Abstract
Background:
The responses of intravenous immunoglobulin (IVIg) or corticosteroids as the initial treatment on pregnancy with ITP were unsatisfactory. This study aimed to assess the safety and effectiveness of prednisone plus IVIg versus prednisone or IVIg in pregnant patients with immune thrombocytopenia (ITP).
Methods:
Between 1 January 2010 and 31 December 2020, 970 pregnancies diagnosed with ITP at 19 collaborative centers in China were reviewed in this observational study. A total of 513 pregnancies (52.89%) received no intervention. Concerning the remaining pregnancies, 151 (33.04%) pregnancies received an initial treatment of prednisone plus IVIg, 105 (22.98%) pregnancies received IVIg alone, and 172 (37.64%) pregnancies only received prednisone.
Results:
Regarding the maternal response to the initial treatment, no differences were found among the three treatment groups (41.1% for prednisone plus IVIg, 33.1% for prednisone, and 38.1% for IVIg). However, a significant difference was observed in the time to response between the prednisone plus IVIg group (4.39 ± 2.54 days) and prednisone group (7.29 ± 5.01 days; p < 0.001), and between the IVIg group (6.71 ± 4.85 days) and prednisone group (p < 0.001). The median prednisone duration in the monotherapy group was 27 days (range, 8–195 days), whereas that in the combination group was 14 days (range, 6–85 days). No significant differences were found among these three treatment groups in neonatal outcomes, particularly concerning the neonatal platelet counts. The time to response in the combination treatment group was shorter than prednisone monotherapy. The duration of prednisone application in combination group was shorter than prednisone monotherapy. The combined therapy showed a lower predelivery platelet transfusion rate than IVIg alone.
Conclusion:
These findings suggest that prednisone plus IVIg may represent a potential combination therapy for pregnant patients with ITP.
Keywords: immune thrombocytopenia, intravenous immunoglobulin, prednisone, pregnant
Introduction
Immune thrombocytopenia (ITP) is a common immune disorder characterized by isolated thrombocytopenia, 1 and its incidence ranges from 1 to 10 in 10,000 pregnant women. 2 ITP accounts for approximately 1–4% of thrombocytopenia in pregnancy3–5 and is the most frequent cause of thrombocytopenia in early pregnancy. 6 Most pregnant women with ITP have mild to moderate thrombocytopenia. An exacerbation or relapse of thrombocytopenia may occur in previously diagnosed women during pregnancy.7,8 The reported rate of postpartum hemorrhage in ITP pregnancies ranges from 1.9 to 23.2%.9–13 Hemorrhage risks may be increased in severe ITP pregnancies. Care et al. 14 reported that the rate of postpartum hemorrhage was 52%, and the rate of severe postpartum hemorrhage was 21% in severe ITP pregnancies.
Approximately 30–35% of pregnant women with ITP require intervention during pregnancy.9,15 Recommendations for the treatment of ITP in pregnancy are primarily based on clinical experience and expert consensus. Loustau et al. 11 conducted a retrospective study of 118 pregnancies with ITP and found that the platelet count decreased significantly in 52% of patients. Treatments were required in 49% of patients during the pregnancy or near delivery. Corticosteroids or intravenous immunoglobulin (IVIg) are recommended as first-line treatments for pregnant women with ITP.6,16,17 Corticosteroids are generally considered safe in pregnant women. However, high-dose corticosteroid therapy is often not tolerable and is sometimes associated with adverse events in the mother and fetus when administered in the first trimester.18–20 IVIg can be used as another first-line treatment option. 21 Prospective randomized studies comparing the treatment for ITP during pregnancy are lacking. To date, studies have been conducted with a concentration on only observational data, and no study has focused on comparing different treatments for pregnant women. The effects of IVIg and corticosteroids on pregnancy outcomes were explored in a retrospective study of 235 pregnancies in 195 women with ITP. In their study, patients were treated with IVIg or corticosteroids as the initial treatment. No significant difference was found regarding maternal platelet counts at delivery. Only 40% of all the pregnancies (treated with either IVIg or corticosteroids) showed a treatment response. 13 No consensus is available on the best treatment for ITP in pregnant women who fail first-line therapy. In addition, many of the treatments frequently used in nonpregnant ITP patients may not be safe during pregnancy. Scant information exists on the safety and efficacy of second-line therapies for ITP during pregnancy. 20 Most immunosuppressive drugs (except for azathioprine), such as danazol and vinca alkaloids, should not be administered to pregnant women because of potential teratogenic effects. 16 Thrombopoietin (TPO) receptor agonists and rituximab are not considered initial therapies in ITP patients. 22 Rituximab is known to be associated with prolonged B-cell lymphocytopenia and the need to delay vaccination in neonates.20,22,23 As for TPO-RAs, it is safe in the second and third trimesters. However, it should be avoided in the first trimester.24,25
The maternal response to initial treatment with IVIg or corticosteroids reported was 38% and 39%, separately, 13 lower than that in nonpregnant patients with ITP. In ITP patients, IVIg can be used with corticosteroids when a more rapid increase in the platelet count is needed. 26 Evidence for the effectiveness of IVIg combined with corticosteroids was concluded in ITP patients without pregnancy. 17 Thus, that evidence could not appropriately represent the patients’ clinical condition in pregnancy. Recommendations for managing ITP in pregnancy suggest that combining therapies (prednisone with IVIg) may lead to a response in patients refractory to either agent alone. 22 Combining first-line therapies is considered effective and can be helpful for patients with inadequate responses to first-line agents.6,16,17,27
Despite being relatively safe in pregnancy, the application of corticosteroids has several limitations and risks, including exacerbation of hypertension, increased blood glucose levels, edema, peptic ulcer disease, and psychosis.6,28 There were also reports of prenatal glucocorticoid exposure on fetal growth and development of immune system. 29 IVIg provides a fast, but often transient, increase in the platelet count and can be used to increase the platelet counts immediately during bleeding or delivery. 26 The risk of adverse reactions to IVIG generally correlates with the dose of IVIG within each course and the rate of infusion.30–32 Thrombosis has not been reported as a frequent adverse event in studies comparing IVIg with corticosteroids in pregnancy. 22 Despite the theoretical risks, thrombosis has not been reported as a frequent adverse event in studies comparing IVIg with corticosteroids in pregnancy. 13 A significant cost is also associated with IVIg compared with prednisone. 26 Scant information is available concerning the usefulness of prednisone plus IVIg to treat obstetric patients with ITP. Therefore, we first assessed the use of combined prednisone and IVIg therapy, prednisone monotherapy, or IVIg monotherapy for ITP pregnancies from national cohort data. Comparisons were made to determine whether the combination of these two agents could maximize their individual efficacy while minimizing adverse effects and assess its possibility as a promising therapeutic strategy for pregnancies with ITP.
Materials and methods
Patients
A retrospective chart review was conducted on pregnant women with ITP from 1 January 2010 through 31 December 2020 at 19 collaborative centers for hematology in China (Supplemental Fig. 1). The pregnant patients either inpatient or outpatient, who met the diagnostic criteria of ITP, were consecutively recruited into our retrospective study. Patients who did not require intervention and had received prednisone plus IVIg, prednisone or IVIg alone as the first-line treatment method16,27 were recruited into this retrospective study. Informed consents to treatment were obtained by all patients or their guardians. This study was approved by the central institutional review board of the Peking University People’s Hospital, Beijing, China (No.11 Xizhimen Street, Xicheng district, Beijing, China) at 2 March 2021. Approval for the study was also obtained from the ethics committees of all other participating centers. This retrospective study involved the analysis of existing data and records; all detailed information of research participants has been de-identified in our article.
