Abstract
Semen Strychni, a traditional Chinese medicine (TCM), has been widely used to treat paraplegia, facial nerve palsy and myasthenia gravis. However, its clinical application is greatly limited due to its fatal toxicity. To investigate the acute toxicity of Semen Strychni and the detoxification effect of licorice, a high-performance liquid chromatography tandem quadrupole time-of-flight mass spectrometry (HPLC-Q-TOF/MS) based urinary metabolomics method was developed in this study. After intraperitoneal injection to rats with Semen Strychni extract, the serum biochemical indexes were changed significantly, the liver and kidney showed severe necrosis and edema. Then the poisoned rat model was subsequently used for metabolomics research. Through principal component analysis (PCA) and orthogonal partial least squares-discriminant analysis (OPLS-DA), we finally identified 19 endogenous differential metabolites involved in amino acid metabolism, glycerophospholipid metabolism, tricarboxylic acid (TCA) cycle, oxidative stress and energy metabolism. In addition, 4 exogenous compounds from Semen Strychni (3 prototypes and 1 metabolite) were also identified in the present study. Results showed that the alterations of 23 compounds caused by Semen Strychni were significantly reversed after licorice treatment, which indicated that restoring the endogenous metabolic disorder and accelerating the metabolism of the main toxic components might be the possible detoxification mechanisms of licorice. This study may provide an integral understanding for the acute toxicity of Semen Strychni and the detoxification effect of licorice, thereby contributing to the clinical use of Semen Strychni and licorice.
Biomarkers and metabolomic pathway provide an integral understanding for the acute toxicity of Semen Strychni and the detoxification effect of licorice.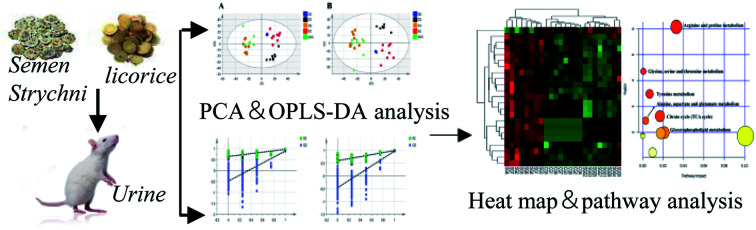
Introduction
Semen Strychni, the mature seeds of Strychnos nux-vomica L., is known for its good therapeutic effects on rheumatism, numbness, paraplegia, bruises and swelling. Phytochemical and modern pharmacological investigations have been demonstrated that brucine and strychnine are its main active ingredients, which possess several pharmacological activities, including analgesia, anti-inflammation, anti-diabetes and anti-angiogenesis.1–3 Although Semen Strychni has a lot of pharmacological effects, its clinical use is still relatively small now. To our knowledge, Semen Strychni is very poisonous and its therapeutic window is narrow. When used in large dosage or for a long time, it can easily cause multiple organ damage, such as liver injury, kidney injury as well as nerve injury.4,5 Thus, it is of great significance to clarify the toxic mechanism and seek a suitable antidote.
Licorice, the root of Glycyrrhiza uralensis Fisch., one of the most popular herbal medicines worldwide, has been widely used as an “Envoy” TCM herb to improve various disorders, especially to alleviate drug toxicity.6,7 Due to the powerful therapeutic effects of licorice, it has attracted extensive attention and increasing therapeutic mechanisms have been proposed in the past years. In TCM prescription, Semen Strychni was often used in combination with licorice to reduce toxicity and potentiate efficacy. Literatures reported that licorice had effects on the absorption and elimination of strychnine and brucine.8 In our previous study, we found there were herb–herb interactions between brucine and licorice. Administration with licorice water extract could decrease the Cmax and AUC of brucine. Meanwhile, the AUC and t1/2 of the main active components of licorice were also changed.9 As can be seen, most of the studies focused on the changes in the prototype compounds of Semen Strychni and the pre-protective effects of licorice, while the investigation about the disturbance of endogenous metabolites caused by Semen Strychni and the detoxification effect of licorice is less to carry out. Moreover, brucine does not fully represent the toxicological effects of Semen Strychni and the mechanism of detoxification also needs further elucidation. Therefore, a urinary metabolomics strategy was applied to complement the above problems in the present work, which may be able to bring valuable insight to the toxicity of Semen Strychni and the therapeutic effect of licorice.
