Abstract
The B-cell lymphoma 2 (BCL-2) inhibitor venetoclax (VEN) in combination with lower-intensity therapy is an efficacious treatment for acute myeloid leukemia (AML). VEN in combination with the hypomethylating agent azacitidine improved rates of response and measurable residual disease (MRD)-negative remissions in addition to overall survival in the pivotal phase 3 VIALE-A trial compared with azacitidine monotherapy and has since emerged as the current standard of care in older or unfit patients with AML. In younger, fit patients with AML, intensive induction and consolidation chemotherapy (IC) is commonly employed as frontline therapy; however, relapse remains the principal cause of treatment failure in approximately 30–40% of patients. Improved IC regimens that increase MRD-negative response rates, result in durable remissions, and enable transition to curative allogeneic hematopoietic stem cell transplantation in appropriate patients remain an area of active inquiry. Preliminary results from trials investigating the combination of VEN with IC have reported promising findings to date, with composite complete remission and MRD-negative remission rates of approximately 89–94% and 82–93%, respectively, correlating with improved 12-month event-free and overall survival compared to historical outcomes with IC. Herein, we discuss ongoing trials investigating VEN in combination with IC in addition to outcomes within specific molecularly defined subgroups; review the molecular mechanisms of sensitivity and resistance to VEN, and highlight future combinations of VEN with novel targeted therapies for the treatment of AML.
Keywords: venetoclax, intensive induction, acute myeloid leukemia
Introduction
Intensive chemotherapy (IC) has been the standard of care treatment for younger, fit patients with acute myeloid leukemia (AML) for over 40 years,1,2 relying on a combination of the pyrimidine analog cytarabine (araC) with anthracycline-based therapy often referred to as the ‘7 + 3’ (7 days of cytarabine + 3 days of daunorubicin) regimen. Response rates with standard IC range from approximately 60–80% in patients younger than 60 years old with long-term survival of approximately 30–40%.1 –3 Even with dose augmentation of standard induction regimens, relapse occurs in approximately 30–40%. 4 Further optimization of treatment strategies remains a priority. 4
Alternative approaches have been developed in attempts to improve upon current therapies. Incorporation of purine analogs such as cladribine and fludarabine into multiagent chemotherapy regimens in combination with anthracycline and cytarabine based regimens have reported impressive results in both the phase 2 setting and compared to intensive chemotherapy controls in randomized phase 3 trials.3,5,6
A single-institution randomized phase 2 study evaluated clofarabine or fludarabine combined with idarubicin and cytarabine (i.e., CIA and. FIA) in a younger AML population (median age 51). Treatment resulted in similar 2-year OS rates of 51% versus 57%, with patients aged < 50 in the FIA arm demonstrating the greatest benefit compared to historical controls receiving idarubicin with cytarabine alone (2-year OS age < 50: 72% versus 36%). 5
A randomized phase 3 trial compared the addition of cladribine and fludarabine to ‘7 + 3’ in 652 younger (median age 47–48 years) with AML. 3 The control arm consisted of daunorubicin 60 mg/m2(D1-3) combined with seven days of continuous infusion cytarabine 200 mg/m2 (D1-7) while the treatment arms added either cladribine (5 mg/m2, D1-5) or fludarabine (25 mg/m2, D1-5) to the ‘7 + 3’ backbone. Treatment with cladribine resulted in higher rate of complete remission (62% versus 51%) and improved 3-year survival (45% versus 33%) compared to patients treated within the 7 + 3 control arm. 3
In the randomized, phase 3 UK-MRC trial comparing daunorubicin + cytarabine (+/- etoposide) compared to fludarabine, cytarabine, granulocyte stimulating factor, and idarubicin (FLAG-IDA), similar remission rates (86% versus 85%) were observed; however, the FLAG-IDA group experienced increased relapse-free survival (45% versus 34%) and decreased relapse rates (34% versus 55%). 6 These results supported further analysis of the addition of fludarabine to traditional induction chemotherapy regimens to improve CR and OS rates.
While these regimens represent progress compared to historical IC for AML, novel approaches to reduce relapse risk and improve long-term survival remain paramount. Minimal residual disease (MRD, measured via multiparameter flow cytometry or polymerase chain reaction) has emerged as an important marker for the assessment of treatment efficacy in AML. MRD-negative remissions correlate with lower relapse rates, improved relapse-free survival, and OS7,8 in patients receiving IC 9 or lower-intensity regimens. 10 MRD also retains its prognostic importance following consolidative allogeneic hematopoietic stem cell transplantation (HSCT).
Attainment of MRD-negative remissions is a critical objective of induction therapy; thus, the development of induction regimens capable of achieving high rates of MRD clearance remains paramount to improving long-term outcomes for patients. 8 In addition, regimens capable of facilitating transition to potentially curative consolidative HSCT in remission remain central to the treatment paradigm of patients with intermediate or adverse risk de novo AML, 11 secondary or therapy-related AML,12,13 or relapsed/refractory (R/R) AML.14,15
Venetoclax (VEN) a potent inhibitor of the antiapoptotic B-cell lymphoma-2 (BCL-2) protein combined with the hypomethylating agents (HMA, azacitidine or decitabine) has emerged as an effective treatment modality in older (age > 75) or unfit patients presenting with newly diagnosed (ND) or R/R-AML. 10 Given the therapeutic potential observed with VEN in combination with HMA’s and preclinical evidence of synergy with VEN and various intensive chemotherapeutics, VEN in combination with IC for the treatment of younger, fit patients with AML represents an area of active investigation. Herein, we review current pre-clinical and clinical experience of venetoclax with IC (VEN + IC) for fit patients and highlight molecular subgroups associated with efficacy and resistance to VEN + IC combinations.
