Abstract
Incense burning is practiced alongside many sacred rituals across different regions of the world. Invariable constituents of incense brands are 21% (by weight) herbal and wood powder, 33% bamboo stick, 35% fragrance material, and 11% adhesive powder. Major incense-combustion outputs include particulate matter (PM), volatile organic content, and polyaromatic hydrocarbons. The relative toxicity of these products is an implicit function of particle size and incomplete combustion, which in turn vary for a specific incense brand. Lately, the attention given to the Air Quality Index by international regulatory bodies has created concern about mounting PM toxicity. The uncharacteristically small physical dimensions of these entities complicates their detection, and with no effect of gravity PM fractions rapidly contribute to oxidative stress, enhancing random biochemical reactions upon being inhaled. Incense burning generates four times the PM extent (45 mg•g−1) of cigarettes (~10 mg•g−1). Several poisonous gases, such as CO, CO2, NO2, and SO2, and the unavoidable challenge of disposing of the burnt incense ash further add to the toxicity. Taken together, these issues demonstrate that incense burning warrants prompt attention. The aim of this article is to highlight the toxicity of incense-combustion materials on the environment and human health. This discussion could be significant in framing future policy regarding ecofriendly incense manufacture and reduced usage.
Keywords: incense combustion, particulate matter, polyaromatic hydrocarbons, volatile organic content, oxidative stress, burnt incense ash
Introduction
Generally in the form of sticks, coils, and cones, incense is used across the globe. Irrespective of cultural beliefs, incense is used in Taoist/Buddhist, Hindu, and Shinto temples and Christian churches. Noted areas of incense use other than religious ceremonies include mosquito repulsion (therapeutic) and fresheners (aesthetic). Materials in incense sticks (ISs) include resins, essential oils, aromatic molecules, and synthetic fragrances.1 The commonest incense-burning sites are temples, which either have limited space for incense burning or are located in the vicinity of residential and commercial areas. Hong Kong has more than 300 registered Chinese temples, out of which 70% alone are in the urban areas, especially in close contact with the residential and commercial areas leading to severe air pollution.2 A 2003 report by the Taiwan Environmental Protection Agency documented a huge 28.7 metric tons of incense being burnt in 92 temples in Kao-Hsiong, resulting in nearly 3580 tons of annual incense consumption.3,4 A further element of surprise is that these statistics correspond to normal days, so are even higher on festive days and ceremonial occasions. The habitual custom of incense burning also consumes significant incense quantities, as reflected by >90% of Chinese residents burning incense every day at home for >20 years.5 These and similar facts (reported elsewhere) suggest domestic use as a major incense source. Table 1 shows the varying incense compositions used in different countries practicing incense burning as a popular custom.4,6 These affect the combustion properties of constituents, providing valuable information for usage-specific preferences. For example, the coil configuration releases more smoke in a fixed time than refills and sticks. Thereby, incense use could be optimised via configuration for user–environment compatibility.
Table 1.
Incense-form shapes and configurations preferred in various countries
| Country | Common names | Shape | Reference |
|---|---|---|---|
| India | Agarbatti, agarwood, dhoop, batti | Sticks, lamps, powder, cones, coils | [2] |
| China | Joss sticks, xiang | Sticks, coils | [2] |
| Japan | Agarwood, ko, kodo, koboku | Cones, sticks | [5] |
| Indonesia | Dupaadalah, batangdupa | Sticks | [5] |
| Jerusalem | Ketoret | Sticks | [4] |
| Tibet | Dhoop | Coils, ropes | [4] |
| Nepal | Dhoop | Ropes | [2,5] |
| Saudi Arabia | Bakhoor, oud | Chips, blocks, powder | [6] |
Incense-use environmental risks arise from its constituent particulate matter (PM), volatile organic content, and several poisonous gases, such as carbon monoxide, nitrogen and sulphur oxides (Table 2). Major risks of particulate fractions (fine and ultrafine particles) emanate from incompletely combusted carbon fractions in incense smoke. These environmental and human health concerns has recently been in the limelight, owing to PM2.5 (≤2.5 μm aerodynamic diameter) involvement in aggravated COVID-19 spread and infection.
Table 2.
Health impact of incense-smoke constituents
| Source/features | Impact on human health | Reference | |
|---|---|---|---|
| Carbon monoxide (CO) | Incomplete combustion of hydrocarbons, wood, incense, cigarette, and fossil fuels | Reduces oxygen-transport ability of blood by forming carboxyhaemoglobin. Even low-level inhalation causes dizziness, headaches, weakness, and nausea. High concentrations can cause severe illness. | [7] |
| Sulphur dioxide (SO2) and nitrogen dioxide (NO2) | May form complex compounds with other pollutants | Reduced work capacity, elevated cardiovascular complications, pulmonary impairment, respiratory illness, lung irritation, and perturbation in self-defence | [8] |
| Volatile organic compounds (VOCs) | Chemicals with low boiling points, evaporate easily at room temperature, and include benzene, xylene, toluene, and isoprene | Ophthalmic inflammation, nose and throat irritation, nausea, vomiting, headaches, asthma exacerbation, dizziness. Chronic exposure leads to cancer, liver damage, and central nervous system damage | [8,9] |
| Aldehydes | Generated by combustion of organic fractions, burning incense generates aerosols, formaldehyde, acrolein, and acetaldehyde | The chief VOCs are distinguished by their irritant effects, low molecular weight, and halogenated aliphatic and unsaturated aldehydes. They affect nasal mucous membranes and oral passages, causing burning sensation, bronchial constriction, and coughing. Exposure to formaldehyde aggravates the risk of cancer and impaired mucociliary clearance. Studies have reported that wood dust and formaldehyde exacerbate nasal cancer risk. | [7] |
| Polycyclic aromatic hydrocarbons (PAHs) | Strongly associated with incense burning, as revealed by studies on Taiwanese temples, Swiss churches | Indoor air had nearly 27 times the PAH extent of outdoor air in a temple study. A Swiss study also revealed PAH prevalence in sedimented dusts generated on incense burning. | [10,11] |
| Diethylphthalate (DEP) | Extensively used as perfume binder in Indian incense, may be emitted in the air on burning | Natively a plasticizer and detergent base, DEP is a potential carcinogen. Sprague Dawley rats fed 50 ppm DEP and 55 ppm ethanol showed altered lipid and enzyme expression in the liver and serum over 120 days. DEP alone severely impaired lipid metabolism, accompanied by liver toxicity. | [12] |
| Particulate matter (PM) | On the basis of penetration depth into the respiratory system, particles of <2.5 μm and 0.1 μm diameter are referred to as fine and ultrafine particles. | Can go as deep as alveoli, posing severest health risks. Incense burning generates four times the PM of cigarettes and is associated with multiple respiratory complications, including lung dysfunction and enhanced oxidative stress by initiating and propagating random reactions. | [1,13] |
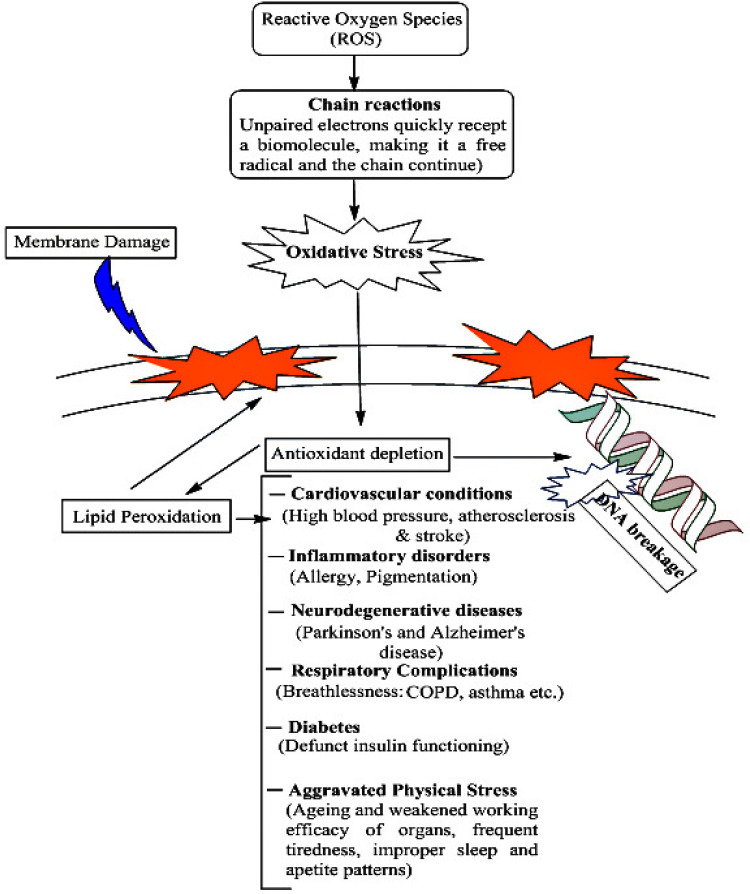
More recently, there has been considerable talk of the deteriorating Air Quality Index, whereby many casualties are being reported due to lacking oxygen. Airborne effluent is characteristically distinct from the vapor phase (having a more specific chemical composition), due to their varying sizes and contribution. The micro- and nanoscale dimensions of aerobic effluent make it gravitationally resistant, thus rendering humans more vulnerable to random oxidative stress enhancement. Oxidative stress in itself is an invitation to manifold adverse physiological events, ranging from aggravated inflammation to respiratory malfunction, neurodegenerative issues, and even cancers14,15 (Figure 1). These risks evidently point to ISs being a mainstream contributor to indoor and outdoor air pollution. Some studies have equated IS inhalation to that of passive smoking, wherein the risks are highly acute and urgent. It is thus of utmost importance to adopt a cautionary approach, particularly when everyone is aware of the severe health and environmental impacts of smoking. The present article thus aims for a broad understanding of incense constituents and their possible risks to human health, with a focus on mechanistic understanding and revisiting past research. A separate section is devoted to the discussion of studies conducted using in vivo analysis.
Figure 1.
Risks of aggravated oxidative stressy, depicting the possibilities of aggravated free-radical activities.
Misconceptions and Health Risks Associated with Incense Burning
Incense use as part of worship across different cultures and religions dates back >2,500 years, and emerged from the burning of resins and aromatic herbs. The flavoring agents of incense are largely plant compounds that impart a characteristic essence to a particular incense variety. The use of incense for reasons other than those of worship is a consequence of easily transported and handled varieties. The ingredients of incense are mostly organic compounds (OCs), along with texture-improving additives, smoothening agents, and stabilizers. Negligence and lack of scientific rationale are the major reasons for multiple cultural beliefs associated with incense burning, principally at the village level. With the compelling need to assess the environmental quality accompanied by progress in micro- and macroscale characterisation techniques, IS composition and properties have been validated. A majority of such studies have been conducted post-2000, and thus the present juncture is significant for IS characterisation. Though the varying proportions of fragrance-enhancing agents and stabilizers have resulted in numerous incense varieties being commercialised, the crude products of ISs invariably comprise several dreadful pollutants.
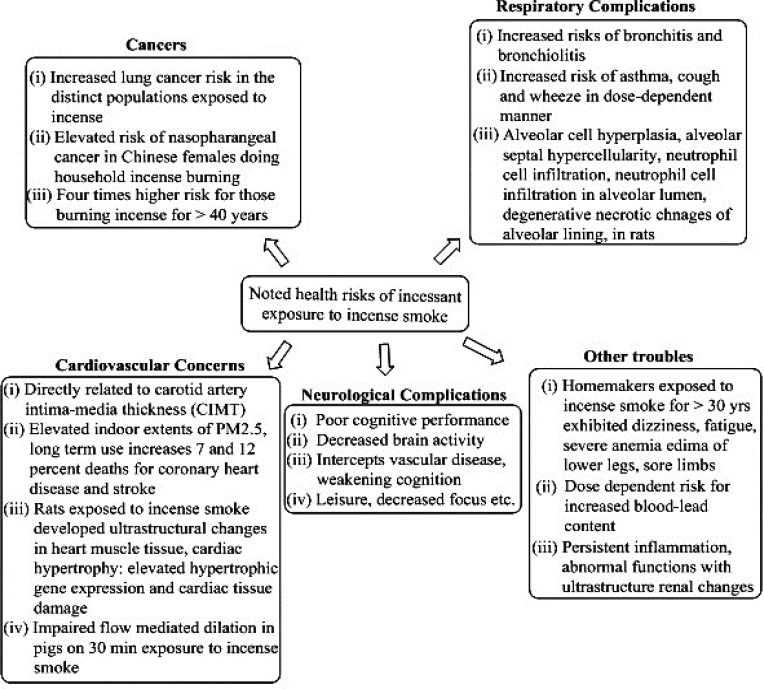
To generate fragrance, incense burns at 200°–300°C, but incomplete combustion of constituent organic material generates many toxic compounds. Studies evaluating several incense brands for such features have revealed a presence of carcinogenic and toxic intermediates in IS emissions.16,17 Figure 2 depicts the various health risks of incense combustion that are substantially due to PM and incomplete combustion of carbon content in the incense. Multiple diseases caused by incense burning are largely due to oxidative stress and respiratory complications. Table 1 has already provided details on deleterious constituent fractions of ISs, including PM, organic entities, and volatile OCs (VOCs). Here, we discuss the findings of some early 2000s studies pertaining to IS characterisation and analysis of PM in ISs. In 2001, Lin et al studied indoor and outdoor exposure to polycyclic aromatic hydrocarbons (PAHs) and total suspended particles (TSPs) in a Taiwanese temple.18 The investigators measured total PAH concentrations and particulate-phase distributions in ambient air inside and outside the temple, along with their connection to heavy traffic.
Figure 2.
Health risks associated with incense combustion. Studies pinpoint indoor air as more contaminated with incense particulate matter than outside air. Major risks present due to oxidative stress and pulmonary inflammation.
Significantly, investigation revealed PAH content in the indoor air of 4739–11,130 ng•m−3. The mean estimate (8888±3598 ng•m−3) was almost 19 times that of the PAH concentration in the outer air (468±156 ng•m−3) but close to the level near the traffic source (81,110 ng•m−3). It was also noticed that particulate-phase PAHs contributed only 3.2% of total PAHs, sufficiently different from those at the intersection (46.1%). These findings suggest that high total PAHs inside the temple had sources different from the traffic. Similarly, TSP concentration in the indoor air was 429–991 μg•m−3, with the mean (790 μg•m−3) being almost three- and elevenfold that of the traffic source (255 μg•m−3) and outside air (72.5 μg•m−3), respectively. Of note, the mean TSP value of 790 μg•m−3 was significantly higher than the yearly geometric mean (130 μg•m−3) and 24-hour mean (250 μg•m−3) benchmarks of the national air-quality standard of Taiwan. Collectively, the findings confirmed high-level exposure of Taiwanese temple occupants to PAHs and TSPs generated via incense burning. A 2002 study by Jetter et al aimed to characterise PM emissions generated by incense burning.1 Interestingly, PM emissions from 23 distinct incense types were analysed using a cyclone filter. Analysis revealed emission rates for PM2.5 of 7–202 mg•h−1 and emission factors of 5–56 mg•g−1 of the incense burnt. Emission rates were also assessed using an electrical low-pressure impactor (ELPI) and a small electrostatic precipitator (ESP), and the rates estimated using the ELPI were always lower than those of the cyclone filter.
