AG plus nimotuzumab with the condition controlled for 2 months.
Table 3.
The systemic chemotherapy and response for metastasis patients in our center.
| First line | Response | PFS a (months) | Second line | Response | PFS b (months) | Third line | Response | PFS c (months) | PFS d (months) | OS (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| CAPOX | PR | 16 | – | – | – | – | – | – | – | 24 |
| GEMOX | PD | 2 | – | – | – | – | – | – | – | 57 |
| FOLFIRINOX | PD | 2 | GP/nimotuzumab | SD | 2 | – | – | – | – | 61 |
| S1 | PR | 23 | – | – | – | – | – | – | – | 66 |
| AG | PD | 3 | FOLFIRINOX | PR | 6 | S1/PD-1 inhibitor | PD | 2 | 2 | 16 |
| GP e | PD | 2 | FOLFIRINOX | PR | 9 | Lenvatinib/PD-1 inhibitor | SD | 4 | – | 17 |
| FOLFIRINOX | PR | 9 | – | – | – | – | – | – | – | 32 |
| FOLFIRINOX e | PR | 18 | GP | PD | 1.5 | Olaparib | PD | 2 | 1 | 39 |
Abbreviations: AG, albumin-bound paclitaxel + gemcitabine; CAPOX, oxaliplatin + capecitabine; FOLFIRINOX, 5-FU + oxaliplatin + leucovorin + irinotecan; GEMOX, gemcitabine + oxaliplatin; GP, gemcitabine + cisplatin; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease; S1, tegafur/gimeracil/potassium.
PFS for first-line chemotherapy.
PFS for second-line chemotherapy.
PFS for the third-line chemotherapy.
PFS for the forth-line chemotherapy.
Patients were detected with BRCA2 mutation.
Two patients with BRCA2 germline mutation had a good response to the FOLFIRINOX regimen and received olaparib treatment. One patient achieved PR to second-line FOLFIRINOX regimen and then received the maintenance olaparib treatment for 5 months with good tolerance. Another patient achieved PR to first-line FOLFIRINOX regimen with PFS (progression-free survival) of 18 months and developed PD to second-line AG regimen and third-line olaparib.
Review of published literature on chemotherapy of metastatic PACC
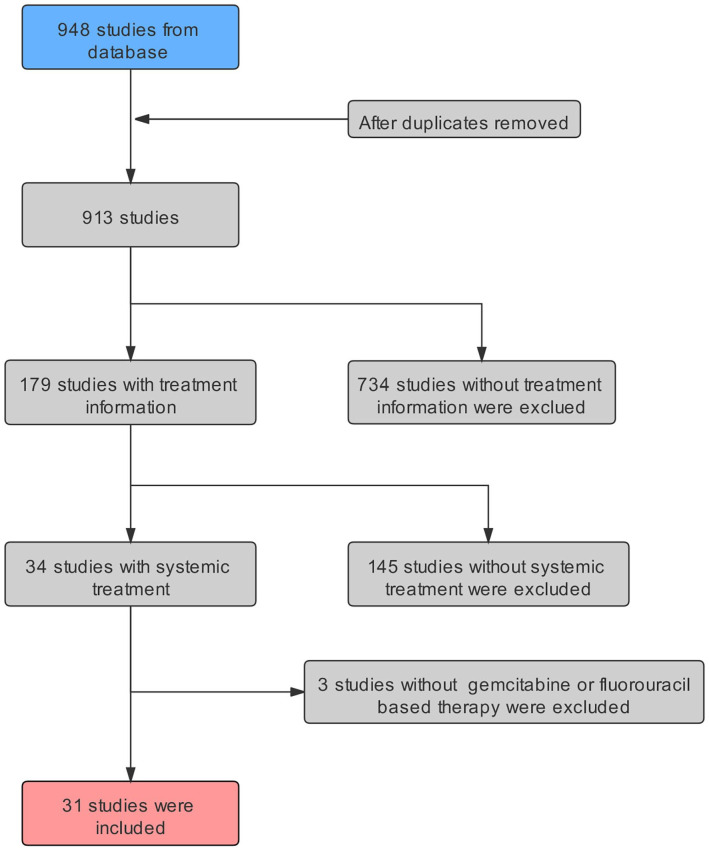
The study involved 32 studies; a total of 86 cases were included. The selection procedure has been shown in Figure 1, and treatment details are given in Table 4. Eighty-six cases received first-line treatment, and 33 out of 86 cases failed to first-line treatment and received second-line therapy. All the enrolled patients were divided into 2 groups: fluoropyrimidine-based regimen group and gemcitabine-based regimen group. The patients receiving gemcitabine plus fluorouracil were classified into the fluoropyrimidine-based regimen group.