Venetoclax, BCL-2, and the intrinsic apoptotic pathway
Mechanism of action of venetoclax
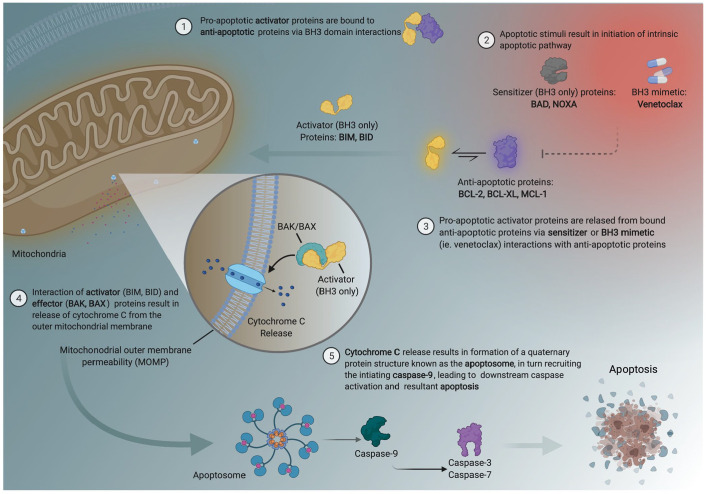
The use of VEN has evolved since early studies identified the role of BCL-2 dependence for leukemic cell survival and has quickly led to a paradigm shift in the treatment of AML.16–18 BCL-2 is a member of the antiapoptotic protein family (BCL-2, BCL-XL, BCL-W, BCL2-A1, and MCL-1) expressing BCL-2 like homology domains 1-4 (BH1-BH4), which when overexpressed inhibit apoptosis. These antiapoptotic proteins in conjunction with the pro-apoptotic activator (BID, BIM, and PUMA), effector (BAK and BAX), and sensitizer (NOXA) proteins comprise the intrinsic apoptotic pathway.19–21
Activation or inhibition of these effectors and activator proteins is governed by BH3 domain interactions between antiapoptotic or proapoptotic BCL-2 family members. BH3 is expressed by all members of the BCL-2 family. All four BH domains are expressed by the suppressor and pore forming proteins (BAK, BAX). However, the activator and sensitizer proapoptotic proteins only contain the BH3 domain. BH3 domain interactions between the sensitizer and antiapoptotic BCL2 family members facilitate apoptosis by enabling activator proteins (now unbound from antiapoptotic BCL2 family proteins) to interact with BAX/BAK on the outer mitochondrial membrane, resulting in pore formation, mitochondrial outer membrane permeabilization, cytochrome C release, caspase activation, and apoptosis (Figure 1). 21
Figure 1.
Intrinsic apoptotic pathway and BCL-2 family biology.
Several BH3 mimetic drugs, ABT-737, venetoclax (ABT-199), and navitoclax (ABT-263), have been developed to bind selectively to the BH3 domain on antiapoptotic proteins thereby displacing proapoptotic proteins and resulting in cell death. 21 ABT-737 selectively binds to BCL-2, BCL-XL, and BCL-W and induces apoptosis in leukemic blast cells through activation of BAX/BAK. Lower platelet counts are observed in the ABT-737-treated subjects, 22 a process thought to be mediated by BCL-XL which plays a role in platelet survival. 23 This was apparent in a phase 1 dose-escalation study of navitoclax (which has a high affinity to both BCL-2 and BCL-XL) in patients with relapsed/refractory lymphoid malignancies, where navitoclax therapy resulted in increased rates of grade 3/4 thrombocytopenia. 20 Unlike navitoclax, VEN is a selective BH3 mimetic preferentially binding BCL-2 with significantly lower affinity for BCL-XL leading to less associated thrombocytopenia when utilized for the treatment of myeloid malignancies.24,25
Mechanisms of resistance to BCL-2 inhibition
Resistance to VEN when used with lower-intensity therapies has been well characterized and often occurs secondary to increased expression of alternative antiapoptotic BCL2 family members (i.e., MCL-1 and BCL-XL), or mutations in genes associated with active signaling or tumor suppressors.26,27 In MCL-1-dependent AML, MCL-1 sequesters the proapoptotic activator BIM, thereby preventing induction of apoptosis. Overexpression of MCL-1 also inhibits the proapoptotic effectors (i.e., BAK and BAX).26,27 Functional studies identified monocytic AML to be particularly associated with increased MCL-1 expression, decreased BCL-2 expression, and resistance to VEN-based therapy. 28 In addition to MCL-1, overexpression of BCL2A1, an antiapoptotic BCL-2 homolog, was significantly expressed in monocytes rendering acute monocytic leukemias (AML-M5) more resistant to VEN treatment.29–31 Investigations of therapies targeting MCL-1 directly, by the addition of cytotoxic chemotherapy to downregulate MCL-1, or through targeting of alternative myeloid transcription programs in AML with monocytic differentiation using VEN in combination with bromodomain and extra-terminal domain inhibitors may prove to be effective strategies to mitigate resistance and relapse to VEN.30,32,33 Several phase I investigations of MCL-1 inhibitors as monotherapy and in combination with venetoclax or other cytotoxic agents are currently ongoing (NCT03218683, NCT05107856, NCT03218683, and NCT03218683).
VEN resistance has also been observed in patients with gene mutations involved in active signaling pathways including FLT3-ITD, RAS, PTPN11. Transduced cell lines overexpressing FLT3-ITD simultaneously increased expression of BCL-XL and MCL-1 conferring resistance to VEN. 34 However, VEN in combination with the FLT3-targeted tyrosine kinase inhibitors midostaurin or gilteritinib resulted in enhanced cell death suggesting FLT3 inhibition may result in downregulation of alternative antiapoptotic BCL2 family proteins, thereby overcoming VEN resistance within this genomic subgroup. 34 Similar resistance mechanisms have been described in KRAS- or PTPN11-mutated AML correlating with VEN resistance. In KRAS-mutated AML, reduced expression of BCL2 and simultaneously increased expression of MCL-1 and BCL2A1 levels were observed. Elimination of KRAS-mutated clones following therapy resulted in restored sensitivity to VEN. PTPN11-mutated samples demonstrated sustained MCL-1 and BCL-XL expression, suggesting combined treatment with MCL-1 or BCL-XL inhibitors may overcome VEN resistance in these cohorts. 31
TP53 mutations also impart resistance to VEN. In patients with TP53-mutated AML treated with VEN in combination with low-dose cytarabine or HMAs, enrichment of TP53-mutated clones at the time of relapse was observed.31,34 Cell viability assays treated with VEN or VEN in combination with low-dose cytarabine or azacitidine similarly confirmed TP53 mutations were associated with resistance to VEN. 34
Venetoclax in combination with intensive chemotherapy in ND-AML
Translational studies of VEN in combination with intensive chemotherapy
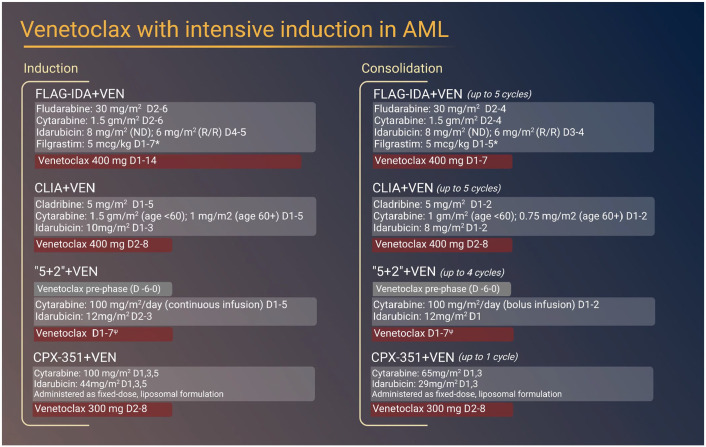
Several impactful preclinical studies demonstrating the synergy of VEN in combination with lower-intensity therapies as well as clinical trials identifying the clinical efficacy of VEN combined with low-intensity chemotherapy including HMA’s or low-dose cytarabine (LDAC) have been recently reported.28,29 While limited, preclinical data also support the use of VEN in combination with cytotoxic chemotherapeutics traditionally used in IC regimens (Figure 2). 26
Figure 2.