In contrast to this, the ESP-estimated rates were constantly greater than those of the cyclone filter, suggesting higher effectiveness of the ESP in computing semi-volatile particle emissions. Particle-size measurement with the ELPI yielded similar distribution patterns for most of the examined incenses. The size distribution was 0.06–2.5 μm aerodynamic diameter, with peaks of 0.26–0.65 μm. The findings yet again suggested that incense emits fine PM to a greater extent than other indoor sources. Analysis using an indoor air-quality model demonstrated that indoor PM2.5 levels could far exceed those of the outdoor concentrations set by the US Environmental Protection Agency’s National Ambient Air Quality Standards (NAAQS). Emissions of CO, NO, and SO2 were also assessed for seven incense types under specific NAAQS conditions, and concentrations were denser indoors than outdoors. This study could be considered highly significant (in terms of incense varieties tested) for elucidating the health risk posed by ISs via inhalation-driven exposure to PM.
Lin et al analysed the contribution of incense burning towards indoor pollution–mediated health issues. The study compared human exposure to PAHs and TSPs inside and outside a Taiwanese temple. Of note, three incense and incense ash (brands) were examined to ascertain the relationship between incense composition and PAH emissions. Using standard methods to determine the extent of 21 PAHs and TSPs inside and outside the temple, the investigators found that indoor mean total PAH, particulate-bound PAH, and TSP concentrations were 6258 ng•m−3, 490 g•g−1, and 1316 g•m−3, respectively. Corresponding outdoor levels were 231 ng•m−3, 245 g•g−1, and 73 g•m−3, with 27- and 18-fold greater PAH and TSP concentration indoors. Amongst the individual PAHs (particulate with gas phase), prominent constituents were acenaphthylene, naphthalene, acenapthene, fluoranthene, and phenanthrene, with 3853, 1264, 349, 243, and 181 ng•m−3 concentrations, respectively. Indoor:outdoor PAH ratios were 5.7–387.9 (median values), suggesting incense burning within the temple was a significant PAH source. From the various literature, it has been found that the PAH level inside the Temple is much higher than in the outside environment. But in comparison to the local traffic intersection and in the graphite electrode–production plant, the PAH level was less. This investigation confirmed that temples were significant PAH- and TSP-generating locations with serious health hazards to local workers.19
Lin et al also reported on metal content–dependent burning characteristics and emission products from nine incense varieties. Studying the effect of metallic contents on burn rate, ash, and particulate-emission factors of combustion, the investigators found the highest Ca and K content (geometric means 8.7 and 2.5 mg•g−1) in the raw materials of incense. After Ca and K, the most abundant metals were Al, Mg, Fe, Na (0.1–1 mg•g−1), and Ba, whereas the lowest values (0.01–0.1 mg•g−1) corresponded to Sr, Mn, Cu, and Zn. Inspection revealed most Ca to be in the form of CaCO3, with similar particulate-generation rates for different incense brands under identical burning conditions. Total suspended particulate emission was inversely correlated with the burn rate of an IS. Further, for a fixed burnt weight of incense, higher ash emission correlated with lower suspended-particulate emission. Thereby, incense with more ash production is better, due to less particulate generation. Another important finding of this study was that for all IS raw materials, a periodic increment in total metallic content (from 0.5% to 2%) can significantly reduce particulate levels (~40%) during combustion.20
The results of quantitative IS profiling in these studies confirm the presence of PM in diverse textures that deteriorates air quality upon being released. Since incense burning has almost attained the form of a habit in most households (more in temples), it is highly urgent to be aware of PM health risks. Though there are several side effects of burning incense sticks, here we have confined its risk mainly to the field of toxicity risks. There is only a subtle line of distinction with reference to the place concerned and the kind of incense being used in a particular location. Risk factors also assume greater proportions if the temple premises are not spacious or adequately ventilated. The studies discussed have unanimously found a greater contribution of indoor air from temples towards PM generation, so preventive norms must be strictly implemented to minimise the health risks.
Risks and Concerns Associated with Incense Burning
The smoke generated from incense burning is injurious to human health and air quality in multiple ways. Sources of these threats are unburnt carbon fractions and other complex residues, which on coming in contact with surrounding air, coalesce and become larger. On being inhaled, such particles have a settling tendency with random toxicity. The greatest risks of these pollutants are to respiratory health, as the respiratory system is the first to encounter these contaminants. Lately though, many curbs have been imposed on incense use, as nearly all brands exported at present pose the risk of pollutant generation.21 Many studies have demonstrated aggravated risk of asthma, respiratory allergies, breathing difficulties, neurological impairment, leukaemia, skin irritation, cardiovascular complications, and lung cancer.8,15,22,23
While Table 1 has already listed the deleterious and environmentally threatening pollutants in ISs along with their ill effects, the specific details of PM are given in Table 3. The foremost complication with PM is its uncertain physical and chemical properties, which is not helped by their minute dimensions. Such dimensions make PM free from the influence of gravity, due to which their interactions are highly random and often culminate in elevated oxidative stress. Numerous studies have listed incense smoke as a major indoor pollutant contributing to cellular toxicity. In terms of characteristic health effects, airborne PM is distinguished on the basis of the relative extent (characteristic particle size) to which it can invade the human respiratory system. Particles >10 μm in diameter comprise a coarse fraction that can be eliminated due to their gravitational settling or via early detection through optical characterisation. Fine particles (PM10) pose a greater threat, as they get segregated in the respiratory passage upon being inhaled. Most hostile are the ones having <2.5 μm diameter (PM2.5), as they can go deep across the alveoli. These entities are the most difficult in terms of characterisation and analysis. Due to their near-nanoscale dimensions, these particles exhibit unpredictable toxicity with random accumulation in biochemical reactions once inside the body. Unburnt fractions of ISs (including soot) are mostly constituted of these particles. There has been increasing interest in the fate of PM2.5 once it invades our respiratory system, owing to these particles’ swift emergence as transmission agents of virulent COVID-19 constituents.24,25 The tiny dimensions of PM2.5 make these particles unpredictable in terms of toxicity via accumulation in biochemical pathways and concomitant enhancement of oxidative stress. In severe cases, accumulation of these particles may result in germ-cell mutations and increased risk of lung and blood cancers.8,15,23
Table 3.
Environmental and human health risks of PM, with the most notable effect being on the respiratory tract
| PM configuration | Environmental threat | Concomitant health risks | Reference |
|---|---|---|---|
| Particles of 2.5–10 μm diameter, referred to as coarse thoracic particles (PM2.5–10) | Random coalescence with aerial pollutants impairs air quality. Little effect of gravity in native state complicates their degradation. Combined with structural toughness, accidental water solubility affects the aquatic habitat by enhanced oxidative stress. | Accumulate in the respiratory system and bloodstream, aggravate asthmatic risks, stimulate nonfatal heart attacks, impair lung function, aggravate airway irritation, coughing, or breathing difficulty | [15] |
| Particles <2.5 μm diameter, referred to as fine particles (PM2.5) | Makes lakes and streams acidic, alters the nutrient balance in aquatic habitats, depletes soil nutrients. Black carbon portion resulting from incomplete combustion has several detrimental effects on health and climate, comprised of carcinogens and diesel-engine exhaust. | Smaller dimensions can make them reach deep into the alveoli, aggravated lung irritation causing liquids and gases to pass through lungs, increased lung-tissue inflammation that affects heart function, clotting-mediated increased stroke risk, increased incidence of lung cancer, and breathlessness. | [23] |
| Ultrafine particles, <0.1 μm in size | Contribute to mounting air pollution, difficult to settle due to small dimensions, can agglomerate with smoke, fog, and dust particles (smog) to increase airborne toxicity, intensify the infection tendency of airborne pathogens and related inflammatory response. | Particles of 50–100 nm ranged particles are highly random in their interactions, interfere in biological reactions via elevating oxidative stress, penetrating deep into the respiratory tract, and even crossing biological barriers, aggravating the pathogenesis of respiratory diseases and complicating their treatment. Adverse inflammatory responses, more risk for those employed in incense-manufacturing industries. | [26] |
The following sections focus on the most severe environmental and health risks posed by incense combustion. These risks are categorised as respiratory issues, cardiovascular complications, neuropsychological disturbances, dermatological concerns, and others of heterogeneous origin. A significant factor contributing to this scenario is aggravated oxidative stress, presumably the root of all degenerative conditions.
Respiratory Issues from Exposure to Incense and Incense-Stick Smoke
PM and VOCs generated from unburnt carbon fractions in ISs easily gain access to deep respiratory system regions and settle there. The end result of these deposits is aggravated oxidative stress, since these materials are not native to the biological environment and consume oxygen for long durations. Ultimately, difficulties in breathing along with disturbed sleep patterns and aggressive fatigue-mediated issues manifest. Certain ISs on the market carry a warning about lung toxicity, owing to a higher proportion of fine particles.27 Numerous studies have highlighted the respiratory complications of unchecked and casual incense combustion. Here, we discuss significant research of the past five decades, foremost amongst which is a 1966 study by Sturton et al. This was conducted in Hong Kong, where a high incidence of nasopharyngeal cancer was observed amongst men who burnt incense compared to other malignant cases (considered controls). The analysis revealed that 74.5% of the nasopharyngeal cancer cases studied and 52% of other malignant cases had been exposed to ISs, which further aggravated the disease.28 In another significant study, Yang et al analysed 4164 elementary schoolchildren in rural Taiwan. Inspection revealed incense burning to be a significant factor, along with mosquito-repellent combustion, in aggravated coughing symptoms.29
Another noteworthy study studied temple workers (due to greater exposure risk to air pollutants) in Kaohsiung, Taiwan. A total of 109 individuals were found to have chronic respiratory symptoms and acute irritation. The investigators concluded that working in a temple posed greater risk to respiratory health, often manifesting as nasal and thoracic allergies, with adjusted odds ratios of 4.14 and 4.5, respectively, for temple workers compared to controls. A key observation of the study was that chronic cough indications were commoner in the temple workers than adjacently located church workers (as controls).30 Alarifi et al exposed rats to an Arabian incense mix for 14 weeks at a rate of 4 g per day. Following completion of exposure, lung tissue were removed from the animals prior to electron-microscopy analysis. Analysis revealed considerable ultrastructural changes in the alveolar pneumocytes. Neutrophil infiltration into alveolar lumina was observed to accompany degenerative and necrotic changes in cells in the alveolar lining. The alveolar walls were thickened owing to collagen-fibril deposits. The researches noted that the ultrastructural changes in pulmonary tissue compromised respiratory efficacy.31 A subsequent study by the same group confirmed this effect in bakhoor (Arabian incense)–exposed rats.32
Studies have also demonstrated that prolonged IS exposure in a closed area heightens lung cancer risk.17,33 Smoke effluent gradually comes into direct contact with the upper respiratory tract, so the lungs are the major organs being affected. Incense-combustion products can aggravate the functioning of growth factors due to oxidative stress, resulting in uncontrolled proliferation of cells.17,34 Prolonged exposure to IS allergens results in aggravated asthmatic symptoms manifesting as bronchial inflammation, persistent wheezing, and cough.4 Lofroth et al analysed PM generation from indoor smoke generated by cigarette smoking, incense burning, frying of experimental lean minced pork, and mosquito-coil burning. Extracts of the analysed materials were mutagenic on the Ames Salmonella test with TA98 and activation and higher response in a microsuspension test with the same strain and activation conditions. Analysis revealed PM from smoking and burning processes 3000–50,000 revertants per gram in the microsuspension assay. An interesting distinction was that emissions of carbon monoxide, isoprene, and benzene in smoking and burning were not detected in frying fumes, suggesting incense burning as a significant indoor pollutant.35 Reddy et al also showed that incense sticks, mosquito-coil burning, and tobacco consumption as chief polluting sources, which mishandling may aggravate the risk of asthma and allergic rhinitis.36 The study noted that incense combustion generated PAH, benzene, carbon monoxide, and PM2.5, recalling an earlier investigation that analysed pollution inside a church in Cardiff during Easter. The air inside the church was found to have a greater proportion of all grades of PM, resulting in 25- to 30-fold enhanced oxidative stress compared to cigarette smoking.37
A recent investigation by Yamamoto et al took note of impaired lung function and increased risk of asthma and investigated the risk of incense smoke causing airway hyperresponsiveness (AHR) and bronchial epithelial barrier function. The study analysed the effect of IS exposure on AHR, expression of multiple tight epithelial junctions, adherens junction–associated mRNAs and lung proteins, and the barrier function of bronchial epithelial cells, assessed by transepithelial electronic resistance, in mice. It was found that exposure of BALB/c mice to ISs aggravated AHR and inflammatory macrophage localisation to BALF suppressed claudin 1, 2, 3, 7, 10b, 12, 15, and 18, occludin, and ZO-1. Exposure to ISs also reduced E-cadherin mRNA expression, causing claudin 2 and ZO1 discontinuity within the lungs, on being examined with protein immunostaining. In agreement with earlier attempts, the exposure of mice to ISs decreased transepithelial electronic resistance and increased ROS in a dose-dependent manner in bronchial epithelial cell cultures. Treatment with antioxidants, N-acetyl-l-cysteine, and β2 agonists provided relief from aggravated oxidative stress symptoms. Amongst other noted distinctions were elevated recruitment of inflammatory macrophages, disruption of tight-junction lung proteins, and damaged epithelial barrier function.38 The comprehensive analysis of this study with triplicate findings, apart from antioxidant-relieved oxidative stress complications, emphasises the health risks of unchecked IS exposure.
Cai and Wong reported an association of ISs (on combustion) from temples and respiratory mortality in Hong Kong. The investigators categorised 366 temples into open, semi-closed, closed, and inactive based on incense-burning patterns. The open domain comprised temples with the largest average area with least quantity. Principle-component analysis revealed that the building, greenery, water density, and temple height were highly influential factors. NO2 and PM2.5 were major pollutants of temple incense burning, which contributed to respiratory mortality. Traffic load was listed as an important factor affecting incense pollution via infiltration into indoor air (in temples), since Hong Kong temples are mostly located on roadsides and nearby residential areas. The building and greenery density were listed as other significant factors in respiratory mortality. The study emphasises a need to have optimum water bodies and greenery, which together function as landscape components for moderating PM concentration. Concerns highlighted in the study included incense being burnt in open areas inside the temple, the location of incense-burning places, and whether there was treatment of discharged pollutants. Since incense composition is an implicit factor in determining the corresponding extent of pollution, temples should use containing having less volatile material with lower carbon levels in order to minimise pollutant emissions.2
Incense combustion also aggravates COPD risk, affecting housewives to a greater extent due to persistent exposure and habitual light of incense in their homes.39,40 COPD is a chronic disorder of the lungs caused extensively by inflammation (response to harmful external-origin stimulus), owing to which it is observed with greater frequency in smokers. The literature is flooded with the effect of cigarette smoke on an individual while only a limited study is carried out on the case of incense smoke and its effect on an individual.4,39 In one such attempt, Guo et al studied the effect of IS exposure in Taiwan, where IS burning is a common religious custom. They concluded that a COPD sufferer should avoid areas where ISs are burnt at least for 1 hour per day, due to the cumulative deleterious effects on the respiratory system. A person with multiple respiratory problems is more prone to incense smoke.39 Based on the aforementioned studies, thereis no doubt about about the risk of IS exposure impairing normal and healthy respiratory function.