Figure 1.
Schematic representation of literature selected.
Table 4.
Palliative chemotherapy for metastatic patients: data from literature.
| Regimen | Response | Author | Publication (year) |
|---|---|---|---|
| First-line regimen | |||
| Gemcitabine-based | |||
| AG | 1SD/2PD | Brunetti et al 11 | 2018 |
| GEM | 1PR/10SD/11PD | Fujii et al 12 /Yokode et al 13 /Seki et al 14 /Brunetti et al 11 /Lowery et al 15 /Simon et al 16 /Yoo et al 17 /Kuji et al 18 /Kruger et al 19 /Toda et al 20 | 2009-2012 2016-2018 |
| GEMOX/GEM + CDDP | 4PR/2SD/2PD | Brunetti et al 11 /Kruger et al 19 /Lowery et al 15 | 2011/2016/2018 |
| GEM + irinotecan | 2SD | Lowery et al 15 | 2011 |
| GEM + erlotinib | 1PR/3PD | Lowery et al 15 /Kruger et al 19 | 2016 |
| Fluoropyrimidine-based | |||
| 5-FU/S1/CAP | 7PR | Yamamoto et al 21 /Morishima et al 22 /Kanemasa et al 23 /Sumiyoshi et al 24 /Yoo et al 17 /Kruger et al 19 | 2010/2012 2013/2015 |
| GEM + 5FU/GEM + S1 | 2CR/4PR/1SD | Nishimizu et al 25 /Fukui et al 26 /Hatata et al 27 /Miyagawa et al 28 /Toda et al 20 /Brunetti et al 11 | 2010/2011 2016/2018 |
| FOLFOX/CAPOX | 4PR/3SD/1PD | Yoo et al 17 /Fontenot et al 29 /Morales et al 30 /Brunetti et al 11 /Kruger et al 19 /Jordan et al 31 | 2013/2016-2018 2020 |
| 5FU + CDDP | 3PR/2SD | Brunetti et al 11 /Butturini et al 5 | 1999/2011 |
| Ukei et al 32 /Jauch et al 33 | 2016/2018 | ||
| GEM + CAP | 1PR/2SD/1PD | Yoo et al 17 /Lowery et al 15 /Sorscher 34 | 2011/2017 |
| PEXG | 5SD | Brunetti et al 11 | 2011 |
| GTX | 1PR/1SD | Lowery et al 15 | 2011 |
| CAPE/temozolomide | 1PD | Callata-Carhuapoma et al 35 | 2015 |
| FOLFIRINOX | 6PR/2SD | Li et al 36 /Yoshihiro et al 37 /Kryklyva et al 38 /Schempf et al 39 /Kruger et al 19 /Pfrommer et al 40 | 2013/2014 2016-2019 |
| Second-line regimen | |||
| Gemcitabine-based | |||
| AG/GEMOX | 1PR/1SD/2PD | Brunetti et al 11 /Lowery et al 15 /Kruger et al 19 | 2011/2016 2018 |
| GEM | 2SD/4PD | Brunetti et al 11 /Yoo et al 17 /Kanemasa et al 23 | 2013/2017/2018 |
| Fluoropyrimidine-based | |||
| S1 | 2PR/3PD | Fujii et al 12 /Yokode et al 13 /Seki et al 14 | 2009/2010/2017 |
| GEM + CAP/GEM + S1 | 1PR/1SD/1PD | Brunetti et al 11 /Lowery et al 15 /Kuji et al 18 | 2011/2018 |
| FOLFIRI | 1PR/2PD | Lowery et al 15 /Morales et al 30 | 2011/2013 |
| FOLFOX | 4PR/1SD | Yoo et al 17 /Brunetti et al 11 /Kruger et al 19 /Simon et al 16 | 2016-2018 |
| FOLFIRINOX | 2PR/1SD | Brunetti et al 11 /Kruger et al 19 /Callata-Carhuapoma et al 35 | 2015/2016/2018 |
Abbreviations: 5FU, 5-fluorouracil; AG, albumin paclitaxel + gemcitabine; CAPE, capecitabine; CAPOX, oxaliplatin + capecitabine; CDDP, cisplatin; FOLFIRI, 5-fluorouracil + leucovorin + irinotecan; FOLFIRINOX, 5-fluorouracil + oxaliplatin + leucovorin + irinotecan; FOLFOX, 5-FU + oxaliplatin + leucovorin; GEM, gemcitabine; GEM + CAP, gemcitabine + capecitabine; GEM + S1, gemcitabine + tegafur/gimeracil/potassium; GEMOX, gemcitabine oxaliplatin; PEXG, cisplatinum + epribicin + capecitabine+ gemcitabine; GTX, gemcitabine + T axotere + capecitabine; PD, progressive disease; PR, partial response; S1, tegafur/gimeracil/potassium; SD, stable disease.