Intensive chemotherapy-based induction and consolidation regimens incorporating venetoclax for the treatment of AML.
*peg-filgrastim permitted to replace filgrastim on D5 (induction) or D3 (consolidation).
ψ venetoclax administered at varying dosage by cohort (A: 50 mg, B: 100 mg, C: 200 mg, D: 400 mg, E: 600 mg).
Synergistic leukemic cell death was observed in both primary AML cell lines and patient samples when treated with VEN in combination with either cytarabine or daunorubicin. 28 Daunorubicin exposure resulted in induced DNA damage and resultant downregulation of MCL-1, in turn increasing intracellular concentrations of the apoptotic activator BIM, and increased apoptosis. Similar findings were demonstrated when cytarabine was combined with VEN.26,35 This preclinical data suggest the synergistic combination of VEN with cytotoxic chemotherapy may result in increased leukemic cell death, resulting in enhanced clinical efficacy. Indeed, early reports of VEN combined with intensive induction and consolidation chemotherapy have preliminarily demonstrated these combinations to be safe and effective in the treatment of patients with ND and R/R-AML based upon preliminary reports of ongoing early-phase trials36–38 (Tables 1 and 2).
Table 1.
Ongoing clinical investigations incorporating venetoclax with intensive chemotherapy.
| Clinical Investigation | Phase | NCT Number |
|---|---|---|
| “7 + 3”+Venetoclax | 1 | NCT03709758 |
| “7 + 3”+Venetoclax | 3 | NCT04628026 |
| FLAG-IDA + Venetoclax | 1b/2 | NCT03214562 |
| CLAG-M + Venetoclax | 1 | NCT04797767 |
| CLIA + Venetoclax | 2 | NCT02115295 |
| FLAVIDA | 2 | NCT03455504 |
| CPX-351 + Venetoclax | 2 | NCT03629171 |
| CPX-351 + Venetoclax (V-FAST) | Ib | NCT04075747 |
Table 2.
Outcomes of contemporary published prospective trials of intensive chemotherapy induction regimens with or without the incorporation of venetoclax.
| Trial | Design | AML type | Age, median (range) | Risk Group | Response | Survival | Early mortality |
|---|---|---|---|---|---|---|---|
| FIA versus CIA | ELN 2010 | 2-year | |||||
| FIA (N = 76) | Phase 2 | ND-AML | 49 (18–66) | int-2/adverse: 58% | ORR: 83%; CR/CRp: 82%; MRD-neg: 65% | 57% | 60 days: 1% |
| CIA (N = 106) | Phase 2 | ND-AML | 53 (20–66) | int-2/adverse: 57% | ORR: 83%; CR/CRp: 80%; MRD-neg: 80% | 51% | 60 days: 4% |
| FLAG-IDA versus ADE | Cytogenetic risk | 8-year | |||||
| FLAG-IDA (N = 635) | Phase 3 | ND-AML | 48 (0–71) | Int: 69%, Adv: 13% | ORR: 86%, CR: 84% | 44% | Induction: 7% |
| ADE (N = 633) | Phase 3 | ND-AML | 48 (0–67) | Int: 72%, Adv: 13% | ORR: 85%, CR: 81% | 37% | Induction: 7% |
| DA versus DAC. versus DAF | SWOG criteria | 3-year | |||||
| DAC (N = 222) | Phase 3 | ND-AML | 48 (18–60) | Int: 52%, Adv: 16% | ORR: 68%, CR: 62% | 45% | Induction: 11% |
| DAF (N = 219) | Phase 3 | ND-AML | 47 (17–60) | Int: 51%, Adv: 18% | ORR: 59%, CR: 55% | 35% | Induction: 9% |
| DA (N = 211) | Phase 3 | ND-AML | 47 (18–60) | Int: 50%, Adv: 18% | ORR: 56%, CR: 51% | 33% | Induction: 10% |
| CPX-351 versus “7 + 3” | Mean (SD) | NCCN criteria | 5-year | ||||
| CPX-351 (N = 153) | Phase 3 | sAML | 67.8 (4.2) | Int: 45%, Adv: 50% | CR/CRi: 48%, CR: 37% | 18% | 60 days: 13.7% |
| “7 + 3” (N = 156) | Phase 3 | sAML | 67.7 (4.1) | Int: 40%, Adv: 57% | CR/CRi: 36%, CR: 30 | 8% | 60 days: 21.2% |
| FLAG-IDA + VEN | ELN 2017 | 1-year | |||||
| ND-AML (N = 29) | Phase 2 | ND-AML | 45 (20–65) | Int: 45%, Adv: 38% | ORR: 97%, CRc:90%, MRD-neg: 96% |
94% | 30 days: 0% |
| R/R-AML (N = 16) | Phase 1b | R/R-AML | 51 (20–73) | Int: 13%, Adv: 50% | ORR: 75%, CRc: 75%, MRD-neg: 58% |
38% | 30 days: 0% |
| R/R-AML (N = 23) | Phase 2 | R/R-AML | 47 (22–66) | Int: 13%, Adv: 61% | ORR: 70%, CRc: 61%, MRD-neg: 79% |
68% | 30 days: 0% |
| CLIA + VEN | ELN 2017 | 1-year | |||||
| Phase 1b/2 | ND-AML | 48 (37–56) | Int: 30%, Adv: 35% | ORR: 94%, CRc: 94%, MRD-neg: 71% |
85% | 60 days: 2% | |
| “5 + 2”+VEN | ELN 2017 | median | |||||
| Phase 1b | ND-AML | 72 (63–80) | Int: 31%, Adv: 49% | ORR: 72%, CR: 41% | 11.2 mo. | 30 days: 6% |
Attenuated cytarabine and idarubicin (i.e., ‘5 + 2’)+venetoclax (CAVEAT Trial)
A phase Ib, open-label, dose escalation and preliminary efficacy study assessing the benefit of venetoclax in combination with an attenuated ‘7 + 3’ regimen consisting of 5 days of cytarabine and 2 days of idarubicin (‘5 + 2’) was conducted in older (median age 72) adults fit for IC with de novo or secondary AML (sAML). Fifty-one patients with predominately European LeukemiaNet (ELN) 39 intermediate and adverse risk disease were enrolled. Twenty-eight (55%) and twenty-three (45%) patients had de novo or sAML, respectively. Patients were enrolled at escalating dose levels of VEN (50 mg, 100 mg, 200 mg, 400 mg, and 600 mg) in combination with 5 + 2 followed by four consolidation cycles (2 + 1 + VEN) (Figure 2).