Cardiovascular Complications
Much evidence has demonstrated the threats and complications that ISs pose to cardiovascular health. The gaseous emissions in ISs, eg, SO2 and NO2, are so deleterious that even nanoscale inhalation can aggravate existing cardiovascular disorders via enhanced levels of oxidative stress. Multiple post-2010 investigations using animal models and individual case histories have created awareness of the cardiovascular risks. A 2011 study on rats revealed an association of IS exposure with adverse metabolic changes of excessive triglycerides and suppressed high-density lipoprotein (cholesterol) levels over time.41 Another major 2011 study focused on endothelial function using flow-mediated dilation in pigs, revealing significant suppression subsequent to 30 minutes’ IS exposure. The impaired response was directly correlated with CO levels within the exposure-chamber boundaries. An absence of similar associations for total particulates and poisonous carboxyhaemoglobin suggested that the gaseous phase that accompanied CO (rather than CO itself) was the cause of acute endothelial dysfunction subsequent to IS exposure.42 In 2012, another study analysed the relationship between endothelial impairment and incense-combustion frequency by exposing human coronary artery endothelial cells to IS combustion products from temples, resulting in significant ET1 increments and suppressed nitric oxide formation. These correlations demonstrate increased cardiovascular disease risk in IS-exposed individuals.43 In a 2014 study, 50 housewives from Taipei were screened, revealing incense was burnt regularly in 48% of homes. A major risk to cardiovascular health from ISs emanates from enhanced PM2.5 generation causing an adverse effect on heart-rate variability (HRV).44
A second 2014 study assessed chronic exposure to daily household incense combustion in a large population-based cohort of middle-aged and elderly Chinese residents. The results revealed a 12% higher chance of developing cardiac disorders, whereas that for stroke and coronary heart disease was 19% and 10% higher, respectively, than former and never-users.15 Overall, 7% of coronary heart disease and 12% of stroke related casualties in this study were due to long-term incense use. These results provide crucial evidence pertaining to IS-mediated cardiovascular risk as exposure to ISs preceded cardiovascular mortality. A 2015 investigation matched the observations of the 2011 study, wherein exposed rats exhibited significant ultrastructural IS-induced changes in heart-muscle tissue and cardiac hypertrophy. These characteristics were accompanied by elevated hypertrophic gene expression and cardiac tissue injury, indicated by considerable enhancement of creatine kinase myocardium-bound lactate dehydrogenase.45 A 2018 study found a positive correlation between incense burning and carotid-artery thickness on multivariate regression analysis following arrest.46 The analysed population in this study comprised 132 Thai–Vietnamese adults aged >35 years. Arterial thickness indirectly ascertains the relative extent of atherosclerosis, confirming the role of indoor incense combustion in the deterioration of native cardiovascular functions. Detailed findings of this study are discussed in Recent Attempts Towards Assessing the Incense Combustion Toxicity, focused on the last 5–7 years’ research on incense-combustion toxicity.
Dermatological and Allergy Concerns
The presence of toxic VOCs and conventionally undetectable PM in ISs heightens vulnerability to multiple sensitive inflammatory stress–driven responses. Major body organs affected by such responses are the eyes, nose, throat, and skin. Accounts of such responses do exist, albeit not in the recent past. For example, a 1987 study reported a 63-year-old accustomed to incense burning for 15 years developing itchy depigmented patches on his left dorsum manus, left shoulder, and abdomen. Careful analysis afterwards revealed incense perfume as the factor responsible for this.47 Another paper in the same year reported a woman with an incense-burning history of 5 years developing pigmented contact dermatitis because of musk ambrette in the incense.48
In 2004, a study reported incense-combustion risk to IgE antibodies in umbilical cord blood in 334 mother–neonate pairs. Investigators noticed enhanced IgE generation in response to excessive Pb exposure, with corresponding Pb levels of 0.14 and 0.21 mg•g−1 in PM2.5 and PM2.5–10, respectively, collected from near a temple in Taiwan. Though experimental proof or mechanistic link between incense exposure and blood-IgE quantity was not found, they discussed the likelihood of Pb emitted from incense burning getting adsorbed followed by its transfer to fetal blood, where it enhanced IgE generation.49 Separate studies conducted on rats have also reported morphological changes in alveolar pneumocytes and neutrophil infiltration into alveolar lumina on exposure to ISs.31,32 Stimulation of resident and recruited inflammatory cells in response to these changes resulted in aggressive activation of multiple mediators, collectively fuelling airway inflammation and remodeling. A 2005 study revealed a manifestation of acute irritation (characterised by eye irritation, nasal secretions, dryness or congestion, throat dryness, and nausea) among a group of temple workers in Kaohsiung, Taiwan. Implicit incense involvement was concluded from the missing similar conditions in the church workers.30
A 2012 study on a Taiwanese birth cohort reported regular incense burning as the most critical indoor factor in the health of 3- to 5-year-old children, irrespective of parental history of allergic and respiratory disorders. Regular incense combustion was listed as a contributing factor towards childhood asthma and allergy, with double the significance in cases of similar conditions in parents.50 In a study carried out in 2019, where 36,541 adults from six big Chinese cities were surveyed and found that about 15.1% of them were burning incense and incense sticks in their daily life routine. Inspection revealed correlationa between incense combustion and aggravated inflammation in eyes (itching or burning sensation) and skin (dry or flushed facial skin, scaling in ears, dermal symptoms in hands characterised by dryness or skin redness).51
Neurodegenerative and Neuropsychological Issues
Although not as serious as that of respiratory and cardiovascular problems, recent studies have demonstrated an association between incense burning and neurological complications. A 2020 study from China presented a 3-year case–control analysis of 515 stroke- and dementia-free adults aged >65 years in Hong Kong.22 Amongst the analysed subjects, incense users were noticed to exhibit poor cognitive performance with suppressed coordination compared to nonusers. Analysis revealed an association of indoor incense burning with suppressed performance across multiple cognitive domains at baseline and the end of third year, apart from reduced default-mode network (DMN) connectivity. Incense-smoke exposure resulted in strong interactions with diabetes mellitus, hyperlipidemia, and white-matter hyperintensity (a noted marker of cerebral small-vessel disease. More details of this study are in Recent Attempts Towards Assessing the Incense Combustion Toxicity, specifically focused on the last 5 year’s research on incense-combustion toxicity.
Another study from China assessed the neural effects of incense exposure on the developing foetus. This cohort study involved nearly 43,000 participants, wherein prenatal exposure to incense was ascertained as significantly associated with early-onset hyperactive behavior in the non–school going children.52 In a Taiwan birth-cohort study, there was delayed the gross motor-milestone achievement in infants born in incense-using homes. The correlation was stronger in mother–infant pairs under persistent exposure, indicating a likely dose–response effect.53 Apart from these impacts, oxidative stress aggravation by inhaled ISs reduces the oxygen supplied to the brain. A number of neurological proteins are adversely affected in terms of their functional behavior. Although there is no experimental evidence at present to demonstrate such correlations, there seems a risk of vulnerable physiology for Alzheimer’s and Parkinson’s disorders. Exercising immediate and utmost caution is mandatory to confront the risk of IS exposure and neurological adversities.
Other Abnormal Conditions
Multiple studies have found correlations of IS exposure with headaches, dizziness, and fatigue. Recurrent episodes of dizziness and headaches subsequent to incense-smoke exposure have been suggested as an outcome of low CO convergence.4 A CO-enriched environment reduces oxygen in the blood, thereby weakening oxygen-transport ability. As a result, red blood cells receive less oxygen, accumulating as oxidative stress in vital organs, including muscles, resulting in fatigue associated with dizziness. In extreme cases, inhaled PM can rupture red blood cells, substantially reducing oxygen transfer to cells and body tissue. Such possibilities are more frequent in places where incense burning is a habit or the surrounding environment is not well ventilated. Unchecked PM accumulation manifests as migraines, headaches, nausea, general discomfort, and unsteadiness.54
Yamamoto et al examined the effect of CS and ISs on testicular function in rats. Rats were grouped into A (n=20), and B (n=10), and C (n=10) cohorts. Group A was exposed to the smoke of 20 cigarettes for 1 hour per day, group B to the smoke of 40 ISs, and group C served as controls over 10 weeks. Nicotine and cotinine levels were examined and a mating test conducted. After 5 days of this exercise, serum concentrations of testosterone prior to and after human chorionic gonadotropin (hCG) stimulation, gonadotropins and epididymal sperm quantity and motility were estimated. In vitro fertilisation was also studied, and nicotine and cotinine were expressed in group A (exposed to CS), but not in B or C. The three groups, however, did not exhibit significant distinctions in basal serum testosterone or gonadotropin concentrations. Surprisingly, the testosterone response to hCG stimulation was considerably lower in group A than the others. Apart from this, group A also exhibited significant reductions in epididymal sperm content, motility, and fertility evaluated in vivo and in vitro. These findings suggest a significant distinction of incense smoke from that of cigarettes, and could be crucial evidence for framing future guidelines regarding incense use. No drastic reductions in spermatogenesis showed that CS toxicants were much lower than incense ones. As such, this study demonstrated a fairness of incense composition with respect to CS, which has stronger polluting ability.55
Similar findings were demonstrated in a 2004 study from Japan, wherein four groups (A–D, each having 20 rats) were exposed to CS and incense smoke. Rats in groups A and B were exposed to cigarette and incense smoke, respectively, for 10 weeks and those in D exposed to CS for 7 weeks. Group C rats were controls. On completion of the experiment, serum-testosterone responses to hCG stimulation, androgen-binding protein activity in testicular cytosols, epididymal sperm motility, oocyte fertilisation rate, oocyte-cleavage rate, and blastocyst-development rate were ascertained subsequent to in vitro fertilisation. These parameters were substantially lower in group A (CS-exposed rats) than the others. Contrary to this, the relative pace of fertilisation, cleavage, and blastocyst development on intracytoplasmic sperm injection (ICSI) were not significantly distinct among the groups. More importantly, the relative proportion of live offspring to those of transfected oocytes (subsequent to IVF and ICSI procedures), was considerably lower in group A rats than the others. This again established CS exposure as detrimental to the secretory efficiency of Leydig and Sertoli cells, resulting in impaired epididymal spermatogenesis.56 IS-exposed rats exhibited no significant risks compared to cigarette smoker–exposed ones, justifying our inference that IS constituents do not pose a similar toxicity threat to cigarettes.
A 2014 study from China evaluated the health risks associated with total PM generated through incense burning. The investigation characterised PM and major chemical components of two incense varieties using an electrical low-pressure impactor and GC-MS. Matching the PM emissions of incense with cigarette smoke, the investigators compared their genotoxicity and cytotoxicity using in vitro assays. Profiling of incense revealed that both PM and smoke mass contributed majorly to ultrafine and fine particles. Manifold irritants and aromatic and toxic compounds were also assessed in particulates, suggesting a significant risk of inflammation and aggravated oxidative stress (discussed earlier). An interesting observation was that application of PM from four ISs and one cigarette sample to Chinese hamster ovary cells induced concentration-dependent responses: the cytotoxicity of total PM from ISs was higher than for CS (3R4F reference cigarette, University of Kentucky). However, analysis of IC50 values revealed a much higher extent for CS than the incense. The authors concluded that CS toxicity was not higher than incense smoke, but the sample was small and there was considerable variability in consumption levels of ISs and cigarettes. The incense generated 64 distinct compounds, and those with >90% matching were evaluated. Though important, the results of this study cannot truly establish that CS is more injurious to health than ISs.57 The observations of milder risk from ISs in the last two studies do not say anything about IS toxicity, but rather acute exposure. Results and corresponding impacts may range considerably (as demonstrated in several other contemporary efforts) once exposure becomes chronic.
