Abstract
The human gut microbiome (GM) is a complex ecosystem that includes numerous prokaryotic and eukaryotic inhabitants. The composition of GM can influence an array of host physiological functions including immune development. Accumulating evidence suggest that several members of non-bacterial microbiota, including protozoa and helminths, that were earlier considered as pathogens, could have a commensal or beneficial relationship with the host. Here we examine the most recent data from omics studies on prokaryota-meiofauna-host interaction as well as the impact of gut parasitome on gut bacterial ecology and its role as ‘immunological driver’ in health and disease to glimpse new therapeutic perspectives.
Keywords: big-data omics, human gut microbiome, meiofauna, prokaryota
Human gut protozoa and helminths: from parasitism to therapy
The human body has a multitude of microorganisms, in a number of at least 1014. The associated microbial communities are called microbiota and the genes encoding it form the microbiome. The vast majority of these microbes inhabits the human gastrointestinal (GI) tract and display an intricate ecosystem of Bacteria, Archaea, and Eukarya, that plays an important role in the maintenance of physiological homeostasis, through the regulation of immunity, the metabolism of nutrients, and the protection against the pathogenic invasion.1,2 When this homeostasis is broken, a deranged state, called dysbiosis, comes up. Thus, the gut microbiome (GM), with its varied influence on physiological functions, can be addressed as a ‘superorganism’. 3
Researchers have emphasized that common eukaryotic parasites colonize the intestine alongside bacterial gut microbiota (BGM).4–6 Preliminary evidence suggests that parasites may play a role in many GI pathologies, such as the irritable bowel syndrome (IBS) and inflammatory bowel diseases (IBD). 7
Intestinal helminth (multicellular worms) and protozoan (single-celled) parasites are responsible for the prevalent parasitic infections in developing countries, whereas in developed countries the protozoan parasites are found to cause acute GI infections. The common helminths that reside in the human gut are Nematodes (roundworms), Cestodes (tapeworms), and Trematodes (flatworms) and their infection is typically due to lack of sanitation. The most common intestinal protozoan parasites include Giardia intestinalis, Entamoeba histolytica, Cyclospora cayetanenensis, and Cryptosporidium spp. 6 Although high morbidity and mortality due to intestinal parasites have been reported in endemic countries (especially in children), indigenous populations show better tolerance than Western populations, which corresponds well with the ‘hygiene hypothesis’.8,9 Intestinal parasites and parasitic secretions have shown to stimulate and train the innate and adaptive immune regulatory pathways, modulating the immune responses. 9 Hence, a new outlook with curative roles have been reported for otherwise notoriously perceived parasites.
A number of studies have suggested beneficial outcomes such as eubiosis related to parasites,10–12 though further advancement may be required. Increasing evidence suggests that specific parasitic species could alleviate the symptoms of IBS, Crohn’s disease, Type 1 diabetes (T1D) and even arthritis. 9 However, there are also studies that support the role of parasites as triggers of some disorders, such as multiple sclerosis (MS). 13
Although there are limited data of the role of gut parasites and their interaction with the BGM, the advent of ‘omic’ technologies has revolutionized the study of gut ‘parasitome’ and has elucidated the relationships among them, the host, and resident prokaryotes, whether pathogens or commensals. 14
The focus of the present review is to discuss recent discoveries on human intestinal parasites that colonizing humans’ enteric niches, and their interaction with the host (beneficial or detrimental) and the other gut microorganisms. We also looked into their immunomodulatory mechanisms, as well as their potential use as therapeutic strategies in human diseases.
Pathogen or commensal citizens of the healthy gut parasitome?
The human GM is composed of a complex community of micro- and macro-organisms. Although majority of the organisms are bacteria, fungi, viruses, protozoans and metazoans also inhabit the intestine tract. 15 The GM can be divided into prokaryotes (bacteria and archaea), bacteriophages, eukaryotic viruses, and the meiofauna (Figure 1).
Figure 1.
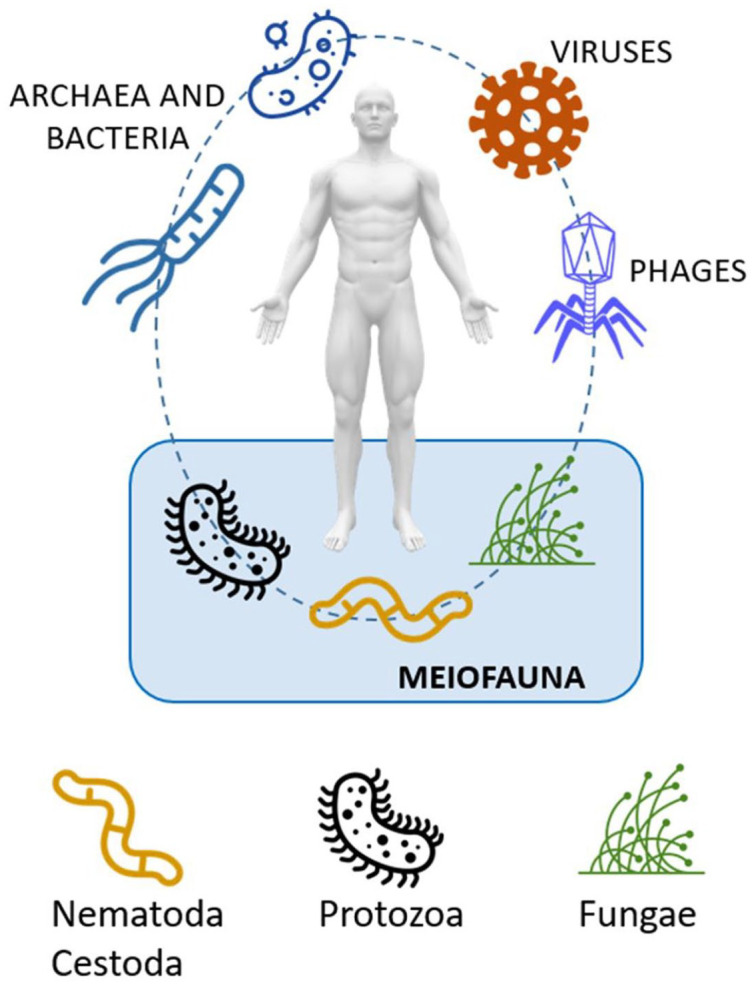
Human gut biome characterized by bacteriome, virome and meiofauna.
The human GI tract is one of the most complex microbial ecosystems on Earth, as it harbors 100 trillion prokaryotic cells (1011 to 1012 cells/mL). 16
The GI tract is also colonized with less abundant microbial eukaryotes, including a fungal microbiome (mycobiome). 17 Combined, these microbial eukaryotes constitute the meiofauna, 18 namely metazoans with body sizes between 45 µm and 1 mm and also eggs and juvenile stages of larger species, including those of parasitic helminths. There is a growing body of evidence showing that eukaryotic parasites are often commensals, and some might even have a positive effect on host health.11,12,19 Lately, many studies on gut protozoan and helminth species have investigated their relationship with BGM. Parasites and microbes have co-evolved along with the intestinal immune system and exhibit a complex interaction network within the human mucosa. 20 Most parasites display strong immunomodulatory characteristics enabling them to colonize and persist in the intestine. Conversely, gut bacteria may also play a role in modulating the pathogenicity of a parasite.
The relationship between several gut parasites and bacteria has been identified in different settings. Escherichia coli can promote hatching of Trichuris muris eggs. 21 Recently, the disruption of mucosal microbiota biofilm has been identified as a pathogenic pathway of Giardia. 22 Moreover, the virulence of Entamoeba histolytica appears to depend on its interaction with gut bacteria. E. histolytica is capable of shifting the BGM toward a pathogenic profile through selective phagocytosis of healthy bacteria such as Lactobacilli. 23 Correspondingly, alterations in BGM induced by helminth-infections could worsen bacterial colitis. 24
Until few years ago, parasites were considered always harmful, but accumulating evidence from parasite-screening studies reveal that several protozoans and helminths, previously thought to be pathogenic, are in fact highly frequent in the gut microbiome of healthy individuals, including some protozoans such as Giardia, Entamoeba, Dientamoeba and Blastocystis.11,12,25 This issue is still controversial, as the evidence is still conflicting.
