Abstract
Objective:
To describe mortality, healthcare resource utilization (HRU), and costs among Medicare beneficiaries with primary Clostridioides difficile infection (pCDI) or recurrent CDI (rCDI), with and without sepsis.
Methods:
We conducted a retrospective observational study of 100% Medicare Fee-for-Service claims from adults aged ⩾ 65 years with ⩾1 CDI episode between 1 January 2009 and 31 December 2017. Patients were continuously enrolled in Medicare Parts A/B/D 12 months before and up to 12 months after pCDI. ICD-9/10 codes defined CDI using ⩾1 inpatient claim, or ⩾1 outpatient claim plus ⩾1 claim for CDI treatment. The pCDI episode ended after 14 days without a CDI claim. rCDI episodes started within 8 weeks from the end of a previous CDI episode. ICD-9/10 codes identified all-cause sepsis over 12 month follow-up.
Results:
Of 497,489 CDI patients, 41.0% (N = 203,888) had sepsis; 57.7% with sepsis died versus 32.4% without sepsis. Among patients with pCDI only (N = 345,893) or ⩾1 rCDI (N = 151,596), 39.2% and 45.1% suffered sepsis, respectively. All-cause hospitalizations were frequent for all cohorts (range: 81–99%). Among patients who died, those with sepsis versus without had more-frequent intensive care unit (ICU) use (pCDI: 29% versus 15%; rCDI: 65% versus 34%), longer hospital stays (pCDI: 12 versus 10 days; rCDI: 12 versus 9 days), and higher per-patient-per-month costs (pCDI: $34,841 versus $22,753; rCDI: $42,269 versus $25,047). In both cohorts, sepsis patients who survived had higher total costs and all-cause HRU than those without sepsis. All p < 0.001 above.
Conclusions:
Sepsis was common among Medicare beneficiaries with CDI. CDI patients with sepsis, especially after an rCDI, experienced higher mortality, HRU, and costs compared with those without sepsis.
Keywords: Clostridioides difficile, Clostridium difficile infection, cost, healthcare resource utilization, recurrent CDI, Medicare, sepsis
Introduction
Clostridioides difficile (formerly Clostridium difficile) is a gram-positive, anaerobic, spore-forming bacillus that causes severe diarrhea and colitis (inflammation of the colon). 1 Clostridioides difficile infection (CDI) is the most common healthcare-associated infection in the United States, affecting an estimated 462,100 persons in 2017.2–4 Risk factors for CDI include older age, recent antibiotic exposure, long length of stay (LOS) in healthcare settings, serious underlying illness, or immunocompromising conditions.5,6 Individuals ⩾65 years are at disproportionately higher risk of CDI, 7 with an incidence of 11.6/1000 discharges compared to 3.5/1000 discharges among adults aged 18–64. 8 The mortality rate is high among older persons with CDI, with one in 11 people aged ⩾65 years dying within 1 month of experiencing CDI, accounting for 93% of CDI-related deaths in older adults.1,9 Among patients with a primary CDI episode (pCDI), up to 35% experience a recurrence (rCDI), and 40–65% of patients with one recurrence will experience two or more recurrences.10–15 rCDI is associated with a higher likelihood of death compared with pCDI, higher healthcare utilization, and results in direct healthcare costs totaling over $2.8 billion annually in the United States (2016 dollars). 16
The high mortality rate among patients with CDI is the result of serious complications that occur from the infection, including sepsis. 17 Previous studies report that sepsis occurs in 16–43% of patients with CDI, with higher frequency after rCDI and for older patients.18–21 This rate is much higher than the 6.0% incidence of sepsis for all hospitalized patients aged ⩾20 years in the United States. 22
Clostridioides difficile rarely survives in the blood since it is an anaerobic bacterium; therefore, sepsis associated with CDI is typically caused by other bacteria and is hypothesized to result from C. difficile breaking down the mucosal barrier of the colon, resulting in bacterial translocation into the bloodstream.23,24 Treatment of sepsis requires antimicrobials, which can subsequently alter the gut microbiota, weakening colonization resistance and propagating the cycle of recurrence of CDI.
Understanding the occurrence of sepsis among those with pCDI and rCDI, and the characteristics that place them at risk may help target therapies to reduce the occurrence of sepsis. Although there are studies in select populations examining epidemiologic and clinical aspects of sepsis among CDI patients,1,13,25,26 there are limited real-world studies describing the added burden of sepsis on healthcare resource utilization (HRU), costs, and mortality specifically among the high-risk elderly Medicare population.
The objective of this study was to describe the occurrence of sepsis among Medicare patients with pCDI only versus ⩾1 rCDI and evaluate mortality, patient characteristics, HRU, and costs among those with and without sepsis, further stratified by those who died versus survived.
Methods
Study design
This retrospective cohort study included beneficiaries from 100% Medicare Fee-For-Service (FFS) claims data administered by the Centers for Medicare and Medicaid Services (CMS). The information is generated through billing and reimbursement processes and included demographic and enrollment information; Medicare Parts A and B covered services; and Part D Prescription Drug Events.
Avalere accessed the 100% FFS claims through a research-focused data use agreement with CMS. The use of these data followed HIPAA requirements for the privacy and security of protected health information. Medicare beneficiaries covered by managed care organizations (Medicare Advantage), which comprised 23–33% of Medicare beneficiaries from 2009 to 2017 (the study period), 27 were not included.
Study population
Patients were eligible for inclusion if they were aged ⩾65 years, with ⩾1 CDI episode from 1 January 2009 to 31 December 2017 and continuously enrolled in Medicare Parts A, B, and D for 12 months before and up to 12 months after the pCDI episode. The index date (occurring between 1 January 2010 and 31 December 2016) was defined as the date of the first qualifying International Classification of Diseases ICD-9-CM or ICD-10-CM diagnosis code for CDI (Supplementary Appendix Table 1). CDI was identified as ⩾1 inpatient claim with a CDI diagnosis, or ⩾1 outpatient claim with CDI diagnosis plus ⩾1 claim for CDI treatment (vancomycin, fidaxomicin, metronidazole, rifaximin, or bezlotoxumab) or fecal microbiota transplant (FMT) (Supplementary Appendix Table 2).