Another investigation from 2014 evaluated testicular degeneration induced by Rhodinol-based incense via monitoring testicular histology, sperm characteristics, and testicular oxidative status. The study was conducted on 24 male adult albino rats 10–12 weeks old weighing 200–230 g subdivided into four groups (A–D) of six rats each. Group A rats served as controls and were exposed to 1 g natural solid freshener. Groups B–D were exposed to 1, 2 and 3 g ISs, respectively, for 30–40 minutes on a daily basis for 62 days. All exposure was through whole-body inhalation. Results demonstrated a considerable suppression in gross anatomical parameters of absolute and relative testicular weight in the 2 and 3 g groups compared to the control group. Incense-exposed rat groups also exhibited diminished basal seminiferous epithelia, testicular atrophy, germinal aplasia, and hypospermatozoa formation. Groups B–D also showed statistically significant decrements in sperm count, sperm motility, and normal sperm morphology and a significant increment in total abnormal sperm morphology compared to controls. Rats exposed to incense also displayed a derangement of oxidative status compared to the control group, with a significant reduction (p <0.05) in activities of superoxide peroxidase, catalase, glutathione, and reduced glutathione and a significant increment in malondialdehyde (a lipid-peroxidation product). These findings collectively suggest that rhodinol-based incense exposure results in testicular derangement in testicular histology, sperm parameters, and oxidative status in albino rats.58
Pollution from IS burning is also known to affect fertility in humans. A 2016 study on such incense-combustion risks was conducted by Chen and Ho on Taiwanese citizens. The study involved ordinary least square and quantile regression analysis of a sample comprising 15,773 term births (>37 gestational weeks, 8216 boys and 75,557 girls) in 2005. Associations were estimated separatelyfor boys and girls. Factors correlated with incense burning were birth weight, head circumference, parental religion, demographics, health characteristics, and pregnancy-related variables. Analysis via fully adjusted ordinary least square regression revealed that prenatal incense burning correlated well with lower birth weight for boys and smaller head circumference for both sexes. On the whole, negative correlations between prenatal incense burning, birth weight (for boys only), and head circumference were more pronounced among the lower quartiles of birth outcomes. Though this study established a critical effect on birth weight and head circumference, a clear-cut explanation to understand the sex-based effects of incense burning requires further research.59
A cohort study in 2018 assessed the risks of incense burning in pregnancy. Citing the dearth of such studies (most on non-pregnant Asian and Arabic subjects), the investigation from China analysed 10,563 pregnant women recruited in Guangzhou. It was conducted between January 2013 and December 2015, and examined correlations between incense burning at home, hypertensive disorders,and blood pressure. Two characteristic findings were a higher prevalence of correlations in women with no smoking history and no dose–response relationship between exposure time and corresponding risk factors. It is interesting to note here that in the absence of CS exposure, the incense threats were substantially greater. This trend could have been due to prior exposure to more toxic CS, as the toxic constituents common to ISs and cigarette are present in low concentrations in the former (highlighted in earlier 1998 and 2004 studies). More details of this study are discussed in Recent Attempts Towards Assessing the Incense Combustion Toxicity.60
The aforementioned research suggests that harms and risks of IS exposure are a consequence of chronic exposure, ie, habitual use for ≥10 years, but more detailed studies are needed to confirm this. PM from polluted air, especially from incense-filled areas, on inhalation invades the respiratory tract and interacts with epithelial proteins.39 PM may interfere with the native organisation of the respiratory airway tract, obstructing airflow and causing obstructive sleep apnea. In a noted 2021 study, Sastry et al assessed incense-combustion effects on wheeze- and sleep-related symptoms among young Indian children.61 ISs were found to be detrimental to the oral microbiota of humans, as also found in other research.62 Renal complications of IS burning have also been reported in studies demonstrating impaired kidney functioning in albino rats aggravating nephrological defects.63 Geng et al reported a risk of end-stage renal disease (ESRD) among Chinese citizens in Singapore who had burnt ISs for a prolonged duration. The study included 63,257 residents aged 45–74 years, and was conducted from 1993 to 1998. Nearly 77% of examined cases were incense users, demonstrating enhanced severity with a usage history of >20 years. The study concluded that long-term daily exposure to ISs could lead to a higher risk of ESRD in the general population.64
Toxicity Risk from Incense Stick–Ash Disposal
IS ash (ISA) generated subsequent to ISs burning is generally very high in volume, but has no immediate significant utility. Its composition varies amongst brands, depending on the fragrance material added. Incense burning is the major source of ISA, the chemical composition of which is a function of varying levels of wood chips, fragrance material, coal powder, and adhesive material in the parent ISs.65 Studies have found it to be a mixture of manifold OCs with amorphous particles due to a majority presence of organic carbon. IS powders are primarily volatile materials comprising oils, perfumes, and several other carbon sources.1,66 Generation of ISA presents a serious waste-disposal issue, particularly in Asian countries (including China, Taiwan, India, and Japan). Since the incense is mainly used for worshiping God or deity, so one cannot prefer the fragrances obtained from various animals after killing or harming them. It is pertinent to mention here that results of previous studies on ISA reuse suggest it is a valuable adsorbent material with efficient absorption of toxic dyes and recalcitrant material from waste water.67
Similar options include the use of ISA as a processed fertilizer additive, filler enhancement in making roads, and composites. However, religious ignorance and pitfalls of governmental policies have made ISA disposal a significant risk to environment quality, with its persistent discharge in rivers and other water bodies.68,69 Even though this practice varies on the basis of religion, Hinduism does not follow this habit to a large extent, albeit no research can be found on this.69 In many Indian temples, ISA is applied on the forehead as a blessing. ISA is compositionally characterised by a high content of Ca, Si, and K (as oxides), Mg, Fe, and Al up to 5%, and traces of Ti, Se, Mn, and Cu.70 In addition to these, ISA also has certain heavy metals (including Cu, Cr, Ni, Co, Cd, As, Hg, Ni, Zn, Mo, and Pb);71 therefore, unchecked ISA disposal into water bodies can deplete the aquatic population. In 2021, Yadav et al extracted iron, calcium, alumina, and silica from ISA waste using magnetic separation, HCl, H2SO4, and NaOH treatments, respectively.69 They moderated the toxicity of native ISA waste through sequential methodology and demonstrated the possibility of safe discharge into water bodies. The following paragraphs illustrate the prospective health risks of ISA heavy metals and metallic oxides to aquatic life.
Risks of Heavy-Metal Exposure to Aquatic Life
Major heavy metals in ISA are Cu, Cr, Ni, Co, Cd, As, Hg, Ni, Zn, Mo, and Pb, with varying levels on the basis of incense type. Irrespective of the net limit, exposure of plants and animals to even minute levels of these moieties is threatening. The major sources of heavy metals in ISA are coal or charcoal powder, facilitating smoother burning.72 Noted physical properties of coal powder in ISA are the moisture content, particle mass, glass composition, and the relative proportion of unburnt carbon particles, which depends on coal-combustion temperature, coal-pulverisation size, and combustion rate.73 Moreover, Indian incense manufacturers include calcium phthalate for smoke minimisation, letting Ca, Fe, Al, and Si prevail as a calcium–ferro–aluminosilicate complex, recoverable using mineral acids and bases.
Often, the risks posed by persistent low heavy-metal content are not noticed in the short term and the damage associated is of a chronic nature. Major complications arising from persistent heavy-metal exposure include genetic mutations, increased risk of cancer development and in extreme cases or long time spans, even entire impairment of one or more organs. Elevated oxidative stress and stone formation are accompanying risks for aquatic life. If water containing these heavy metals is used for irrigation, there is mounting risk of such pollution manifesting in multiple food chains in the vicinity, whereby the ecological balance can be in dire straits. As such, there must be strict legal curbs on discharging ISA and its different complex forms into water bodies. Pretreatment aimed at reducing the recalcitrant pollution load of discarded ISA is significant, for it provides a cost-effective means of recovering multiple industrial materials and conserving nature.
Risks of Alkali Metals (Ca, Mg, and Na)
Though certain amounts of Ca, Mg, K, and Na are needed by living creatures, ISA discarded into water results in an impure source of these moieties. The chemical texture of these materials in ISA is highly injurious to the functioning of our physiological systems, more critically for respiratory and excretory systems. The major threat of disposing ISA in rivers or water bodies is high Ca and Mg concentrations (>50% of their oxides) with low levels (5%–12%) of Na and K oxides, varying by incense brand. Though all these alkali metal oxides form hydroxides on mixing with water, CaO and MgO exclusively form hydroxides with water, which increases the pH of an aquatic habitat. These entities also increase the alkalinity of fresh water, making it unfit for drinking. Both these elements are responsible for increasing the alkalinity and hardness of fresh water, ultimately disturbing an ecological system due to increased pH and alkalinity.69,73 These factors point to a need for careful selection of incense type (characteristic constituents) and subjecting ISA to feasible recovery methods before its disposal. Disturbances posed by increased alkalinity of water resources are highly injurious to the underground water table and agricultural produce. As a result, unattended ISA discharge into water bodies could jeopardise not only life sustenance but also the economy and overall progress.
Recent Attempts to Assess Incense-Combustion Toxicity
The risks of incense burning are mainly attributable to threatening levels of PM comprised of manifold incompletely combusted carbon particles and derivatives. These substances are extremely toxic to human and animal health, and present continued and mounting risks to the environment. As discussed earlier, an IS on burning produces >45 mg•g−1 particulates compared to 10 mg•g−1 for cigarettes. It is thus highly urgent to systematically examine the major health-care abnormalities associated with incense combustion. This could streamline the framing of guidelines and jurisdiction regarding safer incense design and moderation in use on a global scale. Table 4 presents studies related to incense-smoke toxicity in 2015 and 2016. The following paragraphs present a critical discussion of recent in vivo studies drawn from PubMed assessing incense-combustion toxicity.
Table 4.
Incense-smoke toxicity in distinct in vivo experimental settings
| Theme of study | Analysis of subjects | Salient findings | Reference |
|---|---|---|---|
| 2015 | |||
| Short-term effects of oud incense (popular as agarwood perfume in Saudi Arabia and UAE) on larynges and voice acoustics | 72 volunteers (55.6% female) were examined on 5 min exposure to incense smoke, mean age 27.6 years, volunteers sat 1 m from an electrical sensor in a closed room, effects analysed via monitoring throat conditions and breathing difficulties | Exposure-duration severity was noticed in terms of throat malfunction and breathing difficulty. Throat burning was majorly noted in female subjects, but throat dryness and breathing difficulties were observed in both sexes | [74] |
| 2016 | |||
| Chronic effects of incense smoke (bakhur and oud brands) on renal function and architecture | Albino rats, divided into bakhur, oud, and charcoal groups, housed separately, incense exposure via whole-body access by burning 4 g incense on self-burning charcoal, analysed via anaesthetising and euthanising eight rats from each group on days 30, 60, and 90 | Significant enhancements in serum creatinine, blood urea nitrogen, uric acid, interleukin 4, TNFα, ultrastructural changes in kidney, elevated CYP1A1 and CYP1A2 gene expressions | [63] |
| Impact of incense combustion and household exposure on lung function | Adolescents recruited for mass asthma screening, status retrieved via parent/student questionnaires, valid lung function of 5010 students aged 14–16 years assessed through forced vital capacity (FVC) and forced expiratory velocity (FEV1) | Nearly 70.6% of the adolescents exposed to household incense smoke on daily basis exhibited lower FVC and FEV1, sharing bedrooms also decreased FVC and FEV1 | [59] |
| Particle texture of PAHs on burning three incense-stick types (two from Taiwan and one from Japan) assessed for bioreactivity | RAW264.7 murine macrophage cell lines administered with 16 PAHs, 0, 3.125, 6.25, 12.5, 25, 50 and 100 μg•mL−1 incense-particle extracts | PAH 137.84–231.00 pg•μg−1 and total toxic equivalent (6.73–26.30) pg•μg−1. Analysis after 24 h revealed enhanced TNFα and N generation with reduced viability, similar observations for cells treated with particles, harvested via smouldering environment-friendly incense brand | [75] |
The studies discussed are exclusively in vivo analyses and are summarised in Tables 4–7 on yearwise basis. Despite our best attempts to be restrictive, multiple studies on such areas as pollution marginalision, morphology- and particle size–dependent health concerns, and indigenous surveys of rural populations have been included. Studies of this nature are listed in Table 8. In 2015, Mesallam et al studied the short-term effects of oud incense on the larynx function and voice acoustics.74 Oud is an incense analogue used in Saudi Arabia, although it is also popular as agarwood perfume in Saudi Arabia and the United Arab Emirates.76 Volunteers (72 adults, 55.6% females) were assessed after 5 minutes’ oud incense–smoke exposure. They sat 1 m from an electrical sensor in a closed room, nullifying the effects of charcoal burning.74
Table 5.
Salient 2017 studies discussing incense-combustion toxicity across multiple in vivo settings
| Theme of study | Analysed/observed | Salient findings | Reference |
|---|---|---|---|
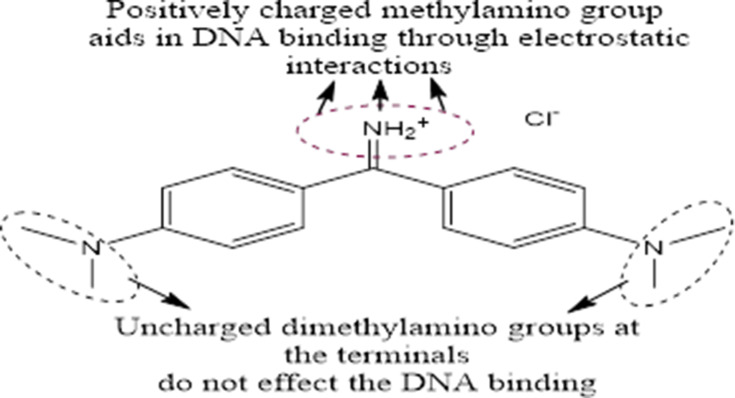
| Role of auramine O (a hydrophilic hydrophobic dye in incense-burnt condensate) in agarwood and sandalwood incense brands in lung cancer aggravation | Non-tumorigenic bronchial epithelial (BEAS2B), cultured in DMEM/F12 medium and human lung adenocarcinoma (CL1-0 and A549) cell lines, cultured in RPMI 1640 media, in vivo activity assessed via matrigel-mixed, AuO-treated, and control A549-cell injection into 5-week-old male BALB/c nude mice, left flank injected with control and right with AuO-treated A549 cells, tumour size monitored weekly using caliper, tumours became visible in 6 weeks | AuO accumulation was observed in nuclei within tumour cells, ascertained via confocal microscopy, no similar observations were noted for BEAS2B cells till 4 hours. Analysis using molecular modelling revealed a key role of AuO methylamino functionality in DNA binding, AuO-treated A549 cells exhibited autophagy (granular surface morphology) with enhanced LC3B II: LC3B I for 5-day AuO-exposed A549 cells. AuO-treated A549 cells exhibited 33 proteins with greater than fivefold expression and ALDH1A1 induced enhanced metastasis. No similar observations were noted for normal BEAS2B lung cells | [77] |
| Study of household incense exposure and aggravated respiratory complications in dogs and rats | 230 dogs and 118 cats assessed for >12 months after incense exposure at National Taiwan University Veterinary Hospital, clinical treatments performed by veterinarians on all animals with an unbiased recording of respiratory disease status and diagnosis factors | Dogs with respiratory disease were vulnerable to incense combustion, key role of body weight, no significant difference in the PM2.5exposure between diseased and normal dogs, age and body condition instrumental in incense risk, household cats with >35 μg•m−3PM2.5 exposure more vulnerable than dogs to respiratory disease, milder outcomes in cats suggested to be due to distinctly programmed metabolic control or gene set-up | [78] |
| Direct consequences of smoking and inhaling herbal incense via smoking tool. Screening of cannabinoid toxicity via hypothermia, analgesia, and akinesia, suppressed locomotor actions in three animal experiments, human CB1-receptor expression changes n Chinese Hamster ovary cells, expressing human CB1 Gα16 and mitochondrial apoaequorin protein | First experiment placed the mice in an activity chamber for 30 min before administering cannabinoid. UR144 was delivered intraperitoneally via 1:1:48 vehicle of ethanol, Kolliphor, and saline. Second group implanted with NanoTag to monitor locomotor activity. Assessed 5 min before and 10 min after administration, revealing hypothermia at 5, 30, and 60 min treatment. Third group analysed for specific time to initiate a movement (akinesia), placing mouse forepaws on a 6 mm–diameter metal bar suspended from 3.5 cm tabletop. Intensity monitored by time taken to bring both forepaws down to the tabletop, with a 1 min cut-off. Tests conducted before and 15, 30, 60 min post-treatment. | UR144 degradant induced considerable hypothermal activities for longer than UR144. Analysis found fourfold agonistic activity for CB1 receptor with UR144 degradant, together suggesting UR144 agonistic activity towards the CB1 receptor. The UR144 degradant induced excitation in rats, noticed via ramping and jumping during initial stages. Hypersthenia in similar mice was monitored using an acceleration sensor. Response was quite similar to cannabinoid administration to drug abusers (resulting in agitation, aggression and tachycardia). CB1 involvement was counter-confirmed by reduced episodes following CB1-receptor agonist, AM251 delivery post–synthetic cannabinoid treatment | [79] |
Table 6.