The overall response rate (ORR: CR + CR with incomplete hematologic recovery [CRi]) among both de novo and sAML patients was 72%, with 41% of patients achieving a true CR. A superior ORR was observed in patients with de novo AML (ORR: 97%) which compared favorably to standard IC utilizing the ‘7 + 3’ regimen (ORR: 60–80%). Though only a minority of patients with NPM1-mutated AML underwent MRD assessment, 83% attained MRD-negative remissions. Patients with sAML demonstrated an ORR of 42% which despite using a reduced schedule of 7 + 3 induction, was similar to current standard of care therapies used in this patient population including CPX-351 (ORR: 48%) and 7 + 3 (ORR: 33%). After a median follow-up of approximately 2 years, median OS for the entire study population was 11.2 months. Significantly longer OS was noted in patients with de novo AML compared with sAML (31.3 versus 6.1 months, p-value < 0.001). Similarly, patients who achieved a CR had a longer median OS compared to patients with a CRi (29.5 versus 6.9 months), albeit this result was not statistically significant (p-value: 0.12).
Common non-hematologic adverse events occurring in ⩾ 10% of study subjects were predominantly infectious, including grade 3 or greater febrile neutropenia (55%, N = 28), sepsis (35%, N = 18), or localized infections (10%, N = 5). The primary hematologic toxicity was thrombocytopenia, particularly during consolidation cycles in patients receiving higher doses of VEN with median time to platelet recovery (i.e., platelet count ⩾ 50 x 109/L) of 39–47 days. Increased hematologic toxicity (including one DLT) was observed in the 600 mg VEN group resulting in a protocol amendment utilizing a lower dose of venetoclax (400 mg on Days 1–14) during consolidation. Despite this adjustment, only one patient had platelet count recovery within 42 days of receiving consolidation therapy. None of the patients treated within the 400–600 mg cohorts who started consolidation were able to complete therapy. Twenty-seven percent of patients transitioned VEN maintenance; however, only six (16%) patients completed all seven planned maintenance cycles (VEN D1-14). Thirty-day all-cause mortality was 6% with all deaths related to sepsis.
These results demonstrated therapy with VEN in combination with IC is feasible in an older, difficult to treat AML population 36 and confirmed the efficacy of combination therapy; In addition, they highlight the potent on-target hematologic toxicities associated with VEN combinations.
Cytarabine and daunorubicin (i.e., ‘7 + 3’) + venetoclax
A phase I dose-escalation trial assessing VEN in combination with standard ‘7 + 3’ induction is currently underway with limited preliminary results reported to date. 3 Patients with de novo AML received daunorubicin 60 mg/m2(Days 2–4) + cytarabine 200 mg/m2 (Days 1–7) induction with VEN administered on Days 1–11 stratified by cohorts receiving escalating doses of VEN (200 mg, 400 mg, and 600 mg). Following two dose-limiting toxicities (DLTs)—one in a 58-year-old patient who developed DIC and the other a 73-year-old patient who died secondary to sepsis, enrollment was restricted to patients aged ⩽ 60 without FLT3 mutations or core-binding factor AML.
Within this younger population, no DLTs were noted in the 200 mg dosing cohort. Three patients were subsequently enrolled in the 400 mg dosing cohort without any additional observed toxicity. A single DLT occurred in the VEN 600 mg dose-escalation cohort (death secondary to septic shock); thus, 400 mg (the current FDA-approved VEN dose for AML in combination with HMAs) was determined to be maximal tolerated dose in combination with ‘7 + 3’ induction. Median time to count recovery (defined as an ANC ⩾ 0.5 x 109/L and a platelet count ⩾ 50 x 109/L) following ‘7 + 3’+VEN induction was 36 days. 40
The overall response rate to ‘7 + 3’ induction with VEN was 100% (n = 10), with 75% (n = 6/8) of patients achieving MRD-negative remissions assessed using multiparameter flow cytometry. Within the initial VEN 200 mg cohort, all seven patients attained a composite CR [CRc; CR: 6, CRi (complete response with incomplete count recovery): 1]; four were MRD-negative. In the VEN 400 mg dose-escalation cohort, 100% achieved a CRc; two patients achieved a MRD-negative CR. Investigations of ‘7 + 3’+ VEN 400 mg induction followed by consolidation using high-dose cytarabine in combination with VEN at 200 mg, 400 mg, or 600 mg dose levels are currently underway. 41
Fludarabine, cytarabine, idarubicin, and G-CSF (FLAG-IDA)+venetoclax
VEN combined with the multiagent induction and consolidation regimen consisting of fludarabine, cytarabine, idarubicin, and granulocyte-colony stimulating factor (FLAG-IDA + VEN) is under evaluation in a phase 1b/2 trial composed of ND (phase 2) and R/R-AML (phase 1b/2) patients. 38 The ND-AML cohort (N = 45) was composed predominantly of patients with European LeukemiaNet (ELN) intermediate (40%) or adverse risk (42%) disease, 42 including twelve (28%) patients with secondary (sAML), treated-secondary (ts-AML), or therapy-related AML (tAML). 38
The ORR to FLAG-IDA + VEN was 98% in this patient cohort, with a CRc (CR, complete response with partial hematologic recovery (CRh) and CRi) rate of 89%. Importantly, 93% of these patients attained an MRD-negative response as measured by multiparameter flow cytometry (MFC), with no significant difference observed between patients with de novo versus sAML/tsAML/tAML (de novo: 93%, sAML/tsAML/tAML: 90%). 42 Eighty-nine percent of patients with ELN adverse risk AML attained a CRc with FLAG-IDA + VEN. After a median study follow-up of 12 months, median event-free (EFS) and overall survival (OS) were not reached. The corresponding 12-month EFS and OS were 77% and 94%, 42 with 69% of patients successfully transitioning to allogeneic HSCT. 38
Cladribine, cytarabine, and idarubicin (CLIA)+venetoclax
VEN in combination with the intensive induction and consolidation regimen comprised of cladribine, idarubicin, and cytarabine (CLIA + VEN) for the treatment of ND acute leukemia (N = 46) or high-risk (defined by the presence of ⩾ 10% blasts or a revised international prognostic scoring system score of ⩾ 2) myelodysplastic syndrome (MDS; N = 4) also demonstrated promising results in a phase 2 study. 37 The patient population predominantly included patients with ELN intermediate (30%) or adverse risk (35%) AML, including a subset of patients (N = 15) with FLT3-ITD and/or TKD-mutated AML who, additionally, received an FDA-approved FLT3 inhibitor (gilteritinib 43 or midostaurin 44 ). Congruent with the results observed with FLAG-IDA + VEN, CLIA + VEN induction resulted in an impressive CR/CRi rate of 94%. Eighty-two percent of patients achieving CR or CRi attained MRD negativity as assessed by MFC. 37
After a median follow-up of approximately 14 months, median EFS and OS were not reached; 12-month EFS and OS were estimated at 68% and 85%, respectively. In the subgroup of patients with ELN adverse risk AML, CLIA + VEN was associated with a 12-month OS of 81%. 37 Sixty-two percent of patients responding to CLIA + VEN received a consolidative HSCT.