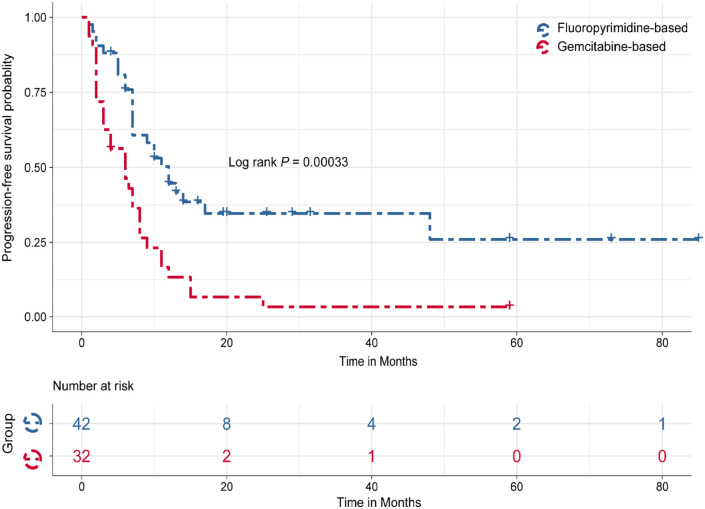
For first-line chemotherapy, the ORR of 86 patients was 39.5%. There were 39 cases in the gemcitabine-based regimen group and 47 subjects in the fluoropyrimidine-based regimen group. The ORR of the fluoropyrimidine-based group (59.6%, 28/47) was higher than that of the gemcitabine-based group (15.4%, 6/39) (P < .001). Eight patients received FOLFIRINOX as first-line chemotherapy, and 6 of them achieved PR. The survival data were available for 74 cases, including 42 patients in the fluoropyrimidine-based group and 32 patients in the gemcitabine-based group. The median PFS and median overall survival (OS) of 74 cases were 8 and 25.4 months, respectively. Median PFS in the fluoropyrimidine-based group were significantly better than the gemcitabine-based group (12 vs 6 months, P < .001; Figure 2).
Figure 2.
Kaplan–Meier plots of progression-free survival stratified by fluorouracil-based therapy and gemcitabine-based therapy for advanced PACC. PACC indicates pancreatic acinar cell carcinoma.
For second-line chemotherapy, the ORR of 29 patients was 37.9%. There were 10 cases in the gemcitabine-based regimen group and 19 cases in the fluoropyrimidine-based regimen group. ORR of the fluoropyrimidine-based group (52.6%, 10/19) was much higher than the gemcitabine-based group (10%, 1/10) (P < .05). The comparison of response between fluoropyrimidine-based regimen and gemcitabine-based regimen for metastatic PACC has been shown in Table 5.
Table 5.
Comparison of response between fluoropyrimidine-based regimen and gemcitabine-based regimen for metastatic PACC.
| Gemcitabine-based | Fluoropyrimidine-based | P value | ||||
|---|---|---|---|---|---|---|
| Cases | ORR (%) | Cases | ORR (%) | |||
| Our study | First line (n = 8) | 3 | 0 (0) | 5 | 4 (80) | >.05 |
| Second line (n = 4) | 2 | 0 (0) | 2 | 2 (100) | >.05 | |
| Literatures | First line (n = 86) | 39 | 6 (15.3) | 47 | 28 (59.6) | <.001 |
| Second line (n = 29) | 10 | 1 (10.0) | 19 | 10 (52.6) | <.05 | |
Abbreviations: ORR, objective response rate; PACC, pancreatic acinar cell carcinoma.