Salient 2018 studies reporting incense-smoke toxicity in distinct in vivo settings
| Theme | Analysed/observed | Salient findings | Reference |
|---|---|---|---|
| Study of long-term incense exposure on carotid intima–media thickness (CIMT) | 132 adults aged ≥35 years, subdivided into unexposed, non–daily exposed and daily exposed groups. Daily and non–daily exposed were distinguished via <5 or >5 day weekly exposure, collected blood samples assessed for total cholesterol, triglycerides, high- and low-density lipoproteins, HbA1c, and high-sensitivity CRP. Before blood collection, body-mass index was determined. All analysis and measurements were done as per American College of Cardiology and Heart Association guidelines. | Higher combined mean CIMT (0.75±0.2 mm) and combined maximum CIMT (0.93±0.23 mm) of left common carotid artery (LCCA) in daily exposed group than non–daily (mean CIMT 0.68±0.15 mm, maximum CIMT 0.85±0.18 mm) and unexposed groups (mean CIMT 0.64±0.11 mm, maximum CIMT 0.80±0.14 mm). Daily exposed group had 0.74±0.21 mm mean and 0.91±0.25 mm maximum. For non–daily and non-exposed groups, levels were 0.70±0.14, 0.88±0.18, 0.67±0.13, and 0.85±0.16 mm. Despite this, no significant difference was observed amongst the three exposure groups. | [46] |
| Study of incense-smoke exposure risks in Romanian citizens with respect to living conditions and household design | Self-reported information on respiratory symptoms in 280 elementary schoolchildren. Categorisation amongst allergy, asthma, and flu-like symptoms. Tobacco smoking, cooking (iron stoves), and poorly controlled indoor climate (random incense burning) identified as indoor pollution sources. | Tobacco smoke aggravated asthma and allergy, living near pesticide-sprayed areas and incense-making industries aggravated risk of asthma, incense combustion more popular than using room fresheners in Romania. Incense-smoke indoor air pollution increased the risk of allergy and flu-like symptoms. | [80] |
| Mechanistic study of aggravated cardiovascular conditions in incense-exposed rats | 7- to 8-week-old male albino rats (Rattus norvegicus) procured from Animal Care Center, College of Pharmacy, King Saud University. Kept in ambient temperature with 12 h light–dark cycle, free water and chow diet. Control (8) and bakhur and oud groups (16 each). Exposure to incense for 30 days via whole-body access on a daily basis. Afterwards, rats from each group were anaesthetised, while the remaining 16 from the two incense-exposed groups were kept away from incense smoke for 30 days and killed after monitoring. Physiological markers assessed via plasma analysis. | Enhanced malondialdehyde with balanced SOD and reduced GSH. Endothelial functional markers: NO decreased and ET1 increased in both incense-exposed groups. Enhanced chemokine and inflammatory mediator expressions, including MCP1, granulocyte macrophage GM-CSF), and all endothelial cell– adhesion molecules. Implicit incense-smoke involvement assessed via reversal of symptoms on 30-day cessation of incense exposure. | [63] |
| Study of incense-smoke exposure on the health of pregnant women, monitored via analysis of hypersensitivity and blood pressure | 10,563 pregnant women from Guangzhou cohort study from January 2013 to December 2015. Information on duration and frequency of incense exposure in early and late pregnancy collected using a questionnaire. Details on outcome variables, including diagnosis of hypersensitive disorders and blood-pressure levels, were retrieved from medical records. | Higher hypersensitivity prevalence in women exposed to incense smoke in late pregnancy, with 1.84 as relative risk factor and 95% confidence, similar observations for blood pressure (before delivery, 1.6 mmHg increment in systolic blood pressure (95% CI 0.4–2.8 mmHg), correlations more significant in women with no history of active/passive smoking. | [60] |
Table 7.
Studies reporting incense-smoke toxicity in varied in vivo settings
| Theme of study | Analysed/observed | Salient findings | Reference | ||
|---|---|---|---|---|---|
| 2019 | |||||
| Analysis of incense-smoke effects on humans via chronic exposure and susceptibility to end-stage renal disease (ESRD) | 63,257 Chinese residents aged 45–74 years, recruited from 1993 to 1998. Information on household incense burning, diet, lifestyle, and medical history was collected via baseline interviews. | 76% of cohort participants followed up for 17.5 years, accounting for 1217 ESRD sufferers. Aggravated risk of ESRD in daily users with >20 year usage history showed an HR of 1.25, while never-users exhibited no significant ESRD risk. Besides PM, groundwater quality, vegetation, and food texture were major ESRD risk factor. | [64] | ||
| 2020 | |||||
| Application of a low-cost sensor, AS-LUNG-P to screen severity of multiple PM2.5 sources of heart-rate variations | 36 nonsmoking Taiwanese individuals aged 20–65 years were assessed | Environmental tobacco smoke, incense burning, and cooking as three PM2.5 sources, with respective generation of respective heart-rate increments of 8.35, 5.85, and 3.52 μg•m−3. Considerable heart-rate variability in healthy adults, even at PM2.5 exposure. | [81] | ||
| Evaluation of the effects of indoor incense combustion on cognition over 3 years, correlation of indoor incense burning with brain’s structural and functional connectivity of default-mode network and fate of incense-combustion materials —vascular marker interactions on cognitive functioning | Older family members with >5 years of incense-burning history for >1 week, levels of six outdoor air pollutants, including fine suspended particulates, O3, SO2, multiple nitrogen oxides, and respiration compatible suspended particulates, were measured on an hourly basis in 13 Hong Kong districts. Cognitive assessment was performed by trained research assistants using Montreal Cognitive Assessment test. Brain MRI was performed at baseline using a 3 T Philips MRI scanner equipped with an 8-channel head coil. | Exposure to indoor incense combustion resulted in poorer cognitive performance over 3 years. Functional brain changes, reduced cognitive resilience via altered functional connectivity, consequential aggressive cognitive decline, and predisposition to poor cognitive functioning were observed. Enhanced manifestation of vascular impairment due to predisposal to poor cognitive functioning. | [22] | ||
| 2021 | |||||
| Study of harmful effects of five tobacco varieties and incense in Hong Kong | Human alveolar epithelial cells (A549) were exposed to PM2.5 released from multiple indoor activities. | Higher emission-factor profile (109.7±36.5 mg•g−1) than incense smoke (97.1±87.3 mg•g−1). Major health issues with both were aggravated oxidative damage, inflammatory aggravation, activation of 8-hydroxydeoxyguanosine, TNFα, and IL6. High–molecular weight PAHs from incense combustion strongly correlated with DNA-damage markers. | [82] | ||
Table 8.
In vitro attempts to evaluate incense-combustion toxicity
| Study theme | Salient observations | Conclusions | Reference |
|---|---|---|---|
| Effect of temperature, relative humidity, and air-exchange rate on emission of 13 VOCs and semi-VOCs amidst incense burning where the release smokes have mainly gaseous phase including highly volatile organic compounds and polyaromatic hydrocarbons | Studies were performed in an environmental test chamber at 20°–130°C, relative humidity 5%–95% and 0.1–2/h air-exchange rate. Inner walls of test chamber were made using electro-polished stainless steel to minimise surface area and potential effects of organic-wall losses. No material of the chamber was plastic. Inlet flow-rate was 30 L•min−1. Incense was placed on a shelf in the centre and ignited using a lighter. | Analysis revealed increased emission with increasing ventilation. Air-exchange rate was the most sensitive parameter affecting formaldehyde, benzene (both carcinogenic), and diisobutyl phthalate (all PAHs) emissions. Temperature was the most sensitive factor for chrysene emission. Continued exposure resulted in benzene and benzo(a)pyrene emissions to close to or higher than air-quality standards. | [83] |
| Quantitative profiling of PM generated from incense smoke in Kanpur temples | PM10 mass concentrations as high as 2184 μg•m–3 was estimated within temple premises, exceeding the CPCB-recommended threshold of 100 μg•m–3. PM10 for the temple with highest attendance was 2336 μg•m–3. Average PM2.5 in the estimated PM10 load was 75%–92%. Data from normal days, so risk could be higher. Majority in accumulation state, particularly in winters. | Incense combustion–generated PM2.5 is a significant contributor to air pollution, with unpredictable and increased risks aggravated by seasonal variations and poor ventilation. | [11] |
| Shape-, texture-, and packaging-based VOC-emission patterns of liquid, mat, and disc configurations of three incense brands | Analysis using GC-MS showed 7.760±4.724, 3.122±0.866, and 1.192±4.724 mg/m3 smoke release for disc, liquid, and mat configurations. 14 VOCs assessed in incense smoke. Most were alkanes, followed by aromatic hydrocarbons and esters. Other major constituents were TSPs, PM10, PM5, PM2.5, allethrin, phenol, benzene, toluene, and xylene, along with multiple aromatic and aliphatic hydrocarbons. | Surface area (disc configuration had highest smoke release) is a key factor affecting homogeneous distribution and combustion of constituents in an incense stick. Most critical effects of incense burning were respiratory complications, inflammatory disorders, impaired nervous system functioning, and DNA damage. Carcinogenic health risk via BTEX (benzene, toluene, ethylbenzene, and xylene) exposure was highest. | [84] |
Analysis was done before and after oud exposure, with concurrent effects of throat burning, throat dryness, and breathing difficulties ascertained. Though no significant distinctions were noted between pre- and post-oud exposure, still 20 candidates exhibited voice and airway effects after exposure. A shortcoming of the study was the small sample and no control group, and the investigators emphasised the need for studying long-term oud-inhalation effects on voice attributes. Significantly, a majority of the subjects were young (mean age 27.6 years). The findings pinpointed serious issues with an increase in exposure duration and advanced age. Noted aspects exhibiting distinctions were frequency variation, jotter shimmer, and noise:harmonic ratio, revealing a sex-independent modulation unlike that of throat burning, which was exclusively in female subjects. On the other hand, throat dryness and breath inadequacy were noted in both sexes. The risks of incense smoke also showed sex sensitivity, with the reason given that most of the women were housewives and burning incense at home during the day.74
In 2016, Hussain et al studied the chronic effects of incense smoke on renal function and architecture.63 The study was conducted on albino rats and monitored via modulated kidney-function markers, oxidative stress, and inflammatory. Modulated kidney morphology and gene expression of CYP1A1 and CYP1A2 were assessed using TEM and RT-PCR. Considerable enhancements in serum creatinine, blood urea nitrogen, uric acid, IL4, and tissue malondialdehyde (TNFα) were seen in the incense smoke–exposed rats. Ultrastructural changes were noted in the kidney, along with elevated CYP1A1 and CYP1A2 expression. These observations suggested oxidative stress and inflammation triggered variations in kidney-function markers. To study the effects of incense, the rats were divided into charcoal, bakhoors and oud groups after 15 days’ acclimatisation. Each group was housed separately so as to nullify cross-exposure. Rats from the bakhoor and oud groups were exposed to incense smoke via whole-body access upon burning 4 g incense on self-burning charcoal. Animals from the charcoal group were exposed to emissions from burning charcoal. For analysis, eight rats from each group were anesthetised and euthanised on days 30, 60, and 90. This study confirmed the predictions of a 2015, wherein long-term exposure was observed to affect non-pulmonary tissue also.74 Therefore, the significance of incense combustion in deteriorating health is indeed crucial and requires urgent attention. The relevance of incense-combustion threat assumes more significance given its use in mosquito repellent. One wonders how a material posing multiple health risks can be ethically justified in worship.1
A 2016 Taiwanese study examined the effects of incense burning and similar household exposure on lung functioning. The subjects were adolescents who contributed in a mass asthma–screening program and whose asthmatic status had been obtained (parentstudent) completed questionnaires. Nearly 10% received lung-function examinations, and valid lung-function data of 5010 students aged 14–16 years were obtained. Analysis was done using forced vital capacity (FVC) and forced expiratory velocity in 1 second (FEV1) comparisons with incense-burning status. Compromised lung function was suggested by decreased FEV1, enhanced FVC, and FEV1:FVC ratio <0.7 Overall, 70.6% of those exposed to incense smoke on a daily basis exhibited significantly lower FVC and FEV1. The statistical significance of correlating parameters was confirmed via negative correlations of FVC and FEV1 with incense-burning exposure. Sharing a bedroom with incense smoke–exposed individuals decreased FVC and FEV1 in unexposed subjects. Correlations of reduced FVC and FEV1 with incense-smoke exposure were inferred by choosing 1000 non-smoking students with no ill effects from asthma or allergic rhinitis. Analysis was conducted as per the standard norms of the American Thoracic Society, wherein height and body weight were noted prior to lung-function assessment for body-weight index.59
Another 2016 study was again from Taiwan, wherein the effects of particle texture of PAHs upon burning three distinct ISs were examined for their bioreactivity in the RAW264.7 murine macrophage cell line. Sixteen PAHs were identified before exposing the macrophages to 0, 3.125, 6.25, 12.5, 25, 50, and 100 μg•mL−1 incense-particle extracts. After 24 hours, cell viability, nitric oxide, and inflammation levels from TNFα expression were measured. The PAHs and total toxic equivalent (TEQ) mass fraction of the incense types were 137.84–231.00 and 6.73–26.30 pg•μg−1, respectively. The incense smoke–exposed RAW264.7 macrophages exhibited enhanced TNFα and NO generation with reduced cell viability. Surprisingly, higher NO and TNFα generation was observed in cells treated with particles harvested from smouldering the environment-friendly incense brand. Enhanced inflammation was positively correlated with the TEQ of fluoranthene, pyrene, benzoanthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, indeno[1,2,3-cd]pyrene (INP), dibenz[a,h] anthracene and benzo[ghi], for which correlation coefficients were 0.64–0.98. This study demonstrated the biologically active nature of incense particle–bound PAHs with the observation that incense with low H:C ratios caused higher bioreactivity.
It is pertinent to note here that smouldering incense generates hugely detrimental pollutants, such as methanol, methane, ammonia, ethylene, and formaldehyde. Such attributes are substantially due to incomplete incense combustion yielding hazardous VOCs, comprising benzene, 1,3-butadiene, furan, and semi-VOCs, including a significant proportion of PAHs. The investigators used their own combustion system for incense burning, wherein airflow was controlled at 6 L per minute and moderate relative humidity maintained 50.3%±0.4% at a constant temperature (27.7°±0.6°C). The temperature (28.0°±0.9°C) and relative humidity (49.2%±1.2%) in the analysis chamber remained constant and the sample inlet was 83 cm above the burning incense tip. The authors analysed environment-friendly incense from Taiwan, very low smoke–generating binchotan charcoal incense from Japan, and standard-smoke laoshan incense from Taiwan. The reason for selecting these incense types was low weight and length variations compared to other available brands. The results were distinguished through lowest H:C ratio, H content, and highest C extent, with higher total PAH and TEQ mass-fraction levels. During burning or smoldering of the eco-friendly incense stick, a much less amount of PAH mass concentration and emission factor was obtained in the particulate phase. Of the 16 PAHs generated, seven were probable human carcinogens, all exhibiting a positive correlation with NO and TNFα.75
In 2017, Tung et al assessed the role of auramine O (AuO), a water-soluble fraction of incense-burnt condensate, via mass spectrometry. AuO is a diarylmethane hydrophobic dye yellow needle-like crystals that is added as a colorant in incense manufacture (Figure 3). Eventually, its thermostable nature makes it an air pollutant. The incense brands chosen were agarwood and sandalwood ISs purchased from vendors in the vicinity of Dajja Jenn Lann temple, while those from a local manufacturer were used as controls, having no AuO content. The cell lines analysed were the non-tumorigenic BEAS2B in DMEM–F12 and the human lung adenocarcinoma cell lines CL1-0 and A549 in RPMI 1640 medium. To examine AuO tumorigenic activity, AuO-treated and control A549 cells were mixed with Matrigel before injection into 5-week-old male BALB/c nude mice. The left flank was injected with the control, while the right was injected with AuO-treated A549 cells. Tumour size was monitored using calipers on a weekly basis once the tumours had become visible after 6 weeks.
