Abstract
The introduction of immune checkpoint inhibitors has changed the therapeutic possibilities for various cancer types. However, despite the success in some entities, a significant fraction of patients does not respond to immune checkpoint inhibitors. A functioning cancer-immunity cycle is needed as the precondition for a clinically meaningful response to immune checkpoint inhibitors. It is assumed that only if each step of the cycle is activated and functioning properly, immune checkpoint inhibitors induce a meaningful immune response. However, an activated cancer-immunity cycle might not be present equally in each patient and cancer type. Ideally, treatment concepts should consider each single step of the cancer-immunity cycle and provide personalized treatment approaches, allowing the adaption to functioning and malfunctioning steps of the individual patient’s specific cancer-immunity cycle. In the following review, we provide an overview of the single steps of the cancer-immunity cycle as well as the impact of malfunctioning steps on the generation of an effective tumor-specific immune response.
Keywords: cancer-immunity cycle, immune checkpoint inhibitors, immune escape, immune resistance, PD-L1, solid cancer
Introduction
The introduction of immune checkpoint inhibitors (ICIs) has revolutionized therapeutic options for cancer as in several entities durable responses could be achieved.1–5 Combinational approaches with chemotherapy or targeted therapies have further increased the fraction of patients profiting from an ICI-based treatment approach.6–10 In contrast to other systemic therapies in metastatic cancer, patients responding to ICI can present with durable responses over years.11–13 Importantly, ICI are not without side effects. Although severe side effects are only occasionally observed, immune-mediated side effects including myocarditis, hypophysitis, and colitis are potentially life threatening.14–18 Further, ICI bear a potential financial burden to the health system, considering the currently still very high treatment costs.
Therefore, ICI should be applied with an optimal cost-effectiveness profile based on reliable, robust biomarkers. Indeed, a significant fraction of patients does not respond and some entities present with primary resistance toward ICI.19–21 The so far intensively studied biomarkers include expression of programmed cell death protein ligand 1 (PD-L1), tumor mutational burden (TMB), antigen load, inflammatory blood biomarkers as well as the gut microbiome.22–25 So far, no single biomarker showed consistent results across entities, underlining the highly complex orchestra of interactions between the immune system and cancer cells.26,27 In the following review, we provide explanation on each step of the cancer-immunity cycle and potential immune escape mechanisms inhibiting a clinically effective immune response.
Overview of the activated cancer-immunity cycle
The cancer-immunity cycle involves several steps initiating a clinically effective T-cell-mediated immune response:26,28
Antigen release from the tumor cells
Antigen uptake by antigen-presenting cells (APCs)
Transport with APCs via the lymph vessel system to the local lymph node
Antigen presentation to naïve T-cells in the local lymph node
Activation of T-cells in the local lymph node
Travel of T-cells via the blood stream to the local tumor site
T-cell-mediated immune response in the tumor microenvironment
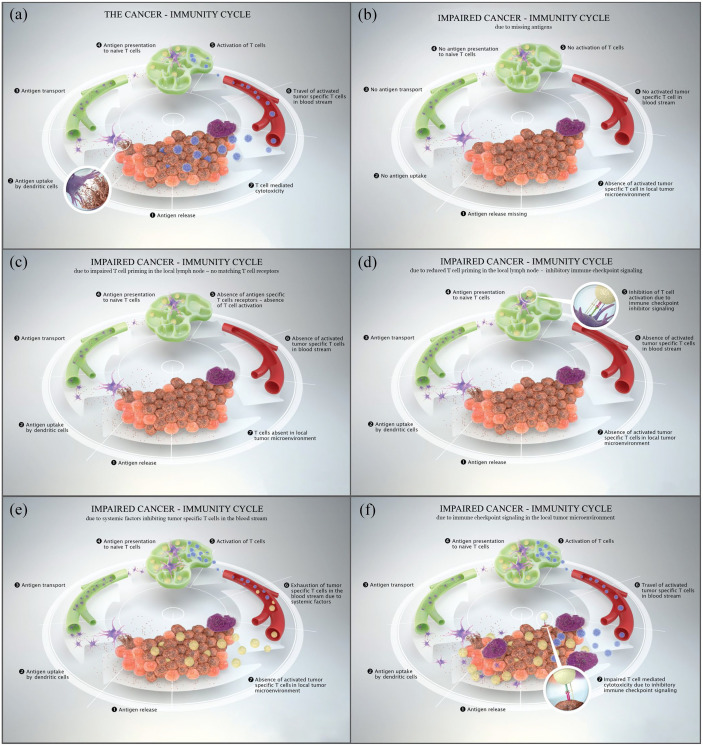
It is assumed that all steps of the cancer-immunity cycle need to function properly to generate an effective immune response (Figure 1(a)). 29 In cancer patients, the cancer-immunity cycle might be impaired resulting in an ineffective sequence and in consequence in the lack of antitumor immunity allowing cancer development and progression. 30
Figure 1.
(a) The cancer-immunity cycle displaying all pivotal steps to generate an effective tumor-specific immune response. (b) Impaired cancer-immunity cycle due to missing antigens. (c) Impaired cancer-immunity cycle due to impaired T-cell priming in the local lymph node – no matching T-cell receptors. (d) Impaired cancer-immunity cycle due to impaired T-cell priming in the local lymph node – immune checkpoint signaling. (e) Impaired cancer-immunity cycle due to systemic factors inhibiting tumor-specific T-cells in the blood stream. (f) Impaired cancer-immunity cycle due to immune checkpoint signaling in the local tumor microenvironment.