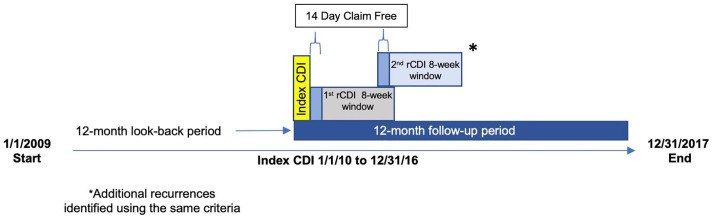
CDI episodes were defined as previously published 28 (Figure 1). An index CDI episode began on the date of the first CDI medical claim and included subsequent medical and drug claims that occurred ⩽14 days from the previous CDI claim as part of the same episode. Each distinct CDI episode ended after a 14-day CDI-claim-free period. An rCDI episode occurred within an 8-week window following the 14-day CDI-claim-free period. CDI events that occurred after the 8-week window were not included because they were not considered an rCDI, in accordance with the Centers for Disease Control and Prevention definition (2019), but rather as new infections. Patients were categorized as having a pCDI only or any (⩾1) rCDI.
Figure 1.
Study design: Definition of index CDI episode, including CDI claims (black bar), the 14-day CDI-claim-free period after last CDI claim (gray bar), and the 8-week period to identify rCDI (yellow bar).
Outcomes
Patients with pCDI or rCDI were further grouped into those who did and did not develop sepsis at any point after index CDI (by ICD-9-CM and ICD-10-CM codes, Supplementary Appendix Table 1). All-cause outcomes among those with and without sepsis included HRU [inpatient admissions, intensive care unit (ICU) stays, mean LOS] and per-patient-per-month (PPPM) costs. Mean (median, SD) time to the first diagnosis of sepsis was calculated for patients with sepsis. All-cause total costs included all medical and pharmacy costs and were reported by expenditure category [inpatient stays, emergency department visits, outpatient services and tests, postacute care, pharmacy, and durable medical equipment (DME)]. All outcome measures were calculated for patients with and without sepsis and stratified by those who died and survived after pCDI or rCDI.
Other variables
Demographic variables including age, sex, geographic region, race/ethnicity, reason for entitlement to Medicare [age or disability/end-stage renal disease (ESRD)], and dual eligibility for Medicaid were measured at index. Clinical variables including Charlson Comorbidity Index (CCI), 29 other medical conditions (ulcerative colitis, Crohn’s disease, type 1 diabetes), indicators of frailty, and procedures and treatments (transplants, gastrointestinal surgery, enteral feeding, and chemotherapy) were measured during the 12-month preindex period. Hospitalizations, LOS, and total costs were measured during the 0–6 months before index, with the goal to capture potential CDI precipitating events.
Data analysis
Descriptive analyses examined the demographic and clinical characteristics, HRU [hospitalization (yes or no), ICU stay (yes or no), LOS], and costs (PPPM) for patients with and without sepsis, stratified by pCDI or rCDI and survival status. Counts and percentages summarized categorical variables, while measures of central tendency (mean [standard deviation], median) summarized continuous variables. p values were calculated using the chi-square test for proportions and t test for means. Costs were adjusted to 2018 dollar values using the medical component of the Consumer Price Index. 30
Multivariate logistic regression models with binomial distribution were used to compare differences in inpatient hospitalization and ICU stays, while generalized linear models with gamma distribution and log link were used to compare LOS and total cost outcomes. Covariates included demographic and clinical variables measured at baseline and were chosen based on baseline differences and clinical relevance. All analyses were conducted with SAS, version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
Demographics
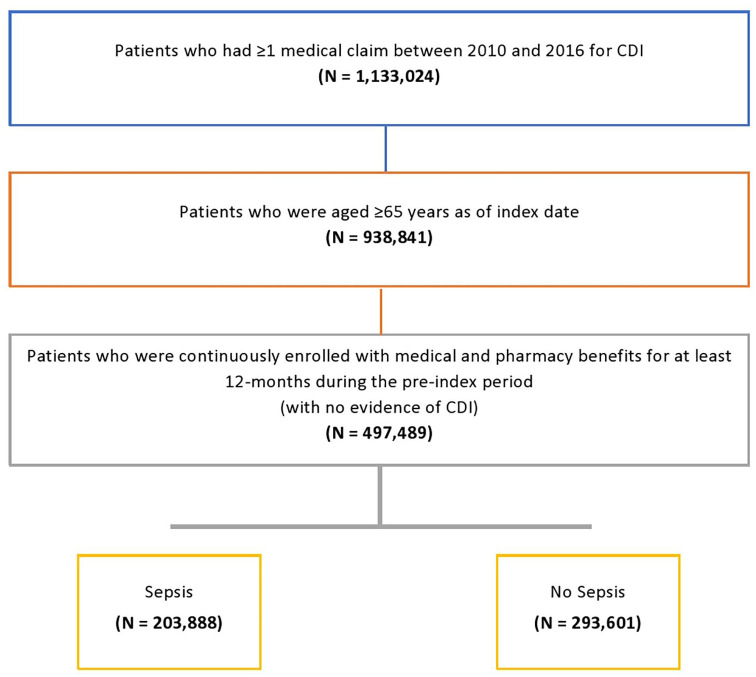
A total of 1,133,024 patients with CDI were identified from 2010 to 2016 (Figure 2). After restricting to those aged ⩾65 years on index date with at least 12 months preindex continuous enrollment, 497,489 patients with CDI were included in the analysis. Among those, 203,888 (41.0%) developed sepsis, and 57.7% of patients with sepsis died during the 12-month follow-up period, compared with 32.4% of patients without sepsis (p < 0.001) (Table 1, Figure 2). Of the 345,893 (69.5%) patients with pCDI only, 39.2% suffered sepsis compared with 45.1% of the 151,596 patients with rCDI. Patients with pCDI and rCDI with sepsis, compared with those without, were younger (range 77.5–80.7 versus 78.3–82.4 years, p < 0.0001), less often female (58.4–64.0% versus 63.7–70.9%, p < 0.0001), more often dual-eligible/low income (40.4–48.0% versus 28.8–38.6%, p < 0.0001), more often Black (range 10.7–14.9% versus 6.2–9.2%, p < 0.0001), or other minority race (5.2–6.3% versus 3.1–4.2%, p < 0.0001), and with higher mean CCI scores (6.0–7.4 versus 4.7–7.0, p < 0.0001).