Adverse events to FLAG-IDA or CLIA with VEN were consistent with those observed in previous trials of intensive induction therapy for AML3,45 with infectious complications predominating. Among patients with ND-AML treated with FLAG-IDA + VEN or CLIA + VEN, febrile neutropenia was reported in 39% and 84%, respectively. Grade 3 or greater infectious complications occurred in 12% of patients treated with CLIA + VEN, while bacteremia and pneumonia occurred in 19% and 24% of patients treated with FLAG-IDA + VEN.37,38 Despite these infectious events, early mortality in patients treated with FLAG-IDA + VEN (30- and 60-day mortality: 0%) or CLIA + VEN (30- and 60-day mortality: 2%) was uncommon.37,38
The addition of VEN into multiagent induction therapy resulted in similar myelosuppression compared to other intensive regimens utilizing an anthracycline and cytarabine backbone with or without a purine analog.3,6,45 Similar to the observed myelosuppression with FLAG-IDA, hematologic recovery (defined as an ANC ⩾ 0.5 x 109/L and a platelet count ⩾ 50 x 109/L) was prolonged following the second cycle of FLAG-IDA + VEN. Median cycle lengths were 31 and 41 days following induction and consolidation, respectively. 42 Similar results were observed with CLIA + VEN for induction, with a median time to hematologic recovery (defined as an ANC ⩾ 1 x 109/L and a platelet count ⩾ 50 x 109/L) of 27 days following induction; only 6% (N = 3) patients had cycle lengths exceeding 45 days. 2
The myelosuppression and infectious complications reported with FLAG-IDA + VEN or CLIA + VEN, similar to other intensive chemotherapy regimens for AML, underscores the importance of access to rigorous supportive care measures necessary for the implementation of these treatment regimens. Treatment should occur at a facility capable of instituting standard antimicrobial prophylaxis (including mold-active antifungal azoles with appropriate FDA-approved VEN dose adjustments), frequent clinical and laboratory assessments, blood product transfusion support, and prompt admission with initiation of broad-spectrum antibiotics at the earliest sign of infection to mitigate these complications and associated mortality.
Thus, the addition of VEN with IC for the treatment of patients with ND-AML is encouraging. Early results of ongoing studies are notable for high response rates ranging from 72% to 97%, with impressive composite CR rates (~ 90%) and survival reported to date. Importantly, VEN-based IC regimens appear particularly effective in irradicating MRD and facilitating transition to consolidative HSCT in fit patients with adverse risk AML. With proper management, these pivotal early trials demonstrate the feasibility, safety, and promise of IC regimens incorporating VEN.
Venetoclax in combination with intensive chemotherapy in R/R-AML
FLAG-IDA + venetoclax
FLAG-IDA + VEN has also been utilized in the high-risk R/R-AML setting with promising early results in the initial 39 patients reported. Thirty-six percent of patients received a prior allogeneic HSCT, 56% had ELN adverse risk AML, and 41% had adverse risk or complex cytogenetics; 30% were in salvage #2 or greater. 38
The initial VEN duration (Days 1–21) and cytarabine dosing (2 g/m2) during induction resulted in prolonged myelosuppression and subsequent infectious related complications within the P1b cohort, prompting a reduction in the duration of VEN (Days 1–14) and dose of cytarabine to (1.5 g/m2). Following dose optimization, the overall response rate was 75% for P1b patients (N = 16) and 70% for patients with R/R-AML treated at the recommended phase 2 dose, 17 with respective CRc (CR + CRh + CRi) rates of 75% and 61%. Seventy-six percent of patients in salvage 1 or 2 attained a CRc. Impressively, 69% of R/R-AML patients in CRc attained MRD negativity.
After a median study follow-up of 12 months, patients treated at the phase 2 dose had a median duration of response and OS that was not reached; median EFS was 11 months. Estimated 12-month EFS and OS rates within this population were 41% and 68%, respectively, 38 representing an improvement compared to historical outcomes in R/R-AML. FLAG-IDA + VEN was effective in patients in salvage 1 or 2 (median OS: 14 months), and within the small, but particularly high-risk subgroup of R/R-AML patients who had received a prior HSCT (median OS: 13 months). Forty-six percent of patients with R/R-AML successfully transitioned to consolidative allogeneic HSCT, including six patients who had relapsed following prior transplantation and received a second allogeneic HSCT at response. 38
Adverse events within the R/R-AML cohorts were consistent with those observed in the ND-AML cohort treated with FLAG-IDA + VEN, with infectious complications predominating. Febrile neutropenia and bacteremia occurred in 51% and 46% of R/R-AML patients, with slightly higher rates of bacteremia observed in the phase 1b cohort compared to the phase 2 cohort (50% versus 43%). 38 While delayed count recovery following cycle 1 or 2 of FLAG-IDA + VEN was more common in patients with R/R-AML or sAML/tAML/ts-AML, median cycle lengths for patients with R/R-AML within the phase 2 cohort were 35, 37, and 39 days for cycles 1, 2, and 3, respectively. Thirty-day mortality and 60-day mortality within this high-risk patient cohort were 0% and 4.4%, respectively.
Fludarabine, cytarabine, and idarubicin (FLAVIDA)+venetoclax
A seven-day course of VEN in combination with fludarabine, cytarabine, and idarubicin (FLAVIDA) also appears to be effective in R/R-AML. 46 FLAVIDA was investigated in thirteen patients with R/R-AML that were predominantly younger (median age 49 years), in first salvage (range: 1–5), and had ELN intermediate and adverse risk (84.6%) AML. Treatment with FLAVIDA resulted in an ORR following induction of 69%, with a median duration of CR/CRi of 7.3 months. Two of these patients achieved MRD negativity. After a median follow-up of 9.3 months, estimated 6-month EFS and OS were 52% and 76%, respectively. 46 Nine patients successfully transitioned to allogeneic HSCT, including two patients who had received prior HSCT.
Common adverse events were similar to those observed with FLAG-IDA + VEN in the R/R setting—77% of patients developed neutropenic fever; 23% developed bacteremia. 46 The median time to neutrophil and platelet recovery (defined as an ANC ⩾ 0.5 x 109/L and a platelet count ⩾ 50 x 109/L) with a seven-day course of FLAVIDA was 33 and 36 days, similar to that observed in a historical cohort of patients treated with FLA-IDA (ANC ⩾ 0.5 x 109/L: 32 days; ⩾ 50 x 109/L: 39 days).
These findings within a R/R-AML population suggest that similar to ND-AML, VEN can be safely incorporated with intensive salvage chemotherapy to improve efficacy in high-risk, relapsed patient populations compared to standard salvage chemotherapy regimens. Importantly, VEN in conjunction with IC enabled the successful transition to allogeneic HSCT in a significant portion of patients with R/R-AML without compromising safety or significantly increasing rates of myelosuppression.