Information from national cohort data was collected on cases of pregnancy in patients who met the diagnostic criteria of ITP in China during a 11-year period.16,17,27 Only patients with primary ITP were identified in our study. The diagnosis of primary ITP were based on the international consensus report on the investigation and management of primary immune thrombocytopenia, 16 updated international consensus report on the investigation and management of primary immune thrombocytopenia, 22 and Chinese guideline on the diagnosis and management of adult primary immune thrombocytopenia (version 2020). 33 To confirm the diagnosis of primary ITP in pregnancy, multidisciplinary team meetings were organized comprising obstetricians, hematologists, and immunologists. The final diagnosis of each case was determined by consensus. Patients with other causes of thrombocytopenia, including gestational thrombocytopenia, preeclampsia, sepsis, hemolysis, elevated liver enzymes, low platelet syndrome, cancer, liver diseases (hepatitis B or C virus infection and cirrhosis), myelodysplasia or dysplasia, drug-induced thrombocytopenia, disseminated intravascular coagulation, antiphospholipid syndrome, thrombotic thrombocytopenic purpura, and systemic lupus erythematosus, were excluded. The Systemic Lupus International Collaborating Clinics criteria for systemic lupus erythematosus 34 and the guidelines for antiphospholipid syndrome 35 were used in the diagnosis and differential diagnosis. In addition, patients with platelet counts more than 70 × 109/l during pregnancy and normal platelet counts afterward were excluded because they could be caused by gestational thrombocytopenia as well.13,36
Data management
Using electronic medical records and institutional databases, data regarding the demographics, treatment response, maternal outcomes, delivery mode, and neonatal outcomes were extracted. The primary end point of the study was the maternal response to treatment, including response and complete response according to the maternal platelet count. The secondary end points included the maternal platelet count at delivery, maternal composite outcomes (including postpartum hemorrhage, predelivery platelet transfusion, peripartum transfusion of any blood product, and postpartum reduction in the hemoglobin concentration of 30 g/l or more), delivery mode, time to response, time to relapse, neonatal platelet counts at birth, and neonatal composite outcomes (including stillbirth, preterm birth, small for gestational age size, or Apgar score < 7 at 5 min). The definitions of maternal treatment responses in terms of the platelet count included the following: (1) no response: less than doubling of the baseline count or lower than 30 × 109/l; (2) response: between 100 and 30 × 109/l and at least doubling of the baseline count; (3) complete response: at least 100 × 109/l.16,17,37 In addition, the time to response was defined as the time between treatment started and response achieved. During the following of the pregnancies with ITP, platelet count usually was checked every 1–4 weeks, depending on the stability of the platelet counts. If platelet counts were found to be <80 ×109/l after week 34, they should be monitored one to three times a week. The World Health Organization (WHO) bleeding scale (0 = no bleeding, 1 = petechiae, 2 = mild blood loss, 3 = gross blood loss, and 4 = debilitating blood loss) was used to define bleeding. Postpartum hemorrhage was defined when patients had undergone an estimated blood loss of 1000 ml or more following cesarean section or 500 ml or more following vaginal delivery. 38
Statistical analysis
Baseline characteristics of the study population were summarized using descriptive statistical methods. Continuous variables were expressed as mean (SD) and were tested for normality distribution with the Kolmogorov–Smirnov test. For comparisons among different treatment groups, one-way analysis of variance (ANOVA) or the Kruskal–Wallis H test was used as appropriate with Bonferroni correction for multiple comparisons. In light of the possible impact of correlated observations owing to repeated pregnancies in same mother, logistic or linear regression models with the generalized estimating equation (GEE) were used. The data were statistically analyzed using GraphPad Prism version 6 (GraphPad Software, San Diego, CA, USA), SPSS version 23.0 for Windows (IBM, Armonk, NY, USA), and R 3.5.1 software. A p value of <0.05 was considered statistically significant. This study conforms to the STROBE statement. 39 A checklist of the STROBE statement for cohort studies is shown in Supplemental Table 1S.
Results
Demographic data
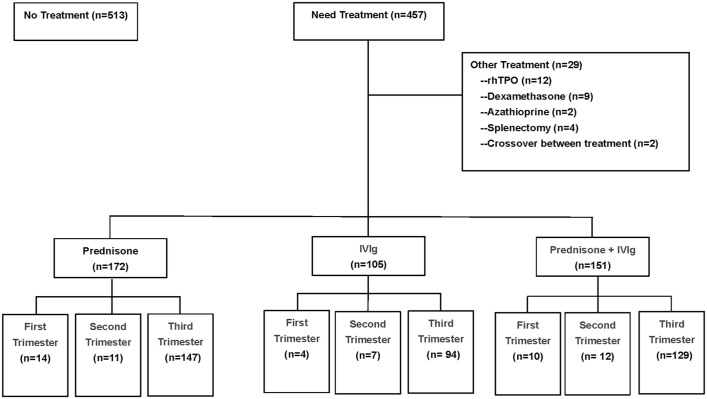
A total of 704 pregnant patients with ITP (970 pregnancies) were reviewed in this study. The intervention was not introduced in 513 pregnancies (513/970; 52.89%). Among the remaining 457 pregnancies, 172 (172/457; 37.64%) had received initial treatment with prednisone alone, 105 (105/457; 22.98%) had received IVIg alone, and 151 (151/457; 33.04%) had received IVIg plus prednisone. The remaining 29 pregnancies had received other treatments, including dexamethasone, recombinant human thrombopoietin, azathioprine, splenectomy, or crossover between treatments. A total of 704 pregnant patients with ITP (970 pregnancies) were reviewed in this study. Among these 704 patients, 567 patients (833 pregnancies) were diagnosed ITP previously. Of them, 137 were first diagnosed with ITP during pregnancy.
Among the 833 pregnancies with a medical history of ITP, 29 received other treatments (including rhTPO, dexamethasone, azathioprine, splenectomy, or crossover between treatments). Of the remaining 804 pregnancies, 428 did not receive any treatment for ITP, 150 received prednisone, 87 received IVIg, and 139 received IVIg plus prednisone as first-line therapy (Supplemental Table S3).
In total, 137 women were newly diagnosed with ITP in our cohort. Among these, 104 (75.9%), 10 (7.3%), and 23 (16.8%) were diagnosed during the first, second, and third trimesters, respectively (Supplemental Table S4).
The median prednisone dose in monotherapy was 25.0 mg/d (range, 5–60 mg/d) for a median duration of 27 days (range, 8–195 days). The mean dose of IVIg used as initial treatment was 1.8 g/kg (range, 1.0–2.0 g/kg) body weight at one cycle. There were 86 patients in the monotherapy group who received 2.0 g/kg of IVIg. The number of pregnant patients who received 1.0, 1.2, and 1.6 g/kg body weight IVIg in the monotherapy group was 12, 5, and 2, respectively. The dose of prednisone in the combined therapy was 25.0 mg/d (range, 5–40 mg/d) for a median duration of 14 days (range, 6–85 days). Patients concomitantly received IVIg at a mean dose of 1.8 g/kg (range, 1.0–2.0 g/kg) body weight in one cycle. A total of 116 patients in the combined treatment group received 2.0 g/kg of IVIg. The number of pregnant patients who received 1.0, 1.2, and 1.6 g/kg body weight IVIg in the combined treatment group was 19, 14, and 2, respectively. As for the IVIg dose, most of the patients enrolled in our study used the higher dose of 2.0 g/kg. Concerning the differences in IVIg dose, we compared the maternal response to different IVIg doses in our cohort. There was no difference among the different IVIg dose groups for all patients using IVIg, either monotherapy or as a combination. Maternal responses to different doses of IVIg are shown in Supplemental Table 2S. Pregnancies were given one to five cycles during the gestation period. Most ITP pregnancies (370/457; 80.96%) started treatment during the third trimester. Twenty-eight and 30 pregnancies began their treatment during the first and second trimesters, respectively (Figure 1). No difference was found in the time of initiation of treatment among the different treatment groups (p = 0.652).
Figure 1.
Patient disposition.
IVIg, intravenous immunoglobulin; rhTPO, recombinant human thrombopoietin.
Maternal characteristics
The patients’ demographic and maternal baseline characteristics are presented in Table 1. No differences were found among the four groups concerning maternal age, the body mass index, or age at ITP diagnosis. The primiparity rates in the different groups were 80.8% (122/151) in the prednisone plus IVIg group, 70.5% (74/105) in the IVIg group, 58.1% (100/172) in the prednisone group, and 58.7% (301/513) in the no-treatment group. More primiparity was observed in the IVIg group and prednisone plus IVIg group than in the prednisone group and nontreatment group (p < 0.001). Regarding the delivery mode, the cesarean section rates of the three treatment groups were 27.3% (47/172) in the prednisone group, 25.7% (27/105) in the IVIg group, and 24.5% (37/151) in the prednisone plus IVIg group. In the no-treatment group, the cesarean section rate was 41.1% (211/513), higher than that in the other treatment groups (p < 0.001). No differences in the rate of operative vaginal delivery were observed between the treated and untreated groups (p = 0.941). The prednisone plus IVIg group had a lower maternal pretreatment platelet count and higher bleeding score than the prednisone or IVIg monotherapy group.
Table 1.