Figure 2.
Study attrition diagram.
Table 1.
Baseline patient characteristics and 0–6 months preindex utilization and costs for CDI patients with and without sepsis.
| Died | Survived | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary CDI only | Any recurrent CDI* | Primary CDI only | Any recurrent CDI* | ||||||||||
| No sepsis | Sepsis | p value | No sepsis | Sepsis | p value | No sepsis | Sepsis | p value | No sepsis | Sepsis | p value | ||
| N = 74,804 | N = 84,093 | <0.0001 | N = 20,332 | N = 33,526 | <0.0001 | N = 135,636 | N = 51,360 | <0.0001 | N = 62,829 | N = 34,909 | <0.0001 | ||
| Age | Mean (SD) | 82.4 (8.5) | 80.7 (8.4) | <0.0001 | 81.8 (8.4) | 79.6 (8.2) | <0.0001 | 78.6 (8.0) | 78.0 (8.0) | <0.0001 | 78.3 (8.0) | 77.5 (7.9) | <0.0001 |
| Sex | Female (%) | 63.7 | 59.9 | <0.0001 | 64.6 | 58.4 | <0.0001 | 70.8 | 64.0 | <0.0001 | 70.9 | 63.6 | <0.0001 |
| Race | White (%) | 86.5 | 79.7 | <0.0001 | 89.1 | 78.9 | <0.0001 | 88.4 | 82.3 | <0.0001 | 90.7 | 84.1 | <0.0001 |
| Black (%) | 9.2 | 14.0 | <0.0001 | 7.8 | 14.9 | <0.0001 | 7.8 | 11.7 | <0.0001 | 6.2 | 10.7 | <0.0001 | |
| Other | 4.2 | 6.3 | <0.0001 | 3.1 | 6.2 | <0.0001 | 3.8 | 6.0 | <0.0001 | 3.1 | 5.2 | <0.0001 | |
| Reason for entitlement | Age (%) | 84.6 | 80.2 | <0.0001 | 84.2 | 77.6 | <0.0001 | 82.5 | 76.2 | <0.0001 | 82.6 | 75.9 | <0.0001 |
| Disability and/or ESRD (%) | 15.4 | 19.8 | <0.0001 | 15.8 | 22.4 | <0.0001 | 17.5 | 23.8 | <0.0001 | 17.4 | 24.1 | <0.0001 | |
| Dual eligible for Medicaid (%) | 37.3 | 43.6 | <0.0001 | 38.6 | 48.0 | <0.0001 | 32.2 | 44.1 | <.0001 | 28.8 | 40.4 | <0.0001 | |
| Census region | Northeast (%) | 21.9 | 23.8 | <0.0001 | 22.3 | 22.9 | 0.0861 | 20.6 | 22.3 | <0.0001 | 21.6 | 22.5 | 0.0013 |
| Midwest (%) | 25.6 | 22.0 | <0.0001 | 27.8 | 23.5 | <0.0001 | 26.2 | 22.5 | <0.0001 | 28.7 | 25.0 | <0.0001 | |
| South (%) | 39.3 | 37.5 | <0.0001 | 37.9 | 37.2 | 0.1028 | 38.7 | 36.4 | <0.0001 | 35.5 | 34.9 | 0.0497 | |
| West (%) | 13.2 | 16.7 | <0.0001 | 12.0 | 16.4 | <0.0001 | 14.4 | 18.7 | <0.0001 | 14.1 | 17.6 | <0.0001 | |
| Charlson comorbidity index, mean (SD) | 7.0 (3.6) | 7.3 (3.6) | <0.0001 | 6.8 (3.6) | 7.4 (3.6) | <0.0001 | 4.7 (3.3) | 6.0 (3.4) | <0.0001 | 4.7 (3.3) | 6.1 (3.5) | <0.0001 | |
| Inpatient hospitalization (%) | 70.7 | 74.7 | <0.0001 | 72.5 | 77.8 | <0.0001 | 50.4 | 62.6 | <0.0001 | 55.5 | 69.6 | <0.0001 | |
| ICU stay (%) | 31.4 | 37.5 | <0.0001 | 31.2 | 40.3 | <0.0001 | 19.2 | 29.0 | <0.0001 | 20.9 | 32.4 | <0.0001 | |
| Inpatient LOS days, mean (SD) | 7.5 (6.8) | 8.7 (8.5) | <0.0001 | 7.1 (6.6) | 8.7 (10.4) | <0.0001 | 6.6 (6.8) | 7.9 (8.6) | <0.00001 | 6.6 (6.8) | 7.8 (7.8) | <0.0001 | |
| Total cost $**, mean (SD) | $55,396 ($106,316) | $69,789 ($125,945) | <0.0001 | $56,713 ($105,311) | $76,055 ($136,611) | <0.0001 | $34,991 ($81,494) | $53,182 ($110,498) | <0.0001 | $39,737 ($90,503) | $60,644 ($118,211) | <0.0001 | |
ESRD, end-stage renal disease; ICU, intensive care unit; LOS, length of stay; SD, standard deviation.
Inflation adjusted to 2018 $US. Includes all medical and pharmacy costs.
p Values were calculated using chi-square test for proportions and t test for means.