Impaired cancer-immunity cycle due to missing antigen release from the tumor
As a first step in the cancer-immunity cycle tumor-specific antigens must be released from dying tumor cells. 31 The tumor-specific antigens are released after necrosis or apoptosis of the tumor cells. Further, the antigens must be captured by APCs, primarily by dendritic cells (DCs), and transported to the local lymph node to start with antigen presentation to naïve T-cells and T-cell priming.32,33 Other types of professional APCs involve macrophages, monocytes, and B cells, whereas thymic epithelial cells and vascular endothelial cells are functioning in antigen presentation as nonprofessional APCs.34–37 An ineffective antigen release, for example, in the absence of dying tumor cells, would prevent an effective antigen presentation and further steps of the cancer-immunity cycle resulting in an ineffective recognition of tumor cells by T-cells (Figure 1(b)).26,29 In consequence, if no antigens are released from tumor cells no immune response in form of a T-cell attack can be generated. 38 In addition, a fully and properly proceeded cancer-immunity cycle inducing the killing of cancer cells would start over with the release of additional tumor-associated antigens which would further restart the cancer-immunity cycle and deepen a tumor-specific T-cell response. 29 The frequently observed correlation of high TMB and response to ICI is explained by a higher availability of tumor neoantigens in the presence of a high mutational burden.39,40 Cancers with microsatellite instability and in consequence a high TMB frequently present with high response rates to ICI-based therapy.41,42 Further, also the configuration of antigens, specifically the antigen-presenting machinery, impacts whether they are taken up by APCs, as human leucocyte antigen (HLA) binding capacity impacts this particular process.43–45 Therefore, another immune evasion pattern is constituted by splitting the antigen-presentation system by either acquired mutations in the HLA component β2 microglobulin or by the loss of the HLA alleles, leading to restricted antigen-presentation and T-cell recognition.46,47
Therapeutically, a cancer vaccination approach is a possible strategy to overcome the lack of antigen release and presentation and is currently widely under investigation.48–50 So far, vaccines have shown some clinical benefit in carcinoma in situ or minimal residual disease.51,52 However, in more advanced tumor stages, therapeutic vaccines have not shown clinical efficacy as monotherapy due to predominating immune evasion mechanisms.53,54 A vaccination approach to increase DC antigen presentation and T-cell activation in consequence, is the transfer of extra-corporal activated DCs. 55 Similar to tumor antigen-based vaccination approaches, DC vaccination failed to have meaningful clinical activity as a monotherapy, however, the combination with ICI in selected patients with proven restriction of DC-based antigen presentation could potentially deliver profit. 56 Further, the combination with chemotherapy and/or radiotherapy might induce the availability of tumor antigens due to the induction of apoptosis/necrosis. 57 In consequence, more tumor antigens are released and available for DC transport and T-cell activation. 58 In line, the combination with chemotherapy and ICI has shown to be clinically more active than ICI alone across several cancer types. 59 The so-called ‘abscopal effect’ refers to the observation that after radiation of a single progressing cancer lesion other distant cancer lesions show response to ICI as well. In detail, the radiotherapy induces tumor cell death which further leads to the release of cancer antigens. 60 Therefore, the implementation of combinations with chemotherapy, radiotherapy, or other vaccination strategies are currently extensively investigated.38,61,62 Further, other novel immune modulating therapeutics include histone deacetylase (HDAC) inhibitors which have been identified to increase the expression of PD-L1, major histocompatibility complex (MHC) class I/II molecules as well as cancer germline mutations and therefore antigen-based tumor immunogenicity.63,64 HDAC inhibitors have shown clinical meaningful results in hematological malignancies such as B-cell lymphomas and their combination with ICI is currently investigated to promote immune responses and potentially show effects in solid tumors as well (Table 1). 63 In terms of safety, HDAC inhibitors are associated with specific class-related side effects such as cardiac effects, that is, the prolongation of QTc interval and in rare cases incidences of arrhythmias, and their management has to be elucidated. 65
Table 1.
Examples of ongoing clinical trials of novel immune-modulating agents targeting steps of the cancer-immunity cycle.
| Step of the cancer-immunity cycle | Mechanism of action/therapeutic target | Malignancy | Clinical trial phase | Examples of clinical trials (NCT number) |
|---|---|---|---|---|
| 1/2/3 | Neoantigen vaccination | Solid tumors, hematologic malignancies | Phase I, II, III |
NCT04397926 NCT05192460 NCT03956056 |
| 1/2/3 | Dendritic cell (DC) vaccination | Solid tumors, hematologic malignancies | Phase I, II, III | NCT04567069NCT03730948 NCT05195619 |
| 1/2 | Histone deacetylase (HDAC) inhibitors | Solid tumors, hematologic malignancies | Phase I, II, III |
NCT04231448 NCT03592472 NCT04651127 |
| 4/5 | Chimeric antigen receptor (CAR) T-cells | Solid tumors, hematologic malignancies | Phase I, II, III |
NCT04257175 NCT05020392 NCT03651128 |
| 4/5 | T-cell receptor (TCR) T-cells | Solid tumors, hematologic malignancies | Phase I, II |
NCT03778814 NCT03441100 NCT05066165 |
| 4/5 | Bispecific T-cell engager (BiTEs) antibodies | Solid tumors, hematologic malignancies | Phase I, II, III |
NCT04631601 NCT04827745 NCT03319940 NCT04260191 |
| 4/5 | Trispecific T-cell engager (TriKEs) antibodies | Solid tumors, hematologic malignancies | Phase I, II |
NCT03214666 NCT03577028 NCT05013554 NCT04184050 |
| 5 | Cytokines involved in T-cell priming (i.e. interferon alpha, chemokines) | Solid tumors, hematologic malignancies | Phase I, II, III |
NCT02506153 NCT04081389 NCT04943679 NCT02634294 |
| 5 | Costimulatory factors for T-cell priming (i.e. CD27, CD40, OX40) | Solid tumors, hematologic malignancies | Phase I, II |
NCT03307746 NCT04081688 NCT03424005 NCT02845323 NCT03414658 NCT03323398 |
| 6 | Interleukin-6 (IL-6) inhibitors | Solid tumors | Phase I, II |
NCT03999749 NCT04452214 |
| 6 | Interleukin 1β (IL-1β) inhibitors | Solid tumors | Phase I | NCT04581343 |
| 1/7 | Poly (ADP-ribose) polymerase (PARP) inhibitors | Solid tumors, hematologic malignancies | Phase I, II, III |
NCT02484404 NCT03061188 NCT03598270 |
| 1/7 | Cyclin-dependent kinase (CDK) 4/6 inhibitors | Solid tumors | Phase I, II |
NCT03041311 NCT04000529 NCT04213404 |
| 7 | Vascular endothelial growth factor (VEGF)-tyrosine kinase inhibitor (TKI) | Solid tumors | Phase I, II, III |
NCT05000294 NCT04493203 NCT04879368 |
| 7 | Wnt inhibitors | Solid tumors | Phase I | NCT01351103 |
| 7 | Viral vectors | Solid tumors, hematologic malignancies | Phase I, II |
NCT05076760 NCT03152318 NCT03004183 |
| 7 | Natural killer cells | Solid tumors, hematologic malignancies | Phase I, II, III |
NCT04590963 NCT05221840 NCT04307329 NCT02921685 |
| 7 | Cancer-associated fibroblasts | Solid tumors | Phase I, II | NCT05064618 |
| 7 | Tumor-associated macrophages | Solid tumors, hematologic malignancies | Phase I, II, III | NCT02339571 |
BiTEs, bispecific T-cell engager; CAR, chimeric antigen receptor; CDK, cyclin-dependent kinase; DC, dendritic cell; HDAC, histone deacetylase; IL, interleukin; PARP, Poly (ADP-ribose) polymerase; TCR, T-cell receptor; TKI, tyrosine kinase inhibitor; TriKEs, trispecific T-cell engager; VEGF, vascular endothelial growth factor.