Some of these parasites, for example, Giardia duodenalis, can cause clinical symptoms, including malabsorptive diarrhea, abdominal cramping, and weight loss, so their general categorization as commensals is arguable (Text Box 1).25,26
Text Box 1.
Blastocystis hominis debate.
| B. hominis, found in human GI tract, varies genetically owing to the 17 subtypes delineated by the small subunit rRNA gene. The subtypes (ST) differ across different types of hosts and is distinct in its prevalence in the world. Subtypes ST1, ST2, ST3 and ST4 are the recurrent subtypes among the nine that infect humans. 27 Despite the understanding of the subtypes, the correlation between parasite virulence and the genetic subtype of the protozoa is still debatable. 28 Infections caused by Blastocystis in humans results in diverse GI signs and symptoms including diarrhea and abdominal pain. Although initially, Blastocystis was considered as an infectious agent, several studies show high prevalence of the protozoan in healthy individuals without causing symptoms. 11 Furthermore, studies on Blastocystis infected individuals with IBS showed decline in pro-inflammatory bacteria, and without IBS showed a decline in anti-inflammatory bacterial spp. (Faecalibacterium prausnitzii), 29 hus, reinstating the correlation of Blastocystis to the pathophysiology of IBS and BGM imbalance. Consistently, Audebert and colleagues found a possible relationship between the presence of Blastocystis and an increase in the diversity and abundance of BGM, mainly observed as increase in the class Clostridia and a reduction in the family Enterobacteriaceae, which suggested that Blastocystis is correlated with a healthy gut microbiota. 30 Blastocystis has also been associated with markers of eubiosis, i.e. low relative abundance of Bacteroides and Clostridial cluster XIVa, and high Prevotella; or high Clostridia and low Enterobacteriaceae.12,31 Such findings suggest that this parasite is a ubiquitous and diverse member of the healthy gut microbiota, and exemplifies long-term host colonization without prompting disease. Hence, Blastocystis is concomitant to the adverse, but certain compelling evidences where a higher microbial richness in the intestines colonized by Blastocystis has been observed, raises questions of its pathogenicity versus beneficial effects to intestinal health. |
However, studies on Giardia infections in children from endemic countries, such as Bangladesh, suggest that they are not associated with diarrhea and that does not affect child development. 32 Similarly, a study by Holtman et al. ruled out Dientamoeba fragilis as a cause of GI symptoms in children. 33
Moreover, the presence of some parasites has been associated with microbiological and/or clinical benefits. Entamoeba spp. (other than Entamoeba histolytica) and Blastocystis spp. in the gut was associated with greater bacterial diversity (a marker of healthy gut microbiome),11,12 and a decrease in the abundance of these organisms has been associated with IBS and IBD, 11 while low prevalence of Blastocystis hominis and Dientamoeba fragilis has been linked to ulcerative colitis. 34 Another interesting metagenomic survey by Beghini et al.showed higher correlation of Blastocystis in healthy control individuals when compared to subjects with obesity, Crohn’s disease and colorectal cancer, suggesting it could be obligatory for host health. 35
The BGM of Blastocystis-positive subjects (alone or along with Dientamoeba fragilis) is characterized by a low relative abundance of Bacteroides and Clostridial cluster XIVa, and high levels of Prevotella. 36 Moreover, Morton and colleagues demonstrated that carriers of Entamoeba displayed prevalence of bacterial taxa that are negatively correlated to autoimmune diseases. 27
Likewise, there is a growing interest to understand the association of helminthic parasites and host health. Helminths have evolved many fascinating molecular mechanisms to regulate host immune response in order to evade expulsion. 37 At the same time, human hosts have co-evolved an immune system (IS) innate response to produce Th2-mediated type 2 immune response in order to minimize helminth virulence and promote intestinal tolerance. 38 Recently, the presence of certain helminths has been correlated with increased BGM diversity, as well as low occurrence of inflammatory and autoimmune diseases.10,38 In endemic countries, for instance in Malaysia, indigenous populations colonized by a variety of helminths including Trichuris spp. (whipworms) and Ascaris spp. (roundworms) showed enrichment of protective bacteria (for instance, Clostridiales) within the gut. 10 In particular, the presence of the Trichuris spp. strongly correlated with an increase in Paraprevotellaceae among the Orang Asli community of semi-urban Malaysia.10,39 The beneficial role of intestinal hookworm Necator americanus was exemplified by trials where deliberate infections with the nematode lead to better gluten tolerance in some patients with celiac disease. 40 Moreover, experiments in Nod2 deficient mice demonstrated that presence of the helminths Trichuris muris and Heligmosomoides polygyrus suppressed symptoms of Crohn’s disease by inhibiting growth of pro-inflammatory Bacteroides spp. Bacterium. 41 The group also investigated BGM in helminth-endemic regions, revealing a higher Clostridiales to Bacteroidales ratio. 41 The ability of helminths to promote protective bacterial species in the gut is already being tested as a novel therapeutic strategy for inflammatory diseases.38,40
In spite of these substantial evidences, the molecular mechanisms by which parasites alter BGM and vice versa remains largely unknown. Besides, we must not forget that these organisms are wired to be pathogenic by nature. Hence, the line between commensalism and pathogenicity is blurred, and the clinical outcome could be determined by many factors related to the host, to the BGM, and to parasites themselves, that are yet to be elucidated.
Issues in the assessment of commensal parasitome
Our lack of knowledge on gut parasitome is in partly due to the delayed interest of research, and also a lack of reliable research tools. It is not always possible to differentiate colonization from invasion and these phenomena also vary depending on the phenotype and parasite strain variability, even within genera. While acute diseases caused by certain parasites (in example, Microsporidia, Cryptosporidium spp., and Giardia intestinalis) usually result in rapid clearance of the parasite and provide long term immunity, some species (for example, beneficial flagellates and immune-tolerant protozoans) establish long term colonization in the host. 19 The potential pathogenicity of a gut parasite is determined by several factors, including the localization of the parasite within the gut (i.e. small intestine or colon, and lumen versus epithelium), 19 host’s age, health status and geography. 12 The sociodemographic and cultural characteristics of particular communities predispose an individual to parasitic tolerance or disease. However, growing evidence point out that the gut microbial environment plays a significant role in influencing clinical manifestations. As mentioned earlier, the bacterial composition of the gut can skew parasite characteristics toward pathogenesis or commensalism. Therefore, the study of host-bacteria-parasites interactions and the understanding of the dynamics of this molecular crosstalk becomes necessary. Formerly, most researches conducted in Western countries produced data geographically biased. It is now well-understood that there is an inherent difference in the diversity of gut parasitome in high- versus low-income countries, that is shaped by factors such as diet, hygiene practices, medications, demography. Indeed, improved sanitization, food sterilization and usage of antibiotics can lead to a lower diversity of parasitic species which in turn affects the bacterial microbiota.11,42 Hence, age, lifestyle, environmental changes, social and demographic factors and public health system are different disease drivers affecting parasitome profiling. 43
The advent of high-throughput quantitative PCR and sequencing techniques have enabled scientists to detect and differentiate the wide-ranging microorganism diversity within the intestine. Nowadays, various experimental methodologies have been adopted to delineate the host-BGM-parasite interactions including parasite life cycle, microbiota surveys and in-vitro or in-vivo experimental models. 12 Surveys majorly rely on targeted metagenomic examination by 16 S rRNA/18 S rRNA analysis and PICRUSt analysis to understand GM composition and function, respectively.12,44 Shotgun metagenomics sequencing allows simultaneous investigation of prokaryotic and eukaryotic genomes, along with GM structural and functional read-outs. 11 However, one of the main limiting factor at present is the lack of metagenomic data available for several human gut eukaryotic parasites. Consequently, metatranscriptomics, metaproteomics, and metabolomics studies present reliable tools to gain better insights on the parasitome. Metabolomics, in particular, can shed light on the modulatory effects of bacterial and parasitic metabolites on each axis of the host-bacteria-parasite network. Thus, while designing experiments and surveys it is always important to take a holistic approach for comprehensive results.