Preindex clinical and economic characteristics
CDI patients who developed sepsis during follow-up had a higher prevalence of comorbid and frailty conditions, transplants, GI surgery, enteral feeding, and chemotherapy in the 12 months prior to index CDI (Supplementary Appendix Table 3). Those who experienced rCDI + sepsis and died had the heaviest burden of preindex conditions and treatments, reflected in the higher HRU and costs during the 0- to 6-month period preceding the index pCDI (Table 1). For example, preindex hospitalizations were higher across the pCDI/rCDI cohorts for those who developed sepsis (range 62.6–77.8% versus 50.4–72.5%, p < 0.0001) with approximately 1 day longer LOS (7.8–8.7 versus 6.6–7.5 days, p < 0.0001). Total healthcare costs were approximately $20,000 higher among patients with sepsis ($53,182–$76,055 versus $34,991–$56,713, p < 0.0001). Patients with any rCDI + sepsis who died had the highest preindex utilization and costs [hospitalizations 77.8%; mean LOS 8.7 days (SD 10.4); mean total costs $76,055 (SD $136,611)].
Postindex HRU and costs
Postindex, the median length of follow-up among those who died varied from a low of 12 days for those with pCDI + sepsis to 88 days for those with rCDI + no sepsis (p < 0.0001) (Table 2). Mean time to first rCDI ranged from 31.6 days for those with no sepsis who died to 35 days for those with sepsis who survived. All-cause hospitalizations were common in all cohorts (range: 81–99%), but among patients who died, patients with sepsis had substantially higher all-cause ICU use (pCDI: 29% versus 15%; rCDI: 65% versus 34%, p < 0.0001) and longer LOS (pCDI: 12.1 versus 10.2 days; rCDI: 11.6 versus 8.8 days, p < 0.0001). All-cause HRU was lower overall among patients who survived; however, those with sepsis who survived had higher ICU use (pCDI: 38.0% versus 14.9%; rCDI: 49.2% versus 19.1%, p < 0.0001) and longer LOS (pCDI: 9.1 versus 6.9 days; rCDI: 9.0 versus 6.6 days, p < 0.0001) compared with those without sepsis.
Table 2.
All-cause unadjusted healthcare resource utilization for CDI patients with and without sepsis: 12-months postindex follow-up.
| Died | Survived | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary CDI only | Any recurrent CDI a | Primary CDI only | Any recurrent CDI a | |||||||||
| No sepsis | Sepsis | p value | No sepsis | Sepsis | p value | No sepsis | Sepsis | p value | No sepsis | Sepsis | p value | |
| N = 74,804 | N = 84,093 | N = 20,332 | N = 33,526 | N = 135,636 | N = 51,360 | N = 62,829 | N = 34,909 | |||||
| Length of follow-up (days),b mean (SD) | 66.1 (91.0) | 60.5 (91.8) | <0.0001 | 121.0 (92.6) | 116.8 (91.5) | <0.0001 | 353.9 (51.4) | 346.8 (64.9) | <0.0001 | 356.5 (43.6) | 351.9 (52.5) | <0.0001 |
| Median | 22.0 | 12.0 | <0.0001 | 88.0 | 84.0 | <0.0001 | 365 | 365 | <0.0001 | 365 | 365 | <0.0001 |
| Time from index CDI to first recurrence (days), mean (SD) | NA | NA | 31.6 (15.3) | 33.5 (15.6) | <0.0001 | NA | NA | 33.3 (20.1) | 35.0 (20.2) | <0.0001 | ||
| Median | NA | NA | 27.0 | 30.0 | <0.0001 | NA | NA | 28.0 | 30.0 | <0.0001 | ||
| Inpatient hospitalization (%) | 91.2 | 95.7 | <0.0001 | 92.2 | 99.2 | <0.0001 | 81.6 | 97.9 | <0.0001 | 82.8 | 99.0 | <0.0001 |
| ICU stay (%) | 15.1 | 29.1 | <0.0001 | 33.8 | 65.0 | <0.0001 | 14.9 | 38.0 | <0.0001 | 19.1 | 49.2 | <0.0001 |
| Inpatient average LOS (days) – mean (SD) | 10.2 (10.3) | 12.1 (13.5) | <0.0001 | 8.8 (7.4) | 11.6 (11.5) | <0.0001 | 6.9 (6.5) | 9.1 (9.1) | <0.0001 | 6.6 (5.7) | 9.0 (7.7) | <0.0001 |
ICU, intensive care unit; LOS, length of stay; NA, not applicable; SD, standard deviation.
Any recurrent CDI during follow-up of up to 12 months.
Mean follow-up time for those who survived is <365 days due to patients who disenrolled for reasons other than death.
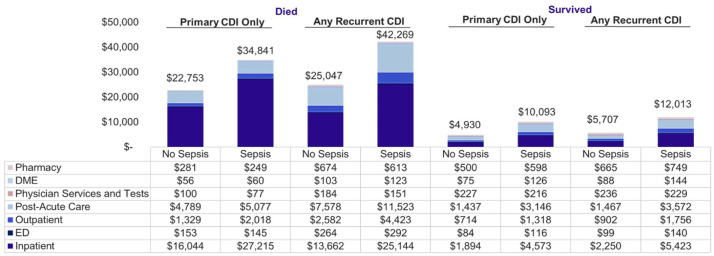
Among patients who died, total healthcare costs were highest among were those with rCDI + sepsis (pCDI: $34,841 versus $22,753; rCDI: $42,269 versus $25,047, p < 0.0001) (Figure 3). Costs for patients who survived were lower but followed a similar pattern for those with and without sepsis (pCDI: $10,093 versus $4930; rCDI: $12,013 versus $5707, p < 0.0001). Inpatient costs accounted for 39–78% of total costs.
Figure 3.
Unadjusted mean healthcare costs (PPPM) by sepsis and mortality status**: 12-months postindex follow-up. Costs were inflation adjusted to 2018 $USD.
**All p < 0.001.
DME, durable medical equipment; ED, emergency department; PPPM: per-patient per-month; USD, US dollars.