Discussion
As PACC is rare, limited information is available. There is no consensus on PACC treatment strategy. More clinical data are needed to improve our understanding of this disease and seek appropriate treatments. The authors collected the data of 22 PACC patients at the cancer center. The authors combined the study results with the published literature to explore the role of adjuvant chemotherapy for non-metastatic disease. They optimized the selection of palliative chemotherapy or targeted therapy for metastatic disease. Compared with gemcitabine-based regimens, fluorouracil-based regimens may be the preferred choice for PACC. Furthermore, the value of olaparib for BRCA germline mutation patients and the role of epidermal growth factor receptor (EGFR) monoclonal antibody combined with chemotherapy for RAS wild-type patients were preliminarily explored.
Pancreatic acinar cell carcinoma may need more aggressive surgical resection because it was considered a more favorable prognosis than PDCA. 9 In the study, 17 non-metastatic patients receiving radical surgery achieved an mDFS of 57 months, similar to that in other clinical reports.1,41 Eight of 9 patients with lymph-vascular invasion or high Ki-67 index (⩾30%) developed metastasis, consistent with other studies. 42 Ki-67 and invasion of lymph-vascular invasion may be prognostic indicators for PACC.
The role of adjuvant chemotherapy or chemoradiotherapy for PACC remains controversial or is considered to be underpowered. Schmidt et al 7 included 865 resected PACC patients from 1985 to 2005 in National Cancer Database (NCDB) to identify the prognostic factors. The results showed that adjuvant therapy was not associated with better outcomes on multivariable analysis and that T classification, tumor size, and nodal status remained non-significant predictors of survival. Pate et al 43 carried out a more contemporary cohort of 298 patients with resectable PACC between 2004 and 2015 from NCDB. The results showed that adjuvant systemic therapy was associated with a significant improvement in OS (hazard ratio [HR]: 0.54, 95% confidence interval [CI]: 0.33-0.89) compared with surgery alone and supported that adjuvant systemic therapy should be given due consideration in patients undergoing resection, mainly when there is evidence of lymph node involvement. Wang et al 44 reported that 14 patients who received radical resection followed by adjuvant chemoradiotherapy had a better outcome than surgery alone. In our study, no DFS benefit of adjuvant chemotherapy was shown. It was speculated that the negative result might be related to the higher proportion of patients with poor prognostic factors such as lymph-vascular invasion or high Ki-67 index in the adjuvant chemotherapy group (62.5% vs 22.2%), inadequate cycles of adjuvant chemotherapy (median cycles of 4), and selection of chemotherapeutic regimen. So far, the literature does not report any study exploring the efficacy of different adjuvant chemotherapy regimens. In this study, the patients receiving fluoropyrimidine-based adjuvant chemotherapy achieved better mDFS than those receiving gemcitabine-based adjuvant chemotherapy (unreached vs 27 months). The value of fluoropyrimidine-based adjuvant chemotherapy may be noteworthy.
Palliative chemotherapy is the primary treatment for metastatic PACC. However, the appreciative chemotherapy regimen remains unclear because the published retrospective and small sample studies or various case reports had significant heterogeneity and a lack of high-quality evidence. Referring to the treatment of PDCC, many physicians were inclined to choose gemcitabine-based regimes with unsatisfied outcomes. A possible underlying mechanism for fluoropyrimidine-based regimen that could be an effective treatment of PACC is the similarity to colon cancer. Research has shown that PACC has none of the gene abnormalities commonly found in PDCC and has gene mutations in the APC gene/β-catenin pathway and genetic progression similar to colon cancer. 17 In the preclinical PACC patient–derived tumor xenograft model, oxaliplatin produced a prolonged durable growth response associated with increased apoptosis, decreased serum lipase level, and increased healthy acinar cells. 45 Yoo et al 46 reported that oxaliplatin-containing chemotherapy against PACC has improved activity compared with gemcitabine. It was indicated that chemotherapeutic agents used in treating colorectal cancer might be effective in ACC of the pancreas. Six out of 7 patients with the FOLFIRINOX regimen achieved PR, while 5 patients with the AG regimen had no response. To further understand the preferred chemotherapy regimen for metastatic PACC, the efficacy of fluoropyrimidine-based regimen and gemcitabine-based regimen were compared after reviewing published literature. The results based on the analysis of 44 patients further supported that fluorouracil-based chemotherapy with higher ORR and improved survival was superior to gemcitabine-based chemotherapy. FOLFIRINOX regimen may be the preferred one for PACC.