Metabolomics is a top-down platform in the field of systems biology, which has been used to analyze the biochemical profile at the small molecule level in an organism.10 One of the major research areas of metabolomics is to investigate the mechanism of drug-induced toxicity, especially the toxicity of TCM.11 Due to multi-component and multi-target properties of TCM, it is difficult to obtain an integral understanding of toxicity by traditional methods. Recent years, a number of studies have been conducted to explore the metabolic mechanisms of TCM-induced nephrotoxicity and hepatotoxicity based on metabolomics strategies, and the satisfactory results indicated the applicability of metabolomics strategies.5,12 Selecting an appropriate analytical platform is critical to the results. Currently, metabolomic analytical platforms used in the majority of studies are nuclear magnetic resonance (NMR), liquid chromatography-mass spectrometry (LC-MS) and gas chromatography-mass spectrometry (GC-MS). Among these platforms, LC-MS is regarded as a staple for its high sensitivity, high resolution and good reproducibility. More importantly, compared with GC, LC allows direct analysis of biological samples without the cumbersome derivatization process, which may cause losses and introduce variability.13 Beyond that, TOF instruments have the best trade-off between scan-time and resolution, making them ideal partners for HPLC.14
In the present study, a HPLC-Q-TOF/MS-based urinary metabolomics strategy accompanied with PCA and OPLS-DA was proposed to analyze metabolic differences between groups and identify potential biomarkers. Moreover, metabolic pathways analysis was utilized to reveal the most relevant pathways relating to the Semen Strychni-induced acute toxicity and deepen the understanding of the detoxification mechanism of licorice. Our research may be able to provide some toxicological information for the safe application of Semen Strychni in clinical practice and offer a significant method to investigate the herb medicines.
Materials and methods
Chemicals and reagents
Formic acid (HPLC grade) was purchased from ROE Scientific Inc. (Orlando, FL, USA). Acetonitrile (HPLC grade) was obtained from ACS Company (Poole, UK). Ultra-pure water was deionized and purified by a Milli-Q system (Millipore, Bedford, MA, USA). All other reagents were of analytical grade and commercially available.
Plant material
Semen Strychni (batch number 201022891) and licorice (batch number 20151021) were obtained from SanXiang Co. Ltd. (Changsha, China). All the herbs were authenticated by Professor Yu-hua Wang (Department of Pharmacy, the Second Xiangya Hospital, Central South University). Voucher specimens of the two herbs were deposited at Department of Pharmacy, the Second Xiangya Hospital.
Extraction of herbs
The extraction procedure of Strychni Semen and licorice was basically in accordance with Wei et al.15 and Zhang et al.,9 respectively. Extraction protocols are described briefly as follows:
The raw Semen Strychni was pulverized and extracted by refluxing with 75% ethanol (1 : 12, w/v, pH 5.0) for three times and 1 h for each time. After filtration with microporous filtering film (0.45 μm), the three filtrates were combined and concentrated by rotary evaporation to remove all ethanol, then adjusted pH of aqueous layer to 6.5 by 0.5 mol L−1 NaOH. Finally, the extracts were diluted with 0.5% carboxymethylcellulose sodium (CMC-Na) solution to a final concentration, which was equivalent to 0.02 g of crude drug per milliliter of extract. Extracts subsequently stored at 4 °C until administration to animals.
The raw licorice was cleaned and macerated with ultra-pure water (1 : 5, w/v) for 12 h, then extracted at 100 °C for two times and 2 h for each time. The two filtrates were combined, concentrated and diluted with 0.5% CMC-Na solution. The final concentration was equivalent to 1.8 g of crude drug per milliliter of extract (contain glycyrrhizic acid (534.75 mg, 0.99%); liquiritigenin (26.1 mg, 0.048%); isoliquiritigenin (7.8 mg, 0.014%), liquiritin (157.8 mg, 0.292%)).
Animal experiments
Thirty-two male Sprague-Dawley rats (200 ± 20 g, 7 weeks old) were purchased from Hunan SJA Laboratory Animal Co. Ltd (Hunan, China). Animals were housed in plastic cages in a climate-controlled room with 12 h of light–dark illumination cycle at 22 ± 2 °C, and a relative humidity of 50 ± 10%. All the animals were acclimated for one week with water and standard rodent food ad libitum. The rats were fasted overnight (12–14 h) before experiments but with free access to water. All animal experiments were approved by the Animal Care & Use Committee of Central South University (SYXK (Xiang) 2015-0017) and carried out following the Regulations on the Administration of Laboratory Animals (Chinese Council).