Figure 3.
DNA binding receptivity of auramine O, a sensitive fluorescent stain added as colourant to incense.
Intracellular AuO persistence was ascertained using confocal microscopy. AuO firstly entered tumour cells and accumulated within nuclei in 2 hours. No such translocation was noticed in BEAS2B cells until 4 hours, suggesting a higher resistance of normal lung cells. Observations of A549 tumour cells revealed a DNA-binding potential for AuO, which was further analysed using molecular modelling. Inspection revealed a major role of AuO methylamino functionality for its DNA binding, remaining unaffected by the dimethylamino group (Figure 3). Similarly, the iminum group of AuO caused no change on its interaction with double-stranded DNA. Isothermal titration calorimetry was used to quantify the DNA-binding affinity of AuO wherein thermodynamic parameters assessed variations in Gibbs free energy in AuO–DNA interaction. Analysis revealed nuclear translocation and DNA-binding potential of AuO, specifically within lung-tumour cells. Autophagy induction in the AuO-treated A549 cells supported these observations, as BEAS2B cells exhibited no morphological change on being maintained in AuO-supplemented culture media. In contrast, the AuO-treated 549 cells had a granular structure at the surface, confirmed by AuO-induced autophagosomes via acridine-orange staining. AuO-treated A549 cells displayed a stronger orange–red conversion of fluorescent signals than BEAS2B cells.
These observations were further supported by LC3B protein expression, registering increments in LC3 puncta contrary to the controls. Increased LC3B II:LC3B I ratio was noticed for 5-day AuO-exposed A549 cells, while no such distinctions were observed for BEAS2B cells. AuO-treated A549 cells showed 33 proteins, with more than fivefold the activity of normal cells. The investigators monitored malignancy in ALDH1A1-knockdown A549 cells, using shRNAs. Eastern blotting confirmed that the shRNAs induced ALDH1A1 expression with considerable AuO suppression that induced migration and invasion in A549 cells. These findings were complemented by suppressed autophagy following ALDH1A1 knockdown, suggesting ALDH1A1 involvement in regulating the AuO-induced lung cancer metastasis.77
In a 2017 study from Taiwan, the effects of indoor air pollutants were studied in 230 dogs and 118 cats. Though incense combustion is an exclusive contributor to indoor air pollutants, several other sources add to deteriorating respiratory conditions. For instance, PM2.5, which gains easy access via the respiratory route, remains trapped in the bronchiolar alveoli and aggravates oxidative stress. The study analysed dogs and cats at the National Taiwan University Veterinary Hospital for >12 months. Clinical treatments were administered by veterinarians on all animals, with an unbiased status recording of respiratory disease and diagnostic factors. Analysis revealed that dogs with respiratory disease were more vulnerable to incense-burning exposure than their counterparts, although there was no significant difference in exposure to PM2.5. The metabolic and physical parameters of body weight, age, and body condition were substantially responsible for the respiratory diseases in dogs. Contrary to this, for cats PM2.5 exhibited a more deleterious effect in terms of respiratory complications. Household cats with >35 μg•m−3 PM2.5 were more vulnerable to respiratory disease than the others (also household), having acceptable PM2.5 levels. Since incense combustion is a significant PM source, the observations suggest its strong role in respiratory complications in cats. The difference observed in dogs might be due to distinctly programmed metabolic control or the genetic set-up for the two. The results of this study present significant insights and also a need for further investigation of incense-combustion effects in animal models.78
A 2017 study from Japan analysed the direct consequences of smoking or inhaling herbal incense using a smoking tool. The study examined cannabinoid toxicity in three animal experiments. The synthetic cannabinoid UR144 generates UR144 degradant on burning. In general, such a response is characterised via hypothermia, analgesia, akinesia, and suppressed locomotor activity. The first animal experiments placed mice in an activity chamber for half an hour before administering the cannabinoid for habituation. The UR144 or UR144 degradant was dissolved in 1:1 ethanol and Kolliphor mixture, which was thereafter diluted with saline to get a 1:1:48 vehicle ratio of ethanol, Kolliphor, and saline. The vehicle (10 mL•kg−1), UR1444, or UR144 degradant was injected intraperitoneally into the mice. A second group of mice were implanted with a Nano-Tag, an instrument that assesses locomotor activity, in their backs. Assessment was made 5 minutes prior to implantation and terminated 10 minutes after administration. Body temperature was used to assess hypothermia grade and rectal temperature as an estimator of total-body heat before and at 5, 30, and 60 minutes after treatment using a small animal warmer and thermometer.
The third experimental setting examined akinesia or catalepsy, the characteristic time needed for initiating a movement. Analysis revealed gentle placement of both mouse forepaws over a 6 mm–diameter metal bar suspended 3.5 cm above a tabletop. Akinesia intensity was assessed by monitoring the time the mouse took to bring both forepaws down to the tabletop, with a 1-minute cut-off. The bar test was conducted before and at 15, 30, and 60 minutes after treatment. Monitoring of altered human CB1-receptor expression was done using Chinese hamster ovary cells expressing the human CB1 receptor Gα16 and mitochondrial apo-aequorin protein. The UR144 degradant induced significant hypothermal activity for longer than UR144. The characteristic features of cannabinoid-mediated pharmacology (hypothermia, diminished spontaneous activity, and catalepsy) act through the CB1 receptor. As such, calcium-immobilisation assays were used to ascertain UR144 degradant binding to the CB1 receptor with a ligand ligation–enhanced intracellular Ca2+. Analysis revealed fourfold agonistic activity for CB1 with the UR144 degradant, suggesting UR144 agonistic activity of CB1.
These observations were supported by in vivo experiments wherein the UR144 degradant induced excitation via ramping and jumping in the initial stages. Hypersthenia-like mouse behavior (vertical movement) was monitored using a biotelemetrical system comprised of an acceleration sensor. UR144 degradant–induced transient excitation was successfully monitored using this system, exhibiting resemblance to the response of cannabinoid-administered drug abusers: agitation, aggression, and tachycardia. The investigators suspected such outcomes were the result of serotonin syndrome–like conditions. The involvement of the CB1 receptor in this study was confirmed by reduced excitation activities following CB1-receptor agonist AM251 delivery following synthetic cannabinoid treatment. This study illustrated the incense-smoke toxicity caused by direct inhalation.79
Interest in incense-smoke toxicity progressed further in 2018 with four significant studies determining toxicity via pathogenesis of manifold pulmonary and non-pulmonary complications. In the first of these, on Thai–Vietnamese subjects, the impact of long-term household incense–smoke exposure was assessed bsed on carotid intima–media thickness (CIMT). CIMT is a frequently used identifier of atherosclerosis and cardiovascular disease risk. Owing to its native insensitivity to short-term impacts, CIMT prevails as a decisive marker to ascertain the long-term cardiovascular risks of air pollution. The study analysed 132 adults aged 35 years or more subdivided into unexposed, non–daily exposed, and daily exposed groups on the basis of long-term household incense use. Participants in the daily and non–daily exposed groups were divided on the basis of <5 or >5-day weekly exposure to incense smoke, respectively.
Collected blood samples were subjected to phlebotomy and assessed for total cholesterol, triglycerides, high-density lipoprotein, low-density lipoprotein, haemoglobin A1c, and high-sensitivity CRP. Prior to blood collection, the volunteers were analysed for body-mass index, ascertained using autobalance. Blood pressure and heart rate were measured using an Omron monitoring device. CIMT measurements were made as per the American College of Cardiology and American Heart Association guidelines using a high-resolution B-mode ultrasound scanner. An adult cardiac 1.8–4.8 MHz linear array transducer with Micro-Convex was used, with offline export of images using Synapse PD-S Viewer version 1.0. Thickness was ascertained as both mean and maximum of the anterior, capturing the media–adventitia interface of far arterial walls. This was validated against histological specimens as representative of true vessel thickness, with 10 mm measurements to the bulb from the common carotid on both common carotid arteries. Mean and maximum CIMT in the left and right common carotid arteries of all participants were averaged to obtain overall mean CIMT and maximum. Analysis revealed higher combined mean CIMT (0.75±0.2 mm) and combined maximum CIMT (0.93±0.23 mm) of the left common carotid artery (LCCA) in the daily exposed group than the non–daily exposed (mean CIMT 0.68±0.15 mm, maximum CIMT 0.85±0.18 mm) and unexposed groups (mean CIMT 0.64±0.11 mm, maximum CIMT 0.80±0.14 mm). For the right common carotid artery (RCCA), the daily exposure group mean CIMT of 0.74±0.21 mm and maximum CIMT of 0.91±0.25 mm. Corresponding levels were 0.70±0.14 mm and 0.88±0.18 mm for the non–daily exposed group and 0.67±0.13 mm and 0.85±0.16 mm for the unexposed group. Despite higher levels for daily incense users, no significant difference was observed amongst the three groups. The study thus revealed a positive correlation between household incense burning and CIMT of the LCCA amongst >35-year-old Thai–Vietnamese, with no similar association for CIMT of the RCCA.46
The second 2018 study focused on incense smoke–exposure risk in Romanian citizens with respect to living conditions and house design. Investigation comprised a subsection of the SINPHONIE project, a Europe-wide cross-sectional study in which the role of indoor air–pollution exposure in occurrence of respiratory diseases was assessed. Statistical analysis was done using self-reported information on respiratory symptoms from 280 Romanian elementary schoolchildren, and categorisations made of allergies, asthma, and flu-like symptoms. Correlations between household living conditions and respiratory health were assessed using multivariate logistic regression controlling for indoor air exposure inside the school premises. Sources contributing to indoor air pollution were tobacco smoking, cooking (use of iron stoves), little control of indoor climate, and the traditional custom of incense burning. While tobacco smoke contributed to aggravated asthma and allergies, living near pesticide-sprayed areas and incense-manufacturing factories also contributed to the occurrence of asthma. Mold and dampness concerns, use of air conditioners, and gas heater/iron stove use were major factors in flu-like symptoms. The study highlighted policy-development findings, in which household environmental exposure was attributed to the deteriorating respiratory health of Romanian citizens. Significantly, the combustion of ISs was found to be more popular in Romania (~12%) compared to air fresheners, aggravating the incidence of allergy-like symptoms. Incense combustion was also assessed as a significant contributor for flulike manifestations. Though triple the allergy risk was noted in houses with frequent incense use, the investigators linked incense-combustion toxicity to household design and the purpose for which ISs were burnt. This was due to no observations of serious health issues in 1995 and 2003 studies on schoolchildren from two other countries. Incense burning was listed as a major contributor to allergies and flu-related symptoms, accompanied by a lack of ventilation in Romanian houses.80
The third study assessed mechanisms of aggravated cardiovascular complications in incense-exposed rats, pointing to ambiguity in prior research. The 7 to 8 weeks old male albino rats 7–8 weeks old were procured from the Animal Care Center, College of Pharmacy, King Saud University. They were kept in ambient temperature on a 12-hour light–dark cycle, with access to free water and chow for the entire duration.The animals were grouped on the basis of incense exposure into control, bakhur, and oud groups, with eight animals in each group. The groups were housed separately to avoid any cross-exposure risk. The rats exposed to the incense smoke were subjected to 30-day whole-body access on a daily basis. After 30 days, the rats in the control group were anaesthetised, while those in the two incense-exposed groups were kept away from incense smoke for 30 days and eventually killed after subsequent 30-day monitoring. To ascertain expression of physiological markers, blood samples were collected from all rats and plasma separated. Inspection revealed considerable enhancement in malondialdehyde, with proportionate SOD and GSH decrements. NO had decreased and ET1 increased significantly in both incense smoke–exposed groups. Elevations were also noted in expression of chemokines and inflammatory mediators, including MCP1 and GM-CSF, along with all endothelial celladhesion molecules. To confirm the implicit role of incense smoke exposure, a 30-day cessation of smoke exposure revealed a reversal in the levels of all physiological markers.63 In sum, this study found that oxidative stress, endothelial dysfunction, and inflammation were possible mechanistic factors in aggravating the toxicity of incense smoke. Such studies are highly crucial databases, and further study correlating the marker expressions observed with varying IS compositions could widen the understanding of component-specific toxicity.
A subsequent study analysed the effect of incense-smoke exposure on the health of pregnant women. The effects of incense were monitored through hypersensitive-disorder severity and blood pressure. The sample comprised 10,563 pregnant women recruited in the Born in Guangzhou cohort study in China from January 2013 to December 2015. Information pertaining to the duration and frequency of incense exposure in early and late pregnancy was collected using a questionnaire. Details on outcome variables, including diagnosis of hypersensitive disorders and blood-pressure levels, at the final antenatal visit before delivery were retrieved from medical records. Exposure–outcome correlations were examined using Poisson regression and general linear models. Prior to each measurement, participants were made to sit upright with back support for 5 minutes. Analysis revealed higher hypersensitive-disorder prevalence in the women exposed to incense smoke in late pregnancy, with relative risk of 1.84 at 95% confidence. Similarly, blood pressure in incense smoke–exposed women before delivery was substantially higher (1.6 mmHg increment in systolic blood pressure, 95% CI 0.4–2.8 mmHg) than the unexposed. Although no significant relationship was found between exposure time and risk of hypersensitive disorders, the correlations were more significant in women with no history of active or passive smoking.60 This observation presents an option for further study to assess associations between smoking and incense smoke chemical profiles. It may be that exposure to the latter has a similar voluntary trigger to that seen in smokers.
A 2019 Singapore study investigated incense-smoke effects on humans via chronic exposure and concurrent risk of ESRD. Subjects were 63,257 Chinese residents aged 45–74 years and recruited from 1993 to 1998. Data on household incense burning, diet, lifestyle, and medical history were collected in baseline interviews. ESRD sufferers were assessed using the Singapore Renal Registry during 2015. Statistical analysis of collected data was was done using Cox proportional-hazard regression analysis to estimate HRs with 95% CIs and correlations with household incense combustion. For a period of about 17.5 years, various participants were under observation and found that 75% were burning incense at their places out of which 1217 suffered from end-stage renal disease, accounting for 1217 ESRD sufferers. The multivariate-adjusted ESRD HR was 1.05 for former and 1.26 for current incense users (P<0.05) compared to never-users. In terms of frequency of incense use, aggravated ESRD risk was observed in daily users with >20-year usage history, leading to an HR of 1.25. On the contrary, the non–daily incense users (<20 years) exhibited no significant ESRD risk. As such, this study demonstrated long-term household incense-smoke exposure was a contributing factor to enhanced ESRD risk.64 However, the PM fraction of incense smoke does mean it is the sole ESRD risk factor, as possible contributions may come from similar particulate effluent. Secondly, the groundwater quality, vegetation, and food texture of the study location also emerged as crucial factors in ESRD aggravation, since renal health is related to metabolism and excretion.