Impaired cancer-immune cycle due to impaired T-cell priming in the local lymph node
The presentation of tumor-specific antigens to naïve T-cells triggers the priming and activation of effector T-cells. Importantly, the distribution of effector T-cells and regulatory T-cells is critically influencing the effectiveness of the immune response. 29 As most tumor antigens derive from self-antigens, tumor-specific antigens could potentially not get properly recognized by APCs or T-cells as ‘foreign’ enough and could be considered as ‘self’. This would lead to the priming and activation of regulatory T-cells rather than effector T-cells. Therefore, this process must be accompanied by immunogenic signals like proinflammatory cytokines (i.e. interleukin-1, interferon α, tumor-necrosis factor α) and costimulatory factors (i.e. CD27, OX40) to promote immunity and not lead to tolerance against the specific antigens.29,66–68
Furthermore, within the local lymph node matching T-cell receptors (TCRs) for each mature T-cell have to be activated. 69 If no matches are present, that is, due to a low TCR repertoire diversity, no immune response can be generated (Figure 1(c)). Indeed, the TCR repertoire has been identified as a potential multidimensional biomarker for response assessment of immunotherapeutics as a richer diversity of the TCR repertoire was associated with beneficial outcome in metastatic melanoma patients treated with the cytotoxic T-lymphocyte antigen 4 (CTLA4) inhibitor ipilimumab.70,71 Moreover, immune checkpoint blockade has shown to impact the TCR repertoire diversity and therefore to boost the antitumor immunity at this specific step in the cancer-immunity cycle. 72 Further, the binding affinity between tumor antigen–MHC complexes and TCRs plays an important role in the activation of antitumor activity. TCRs binding to tumor-specific antigens have been described to have a significantly lower binding affinity than TCRs binding to viral antigens. 73 This observation implies an acquired tolerance to self-antigens that consequently dampens the antitumor activity of ‘native’ T-cells.
Adoptive T-cell therapy is a potential therapeutic approach to overcome the lack of insufficient effector T-cell activation by reinfusing genetically modified autologous T-cells into the patient. 29 Chimeric antigen receptor (CAR) T-cells targeting a tumor surface antigen have shown clinical benefit in hematologic malignancies for instance in forms of leukemia or lymphoma.74–76 A similar approach with targeting recombinant antigen-specific TCR α and β chains is under investigation and aims to bypass immune tolerance by molecularly engineered higher affinities for the targeted antigens. 77 This method aims to supply the immune system with large quantities of specifically tumor-antigen targeting T-cells. So far, this approach has not achieved sufficient clinical efficacy in solid tumors and is under further investigation. 78 Specifically, acquired resistance to transferred monospecific T-cells as a result of antigenic drift as well as potential severe and life-threatening side effects, that is, cytokine release storm, have to be addressed before aiming for wider clinical usage in solid tumors. 79 Cytokine release syndrome (CRS) manifests as a strong systemic immune reaction with hypotension, hyperpyrexia, and acute respiratory distress as a consequence of released cytokines by immune cells. 80 Further, bispecific and trispecific T-cell engager (BiTE, TriTE) as well as bispecific and trispecific killer cell engager (BiKE, TriKE) multispecific antibodies (msABs) are linkers between endogenous cytotoxic T/NK cells and antigens expressed by cancer cells with two or three binding domains, respectively.81,82 The consecutive binding of the cytotoxic T/NK cells and the cancer antigens leads to T-cell proliferation and potentiation of the T-cell-mediated immune response and increased tumor specificity. 82 MsABs are currently under clinical investigation in Phase I–III trials and bear the potential to overcome escape mutations and to access personalized immunotherapy approaches (NCT04631601, NCT03319940, NCT03214666; Table 1).81,83 In addition, also cytokines and costimulatory factors involved in T-cell priming and activation can be therapeutically targeted and are under clinical investigations (NCT03307746, NCT03323398, NCT02506153; Table 1). 84
Another important immune escape mechanism taking place in the local lymph node is the control of T-cell activation by additional inhibitory immune checkpoints as they can prevent the activation of T-cells in the lymph node by binding to their ligands (Figure 1(d)). 29
The CTLA4 inhibitory antibody ipilimumab blocks CTLA4 and therefore prevents the interaction with its ligands CD80 and CD86 in the local lymph node. In consequence, the negative regulation and prohibition of T-cell activation is blocked resulting in the expansion of T-cells. The disinhibited T-cell expansion also leads to development of autoreactive T-cells explaining the in comparison higher amount of immune-related toxicities associated with ipilimumab therapy. 85 Nevertheless, the clinical responses have aroused the pursuit of other targetable immune checkpoints and led to the implementation of programmed cell death protein 1 (PD-1) and PD-L1 targeting monoclonal antibodies, that is, pembrolizumab, nivolumab, and atezolizumab which are now in broad clinical practice as approved first-line monotherapies or combinational therapies in different metastatic solid cancers. 86 Immune checkpoint inhibition specifically targets the mechanism in cancers that inhibit an effective immune response rather than nonspecific activation of the immune system potentially leading to autoimmunity or unwanted severe side effects. Importantly, dual immune checkpoint blockade with the CTLA-4-targeting agent ipilimumab in combination with the PD-1-targeting agent nivolumab has shown complementary modes of action and therefore beneficial effects in clinical trials and is nowadays an approved first-line regimen in solid tumors.87,88 Noteworthy, the dual checkpoint blockade with ipilimumab and nivolumab is associated with a higher incidence of immune-related adverse events compared to ICI monotherapy, including rash, colitis, diarrhea, pneumonitis, and hepatitis and a clinical as well as laboratory–chemical observation during therapy is important. 89
Impaired cancer-immune cycle due to systemic factors inhibiting tumor-specific T-cells in the blood stream
Activated effector T-cells have to travel via the blood stream to the local tumor site. However, factors within the blood stream could impact the sustainability of activated T-cells (Figure 1(e)). Systemic inflammation is triggered by cytokines, immune cells, and acute phase proteins. The circulation of immune cells, that is, neutrophils, can be increased as a result of cancer-mediated myelopoiesis. Further, neutrophils were described to dampen the T-cell-mediated antitumor response by the secretion of inhibitory factors such as arginase, nitric oxide synthase (NOS), and phagocytosis-associated oxidases (PHOXs), which further generates reactive oxygen species (ROS) and inhibits T-cell activation. 90 In line, elevated systemic inflammation markers as for example the neutrophil-to-lymphocyte ratio (NLR) and the acute phase protein C-reactive protein (CRP) correlated with poor treatment response and worse overall survival in different solid cancers.91–94 Importantly, the clinical use of steroids was shown to be associated with higher levels of neutrophils and NLR counts and a simultaneously worse outcome in non-small-cell lung cancer (NSCLC) patients treated with ICI. 95 Further, CRP and different cytokines were shown to impact the generated tumor specific immune response, resulting in impaired response to immune modulating therapies.96,97 Elevated systemic CRP levels were found to negatively correlate with levels of CD4+-infiltrating lymphocytes in the tumor microenvironment indicating a close link of local and systemic inflammatory responses. 98 Elevated CRP serum levels were further associated with expression of PD-L1 which contributes to immunosuppression in the tumor microenvironment. 99 Circulating proinflammatory cytokines such as interleukin-6 (IL-6), IL-1, tumor necrosis factor alpha (TNF-α) and transforming growth factor beta (TGF-β) are on the one hand triggering CRP expression and on the other hand were also described to upregulate PD-L1 expression themselves.99,100