The molecular feature of PACC is different from other pancreatic cancers. 17 Typical genetic alterations observed in PDAC usually are not detected or rarely occur in ACC, that is, mutations in KRAS, TP53, CDKN2A, and SMAD4. In our study, 4 patients underwent NGS and showed MSS, wild-type RAS/BRAF, and low TMB. Due to their pivotal role in maintaining genome integrity, BRCA1/2-deficient tumors are susceptible to therapies introducing cross-linking and DNA damage, namely, platinum-based chemotherapies and PARP inhibitors.17,47,48 Olaparib, an oral PARP inhibitor, has been approved for the maintenance treatment of adult patients with germline or BRCA-mutated advanced ovarian cancer, 49 breast cancer, 50 and pancreatic cancer. 51 In our study, molecular analysis revealed germline BRCA2 in 2 patients with a familial history of breast and pancreatic cancer and achieved PR to first-line FOLFIRINOX regimen. The authors preliminarily explored the value of olaparib maintenance treatment for 1 patient who had responded to the first-line FOLFIRINOX regimen and then received olaparib for 5 months with good tolerance. It may be crucial to establish the link between ACC and BRCA1/2 mutations, given the importance of recognizing potentially hereditary tumors and identifying patients who may benefit from platinum-based chemotherapy and targeted therapy.
The study has some limitations. First, it is a retrospective study from a single institution which resulted in an inherent selective bias. Second, the relatively small sample size and the imbalance in subgroups resulted in the deviation of analyses. Last, the meta-analysis lacks genomics and there were no criteria for the search engine to check the literature.
Conclusion
It was further confirmed that PACC is different from PDCC in clinicopathological and molecular characteristics and should have different management strategies and chemotherapy choices. Ki-67 and invasion of lymph-vascular invasion may be prognostic indicators for PACC. Although the benefit of adjuvant chemotherapy remained unclear, the value of fluoropyrimidine-based chemotherapy deserves attention. For patients with metastasis, fluorouracil-based chemotherapy such as FOLFIRINOX could be a preferred treatment, but more studies are needed for small sample size in this study. The maintenance treatment of PARP inhibitors after effective platinum-containing treatment for BRAC mutation patients should be explored further.
Acknowledgments
We would like to thank all the subjects who participated in this study.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The manuscript has received preprint service on Research Square—https://www.researchsquare.com/article/rs-177880/v1.
Author Contributions: F.-H.W. designed the study; J.-Y.X. and W.-L.G. performed data analysis and wrote the manuscript; X.-L.W., W.-J.S., C.R., S.-X.L., S.-P.L., and Y.-H.L. collected and interpreted data; and F.-H.W. and M.-Z.Q. reviewed and revised.
Availability of Data and Materials: Main data are shown in this article and additional data about this study could be obtained from the corresponding author on reasonable request.
Ethical Approval and Consent to Participate: The study protocol was approved by the institutional ethics committee of Sun Yat-sen University Cancer Center (approval number: B2022-050-01, approval date: July 19, 2021), and was carried out in full compliance with the principles of the “Declaration of Helsinki” (current revision) and “Good Clinical Practice” guideline. Written informed consent was obtained from all participants before the start of treatment or any study-related procedures.