Liposomal daunorubicin and cytarabine (i.e., CPX-351)+venetoclax
VEN has also been investigated with the dual drug liposomal formulation of cytarabine and daunorubicin administered at a fixed 5:1 molar ratio (CPX-351)45,47 in a heavily pretreated (median of 2 prior therapies) R/R-AML population. Fifty percent (N = 9) of enrolled patients harbored a complex karyotype, and six (33%) had TP53-mutated AML. Due to cytopenias (notably on-target neutropenia and thrombocytopenia), an attenuated schedule of VEN (Days 2–8 rather than 2–21 of a 28-day cycle) in combination with CPX-351 is now under evaluation. 47 In the initial 18 patients reported to date (17 of whom had R/R-AML), the ORR was 44%; 37% (N = 6) attained a CR/CRi. Notably, the cohort included six patients with R/R-AML who had received prior VEN-based treatment. Within this small subgroup, the ORR (17%) was more modest. The sole patient reported to date with ND-AML attained an MRD-negative CR following induction. In the entire study cohort, the median OS was 6.4 months, while median OS and relapse-free survival were not reached among responding patients at the time of analysis. Thirty-day mortality and 60-day mortality were 11% and 22%, respectively.
The observed efficacy and toxicity profile in this adverse risk patient population are notable and warrant follow-up of ongoing dose-expansion cohorts in both ND and R/R-AML in order to define which patients may derive the most benefit from this potent combination. 47
Molecular determinates of response to venetoclax based induction regimens in AML
Intensive chemotherapy incorporating VEN evokes high initial response rates across all genomic subgroups in AML. In patients with ND-AML, FLAG-IDA + VEN resulted in CRc (CR + CRi + CRh) rates of 88%, 89%, and 89% in patients with ELN favorable, intermediate, or adverse risk AML. 42 Similarly, patients treated with CLIA + VEN with ELN favorable, intermediate, or adverse risk AML demonstrated 12-month survival rates of 78%, 93%, and 81%, respectively. 37
Analysis of molecular correlates of blast reduction within the CAVEAT trial identified marked reductions in bone marrow blasts in patients with ND-AML and mutations in NPM1 (56% reduction), IDH2 (55% reduction), or SRSF2 (47% reduction) following a 7-day VEN pre-phase prior to combining the ‘5 + 2’ schedule of idarubicin and cytarabine. 36 Consequently, patients with NPM1-, IDH2-, or SRSF2-mutated AML demonstrated favorable median OS (NPM1: 13.2 months; IDH2: not reached; and SRSF2: 31.3 months). 36 Similar to patients with ND-AML, patients with R/R-AML harboring mutations in NPM1, IDH1, or IDH2 demonstrated favorable outcomes to FLAG-IDA + VEN treatment. 38 Within this subgroup, the CRc rate was 100% and 12-month survival was 83%, with 71% of patients able to transition to HSCT with curative intent.10,34,38,48
Patients with ND-AML with mutations in signaling pathway genes (FLT3-ITD/TKD, RAS, PTPN11) or TP53 demonstrated more modest reductions in bone marrow blasts during the VEN pre-phase (17% and 7%, respectively) of the CAVEAT trial, correlating with inferior survival in patients with FLT3-ITD (median OS: 3.6 months) or TP53-mutated AML (median OS: 5.5 months), respectively. 14 Whereas mutations in signaling pathway genes (K/NRAS, PTPN11, FLT3, CBL, and KIT) were not prognostic in the frontline setting with FLAG-IDA + VEN, inferior survival was observed in R/R patients with such mutations compared to patients with wild-type signaling genes (median OS: 6 versus 16 months). A similar phenomenon was observed in patients with R/R-AML where mutations in tumor suppressor genes (TP53, WT1, FBXW7, or PHF6) were associated with significantly lower CRc rates (38%) and inferior survival (median OS: 7 months) with FLAG-IDA + VEN treatment in the R/R-AML setting. 17
TP53 mutations in particular appear to correlate with VEN resistance,29,31 with translational studies demonstrating intact p53 function integral to sustained VEN efficacy. 49 Single-cell sequencing analysis of four non-responding patients treated on the CAVEAT trial revealed outgrowth of resistant TP53-mutated subclones (some with cooperative signaling mutations) during the 7-day VEN pre-phase, believed in part due to an increased threshold for activation of the apoptotic effectors BAX and BAK. 49 BCL-2 inhibition combined with cytotoxic chemotherapy (cytarabine or decitabine) did not reduce outgrowth of TP53-mutated clones. Conversely, co-targeting of BCL-2 and the alternative antiapoptotic BH3-family protein MCL-1 resulted in reduced TP53-mutated clone size and improved survival in murine models, 49 further suggesting targeted combinations may prove more effective in TP53-mutated AML than VEN combined with cytotoxic induction chemotherapy.
Clinical experience supports the preclinical data. Ten patients (ND-AML:3; R/R-AML: 7) with TP53-mutated AML were included in the initial FLAG-IDA + VEN report; 40% were refractory to induction therapy. Median OS was 9 and 3.2 months in patients with ND and R/R-AML and TP53 mutations, respectively. 38 Persistent TP53 mutations were detected in 100% of patients who attained an MRD-negative CRc. 38 Of the two non-responding patients treated with CLIA + VEN, one harbored a TP53 mutation. 37
Other molecular aberrations necessitate more follow-up to determine their predictive impact with VEN-based treatment. Intriguingly, one patient with ELN favorable risk AML refractory to CLIA + VEN harbored a RUNX1-RUNXT1 t(8;21)(q22; q22) translocation consistent with core-binding factor AML, which may impart resistance to VEN based on preclinical data.29,31 In R/R-AML patients treated with FLAG-IDA + VEN, favorable risk (i.e., core-binding factor) cytogenetics also associated with an unexpectedly low median EFS (4 versus 7 months) and OS (7.6 versus 11 months) in comparison with patients with adverse or complex cytogenetics. However, a similar finding was not observed in pediatric patients with R/R core-binding factor AML (N = 6) treated with ‘7 + 3’+VEN where 83% (n = 5) attained a CR/CRi. 50 Of interest, the sole non-responding patient demonstrated mixed BCL-2 and BCL-XL dependence on BH3 profiling, suggesting variation in antiapoptotic protein dependency may exist in this leukemic subtype.
Conversely, patients with KMT2A-rearranged AML (traditionally considered an adverse cytogenetic feature) 51 also appear to respond favorably to VEN in combination with intensive chemotherapy. In patients treated with FLAG-IDA + VEN, 100% (N = 7; R/R-AML: 4, ND-AML:3) of patients with KMT2A-rearranged AML attained a CRc, with 80% attaining a MRD-negative CRc based on reverse transcriptase-polymerase chain reaction; 38 12-month OS in this molecular subgroup was an impressive 80%.
As VEN-based therapies continue to evolve in the treatment of both young and older patients with AML, a deeper understanding of the effects imparted by VEN in combination with various therapy backbones at the molecular and cellular level is warranted, necessitating larger correlative analyses of patients treated with VEN-based induction regimens to help confirm and expand these preliminary findings.
Future directions
The efficacy signals emanating from the early results of VEN-containing IC regimens are promising. Future work will further clarify clinical and molecular patient subgroups that benefit most from these treatments.