The inhibition of proinflammatory cytokines might be a possible way of potentiating the antitumor immune response. Here, the inhibition of IL-6 was reported to have a beneficial effect on the efficacy of anti-PD-L1 therapy in preclinical studies and is now investigated in a phase I–II clinical trial in combination with PD-1 targeting therapy (NCT04191421).101,102 Another preclinical study showed a decline in monocyte recruitment and macrophages differentiation accompanied with a high proportion of IL-12 secreting DCs cherishing the antitumor immunity by activation of CD8+ T-cells in the tumor microenvironment in IL-1β deficient mice. 103 Therefore, the blocking of IL-1β, that is, with the anti-IL-1β antibody canakinumab is currently under investigation in clinical trials as combinational immunotherapy with anti-PD-1 therapy in NSCLC patients (NCT03631199). 96 Regarding the safety profile of canakinumab, its usage in rheumatologic diseases has already revealed its tolerable side effect profile with infections, especially of the upper respiratory tract, as most common adverse event. 104 In conclusion, many targets for systemic inflammatory molecules are under preclinical and clinical investigation and will potentially form components in the future immunotherapy.
Impaired cancer-immunity cycle due to immune checkpoint signaling in the local tumor microenvironment
Within the local tumor microenvironment, the composition of the vascular structures and the cytokine gradient impacts the efficacy of T-cell influx (‘homing’) and further the antitumor immune response. Pathologically composed and activated endothelial cells were shown to hamper the effective influx of T-cells. 105 Once arrived in the local tumor microenvironment, the tumor specific activated T-cells face several immune-suppressive factors. Tumor cells themselves or tumor-infiltrating lymphocytes can express immune checkpoints such as PD-1 and their ligands (PD-L1) inhibiting the immune response and acting as an ‘immunostat’ (Figure 1(f)).27,86 Further immune checkpoints expressed on tumor cells are lymphocyte-activation gene 3 (LAG3) or T-cell immunoglobulin and mucin-domain containing-3 (TIM3). 28 In addition, immune-modulating molecules such as indoleamine 2,3-dioxygenase (IDO) as well as cytokines (i.e. IL-6, IL-10, TGFβ) released by tumor cells can prevent effective T-cell action and even generate intratumoral T-cell exhaustion.106,107 Strategies to prevent or reverse T-cell exhaustion by a multimodal and combinational therapy approach are, therefore, needed. Further, myeloid-derived suppressor cells (MDSCs) within the tumor microenvironment as well as tumor-associated macrophages, specifically M2 macrophages, can add to the immune suppression and the evasion of tumor cells.108,109 Importantly, the composition of tumor-infiltrating lymphocyte subsets forming the local inflammatory microenvironment, including CD8+ and CD3+ effector T-cells, CD45RO+ memory T-cells and FOXP3+ regulatory T-cells, determines the capacity of either antitumor or tumor-promoting inflammatory responses.107,110 FOXP3 + regulatory T-cells function as immune-suppressive immune cells by secreting inhibitory cytokines (i.e. IL-10, TGF-β).
The application of ICI targeting the individual route of immune evasion of a given patient needs to be addressed. The combination with chemotherapy was shown to potentially increase PD-L1 expression on tumor cells and thereby improve the response potential to PD-1 axis directed ICI therapy and combinational therapies are in clinical use for different solid cancers.111–113 Besides chemotherapy, also combinational inhibition of vascular endothelial growth factor (VEGF) with antiangiogenic agents such as the antibody bevacizumab or VEGF-targeting tyrosine kinase inhibitors (TKIs) may potentially boost the antitumor immune response of anti-PD-1 blockade by facilitating T-cell infiltration into the tumor microenvironment and is under investigation (NCT03396926, NCT04879368; Table 1). Further, combinational approaches with the epidermal growth factor receptor (EGFR)-targeting antibody cetuximab with PD-1-targeting ICIs are postulated to have synergistic effects in antitumor immunity. 114 In addition, targeting the Wnt/β-catenin signaling pathway in combination with PD-1 targeting ICI has been suggested to improve T-cell priming and T-cell infiltration into the tumor microenvironment since the Wnt/β-catenin pathway was associated with modulation of dendritic cells, tumor-associated macrophages as well as regulatory T-cell infiltration and is tested in early clinical trials (NCT01351103). 115 Also immune-suppressive cytokines can be specifically targeted and are under investigation in early clinical trials such as antibodies targeting IDO (i.e. NCT03854032, NCT03915405) IL-6/ IL-6R (i.e. NCT04191421, NCT04691817), IL-10 (i.e. NCT03382912, NCT02009449), and TGFβ (i.e. NCT04429542) as combinational immunotherapies in advanced solid cancers. 116 In addition, preclinical and clinical studies suggest a combinational approach of inhibiting DNA damage repair (DDR) pathways with, that is, poly (ADP-ribose) polymerase (PARP) inhibitors or cyclin-dependent kinase 4/6 (CDK4/6) inhibitors with PD-1/PD-L1-axis targeting agents since a dysfunctioning DDR has been postulated to play a role in the activation of the host’s immune system. 117 PARP inhibitors, for example, have been described to enhance the antitumor immunity via STING pathway activation leading to an increased chemokine recruitment and further induced cytotoxic T-cell functioning. 118 In line, mismatch repair (MMR) deficiency is an established biomarker for the use of ICI therapy since MMR-deficiency leads to new somatic mutations and an increase in neoantigens reinforcing the cancer-immunity cycle. 119 Further, other cell types within the inflammatory microenvironment of tumors like the innate natural killer (NK) cells, tumor-associated macrophages as well as cancer-associated fibroblasts can be therapeutically addressed (Table 1). Finally, replicative oncolytic viral vectors that are locally injected into tumors providing local immunostimulating signals are currently under early clinical investigation (NCT05076760; Table 1). Various combinational immunotherapy approaches are currently under investigation in clinical trials (i.e. NCT04301778, NCT02829723) and might improve the generated antitumor immune response.27,120,121 Importantly, new class-specific immune-related side effects may occur with novel immune-modulating or combinational therapeutics, therefore, clear treatment guidelines and clinical experience are needed to assure the safety and quality of life of patients. Table 1 summarizes immune-modulating therapeutic approaches under clinical investigation targeting the cancer-immunity cycle as monotherapy or combinational therapy with ICI.
