Abstract
Macrophages (Mφs) play a crucial role in the development of atherosclerosis by engulfing modified LDL particles and forming foam cells, the hallmark of atherosclerosis. Many studies suggest that myeloperoxidase-oxidized LDL (Mox-LDL) is an important pathophysiological model for LDL modification in vivo. Classically (M1) and alternatively activated (M2) Mφs are both implicated in the process of atherogenesis. Mφs are highly plastic cells whereby they undergo repolarization from M1 to M2 and vice versa. Since little is known about the effects of Mox-LDL on Mφ polarization and repolarization, our study aimed at evaluating the in vitro effects of Mox-LDL at this level through making use of the well-established model of human THP-1-derived Mφs. Resting M0-Mφs were polarized toward M1- and M2-Mφs, then M0-, M1- and M2-Mφs were all treated with physiological concentrations of Mox-LDL to assess the effect of Mox-LDL treatment on Mφ polarization and repolarization. Treatment of M0-Mφs with a physiological concentration of Mox-LDL had no significant effects at the level of their polarization. However, treatment of M1-Mφs with Mox-LDL resulted in a significant reduction in their IL-10 cytokine secretion. Our results point to a potential role of Mox-LDL in increasing the pro-inflammatory state in Mφs through reducing the release of the anti-inflammatory cytokine, IL-10.
Keywords: mox-LDL, atherosclerosis, macrophage polarization, macrophage repolarization, THP-1, pro-inflammatory, anti-inflammatory
Introduction
Atherosclerosis is a clinical condition for which multiple genetic and environmental causal factors have been proposed. The accumulation of foam cells, macrophages (Mφs) that have engulfed large amounts of modified low density lipoprotein (LDL) particles is a key process that results in the thickening of the arterial wall which is seen during the course of atherosclerosis.1,2 Observations also suggest that myeloperoxidase (MPO), a protein secreted by immune cells, is a major physiological player in generating modified/oxidized LDL molecules via the production of hypochlorous acid (HOCl) from H2O2 and chloride.3–5 Several studies have shown that human atherosclerotic lesions contain HOCl-modified LDLs, which are located in vascular cells as well as in extracellular spaces. 4 Other studies have demonstrated that patients with MPO-deficiency have reduced risk of cardiovascular disease.5,6 In human atheroma plaques, markers for both M1- and M2-Mφs have been detected throughout all stages of disease progression. 7 Similarly, murine models have confirmed the presence of M1- and M2-Mφ markers in mouse atherosclerotic lesions and it has been suggested that a shift from M2 to M1 phenotype occurs while the plaque is progressing.8,9 Mφs possess diverse phenotypic and functional properties and thus have the ability to respond to various environmental stimuli. 10 Mφs are polarized into the M1 phenotype by Th1 cytokines such as IFN-γ and TNF-α and/or by a microbial stimulus such as lipopolysaccharide (LPS). At the other extreme, Mφs are polarized towards the M2 phenotype by Th2 cytokines mainly IL-4 and IL-13, as well as by other anti-inflammatory cytokines [i.e. TGF-β and IL-10], glucocorticoids, or immune complexes.11,12 Polarized M2-Mφs do not constitute a uniform population and are subcategorized into M2a, M2b, M2c, and M2d whereby M2a is the most well-described polarized M2-Mφ population. 13 M1-Mφs secrete high levels of pro-inflammatory cytokines (i.e. IL-1β, IL-6, IL-12, IL-23, and TNF-α) and play a crucial role in the elimination of pathogens and tumor cells.11,12 In contrast, M2-Mφs secrete high levels of anti-inflammatory cytokines (i.e. IL-10 and TGF-β) and are involved in promoting tissue repair and remodeling, inducing angiogenesis, clearing parasites and worms, and promoting matrix deposition. 13 Atheroma plaque-associated Mφs express M1- and M2-Mφ phenotypic states, in addition to Mφ states and features that are intermediate between the two extreme polarization states of Mφs. 14 However, it remains controversial whether Mφs with intermediate features are in a transition state between the two extreme polarization states or they are distinct populations with specific functions. 14 Inside human atherosclerotic lesions and within the plaque, there is a significant difference in the spatial distribution of polarized Mφs. For instance, M1-Mφs dominate the plaque shoulder region which is a significant predilection site for plaque rupture while M2-Mφs is the main Mφ subset present in the perivascular adventitial tissue. 15 Moreover, fibrous cap Mφs show similar numbers of M1- and M2-Mφs. 15 Of note, Mφ polarization states were also reported to differ between the plaques of symptomatic and asymptomatic patients where symptomatic plaques express significantly higher amounts of M1-Mφs and Th-1 cytokines and asymptomatic plaques expressed significantly more M2-Mφs and Th-2 cytokines. 16 Mφs were found to have the ability to dynamically shift their phenotypes in response to microenvironmental and intracellular cues. This ability to change phenotypic polarized sates is referred to as “repolarization” and it gives Mφs their plasticity. 14 In vitro, polarized Mφs completely reverted from their initial phenotype to the opposite phenotype after stimulation with the opposite polarizing stimulants. 9
Since little is known about the effect of MPO oxidized-LDL (Mox-LDL) on Mφs, this study aimed at evaluating the in vitro effects of Mox-LDL on the polarization of resting M0-Mφs as well as on the repolarization of M1- and M2-Mφs through making use of the well-established model of human THP-1-derived Mφs. 17 Our results point to a potential role for Mox-LDL in increasing the pro-inflammatory state in Mφs by reducing the anti-inflammatory cytokine IL-10.
Materials and methods
Maintenance of THP-1 monocytes
THP-1 cells (kindly provided by Dr Marwan El-Sabban, American University of Beirut) were cultured in growth medium (GM) made up of Roswell Park Memorial Institute medium (RPMI) 1640 medium (Sigma Aldrich, Darmstadt, Germany), supplemented with 10% heat inactivated fetal bovine serum (FBS), 1% penicillin/streptomycin mixture, and 1% L-glutamine (Sigma Aldrich). Cells were maintained at 37˚C in a humidified 5% CO2 incubator at 37˚C. THP-1 cells were used between passages 10–30. Cells were checked for viability using the Trypan blue exclusion method throughout the maintenance of THP-1 cells in culture. 18
THP-1 monocytes differentiation into M0-Mφs
To differentiate THP-1 cells into M0-Mφs, THP-1 cells were seeded in a 6-well culture plate at a density of 1 × 106 cells/well. Cells were either left untreated or treated with phorbol 12-myristate 13-acetate (phorbol 12-myristate 13-acetate (PMA), Sigma Aldrich) at a concentration of 150 nM for a period of 48 h. 19 Following differentiation, adherent M0-Mφs were washed twice with GM and cells were subjected to a resting period for an additional 24 h period in GM to obtain resting M0-Mφs. 17 Meanwhile, THP-1 monocytes were replenished with fresh GM.