Other issues in the evaluation of parasites could rely in other steps of analysis, including protocols of genome extractions. In a recent comparative evaluation of five commercial methods, the combination of combining chemical, enzymatic and/or mechanical lysis procedures at temperatures > 56°C were proven more effective in releasing DNA from Cryptosporidium oocysts. 28 However, extraction protocols using strong homogeneization/lysis steps for fungi and protists may bring to degradation and release of bacterial DNA. In conclusion, several steps of the analysis process, from DNA extraction to sequencing, may accoung of discrepancies in findings of gut microbial analyses.
The Parasitome as an immunological driver
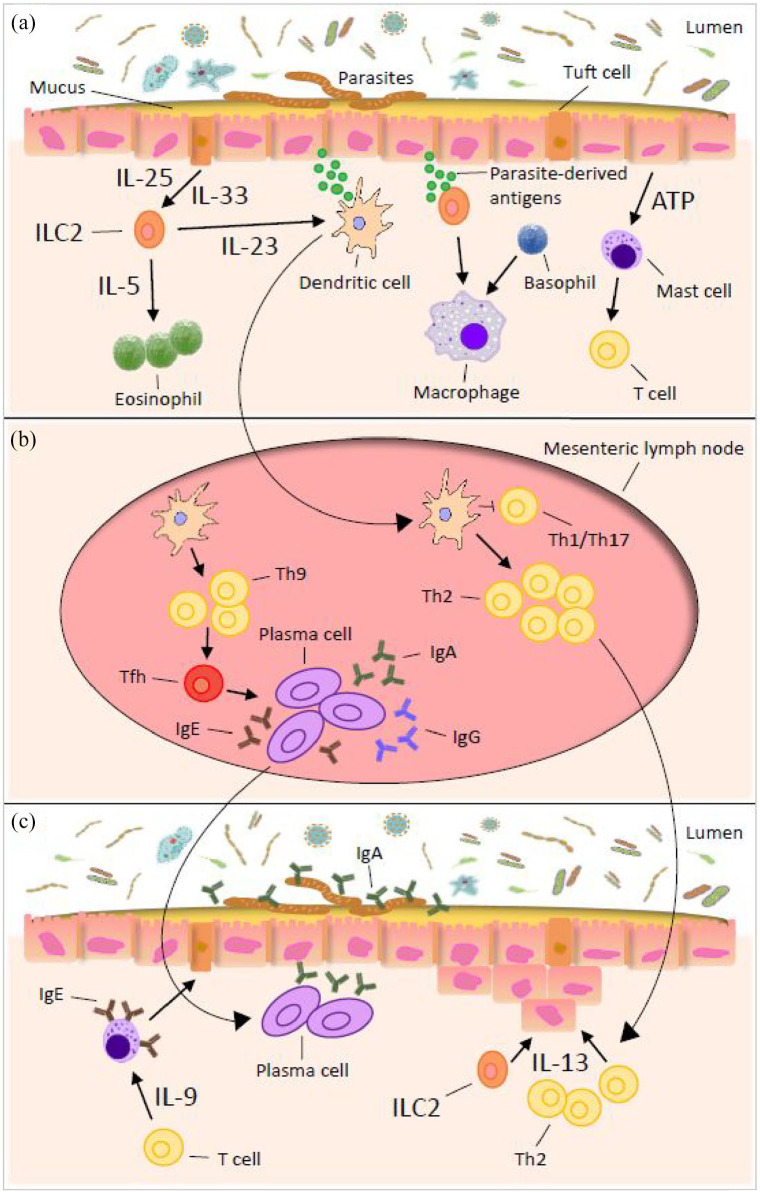
A growing body of evidence suggests that gut parasites may potentially have a deep impact on our immune system (IS) (Figure 2).
Figure 2.
Immune response to gastrointestinal nematode infections. (a) Detection and transmission phase in the intestinal epithelial layer. Type 2 immunity against nematodes results from recognition of parasite-derived antigens that are continuously secreted during infection. Once a parasite has been detected by the epithelium and/or other nonhematopoietic cells, the signal is transmitted to cells of the innate immune system and an inflammatory cascade can be initiated. (b) Induction of immunity in the lymphoid tissue. Once the innate immune system has been alerted to the presence of nematodes, it propagates the signal to the mesenteric lymph nodes for an adaptive immune response. This role is mainly performed by the dendritic cells whose determine the T and B cell activation. (c) Expulsion of the parasites from the intestine and resolution of the response.
Apart from the specific host immune responses they elicit, chronic infections might lead to alteration of immune response toward bystander pathogens, allergens and vaccines. Both protozoans and helminths activate the host innate and adaptive immune system (IIS and AIS) via immunological pathways involved in parasite recognition. It has been evidenced that IIS activation occurs through toll-like receptors (TLRs), macrophages and neutrophils, and AIS activation through T-cells and antibodies. Conversely, parasites can inhibit the IS by means of their molecular secretions. Due to the extensive repertoire of gut parasites with unique molecular pathogenicity, it is difficult to generalize the immunological effect of each organism.
Parasites as IS activators or enhancers
Protective immune response to most parasites often occurs in the case of mucosal invasion and this mainly involves secretion of cytokines by the intestinal epithelial cells (IECs). 7 Specific cytokines recruit innate immune cells including neutrophils and macrophages, leading to subsequent effector responses. Recently, tuft cells were also identified as early responders to protozoan and helminth infections. 45
Activation of TLRs has been evidenced in protozoans such as Giardia and E. histolytica. 28 TLRs affect the functioning of antigen-presenting cells (macrophages, dendritic cells, etc.) dictating the downstream pathways involved in AIS. Activation of the AIS involves Th1 or Th2 mediated response by triggering production of pathogen-specific CD8 + T-cells and IgM, IgG, IgA antibodies. IFNγ, a Th1 cytokine recently identified as a key player in host inflammatory response induced by apicomplexan pathogens, drives IIS and AIS by inducing production of antimicrobial peptides (AMPs), nitric oxide (NO), reactive oxygen species (ROS) and Th1 cytokines. 28 In another example, excretory/secretory products (ESPs) from Giardia trophozoites demonstrated activation of MAPK and NF-κB pro-inflammatory pathways resulting in cytokine production including TNF. 46 Lately, stimulation of nod-like receptors (NLRs) and inflammasomes have also been described in E. histolytica infection models. 47
As for helminth infections, a type 2 immune response is usually observed. Recognition by the IS can in part be triggered by epithelial damages, as exemplified in the case of H. polygyrus or Nippostrongylus brasiliensis. This leads to release of damage-associated molecular patterns (notably ATP and uric acid), proallergic alarmin cytokines (in example IL-33 and IL-25) and thymic stromal lymphopoietin, along with general inflammatory cytokines such as IL-1α and granulocyte-macrophage colony-stimulating factor (GM-CSF). The alarmin cytokines IL-33 and IL-25 are potent activators of the type 2 responses, by promoting activation and differentiation of type 2 innate and adaptive lymphoid cells.37,48 Moreover, helminths (eg. Schistosoma mansoni and Ascaris lumbricoides) activate TLR2/4 and TLR9 pathways to promote type 2 response. 49 Another noteworthy experiment by Dubey et al. established that increased antibody response to helminth infection is due to the de-novo B-cell follicle formation in an IL-4Ra-dependent manner. They found that chronic H. polygyrus bakeri infection in mice leads to IL-4Ra signaling, which promotes lymphotoxin expression by hematopoietic cells thereby causing remodeling and proliferation of stromal cells. This mechanism could probably help against constant re-infection and keep a check on the parasite burden. 50
More recently, the succinates produced by some parasites, both helminth and protozoan (Nippostrongylus brasiliensis and Tritrichomonad) has been proven to be one of the molecules activating the tuft cells-mediated immune response. 51 Activated tuft cells secrete IL-25, which in turn induces type 2 ILC2s to produce IL-13. In a positive feedback loop, IL-13 and TRMP5 induces differentiation of epithelial cells (IECs) into more tuft cells. 51 Moreover, Solaymani-Mohammadi et al. investigated the role of IL-21/IL-21R signaling pathway in both protozoan and helminth infections. Finally, IL-21R seems to be upregulated in parasitized organs leading to IL-21/IL-21R induced IFNγ-mediated inflammatory pathways. 52
Parasites as IS suppressors
The immune response to parasites can vary markedly in acute versus chronic infections. Gut parasites have developed a number of molecular mechanisms to evade or moderate the host IIS and AIS, that can ultimately lead to establishment of chronic immune disorders, or on the other hand, a chronic asymptomatic commensal/symbiotic relationship.