ORCID iDs: Jian-Ying Xu  https://orcid.org/0000-0003-3409-9873
https://orcid.org/0000-0003-3409-9873
Wen-Jie Shi  https://orcid.org/0000-0002-4588-4044
https://orcid.org/0000-0002-4588-4044
References
- 1. Holen KD, Klimstra DS, Hummer A, et al. Clinical characteristics and outcomes from an institutional series of acinar cell carcinoma of the pancreas and related tumors. J Clin Oncol. 2002;20:4673-4678. [DOI] [PubMed] [Google Scholar]
- 2. Thompson ED, Wood LD. Pancreatic neoplasms with acinar differentiation: a review of pathologic and molecular features. Arch Pathol Lab Med. 2020;144:808-815. [DOI] [PubMed] [Google Scholar]
- 3. Mortenson MM, Katz MH, Tamm EP, et al. Current diagnosis and management of unusual pancreatic tumors. Am J Surg. 2008;196:100-113. [DOI] [PubMed] [Google Scholar]
- 4. Al-Hader A, Al-Rohil RN, Han H, Von Hoff D. Pancreatic acinar cell carcinoma: a review on molecular profiling of patient tumors. World J Gastroenterol. 2017;23:7945-7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Butturini G, Pisano M, Scarpa A, D’Onofrio M, Auriemma A, Bassi C. Aggressive approach to acinar cell carcinoma of the pancreas: a single-institution experience and a literature review. Langenbecks Arch Surg. 2011;396:363-369. [DOI] [PubMed] [Google Scholar]
- 6. Wisnoski NC, Townsend CM, Jr, Nealon WH, Freeman JL, Riall TS. 672 patients with acinar cell carcinoma of the pancreas: a population-based comparison to pancreatic adenocarcinoma. Surgery. 2008;144:141-148. [DOI] [PubMed] [Google Scholar]
- 7. Schmidt CM, Matos JM, Bentrem DJ, Talamonti MS, Lillemoe KD, Bilimoria KY. Acinar cell carcinoma of the pancreas in the United States: prognostic factors and comparison to ductal adenocarcinoma. J Gastrointest Surg. 2008;12:2078-2086. [DOI] [PubMed] [Google Scholar]
- 8. Kitagami H, Kondo S, Hirano S, Kawakami H, Egawa S, Tanaka M. Acinar cell carcinoma of the pancreas: clinical analysis of 115 patients from pancreatic cancer registry of Japan pancreas society. Pancreas. 2007;35:42-46. [DOI] [PubMed] [Google Scholar]
- 9. Matos JM, Schmidt CM, Turrini O, et al. Pancreatic acinar cell carcinoma: a multi-institutional study. J Gastrointest Surg. 2009;13:1495-1502. [DOI] [PubMed] [Google Scholar]
- 10. Klimstra DS, Heffess CS, Oertel JE, Rosai J. Acinar cell carcinoma of the pancreas. A clinicopathologic study of 28 cases. Am J Surg Pathol. 1992;16:815-837. [DOI] [PubMed] [Google Scholar]
- 11. Brunetti O, Aprile G, Marchetti P, et al. Systemic chemotherapy for advanced rare pancreatic histotype tumors: a retrospective multicenter analysis. Pancreas. 2018;47:759-771. [DOI] [PubMed] [Google Scholar]
- 12. Fujii M, Sato H, Ogasawara T, et al. [A case of liver metastasis of pancreatic acinar cell carcinoma treated with S-1 and intra-arterial CDDP combination therapy]. Gan To Kagaku Ryoho. 2010;37:1987-1990. [PubMed] [Google Scholar]
- 13. Yokode M, Itai R, Yamashita Y, Zen Y. A case report of mixed acinar-endocrine carcinoma of the pancreas treated with S-1 chemotherapy: does it work or induce endocrine differentiation? Medicine. 2017;96:e8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seki Y, Okusaka T, Ikeda M, Morizane C, Ueno H. Four cases of pancreatic acinar cell carcinoma treated with gemcitabine or s-1 as a single agent. Jpn J Clin Oncol. 2009;39:751-755. [DOI] [PubMed] [Google Scholar]
- 15. Lowery MA, Klimstra DS, Shia J, et al. Acinar cell carcinoma of the pancreas: new genetic and treatment insights into a rare malignancy. Oncologist. 2011;16:1714-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simon M, Bioulac-Sage P, Trillaud H, Blanc JF. Folfox regimen in pancreatic acinar cell carcinoma: case report and review of the literature. Acta Oncol. 2012;51:403-405. [DOI] [PubMed] [Google Scholar]