Patients’ demographic and maternal baseline characteristics.
| No treatment (n = 513) |
Pre. plus IVIg (n = 151) |
Pre. (n = 172) |
IVIg (n = 105) |
p value | ||||
|---|---|---|---|---|---|---|---|---|
| All groups | IVIg versus pre | Pre. plus IVIg versus IVIg | Pre. plus IVIg versus pre. | |||||
| Maternal age (years), mean (SD) | 29.47 (4.63) | 29.31 (4.56) | 29.22 (4.50) | 29.20 (4.36) | 0.956 | 0.979 | 0.851 | 0.849 |
| BMI (kg/m2), mean (SD) | 23.40 (3.63) | 23.27 (3.02) | 23.59 (3.41) | 23.51 (2.90) | 0.706 | 0.841 | 0.589 | 0.403 |
| Primiparity, n (%) | 301 (58.7) | 122 (80.8) | 100 (58.1) | 74 (70.5) | <0.001 | 0.036 | 0.088 | <0.001 |
| Age at ITP diagnosis, mean (SD) | 24.89 (5.30) | 24.59 (6.75) | 24.08 (5.59) | 24.67 (6.33) | 0.623 | 0.408 | 0.916 | 0.424 |
| Maternal pretreatment platelet count (×109/l), mean (SD) | NA | 13.51 (9.99) | 20.99 (10.96) | 16.57 (10.10) | <0.001 | 0.001 | 0.020 | <0.001 |
| Gestational age at platelet nadir (wk), median (range) | 33.5 (8, 40) | 28.0 (7, 41) | 32.0 (8, 40) | 30.5 (9, 40) | <0.001 | 0.087 | 0.032 | <0.001 |
| Operative vaginal delivery (n/N) (%) | 22/302 (7.3) | 8/114 (7.0) | 9/125 (7.2) | 5/78 (6.4) | 0.941 | 0.833 | 0.873 | 0.957 |
| Cesarean section, n (%) | 211 (41.1) | 37 (24.5) | 47 (27.3) | 27 (25.7) | <0.001 | 0.781 | 0.839 | 0.589 |
| Bleeding score (%) | <0.001 | 0.050 | 0.019 | <0.001 | ||||
| 0 | 454 (88.5) | 102 (67.5) | 146 (84.9) | 80 (76.2) | ||||
| 1 | 59 (11.5) | 46 (30.5) | 23 (13.4) | 23 (21.9) | ||||
| 2 | 0 (0.0) | 2 (1.3) | 2 (1.2) | 0 (0.0) | ||||
| 3 | 0 (0.0) | 1 (0.7) | 1 (0.6) | 2 (1.9) | ||||
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||
BMI, body mass index; ITP, immune thrombocytopenia; IVIg, intravenous immunoglobulin; NA, not applicable; pre., prednisone; SD, standard deviation.
Antepartum hemorrhage was observed in 21 (4.1%) pregnancies from no-treatment group, and 18 (4.2%) in treated pregnancies. There were no differences been observed in antepartum hemorrhage between pregnancies with or without treatment (Table 2). Among the three treated groups, antepartum hemorrhages were observed in five (2.9%) from prednisone group, five (4.8%) from IVIg group, and eight (5.3%) from prednisone plus IVIg group, respectively (Table 3).
Table 2.
Maternal outcomes of treated group and no-treatment group.
| No treatment (n = 513) |
Treated (n = 428) |
p value | |
|---|---|---|---|
| Maternal platelet count at delivery (×109/l), mean (SD) | 109.20 (29.76) | 66.16 (36.57) | <0.001 |
| Antepartum hemorrhage, n (%) | 21 (4.1) | 18 (4.2) | 0.358 |
| Postpartum hemorrhage, n (%) | 15 (2.9) | 17 (4.0) | 0.042 |
| Predelivery platelet transfusion, n (%) | 18 (3.5) | 44 (10.3) | <0.001 |
| Peripartum transfusion: any blood product, n (%) | 20 (3.9) | 52 (12.1) | <0.001 |
| Hemoglobin drop > 30 g/l after delivery | 44 (8.6) | 49 (11.4) | 0.053 |
SD, standard deviation.
Table 3.
Maternal outcomes among different treatment.
| Pre. plus IVIg (n = 151) |
Pre. (n = 172) |
IVIg (n = 105) |
p value | |||
|---|---|---|---|---|---|---|
| IVIg versus Pre | Pre. plus IVIg versus IVIg | Pre. plus IVIg versus pre. | ||||
| Maternal response to initial treatment, (%) | 62 (41.1) | 57 (33.1) | 40 (38.1) | 0.960 | 0.634 | 0.550 |
| Maternal platelet count at delivery (×109/l), mean (SD) | 63.57 (41.06) | 67.61 (33.53) | 67.52 (34.59) | 0.984 | 0.337 | 0.265 |
| Antepartum hemorrhage, n (%) | 8 (5.3) | 5 (2.9) | 5 (4.8) | 0.458 | 0.834 | 0.288 |
| Postpartum hemorrhage, n (%) | 9 (6.0) | 6 (3.5) | 2 (1.9) | 0.481 | 0.079 | 0.222 |
| Pre-delivery platelet transfusion, n (%) | 11 (7.3) | 18 (10.5) | 15 (14.3) | 0.227 | 0.031 | 0.264 |
| Peripartum transfusion: any blood product, n (%) | 11 (7.3) | 23 (13.4) | 18 (17.1) | 0.262 | 0.004 | 0.045 |
| Hemoglobin drop > 30 g/l after delivery | 20 (13.2) | 12 (7.0) | 17 (16.2) | 0.014 | 0.442 | 0.063 |
IVIg, intravenous immunoglobulin; Pre., prednisone; SD, standard deviation.
Neonatal characteristics
Regarding the neonatal characteristics, 974 live births occurred (974 neonates: 951 pregnancies, including 23 twin pregnancies) with 19 intrauterine deaths. No newborn died after birth in this study. The cord blood platelet counts were measured in 641 (641/951; 67.4%) neonates. In the prednisone plus IVIg group, 23/103 (22.3%) neonates showed a platelet count <100 × 109/l; the rate was 21/110 (19.1%) in the prednisone group and 9/66 (13.6%) in the IVIg group. Among these three treatment groups, no significant difference was observed regarding the neonatal outcomes, particularly the neonatal platelet counts (Table 4).
Table 4.
Neonatal outcomes according to treatment strategy.
| No treatment (n = 513) |
Pre. plus IVIg (n = 151) |
Pre. (n = 172) |
IVIg (n = 105) |
p value | ||||
|---|---|---|---|---|---|---|---|---|
| All groups | IVIg versus pre | Pre. plus IVIg versus IVIg | Pre. plus IVIg versus pre. | |||||
| Stillbirth, (%) | 7 (1.4) | 2 (1.3) | 5 (2.9) | 5 (4.8) | 0.065 | 0.303 | 0.063 | 0.329 |
| Preterm birth < 37 weeks, (%) | 27 (5.3) | 11 (7.3) | 16 (9.3) | 10 (9.5) | <0.001 | 0.227 | 0.131 | 0.264 |
| Preterm birth < 34 weeks, (%) | 4 (1.0) | 2 (1.3) | 5 (2.9) | 3 (3.0) | 0.282 | 0.952 | 0.294 | 0.252 |
| Birth weight, mean (SD) | 3146.26 (587.71) | 3067.24 (555.53) | 3048.40 (533.90) | 2930.00 (558.86) | 0.247 | 0.101 | 0.064 | 0.773 |
| Small for gestational age n (%) | 18 (3.5) | 6 (4.0) | 4 (2.3) | 5 (4.8) | 0.690 | 0.295 | 0.747 | 0.427 |
| Apgar score < 7 at 5 min, (%) | 22 (4.3) | 8 (5.3) | 9 (5.2) | 5 (4.8) | 0.143 | 0.136 | 0.764 | 0.146 |
| Neonatal platelet count (×109/l), mean (SD) | 236.20 (107.20) | 199.86 (111.55) | 209.10 (115.61) | 218.28 (115.27) | 0.019 | 0.593 | 0.289 | 0.542 |
IVIg, intravenous immunoglobulin; Pre., prednisone; SD, standard deviation.
Outcomes
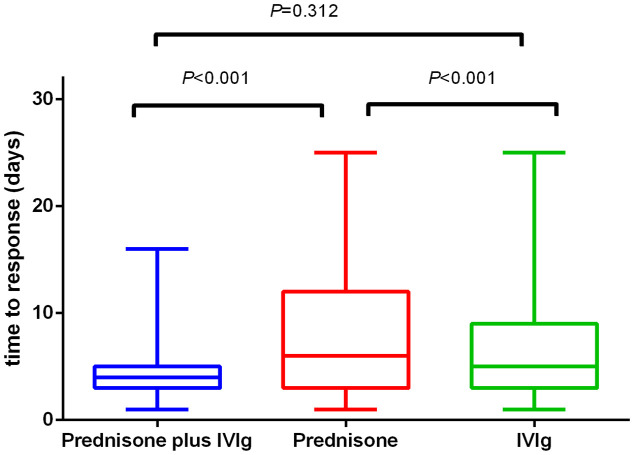
Regarding the maternal response (either complete response or response) to the initial treatment and postpartum hemorrhage, no differences were observed among the three treatment groups (Table 3). Overall, bleeding-related variables, including predelivery platelet transfusion, maternal platelet counts at delivery, postpartum hemorrhage, and peripartum transfusion of any blood product, differed between the no-treatment group and treatment group (Table 2). The time to response of the three treatment groups was 7.29 ± 5.01 days in the prednisone group, 6.71 ± 4.85 days in the IVIg group, and 4.39 ± 2.54 days in the prednisone plus IVIg group. The time to response of the prednisone plus IVIg group and IVIg group was shorter than that of the prednisone alone group (p < 0.001; Figure 2). No significant difference was found between the prednisone plus IVIg group and IVIg alone group (p = 0.312; Figure 2). The predelivery platelet transfusion of the IVIg monotherapy group was greater than that of the prednisone plus IVIg group (p = 0.031; Table 3). Fewer peripartum transfusions of any blood product were performed in the prednisone plus IVIg group than in the other treatment groups (p = 0.004 and 0.045, compared with IVIg alone and prednisone alone, respectively; Table 3).