The mean [median (SD)] time to sepsis following a pCDI episode was 33 [0 (77)] days and following rCDI was 52 days [9 (81)]. For patients with pCDI, 83.3% experienced sepsis within 60 days of CDI index, while 54.4% of rCDI patients experienced sepsis within 60 days of the first rCDI episode. Among patients who died, after adjusting for demographic and clinical characteristics (Table 3), patients with pCDI + sepsis had more than twice the odds of ⩾1 inpatient stay [odds ratio (OR) 2.4 (95% confidence interval [CI] 2.3–2.5)] with slightly longer mean LOS (12.4 versus 11.3 days, p < 0.0001) and twice the odds of an ICU stay [OR 2.2 (95% CI 2.1–2.3)] compared with those without sepsis. Patients with rCDI + sepsis who died had 10× the odds of an inpatient stay [OR 10.6 (95% CI 9.3–12.2)] with longer LOS (11.7 versus 9.6 days, p < 0.0001), and 3× the odds of an ICU stay [OR 3.4 (95% CI 3.3–3.5)] compared with those without sepsis who died. Patients with sepsis who died also had significantly higher adjusted costs compared with those without sepsis who died (pCDI: $10,721 higher PPPM; rCDI: $16,003 higher PPPM, p < 0.0001).
Table 3.
All-cause adjusted a healthcare resource utilization and costs among Medicare patients with and without sepsis: 12-months postindex follow-up.
| Primary CDI only | Any recurrent CDI b | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Died | Died No Sepsis (Ref), N = 74,804 | Died Sepsis, N = 84,093 |
OR difference (95% CI) | p value | Died No Sepsis (Ref), N = 20,332 | Died sepsis, N = 33,526 | OR difference (95% CI) | p value | |
| Inpatient hospitalization (yes: ⩾ 1) | % | 91.7 | 96.4 | 2.4 (2.3, 2.5) | <0.0001 | 95.5 | 99.6 | 10.6 (9.3, 12.2) | <0.0001 |
| ICU stay (yes: ⩾1) | % | 16.4 | 30.0 | 2.2 (2.1, 2.3) | <0.0001 | 38.9 | 68.2 | 3.4 (3.3, 3.5) | <0.0001 |
| LOS (days) | Mean | 11.3 | 12.4 | 1.1 (1.1, 1.1) | <0.0001 | 9.6 | 11.7 | 2.1 (2.1, 2.1) | <0.0001 |
| Total cost (PPPM) c | Mean | $26,771 | $37,492 | $10,721 ($10,652, $10,792) | <0.0001 | $29,396 | $45,399 | $16,003 ($15,867, $16,138) | <0.0001 |
| Survived | Survived No sepsis (Ref), N = 135,636 |
Survived Sepsis, N = 51,360 |
OR difference (95% CI) | p value | Survived No sepsis (Ref), N = 62,829 | Survived Sepsis, N = 34,909 | OR difference (95% CI) | p value | |
| Inpatient hospitalization (yes: ⩾1) | % | 86.3 | 98.4 | 9.5 (8.9, 10.1) | <0.0001 | 88.6 | 99.3 | 18.5 (16.6, 20.7) | <0.0001 |
| ICU stay (yes: ⩾1) | % | 15.3 | 34.7 | 3.0 (2.9, 3.0) | <0.0001 | 21.0 | 48.1 | 3.5 (3.4, 3.6) | <0.0001 |
| LOS (days) | Mean | 7.2 | 9.0 | 1.8 (1.8, 1.8) | <0.0001 | 6.9 | 8.8 | 1.9 (1.9, 1.9) | <0.0001 |
| Total cost (PPPM) c | Mean | $5,551 | $9,567 | $4,016 ($3,970, $4,063) |
<0.0001 | $6,611 | $11,818 | $5,207 ($5,138, $5,277) | <0.0001 |
Logistic regression models with binomial distribution used for hospitalization and ICU outcomes; generalized linear models with gamma distribution and log link used for LOS and cost outcomes. Adjusted for age at index date (65–74, 75–84, and 85+ years), gender, race (White, Black, and other/unknown), geographic census region (MW, NE, S, and W), dual eligible, original reason for entitlement to Medicare (aged, disabled, and ESRD), CCI score (continuous), myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic obstructive pulmonary disease, rheumatologic disease, peptic ulcer disease, mild liver disease, moderate/severe liver disease, diabetes without complications, diabetes with complications, hemiplegia or paraplegia, renal disease, malignancy, metastatic solid tumor, ulcerative colitis, Crohn’s disease, type 1 diabetes, abnormality of gait, abnormal loss of weight and underweight, adult failure to thrive, debility, difficulty in walking, fall, muscle weakness, pressure ulcer, transplants, gastrointestinal surgery, enteral feeding, and chemotherapy.
Any recurrent CDI during follow-up of up to 12 months.
Inflation adjusted to 2018 $US.
CI, confidence interval; ICU, intensive care unit; LOS, length of stay; OR, odds ratio; PPPM, per-patient per-month.
Adjusted results for CDI patients who survived followed a similar pattern to those who died. Surviving patients with pCDI + sepsis had 9.5× the odds of ⩾1 inpatient stay [OR 9.5 (95% CI 8.9–10.1)] with longer LOS (9.0 versus 7.2 days, p < 0.0001), and 3× more likely to have an ICU stay [OR 3.0 (95% CI 2.9–3.0)] compared with those without sepsis. Patients with rCDI + sepsis were 18× more likely to have an inpatient stay [OR 18.5 (95% CI 16.6–20.7)] with longer LOS (8.8 versus 6.9 days, p < 0.0001), and 3.5× more likely to have an ICU stay [OR 3.5 (95% CI 3.4–3.6)] compared with those without sepsis. Surviving patients with sepsis had significantly higher adjusted costs compared with those without sepsis (pCDI: $4016 higher; rCDI: $5207 higher, p < 0.0001).