- 17. Abraham SC, Wu TT, Hruban RH, et al. Genetic and immunohistochemical analysis of pancreatic acinar cell carcinoma: frequent allelic loss on chromosome 11p and alterations in the APC/beta-catenin pathway. Am J Pathol. 2002;160:953-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuji M, Yamamoto Y, Tani C, Kenno S, Kobayashi T. [A case of advanced pancreatic cancer responding well to s-1/gemcitabine combination therapy after gemcitabine therapy]. Gan To Kagaku Ryoho. 2011;38:853-855. [PubMed] [Google Scholar]
- 19. Kruger S, Haas M, Burger PJ, et al. Acinar cell carcinoma of the pancreas: a rare disease with different diagnostic and therapeutic implications than ductal adenocarcinoma. J Cancer Res Clin Oncol. 2016;142:2585-2591. [DOI] [PubMed] [Google Scholar]
- 20. Toda H, Kurahara H, Maemura K, et al. [A case of curative resection for advanced pancreatic acinar cell carcinoma with liver metastasis and involvement of the superior mesenteric artery after chemoradiotherapy following systemic chemotherapy]. Gan To Kagaku Ryoho. 2016;43:2071-2073. [PubMed] [Google Scholar]
- 21. Yamamoto T, Ohzato H, Fukunaga M, Imamura H, Furukawa H. Acinar cell carcinoma of the pancreas: a possible role of S-1 as chemotherapy for acinar cell carcinoma. A case report. JOP. 2012;13:87-90. [PubMed] [Google Scholar]
- 22. Morishima K, Hyodo M, Nihei Y, Sata N, Yasuda Y. [A case of acinar cell carcinoma of pancreas with liver metastases treated effectively by s-1]. Gan To Kagaku Ryoho. 2010;37:127-129. [PubMed] [Google Scholar]
- 23. Kanemasa Y, Kamisawa T, Tabata T, et al. Mixed acinar-endocrine carcinoma of the pancreas treated with S-1. Clin J Gastroenterol. 2013;6:459-464. [DOI] [PubMed] [Google Scholar]
- 24. Sumiyoshi T, Shima Y, Okabayashi T, et al. Long-term survival following pancreatectomy and s-1 chemotherapy for pancreatic acinar cell carcinoma with peritoneal dissemination: a case report and literature review. Medicine (Baltimore). 2015;94:e378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nishimizu T, Minemura M, Kajiura S, et al. [A case of pancreatic acinar cell carcinoma with a giant liver metastasis successfully treated with combination of gemcitabine and peroral S-1]. Gan To Kagaku Ryoho. 2011;38:309-312. [PubMed] [Google Scholar]
- 26. Fukui H, Kou C, Matsumoto T, Matsumoto M. [S-1+gemcitabine (GEM) therapy effective in a case of pancreatic body cancer with multiple liver metastasis]. Gan To Kagaku Ryoho. 2010;37:1775-1778. [PubMed] [Google Scholar]
- 27. Hatata T, Takaya S, Taniguchi K, Naka T, Kondo A, Ikeguchi M. [A case of complete response of gemcitabine (GEM) monotherapy-refractive liver metastatic pancreatic cancer treated with GEM+S-1 combined chemotherapy]. Gan To Kagaku Ryoho. 2011;38:109-112. [PubMed] [Google Scholar]
- 28. Miyagawa K, Yata Y, Yamaoka N, Sagara Y. [A case of complete response (CR) to combination therapy of S-1 and gemcitabine (GEM) for unresectable pancreatic cancer]. Gan To Kagaku Ryoho. 2010;37:1145-1147. [PubMed] [Google Scholar]
- 29. Fontenot J, Spieler B, Hudson C, Boulmay B. Pancreatic acinar cell carcinoma—literature review and case report of a 56-year-old man presenting with abdominal pain. Radiol Case Rep. 2020;15:39-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morales M, Cabrera MA, Maeso MD, Ferrer-López N. Use of panitumumab in the treatment of acinar cell carcinoma of the pancreas: a case report. Oncol Lett. 2013;5:969-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jordan EJ, Basturk O, Shia J, et al. Case report: primary acinar cell carcinoma of the liver treated with multimodality therapy. J Gastrointest Oncol. 2017;8:E65-E72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ukei T, Okagawa K, Uemura Y, et al. Effective intra-arterial chemotherapy for acinar cell carcinoma of the pancreas. Dig Surg. 1999;16:76-79. [DOI] [PubMed] [Google Scholar]
- 33. Jauch SF, Morris VK, Jensen CT, Kaseb AO. Multimodal approach and long-term survival in a patient with recurrent metastatic acinar cell carcinoma of the pancreas: a case report. Pancreatology. 2016;16:153-156. [DOI] [PubMed] [Google Scholar]