Figure 2.
Box plots of the time to response of patients with an initial response to prednisone plus IVIg, prednisone, or IVIg alone. The time to response in the prednisone plus IVIg group and IVIg alone group was significantly shorter than that in the prednisone alone group. No significant difference was found between the prednisone plus IVIg group and IVIg alone group.
Relationship analysis
In addition, the neonatal platelet counts and maternal pretreatment platelet counts revealed no correlation (correlation coefficient = 0.00086; Supplemental Fig. 2A). No correlation was found between the neonatal platelet counts and maternal pretreatment platelet counts for all treatment groups (Supplemental Fig. 2B). No correlation was also found between the maternal platelet count at delivery and neonatal platelet count in all pregnancies (correlation coefficient = 0.019, Supplemental Fig. 2 C) or in different groups (Supplemental Fig. 2D).
Discussion
Our retrospective study of 970 pregnancies with ITP was conducted to evaluate the efficacy and safety of prednisone plus IVIg versus prednisone or IVIg monotherapy as initial treatment. No differences were found in the maternal treatment response or adverse events among the three treatment groups. Importantly, maternal ITP patients who had received combination therapy showed a shorter time to response than those who had received prednisone monotherapy. In addition, the combination therapy group had a lower predelivery platelet transfusion rate than monotherapy group. These findings may have clinical implications and deserve further study.
Corticosteroids or IVIg are the first-line treatment options for ITP in pregnancy recommended by the guidelines or concensus.20,22,26 Corticosteroids are generally considered to be safe in pregnancy. Several case reports and small series have reported use of TPO-RAs in pregnant women in retrospective data.24,25,40 It is recommended that only under some circumstances, second-line medications including romiplostim and rituximab can serve as therapeutic options for refractory immune thrombocytopenia in pregnancy.20,22–25,41 Prednisone, one of the first-line therapies for ITP during pregnancy, can exacerbate hypertension, induce hyperglycemia, weight gain, and contribute to adverse pregnancy outcomes.16,20,42 IVIg usually affects the maternal platelet count quickly. The platelet response is usually achieved within an average of 2 days after infusion. 13 However, because of its potential effects on acute hemolysis, high cost, and need for intravenous administration, its application remains limited.6,43 In practice, IVIg plus prednisone has been used in some pregnancies with ITP. To evaluate the efficacy and safety of prednisone or IVIg alone or in combination in pregnancies with ITP, we conducted a retrospective analysis in this study. Most of the current reports are based on observational studies that examine existing epidemiological evidence or report the results of a single treatment. Therefore, while advice relies on experience, this study aimed to outline a reliable approach to manage ITP during pregnancy.
The first-line therapy of pregnant patients with ITP does not notably differ from nonpregnant women who are diagnosed with ITP.6,16,17 Corticosteroids are the first-line treatment for nonpregnant patients with ITP, with an initial response rate of 70–80%. 16 The response rate for IVIg has been reported to be up to 80%. 16 To date, many studies have discussed treatment for pregnant patients with ITP,11,44–46 while the response rates have not been covered. Webert et al. 9 reported a platelet count response in 3/8 (38%) corticosteroid-treated and 11/20 (55%) IVIg-treated pregnancies. The response rate to IVIg in our study was lower (40/105 (38.1%) than that in Webert’s study. Sun et al. 13 reported a platelet count response of 39% for corticosteroid-treated cases and 38% for IVIg-treated pregnancies. In this study, the maternal response to initial treatment was 41.1% in the prednisone plus IVIg group, 33.1% in the prednisone group, and 38.1% in the IVIg group. No difference was found among the treatment groups.
To safely and timely manage pregnant patients with ITP, obstetricians, hematologists, immunologists, and anesthetists should collaborate. Assessing the risk of maternal hemorrhage is crucial. Most of the treatment recommendations have been based on limited and inconsistent observational reports. No consensus are available regarding the dosing of prednisone used in ITP pregnancies. Some international consensuses recommend an initial dose of 1 mg/kg, 47 and some suggest starting at 40–50 mg daily. 13 Because no definite evidence supports that high doses of corticosteroids are more efficacious in pregnancy, 48 some experts recommended a lower dose of 0.25–0.5 mg/kg daily to minimize steroid-related adverse effects.5,49,50 In our study, the median prednisone dose in monotherapy was 25 mg/d (range, 5–60 mg/d). The median dose of prednisone in the combined group was 25 mg/d (range, 5–40 mg/d).
In nonpregnant individuals, the approximate time to respond to prednisone is several days to several weeks. The time to response for IVIg is typically 2–4 days, even within 24 h. Consistent with previous studies, we reported a significantly faster response to prednisone plus IVIg therapy (time to response, 4.39 ± 2.54 days) and IVIg (time to response, 6.71 ± 4.85 days) than to prednisone monotherapy (time to response, 7.29 ± 5.01 days). The median duration of prednisone in monotherapy was 27 days (range, 8–195 days) and 14 days (range, 6–85 days) in the combination therapy group. The duration of prednisone in the combination treatment group was shorter than that in the prednisone monotherapy group. The application of IVIg in combination therapy might result in an improved time to response and shortened prednisone treatment duration.
Platelet transfusions may be helpful and must not be postponed in cases of life-threatening bleeding, especially intracranial hemorrhage (ICH). 22 Platelet transfusions are also considered if an emergency cesarean section is required with a platelet count below 50 × 109/l despite other treatment measures. 51 The platelet lifespan is short following transfusion. 52 Usually, the platelet counts will decrease to the baseline level approximately 1 week after platelet transfusion. 53 Concurrent administration of platelet transfusions and IVIg was associated with resolution of bleeding, rapid restoration of adequate platelet counts, and minimal side effects. 16 In our study, the predelivery platelet transfusion of the IVIg monotherapy group was greater than that of the prednisone plus IVIg group. This effect might benefit from the treatment of prednisone combined with IVIg, which could maintain platelet levels more persistently than IVIg alone.
Our study was the largest cohort study to compare the effectiveness and safety of combination of IVIg and prednisone with prednisone or IVIg monotherapy in pregnancies with ITP. The sample size with data capture from 19 centers from national wide in China was large for pregnancies with ITP. Findings from this study add to the literature on treatment of ITP in pregnancies in two ways. First, the combination treatment group may yield a shorter response time and a shorter durations of prednisone application when compared with prednisone monotherapy group. Second, while compared with IVIg monotherapy, the combination therapy group had a lower predelivery platelet transfusion rate and a lower peripartum transfusions of any blood product during observational period.
The main limitation of our study is its retrospective nature. As in all observational studies, our results may be impacted by confounding (e.g. incomplete control for confounding due to missing data/variables, and other residual confounding). Secondarily, there was no a priori power calculation in our retrospective study. We have included all the subjects meeting the criterion of our study from 19 centers in China during a 11-year period. The conclusions of our retrospective study still need further research. Not all data points were collected at the same time for each patient and possible over- and underreporting of events. The inclusion of consecutive pregnancies who met the diagnostic criteria of ITP from different areas in China ensured that the data collected were representative. No patient was excluded based on treatment adherence or outcome. Available data were limited to information in each patient’s medical record. As such, there was the potential for documentation bias. The sample size of our study was large, but the rate of maternal hemorrhage was low, which limits the evaluation of the treatment effects of bleeding events.
Conclusion
This observational study prompted that combination treatment with prednisone and IVIg in pregnancies with ITP might be similar to prednisone or IVIg alone regarding the maternal response. However, the combined therapy showed a faster time to response and a shorter duration of prednisone administration compared with prednisone monotherapy and a lower predelivery platelet transfusion compared with IVIg alone. Prospective studies need to be conducted to better identify the best treatment and optimum dose in maternal ITP patients.