Protozoans are known to produce numerous types of virulence factors that can directly weaken the host immune response. For instance, E. histolytica can downregulate IL-1b expression by producing monocyte locomotion inhibitory factors. 47 Similarly, Giardia can inhibit nitric oxyde(NO) production by (i) competing with arginine, the substrate for NO synthase, and also (ii) by secreting flavohemoglobins that has NO reductase activity. 53 Moreover, there is increasing evidence that chronic Giardia infection can downregulate inflammatory pathways. In cattle, Giardia infections were shown to upregulate anti-inflammatory transcription factor peroxisome proliferation activation receptor γ (PPARγ). 46 tSeveral Giardia proteases can cleave pro-inflammatory NF-κB transcription factor. 53 Furthermore, the upregulation of T-regs have been reported as an immunosuppressive strategy in many protozoans including Leishmania major and Toxoplasma gondii. 54 Correspondingly, the anti-inflammatory IL-10 cytokine is often upregulated in leishmaniasis and toxoplasmosis, and also in other protozoan diseases including malaria and trypanosomiasis. 54 Downregulation of TLRs, particularly TLR2, is an alternative strategy confirmed in Trypanosome spp. and E. histolytica. 49 Taken together, in general, the localization of these protozoans in the case of chronic asymptomatic infections are yet to be ascertained. Most probably, they could be floating within the intestinal lumen to exert prolonged immunosuppressive effects.
Similarly, chronic infections caused by helminths also tend to decrease pro-inflammatory cytokines. 55 Asymptomatic helminth carriers show specific unresponsiveness, providing evidence of immunosuppression. During asymptomatic infections, the immunosuppressive T-cell subset, Tregs, are significantly increased by the parasite and molecular balances are altered. A relative increase in IL-4 compared to IL-17 and IFN-γ is observed, and elevated levels of regulatory IL-10 and TGF-b. In this context, it has also been shown that Treg cells drive the isotype switch from the pro-allergenic/inflammatory IgE to non-inflammatory IgG4. Some filarial parasites also downregulate the effector cell population (they require restimulation for activation), introducing one more level of immune downregulation.37,48 Another intriguing strategy of immunosuppression has been shown by Schistosoma, wherein repression of TLR genes occur by week 8 of infection. 49 Again, in H. polygyrus, the ESP Alarmin Release Inhibitor (HpARI), is responsible for attenuating IL-33 pathway resulting in dampened type 2 responses and inflammation. 56 As reviewed by Maizels et al., several other ESPs have been discovered that are known to downregulate host IS. 57
In totality, other resident bacteria and parasite commensals can have an antagonistic or synergistic immunosuppressive effect. For example, bacterial species such as Escherichia coli and Yersinia spp. also exhibit NF-κB inhibitory mechanisms. 53 Hence, the final outcome could be a balancing act between parasite, bacteria and host.
Parasites as IS regulators: consequences in clinical settings
The immune modulatory mechanisms by parasites have shown to influence the host IS in the context of vaccines, allergy, infectious diseases, inflammatory diseases, metabolic disorders and others.
Studies showed an increased number of immune cells and as well as levels of inflammatory cytokines in presence of the commensal protist Tritrichomonas musculis in mice that conferred protection against mucosal infection by Salmonella typhimurium. 58 They found that this was attained by the activation of the host epithelial inflammasome and inducing IL-18 production, the latter promoting T helper Th1 and Th17 immunity via dendritic cells, without inducing any tissue damage. 58
Helminths have been designated as ‘master regulators’ of host IS so as to warrant a lifelong mutual relationship. The steering of immune response in the Th2 direction, thereby muting Th1/Th17 pathway has been strongly correlated to a low risk of autoimmune diseases. On the other hand, they are also capable of weakening the host protective Th2 immune response via expansion of regulatory cells, anti-inflammatory cytokines and antibody isotype switching. Since allergies are typically IgE-mediated, this modified Th2 response has been linked to alleviation of allergic symptoms in experimental animal models carrying Schistosoma mansoni, H. polygyrus and Trichinella spiralis among other helminths. Moreover, helminth protease inhibitors (e.g. cystatins and serpins) and numerous other ESPs are known to interfere with antigen-presentation, complement pathways and inflammation which has been reviewed elsewhere.57,59
Helminth colonization can also influence the human response to pathogenic infection. The presence of Ascaris lumbricoides, Trichuris trichiura and Strongyloides stercoralis have shown a protective role against malaria, 60 whereas helminths show a synergistic effect in the progression of tuberculosis. 61 Another recent experiment in mice suggest that chronic gut-restricted H. polygyrus infection can enhance neutrophil responses to Pseudomonas aeruginosa infection. 62 Furthermore, downregulation of TLR and other pattern recognition receptors (PRRs), along with a low-key Th1/Th17 response can lead to insufficient immune response to vaccines. This has been well studied in the case of vaccines against Mycobacterium tuberculosis (BCG vaccine) where, deworming can reverse this effect. 61 Correspondingly, a study by Haben et al. indicate intervention of follicular T helper cells (TFH) induction by helminths as another mechanism that dampens vaccine efficacy. 63
In addition, chronic parasitic colonization, mainly helminths, has shown immunomodulatory implications in a wide array of pathologies, including inflammatory disorders (Text Box 2), co-infections, diabetes and cancer (Text Box 3). The majority of these studies have been focused on helminths, and data on protozoans are quite limited.
Text Box 2.