Discussion
This study found that sepsis is a common complication of CDI among Medicare beneficiaries aged ⩾65 years, resulting in higher all-cause mortality, healthcare resource utilization, and costs. Those with sepsis and rCDI experienced the heaviest clinical and economic burden, with those who died experiencing the highest burden of all. Patients who developed sepsis during follow-up had more comorbidities and HRU prior to their index CDI and were more likely to be Black or other minority, disabled, and dual eligible for Medicaid (indicating low-income status). Together, these data show that CDI and sepsis are a deadly combination among older patients who also have a higher risk for CDI and sepsis.
Although previous studies have shown that CDI is an important risk factor for sepsis, 31 this study found that sepsis in patients with CDI is more common in the Medicare population than previously reported in other populations and has a more significant impact on HRU, costs, and mortality. Our study revealed that 41.0% of older patients with CDI and 45.1% of those with rCDI developed sepsis. Other studies have reported lower rates, possibly because they reflect younger populations and shorter follow-up times. A study of patients treated with mechanical ventilation for >48 h within ICUs reported septic shock affected 34.7% of CDI patients. 21 A retrospective study found that 18.3% of patients with CDI developed a bloodstream infection (BSI) within 30 days of CDI. 18 A single-center study of patients with rCDI found a 22% BSI rate after CDI treatment with antibiotics. 20 In a claims study among a younger population (mean age 48 years) with CDI, sepsis occurred in 16.5%, 27.3%, 33.1%, and 43.3% of patients after 0, 1, 2, and 3+ rCDI episodes, respectively, during the 12-month follow-up, 19 supporting our finding of increased incidence among those with rCDI. The mean time to CDI recurrence in that study was approximately 30 days, similar to our study (range 31.6–35.0 days).
The mortality rate after sepsis was high in the Medicare population, with 57.7% of sepsis patients dying within 12 months of index CDI. Prior studies have reported lower mortality rates among CDI patients with sepsis but had shorter follow-up times and focused on specific populations. Ianiro et al. 20 reported a 90-day mortality rate of 52.5% for those who developed a BSI after CDI. A study of all hospitals in the United States that performed elective open abdominal vascular operations among the Medicare population found 30% 30-day mortality rates after discharge for CDI + sepsis. 32 It is clear that patients with CDI frequently experience sepsis afterward, and our study further reinforces that burden in the older Medicare population. Considering that almost six in 10 Medicare beneficiaries with CDI + sepsis will die within 12 months, the urgency for optimized therapy to decrease recurrences and diminish the future risk of poor outcomes takes on added importance.
Coinciding with the heavy clinical burden of sepsis were higher HRU and costs, especially for those with rCDI and those who died. Comparable utilization and economic outcomes specific to patients with CDI and sepsis are lacking, but CDI studies overall support our results, reporting that CDI is associated with high healthcare costs, driven primarily by inpatient costs, 33 with rCDI costing more than pCDI. 34 A claims-based study from 2010 to 2017 of patients aged 18–64 years found mean annual direct medical costs per patient were $71,980 for pCDI and $207,733 for those with ⩾3 rCDI. 35 Use of emerging therapies (i.e. fidaxomicin, bezlotoxumab, and fecal microbiota transplantation) were low in our study, with <5% of patients in any subgroup having a claim for one of these treatments; therefore, while the per use cost of these therapies may have been higher than other CDI treatments, the overall contribution of the emerging therapies to the 12-month follow-up costs is not expected to be a substantial component.
Previous studies have reported a LOS of 6–11 days for CDI patients in the United States,34,36 which is similar to the LOS reported in our study for patients without sepsis (6.6–10.2 days), but lower than the LOS for patients with sepsis (9.0–12.1 days). The longer patients stay in the hospital, the more likely they are to suffer from other inpatient complications, such as deep vein thrombosis and other healthcare-associated infections. Our data also showed that if primary CDI is occurring alone, it is possible to treat as outpatient for a small subset of patients, but when it is occurring with sepsis, hospitalization is almost always needed. Prevention of CDI is the key to avoiding hospitalized patients from having unnecessary additional days and avoiding hospital readmissions, which could occur due to CDI and sepsis.
Prevention of CDI has been a national priority for a number of years, 37 with efforts to enhance infection control measures and improve antimicrobial stewardship across healthcare settings. While prevention is a way to limit incidence, many patients still acquire CDI. Understanding the risk factors associated with CDI occurrence, recurrence, and poor outcomes will help clinicians triage those patients who might require more aggressive therapeutics to lower their risks of CDI. In our study, racial/ethnic minorities, those with disabilities, and low-income patients were particularly susceptible to poor outcomes. These groups also tend to have more medical comorbidities and potentially less access to outpatient care than those in other demographics. Clinicians should focus on these groups to ensure an optimal plan of care for patients to receive needed therapies and complete recommended treatments. The key prevention point is to reduce the risk of recurrent CDI due to the heightened patient harm. Our study findings may help justify personnel time to bolster follow-up for patients who had primary CDI, and to establish appropriate discharge protocol aiming at reducing risk of future rCDI. At-risk patients can benefit from a diverse clinical team contributing to the transition of care following hospital discharge, ensuring the best outcomes possible. More aggressive treatment at an earlier point in the disease can prevent future complications, which harm the patients and are expensive.
This study is strengthened by its large representative sample of older Medicare beneficiaries, who comprise the largest proportion of CDI patients in the United States, and the use of comprehensive Medicare claims. There are limitations of Medicare claims data that do not impact this analysis but may limit the generalizability of results. The Medicare FFS population may not reflect the experience of CDI patients with other insurance, such as Medicare Advantage, 38 Medicaid, or commercial coverage. The identification of CDI and sepsis relies on accurate reporting of diagnosis codes and treatments on claims; therefore, some misclassification is possible. Furthermore, claims data only capture services that have billing codes; reimbursement for services not paid for by Medicare, such as those paid for by Medigap coverage, is not included. Care provided in settings such as the Veterans Administration or long-term skilled nursing homes is not included. This study type cannot be used to determine cause and effect (i.e., whether sepsis was attributable to prior CDI), and there is no way to control for residual, unmeasured confounding when comparing those with and without sepsis.