Supplemental Material
Supplemental material, sj-docx-1-tah-10.1177_20406207221095226 for Prednisone plus IVIg compared with prednisone or IVIg for immune thrombocytopenia in pregnancy: a national retrospective cohort study by Xiao-Lu Zhu, Ru Feng, Qiu-Sha Huang, Mei-Ying Liang, Ming Jiang, Hui Liu, Yi Liu, Hong-Xia Yao, Lei Zhang, Shen-Xian Qian, Tong-Hua Yang, Jing-Yu Zhang, Xu-Liang Shen, Lin-Hua Yang, Jian-Da Hu, Ren-Wei Huang, Zhong-Xing Jiang, Jing-Wen Wang, Hong-Yu Zhang, Zhen Xiao, Si-Yan Zhan, Hui-Xin Liu, Xing-Lin Wang, Ying-Jun Chang, Yu Wang, Yuan Kong, Lan-Ping Xu, Kai-Yan Liu, Xiao-Hong Zhang, Cheng-Hong Yin, Yue-Ying Li, Qian-Fei Wang, Jian-Liu Wang, Xiao-Jun Huang and Xiao-Hui Zhang in Therapeutic Advances in Hematology
Supplemental material, sj-tiff-2-tah-10.1177_20406207221095226 for Prednisone plus IVIg compared with prednisone or IVIg for immune thrombocytopenia in pregnancy: a national retrospective cohort study by Xiao-Lu Zhu, Ru Feng, Qiu-Sha Huang, Mei-Ying Liang, Ming Jiang, Hui Liu, Yi Liu, Hong-Xia Yao, Lei Zhang, Shen-Xian Qian, Tong-Hua Yang, Jing-Yu Zhang, Xu-Liang Shen, Lin-Hua Yang, Jian-Da Hu, Ren-Wei Huang, Zhong-Xing Jiang, Jing-Wen Wang, Hong-Yu Zhang, Zhen Xiao, Si-Yan Zhan, Hui-Xin Liu, Xing-Lin Wang, Ying-Jun Chang, Yu Wang, Yuan Kong, Lan-Ping Xu, Kai-Yan Liu, Xiao-Hong Zhang, Cheng-Hong Yin, Yue-Ying Li, Qian-Fei Wang, Jian-Liu Wang, Xiao-Jun Huang and Xiao-Hui Zhang in Therapeutic Advances in Hematology
Supplemental material, sj-tiff-3-tah-10.1177_20406207221095226 for Prednisone plus IVIg compared with prednisone or IVIg for immune thrombocytopenia in pregnancy: a national retrospective cohort study by Xiao-Lu Zhu, Ru Feng, Qiu-Sha Huang, Mei-Ying Liang, Ming Jiang, Hui Liu, Yi Liu, Hong-Xia Yao, Lei Zhang, Shen-Xian Qian, Tong-Hua Yang, Jing-Yu Zhang, Xu-Liang Shen, Lin-Hua Yang, Jian-Da Hu, Ren-Wei Huang, Zhong-Xing Jiang, Jing-Wen Wang, Hong-Yu Zhang, Zhen Xiao, Si-Yan Zhan, Hui-Xin Liu, Xing-Lin Wang, Ying-Jun Chang, Yu Wang, Yuan Kong, Lan-Ping Xu, Kai-Yan Liu, Xiao-Hong Zhang, Cheng-Hong Yin, Yue-Ying Li, Qian-Fei Wang, Jian-Liu Wang, Xiao-Jun Huang and Xiao-Hui Zhang in Therapeutic Advances in Hematology
Supplemental material, sj-tiff-4-tah-10.1177_20406207221095226 for Prednisone plus IVIg compared with prednisone or IVIg for immune thrombocytopenia in pregnancy: a national retrospective cohort study by Xiao-Lu Zhu, Ru Feng, Qiu-Sha Huang, Mei-Ying Liang, Ming Jiang, Hui Liu, Yi Liu, Hong-Xia Yao, Lei Zhang, Shen-Xian Qian, Tong-Hua Yang, Jing-Yu Zhang, Xu-Liang Shen, Lin-Hua Yang, Jian-Da Hu, Ren-Wei Huang, Zhong-Xing Jiang, Jing-Wen Wang, Hong-Yu Zhang, Zhen Xiao, Si-Yan Zhan, Hui-Xin Liu, Xing-Lin Wang, Ying-Jun Chang, Yu Wang, Yuan Kong, Lan-Ping Xu, Kai-Yan Liu, Xiao-Hong Zhang, Cheng-Hong Yin, Yue-Ying Li, Qian-Fei Wang, Jian-Liu Wang, Xiao-Jun Huang and Xiao-Hui Zhang in Therapeutic Advances in Hematology
Supplemental material, sj-tiff-5-tah-10.1177_20406207221095226 for Prednisone plus IVIg compared with prednisone or IVIg for immune thrombocytopenia in pregnancy: a national retrospective cohort study by Xiao-Lu Zhu, Ru Feng, Qiu-Sha Huang, Mei-Ying Liang, Ming Jiang, Hui Liu, Yi Liu, Hong-Xia Yao, Lei Zhang, Shen-Xian Qian, Tong-Hua Yang, Jing-Yu Zhang, Xu-Liang Shen, Lin-Hua Yang, Jian-Da Hu, Ren-Wei Huang, Zhong-Xing Jiang, Jing-Wen Wang, Hong-Yu Zhang, Zhen Xiao, Si-Yan Zhan, Hui-Xin Liu, Xing-Lin Wang, Ying-Jun Chang, Yu Wang, Yuan Kong, Lan-Ping Xu, Kai-Yan Liu, Xiao-Hong Zhang, Cheng-Hong Yin, Yue-Ying Li, Qian-Fei Wang, Jian-Liu Wang, Xiao-Jun Huang and Xiao-Hui Zhang in Therapeutic Advances in Hematology
Supplemental material, sj-tif-6-tah-10.1177_20406207221095226 for Prednisone plus IVIg compared with prednisone or IVIg for immune thrombocytopenia in pregnancy: a national retrospective cohort study by Xiao-Lu Zhu, Ru Feng, Qiu-Sha Huang, Mei-Ying Liang, Ming Jiang, Hui Liu, Yi Liu, Hong-Xia Yao, Lei Zhang, Shen-Xian Qian, Tong-Hua Yang, Jing-Yu Zhang, Xu-Liang Shen, Lin-Hua Yang, Jian-Da Hu, Ren-Wei Huang, Zhong-Xing Jiang, Jing-Wen Wang, Hong-Yu Zhang, Zhen Xiao, Si-Yan Zhan, Hui-Xin Liu, Xing-Lin Wang, Ying-Jun Chang, Yu Wang, Yuan Kong, Lan-Ping Xu, Kai-Yan Liu, Xiao-Hong Zhang, Cheng-Hong Yin, Yue-Ying Li, Qian-Fei Wang, Jian-Liu Wang, Xiao-Jun Huang and Xiao-Hui Zhang in Therapeutic Advances in Hematology
Supplemental material, sj-docx-7-tah-10.1177_20406207221095226 for Prednisone plus IVIg compared with prednisone or IVIg for immune thrombocytopenia in pregnancy: a national retrospective cohort study by Xiao-Lu Zhu, Ru Feng, Qiu-Sha Huang, Mei-Ying Liang, Ming Jiang, Hui Liu, Yi Liu, Hong-Xia Yao, Lei Zhang, Shen-Xian Qian, Tong-Hua Yang, Jing-Yu Zhang, Xu-Liang Shen, Lin-Hua Yang, Jian-Da Hu, Ren-Wei Huang, Zhong-Xing Jiang, Jing-Wen Wang, Hong-Yu Zhang, Zhen Xiao, Si-Yan Zhan, Hui-Xin Liu, Xing-Lin Wang, Ying-Jun Chang, Yu Wang, Yuan Kong, Lan-Ping Xu, Kai-Yan Liu, Xiao-Hong Zhang, Cheng-Hong Yin, Yue-Ying Li, Qian-Fei Wang, Jian-Liu Wang, Xiao-Jun Huang and Xiao-Hui Zhang in Therapeutic Advances in Hematology
Footnotes
Author contributions: Xiao-Lu Zhu: Data curation; Writing – original draft.
Ru Feng: Conceptualization; Writing – original draft.
Qiu-Sha Huang: Data curation; Formal analysis.
Mei-Ying Liang: Resources.
Ming Jiang: Data curation.
Hui Liu: Data curation.
Yi Liu: Data curation.
Hong-Xia Yao: Data curation.
Lei Zhang: Data curation.
Shen-Xian Qian: Data curation.
Tong-Hua Yang: Data curation.
Jing-Yu Zhang: Data curation.
Xu-Liang Shen: Data curation.
Lin-Hua Yang: Data curation.
Jian-Da Hu: Data curation.
Ren-Wei Huang: Data curation.
Zhong-Xing Jiang: Data curation.
Jing-Wen Wang: Data curation.
Hong-Yu Zhang: Data curation.
Zhen Xiao: Data curation.
Si-Yan Zhan: Methodology.
Hui-Xin Liu: Software.
Xing-Lin Wang: Resources.
Ying-Jun Chang: Formal analysis.
Yu Wang: Methodology.
Yuan Kong: Investigation.
Lan-Ping Xu: Resources.
Kai-Yan Liu: Validation.
Xiao-Hong Zhang: Visualization.
Cheng-Hong Yin: Investigation.
Yue-Ying Li: Methodology; Supervision.