Polarization of M0-Mφs
M0-Mφs were polarized into M1-Mφs by treatment with 20 ng/ml of IFN-γ (Invitrogen, Thermo Fisher, Waltham, Massachusetts, USA) and 100 ng/ml of LPS (InvivoGen, Toulouse, France) for 24 or 48 h. On the other hand, M0-Mφs were polarized into M2-Mφs by incubation with 20 ng/ml of IL-13 (Gibco, Thermo Fisher, Waltham, Massachusetts, USA) and 20 ng/ml of IL-4 (Invitrogen) for 24 or 48 h.
Mox-LDL preparation
Mox-LDL was generated by mixing 1.6 mg of native LDL (final concentration: 0.8 mg/ml) (Invitrogen), with 8 µl of 1 M HCl (final concentration: 4 mM), 45 µl of MPO (final concentration: 250 nM), and 40 µl of 50 mM H2O2 (final concentration: 1 mM). The volume was adjusted to 2 ml using Dulbecco's phosphate-buffered saline (DPBS, pH 7.4, Sigma Aldrich) containing 1 g/l of ethylenediaminetetraacetic acid (EDTA). Afterwards, Mox-LDL was desalted to remove impurities. 20
Mox-LDL treatment of Mφs
M0-, M1-, and M2-Mφs were treated with Mox-LDL at a concentration of 50 μg/ml for 24 h, as previously reported and which reflects what may happen under physiological conditions in vivo. 21 Thus, they will be referred to as Mox-LDL-Mφs, Mox-LDL/M1-Mφs and Mox-LDL/M2-Mφs, respectively.
Detachment of Mφs
To collect adherent Mφs, cells were first washed twice with warm DPBS and then incubated with accutase solution (Gibco) for 5–10 min at 37˚C. Detached cells were collected by centrifugation at 270 g at 4˚C for 5 min.
Propidium iodide (Pi) cell viability assay
PI cell viability assay was used to assess the viability of M0-Mφs post-M1 and M2-Mφ polarization as well as post-Mφ treatment with Mox-LDL. Cells were stained with 50 μg/ml PI (Invitrogen) for 10 min at room temperature in the dark. Samples were run on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA). and data were analyzed using CellQuest Pro software version 5.1 (BD Biosciences). Viable and dead cell populations were identified as PI- and PI+ cells, respectively. A total of 10,000 single cell events were measured for each sample.
Morphological examination of THP-1 monocytes and Mφs
Phase-contrast images ( × 400 total magnification) of THP-1 monocytes and different Mφ types were obtained using a phase contrast inverted microscope (Leica microsystems, Wetzlar, Germany).
Immunophenotyping of THP-1 monocytes and Mφs
THP-1 monocytes and different Mφ types were collected and incubated at a density of 1 × 105 cells in 100 μl of blocking buffer (DPBS + 2.5% FBS) for 15 min at 4˚C. Cells were then incubated with phycoerythrin (PE)-conjugated mouse anti-human CD11b, allophycocyanin (APC)-conjugated mouse anti-human CD11c, APC-conjugated mouse anti-human CD80, or APC-conjugated mouse anti-human CD209 (BD Biosciences) for 30 min at 4˚C. Cells were also stained with isotype-matched control Abs which included: APC-conjugated IgG1, IgG2a, IgG2b, or PE conjugated mouse IgG1 (BD Biosciences). Following incubation, cells were washed twice with cold DPBS and finally re-suspended in 500 µl cold DPBS and run on a flow cytometer within 30 min.
Flow cytometry analysis
Surface expression levels of receptors were analyzed on THP-1 monocytes and different Mφ types using a FACSCalibur flow cytometer (BD Biosciences) and CellQuest Pro software 5.1 (BD Biosciences). Surface receptor expression levels were reported either as percentage of receptor-positive cells or as raw geometric mean fluorescence intensity (MFI) of receptor-positive cells. In addition, forward scatter (FSC) and side scatter (SSC) properties of cells were analyzed. A total of 10,000 single cell events were measured for each sample.
ELISA
Supernatants from M0-, M1-, M2-, Mox-LDL, Mox-LDL/M1-, and Mox-LDL/M2-Mφ cultures were collected and stored at -80˚C for later cytokine analysis. IL-6 and IL-10 levels in Mφ culture supernatants were measured using commercially available sandwich ELISA kits (Invitrogen). Samples were processed according to the manufacturer's instructions in duplicates and measured at 450 nm on a micro-plate reader (Biotek, Winooski, VT, USA).
Statistical analysis
Statistical analysis was performed using GraphPad Prism software (version 6.0; GraphPad Software, San Diego, CA, USA). Data are expressed as the mean ± standard error mean (SEM). Statistical significance was conducted using unpaired t-test for experiments consisting of only two groups or one-way ANOVA followed Tukey's multiple comparison post hoc test for experiments with 3 or more groups. Differences were considered statistically significant at P < 0.05.
Results
The effect of PMA-induced differentiation of THP-1 monocytes into M0-Mφs on cell morphology, granularity and Cd11b/Cd11c surface expression
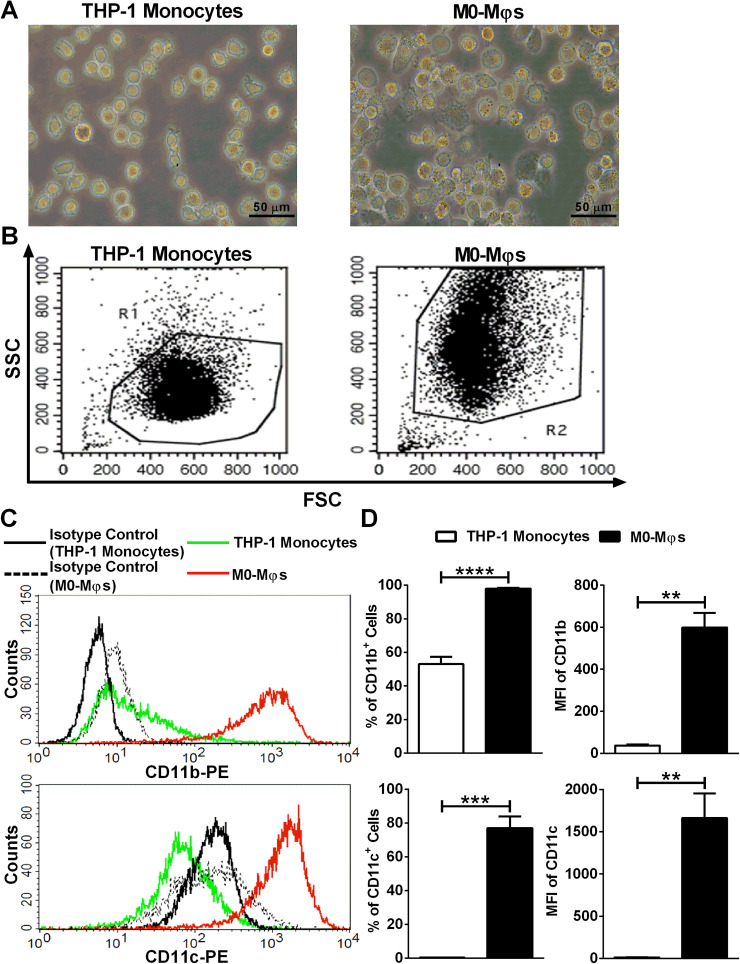
For the confirmation of PMA-induced differentiation of THP-1 monocytes into M0-Mφs, cells were visualized under an inverted phase contrast microscope for the assessment of morphology. PMA treatment of THP-1 monocytes was shown to increase the size of THP-1 monocytes, and to promote their adherence to the bottom of culture plate, whereby both characteristics are indicative of the Mφ phenotype (Figure 1A). Moreover, upon PMA-induced differentiation of THP-1 monocytes to M0-Mφs, flow cytometry analysis revealed a significant increase in SSC properties of THP-1-derived M0-Mφs in comparison with THP-1 monocytes (Figure 1B). In order to further confirm the PMA-induced differentiation of THP-1 monocytes into M0-Mφs, CD11b and CD11c surface expression levels were analyzed, and the data showed a statistically significant increase in the percentage of cells bearing those relevant markers upon the differentiation of THP-1 monocytes into M0-Mφs (Figures 1C and 1D).