Amongst the major 2020 in vivo studies analysing incense-smoke hazards, the first used a low-cost sensor, the AS-Lung-P, to assess the severity of multiple PM2.5 sources (generated by incense combustion) based on heart-rate variations. A total of 36 non-smoking Taiwanese individuals aged 20–65 years wore an AS-Lung-P and RootiRx for 2–4 days in summer and winter. Inspection revealed environmental tobacco smoke, incense burning and cooking as the top three PM2.5 sources, with the respective heart-rate increments of 8.35, 5.85, and 3.52 μg•m−3. A notable distinction was considerable HRV in healthy adults, even at low PM2.5 exposure. The sensor is workable via two modules: a wearable environmental device for analysing PM2.5 and a certified medical version equipped for HRV screening.
Subjects were selected from all of Taiwan except the western region, as it has varying PM2.5 exposure. Inclusion criteria were agreement to complete questionnaires, wearing the sensors, being >20 years in age, and no history of smoking or cardiovascular complications. Each subject was made to wear an AS-Lung-P near the chest with a strap or carry it in a bag with a protruding inlet for 2–4 days in different weather. Other than incense smoke (religious or mosquito-repellent), exposure sources included vehicle emissions, environmental tobacco smoke, cooking, and burning of agricultural waste. Various factors affected the HRV upon exposure to the studied stimuli, with vehicle emissions as the major contributor, not more localised tobacco-smoke exposure. The results remained varied for home, office, and other surroundings. For incense burning, respondents generally had household exposure to such activities such as static leisure, eating, and worshipping. They were instructed via telephone to monitor their stimuli at appropriate intervals. Analysis focused on mean exposure and inhalation levels, and correlations were statistically significant for each factor. This study also listed incense smoke as a significant PM2.5 source, exposure to which resulted in HRV.81
A 2020 study assessed incense-combustion impacts on cognitive functioning and neurological connectivity. Specific objectives were to ascertain the effects of indoor incense combustion on cognition over 3 years, unearth the association of indoor incense burning with the structural and functional connectivity of the DMN, and investigate the fate of incense combustion material–vascular marker interactions on cognitive functioning. The study population chosen had no history of stroke, dementia, or related conditions. Incense impact was ascertained by focusing on the older members of families with >5 years’ incense-burning history more than once a week. A 200–800 ft2 (19–74 m2) apartment with one living room and one to three bedrooms was chosen as the typical indoor household environment. To monitor incense-specific impact, levels of six outdoor air pollutants, including fine suspended particulates, O3, SO2, nitrogen oxides, and respiration-feasible suspended particulates, were measured on an hourly basis in 13 districts of Hong Kong. Cognitive assessment was done by trained research assistants using the Montreal Cognitive Assessment tool, which covers cognitive domains of learning, memory, executive and visuospatial function, language, attention, working memory, abstraction, and orientation. The tool is sensitive to mild cognitive dysfunction and cognitive change monitored over time. The total score was used as a global cognitive function index, and domain scores for memory, executive and visuospatial function, language, and attention were determined using an earlier standardised method.
Brain MRI was performed at baseline using a 3 T Philips MRI scanner equipped with an eight-channel head coil. The study analysed 515 participants. The mean age of non–incense users was 1.1 years older than their counterparts, and they were less susceptible to hypertension. Analysis revealed that regular exposure to indoor incense combustion resulted in poorer cognitive domain scores over a 3-year duration. Though users of incense did not show significant structural brain changes in terms of small vessel–disease lesions (imaging biomarker for Alzheimer’s disease), the impact of incense burning on brain functioning was evident via DMN functional connectivity. In general, the DMN is active in the resting state and during introspective, self-referential processing. Switching between the DMN and active brain networks regulates manifold cognitive events, and impairment in this area is a hallmark of multiple neurological and psychiatric conditions. Analysis revealed indoor incense burning as a factor behind functional brain changes, reducing cognitive resilience via altered functional connectivity and consequential vulnerability to future cognitive decline. Though the frequency of diabetes mellitus and hyperlipidaemia showed no significant changes, incense burning modulated the manifestation of these vascular complications, because of predisposition to poor cognitive functioning. This attenuation unearthed a decisive role of incense burning as a risk factor of vascular cognitive impairment. This suggests that further research on interactions between incense burning and cognitive change is needed.22
In 2021, Niu et al examined the harmful effects of five tobacco varieties and incense in Hong Kong using an environmental chamber. To monitor toxicity, A549 cells were exposed to PM2.5 released from several indoor activities. As expected, emission-factor profiling of environmental tobacco smoke showed higher values (109.7±36.5 mg•g−1) than incense smoke (97.1±87.3 mg•g−1). Major effects from both sources were oxidative damage, inflammatory aggravation, and activation of 8-hydroxydeoxyguanosine, TNFα, and IL6. High–molecular weight PAHs from incense combustion exhibited stronger correlations with DNA-damage markers, while almost all indoor PAH sources contributed to aggravated inflammation. Tobacco smoking and incense burning posed critical risks to the alveolar epithelium within the distal human respiratory tract, indicating enhanced cytotoxicity and lung cancer risk.82
Conclusion
We have presented a comprehensive account of incense-combustion risks affecting human health and the environment. The major health risks posed by incense combustion are respiratory and cardiovascular complications and a substantial proportion of allergic and dermatological issues. The choice of incense brand has a significant bearing on the nature of incense-combustion products. Therefore, policymakers and users should investigate the composition of the incense brand being used for worship. Apart from this, indirect toxicity of incense-combustion end products is a menace, and there is no proper documentation on its disposal. Appropriate caution must be exercised to reduce their deleterious content so that incense by-products can be disposed of without harming the environment. Since incense use for worship is a long-standing tradition, it would be better if incense brands for this purpose were made with easily degradable constituents and atoxic fragrance additives. A crucial inference drawn from our discussion is that PM is the most significant constituent affecting health, due to its minute dimensions. Future incense manufacturing should ensure that no unburnt carbon material or residue prevails upon combustion.
Funding Statement
The authors gratefully acknowledge the Deanship of Scientific Research, King Khalid University (KKU), Abha-61421, Asir, Kingdom of Saudi Arabia for funding this research work under the grant number RGP.2/140/43. This work was funded by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) of the Republic of Korea (No. 20210310100020).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article was submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Krishna Kumar Yadav reports nonfinancial support from Madhyanchal Professional University, Bhopal, Madhya Pradesh during the conduct of the study. The authors report no other potential conflicts of interest for this work.
References
- 1.Jetter JJ, Guo Z, McBrian JA, Flynn MR. Characterization of emissions from burning incense. Sci Total Environ. 2002;295:51–67. doi: 10.1016/S0048-9697(02)00043-8 [DOI] [PubMed] [Google Scholar]
- 2.Cai WH, Wong PPY. Associations between incense-burning temples and respiratory mortality in Hong Kong. Atmosphere. 2021;12:774. doi: 10.3390/atmos12060774 [DOI] [Google Scholar]
- 3.Bureau KHCEP. Reduction of pollutants in temples. In: Total Inventory Control of Air Pollutants and the Guidance Program for Reduction. Kao-Hsiong, Taiwan: Bureau KHCEP; 2003:6–9.7. [Google Scholar]
- 4.Lin TC, Krishnaswamy G, Chi DS. Incense smoke: clinical, structural and molecular effects on airway disease. Clin Mol Allergy. 2008;6:1–9. doi: 10.1186/1476-7961-6-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apte K, Salvi S. Household air pollution and its effects on health. F1000Res. 2016;5:F1000 Faculty Rev–2593. doi: 10.12688/f1000research.7552.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moeran B. Making scents of smell: manufacturing and consuming incense in Japan. Hum Organ. 2009;68:439–450. doi: 10.17730/humo.68.4.q570w7g1684u412t [DOI] [Google Scholar]
- 7.Lin JM, Tang CS. Characterization and aliphatic aldehyde content of particulates in Chinese c. Bulletin of Environ Contam Toxicol. 1994;53:895–901. doi: 10.1007/BF00196221 [DOI] [PubMed] [Google Scholar]
- 8.Shrestha O. Incense stick: an overlooked source of health hazard. JNMA J Nepal Med Assoc. 2020;58:823–825. doi: 10.31729/jnma.5286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Chen W, Li J, Yu S, Zhao W. VOCs and particulate pollution due to incense burning in temples, China. Procedia Eng. 2015;121:992–1000. doi: 10.1016/j.proeng.2015.09.067 [DOI] [Google Scholar]
- 10.Bootdee S, Chantara S, Prapamontol T. Determination of PM2.5 and polycyclic aromatic hydrocarbons from incense burning emission at shrine for health risk assessment. Atmos Pollut Res. 2016;7:680–689. doi: 10.1016/j.apr.2016.03.002 [DOI] [Google Scholar]
- 11.Goel A, Wathore R, Chakraborty T, Agrawal M. Characteristics of exposure to particles due to incense burning inside temples in Kanpur, India. Aerosol Air Qual Res. 2017;17:608–615. doi: 10.4209/aaqr.2016.04.0146 [DOI] [Google Scholar]
- 12.Wang IJ, Tsai CH, Chen CH, Tung KY, Lee YL. Glutathione S-transferase, incense burning and asthma in children. Eur Resp J. 2011;37:1371. doi: 10.1183/09031936.00137210 [DOI] [PubMed] [Google Scholar]
- 13.Ji X, Bihan OL, Ramalho O, et al. Characterization of particles emitted by incense burning in an experimental house. Indoor Air. 2010;20:147–158. [DOI] [PubMed] [Google Scholar]
- 14.Chen YC, Ho WC, Yu YH. Adolescent lung function associated with incense burning and other environmental exposures at home. Indoor Air. 2017;27:746–752. doi: 10.1111/ina.1235513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan A, Clark ML, Ang LW, Yu MC, Yuan JM, Koh WP. Incense use and cardiovascular mortality among Chinese in Singapore: the Singapore Chinese Health Study. Environ Health Perspect. 2014;122:1279–1284. doi: 10.1289/ehp.1307662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang HL, Kuo ML, Lin JM. Mutagenic activity of incense smoke in comparison to formaldehyde and acetaldehyde in Salmonella typhimurium TA102. Bull Environ Contam Toxicol. 1997;58:394–401. doi: 10.1007/s00128990034781 [DOI] [PubMed] [Google Scholar]
- 17.Navasumrit P, ArayAsiri M, Hiang OM, et al. Potential health effects of exposure to carcinogenic compounds in incense smoke in temple workers. Chem Biol Interact. 2008;173:19–31. doi: 10.1016/j.cbi.2008.02.004119 [DOI] [PubMed] [Google Scholar]
- 18.Lin TC, Chang FH, Hsieh JH, Chao HR, Chao MR. Environmental exposure to polycyclic aromatic hydrocarbons and total suspended particulates in a Taiwanese temple. Bull Environ Contam Toxicol. 2001;67:332–338. doi: 10.1007/s001280129 [DOI] [PubMed] [Google Scholar]
- 19.Lin TC, Chang FH, Hsieh JH, Chao HR, Chao MR. Characteristics of polycyclic aromatic hydrocarbons and total suspended particulate in indoor and outdoor atmosphere of a Taiwanese temple. J Hazardous Mater A. 2002;95:1–12. doi: 10.1016/S0304-3894(02)00146-2 [DOI] [PubMed] [Google Scholar]
- 20.Lin TC, Yang CR, Chang FH. Burning characteristics and emission products related to metallic content in incense. J Hazardous Mater. 2007;140:165–172. doi: 10.1016/j.jhazmat.2006.06.052 [DOI] [PubMed] [Google Scholar]
- 21.Pastor-Nieto MA, Gatica-Ortega ME. Ubiquity, hazardous effects, and risk assessment of fragrances in consumer products. Curr Treat Options Allergy. 2021;8:21–41. doi: 10.1007/s40521-020-00275-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong A, Lou W, Ho KF, et al. Indoor incense burning impacts cognitive functions and brain functional connectivity in community older adults. Sci Rep. 2020;10:7090. doi: 10.1038/s41598-020-63568-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friborg JT, Yuan JM, Wang R, Koh WP, Lee HP, Yu MC. Incense use and respiratory tract carcinomas. Cancer. 2008;113:1676–1684. doi: 10.1002/cncr.23788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nor NSM, Yip CW, Ibrahim N, et al. Particulate matter (PM2.5) as a potential SARS-CoV-2 carrier. Sci Rep. 2021;2508. doi: 10.1038/s41598-021-81935-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czwojdzinska M, Terpinska M, Kuzniarski A, Placzkowska S, Piwowar A. Exposure to PM2.5 and PM10 and COVID-19 infection rates and mortality: a one-year observational study in Poland. Biomed J. 2021. doi: 10.1016/j.bj.2021.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schraufnagel DE. The health effects of ultrafine particles. Exp Mol Med. 2020;52:311–317. doi: 10.1038/s12276-020-0403-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Illegal mosquito repellent incense sticks are dangerous and poisonous. Think before you buy them!!!! (Article discusses the regulations of Central Insecticide board (CIB) and Home Insect Control Association (HICA)). Available from: https://www.hindustantimes.com/brand-post/illegal-mosquito-repellent-incense-sticks-are-dangerous-and-poisonous-think-before-you-buy-them/story-X5f7B6XiGeFa7XMUmdSckM.html. Accessed March 29, 2022.