Conclusion
All steps of the cancer-immunity cycle can show impaired functioning, resulting in its ineffective sequence and in consequence the reduction of the tumor-specific immune response. Indeed, immune escape and resistance mechanisms might overpower ICI monotherapy and combinational therapies targeting several steps in the cancer-immunity cycle might be needed to achieve a meaningful immune response in cancer patients. A personalized biomarker approach is warranted to identify the impairment of a given patient. Based on the biomarker analysis, targeted combinational treatments should be applied. Many promising therapeutic strategies for novel immune-modulating therapies as well as their combinations and optimal sequences are currently in clinical examinations. However, severe immune-related side effects may be the result of disinhibiting the brakes of the immune system and their management has to be contemplated. To conclude, in the future, understanding the patient’s specific configuration of immune system–cancer cell interactions as well as the specific underlying immune escape mechanism will be needed to guide personalized treatment options of immunotherapy in cancer patients.
Acknowledgments
This review was performed within the PhD thesis of Angelika M. Starzer with the title ‘Immune monitoring in cancer patients’ in the N790 doctoral program at the Medical University Vienna, Austria. The financial support by the Austrian Federal Ministry for Digital and Economic Affairs, the National Foundation for Research, Technology and Development and the Christian Doppler Research Association is gratefully acknowledged.
Footnotes
Author contribution(s): Angelika M. Starzer: Conceptualization; Investigation; Methodology; Visualization; Writing – original draft.
Matthias Preusser: Conceptualization; Funding acquisition; Project administration; Resources; Supervision; Validation; Writing – review & editing.
Anna S. Berghoff: Conceptualization; Funding acquisition; Methodology; Project administration; Resources; Supervision; Visualization; Writing – original draft; Writing – review & editing.
ORCID iD: Angelika M. Starzer  https://orcid.org/0000-0001-5867-8461
https://orcid.org/0000-0001-5867-8461
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The review was supported by the research budget of the Medical University of Vienna, the Austrian Federal Ministry for Digital and Economic Affairs, the National Foundation for Research, Technology, and Development, and the Christian Doppler Research Association.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AMS has received lecture honoraria from Astra Zeneca and travel support from PharmaMar. MP has received honoraria for lectures, consultation or advisory board participation from the following for-profit companies: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, and Tocagen. The following for-profit companies have supported clinical trials and contracted research conducted by MP with payments made to his institution: Böhringer-Ingelheim, Bristol-Myers Squibb, Roche, Daiichi Sankyo, Merck Sharp & Dome, Novocure, GlaxoSmithKline, and AbbVie. ASB has research support from Daiichi Sankyo and Roche, honoraria for lectures, consultation, or advisory board participation from Roche Bristol-Meyers Squibb, Merck, Daiichi Sankyo, as well as travel support from Roche, Amgen, Daiichi Sankyo, and AbbVie.
Contributor Information
Angelika M. Starzer, Division of Oncology, Department of Medicine I, Medical University of Vienna, Vienna, Austria Christian Doppler Laboratory for Personalized Immunotherapy, Department of Medicine I, Medical University of Vienna, Vienna, Austria.
Matthias Preusser, Division of Oncology, Department of Medicine I, Medical University of Vienna, Vienna, Austria; Christian Doppler Laboratory for Personalized Immunotherapy, Department of Medicine I, Medical University of Vienna, Vienna, Austria.
Anna S. Berghoff, Division of Oncology, Department of Medicine I, Medical University of Vienna, Waehringer Guertel 18-20, 1090 Vienna, Austria; Christian Doppler Laboratory for Personalized Immunotherapy, Department of Medicine I, Medical University of Vienna, Vienna, Austria.