Patients with sAML have favorable responses with FLAG-IDA + VEN and CLIA + VEN, suggesting both may serve a role as frontline induction regimens given the notable activity within this high-risk patient subgroup, akin to the efficacy observed with CPX-351. 45 Frontline VEN-based regimens do not appear to have increased activity in patients with TP53-mutated AML; however, marked activity was noted in patients with intermediate- or adverse-risk AML, including subgroups of patients with other adverse-risk features (i.e., ASXL1, RUNX1, KMT2A-rearranged, adverse/complex cytogenetics without TP53 mutations). It seems plausible that within these patient populations, the use of VEN-containing regimens could further improve outcomes compared to historical standard of care regimens and should be considered for use. An ongoing phase 3 trial (NCT04628026) of 7 + 3 versus 7 + 3 + VEN will help validate these findings.
In the salvage setting, the high rate of MRD-negative remissions and successful transition to HSCT in patients with R/R-AML treated with FLAG-IDA + VEN provides evidence about the efficacy of the regimen in this difficult to treat patient population, where its use should be considered alongside other commonly used conventional cytotoxic chemotherapy-based salvage regimens (i.e., FLAG-IDA and MEC). Prospective randomized investigations establishing the efficacy of VEN with IC in the salvage setting would be a welcome addition to confirm the early efficacy signals reported in phase 2 investigations of R/R-AML.
An additional ensuing question of interest is whether HSCT may be omitted in certain patients who attain MRD-negative remissions, such as patients with ELN intermediate-risk AML, where exactly which patients benefit from consolidative HSCT remains undefined. Data presented at the 2021 Society of Hematologic Oncology found no significant difference in EFS or OS with the use of HSCT in patients treated with FLAG-IDA + VEN. 52 Whether these potent induction regimens incorporating VEN induce deeper responses and can effectively cure select patients and spare the use of consolidative HSCT remains a much-anticipated question.
Conclusions
Venetoclax is now an established standard of care in older, unfit patients with AML, and emerging data suggest a promising role when utilized in combination with induction and consolidation therapy in younger, fit patients. Early results from ongoing phase 1/2 studies indicate high rates of response and eradication of measurable residual disease in patients treated with VEN combinations. When indicated these potent regimens enable the successful transition to curative HSCT in both ND and R/R-AML and appear to improve upon historical standard of care therapies in several traditionally adverse-risk patient subgroups, suggesting VEN combinations may partially abrogate the negative prognostic impact of these adverse-risk defining molecular and cytogenetic characteristics.
Clinical investigations currently underway will aid in determining the optimal dosing and duration of IC and VEN combinations, thereby minimizing untoward toxicities including observed myelosuppression and infectious complications while maintaining therapeutic efficacy. Ongoing translational studies will undoubtedly continue to unravel the complex cellular and molecular mechanisms underpinning resistance to IC in combination with VEN, enabling more precise and individualized therapy. Expanded results of these ongoing early-phase trials in addition to trials implementing additional targeted therapies in combination with VEN will provide a pivotal path forward to guide therapy and further improve outcomes in patients with AML.
Footnotes
Author contributions: Curtis A. Lachowiez: Data curation; Formal analysis; Visualization; Writing – original draft; Writing – review & editing.
Himachandana Atluri: Formal analysis; Writing – original draft; Writing – review & editing.
Courtney D. DiNardo: Conceptualization; Data curation; Investigation; Supervision; Visualization; Writing – original draft; Writing – review & editing.
ORCID iD: Courtney D. DiNardo  https://orcid.org/0000-0001-9003-0390
https://orcid.org/0000-0001-9003-0390
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared the following potential conflicts of interest for the research, authorship, and/or publication of this article: Curtis A. Lachowiez and Himachandana Atluri: No conflicts of interest Courtney D. DiNardo: GlaxoSmithKline: Membership on an entity’s Board of Directors or advisory committees; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity’s Board of Directors or advisory committees; ImmuneOnc: Honoraria, Research Funding; Forma: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; AbbVie: Consultancy, Research Funding; Agios/Servier: Consultancy, Honoraria, Research Funding; Takeda: Honoraria; Novartis: Honoraria; Foghorn: Honoraria, Research Funding; Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding
Contributor Information
Curtis A. Lachowiez, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Himachandana Atluri, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Courtney D. DiNardo, Department of Leukemia, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA.
References
- 1. Kantarjian H, Kadia T, DiNardo C, et al. Acute myeloid leukemia: current progress and future directions. Blood Cancer J 2021; 11: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med 2015; 373: 1136–1152. [DOI] [PubMed] [Google Scholar]
- 3. Holowiecki J, Grosicki S, Giebel S, et al. Cladribine, but not fludarabine, added to daunorubicin and cytarabine during induction prolongs survival of patients with acute myeloid leukemia: a multicenter, randomized phase III study. J Clin Oncol 2012; 30: 2441–2448. [DOI] [PubMed] [Google Scholar]
- 4. Burnett AK, Russell NH, Hills RK, et al. A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients. Blood 2015; 125: 3878–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jabbour E, Short NJ, Ravandi F, et al. A randomized phase 2 study of idarubicin and cytarabine with clofarabine or fludarabine in patients with newly diagnosed acute myeloid leukemia. Cancer 2017; 123: 4430–4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burnett AK, Russell NH, Hills RK, et al. Optimization of chemotherapy for younger patients with acute myeloid leukemia: results of the medical research council AML15 trial. J Clin Oncol 2013; 31: 3360–3368. [DOI] [PubMed] [Google Scholar]
- 7. Short NJ, Rafei H, Daver N, et al. Prognostic impact of complete remission with MRD negativity in patients with relapsed or refractory AML. Blood Adv 2020; 4: 6117–6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Short NJ, Zhou S, Fu C, et al. Association of measurable residual disease with survival outcomes in patients with acute myeloid leukemia: a systematic review and meta-analysis. JAMA Oncol 2020; 6: 1890–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jongen-Lavrencic M, Grob T, Hanekamp D, et al. Molecular minimal residual disease in acute myeloid leukemia. N Engl J Med 2018; 378: 1189–1199. [DOI] [PubMed] [Google Scholar]
- 10. DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med 2020; 383: 617–629. [DOI] [PubMed] [Google Scholar]
- 11. Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA 2009; 301: 2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuykendall A, Duployez N, Boissel N, et al. Acute myeloid leukemia: the good, the bad, and the ugly. Am Soc Clin Oncol Educ Book 2018; 38: 555–573. [DOI] [PubMed] [Google Scholar]
- 13. Michelis FV, Atenafu EG, Gupta V, et al. Comparable outcomes post allogeneic hematopoietic cell transplant for patients with de novo or secondary acute myeloid leukemia in first remission. Bone Marrow Transpl 2015; 50: 907–913. [DOI] [PubMed] [Google Scholar]