Figure 1.
Validation of PMA-induced differentiation of THP-1 monocytes into M0-Mφs. (A) Representative phase contrast inverted microscopy images of THP-1 monocytes and M0-Mφs under × 400 magnification (scale bar: 50 μm). (B) Representative flow cytometry dot plots showing the forward-scatter (FSC) and side-scatter (SSC) properties of THP-1 monocytes and M0-Mφs. (C) Representative flow cytometry histogram plots demonstrating the surface expression of CD11b and CD11c on THP-1 monocytes and M0-Mφs. (D) Column bars representing mean values of the percentage (%) and geometric mean fluorescence intensity (MFI) of CD11b+ and CD11c+ cells from 3 independent experiments (with each condition performed in duplicate). Error bars represent SEM. Statistically significant differences were determined by unpaired t-test **P < 0.01; ***P < 0.001; ****P < 0.0001.
Effect of polarization and Mox-LDL treatment on the morphology and viability of M0-Mφs
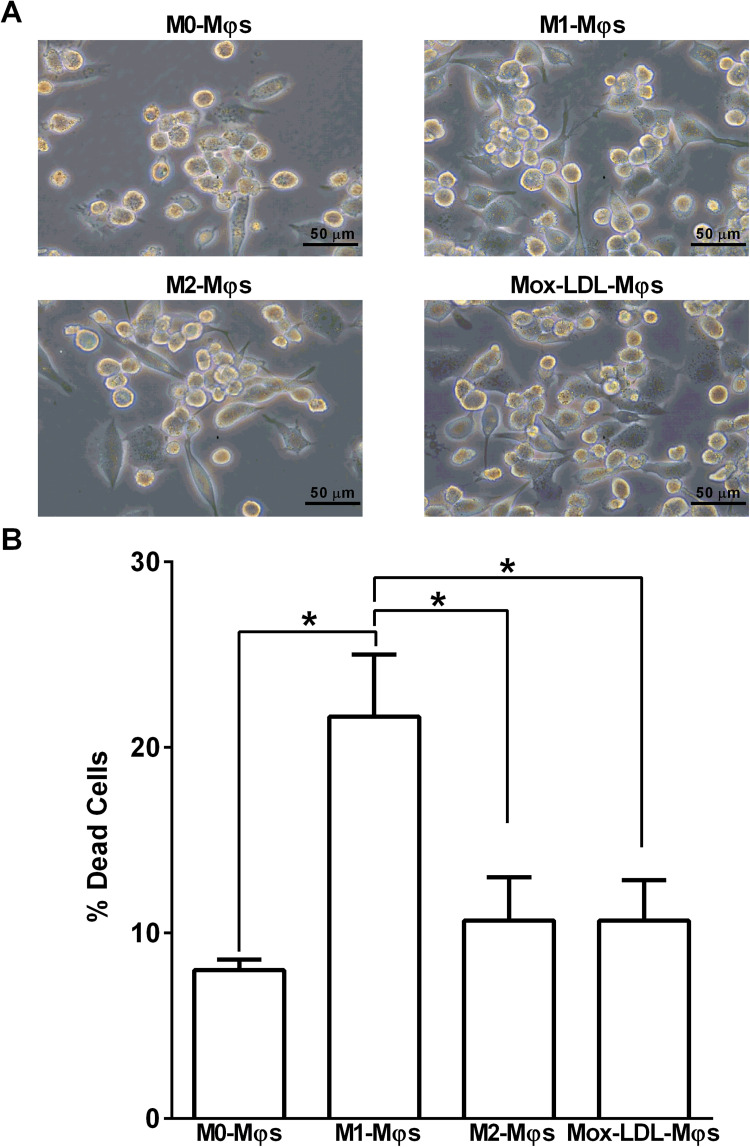
Changes in M0-Mφs morphology were observed following their polarization towards M1-Mφ or M2-Mφ phenotype, as well as upon their treatment with Mox-LDL. Unpolarized/uncommitted M0-Mφs displayed a mixture of round- and spindle-shaped cells with the former being more abundant. However, M0-Mφs exhibited very similar changes following their 24 h culture in M1- or M2-Mφ polarizing conditions or upon their treatment with Mox-LDL (Mox-LDL-Mφ) whereby spindle-shaped cells were more abundant than round-shaped cells (Figure 2A). To confirm that M0-Mφs treatment with Mox-LDL and their polarization did not severely affect cell viability, all Mφ types were stained with PI and analyzed by flow cytometry. M0-Mφs showed ∼8% of spontaneous cell death, whereas polarized M1-Mφs demonstrated a significantly (P < 0.05) higher % of dead cells (22%) as compared to M0-Mφs. In contrast, polarization of M0-Mφs towards the M2 phenotype did not induce any significant increase in cell death. Mox-LDL treatment of M0-Mφs resulted in ∼11% of dead cells, thus indicating that Mox-LDL treatment of M0-Mφs did cause a significant increase in M0-Mφs cell death (Figure 2B).
Figure 2.
Effect of Mox-LDL treatment on the morphology and viability of M0-Mφs. (A) Representative phase contrast inverted microscopy images of M0-Mφs, M1-Mφs, M2-Mφs, and Mox-LDL-treated M0-Mφs (Mox-LDL-Mφs) under × 400 magnification (scale bar: 50 μm). (B) Column bars demonstrating mean values of the percentage (%) of dead (PI-positive cells) M0-Mφs, M1-Mφs, M2-Mφs, and Mox-LDL-treated (at a concentration of 50 μg/ml for 24 h) M0-Mφs from 3 independent experiments (with each condition performed in duplicate). Error bars represent SEM. Statistically significant differences were determined by one-way ANOVA followed by Tukey's multiple comparison post hoc test *P < 0.05.