References
- 1. Alsina M, Miquel JM, Diez M, et al. How I treat gastric adenocarcinoma. ESMO Open 2019; 4: e000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nishio M, Hida T, Atagi S, et al. Multicentre phase II study of nivolumab in Japanese patients with advanced or recurrent non-squamous non-small cell lung cancer. ESMO Open 2017; 1: e000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aguiar PN, Jr, De Mello RA, Barreto CMN, et al. Immune checkpoint inhibitors for advanced non-small cell lung cancer: emerging sequencing for new treatment targets. ESMO Open 2017; 2: e000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Marinis F, Ciardiello F, Baas P, et al. 30 Immunotherapy in advanced NSCLC-from the ‘tsunami’ of therapeutic knowledge to a clinical practice algorithm: results from an international expert panel meeting of the Italian Association of Thoracic Oncology (AIOT). ESMO Open 2018; 3: e000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vansteenkiste JF. Immunotherapy in lung cancer. ESMO Open 2018; 3: e000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Okusaka T, Ikeda M. Immunotherapy for hepatocellular carcinoma: current status and future perspectives. ESMO Open 2018; 3(Suppl. 1): e000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park JH, Ahn JH, Kim SB. How shall we treat early triple-negative breast cancer (TNBC): from the current standard to upcoming immuno-molecular strategies. ESMO Open 2018; 3(Suppl. 1): e000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rodriguez-Vida A, Hutson TE, Bellmunt J, et al. New treatment options for metastatic renal cell carcinoma. ESMO Open 2017; 2: e000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Economopoulou P, Kotsantis I, Psyrri A. The promise of immunotherapy in head and neck squamous cell carcinoma: combinatorial immunotherapy approaches. ESMO Open 2016; 1: e000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Berghoff AS, Bellosillo B, Caux C, et al. Immune checkpoint inhibitor treatment in patients with oncogene- addicted non-small cell lung cancer (NSCLC): summary of a multidisciplinary round-table discussion. ESMO Open 2019; 4: e000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 2015; 33: 1889–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paglialunga L, Salih Z, Ricciuti B, et al. Immune checkpoint blockade in small cell lung cancer: is there a light at the end of the tunnel? ESMO Open 2016; 1: e000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Everest L, Shah M, Chan KKW. Comparison of long-term survival benefits in trials of immune checkpoint inhibitor vs non-immune checkpoint inhibitor anticancer agents using ASCO value framework and ESMO magnitude of clinical benefit scale. JAMA Netw Open 2019; 2: e196803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Geukes Foppen MH, Rozeman EA, van Wilpe S, et al. Immune checkpoint inhibition-related colitis: symptoms, endoscopic features, histology and response to management. ESMO Open 2018; 3:e000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Varricchi G, Galdiero MR, Marone G, et al. Cardiotoxicity of immune checkpoint inhibitors. ESMO Open 2017; 2: e000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doherty GJ, Duckworth AM, Davies SE, et al. Severe steroid-resistant anti-PD1 T-cell checkpoint inhibitor-induced hepatotoxicity driven by biliary injury. ESMO Open 2017; 2: e000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kastrisiou M, Kostadima FL, Kefas A, et al. Nivolumab-induced hypothyroidism and selective pituitary insufficiency in a patient with lung adenocarcinoma: a case report and review of the literature. ESMO Open 2017; 2: e000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aya F, Gaba L, Victoria I, et al. Life-threatening colitis and complete response with ipilimumab in a patient with metastatic BRAF-mutant melanoma and rheumatoid arthritis. ESMO Open 2016; 1: e000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vansteenkiste JF. Immune checkpoint inhibitors for every patient with non-small cell lung cancer? Update on immunotherapy in patients with lung cancer. ESMO Open 2018; 3: e000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haanen JBAG. Questions asked in the everyday practice: immune checkpoint inhibitors. ESMO Open 2018; 3: e000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thungappa S, Ferri J, Caglevic C, et al. Immune checkpoint inhibitors in lung cancer: the holy grail has not yet been found. ESMO Open 2017; 2: e000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kang BW, Chau I. Current status and future potential of predictive biomarkers for immune checkpoint inhibitors in gastric cancer. ESMO Open 2020; 5: e000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 2016; 17: e542–e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu J, Armstrong AJ, Friedlander TW, et al. Biomarkers of immunotherapy in urothelial and renal cell carcinoma: PD-L1, tumor mutational burden, and beyond. J Immunother Cancer 2018; 6: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science 2018; 359: 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Preusser M, Berghoff AS, Thallinger C, et al. Cancer immune cycle: a video introduction to the interaction between cancer and the immune system. ESMO Open 2016; 1: e000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Preusser M, Berghoff AS, Thallinger C, et al. Educational video: the role of PD-L1 in the local tumour microenvironment. ESMO Open 2016; 1: e000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Granier C, De Guillebon E, Blanc C, et al. Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer. ESMO Open 2017; 2: e000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013; 39: 1–10. [DOI] [PubMed] [Google Scholar]
- 30. Kim JM, Chen DS. Immune escape to PD-L1/PD-1 blockade: seven steps to success (or failure). Ann Oncol 2016; 27: 1492–1504. [DOI] [PubMed] [Google Scholar]
- 31. Gamrekelashvili J, Greten TF, Korangy F. Immunogenicity of necrotic cell death. Cell Mol Life Sci 2015; 72: 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Théry C, Amigorena S. The cell biology of antigen presentation in dendritic cells. Curr Opin Immunol 2001; 13: 45–51. [DOI] [PubMed] [Google Scholar]
- 33. Lee MY, Jeon JW, Sievers C, et al. Antigen processing and presentation in cancer immunotherapy. J Immunother Cancer 2020; 8: e001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jakubzick CV, Randolph GJ, Henson PM. Monocyte differentiation and antigen-presenting functions. Nat Rev Immunol 2017; 17: 349–362. [DOI] [PubMed] [Google Scholar]
- 35. Chen X, Jensen PE. The role of B lymphocytes as antigen-presenting cells. Arch Immunol Ther Exp 2008; 56: 77–83. [DOI] [PubMed] [Google Scholar]
- 36. Alexandropoulos K, Danzl NM. Thymic epithelial cells: antigen presenting cells that regulate T cell repertoire and tolerance development. Immunol Res 2012; 54: 177–190. [DOI] [PubMed] [Google Scholar]
- 37. Shao Y, Saredy J, Yang WY, et al. Vascular endothelial cells and innate immunity. Arterioscler Thromb Vasc Biol 2020; 40: E138–E152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gubin MM, Zhang X, Schuster H, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 2014; 515: 577–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015; 348: 69–74. [DOI] [PubMed] [Google Scholar]