Rats were randomly divided into four groups (n = 8) as follows:
Blank group (CG): rats were administrated the vehicle (0.5% CMC/Na solution) via intraperitoneal injection and 30 min later, a gavage of vehicle (0.5% CMC/Na solution) was given.
Semen Strychni extract group (SG): rats were administrated the Semen Strychni extract (0.2 g kg−1) via intraperitoneal injection and 30 min later, a gavage of vehicle (0.5% CMC/Na solution) was given.
Detoxification group (SGG): rats were administrated the Semen Strychni extract (0.2 g kg−1) by intraperitoneal injection and 30 min later, a gavage of licorice extract (18 g kg−1) was given.
Licorice extract group (GG): rats were administrated the vehicle (0.5% CMC/Na solution) via intraperitoneal injection and 30 min later, a gavage of licorice extract (18 g kg−1) was given.
The dosage setting of licorice is based on the clinical detoxification dose of licorice in adults (about 200 g). The dosage of Strychni Semen was set with reference to the Chinese Pharmacopoeia (0.3–0.6 g per day) and the pre-experimental screening.
Samples collection and pretreatment
24 h urine was collected from all rats in individual metabolic cages after the first administration. All urine samples were immediately centrifuged at 15 000 rpm for 10 min, then the supernatants were stored at −80 °C until analysis. The blood collection was performed right after urine collection. All blood samples were then centrifuged at 3500 rpm for 10 min at 4 °C. The separated supernatants were stored at −80 °C until analysis. After rats were sacrificed, the liver and kidney tissues were immediately removed and fixed in 10% formalin for histological study.
Biochemical assay and histological study
Several serum biochemical indexes, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (CREA), glucose (GLU), serum urea nitrogen (BUN), total protein (TP), albumin (ALB), globulin (GLO), triglyceride (TG) and total cholesterol (CHOL), were evaluated through a Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan) in the Second Xiangya Hospital. The operation procedure is as follows: enter the routine work screen, then select the specimen type, input sample number, select sample volume, etc. Save the information and press OK to complete specimen registration. Then, according to the pre-registered position, place the specimens on the specimen shelf in order, start the measurement, and finally print the data.
Liver and kidney tissues were fixed in 10% formalin for at least 24 h, then dehydrated, embedded in paraffin. Sections of 4 μm were cut and stained with hematoxylin and eosin (H&E) for light microscopic examinations.
Urine sample preparation and HPLC-MS condition
Prior to analysis, the urine samples were thawed at room temperature and centrifuged at 15 000 rpm for 10 min at 4 °C, then filtered with microporous membrane (0.22 μm). Finally, the prepared samples were transferred to the injection vials for HPLC-Q-TOF/MS analysis. Quality control (QC) samples were prepared by mixing an equal amount from each urine sample, then the following procedures were in accordance with real samples.16
Chromatographic separation was performed on an HPLC system (Agilent 1290, Agilent Technologies, CA, USA) equipped with an Ultimate AQ-C18 column (3.0 μm, 3.0 × 100 mm, Welch Materials Inc, MD, USA). The mobile phase was a gradient elution of solution A (0.1% formic acid aqueous) and solution B (acetonitrile). The following gradient program was used: 0–5 min, 3% B; 5–30 min, 3–25% B; 30–32 min, 25% B; 32–32.5 min, 25–60% B, 32.5–34 min, 60% B in positive mode; 0–10 min, 3% B; 10–40 min, 3–25% B; 40–42 min, 25% B; 42–50.5 min, 25–60% B, 50.5–53 min, 60% B in negative mode. An additional 8.5 min elution was performed to ensure column equilibration. The temperature of column and auto sampler was kept at 40 °C and 4 °C, respectively. The flow rate was 0.4 mL min−1. The injection volume in positive and negative modes were 5 μL and 2 μL, respectively.
The HPLC system was connected to a Q-TOF/MS (Agilent 6540 Q-TOF/MS, Agilent Technologies, CA, USA) coupled with electrospray ionization (ESI) source. The MS conditions in positive mode were as follows: capillary voltage, 4500 V; nebulizer pressure, 20 psig; drying gas flow, 11 L min−1; drying gas temperature, 350 °C; fragmentor voltage, 180 V; skimmer voltage, 45 V; OctopoleRFPeak, 750 V; mass range, 50–1000. Reference masses (m/z 121.050873 and m/z 922.009798) were used to calibrate the exact mass measurement. Collision energy was 20 eV, 30 eV, 40 eV, and collision gas was high purity nitrogen. Parameters in negative mode were same as those in positive mode, except that capillary voltage was reset at 4000 V, reference masses (m/z 112.985587 and m/z 1033.988109) were chosen for calibrating the exact mass measurement.