Effect of Mox-LDL on M0-Mφs polarization
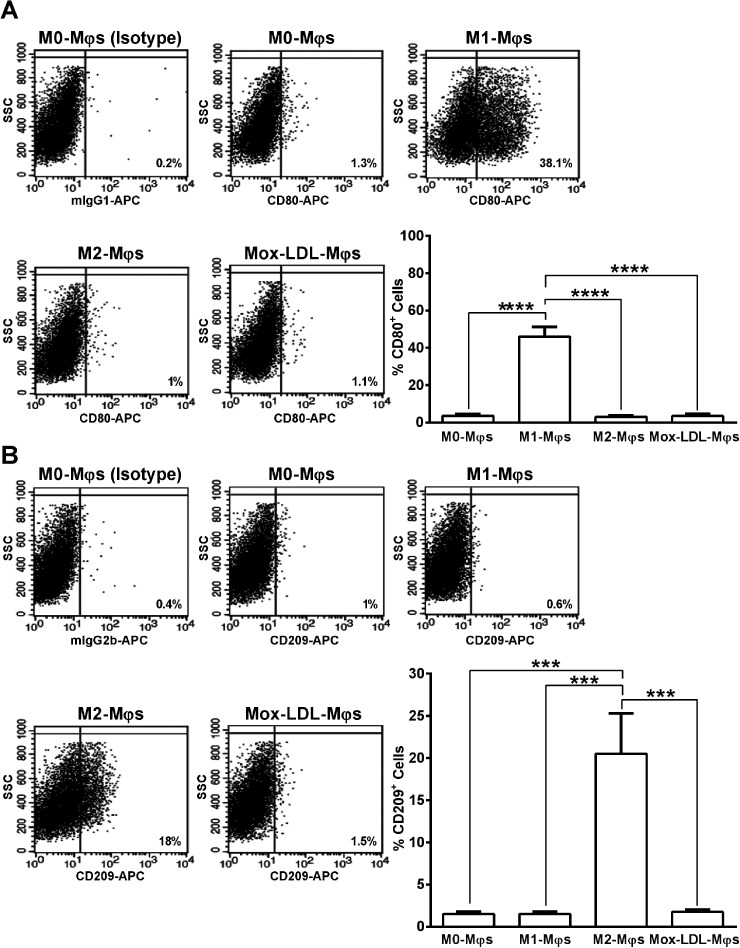
To determine the impact of Mox-LDL on M0-Mφs polarization, the surface expression of CD80 and CD209 receptors was analyzed on Mox-LDL-Mφs and was compared to M1- and M2-polarized Mφs. Expression levels of CD80 and CD209 are known to be up-regulated upon the polarization of THP-1-derived M0-Mφs into M1-Mφs 22 and M2-Mφs, 23 respectively. A concentration-response analysis was carried out to evaluate the effect of 24 h stimulation of M0-Mφs with increasing concentrations (25, 50 and 100 µg/ml) of Mox-LDL on their surface expression of CD80 and CD209. No significant differences in the percentages of CD80+ and CD209+ cells were observed between control unstimulated M0-Mφs and M0-Mφs stimulated with the three different Mox-LDL concentrations (Supplementary Figure S1). Accordingly, the 50 µg/ml intermediate concentration of Mox-LDL, which also represents the physiological concentration, 21 was used in the following experiments. As expected, M0-Mφs stimulation with LPS and IFN-γ (M1-Mφ polarizing agents) resulted in a significant (P < 0.0001) up-regulation in the percentage (∼15-fold) of CD80 positive cells, as compared to M0-, M2-, and Mox-LDL-Mφs (Figure 3A). Moreover, treatment of M0-Mφs with Mox-LDL had no significant effect on their CD80 expression whereby they presented a similar profile to that of M0- and M2-Mφs (Figure 3A). On the other hand, stimulation of M0-Mφs with IL-4 and IL-13 (M2-Mφ polarizing agents) showed a significant (P < 0.001) ∼13-fold increase in the percentage of CD209 positive cells for M2-Mφs versus M0-, M1-, and Mox-LDL-Mφs (Figure 3B). Furthermore, exposure of M0-Mφs to Mox-LDL had no effect on their CD209 expression and treated Mφs expressed CD209 at levels comparable to those of M0- and M1-Mφs (Figure 3B).
Figure 3.
Analysis of expression of M1-Mφ and M2-Mφ signature receptors on Mox-LDL-treated M0-Mφs. Representative flow cytometry dot plots and column bars demonstrating the surface expression of (A) CD80 (M1-φ marker) and (B) CD209 (M2-φ marker) on M0-Mφs, M1-Mφs, M2-Mφs and M0-Mφs treated with 50 μg/ml Mox-LDL for 24 h (Mox-LDL-Mφs). Column bars represent mean values of the percentage of CD80+ or CD209+ cells from 4 independent experiments (with each condition performed in duplicate). Error bars represent SEM. One-way ANOVA followed by Tukey's multiple comparison post hoc test was used to calculate statistical significance. ***P < 0.001; ****P < 0.0001.
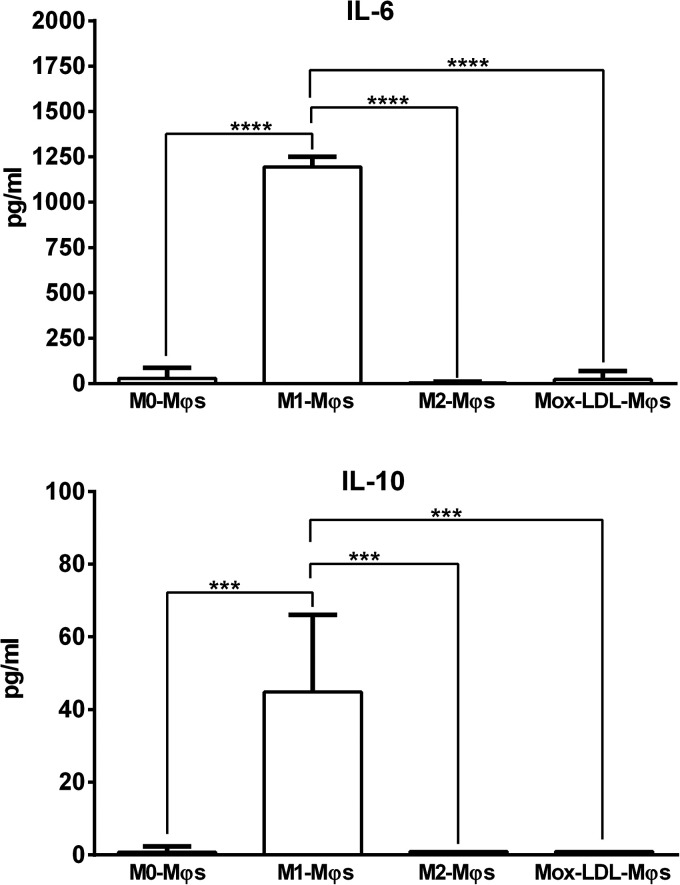
Oxidized LDL has been previously reported to stimulate THP-1 monocytes or THP-1-derived M0-Mφs to secrete certain M1-Mφ- and M2-Mφ-associated cytokines such as IL-6 and IL-10, respectively. 24 M0-Mφs exposed to a physiological concentration of Mox-LDL for 24 h were assessed for their IL-6 and IL-10 secreted levels that were compared to those produced by M0-, M1- and M2-Mφs. We found that treatment of M0-Mφs with Mox-LDL did not affect their IL-6 and IL-10 production patterns (Figure 4). Unsurprisingly, M1-Mφs produced significantly higher levels of IL-6 (P < 0.0001) than M0-Mφs, M2-Mφs and Mox-LDL-Mφs did (Figure 4). Surprisingly, IL-10 was released in significantly (P < 0.001) greater amounts by M1-Mφs than by M0-Mφs, M2-Mφs and Mox-LDL-Mφs which produced very little or undetectable amounts of IL-10 (Figure 4). Overall, it can be suggested that under the tested conditions, Mox-LDL is unable to polarize M0-Mφs either towards a pro-inflammatory M1- or towards an anti-inflammatory M2-Mφ phenotype.