- 40. McGranahan N, Furness AJS, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016; 351: 1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vranic S. Microsatellite instability status predicts response to anti-PD-1/PD-L1 therapy regardless the histotype: a comment on recent advances. Bosn J Basic Med Sci 2017; 17: 274–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kok M, Chalabi M, Haanen J. How I treat MSI cancers with advanced disease. ESMO Open 2019; 4(Suppl. 2): e000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wolf Y, Samuels Y. Cancer research in the era of immunogenomics. ESMO Open 2018; 3: e000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Łuksza M, Riaz N, Makarov V, et al. A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature 2017; 551: 517–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Verdegaal EME, De Miranda NFCC, Visser M, et al. Neoantigen landscape dynamics during human melanoma-T cell interactions. Nature 2016; 536: 91–95. [DOI] [PubMed] [Google Scholar]
- 46. Sade-Feldman M, Jiao YJ, Chen JH, et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat Commun 2017; 8: 1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McGranahan N, Rosenthal R, Hiley CT, et al. Allele-specific HLA loss and immune escape in lung cancer evolution. Cell 2017; 171: 1259–1271.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. van der Burg SH, Arens R, Ossendorp F, et al. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer 2016; 16: 219–233. [DOI] [PubMed] [Google Scholar]
- 49. Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013; 39: 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. De Mattos-Arruda L, Blanco-Heredia J, Aguilar-Gurrieri C, et al. New emerging targets in cancer immunotherapy: the role of neoantigens. ESMO Open 2020; 4(Suppl. 3): e000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Melief CJM, Van Hall T, Arens R, et al. Therapeutic cancer vaccines. J Clin Invest 2015; 125: 3401–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kenter GG, Welters MJP, Valentijn ARPM, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med 2009; 361: 1838–1847. [DOI] [PubMed] [Google Scholar]
- 53. Miles D, Roché H, Martin M, et al. Phase III multicenter clinical trial of the sialyl-TN (STn)-keyhole limpet hemocyanin (KLH) vaccine for metastatic breast cancer. Oncologist 2011; 16: 1092–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Butts C, Socinski MA, Mitchell PL, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol 2014; 15: 59–68. [DOI] [PubMed] [Google Scholar]
- 55. Mastelic-Gavillet B, Balint K, Boudousquie C, et al. Personalized dendritic cell vaccines-recent breakthroughs and encouraging clinical results. Front Immunol 2019; 10: 766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vasaturo A, Di Blasio S, Peeters DGA, et al. Clinical implications of co-inhibitory molecule expression in the tumor microenvironment for DC vaccination: a game of stop and go. Front Immunol 2013; 4: 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lin W, Xu Y, Chen X, et al. Radiation-induced small extracellular vesicles as ‘carriages’ promote tumor antigen release and trigger antitumor immunity. Theranostics 2020; 10: 4871–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chi KH, Liu SJ, Li CP, et al. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J Immunother 2005; 28: 129–135. [DOI] [PubMed] [Google Scholar]
- 59. Calmeiro J, Carrascal MA, Tavares AR, et al. Pharmacological combination of nivolumab with dendritic cell vaccines in cancer immunotherapy: an overview. Pharmacol Res 2021; 164: 105309. [DOI] [PubMed] [Google Scholar]
- 60. Wang S, Yu H, He R, et al. Exposure to low-dose radiation enhanced the antitumor effect of a dendritic cell vaccine. Dose Response 2019; 17: 1559325819832144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Van Der Sluis TC, Van Duikeren S, Huppelschoten S, et al. Vaccine-Induced tumor necrosis factor-producing T cells synergize with cisplatin to promote tumor cell death. Clin Cancer Res 2015; 21: 781–794. [DOI] [PubMed] [Google Scholar]
- 62. Mkrtichyan M, Chong N, Abu Eid R, et al. Anti-PD-1 antibody significantly increases therapeutic efficacy of Listeria monocytogenes (Lm)-LLO immunotherapy. J Immunother Cancer 2013; 1: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang X, Waschke BC, Woolaver RA, et al. HDAC inhibitors overcome immunotherapy resistance in B-cell lymphoma. Protein Cell 2020; 11: 472–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang X, Waschke BC, Woolaver RA, et al. Histone deacetylase inhibition sensitizes PD1 blockade-resistant B-cell lymphomas. Cancer Immunol Res 2019; 7: 1318–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Shah RR. Safety and tolerability of histone deacetylase (HDAC) inhibitors in oncology. Drug Saf 2019; 42: 235–245. [DOI] [PubMed] [Google Scholar]
- 66. Ferguson TA, Choi J, Green DR. Armed response: how dying cells influence T-cell functions. Immunol Rev 2011; 241: 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Starzer AM, Berghoff AS. New emerging targets in cancer immunotherapy: CD27 (TNFRSF7). ESMO Open 2020; 4(Suppl. 3): e000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Alves Costa Silva C, Facchinetti F, Routy B, et al. New pathways in immune stimulation: targeting OX40. ESMO Open 2020; 5: e000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Aversa I, Malanga D, Fiume G, et al. Molecular T-cell repertoire analysis as source of prognostic and predictive biomarkers for checkpoint blockade immunotherapy. Int J Mol Sci 2020; 21: 2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Postow MA, Manuel M, Wong P, et al. Peripheral T cell receptor diversity is associated with clinical outcomes following ipilimumab treatment in metastatic melanoma. J Immunother Cancer 2015; 3: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515: 568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Robert L, Tsoi J, Wang X, et al. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clin Cancer Res 2014; 20: 2424–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Aleksic M, Liddy N, Molloy PE, et al. Different affinity windows for virus and cancer-specific T-cell receptors: implications for therapeutic strategies. Eur J Immunol 2012; 42: 3174–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor–modified T cells for acute lymphoid leukemia. N Engl J Med 2013; 368: 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Laport GG, Levine BL, Stadtmauer EA, et al. Adoptive transfer of costimulated T cells induces lymphocytosis in patients with relapsed/refractory non-Hodgkin lymphoma following CD34 +-selected hematopoietic cell transplantation. Blood 2003; 102: 2004–2013. [DOI] [PubMed] [Google Scholar]
- 76. Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013; 5: 177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chervin AS, Aggen DH, Raseman JM, et al. Engineering higher affinity T cell receptors using a T cell display system. J Immunol Methods 2008; 339: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Newick K, O’Brien S, Moon E, et al. CAR T cell therapy for solid tumors. Annu Rev Med 2017; 68: 139–152. [DOI] [PubMed] [Google Scholar]
- 79. Rath JA, Arber C. Engineering strategies to enhance TCR-based adoptive T cell therapy. Cells 2020; 9: 1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chen H, Wang F, Zhang P, et al. Management of cytokine release syndrome related to CAR-T cell therapy. Front Med 2019; 13: 610–617. [DOI] [PubMed] [Google Scholar]
- 81. Einsele H, Borghaei H, Orlowski RZ, et al. The BiTE (bispecific T-cell engager) platform: development and future potential of a targeted immuno-oncology therapy across tumor types. Cancer 2020; 126: 3192–3201. [DOI] [PubMed] [Google Scholar]