Parasites in IBS and IBD.
| Evidences show that a direct correlation exist between loss of parasite colonization and a rise in autoimmune disorders such as IBD and IBS in the individuals living in developed countries. IBD and IBS qualify as the most important immune-mediated intestinal conditions. 9 IBS is described as a common, long-term condition of the digestive system characterized by bloating, diarrhea and/or constipation. IBS cases reported to occur following enteric infection with different pathogens are called post-infectious IBS (PI-IBS) with symptoms shown to persist for ⩾ 10 years following the infectious enteritis episode. 64 Plausible role of B. hominis, C. parvum and Giardia spp. in patients with diarrhea-predominant-IBS has been suggested along with bowel dysfunction due to the penetration of mucosal layer.6,65 In the case of the nematode Trichinella, IBS is seen as a secondary syndrome. Also, Dientamoeba fragilis and Haplorchis taichui are considered etiologic agents for IBS-like symptoms. 6 IBD is described as an idiopathic, chronic, and recurring inflammatory disease of the GI tract characterized by ulcerative colitis (UC) and Crohn’s disease. The symptoms in this disorder appear as chronic abdominal pain and changed bowel habits. Several investigations found out that intervention in the gut microflora contributes to the etiology which is otherwise unclear. 6 In IBD, we observe a shift from mostly strict anaerobic bacteria to facultative anaerobes (notably Enterobacteriaceae). The negative association between Blastocystis and Bacteroides can be explained by the fact that Bacteroides contribute less to butyrate production (which is then used in a reaction consuming oxygen, maintaining an anaerobic environment in eubiosis) than Firmicutes. 37 Studies showed that Tritrichomonas musculis infections increasing inflammation level can lead to T-cell mediated colitis.31,58 The frequent occurrence of Blastocystosis in patients with IBD have been reported. 6 Moreover, studies demonstrate that T. gondii infection influences small intestine necrosis and cell death in sensitive patients with IBD. 6 Contrary to the protozoans’ negative impact, helminthes such as Trichuris suis, Trichuris muris, Nippostrongylus brasiliensis and Trichinella spiralis appear to harmonize the symptoms of IBD and ameliorate host immunity in various experiments.6,9 However, mice studies on Citrobacter rodentium-induced colitis, Heligmosomoides polygyrus was shown to exacerbate the symptoms through a Th2-mediated GM composition alteration, underlining once more the effects of helminths on immune pathways, microbiota composition and severity of GI tract disease at the same time. 24 |
Text Box 3.
Parasites and GI cancerogenesis.
| The International Agency for Research on Cancer (IARC) identifies parasites as one of the infectious agent to cause cancer disease in humans. Research on understanding the oncogenic potential of certain parasites have gained traction in the recent years. Several protozoan and helminth species have been associated with GI carcinomas including gastric, colorectal and liver cancer. Chronic infections by T. muris, Platynosomum fastosum, C. parvum, T. gondii, Trichomonas vaginalis, Fasciola gigantica and Strongyloides stercoralis have shown inflammation driven generation reactive oxygen species (ROS) that can lead to carcinogenic mutations. 66 T. muris and C. parvum is reported to be concomitant with exacerbated intestinal tumors. 66 Recently, C. parvum induced ileocecal tumors was shown to be caused by alteration of several intracellular pathways including the Wnt-signaling pathway. 67 The causal link between (i) liver flukes (such as Opisthorchis viverrini and Clonorchis sinensis) and cholangiocarcinoma, and (ii) blood flukes (such as Schistosoma japonicum and S. mansoni) and colorectal cancer has been documented. 68 Chudnovskiy et al. observed that Tritrichomonas musculis can induce colorectal carcinoma in mice. 58 Some studies have suggested Blastocystis spp. as a causative agent of colorectal cancer (80%) by facilitating proliferation of colorectal cancer cells and down-regulation of the host immune cell response.69,70 Moreover, certain microbiome signatures have been described in different cancers, but the role of gut parasites in the BGM-cancer axis remains unexplored (see Outstanding Questions). 71 Future experimental and population studies can pave way for better comprehension of the association between parasites and cancer. This will also help in exploring reliable clinical biomarkers for diagnostics and prognostics. The possibility of helminths and their secretory products as therapeutics is also being investigated (see Text Box 4). |
Parasites are involved in IS development during early life
There is an established association between lower incidence of autoimmune disorders and higher prevalence of parasites infections, 9 supporting the hygiene hypothesis. This hypothesis could be explained by a change in the healthy components of the gut ecosystem over time, leading to a lack of training of the IS. 51 This ‘training’ is now known to happen not only during the early years of life, but even before that, in the prenatal stage.
The involvement of TLRs in the IS training, activated by both commensals and pathogens is supported by consolidated experimental data. 9 Consistently with this notion, studies prove that TLR agonists have the ability to train the fetal IIS during pregnancy. In utero exposure to HIV TLR agonists show an enhanced cytokine profile, whereas malarial TLR agonists have shown a reduction in IIS cytokines. 72 Besides, the development of fetal AIS is dependent on the IIS evoked by maternal inflammation. Interestingly, data shows immunosuppressive effects of some parasites, notably an increased IS tolerance of the newborn from a helminth-infected mother, that appears to lead to increased susceptibility to further infections, up to 17 years later.37,72 Conversely, this immune tolerance lowers the risk of autoimmune and atopic diseases. Likewise, in urbanized settings where helminth infections are negligible, other pathogens, including T. gondii, can bring about the same effect. 72 To sum up, maternal parasite infection can sensitize neonatal IS development, probably via a mechanism involving fetal hematopoietic stem cells. 72 In this perspective, the trajectory of fetal IS development could be determined by the degree of maternal infection, the duration and co-infections, among other unknown factors. Deworming prior to vaccination in children (especially in endemic countries) could be more effective in the development of AIS. Although several associations between parasites and IS development have been found, the exact molecular mechanisms are yet to be unfolded.
Where does the bacterial microbiota fit in? Parasite-bacteria cross-talks in colonization and infection processes
The interaction between GBM and parasite appear to influence gut colonization and infection processes.
Numerous research groups have investigated the complex interaction between gut parasites and common bacterial/viral infectious diseases. Certain commensal parasites have shown to affect viral pathogenicity, for instance, disease aggravation in the case of Schistosoma mansoni and hepatitis C manifestations. 60 A similar controversial relationship has been established in several parasite-bacterial co-infections. Pseudomonas aeruginosa and Escherichia coli have shown to inhibit Plasmodium falciparum life cycle, 60 while helminths are most likely to aggravate clinical manifestations of Mycobacterium tuberculosis and Salmonella infections.60,61
Researches have also shown that the interaction between Entamoeba species and intestinal bacteria plays a significant role in the regulation of amebic virulence and coculture with E. coli can lead to either an increased or decreased virulence of E. histolytica depending on the host background.73,31 Studies show a correlation between a high parasite burden in presence of Prevotella copri and symptomatic infections with E. histolytica. 12 Lactobacillus casei and Enterococcus alone inhibits E. histolytica survival by 71%, with synergic effect. 31 Moreover, Lactobacillus species (L. johnsonii, L. casei and L. rhamnosus) promote Giardia clearance, while bacteriocins produced by L. acidophilus (P106) and L. plantarum (P164) decrease parasite adhesion. 74
Concluding remarks and future perspectives
Over the last two decades, helminths and protozoans, previously considered only as pathogens, have been being increasingly suggested to be also commensal, protective or even curative microorganisms. These findings essentially point out that most of these primarily-considered ‘parasites’ could have an evolutionary history, tipping the balance toward commensalism, where they have adapted to live off the host without causing any harm. But then, it is important to keep in mind that for most of these data, there are other studies that have found opposite results. A likely explanation for these differences can be attributed to various parameters affecting the study design and protocols. One of the major factors is the host genetic background, which has a major influence on susceptibility to a given microbe. Epidemiological studies have been able to successfully link unique gene loci to parasitosis susceptibility. 75 Second, the method of analysis which includes the type of sample used for sequencing, the workflow standardization, and lab to lab or operator variability, can also create a bias in the results. Moreover, the majority of the studies rely on fecal samples, which does not give enough information of the localization of the parasite within the GI tract.
Nonetheless, even though the changes induced by parasitic infection can differ from one study to another, the broad mechanisms involved (Th1/Th2/Treg balances in immunity, bacterial composition modifications) remain consistent. Hence, the wide spectrum implications of human gut parasites are no longer debated. The new therapeutic applications allow a wide scope of use of parasites. While bacteria are already in common use for pre- and probiotic therapies, parasitic therapy is still preliminary (Text Box 4). Further characterization of the human gut-parasitome is needed, and this will require time and new tools. The -omics analysis ((meta)genomics, (meta)transcriptomics, (meta)proteomics, (meta)metabolomics) represent a robust tool for such type of studies. 14
Text Box 4.