Sepsis occurred commonly among Medicare beneficiaries with CDI, and CDI patients with sepsis were much more likely to die than those without sepsis. CDI patients with sepsis, and especially those with recurring CDI, have substantially higher healthcare resource utilization and costs compared with patients without sepsis. Strategies to reduce CDI may be an effective path to reducing the occurrence of sepsis and thereby risk of death, in addition to lowering the added clinical and economic burden among the growing elderly Medicare population.
Supplemental Material
Supplemental material, sj-docx-1-tai-10.1177_20499361221095679 for Mortality, healthcare resource utilization, and cost among Medicare beneficiaries with Clostridioides difficile infection with and without sepsis by Alpesh Amin, Winnie W. Nelson, Jill Dreyfus, Anny C. Wong, Iman Mohammadi, Christie Teigland, David N. Dahdal and Paul Feuerstadt in Therapeutic Advances in Infectious Disease
Acknowledgments
Editorial support was provided by Agnella Izzo Matic, PhD, CMPP and was funded by Ferring Pharmaceuticals Inc.
Footnotes
Ethics approval and consent to participate: This study was exempt from institutional review board approval, as it did not involve any interventional biomedical research with human participants. The data used were de-identified medical and pharmacy claims data, and they were obtained by HIPAA-compliant methods. As such, informed consent was not required.
Previous presentation: Portions of this work were presented as a poster at Digestive Disease Week (DDW) 2021 (virtual from 21 to 23 May 2021).
Author contribution(s): Alpesh Amin: Investigation; Validation; Writing – original draft; Writing – review & editing.
Winnie W. Nelson: Conceptualization; Data curation; Supervision; Writing – original draft; Writing – review & editing.
Jill Dreyfus: Data curation; Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Anny C. Wong: Data curation; Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Iman Mohammadi: Data curation; Formal analysis; Methodology; Validation; Visualization; Writing – original draft; Writing – review & editing.
Christie Teigland: Data curation; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
David N. Dahdal: Data curation; Project administration; Validation; Writing – original draft; Writing – review & editing.
Paul Feuerstadt: Data curation; Investigation; Validation; Writing – original draft; Writing – review & editing.
ORCID iD: Alpesh Amin  https://orcid.org/0000-0002-9790-0245
https://orcid.org/0000-0002-9790-0245
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this study was provided by Ferring Pharmaceuticals Inc.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DD and WWN were employees of Ferring Pharmaceuticals at the time of the research and manuscript development. IM and CT are employees of Avalere Health. JD and AW were employees of Avalere Health at the time of the research and manuscript development. PF: Merck and Co.: Speakers Bureau; Ferring/Rebiotix: Consulting, Advisory Board; SERES Therapeutics: Advisory Board; Takeda Pharmaceuticals: Advisory Board. AA: PI or co-I of clinical trials sponsored by NIH/NIAID, NeuroRx Pharma, Pulmotect, Blade Therpeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, Alexion. He has served as a speaker and/or consultant for BMS, Pfizer, BI, Portola, Sunovion, Mylan, Salix, Alexion, AstraZeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achogen LaJolla, Ferring, Seres, Millenium, HeartRite, Aseptiscope, Sprightly.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Alpesh Amin, UCI Medical Center, 101 The City Drive, City Tower, Suite 500, Orange, CA 92868, USA.
Winnie W. Nelson, Ferring Pharmaceuticals Inc., Parsippany, NJ, USA
Jill Dreyfus, Avalere Health, Washington, DC, USA.
Anny C. Wong, Avalere Health, Washington, DC, USA
Iman Mohammadi, Avalere Health, Washington, DC, USA.
Christie Teigland, Avalere Health, Washington, DC, USA.
David N. Dahdal, Ferring Pharmaceuticals Inc., Parsippany, NJ, USA
Paul Feuerstadt, PACT Gastroenterology Center, Hamden, CT, USA; Yale School of Medicine, Yale University, New Haven, CT, USA.
References
- 1. Depestel DD, Aronoff DM. Epidemiology of Clostridium difficile infection. J Pharm Pract 2013; 26: 464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ma GK, Brensinger CM, Wu Q, et al. Increasing incidence of multiply recurrent Clostridium difficile infection in the United States: a cohort study. Ann Intern Med 2017; 167: 152–158. [DOI] [PubMed] [Google Scholar]
- 3. Guh AY, Mu Y, Winston LG, et al. Trends in U.S. burden of Clostridioides difficile infection and outcomes. N Engl J Med 2020; 382: 1320–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Magill SS, O’Leary E, Janelle SJ, et al. Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med 2018; 379: 1732–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnson S. Recurrent Clostridium difficile infection: causality and therapeutic approaches. Int J Antimicrob Agents 2009; 33(Suppl. 1): S33–S36. [DOI] [PubMed] [Google Scholar]
- 6. Loo VG, Bourgault A-M, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med 2011; 365: 1693–1703. [DOI] [PubMed] [Google Scholar]
- 7. Balsells E, Shi T, Leese C, et al. Global burden of Clostridium difficile infections: a systematic review and meta-analysis. J Glob Health 2019; 9: 010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pechal A, Lin K, Allen S, et al. National age group trends in Clostridium difficile infection incidence and health outcomes in United States Community Hospitals. BMC Infect Dis 2016; 16: 682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention. Clostridioides difficile infection, 2019, https://www.cdc.gov/hai/organisms/cdiff/cdiff_infect.html (accessed 1 March 2021).