- 82. Elshiaty M, Schindler H, Christopoulos P. Principles and current clinical landscape of multispecific antibodies against cancer. Int J Mol Sci 2021; 22: 5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tapia-Galisteo A, Sánchez Rodríguez Í, Aguilar-Sopeña O, et al. Trispecific T-cell engagers for dual tumor-targeting of colorectal cancer. Oncoimmunology 2022; 11: 2034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Burris HA, Infante JR, Ansell SM, et al. Safety and activity of varlilumab, a novel and first-in-class agonist anti-CD27 antibody, in patients with advanced solid tumors. J Clin Oncol 2017; 35: 2028–2036. [DOI] [PubMed] [Google Scholar]
- 85. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy-inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res 2012; 18: 6580–6587. [DOI] [PubMed] [Google Scholar]
- 87. Baas P, Scherpereel A, Nowak AK, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 2021; 397: 375–386. [DOI] [PubMed] [Google Scholar]
- 88. Albiges L, Tannir NM, Burotto M, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 2020; 5: e001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhou S, Khanal S, Zhang H. Risk of immune-related adverse events associated with ipilimumab-plus-nivolumab and nivolumab therapy in cancer patients. Ther Clin Risk Manag 2019; 15: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Müller I, Munder M, Kropf P, et al. Polymorphonuclear neutrophils and T lymphocytes: strange bedfellows or brothers in arms? Trends Immunol 2009; 30: 522–530. [DOI] [PubMed] [Google Scholar]
- 91. Xie Q-K, Chen P, Hu W-M, et al. The systemic immune-inflammation index is an independent predictor of survival for metastatic colorectal cancer and its association with the lymphocytic response to the tumor. J Transl Med 2018; 16: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Scott HR, McMillan DC, Forrest LM, et al. The systemic inflammatory response, weight loss, performance status and survival in patients with inoperable non-small cell lung cancer. Br J Cancer 2002; 87: 264–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Guthrie GJ, Charles KA, Roxburgh CS, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013; 88: 218–230. [DOI] [PubMed] [Google Scholar]
- 94. McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 2013; 39: 534–540. [DOI] [PubMed] [Google Scholar]
- 95. Fucà G, Galli G, Poggi M, et al. Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. ESMO Open 2019; 4: e000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Iivanainen S, Ahvonen J, Knuuttila A, et al. Elevated CRP levels indicate poor progression-free and overall survival on cancer patients treated with PD-1 inhibitors. ESMO Open 2019; 4: e000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Naqash AR, Stroud CRG, Butt MU, et al. Co-relation of overall survival with peripheral blood-based inflammatory biomarkers in advanced stage non-small cell lung cancer treated with anti-programmed cell death-1 therapy: results from a single institutional database. Acta Oncol 2018; 57: 867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Canna K, McArdle PA, McMillan DC, et al. The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br J Cancer 2005; 92: 651–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Akamine T, Takada K, Toyokawa G, et al. Association of preoperative serum CRP with PD-L1 expression in 508 patients with non-small cell lung cancer: a comprehensive analysis of systemic inflammatory markers. Surg Oncol 2018; 27: 88–94. [DOI] [PubMed] [Google Scholar]
- 100. Chen MF, Chen PT, Chen WC, et al. The role of PD-L1 in the radiation response and prognosis for esophageal squamous cell carcinoma related to IL-6 and T-cell immunosuppression. Oncotarget 2016; 7: 7913–7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Liu H, Shen J, Lu K. IL-6 and PD-L1 blockade combination inhibits hepatocellular carcinoma cancer development in mouse model. Biochem Biophys Res Commun 2017; 486: 239–244. [DOI] [PubMed] [Google Scholar]
- 102. Masjedi A, Hashemi V, Hojjat-Farsangi M, et al. The significant role of interleukin-6 and its signaling pathway in the immunopathogenesis and treatment of breast cancer. Biomed Pharmacother 2018; 108: 1415–1424. [DOI] [PubMed] [Google Scholar]
- 103. Kaplanov I, Carmi Y, Kornetsky R, et al. Blocking IL-1β reverses the immunosuppression in mouse breast cancer and synergizes with anti–PD-1 for tumor abrogation. Proc Natl Acad Sci USA 2019; 116: 1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sota J, Vitale A, Insalaco A, et al. Safety profile of the interleukin-1 inhibitors anakinra and canakinumab in real-life clinical practice: a nationwide multicenter retrospective observational study. Clin Rheumatol 2018; 37: 2233–2240. [DOI] [PubMed] [Google Scholar]
- 105. Missiaen R, Mazzone M, Bergers G. The reciprocal function and regulation of tumor vessels and immune cells offers new therapeutic opportunities in cancer. Semin Cancer Biol 2018; 52: 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Chen DS, Mellman I. Elements of cancer immunity and the cancer–immune set point. Nature 2017; 541: 321–330. [DOI] [PubMed] [Google Scholar]
- 107. Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity 2013; 39: 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Jinushi M, Komohara Y. Tumor-associated macrophages as an emerging target against tumors: creating a new path from bench to bedside. Biochim Biophys Acta 2015; 1855: 123–130. [DOI] [PubMed] [Google Scholar]
- 109. Chanmee T, Ontong P, Konno K, et al. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers 2014; 6: 1670–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Roxburgh CSD, McMillan DC. The role of the in situ local inflammatory response in predicting recurrence and survival in patients with primary operable colorectal cancer. Cancer Treat Rev 2012; 38: 451–466. [DOI] [PubMed] [Google Scholar]
- 111. Leduc C, Adam J, Louvet E, et al. TPF induction chemotherapy increases PD-L1 expression in tumour cells and immune cells in head and neck squamous cell carcinoma. ESMO Open 2018; 3: e000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non–small-cell lung cancer. J Clin Oncol 2020; 38: 1505–1517. [DOI] [PubMed] [Google Scholar]
- 113. Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2020; 21: 44–59. [DOI] [PubMed] [Google Scholar]
- 114. Sacco AG, Chen R, Worden FP, et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: an open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol 2021; 22: 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Pai SG, Carneiro BA, Mota JM, et al. Wnt/beta-catenin pathway: modulating anticancer immune response. J Hematol Oncol 2017; 10: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Naing A, Wong DJ, Infante JR, et al. Pegilodecakin combined with pembrolizumab or nivolumab for patients with advanced solid tumours (IVY): a multicentre, multicohort, open-label, phase 1b trial. Lancet Oncol 2019; 20: 1544–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Sun W, Zhang Q, Wang R, et al. Targeting DNA damage repair for immune checkpoint inhibition: mechanisms and potential clinical applications. Front Oncol 2021; 11: 648687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sen T, Rodriguez BL, Chen L, et al. Targeting DNA damage response promotes antitumor immunity through STING-mediated T-cell activation in small cell lung cancer. Cancer Discov 2019; 9: 646–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Germano G, Amirouchene-Angelozzi N, Rospo G, et al. The clinical impact of the genomic landscape of mismatch repair-deficient cancers. Cancer Discov 2018; 8: 1518–1528. [DOI] [PubMed] [Google Scholar]
- 120. Halama N. Macrophage repolarisation therapy in colorectal cancer. ESMO Open 2018; 3: e000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Tartour E. New therapeutic targets in the inflammatory microenvironment. ESMO Open 2018; 3: e000310. [DOI] [PMC free article] [PubMed] [Google Scholar]