Therapeutic perspectives.
| Targeting the GM and tweaking the cross talk between bacteria, protozoans and helminths is regarded as a prospective therapeutic strategy for the treatment of several pathologies. In the case of pathogenic protozoan invasions, for example, E. histolytica, G. duodenalis, Cryptosporidium spp. or Toxoplasma spp., interventions such as probiotics, prebiotics or fecal microbiota transplantation (FMT) can be introduced to enhance microbiota diversity and change the course of pathogenesis and the disease outcomes in the host. 76 In addition, protozoans such as Blastocystis spp. can be a likely therapeutic choice in treating chronic inflammatory conditions as they exhibit the anti-inflammatory potential and is seen to increase the bacterial diversity. 28 Lately, the therapeutic use of helminths has gained a lot of attention. This is popularly known as “helminth therapy” (HT) which demonstrates the innate ability of helminths to alter immune response from Th1 to Th2/Treg. 77 HT and helminth-derived product therapy (HDPT) utilizes live helminths and ESP components for immuno-modulation. Many animal studies have shown promising results to treat or prevent inflammatory diseases such as IBD, T1D, MS, rheumatoid arthritis (RA) and asthma. For example, schistosome glutathione S-transferase (P28GST) improves intestinal inflammation in experimental colitis and Fasciola hepatica mitigates experimental autoimmune encephalomyelitis (EAE) and MS. 77 Similarly, ES-62, a glycoprotein from the filarial nematode Acanthocheilonema vitae, is a potent pharmacological molecule that can be used for asthma, lung fibrosis and RA. 78 Likewise, metabolism and weight regulation are in part immunologically regulated, hence helminth infections such as Nippostrongylus brasiliensis and S. mansoni, once again display a therapeutic perspective since it was shown that they prevent glucose intolerance through adipose tissue eosinophils and type 2 macrophages activation. 37 Furthermore, a number of animal studies have evidenced that helminth infections (S. mansoni, Trichinella spiralis and Filaria spp.,) and helminth derived products can prevent onset or cure T1D, possibly by repressing Th1 immune response. 79 In cases where parasite worms worsen the diagnosis, such as H. polygyrus in Citrobacter colitis, 26 the parasite itself could be targeted in therapy. Another interesting scenario is the proposition of T. musculis as a “protistic antibiotic” as its protection against Salmonella infection through IL-18 pathways has already been shown.58,31 Although helminth based therapies seem transcendental and few human trials are underway, several hurdles remain. 77 One of the major shortcoming is the tumor promoting activity of ESPs. 80 Moreover, the use of live helminths remain contentious because of the lack of comprehensive understanding of mechanism of disease prevention and inhibition. |
In this context, the application of ‘big data’ methodologies to GM may boost this intervention. The introduction of sequencing technologies has revolutionized the field, enabling investigators to characterize microbial communities directly from feces. By utilizing larger datasets, researchers are able to design large-scale studies to ask (and answer) complex questions. Metadata associated with samples is becoming an increasingly large contributor to microbiome big data and the challenges associated with streamlining data analysis. The successful application of big microbiome dataset analysis has already provided relevant insights for other areas of research such as epidemiology, agriculture, and healthcare. Since gut microbiota composition differs widely according to host genetics, diet, lifestyle and geographical location, we stress on the fact that the application of big data methodologies to gut microbiota could be of utmost importance in order to have a wide understanding of various complex diseases.
In recent times, the application of microbial-based big data in patients with cancer has gained considerable attention. Current microbiota-based data (largely gut bacteriome) that are available in patients with cancer (and patients undergoing immunotherapy) are interesting, but is not yet definitive for translation to clinical practice.71,81,82 A similar approach in gut parasitology is also of growing interest. The gathering of big microbiome-based databases could therefore be matched with other types of information (including lifestyle of patients and biology of tumors), that can help in identifying microbial patterns associated with increased risk of cancer and modifying them through therapeutic microbiota manipulation (including diet, antibiotics, prebiotics, probiotics, fecal microbiota transplantation (FMT)). 29 To date, a relevant rate of patients stop chemotherapy because of toxicity and side effects (including diarrhea). Moreover, immunotherapy, although promising, provides only partial efficacy in several cancers. As gut microbiota appears to be deeply involved in both pathways, its modulation could be of utmost importance. 83 The connection of big microbiome-based databases with safety and efficacy of different cancer therapies could target the therapeutic modulation of gut microbiota and improve outcomes in these patients. Specifically, parasites, by their capacity to modulate host immunity, have been hypothesized to play a potential role in modulating cancer immunotherapy. 84
To conclude, effective translation of clinical results from bench to bedside requires advancement of patient-centric solutions through collaborative efforts between research organizations, pharmaceutical industries, regulatory bodies and other stakeholders. The determination of optimal approaches that appropriately modulate the patient’s GM need to be well-defined, taking into account lifestyle, socio-economic, demographic, geographic and public health parameters. Moreover, in this data driven era, further connections with other big data and genome-wide association links, as well as studies from a holobiont perspective, are advocated.
Footnotes
Author contribution(s): Gianluca Ianiro: Conceptualization; Data curation; Formal analysis; Investigation; Supervision; Validation; Writing – original draft; Writing – review & editing.
Andrea Iorio: Formal analysis; Investigation; Validation; Writing – original draft; Writing – review & editing.
Serena Porcari: Formal analysis; Investigation; Writing – original draft; Writing – review & editing.
Luca Masucci: Investigation; Writing – review & editing.
Maurizio Sanguinetti: Critical review; Comment; Revision of the manuscript.
Carlo Federico Perno: Investigation; Validation; Writing – review & editing.
Antonio Gasbarrini: Investigation; Validation; Writing – review & editing.
Lorenza Putignani: Conceptualization; Data curation; Formal analysis; Investigation; Supervision; Validation; Writing – original draft; Writing – review & editing.
Giovanni Cammarota: Conceptualization; Methodology; Supervision; Validation; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Gianluca Ianiro  https://orcid.org/0000-0002-8318-0515
https://orcid.org/0000-0002-8318-0515
Contributor Information
Gianluca Ianiro, Gastroenterology Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Università Cattolica del Sacro Cuore, Largo A. Gemelli 8, Rome 00168, Italy.
Andrea Iorio, Department of Diagnostic and Laboratory Medicine, Unit of Parasitology and Multimodal Laboratory Medicine Research Area, Unit of Human Microbiome, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy.
Serena Porcari, Gastroenterology Unit, Fondazione Policlinico Gemelli IRCCS, Roma, Italy.
Luca Masucci, Microbiology Unit, Fondazione Policlinico Universitario ‘A. Gemelli’ IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy.
Maurizio Sanguinetti, Microbiology Unit, Fondazione Policlinico Universitario ‘A. Gemelli’ IRCCS, Università Cattolica del Sacro Cuore, Rome, Italy.
Carlo Federico Perno, Department of Diagnostic and Laboratory Medicine, Unit of Microbiology and Diagnostic Immunology, and Multimodal Laboratory Medicine Research Area, Ospedale Pediatrico Bambino Gesù, Roma, Italy.
Antonio Gasbarrini, Gastroenterology Unit, Fondazione Policlinico Gemelli IRCCS, Roma, Italy.
Lorenza Putignani, Department of Diagnostic and Laboratory Medicine, Unit of Parasitology and Multimodal Laboratory Medicine Research Area, Unit of Human Microbiome, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy.
Giovanni Cammarota, Gastroenterology Unit, Fondazione Policlinico Gemelli IRCCS, Roma, Italy.