Qian-Fei Wang: Methodology; Supervision.
Jian-Liu Wang: Supervision; Writing – review & editing.
Xiao-Jun Huang: Writing – review & editing.
Xiao-Hui Zhang: Project administration.
ORCID iD: Xiao-Hui Zhang  https://orcid.org/0000-0003-4730-256X
https://orcid.org/0000-0003-4730-256X
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Key Research and Development Program of China (no. 2021YFC2500300), the National Natural Science Foundation of China (no. 81970113), the Key Program of National Natural Science Foundation of China (no. 81730004), and the Capital Health Research and Development of Special (No. 2022-1-4082). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Xiao-Lu Zhu, Peking University People’s Hospital, Beijing, P.R. China; Peking University Institute of Hematology, Beijing, P.R. China; National Clinical Research Center for Hematologic Disease, Beijing, P.R. China; Collaborative Innovation Center of Hematology, Beijing, P.R. China; Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Peking University People’s Hospital, Beijing, P.R. China.
Ru Feng, Departments of Hematology, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing, P.R. China.
Qiu-Sha Huang, Peking University People’s Hospital, Beijing, P.R. China; Peking University Institute of Hematology, Beijing, P.R. China; National Clinical Research Center for Hematologic Disease, Beijing, P.R. China; Collaborative Innovation Center of Hematology, Beijing, P.R. China; Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Peking University People’s Hospital, Beijing, P.R. China.
Mei-Ying Liang, Department of Obstetrics and Gynecology, Peking University People’s Hospital, Beijing, P.R. China.
Ming Jiang, Center of Hematologic Diseases, First Affiliated Hospital of Xinjiang Medical University, Ürümqi, P.R. China.
Hui Liu, Departments of Hematology, Beijing Hospital, National Center of Gerontology, Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing, P.R. China.
Yi Liu, Department of Hematology, Navy General Hospital, Beijing, P.R. China.
Hong-Xia Yao, Department of Hematology, People’s Hospital of Hainan Province, Haikou, P.R. China.
Lei Zhang, State Key Laboratory of Experimental Hematology, Institute of Hematology and Blood Disease Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, P.R. China.
Shen-Xian Qian, Department of Hematology, First People’s Hospital of Hangzhou, Hangzhou, P.R. China.
Tong-Hua Yang, Department of Hematology, First People’s Hospital of Yunnan Province, Kunming, P.R. China.
Jing-Yu Zhang, Department of Hematology, Hebei Institute of Hematology, The Second Hospital of Hebei Medical University, Shijiazhuang, P.R. China.
Xu-Liang Shen, Department of Hematology, He Ping Central Hospital of the Changzhi Medical College, Changzhi, P.R. China.
Lin-Hua Yang, Department of Hematology, Second Hospital of Shanxi Medical University, Taiyuan, P.R. China.
Jian-Da Hu, Fujian Institute of Hematology, Fujian Provincial Key Laboratory of Hematology, Fujian Medical University Union Hospital, Fuzhou, P.R. China.
Ren-Wei Huang, Department of Hematology, Third Affiliated Hospital of Southern Medical University, Guangzhou, P.R. China.
Zhong-Xing Jiang, Department of Hematology, First Affiliated Hospital of Zhengzhou University, Zhengzhou, P.R. China.
Jing-Wen Wang, Department of Hematology, Beijing Tongren Hospital, Beijing, P.R. China.
Hong-Yu Zhang, Department of Hematology, Peking University Shenzhen Hospital, Shenzhen, P.R. China.
Zhen Xiao, Department of Hematology, Affiliated Hospital of Inner Mongolia Medical University, Hohhot, P.R. China.
Si-Yan Zhan, Department of Epidemiology and Biostatistics, School of Public Health, Peking University Health Science Center, Beijing, P.R. China.
Hui-Xin Liu, Department of Clinical Epidemiology, Peking University People’s Hospital, Beijing, P.R. China.
Xing-Lin Wang, Peking University People’s Hospital, Beijing, P.R. China; Peking University Institute of Hematology, Beijing, P.R. China; National Clinical Research Center for Hematologic Disease, Beijing, P.R. China; Collaborative Innovation Center of Hematology, Beijing, P.R. China; Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Peking University People’s Hospital, Beijing, P.R. China.
Ying-Jun Chang, Peking University People’s Hospital, Beijing, P.R. China; Peking University Institute of Hematology, Beijing, P.R. China; National Clinical Research Center for Hematologic Disease, Beijing, P.R. China; Collaborative Innovation Center of Hematology, Beijing, P.R. China; Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Peking University People’s Hospital, Beijing, P.R. China.
Yu Wang, Peking University People’s Hospital, Beijing, P.R. China; Peking University Institute of Hematology, Beijing, P.R. China; National Clinical Research Center for Hematologic Disease, Beijing, P.R. China; Collaborative Innovation Center of Hematology, Beijing, P.R. China; Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Peking University People’s Hospital, Beijing, P.R. China.
Yuan Kong, Peking University People’s Hospital, Beijing, P.R. China; Peking University Institute of Hematology, Beijing, P.R. China; National Clinical Research Center for Hematologic Disease, Beijing, P.R. China; Collaborative Innovation Center of Hematology, Beijing, P.R. China; Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Peking University People’s Hospital, Beijing, P.R. China.
Lan-Ping Xu, Peking University People’s Hospital, Beijing, P.R. China; Peking University Institute of Hematology, Beijing, P.R. China; National Clinical Research Center for Hematologic Disease, Beijing, P.R. China; Collaborative Innovation Center of Hematology, Beijing, P.R. China; Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Peking University People’s Hospital, Beijing, P.R. China.
Kai-Yan Liu, Peking University People’s Hospital, Beijing, P.R. China; Peking University Institute of Hematology, Beijing, P.R. China; National Clinical Research Center for Hematologic Disease, Beijing, P.R. China; Collaborative Innovation Center of Hematology, Beijing, P.R. China; Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Peking University People’s Hospital, Beijing, P.R. China.
Xiao-Hong Zhang, Department of Obstetrics and Gynecology, Peking University People’s Hospital, Beijing, P.R. China.
Cheng-Hong Yin, Department of Internal Medicine, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing, P.R. China.
Yue-Ying Li, CAS Key Laboratory of Genomic and Precision Medicine, Collaborative Innovation Center of Genetics and Development, Beijing Institute of Genomics, Chinese Academy of Sciences, China National Center for Bioinformation, Beijing, P.R. China.
Qian-Fei Wang, CAS Key Laboratory of Genomic and Precision Medicine, Collaborative Innovation Center of Genetics and Development, Beijing Institute of Genomics, Chinese Academy of Sciences, China National Center for Bioinformation, Beijing, P.R. China.
Jian-Liu Wang, Department of Obstetrics and Gynecology, Peking University People’s Hospital, Beijing, P.R. China.
Xiao-Jun Huang, Peking University People’s Hospital, Beijing, P.R. China; Peking University Institute of Hematology, Beijing, P.R. China; National Clinical Research Center for Hematologic Disease, Beijing, P.R. China; Collaborative Innovation Center of Hematology, Beijing, P.R. China; Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Peking University People’s Hospital, Beijing, P.R. China.
Xiao-Hui Zhang, Peking University People’s Hospital, Peking University Institute of Hematology, No. 11 Xizhimen South Street, Xicheng District, Beijing 100044, P.R. China; National Clinical Research Center for Hematologic Disease, Beijing, P.R. China; Collaborative Innovation Center of Hematology, Beijing, P.R. China; Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, Beijing, P.R. China.