- 34. Sorscher SM. Acinar cell carcinoma responding to carboplatin/etoposide chemotherapy. J Gastrointest Cancer. 2012;43:S2-S3. [DOI] [PubMed] [Google Scholar]
- 35. Callata-Carhuapoma HR, Pato Cour E, Garcia-Paredes B, et al. Pancreatic acinar cell carcinoma with bilateral ovarian metastases, panniculitis and polyarthritis treated with folfirinox chemotherapy regimen. A case report and review of the literature. Pancreatology. 2015;15:440-444. [DOI] [PubMed] [Google Scholar]
- 36. Li M, Mou Y, Hou S, Cao D, Li A. Response of germline BRCA2-mutated advanced pancreatic acinar cell carcinoma to olaparib: a case report. Medicine (Baltimore). 2018;97:e13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yoshihiro T, Nio K, Tsuchihashi K, et al. Pancreatic acinar cell carcinoma presenting with panniculitis, successfully treated with folfirinox: a case report. Mol Clin Oncol. 2017;6:866-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kryklyva V, Haj Mohammad N, Morsink FHM, et al. Pancreatic acinar cell carcinoma is associated with BRCA2 germline mutations: a case report and literature review. Cancer Biol Ther. 2019;20:949-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schempf U, Sipos B, König C, Malek NP, Bitzer M, Plentz RR. Folfirinox as first-line treatment for unresectable acinar cell carcinoma of the pancreas: a case report. Z Gastroenterol. 2014;52:200-203. [DOI] [PubMed] [Google Scholar]
- 40. Pfrommer S, Weber A, Dutkowski P, et al. Successful salvage chemotherapy with folfirinox for recurrent mixed acinar cell carcinoma and ductal adenocarcinoma of the pancreas in an adolescent patient. Case Rep Oncol. 2013;6:497-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seth AK, Argani P, Campbell KA, et al. Acinar cell carcinoma of the pancreas: an institutional series of resected patients and review of the current literature. J Gastrointest Surg. 2008;12:1061-1067. [DOI] [PubMed] [Google Scholar]
- 42. La Rosa S, Adsay V, Albarello L, et al. Clinicopathologic study of 62 acinar cell carcinomas of the pancreas: insights into the morphology and immunophenotype and search for prognostic markers. Am J Surg Pathol. 2012;36:1782-1795. [DOI] [PubMed] [Google Scholar]
- 43. Patel DJ, Lutfi W, Sweigert P, et al. Clinically resectable acinar cell carcinoma of the pancreas: is there a benefit to adjuvant systemic therapy? Am J Surg. 2020;219:522-526. [DOI] [PubMed] [Google Scholar]
- 44. Wang Y, Wang S, Zhou X, et al. Acinar cell carcinoma: a report of 19 cases with a brief review of the literature. World J Surg Oncol. 2016;14:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hall JC, Marlow LA, Mathias AC, et al. Novel patient-derived xenograft mouse model for pancreatic acinar cell carcinoma demonstrates single agent activity of oxaliplatin. J Transl Med. 2016;14:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yoo C, Kim BJ, Kim KP, et al. Efficacy of chemotherapy in patients with unresectable or metastatic pancreatic acinar cell carcinoma: potentially improved efficacy with oxaliplatin-containing regimen. Cancer Res Treat. 2017;49:759-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wattenberg MM, Asch D, Yu S, et al. Platinum response characteristics of patients with pancreatic ductal adenocarcinoma and a germline BRCA1, BRCA2 or PALB2 mutation. Br J Cancer. 2020;122:333-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chmielecki J, Hutchinson KE, Frampton GM, et al. Comprehensive genomic profiling of pancreatic acinar cell carcinomas identifies recurrent RAF fusions and frequent inactivation of DNA repair genes. Cancer Discov. 2014;4:1398-1405. [DOI] [PubMed] [Google Scholar]
- 49. Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505. [DOI] [PubMed] [Google Scholar]
- 50. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:1700. [DOI] [PubMed] [Google Scholar]
- 51. Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381:317-327. [DOI] [PMC free article] [PubMed] [Google Scholar]