- 10. Cornely OA, Miller MA, Louie TJ, et al. Treatment of first recurrence of Clostridium difficile infection: fidaxomicin versus vancomycin. Clin Infect Dis 2012; 55(Suppl. 2): S154–S161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deshpande A, Pasupuleti V, Thota P, et al. Risk factors for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 2015; 36: 452–460. [DOI] [PubMed] [Google Scholar]
- 12. Garey KW, Sethi S, Yadav Y, et al. Meta-analysis to assess risk factors for recurrent Clostridium difficile infection. J Hosp Infect 2008; 70: 298–304. [DOI] [PubMed] [Google Scholar]
- 13. Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med 2015; 372: 1539–1548. [DOI] [PubMed] [Google Scholar]
- 14. Smits WK, Lyras D, Borden Lacy D, et al. Clostridium difficile infection. Nat Rev Dis Primers 2016; 2: 16020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leong C, Zelenitsky S. Treatment strategies for recurrent Clostridium difficile infection. Can J Hosp Pharm 2013; 66: 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rodrigues R, Barber GE, Ananthakrishnan AN. A comprehensive study of costs associated with recurrent Clostridium difficile infection. Infect Control Hosp Epidemiol 2017; 38: 196–202. [DOI] [PubMed] [Google Scholar]
- 17. Kwon JH, Olsen MA, Dubberke ER. The morbidity, mortality, and costs associated with Clostridium difficile infection. Infect Dis Clin North Am 2015; 29: 123–134. [DOI] [PubMed] [Google Scholar]
- 18. Falcone M, Russo A, Iraci F, et al. Risk factors and outcomes for bloodstream infections secondary to Clostridium difficile infection. Antimicrob Agents Chemother 2016; 60: 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feuerstadt P, Boules M, Stong L, et al. Clinical complications in patients with primary and recurrent Clostridioides difficile infection: a real-world data analysis. SAGE Open Med 2021; 9: 2050312120986733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ianiro G, Murri R, Sciumè GD, et al. Incidence of bloodstream infections, length of hospital stay, and survival in patients with recurrent clostridioides difficile infection treated with fecal microbiota transplantation or antibiotics: a prospective cohort study. Ann Intern Med 2019; 171: 695–702. [DOI] [PubMed] [Google Scholar]
- 21. Micek ST, Schramm G, Morrow L, et al. Clostridium difficile infection: a multicenter study of epidemiology and outcomes in mechanically ventilated patients. Crit Care Med 2013; 41: 1968–1975. [DOI] [PubMed] [Google Scholar]
- 22. Rhee C, Dantes R, Epstein L, et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA 2017; 318: 1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chatila W, Manthous CA. Clostridium difficile causing sepsis and an acute abdomen in critically ill patients. Crit Care Med 1995; 23: 1146–1150. [DOI] [PubMed] [Google Scholar]
- 24. Baggs J, Jernigan JA, Halpin AL, et al. Risk of subsequent sepsis within 90 days after a hospital stay by type of antibiotic exposure. Clin Infect Dis 2018; 66: 1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis 2012; 55(Suppl. 2): S88–S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fehér C, Múñez Rubio E, Merino Amador P, et al. The efficacy of fidaxomicin in the treatment of Clostridium difficile infection in a real-world clinical setting: a Spanish multi-centre retrospective cohort. Eur J Clin Microbiol Infect Dis 2017; 36: 295–303. [DOI] [PubMed] [Google Scholar]
- 27. Jacobson G, Freed M, Damico A, et al. A dozen facts about medicare advantage in 2019, 2019, http://files.kff.org/attachment/Data-Note-A-Dozen-Facts-About-Medicare-Advantage-in-2019 (accessed 1 March 2021). [Google Scholar]
- 28. Nelson W, Scott TA, Boules M, et al. Health care resource utilization and costs of recurrent Clostridioides Difficile infection in the elderly: a real-world claims analysis. J Manag Care Spec Pharm 2021; 27: 828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45: 613–619. [DOI] [PubMed] [Google Scholar]
- 30. US Bureau of Labor Statistics. Measuring price change in the CPI: medical care, 2020, https://www.bls.gov/cpi/factsheets/medical-care.htm (accessed 1 March 2021).
- 31. Prescott HC, Dickson RP, Rogers MAM, et al. Hospitalization type and subsequent severe sepsis. Am J Respir Crit Care Med 2015; 192: 581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vogel TR, Dombrovskiy VY, Lowry SF. Impact of infectious complications after elective surgery on hospital readmission and late deaths in the U.S. medicare population. Surg Infect (Larchmt) 2012; 13: 307–311. [DOI] [PubMed] [Google Scholar]
- 33. Desai K, Gupta SB, Dubberke ER, et al. Epidemiological and economic burden of Clostridium difficile in the United States: estimates from a modeling approach. BMC Infect Dis 2016; 16: 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shah DN, Aitken SL, Barragan LF, et al. Economic burden of primary compared with recurrent Clostridium difficile infection in hospitalized patients: a prospective cohort study. J Hosp Infect 2016; 93: 286–289. [DOI] [PubMed] [Google Scholar]
- 35. Feuerstadt P, Stong L, Dahdal DN, et al. Healthcare resource utilization and direct medical costs associated with index and recurrent Clostridioides difficile infection: a real-world data analysis. J Med Econ 2020; 23: 603–609. [DOI] [PubMed] [Google Scholar]
- 36. Aitken SL, Joseph TB, Shah DN, et al. Healthcare resource utilization for recurrent Clostridium difficile infection in a large university hospital in Houston, Texas. PLoS ONE 2014; 9: e102848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Department of Health and Human Services. National action plan to prevent health care-associated infections, 2016, https://health.gov/our-work/health-care-quality/health-care-associated-infections/national-hai-action-plan (accessed 1 March 2021).
- 38. Virnig B. Strengths and limitations of CMS administrative data in research. Research Data Assistance Center, 2018, https://www.resdac.org/articles/strengths-and-limitations-cms-administrative-data-research (accessed 1 March 2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tai-10.1177_20499361221095679 for Mortality, healthcare resource utilization, and cost among Medicare beneficiaries with Clostridioides difficile infection with and without sepsis by Alpesh Amin, Winnie W. Nelson, Jill Dreyfus, Anny C. Wong, Iman Mohammadi, Christie Teigland, David N. Dahdal and Paul Feuerstadt in Therapeutic Advances in Infectious Disease