Figure 4.
Measurement of M1-Mφ and M2-Mφ signature cytokines produced by Mox-LDL-treated M0-Mφs. (A) IL-6 (M1-φ marker) and (B) IL-10 (M2-φ marker) levels in the culture supernatants of M0-Mφs, M1-φs, M2-Mφs, and Mox-LDL-treated (at a concentration of 50 μg/ml for 24 h) M0-Mφs (Mox-LDL-Mφs) as measured by ELISA. Column bars represent mean values of 4 and 3 independent experiments (with each condition performed in duplicate) for IL-6 and IL-10 levels, respectively. Error bars represent SEM. One-way ANOVA followed by Tukey's multiple comparison post hoc test was used to calculate statistical significance. *** P < 0.001; **** P < 0.0001.
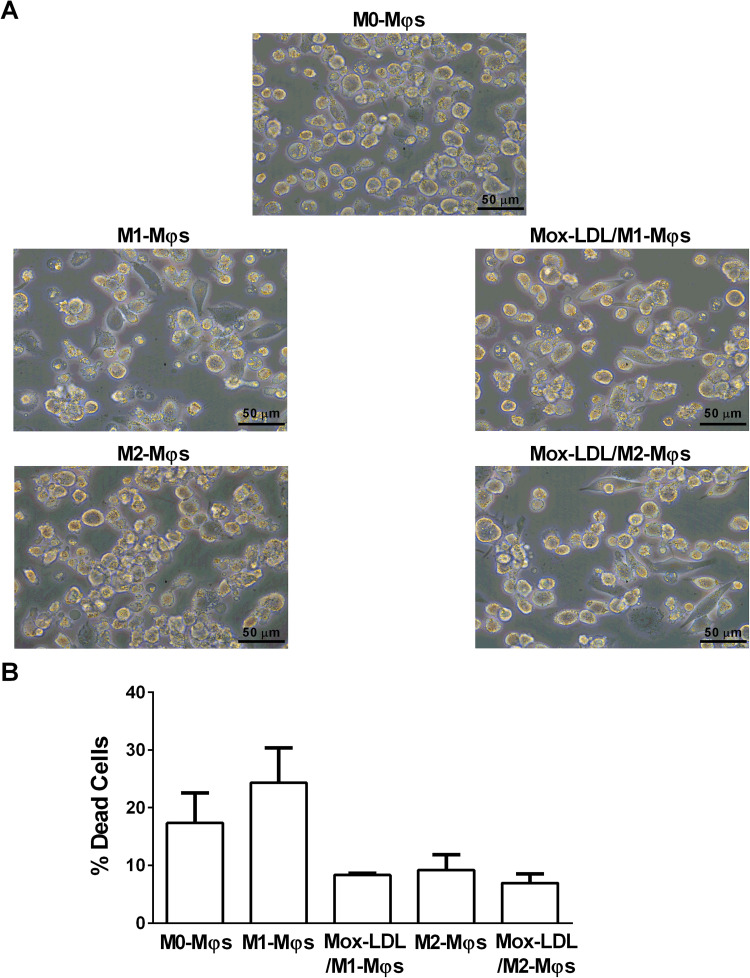
Effect of Mox-LDL treatment on the morphology and viability of M1- and M2-Mφs
Microscopic analysis was performed so as to observe whether treatment of M1- and M2-Mφs with Mox-LDL would have any impact on their morphology. No significant morphological differences were noted between Mox-LDL/M1-Mφs and M1-Mφs as well as between Mox-LDL/M2-Mφs and M2-Mφs whereby untreated and Mox-LDL-treated M1 and M2-Mφs of round- and spindle-shaped cells (Figure 5A). PI viability assay was carried out to assess any potential effect of Mox-LDL treatment on the viability of polarized M1- and M2-Mφs. The % of dead cells among M1-Mφs, Mox-LDL/M1-Mφs, M2-Mφs and Mox-LDL/M2-Mφs was ∼24%, ∼8.5%, ∼9% and ∼7%, respectively (Figure 5B). Hence, Mox-LDL treatment did not lead to a significant increase in cell death among M1- and M2-Mφs.
Figure 5.
Effect of Mox-LDL treatment on the morphology and viability of M1-Mφs and M2-Mφs. A) Representative phase contrast inverted microscopy images of M0-Mφs, M1-Mφs, M2-Mφs, and Mox-LDL-treated (at a concentration of 50 μg/ml for 24 h) M1-Mφs (Mox-LDL/M1-Mφs) and M2-Mφs (Mox-LDL/M2-Mφs) under × 400 magnification (scale bar: 50 μm). B) Column bars demonstrating mean values of the percentage (%) of dead (PI-positive cells) M0-Mφs, M1-Mφs, M2-Mφs, Mox-LDL/M1-Mφs and Mox-LDL/M2-Mφs from 3 independent experiments (with each condition performed in duplicate). Error bars represent SEM. Statistically significant differences were determined by one-way ANOVA followed by Tukey's multiple comparison post hoc test.