- 14. Schlenk RF, Döhner K, Mack S, et al. Prospective evaluation of allogeneic hematopoietic stem-cell transplantation from matched related and matched unrelated donors in younger adults with high-risk acute myeloid leukemia: German-Austrian Trial AMLHD98A. J Clin Oncol 2010; 28: 4642–4648. [DOI] [PubMed] [Google Scholar]
- 15. Duval M, Klein JP, He W, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol 2010; 28: 3730–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andreeff M, Jiang S, Zhang X, et al. Expression of Bcl-2-related genes in normal and AML progenitors: changes induced by chemotherapy and retinoic acid. Leukemia 1999; 13: 1881–1892. [DOI] [PubMed] [Google Scholar]
- 17. Konopleva M, Pollyea DA, Potluri J, et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov 2016; 6: 1106–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vo T-T, Ryan J, Carrasco R, et al. Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell 2012; 151: 344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pollyea DA, Amaya M, Strati P, et al. Venetoclax for AML: changing the treatment paradigm. Blood Adv 2019; 3: 4326–4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Teh TC, Nguyen NY, Moujalled DM, et al. Enhancing venetoclax activity in acute myeloid leukemia by co-targeting MCL1. Leukemia 2018; 32: 303–312. [DOI] [PubMed] [Google Scholar]
- 21. Kale J, Osterlund EJ, Andrews DW. BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ 2018; 25: 65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Konopleva M, Contractor R, Tsao T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell 2006; 10: 375–388. [DOI] [PubMed] [Google Scholar]
- 23. Wilson WH, O’Connor OA, Czuczman MS, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol 2010; 11: 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Certo M, Del Gaizo Moore V, Nishino M, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 2006; 9: 351–365. [DOI] [PubMed] [Google Scholar]
- 25. Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med 2013; 19: 202–208. [DOI] [PubMed] [Google Scholar]
- 26. Niu X, Zhao J, Ma J, et al. Binding of released Bim to Mcl-1 is a mechanism of intrinsic resistance to ABT-199 which can be overcome by combination with daunorubicin or cytarabine in AML cells. Clin Cancer Res 2016; 22: 4440–4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yue X, Chen Q, He J. Combination strategies to overcome resistance to the BCL2 inhibitor venetoclax in hematologic malignancies. Cancer Cell Int 2020; 20: 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pei S, Pollyea DA, Gustafson A, et al. Monocytic subclones confer resistance to venetoclax-based therapy in patients with acute myeloid leukemia. Cancer Discov 2020; 10: 536–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bisaillon R, Moison C, Thiollier C, et al. Genetic characterization of ABT-199 sensitivity in human AML. Leukemia 2020; 34: 63–74. [DOI] [PubMed] [Google Scholar]
- 30. Wang H, Guo M, Wei H, et al. Targeting MCL-1 in cancer: current status and perspectives. J Hematol Oncol 2021; 14: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang H, Nakauchi Y, Köhnke T, et al. Integrated analysis of patient samples identifies biomarkers for venetoclax efficacy and combination strategies in acute myeloid leukemia. Nat Cancer 2020; 1: 826–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wei AH, Roberts AW, Spencer A, et al. Targeting MCL-1 in hematologic malignancies: rationale and progress. Blood Rev 2020; 44: 100672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Romine KA, Nechiporuk T, Bottomly D, et al. Monocytic differentiation and AHR signaling as primary nodes of BET inhibitor response in acute myeloid leukemia. Blood Cancer Discov 2021; 2: 518–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. DiNardo CD, Tiong IS, Quaglieri A, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood 2020; 135: 791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bose P, Gandhi V, Konopleva M. Pathways and mechanisms of venetoclax resistance. Leuk Lymph 2017; 58: 2026–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chua CC, Roberts AW, Reynolds J, et al. Chemotherapy and venetoclax in elderly acute myeloid leukemia trial (CAVEAT): a phase Ib dose-escalation study of venetoclax combined with modified intensive chemotherapy. J Clin Oncol 2020; 38: 3506–3517. [DOI] [PubMed] [Google Scholar]
- 37. Kadia TM, Reville PK, Borthakur G, et al. Venetoclax plus intensive chemotherapy with cladribine, idarubicin, and cytarabine in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: a cohort from a single-centre, single-arm, phase 2 trial. Lancet Haematol 2021; 8: e552–e561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. DiNardo CD, Lachowiez CA, Takahashi K, et al. Venetoclax combined with FLAG-IDA induction and consolidation in newly diagnosed and relapsed or refractory acute myeloid leukemia. J Clin Oncol 2021; 39: 2768–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017; 129: 424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stone RM, DeAngelo DJ, Galinsky I, et al. Phase I trial of escalating doses of the Bcl-2 inhibitor venetoclax in combination with daunorubicin/cytarabine induction and high dose cytarabine consolidation in previously untreated adults with acute myeloid leukemia (AML). Blood 2019; 134: 3908–3908. [Google Scholar]
- 41. Stone RM, DeAngelo DJ, Letai AG, et al. Maximal tolerated dose of the BCL-2 inhibitor venetoclax in combination with daunorubicin/cytarabine induction in previously untreated adults with acute myeloid leukemia (AML). Blood 2020; 136: 40–41. [Google Scholar]
- 42. Lachowiez C, DiNardo CD, Takahashi K, et al. Venetoclax combined with FLAG-IDA Induction and consolidation in newly diagnosed acute myeloid leukemia. Blood 2021; 138: 701–701. [DOI] [PubMed] [Google Scholar]
- 43. Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med 2019; 381: 1728–1740. [DOI] [PubMed] [Google Scholar]
- 44. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med 2017; 377: 454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol 2018; 36: 2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shahswar R, Beutel G, Klement P, et al. FLA-IDA salvage chemotherapy combined with a seven-day course of venetoclax (FLAVIDA) in patients with relapsed/refractory acute leukaemia. Br J Haematol 2020; 188: e11–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kadia T, Borthakur G, Takahashi K, et al. Phase II study of CPX-351 plus venetoclax in patients with acute myeloid leukemia (AML). Blood 2020; 136: 20–22. [Google Scholar]
- 48. Lachowiez CA, Loghavi S, Kadia TM, et al. Outcomes of older patients with NPM1-mutated AML: current treatments and the promise of venetoclax-based regimens. Blood Adv 2020; 4: 1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thijssen R, Diepstraten ST, Moujalled D, et al. Intact TP-53 function is essential for sustaining durable responses to BH3-mimetic drugs in leukemias. Blood 2021; 137: 2721–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Karol SE, Alexander TB, Budhraja A, et al. Venetoclax in combination with cytarabine with or without idarubicin in children with relapsed or refractory acute myeloid leukaemia: a phase 1, dose-escalation study. Lancet Oncol 2020; 21: 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Issa GC, Zarka J, Sasaki K, et al. Predictors of outcomes in adults with acute myeloid leukemia and KMT2A rearrangements. Blood Cancer J 2021; 11: 162–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lachowiez C, Takahashi K, Loghavi S, et al. Oral abstract: AML-204: venetoclax combined with FLAG-IDA induction and consolidation in newly diagnosed acute myeloid leukemia. Clin Lymph Myel Leuk 2021; 21: S201. [Google Scholar]