References
- 1. Riaz Rajoka MS, Shi J, Mehwish HM, et al. Interaction between diet composition and gut microbiota and its impact on gastrointestinal tract health. Food Sci Hum Well 2017; 6: 121–130. [Google Scholar]
- 2. Parkar SG, Kalsbeek A, Cheeseman JF. Potential role for the gut microbiota in modulating host circadian rhythms and metabolic health. Microorganisms 2019; 7: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salvucci E. The human-microbiome superorganism and its modulation to restore health. Int J Food Sci Nutr 2019; 70: 781–795. [DOI] [PubMed] [Google Scholar]
- 4. Scanlan PD, Marchesi JR. Micro-eukaryotic diversity of the human distal gut microbiota: qualitative assessment using culture-dependent and -independent analysis of faeces. ISME J 2008; 2: 1183–1193. [DOI] [PubMed] [Google Scholar]
- 5. Burgess SL, Petri WA., Jr. The intestinal bacterial microbiome and E. histolytica infection. Curr Trop Med Rep 2016; 3: 71–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hollm-Delgado MG, Gilman RH, Bern C, et al. Lack of an adverse effect of Giardia intestinalis infection on the health of Peruvian children. Am J Epidemiol 2008; 168: 647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Panelli S, Epis S, Cococcioni L, et al. Inflammatory bowel diseases, the hygiene hypothesis and the other side of the microbiota: parasites and fungi. Pharmacol Res 2020; 159: 104962. [DOI] [PubMed] [Google Scholar]
- 8. King IL, Li Y. Host-parasite interactions promote disease tolerance to intestinal helminth infection. Front Immunol 2018; 9: 2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bach JF. The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat Rev Immunol 2018; 18: 105–120. [DOI] [PubMed] [Google Scholar]
- 10. Lee SC, Tang MS, Lim YA, et al. Helminth colonization is associated with increased diversity of the gut microbiota. Plos Negl Trop Dis 2014; 8: e2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chabé M, Lokmer A, Ségurel L. Gut Protozoa: friends or foes of the human gut microbiota. Trends Parasitol 2017; 33: 925–934. [DOI] [PubMed] [Google Scholar]
- 12. Stensvold CR, van der Giezen M. Associations between gut microbiota and common luminal intestinal parasites. Trends Parasitol 2018; 34: 369–377. [DOI] [PubMed] [Google Scholar]
- 13. Tanasescu R, Constantinescu CS. Helminth therapy for MS. Curr Top Behav Neurosci 2015; 26: 195–220. [DOI] [PubMed] [Google Scholar]
- 14. Marzano V, Mancinelli L, Bracaglia G, et al. ‘Omic’ investigations of protozoa and worms for a deeper understanding of the human gut ‘parasitome’. PLoS Negl Trop Dis 2017; 11: e0005916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van de Guchte M, Blottière HM, Doré J. Humans as holobionts: implications for prevention and therapy. Microbiome 2018; 6: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hillman ET, Lu H, Yao T, et al. Microbial ecology along the gastrointestinal tract. Microbes Environ 2017; 32: 300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ianiro G, Bruno G, Lopetuso L, et al. Role of yeasts in healthy and impaired gut microbiota: the gut mycome. Curr Pharm Des 2014; 20: 4565–4569. [DOI] [PubMed] [Google Scholar]
- 18. Giere O. Meiobenthology. The microscopic motile fauna of aquatic sediments. 2nd ed. Berlin; Heidelberg: Springer, 2009. [Google Scholar]
- 19. Lukeš J, Stensvold CR, Jirků-Pomajbíková K, et al. Are human intestinal eukaryotes beneficial or commensals? PLoS Pathog 2015; 11: e1005039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res 2020; 30: 492–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Midha A, Ebner F, Schlosser-Brandenburg J, et al. Trilateral relationship: ascaris, microbiota, and host cells. Trends Parasitol 2021; 37: 251–262. [DOI] [PubMed] [Google Scholar]
- 22. Beatty JK, Akierman SV, Motta JP, et al. Giardia duodenalis induces pathogenic dysbiosis of human intestinal microbiota biofilms. Int J Parasitol 2017; 47: 311–326. [DOI] [PubMed] [Google Scholar]
- 23. Iyer LR, Verma AK, Paul J, et al. Phagocytosis of gut bacteria by entamoeba histolytica. Front Cell Infect Microbiol 2019; 9: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Su C, Su L, Li Y, et al. Helminth-induced alterations of the gut microbiota exacerbate bacterial colitis. Mucosal Immunol 2018; 11: 144–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Einarsson E, Ma’ayeh S, Svärd SG. An up-date on giardia and giardiasis. Curr Opin Microbiol 2016; 34: 47–52. [DOI] [PubMed] [Google Scholar]
- 26. Bartelt LA, Platts-Mills JA. Giardia: a pathogen or commensal for children in high-prevalence settings? Curr Opin Infect Dis 2016; 29: 502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morton ER, Lynch J, Froment A, et al. Variation in rural African gut microbiota is strongly correlated with colonization by entamoeba and subsistence. PLoS Genet 2015; 11: e1005658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paulos S, Mateo M, de Lucio A, et al. Evaluation of five commercial methods for the extraction and purification of DNA from human faecal samples for downstream molecular detection of the enteric protozoan parasites Cryptosporidium spp., Giardia duodenalis, and Entamoeba spp. J Microbiol Methods 2016; 127: 68–73. [DOI] [PubMed] [Google Scholar]
- 29. Ianiro G, Bibbò S, Gasbarrini A, et al. Therapeutic modulation of gut microbiota: current clinical applications and future perspectives. Curr Drug Targets 2014; 15: 762–770. [DOI] [PubMed] [Google Scholar]
- 30. Audebert C, Even G, Cian A, et al. Colonization with the enteric protozoa Blastocystis is associated with increased diversity of human gut bacterial microbiota. Sci Rep 2016; 6: 25255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Burgess SL, Gilchrist CA, Lynn TC, et al. Parasitic protozoa and interactions with the host intestinal microbiota. Infect Immun 2017; 85: e00101–e00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Donowitz JR, Alam M, Kabir M, et al. A prospective longitudinal cohort to investigate the effects of early life Giardiasis on growth and all cause diarrhea. Clin Infect Dis 2016; 63: 792–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Holtman GA, Kranenberg JJ, Blanker MH, et al. Dientamoeba fragilis colonization is not associated with gastrointestinal symptoms in children at primary care level. Fam Pract 2017; 34: 25–29. [DOI] [PubMed] [Google Scholar]
- 34. Petersen AM, Stensvold CR, Mirsepasi H, et al. Active ulcerative colitis associated with low prevalence of Blastocystis and Dientamoeba fragilis infection. Scand J Gastroenterol 2013; 48: 638–639. [DOI] [PubMed] [Google Scholar]
- 35. Beghini F, Pasolli E, Truong TD, et al. Large-scale comparative metagenomics of Blastocystis, a common member of the human gut microbiome. ISME J 2017; 11: 2848–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O’Brien Andersen L, Karim AB, Roager HM, et al. Associations between common intestinal parasites and bacteria in humans as revealed by qPCR. Eur J Clin Microbiol Infect Dis 2016; 35: 1427–1431. [DOI] [PubMed] [Google Scholar]
- 37. Maizels RM, Smits HH, McSorley HJ. Modulation of host immunity by Helminths: the expanding repertoire of parasite effector molecules. Immunity 2018; 49: 801–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Loke P, Lim YA. Helminths and the microbiota: parts of the hygiene hypothesis. Parasite Immunol 2015; 37: 314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee SC, Tang MS, Easton AV, et al. Linking the effects of helminth infection, diet and the gut microbiota with human whole-blood signatures. Plos Pathog 2019; 15: e1008066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sipahi AM, Baptista DM. Helminths as an alternative therapy for intestinal diseases. World J Gastroenterol 2017; 23: 6009–6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ramanan D, Bowcutt R, Lee SC, et al. Helminth infection promotes colonization resistance via type 2 immunity. Science 2016; 352: 608–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Parfrey LW, Walters WA, Lauber CL, et al. Communities of microbial eukaryotes in the mammalian gut within the context of environmental eukaryotic diversity. Front Microbiol 2014; 5: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Suk JE, Semenza JC. Future infectious disease threats to Europe. Am J Public Health 2011; 101: 2068–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sun S, Jones RB, Fodor AA. Inference-based accuracy of metagenome prediction tools varies across sample types and functional categories. Microbiome 2020; 8: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Howitt MR, Lavoie S, Michaud M, et al. Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 2016; 351: 1329–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cotton JA, Amat CB, Buret AG. Disruptions of host immunity and inflammation by Giardia duodenalis: potential consequences for co-infections in the gastro-intestinal tract. Pathogens 2015; 4: 764–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gurung P, Kanneganti TD. Immune responses against protozoan parasites: a focus on the emerging role of nod-like receptors. Cell Mol Life Sci 2016; 73: 3035–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gazzinelli-Guimaraes PH, Nutman TB. Helminth parasites and immune regulation. F1000res 2018; 7: F1000FacultyRev-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rajasekaran S, Anuradha R, Bethunaickan R. TLR specific immune responses against helminth infections. J Parasitol Res 2017; 2017: 6865789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dubey LK, Lebon L, Mosconi I, et al. Lymphotoxin-dependent B cell-FRC crosstalk promotes de novo follicle formation and antibody production following intestinal helminth infection. Cell Rep 2016; 15: 1527–1541. [DOI] [PubMed] [Google Scholar]
- 51. Loke P, Cadwell K. Getting a taste for parasites in the gut. Immunity 2018; 49: 16–18. [DOI] [PubMed] [Google Scholar]
- 52. Solaymani-Mohammadi S, Eckmann L, Singer SM. Interleukin (IL)-21 in inflammation and immunity during parasitic diseases. Front Cell Infect Microbiol 2019; 9: 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Faria CP, Neves BM, Lourenço Cruz Á MT, et al. Giardia lamblia decreases NF-κB p65RelA protein levels and modulates LPS-induced pro-inflammatory response in macrophages. Sci Rep 2020; 10: 6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Engwerda CR, Ng SS, Bunn PT. The regulation of CD4(+) T cell responses during protozoan infections. Front Immunol 2014; 5: 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Guivier E, Bellenger J, Sorci G, et al. Helminth interaction with the host immune system: short-term benefits and costs in relation to the infectious environment. Am Nat 2016; 188: 253–263. [DOI] [PubMed] [Google Scholar]
- 56. Osbourn M, Soares DC, Vacca F, et al. HpARI protein secreted by a helminth parasite suppresses interleukin-33. Immunity 2017; 47: 739–751.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Maizels RM, McSorley HJ. Regulation of the host immune system by helminth parasites. J Allergy Clin Immunol 2016; 138: 666–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chudnovskiy A, Mortha A, Kana V, et al. Host-protozoan interactions protect from mucosal infections through activation of the inflammasome. Cell 2016; 167: 444–456.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zakeri A, Hansen EP, Andersen SD, et al. Immunomodulation by helminths: intracellular pathways and extracellular vesicles. Front Immunol 2018; 9: 2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shen SS, Qu XY, Zhang WZ, et al. Infection against infection: parasite antagonism against parasites, viruses and bacteria. Infect Dis Poverty 2019; 8: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lang R, Schick J. Review: impact of helminth infection on antimycobacterial immunity – a focus on the macrophage. Front Immunol 2017; 8: 1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Long SR, Lanter BB, Pazos MA, et al. Intestinal helminth infection enhances bacteria-induced recruitment of neutrophils to the airspace. Sci Rep 2019; 9: 15703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Haben I, Hartmann W, Breloer M. Nematode-induced interference with vaccination efficacy targets follicular T helper cell induction and is preserved after termination of infection. Plos Negl Trop Dis 2014; 8: e3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Klem F, Wadhwa A, Prokop LJ, et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: a systematic review and meta-analysis. Gastroenterology 2017; 152(5): 1042–1054.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jadallah KA, Nimri LF, Ghanem RA. Protozoan parasites in irritable bowel syndrome: a case-control study. World J Gastrointest Pharmacol Ther 2017; 8: 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Machicado C, Marcos LA. Carcinogenesis associated with parasites other than Schistosoma, Opisthorchis and Clonorchis: a systematic review. Int J Cancer 2016; 138: 2915–2921. [DOI] [PubMed] [Google Scholar]
- 67. Sawant M, Baydoun M, Creusy C, et al. Cryptosporidium and colon cancer: cause or consequence? Microorganisms 2020; 8: 1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Scholte LLS, Pascoal-Xavier MA, Nahum LA. Helminths and cancers from the evolutionary perspective. Front Med (Lausanne) 2018; 5: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Toychiev A, Abdujapparov S, Imamov A, et al. Intestinal helminths and protozoan infections in patients with colorectal cancer: prevalence and possible association with cancer pathogenesis. Parasitol Res 2018; 117: 3715–3723. [DOI] [PubMed] [Google Scholar]
- 70. Zhang N, Zhang H, Yu Y, et al. High prevalence of Pentatrichomonas hominis infection in gastrointestinal cancer patients. Parasit Vectors 2019; 12: 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cammarota G, Ianiro G, Ahern A, et al. Gut microbiome, big data and machine learning to promote precision medicine for cancer. Nat Rev Gastroenterol Hepatol 2020; 17: 635–648. [DOI] [PubMed] [Google Scholar]
- 72. Apostol AC, Jensen KDC, Beaudin AE. Training the fetal immune system through maternal inflammation – a layered hygiene hypothesis. Front Immunol 2020; 11: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Reyna-Fabián ME, Zermeño V, Ximénez C, et al. Analysis of the bacterial diversity in liver abscess: differences between pyogenic and amebic abscesses. Am J Trop Med Hyg 2016; 94: 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Amer EI, Mossallam SF, Mahrous H. Therapeutic enhancement of newly derived bacteriocins against Giardia lamblia. Exp Parasitol 2014; 146: 52–63. [DOI] [PubMed] [Google Scholar]
- 75. Mangano VD, Modiano D. Host genetics and parasitic infections. Clin Microbiol Infect 2014; 20: 1265–1275. [DOI] [PubMed] [Google Scholar]
- 76. Ipci K, Altıntoprak N, Muluk NB, et al. The possible mechanisms of the human microbiome in allergic diseases. Eur Arch Otorhinolaryngol 2017; 274: 617–626. [DOI] [PubMed] [Google Scholar]
- 77. Smallwood TB, Giacomin PR, Loukas A, et al. Helminth immunomodulation in autoimmune disease. Front Immunol 2017; 8: 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bohnacker S, Troisi F, de Los Reyes Jiménez M, et al. What can parasites tell us about the pathogenesis and treatment of asthma and allergic diseases. Front Immunol 2020; 11: 2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tang CL, Zou JN, Zhang RH, et al. Helminths protect against type 1 diabetes: effects and mechanisms. Parasitol Res 2019; 118: 1087–1094. [DOI] [PubMed] [Google Scholar]
- 80. Maruszewska-Cheruiyot M, Donskow-Łysoniewska K, Doligalska M. Helminth therapy: advances in the use of parasitic worms against inflammatory bowel diseases and its challenges. Helminthologia 2018; 55: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wong SH, Kwong TNY, Wu CY, et al. Clinical applications of gut microbiota in cancer biology. Semin Cancer Biol 2019; 55: 28–36. [DOI] [PubMed] [Google Scholar]
- 82. Cheng WY, Wu CY, Yu J. The role of gut microbiota in cancer treatment: friend or foe? Gut 2020; 69: 1867–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ianiro G, Rossi E, Thomas AM, et al. Faecal microbiota transplantation for the treatment of diarrhoea induced by tyrosine-kinase inhibitors in patients with metastatic renal cell carcinoma. Nat Commun 2020; 11: 4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yousofi Darani H, Yousefi M, Safari M, et al. Parasites and immunotherapy: with or against? J Parasit Dis 2016; 40: 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]