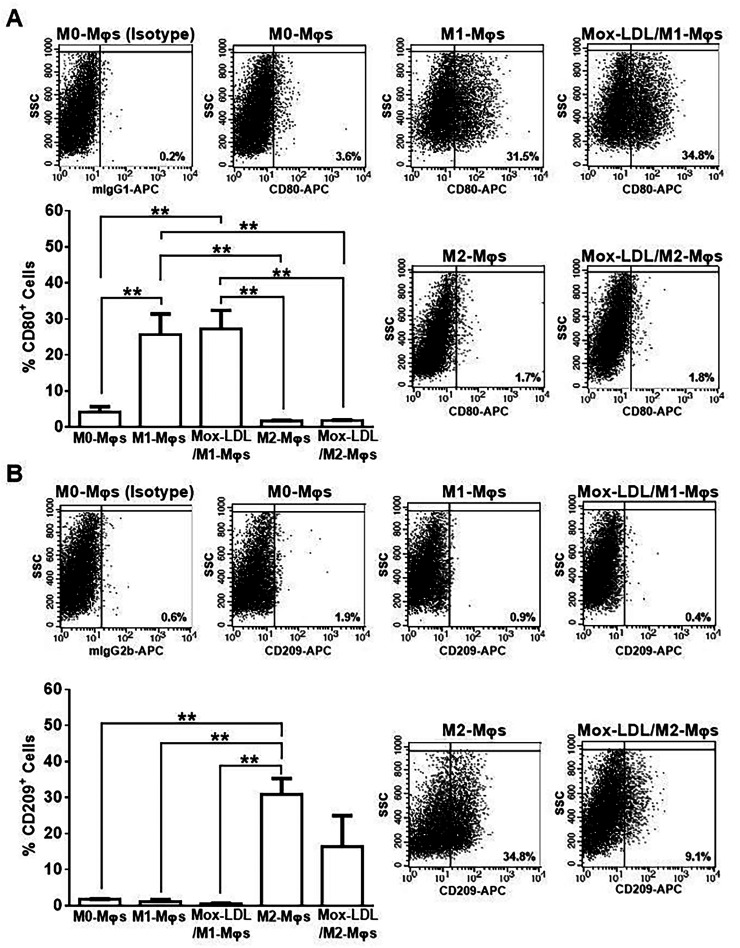
Effect of Mox-LDL on M1-Mφ and M2-Mφ repolarization
To investigate the ability of Mox-LDL to switch/repolarize the phenotype of polarized M- Mφs, Mox-LDL/M1-Mφs and Mox-LDL/M2-Mφs were evaluated for their expression of M1- and M2-Mφ-associated surface receptors. Mox-LDL treatment of M1- and M2-Mφs for 24 h did not influence their surface expression of the M1-Mφ associated marker, CD80, whereby the % of CD80+ cells among M1-Mφs, Mox-LDL/M1-Mφs, M2-Mφs and Mox-LDL/M2-Mφs was ∼25.7%, ∼27.3%, 1.7% and 1.8%, respectively (Figure 6A). In a similar manner, Mox-LDL treatment of M1-Mφs did not influence their surface expression of the M2-Mφ associated marker, CD209, since M1-Mφs and Mox-LDL/M1-Mφs had a similar % of CD209+ cells among them (Figure 6B). In contrast, there was a trend towards a reduction in the % of CD209+ cells among Mox-LDL/M2-Mφs (∼16.4%) as compared to M2-Mφs (30.9%) (Figure 6B).
Figure 6.
Analysis of expression of M1-Mφ and M2-Mφ signature receptors on Mox-LDL-treated M1-Mφs and M2-Mφs. Representative flow cytometry dot plots and column bars demonstrating the surface expression of (A) CD80 (M1 marker) and (B) CD209 (M2 marker) on M0-Mφs, M1-Mφs, M2-Mφs and Mox-LDL-treated (at a concentration of 50 μg/ml for 24 h) M1-Mφs (Mox-LDL/M1-Mφs) and M2-Mφs (Mox-LDL/M2-Mφs). Column bars represent mean values of the percentage (%) of CD80+ or CD209+ cells from 3 independent experiments (with each condition performed in duplicate). Error bars represent SEM. One-way ANOVA followed by Tukey's multiple comparison post hoc test was used to calculate statistical significance. **P < 0.01.
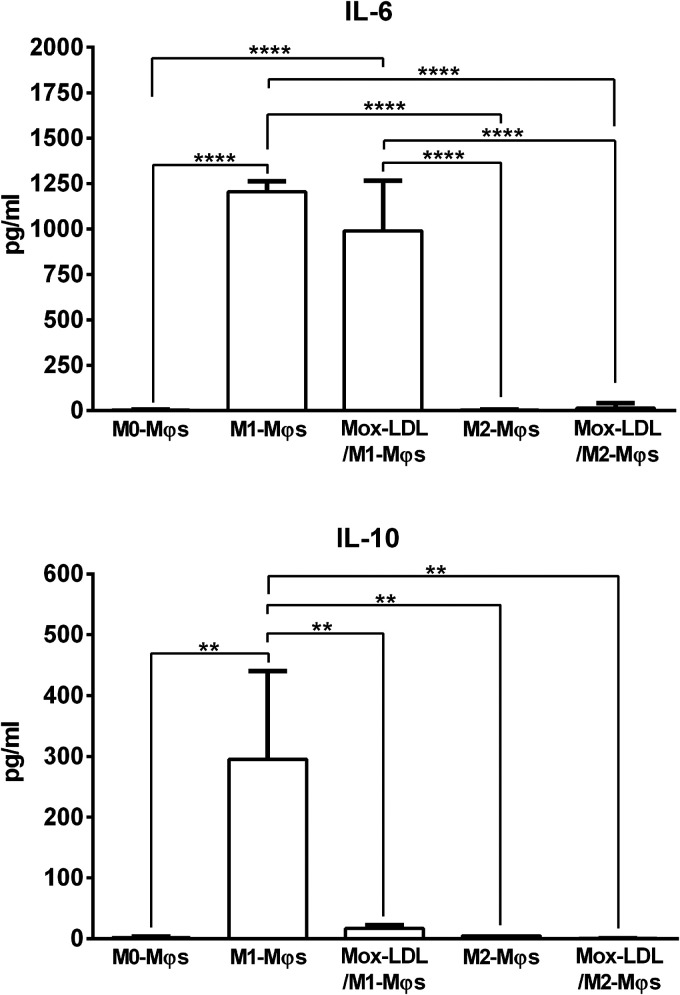
To further determine the capacity of Mox-LDL to repolarize Mφs, we characterized the pro-inflammatory (IL-6) and anti-inflammatory (IL-10) cytokine secretion profiles of Mox-LDL-treated M1-Mφs and M2-Mφs. Analysis of cytokine release profiles clearly demonstrated that Mox-LDL/M1-Mφs and Mox-LDL/M2-Mφs produced the M1-Mφ associated cytokine, IL-6, at levels similar to those secreted by M1-Mφs and M2-Mφs, respectively (Figure 7). Remarkably, Mox-LDL/M1-Mφs exhibited significantly (P < 0.001) reduced secretion levels of IL-10 compared to M1-Mφs (Figure 7). The low secretion of IL-10 by M2-Mφs was not altered upon Mox-LDL treatment whereby Mox-LDL/M2-Mφs maintained their low IL-10 production (Figure 7).
Figure 7.
Measurement of M1-Mφ and M2-Mφ signature cytokines produced by Mox-LDL-treated M1-Mφs and M2-Mφs. (A) IL-6 (M1-φ marker) and (B) IL-10 (M2-φ marker) levels in the culture supernatants of M0-Mφs, M1-φs, M2-Mφs, and Mox-LDL-treated (at a concentration of 50 μg/ml for 24 h) M1-Mφs (Mox-LDL/M1-Mφs) and M2-Mφs (Mox-LDL/M2-Mφs) as measured by ELISA. Column bars represent mean values of 4 independent experiments (with each condition performed in duplicate). Error bars represent SEM. One-way ANOVA followed by Tukey's multiple comparison post hoc test was used to calculate statistical significance. ** P < 0.01; **** P < 0.0001.