References
- 1. Toltl LJ, Nazi I, Jafari R, et al. Piecing together the humoral and cellular mechanisms of immune thrombocytopenia. Semin Thromb Hemost 2011; 37: 631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gill KK, Kelton JG. Management of idiopathic thrombocytopenic purpura in pregnancy. Semin Hematol 2000; 37: 275–289. [DOI] [PubMed] [Google Scholar]
- 3. Sainio S, Kekomaki R, Riikonen S, et al. Maternal thrombocytopenia at term: a population-based study. Acta Obstet Gynecol Scand 2000; 79: 744–749. [PubMed] [Google Scholar]
- 4. Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med 2002; 346: 995–1008. [DOI] [PubMed] [Google Scholar]
- 5. Eslick R, McLintock C. Managing ITP and thrombocytopenia in pregnancy. Platelets 2020; 31: 300–306. [DOI] [PubMed] [Google Scholar]
- 6. Gernsheimer T, James AH, Stasi R. How I treat thrombocytopenia in pregnancy. Blood 2013; 121: 38–47. [DOI] [PubMed] [Google Scholar]
- 7. Won YW, Moon W, Yun YS, et al. Clinical aspects of pregnancy and delivery in patients with chronic idiopathic thrombocytopenic purpura (ITP). Korean J Intern Med 2005; 20: 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fujimura K, Harada Y, Fujimoto T, et al. Nationwide study of idiopathic thrombocytopenic purpura in pregnant women and the clinical influence on neonates. Int J Hematol 2002; 75: 426–433. [DOI] [PubMed] [Google Scholar]
- 9. Webert KE, Mittal R, Sigouin C, et al. A retrospective 11-year analysis of obstetric patients with idiopathic thrombocytopenic purpura. Blood 2003; 102: 4306–4311. [DOI] [PubMed] [Google Scholar]
- 10. Yuce T, Acar D, Kalafat E, et al. Thrombocytopenia in pregnancy: do the time of diagnosis and delivery route affect pregnancy outcome in parturients with idiopathic thrombocytopenic purpura? Int J Hematol 2014; 100: 540–544. [DOI] [PubMed] [Google Scholar]
- 11. Loustau V, Debouverie O, Canoui-Poitrine F, et al. Effect of pregnancy on the course of immune thrombocytopenia: a retrospective study of 118 pregnancies in 82 women. Br J Haematol 2014; 166: 929–935. [DOI] [PubMed] [Google Scholar]
- 12. Belkin A, Levy A, Sheiner E. Perinatal outcomes and complications of pregnancy in women with immune thrombocytopenic purpura. J Matern Fetal Neonatal Med 2009; 22: 1081–1085. [DOI] [PubMed] [Google Scholar]
- 13. Sun D, Shehata N, Ye XY, et al. Corticosteroids compared with intravenous immunoglobulin for the treatment of immune thrombocytopenia in pregnancy. Blood 2016; 128: 1329–1335. [DOI] [PubMed] [Google Scholar]
- 14. Care A, Pavord S, Knight M, et al. Severe primary autoimmune thrombocytopenia in pregnancy: a national cohort study. BJOG 2018; 125: 604–612. [DOI] [PubMed] [Google Scholar]
- 15. Veneri D, Franchini M, Raffaelli R, et al. Idiopathic thrombocytopenic purpura in pregnancy: analysis of 43 consecutive cases followed at a single Italian institution. Ann Hematol 2006; 85: 552–554. [DOI] [PubMed] [Google Scholar]
- 16. Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 2010; 115: 168–186. [DOI] [PubMed] [Google Scholar]
- 17. Neunert C, Lim W, Crowther M, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011; 117: 4190–4207. [DOI] [PubMed] [Google Scholar]
- 18. Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology 2000; 62: 385–392. [DOI] [PubMed] [Google Scholar]
- 19. Yildirim Y, Tinar S, Oner RS, et al. Gestational diabetes mellitus in patients receiving long-term corticosteroid therapy during pregnancy. J Perinat Med 2006; 34: 280–284. [DOI] [PubMed] [Google Scholar]
- 20. Pishko AM, Levine LD, Cines DB. Thrombocytopenia in pregnancy: diagnosis and approach to management. Blood Rev 2020; 40: 100638. [DOI] [PubMed] [Google Scholar]
- 21. Cines DB, Levine LD. Thrombocytopenia in pregnancy. Blood 2017; 130: 2271–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Provan D, Arnold DM, Bussel JB, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv 2019; 3: 3780–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chakravarty EF, Murray ER, Kelman A, et al. Pregnancy outcomes after maternal exposure to rituximab. Blood 2011; 117: 1499–1506. [DOI] [PubMed] [Google Scholar]
- 24. Agarwal N, Mangla A. Thrombopoietin receptor agonist for treatment of immune thrombocytopenia in pregnancy: a narrative review. Ther Adv Hematol 2021; 12: 20406207211001139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Michel M, Ruggeri M, Gonzalez-Lopez TJ, et al. Use of thrombopoietin receptor agonists for immune thrombocytopenia in pregnancy: results from a multicenter study. Blood 2020; 136: 3056–3061. [DOI] [PubMed] [Google Scholar]
- 26. Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv 2019; 3: 3829–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Letsky EA, Greaves M. Guidelines on the investigation and management of thrombocytopenia in pregnancy and neonatal alloimmune thrombocytopenia. Maternal and Neonatal Haemostasis Working Party of the Haemostasis and Thrombosis Task Force of the British Society for Haematology. Br J Haematol 1996; 95: 21–26. [PubMed] [Google Scholar]
- 28. Laskin CA, Bombardier C, Hannah ME, et al. Prednisone and aspirin in women with autoantibodies and unexplained recurrent fetal loss. N Engl J Med 1997; 337: 148–153. [DOI] [PubMed] [Google Scholar]
- 29. Solano ME, Arck PC. Steroids, pregnancy and fetal development. Front Immunol 2019; 10: 3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stiehm ER. Adverse effects of human immunoglobulin therapy. Transfus Med Rev 2013; 27: 171–178. [DOI] [PubMed] [Google Scholar]
- 31. Guo Y, Tian X, Wang X, et al. Adverse effects of immunoglobulin therapy. Front Immunol 2018; 9: 1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol 2017; 139(Suppl. 3): S1–S46. [DOI] [PubMed] [Google Scholar]
- 33. Thrombosis and Hemostasis Group, Chinese Society of Hematology, Chinese Medical Association. [Chinese guideline on the diagnosis and management of adult primary immune thrombocytopenia (version 2020)]. Zhonghua Xue Ye Xue Za Zhi 2020; 41: 617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Petri M, Orbai AM, Alarcon GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012; 64: 2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Keeling D, Mackie I, Moore GW, et al. Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol 2012; 157: 47–58. [DOI] [PubMed] [Google Scholar]
- 36. Kelton JG. Idiopathic thrombocytopenic purpura complicating pregnancy. Blood Rev 2002; 16: 43–46. [DOI] [PubMed] [Google Scholar]
- 37. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an International Working Group. Blood 2009; 113: 2386–2393. [DOI] [PubMed] [Google Scholar]
- 38. Leduc D, Senikas V, Lalonde AB, et al. Active management of the third stage of labour: prevention and treatment of postpartum hemorrhage. J Obstet Gynaecol Can 2009; 31: 980–993. [DOI] [PubMed] [Google Scholar]
- 39. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453–1457. [DOI] [PubMed] [Google Scholar]
- 40. Rosa Maria RN, Laura RL, Angeles PB, et al. Use of Romiplostim during pregnancy as a rescue therapy in primary immune thrombocytopenia: literature review and case description. Platelets 2020; 31: 403–406. [DOI] [PubMed] [Google Scholar]
- 41. Kong Z, Qin P, Xiao S, et al. A novel recombinant human thrombopoietin therapy for the management of immune thrombocytopenia in pregnancy. Blood 2017; 130: 1097–1103. [DOI] [PubMed] [Google Scholar]
- 42. Xu X, Liang MY, Dou S, et al. Evaluation of glucocorticoid compared with immunoglobulin therapy of severe immune thrombocytopenia during pregnancy: response rate and complication. Am J Reprod Immunol 2018; 80: e13000. [DOI] [PubMed] [Google Scholar]