- 28.Sturton SD, Wen HL, Sturton OG. Etiology of cancer of the nasopharynx. Cancer. 1966;19:1666–1669. doi: [DOI] [PubMed] [Google Scholar]
- 29.Yang CY, Chiu JF, Cheng MF, Lin MC. Effects of indoor environmental factors on respiratory health of children in a subtropical climate. Environ Res. 1997;75:49–55. doi: 10.1006/enrs.1997.3774 [DOI] [PubMed] [Google Scholar]
- 30.Ho CK, Tseng WR, Yang CY. Adverse respiratory and irritant health effects in temple workers in Taiwan. J Toxicol Environ Health A. 2005;68:1465–1470. doi: 10.1080/15287390590967405 [DOI] [PubMed] [Google Scholar]
- 31.Alarifi SA, Mubarak MM, Alokail MS. Ultrastructure of the pulmonary alveolar cells of rats exposed to Arabian mix incense (Ma`amoul). J Biol Sci. 2004;4:694–699. doi: 10.3923/jbs.2004.694.69963 [DOI] [Google Scholar]
- 32.Alarifi SA, Mubarak MM, Alokail MS. Ultrastructural changes of pneumocytes of rat exposed to Arabian incense (Bakhour). Saudi Med J. 2004;25:1689–1693. [PubMed] [Google Scholar]
- 33.Wang B, Lee SC, Ho KF, Kang YM. Characteristics of emissions of air pollutants from burning of incense in temples. Hong Kong Sci Tot Env. 2007;377:52–60. doi: 10.1016/j.scitotenv.2007.01.099 [DOI] [PubMed] [Google Scholar]
- 34.Sholih M, Perwitasari D, Hendriani R, et al. Knowledge, attitudes, and practices of lung cancer risk factors in West Bandung Society. J Pharm Bioallied Sci. 2019;11:574–579. doi: 10.4103/jpbs.JPBS_213_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Löfroth G, Stensman C, Brandhorst-Satzkorn M. Indoor sources of mutagenic aerosol particulate matter: smoking, cooking and incense burning. Mutat Res. 1991;261:21–28. doi: 10.1016/0165-1218(91)90094-3 [DOI] [PubMed] [Google Scholar]
- 36.Reddy KRBK, Gupta N, Bhattacharya BG, et al. Impact of air pollution on allergic rhinitis and asthma: consensus statement by Indian Academy of Pediatrics. Indian Pediatr. 2021;58:766–770. doi: 10.1007/s13312-021-2288-1 [DOI] [PubMed] [Google Scholar]
- 37.Chuang HC, Jones T, Berube K. Combustion particles emitted during church services: implication for human respiratory health. Environ Int. 2012;40:137–142. doi: 10.1016/j.envint.2011.07.009 [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto N, Kan-o K, Tatsuta M, et al. Incense smoke-induced oxidative stress disrupts tight junctions and bronchial epithelial barrier integrity and induces airway hyperresponsiveness in mouse lungs. Sci Rep. 2021;11:7222. doi: 10.1038/s41598-021-86745-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo SE, Chi MC, Lin CM, Yang TM. Contributions of burning incense on indoor air pollution levels and on the health status of patients with chronic obstructive pulmonary disease. Peer J. 2020;8:e9768–e9768. doi: 10.7717/peerj.9768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laniado-Laborín R. Smoking and chronic obstructive pulmonary disease (COPD). Parallel epidemics of the 21 century. Int J Env Res Public Health. 2009;6:209–224. doi: 10.3390/ijerph6010209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alokail MS, Al-Daghri NM, Alarifi SA, Draz HM, Hussain T, Yakout SM. Long-term exposure to incense smoke alters metabolism in Wistar albino rats. Cell Biochem Funct. 2011;29:96–101. doi: 10.1002/cbf.1726 [DOI] [PubMed] [Google Scholar]
- 42.Weber LP, Al-Diss A, Marit JS, German TN, Terletski SD. Role of carbon monoxide in impaired endothelial function mediated by acute second-hand tobacco, incense, and candle smoke exposures. Environ Toxicol Pharmacol. 2011;31:453–459. doi: 10.1016/j [DOI] [PubMed] [Google Scholar]
- 43.Lin LY, Lin HY, Chen HW, Su TL, Huang LC, Chuang KJ. Effects of temple particles on inflammation and endothelial cell response. Sci Total Environ. 2012;414:68–72. doi: 10.1016/j.scitotenv.2011.08.05071 [DOI] [PubMed] [Google Scholar]
- 44.Huang YL, Chen HW, Han BC, et al. Personal exposure to household particulate matter, household activities and heart rate variability among housewives. PLoS One. 2014;9:e89969. doi: 10.1371/journal.pone.008996967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Attas OS, Hussain T, Ahmed M, et al. Ultrastructural changes, increased oxidative stress, inflammation, and altered cardiac hypertrophic gene expressions in heart tissues of rats exposed to incense smoke. Environ Sci Pollut Res Int. 2015;22:10083–10093. doi: 10.1007/s11356-015-4212-569 [DOI] [PubMed] [Google Scholar]
- 46.Kammoolkon R, Taneepanichskul N, Pitaknoppakul N, Lertmaharit S, Lohsoonthorn V. Incense smoke and increasing carotid intima media thickness: a cross-sectional study of the Thai-Vietnamese community. Asia Pac J Public Health. 2018;30:178–187. doi: 10.1177/101053951775393065 [DOI] [PubMed] [Google Scholar]
- 47.Hayakawa R, Matsunaga K, Arima Y. Depigmented contact dermatitis due to incense. Contact Dermatitis. 1987;16:272–274. doi: 10.1111/j.1600-0536.1987.tb01451.x [DOI] [PubMed] [Google Scholar]
- 48.Hayakawa R, Matsunaga K, Arima Y. Airborne pigmented contact dermatitis due to musk ambrette in incense. Contact Dermatitis. 1987;16:96–98. doi: 10.1111/j.1600-0536.1987 [DOI] [PubMed] [Google Scholar]
- 49.Lin CY, Wen HJ, Lee YL, Guo YL. Are maternal psychosocial factors associated with cord immunoglobulin E in addition to family atopic history and mother immunoglobulin E? Clin Exp Allergy. 2004;34:548–554. doi: 10.1111/j.1365-2222.2004.1928.x [DOI] [PubMed] [Google Scholar]
- 50.Hsu NY, Wang JY, Wu PC, Su HJ. Paternal heredity and housing characteristics affect childhood asthma and allergy morbidity. Arch Environ Occup Health. 2012;67:155–162. doi: 10.1080/19338244.2011.598890 [DOI] [PubMed] [Google Scholar]
- 51.Norbäck D, Zhang X, Fan Q, et al. Home environment and health: domestic risk factors for rhinitis, throat symptoms and non-respiratory symptoms among adults across China. Sci Tot Environ. 2019;681:320–330. doi: 10.1016/j.scitotenv.2019.05.08454 [DOI] [PubMed] [Google Scholar]
- 52.Fang XY, Strodl E, Liu BQ, et al. Association between prenatal exposure to household inhalants exposure and ADHD-like behaviors at around 3 years of age: findings from Shenzhen Longhua Child Cohort Study. Environ Res. 2019;177:108612. doi: 10.1016/j.envres.2019.108612 [DOI] [PubMed] [Google Scholar]
- 53.Wei CF, Chen MH, Lin CC, et al. Household incense burning and infant gross motor development: results from the Taiwan Birth Cohort Study. Environ Int. 2018;115:110–116. doi: 10.1016/j [DOI] [PubMed] [Google Scholar]
- 54.World Health Organization. Health effects of particulate matter, Policy Implications for countries in Eastern Europe, Caucasus and Central Asia; 2013. ISBN: 9789289000017.
- 55.Yamamoto Y, Isoyama E, Sofikitis N, Miyagawa I. Effects of smoking on testicular function and fertilizing potential in rats. Urol Res. 1998;26:45–48. doi: 10.1007/s002400050022 [DOI] [PubMed] [Google Scholar]
- 56.Kapawa A, Giannakis D, Tsoukanelis K, et al. Effects of paternal cigarette smoking on testicular function, sperm fertilizing capacity, embryonic development and blastocyst capacity for implantation in rats. Andrologia. 2004;36:57–68. doi: 10.1111/j.1439-0272.2004.00605.x [DOI] [PubMed] [Google Scholar]
- 57.Zhou R, An Q, Pan XW, Yang B, Hu J, Wang YH. Higher cytotoxicity and genotoxicity of burning incense than cigarette. Environ Chem Lett. 2015;13:465–471. doi: 10.1007/s10311-015-0521-7 [DOI] [Google Scholar]
- 58.Akingbade AM, Saalu LC, Oyebanji OO, Oyeniran DA, AKande OO, Akunna GG. Rhodinol-based incense testiculotoxicity in Albino rats: testicular histology, spermatogenic and biochemical evaluations. J Pharmacol Toxicol. 2014;9:68–81. doi: 10.3923/jpt.2014.68.81 [DOI] [Google Scholar]
- 59.Chen LY, Ho C. Incense burning during pregnancy and birth weight and head circumference among term births: the Taiwan Birth Cohort Study. Env Health Persp. 2016;124:1487–1492. doi: 10.1289/ehp.1509922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He JR, Wei DM, Chan FF, et al. Associations between maternal exposure to incense burning and blood pressure during pregnancy. Sci Tot Env. 2018;610-611:1421–1427. doi: 10.1016/j.scitotenv.2017.08.134 [DOI] [PubMed] [Google Scholar]
- 61.Sastry J, Agawane S, Rajan M, Black K, Laumbach R, Ramagopal M. The effect of the indoor environment on wheeze- and sleep-related symptoms in young Indian children. Lung India. 2021;38:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vallès Y, Inman CK, Peters BA, et al. Incense burning is associated with human oral microbiota composition. Sci Rep. 2019;9:10039. doi: 10.1038/s41598-019-46353-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hussain T, Al-Attas OS, Alrokayan SA, et al. Deleterious effects of incense smoke exposure on kidney function and architecture in male albino rats. Inhal Toxicol. 2016;28:364–373. doi: 10.1080/08958378.2016.1179372 [DOI] [PubMed] [Google Scholar]
- 64.Geng TT, Jafar TH, Yuan JM, Koh WP. Long-term incense use and the risk of end-stage renal disease among Chinese in Singapore: the Singapore Chinese health study. BMC Nephrol. 2019;20. doi: 10.1186/s12882-018-1186-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yadav VK, Singh B, Choudhary N. Characterization of Indian incense stick powders for their physical, chemical and mineralogical properties. World J Environ Biosci. 2020;9:39–43. [Google Scholar]
- 66.Balasubramanian N. Scented oils and perfumes chemical technology in antiquity. Am Chem Soc. 2015;1211:219–244. [Google Scholar]
- 67.Jain SN, Tamboli SR, Sutar DS, Mawal VN, Shaikh AA, Prajapati AA. Incense stick ash as a novel and sustainable adsorbent for sequestration of Victoria Blue from aqueous phase. Sustainable Chem Pharm. 2020;15:100199. doi: 10.1016/j.scp.2019.100199 [DOI] [Google Scholar]
- 68.Mishra S, Singh AL, Tiwary D. Studies of physico-chemical status of the ponds at Varanasi Holy City under anthropogenic influences. Int J Environ Res Dev. 2018;4:261–268. [Google Scholar]
- 69.Yadav VK, Yadav KK, Ahmed EM, et al. Transformation of hazardous sacred incense sticks ash waste into less toxic product by sequential approach prior to their disposal into the water bodies. Env Sc Pollut Res Int. 2021. doi: 10.1007/s11356-021-15009-8 [DOI] [PubMed] [Google Scholar]
- 70.Khezri B, Chan YY, Tiong LYD, Webster RD. Annual air pollution caused by the Hungry Ghost Festival. Environ Sci Process Impacts. 2015;17:1578–1586. doi: 10.1039/c5em00312a [DOI] [PubMed] [Google Scholar]
- 71.Fang GC, Chang CN, Chu CC, et al. Fine (PM2.5), coarse (PM2.5-10), and metallic elements of suspended particulates for incense burning at Tzu Yun Yen temple in central Taiwan. Chemosphere. 2003;51:983–991. doi: 10.1016/S0045-6535(03)00124-3 [DOI] [PubMed] [Google Scholar]
- 72.Balaram V. Rare earth elements: a review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci Front. 2019;10:1285–1303. doi: 10.1016/j.gsf.2018.12.005 [DOI] [Google Scholar]
- 73.Yadav VK, Gnanamoorthy G, Cabral-Pinto MMS, et al. Variations and similarities in structural, chemical, and elemental properties on the ashes derived from the coal due to their combustion in open and controlled manner. Environ Sci Pollut Res Int. 2021;28:32609–32625. doi: 10.1007/s11356-021-12989-5 [DOI] [PubMed] [Google Scholar]
- 74.Mesallam TA, Farahat M, Shoeib R, et al. Acute effects of inhaling Oud incense on voice of Saudi adults. Ann Saudi Med. 2015;35:111–119. doi: 10.5144/0256-4947.2015.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang TT, Ho SC, Chuang LT, Chuang HC, Li YT, Wu JJ. Characterization of particulate-phase polycyclic aromatic hydrocarbons emitted from incense burning and their bioreactivity in RAW264.7 macrophage. Env Pollut. 2017;220:1190–1198. doi: 10.1016/j.envpol.2016.11.016 [DOI] [PubMed] [Google Scholar]
- 76.Elsayed Y, Dalibalta S, Gomes I, Fernandes N, Alqtaishat F. Chemical composition and potential health risks of raw Arabian incense (Bakhour). J Saudi Chem Soc. 2016;20:465–473. doi: 10.1016/j.jscs.2014.10.005 [DOI] [Google Scholar]
- 77.Tung JC, Huang WC, Yang JC, et al. an incense smoke ingredient, promotes lung cancer malignancy. Envir Toxicol. 2017;32:2379–2391. doi: 10.1002/tox.22451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin CH, Lo PY, Wu HD, Chang C, Wang LC. Association between indoor air pollution and respiratory disease in companion dogs and cats. J Vet Int Med. 2018;32:1259–1267. doi: 10.1111/jvim.15143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaizaki-Mitsumoto A, Hataoka K, Funada M, Odanaka Y, Kumamoto H, Numazawa S. Pyrolysis of UR-144, a synthetic cannabinoid, augments an affinity to human CB1 receptor and cannabimimetic effects in mice. J Toxicol Sci. 2017;42:335–341. doi: 10.2131/jts.42.335 [DOI] [PubMed] [Google Scholar]
- 80.Lu Y, Lin S, Lawrence WR, et al. Evidence from SINPHONIE project: impact of home environmental exposures on respiratory health among school-age children in Romania. Sc Tot Environ. 2018;621:75–84. doi: 10.1016/j.scitotenv.2017.11.157 [DOI] [PubMed] [Google Scholar]
- 81.Lung SC, Wang WV, Wen TJ, Liu C, Hu S. A versatile low-cost sensing device for assessing PM2.5 spatiotemporal variation and quantifying source contribution. Sci Tot Environ. 2020;716:137145. doi: 10.1016/j.scitotenv.2020.137145 [DOI] [PubMed] [Google Scholar]
- 82.Niu X, Jones T, BéruBé K, Chuang HC, Sun J, Ho KF. The oxidative capacity of indoor source combustion derived particulate matter and resulting respiratory toxicity. Sci Tot Environ. 2021;767:144391. doi: 10.1016/j.scitotenv.2020.144391 [DOI] [PubMed] [Google Scholar]
- 83.Manoukian A, Buiron D, Temime-Roussel B, Wortham H, Quivet E. Measurements of VOC/SVOC emission factors from burning incenses in an environmental test chamber: influence of temperature, relative humidity, and air exchange rate. Environ Sci Pollut Res. 2016;23:6300–6311. doi: 10.1007/s11356-015-5819-2 [DOI] [PubMed] [Google Scholar]
- 84.Lu F, Li S, Shen B, et al. The emission characteristic of VOCs and the toxicity of BTEX from different mosquito-repellent incenses. J Hazard Mater. 2020;384:121428. doi: 10.1016/j.jhazmat.2019.121428 [DOI] [PubMed] [Google Scholar]