Discussion
Mox-LDL is a patho-physiological model for LDL oxidation in vivo and is endowed with pro-inflammatory properties. 25 A previous study has demonstrated the ability of Mox-LDL to increase IL-8 and TNF-α production by THP-1 monocytes, 26 however, the role of Mox-LDL in Mφ phenotypic polarization and repolarization was still unidentified. Knowing that Mox-LDL particles are pro-inflammatory, the present study investigated the potential in vitro effect of Mox-LDL on the polarization of uncommitted M0-Mφs as well as on the phenotypic switching of fully polarized M1- and M2-Mφs using the human THP-derived Mφ model. M0-Mφs derived from the PMA-treated monocytic THP-1 cells have been employed as a reliable cellular model to study the biology of Mφs as well as their role in various inflammatory diseases.17,27 Mφs play an important role in inflammation and their contribution to the process of atherogenesis is well documented. Monocytes are among the first cells that arrive to the site of inflammation in atherosclerotic lesions. Upon their activation, monocytes differentiate into Mφs that produce reactive oxygen species (ROS), thus leading to the conversion of LDL into a high-uptake form that is involved in transformation of Mφs into foam cells. 20
A lot of disparities can be spotted in the scientific literature in terms of the protocols adopted for the differentiation of THP-1 monocytes into M0-Mφs as well as for the polarization of M0-Mφs towards the M1 and M2 types. All those protocols differ in the used PMA concentration and differentiation duration. In our study, we adopted a differentiation protocol that requires a 48 h-treatment period with PMA followed by a resting period of 24 h. 28 We successfully drove the differentiation of THP-1 monocytes into M0-Mφs and this was confirmed through the significant increase in M0-Mφs size and granularity as well as in their surface expression of CD11b and CD11c when compared to THP-1 monocytes. These hallmarks of THP-1 monocyte to M0-Mφ differentiation have been previously reported in multiple studies.23,27,29 Mφs are renowned for their plasticity, and hence, they are capable of switching functions in response to microenvironmental cues which can induce diverse Mφ activated phenotypes with “classically activated” M1-Mφs and “alternatively activated” M2-Mφs representing the two extremes. 29 Atherogenesis is a very complex process and it involves inflammatory and oxidative stress pathways where Mφs play a critical role in the chronic inflammatory response in the developing atherosclerotic plaque. 30 Both M1- and M2-Mφs are known to exist in diverse stages of the human atherosclerotic plaques. 1 Various studies have analyzed the temporal distribution of M1-Mφs and M2-Mφs in atherosclerosis stages whereby M2-Mφs were found in early atherosclerotic stages while M1-Mφs where found in the middle and late stages of atherosclerosis. 31 In this study, M0-Mφs were polarized towards the M1-Mφ type by stimulation with LPS and IFN-γ and towards the M2-Mφ type by stimulation with IL-4 and IL-13. 19 In order to check whether Mox-LDL favors the polarization of M0-Mφs towards the M1- or M2-Mφ phenotype, we treated M0-Mφs with Mox-LDL for 24 h to generate Mox-LDL-Mφs which were analyzed for their surface expression and cytokine release of signature M1-Mφ (CD80 and IL-6) and M2-Mφ (CD209 and IL-10) markers.22,23 Our results clearly demonstrated that Mox-LDL-Mφs expressed CD80 and CD209 at levels that were similar to those of M0-Mφs/M2-Mφs and M0-Mφs/M1-Mφs, respectively. Moreover, Mox-LDL-Mφs exhibited IL-6 and IL-10 release patterns that were identical to M0-Mφs and M2-Mφs. Based on our experimental model, Mox-LDL failed to induce the surface expression of CD80 and CD209 on M0-Mφs. Furthermore, Mox-LDL did not stimulate IL-6 and IL-10 production by M0-Mφs, thus indicating the inability of Mox-LDL in driving the polarization of M0-Mφs towards the M1 or M2 phenotype. One intriguing observation from our study was the high IL-10 release by M1-Mφs as compared to M2-Mφs. Such a finding contradicts the already known fact about IL-10 as being an M2-Mφ-assoicated anti-inflammatory cytokine. However, a previous study has reported a similar observation whereby M1-Mφs produced high levels of IL-10 which might be related to an autocrine and paracrine control of M1 metabolic programing in Mφs. 32 On the same line, another study has shown that IL-10 mRNA expression was significantly higher in M1-Mφs relative to M2-Mφs. 22
Fully polarized Mφs are known to retain their plasticity and can switch from a pro-inflammatory phenotype (M1) to an anti-inflammatory phenotype (M2) or vice versa. This unique ability of Mφs to switch polarized sates is referred to as “repolarization”. 14 In order to study the effect of Mox-LDL on the repolarization of Mφs, M1- and M2-Mφs were treated with Mox-LDL to generate Mox-LDL/M1-Mφs and Mox-LDL/M2-Mφs, respectively. Both Mφ types were characterized for their CD80/CD209 surface expression and IL-6/IL-10 release patterns. We found that treatment of M1- and M2-Mφs with Mox-LDL had no significant effect on the viability of cells. This is in line with our previous study performed on human aortic endothelial cells (HAEC), which showed that Mox-LDL treatment did not induce any significant effect on cell viability. 33 Conversely, another study has revealed the ability of a different form of oxidized LDL, copper oxidized LDL (Cu-oxLDL), to induce a significant reduction in the viability THP-1-derived M2-Mφs when used at a concentration of 1 or 40 μg/ml. 34 While Mox-LDL treatment of M1-Mφs (Mox-LDL/M1-Mφs) and M2-Mφs (Mox-LDL/M2-Mφs) did not result in any alteration in their surface expression of CD80 or IL-6 release, it did result in a decrease in CD209 expression on Mox-LDL/M2-Mφs; however, this decrease did not attain statistical significance. Moreover, Mox-LDL treatment failed to modulate CD209 surface expression on Mox-LDL/M1-Mφs and IL-10 release by Mox-LDL/M2-Mφs. Interestingly, upon treatment of M1-Mφs with Mox-LDL, we observed a significant reduction in IL-10 secretion by Mox-LDL/M1-Mφs. We believe that the Mox-LDL treatment may have induced a repolarization shift to a certain degree in M1-Mφs, by decreasing the secretion of the anti-inflammatory cytokine, IL-10, and hence altering the phenotypic profile of these Mφs toward a more pro-inflammatory profile. It has been previously shown that Cu-oxLDL can reduce the functional phenotype of primary human monocyte derived M2-Mφs through switching their phenotype towards a pro-inflammatory M1-Mφ type that exhibited elevated secretion of IL-6 and IL-8 and down-regulated expression of IL-10 following exposure to LPS. On the other hand, Cu-oxLDL had no effect on the phenotype of primary human monocyte derived M1-Mφs. 35 It was also reported that pretreatment of THP-1-derived M0-Mφs with Cu-oxLDL followed by LPS stimulation resulted in an increase in IL-10 production and a decrease in IL-6 release as compared to non-pre-treated/LPS-stimulated M0-Mφs. 24
Results from the current study showed that Mox-LDL failed to drive the polarization of THP-1-derived M0-Mφs. However, Mox-LDL demonstrated a potential to increase the pro-inflammatory state in Mφs by reducing the secretion of the anti-inflammatory M2-acssociated cytokine, IL-10, and by showing a tendency to downregulate the surface expression of the M2-Mφ associated marker, CD209.
Future studies should aim at testing Mox-LDL potential effect on M1 and M2 functions in vitro and their relation to what happens during atherogenesis e.g., lipid uptake, reactive oxygen species production. Generally, the microenvironment of atherosclerotic lesions involves an abundance of Mφs with different polarized phenotypic states that are profoundly associated with lesion progression. Hence, we hope that our findings will pave the way for future investigations that would dig further into the effect of Mox-LDL on Mφ biology to gain better insights into the immunological processes behind atherosclerosis development.