- 43. Bussel JB, Berkowitz RL, Lynch L, et al. Antenatal management of alloimmune thrombocytopenia with intravenous gamma-globulin: a randomized trial of the addition of low-dose steroid to intravenous gamma-globulin. Am J Obstet Gynecol 1996; 174: 1414–1423. [DOI] [PubMed] [Google Scholar]
- 44. Kasai J, Aoki S, Kamiya N, et al. Clinical features of gestational thrombocytopenia difficult to differentiate from immune thrombocytopenia diagnosed during pregnancy. J Obstet Gynaecol Res 2015; 41: 44–49. [DOI] [PubMed] [Google Scholar]
- 45. Hachisuga K, Hidaka N, Fujita Y, et al. Can we predict neonatal thrombocytopenia in offspring of women with idiopathic thrombocytopenic purpura? Blood Res 2014; 49: 259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhou F, Xu T, Deng C, et al. Severe thrombocytopenia in pregnancy: a case series from west China. Clin Exp Med 2019; 19: 495–503. [DOI] [PubMed] [Google Scholar]
- 47. British Committee for Standards in Haematology General Haematology Task Force. Guidelines for the investigation and management of idiopathic thrombocytopenic purpura in adults, children and in pregnancy. Br J Haematol 2003; 120: 574–596. [DOI] [PubMed] [Google Scholar]
- 48. Christiaens GC, Nieuwenhuis HK, von dem Borne AE, et al. Idiopathic thrombocytopenic purpura in pregnancy: a randomized trial on the effect of antenatal low dose corticosteroids on neonatal platelet count. Br J Obstet Gynaecol 1990; 97: 893–898. [DOI] [PubMed] [Google Scholar]
- 49. Halici-Ozturk F, Ozturk M, Yakistiran B, et al. Severe thrombocytopenia in pregnancy: a retrospective study. Blood Coagul Fibrinolysis 2020; 31: 517–521. [DOI] [PubMed] [Google Scholar]
- 50. Wang X, Xu Y, Luo W, et al. Thrombocytopenia in pregnancy with different diagnoses: differential clinical features, treatments, and outcomes. Medicine 2017; 96: e7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Huang QS, Zhu XL, Qu QY, et al. Prediction of postpartum hemorrhage in pregnant women with immune thrombocytopenia: development and validation of the MONITOR model in a nationwide multicenter study. Am J Hematol 2021; 96: 561–570. [DOI] [PubMed] [Google Scholar]
- 52. Fogerty AE. Thrombocytopenia in pregnancy: mechanisms and management. Transfus Med Rev 2018; 32: 225–229. [DOI] [PubMed] [Google Scholar]
- 53. Jones RM, de Lloyd L, Kealaher EJ, et al. Platelet count and transfusion requirements during moderate or severe postpartum haemorrhage. Anaesthesia 2016; 71: 648–656. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tah-10.1177_20406207221095226 for Prednisone plus IVIg compared with prednisone or IVIg for immune thrombocytopenia in pregnancy: a national retrospective cohort study by Xiao-Lu Zhu, Ru Feng, Qiu-Sha Huang, Mei-Ying Liang, Ming Jiang, Hui Liu, Yi Liu, Hong-Xia Yao, Lei Zhang, Shen-Xian Qian, Tong-Hua Yang, Jing-Yu Zhang, Xu-Liang Shen, Lin-Hua Yang, Jian-Da Hu, Ren-Wei Huang, Zhong-Xing Jiang, Jing-Wen Wang, Hong-Yu Zhang, Zhen Xiao, Si-Yan Zhan, Hui-Xin Liu, Xing-Lin Wang, Ying-Jun Chang, Yu Wang, Yuan Kong, Lan-Ping Xu, Kai-Yan Liu, Xiao-Hong Zhang, Cheng-Hong Yin, Yue-Ying Li, Qian-Fei Wang, Jian-Liu Wang, Xiao-Jun Huang and Xiao-Hui Zhang in Therapeutic Advances in Hematology
Supplemental material, sj-tiff-2-tah-10.1177_20406207221095226 for Prednisone plus IVIg compared with prednisone or IVIg for immune thrombocytopenia in pregnancy: a national retrospective cohort study by Xiao-Lu Zhu, Ru Feng, Qiu-Sha Huang, Mei-Ying Liang, Ming Jiang, Hui Liu, Yi Liu, Hong-Xia Yao, Lei Zhang, Shen-Xian Qian, Tong-Hua Yang, Jing-Yu Zhang, Xu-Liang Shen, Lin-Hua Yang, Jian-Da Hu, Ren-Wei Huang, Zhong-Xing Jiang, Jing-Wen Wang, Hong-Yu Zhang, Zhen Xiao, Si-Yan Zhan, Hui-Xin Liu, Xing-Lin Wang, Ying-Jun Chang, Yu Wang, Yuan Kong, Lan-Ping Xu, Kai-Yan Liu, Xiao-Hong Zhang, Cheng-Hong Yin, Yue-Ying Li, Qian-Fei Wang, Jian-Liu Wang, Xiao-Jun Huang and Xiao-Hui Zhang in Therapeutic Advances in Hematology
Supplemental material, sj-tiff-3-tah-10.1177_20406207221095226 for Prednisone plus IVIg compared with prednisone or IVIg for immune thrombocytopenia in pregnancy: a national retrospective cohort study by Xiao-Lu Zhu, Ru Feng, Qiu-Sha Huang, Mei-Ying Liang, Ming Jiang, Hui Liu, Yi Liu, Hong-Xia Yao, Lei Zhang, Shen-Xian Qian, Tong-Hua Yang, Jing-Yu Zhang, Xu-Liang Shen, Lin-Hua Yang, Jian-Da Hu, Ren-Wei Huang, Zhong-Xing Jiang, Jing-Wen Wang, Hong-Yu Zhang, Zhen Xiao, Si-Yan Zhan, Hui-Xin Liu, Xing-Lin Wang, Ying-Jun Chang, Yu Wang, Yuan Kong, Lan-Ping Xu, Kai-Yan Liu, Xiao-Hong Zhang, Cheng-Hong Yin, Yue-Ying Li, Qian-Fei Wang, Jian-Liu Wang, Xiao-Jun Huang and Xiao-Hui Zhang in Therapeutic Advances in Hematology
Supplemental material, sj-tiff-4-tah-10.1177_20406207221095226 for Prednisone plus IVIg compared with prednisone or IVIg for immune thrombocytopenia in pregnancy: a national retrospective cohort study by Xiao-Lu Zhu, Ru Feng, Qiu-Sha Huang, Mei-Ying Liang, Ming Jiang, Hui Liu, Yi Liu, Hong-Xia Yao, Lei Zhang, Shen-Xian Qian, Tong-Hua Yang, Jing-Yu Zhang, Xu-Liang Shen, Lin-Hua Yang, Jian-Da Hu, Ren-Wei Huang, Zhong-Xing Jiang, Jing-Wen Wang, Hong-Yu Zhang, Zhen Xiao, Si-Yan Zhan, Hui-Xin Liu, Xing-Lin Wang, Ying-Jun Chang, Yu Wang, Yuan Kong, Lan-Ping Xu, Kai-Yan Liu, Xiao-Hong Zhang, Cheng-Hong Yin, Yue-Ying Li, Qian-Fei Wang, Jian-Liu Wang, Xiao-Jun Huang and Xiao-Hui Zhang in Therapeutic Advances in Hematology
Supplemental material, sj-tiff-5-tah-10.1177_20406207221095226 for Prednisone plus IVIg compared with prednisone or IVIg for immune thrombocytopenia in pregnancy: a national retrospective cohort study by Xiao-Lu Zhu, Ru Feng, Qiu-Sha Huang, Mei-Ying Liang, Ming Jiang, Hui Liu, Yi Liu, Hong-Xia Yao, Lei Zhang, Shen-Xian Qian, Tong-Hua Yang, Jing-Yu Zhang, Xu-Liang Shen, Lin-Hua Yang, Jian-Da Hu, Ren-Wei Huang, Zhong-Xing Jiang, Jing-Wen Wang, Hong-Yu Zhang, Zhen Xiao, Si-Yan Zhan, Hui-Xin Liu, Xing-Lin Wang, Ying-Jun Chang, Yu Wang, Yuan Kong, Lan-Ping Xu, Kai-Yan Liu, Xiao-Hong Zhang, Cheng-Hong Yin, Yue-Ying Li, Qian-Fei Wang, Jian-Liu Wang, Xiao-Jun Huang and Xiao-Hui Zhang in Therapeutic Advances in Hematology
Supplemental material, sj-tif-6-tah-10.1177_20406207221095226 for Prednisone plus IVIg compared with prednisone or IVIg for immune thrombocytopenia in pregnancy: a national retrospective cohort study by Xiao-Lu Zhu, Ru Feng, Qiu-Sha Huang, Mei-Ying Liang, Ming Jiang, Hui Liu, Yi Liu, Hong-Xia Yao, Lei Zhang, Shen-Xian Qian, Tong-Hua Yang, Jing-Yu Zhang, Xu-Liang Shen, Lin-Hua Yang, Jian-Da Hu, Ren-Wei Huang, Zhong-Xing Jiang, Jing-Wen Wang, Hong-Yu Zhang, Zhen Xiao, Si-Yan Zhan, Hui-Xin Liu, Xing-Lin Wang, Ying-Jun Chang, Yu Wang, Yuan Kong, Lan-Ping Xu, Kai-Yan Liu, Xiao-Hong Zhang, Cheng-Hong Yin, Yue-Ying Li, Qian-Fei Wang, Jian-Liu Wang, Xiao-Jun Huang and Xiao-Hui Zhang in Therapeutic Advances in Hematology
Supplemental material, sj-docx-7-tah-10.1177_20406207221095226 for Prednisone plus IVIg compared with prednisone or IVIg for immune thrombocytopenia in pregnancy: a national retrospective cohort study by Xiao-Lu Zhu, Ru Feng, Qiu-Sha Huang, Mei-Ying Liang, Ming Jiang, Hui Liu, Yi Liu, Hong-Xia Yao, Lei Zhang, Shen-Xian Qian, Tong-Hua Yang, Jing-Yu Zhang, Xu-Liang Shen, Lin-Hua Yang, Jian-Da Hu, Ren-Wei Huang, Zhong-Xing Jiang, Jing-Wen Wang, Hong-Yu Zhang, Zhen Xiao, Si-Yan Zhan, Hui-Xin Liu, Xing-Lin Wang, Ying-Jun Chang, Yu Wang, Yuan Kong, Lan-Ping Xu, Kai-Yan Liu, Xiao-Hong Zhang, Cheng-Hong Yin, Yue-Ying Li, Qian-Fei Wang, Jian-Liu Wang, Xiao-Jun Huang and Xiao-Hui Zhang in Therapeutic Advances in Hematology