Supplemental Material
Supplemental material, sj-docx-1-ini-10.1177_17534259221090679 for The effect of myeloperoxidase-oxidized LDL on THP-1 macrophage polarization and repolarization by Samer Bazzi, Christian Frangie, Eliana Azar and Jalil Daher in Innate Immunity
Footnotes
Acknowledgements: The author would sincerely like to thank the University of Balamand for their unbounded support.
Data availability statement: All data generated or analyzed during this study are included in this published article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jalil Daher https://orcid.org/0000-0001-5899-1685
Supplemental material: Supplemental material for this article is available online.
References
- 1.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011; 145: 341–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol 2010; 7: 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daugherty A, Dunn JL, Rateri DL, et al. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest 1994; 94: 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malle E, Waeg G, Schreiber R, et al. Immunohistochemical evidence for the myeloperoxidase/H2O2/halide system in human atherosclerotic lesions: colocalization of myeloperoxidase and hypochlorite-modified proteins. Eur J Biochem 2000; 267: 4495–4503. [DOI] [PubMed] [Google Scholar]
- 5.Zhang R, Brennan ML, Fu X, et al. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA 2001; 286: 2136–2142. [DOI] [PubMed] [Google Scholar]
- 6.Kutter D, Devaquet P, Vanderstocken G, et al. Consequences of total and subtotal myeloperoxidase deficiency: risk or benefit? Acta Haematol 2000; 104: 10–15. [DOI] [PubMed] [Google Scholar]
- 7.Waldo SW, Li Y, Buono C, et al. Heterogeneity of human macrophages in culture and in atherosclerotic plaques. Am J Pathol 2008; 172: 1112–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadl A, Meher AK, Sharma PR, et al. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res 2010; 107: 737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khallou-Laschet J, Varthaman A, Fornasa G, et al. Macrophage plasticity in experimental atherosclerosis. PLoS One 2010; 5: e8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasheed A, Rayner KJ. Macrophage responses to environmental stimuli during homeostasis and disease. Endocr Rev 2021; 42: 407–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biswas SK, Chittezhath M, Shalova IN, et al. Macrophage polarization and plasticity in health and disease. Immunol Res 2012; 53: 11–24. [DOI] [PubMed] [Google Scholar]
- 12.Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol 2018; 233: 6425–6440. [DOI] [PubMed] [Google Scholar]
- 13.Huang X, Li Y, Fu M, et al. Polarizing macrophages in vitro. Methods Mol Biol 2018; 1784: 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leitinger N, Schulman IG. Phenotypic polarization of macrophages in atherosclerosis. Arterioscler Thromb Vasc Biol 2013; 33: 1120–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoger JL, Gijbels MJ, van der Velden S, et al. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis 2012; 225: 461–468. [DOI] [PubMed] [Google Scholar]
- 16.de Gaetano M, Crean D, Barry M, et al. M1- and M2-type macrophage responses are predictive of adverse outcomes in human atherosclerosis. Front Immunol 2016; 7: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chanput W, Mes JJ, Wichers HJ. THP-1 cell line: an in vitro cell model for immune modulation approach. Int Immunopharmacol 2014; 23: 37–45. [DOI] [PubMed] [Google Scholar]
- 18.Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol 2015; 111: A3.B.1–A3.B.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Genin M, Clement F, Fattaccioli A, et al. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 2015; 15: 577–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delporte C, Van Antwerpen P, Vanhamme L, et al. Low-density lipoprotein modified by myeloperoxidase in inflammatory pathways and clinical studies. Mediators Inflamm 2013; 2013: 971579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zouaoui Boudjeltia K, Daher J, Van Antwerpen P, et al. Exposure of endothelial cells to physiological levels of myeloperoxidase-modified LDL delays pericellular fibrinolysis. PLoS One 2012; 7: e38810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiratori H, Feinweber C, Luckhardt S, et al. THP-1 and human peripheral blood mononuclear cell-derived macrophages differ in their capacity to polarize in vitro. Mol Immunol 2017; 88: 58–68. [DOI] [PubMed] [Google Scholar]
- 23.Surdziel E, Clay I, Nigsch F, et al. Multidimensional pooled shRNA screens in human THP-1 cells identify candidate modulators of macrophage polarization. PLoS One 2017; 12: e0183679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rios FJ, Koga MM, Pecenin M, et al. Oxidized LDL induces alternative macrophage phenotype through activation of CD36 and PAFR. Mediators Inflamm 2013; 2013: 198193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daher J, Martin M, Rousseau A, et al. Myeloperoxidase oxidized LDL interferes with endothelial cell motility through miR-22 and heme oxygenase 1 induction: possible involvement in reendothelialization of vascular injuries. Mediators Inflamm 2014; 2014: 134635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boudjeltia KZ, Legssyer I, Van Antwerpen P, et al. Triggering of inflammatory response by myeloperoxidase-oxidized LDL. Biochem Cell Biol 2006; 84: 805–812. [DOI] [PubMed] [Google Scholar]
- 27.Aldo PB, Craveiro V, Guller S, et al. Effect of culture conditions on the phenotype of THP-1 monocyte cell line. Am J Reprod Immunol 2013; 70: 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tedesco S, De Majo F, Kim J, et al. Convenience versus biological significance: are PMA-differentiated THP-1 cells a reliable substitute for blood-derived macrophages when studying in vitro polarization? Front Pharmacol 2018; 9: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forrester MA, Wassall HJ, Hall LS, et al. Similarities and differences in surface receptor expression by THP-1 monocytes and differentiated macrophages polarized using seven different conditioning regimens. Cell Immunol 2018; 332: 58–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mikita T, Porter G, Lawn RM, et al. Oxidized low density lipoprotein exposure alters the transcriptional response of macrophages to inflammatory stimulus. J Biol Chem 2001; 276: 45729–45739. [DOI] [PubMed] [Google Scholar]
- 31.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 2013; 13: 709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baseler WA, Davies LC, Quigley L, et al. Autocrine IL-10 functions as a rheostat for M1 macrophage glycolytic commitment by tuning nitric oxide production. Redox Biol 2016; 10: 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samad G E, Bazzi S, Karam M, et al. Effect of myeloperoxidase modified LDL on bovine and human aortic endothelial cells. Exp Ther Med 2019; 18: 4567–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isa SA, Ruffino JS, Ahluwalia M, et al. M2 macrophages exhibit higher sensitivity to oxLDL-induced lipotoxicity than other monocyte/macrophage subtypes. Lipids Health Dis 2011; 10: 229–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Tits LJ, Stienstra R, van Lent PL, et al. Oxidized LDL enhances pro-inflammatory responses of alternatively activated M2 macrophages: a crucial role for kruppel-like factor 2. Atherosclerosis 2011; 214: 345–349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ini-10.1177_17534259221090679 for The effect of myeloperoxidase-oxidized LDL on THP-1 macrophage polarization and repolarization by Samer Bazzi, Christian Frangie, Eliana Azar and Jalil Daher in Innate Immunity