Before analyzing, five QC samples were injected to equilibrate the system. Moreover, a QC sample was injected after each eight real sample to evaluate the stability of the system during the analysis.
Data processing and pattern recognition
All HPLC-MS raw data files (.d) were first processed by MassHunter Profinder (B.07.00, Agilent Technologies, CA, USA) for batch recursive feature extraction, then generated a three-dimensional data matrix, including retention time, m/z value, and peak area. Total chromatographic area normalization was used to reduce the deviation and correct mass spectrometry response.
The normalized data was imported into the SIMCA-P program (version 14.1, Umetrics, Umea, Sweden) for PCA and OPLS-DA analysis. The results of PCA and OPLS-DA were presented in the forms of scores plot and variable importance for the prediction (VIP) plot. VIP values was used to measure the explanatory power of each metabolite on the classification of samples, thus assisting biomarker screening.17 The fitness and prediction ability of OPLS-DA model were evaluated with parameters R2Y and Q2. For identifying the structure of candidate biomarkers, its accurate mass was used to compare with the included date in the online metabolite databases (HMDB, http://www.hmdb.ca/; METLIN, http://metlin.scripps.edu/; MassBank, http://www.massbank.jp) under the molecular weight tolerance was controlled within ±10 ppm. When there was more than one feedback result from the database, the judgment was made by comparing the theoretical secondary spectrum with the actual secondary spectrum (fragment ions). The final identification of metabolites was based on a combination of accurate mass, elemental composition, fragment information and chromatographic retention behavior. MetaboAnalyst 4.0 (http://www.metaboanalyst.ca/) was employed to reveal the metabolic pathways related to the putative biomarkers.18
Statistical analysis
Values are presented as mean ± standard deviation (SD). For the statistics of biochemical date, one-way analysis of variance (ANOVA) followed by least significant difference (LSD) test (SPSS 18.0) was used to evaluate differences between groups. Student's t-test was performed for metabolites screening. P < 0.05 were considered to be statistically significant.
Results
Biochemical indexes
Several biochemical indexes in the serum were measured to assess the acute toxicity of Semen Strychni and the detoxification effect of licorice. As shown in Table 1, a significantly decreased serum TP and ALB levels was observed in the poisoned rats in comparison with those in the control group rats, which reflected the impairment in liver tissue after a single dose of. Nevertheless, the effect of licorice on TP and ALB is not obvious. When compared with the CG, the levels of CREA and GLU in the SG were significantly increased. Besides, there was a significant difference of the CREA level between the SG and the SGG, which indicated that licorice relieved kidney damage caused by Semen Strychni. However, it is worth noting that ALT and AST, as the conventional liver function indexes, were not elevated after Semen Strychni treatment.
Effects of Semen Strychni and licorice on serum biochemical indexes in rats. (Results were presented as the mean ± SD, n = 6).
| Biochemical indexes | CG | SG | SGG | GG |
|---|---|---|---|---|
| ALT (U L−1) | 41.93 ± 2.71 | 28.25 ± 3.73b | 29.98 ± 6.63 | 35.83 ± 9.18 |
| AST (U L−1) | 132.75 ± 46.72 | 109.25 ± 16.33 | 107.80 ± 29.52 | 171.73 ± 54.34 |
| TP (g L−1) | 53.38 ± 1.89 | 50.55 ± 0.83a | 51.03 ± 2.47 | 56.98 ± 2.44 |
| ALB (g L−1) | 22.42 ± 0.93 | 20.77 ± 0.42b | 20.72 ± 0.95 | 22.35 ± 0.90 |
| GLO (g L−1) | 30.97 ± 1.06 | 29.78 ± 0.63 | 30.32 ± 1.64 | 34.63 ± 1.62 |
| BUN (mmol L−1) | 3.49 ± 1.09 | 4.73 ± 0.88 | 5.88 ± 1.26 | 5.47 ± 1.14 |
| CREA (μmol L−1) | 19.43 ± 2.71 | 27.20 ± 2.09b | 23.38 ± 3.58c | 22.27 ± 3.40 |
| TG (mmol L−1) | 0.71 ± 0.29 | 0.57 ± 0.16 | 1.49 ± 0.91c | 1.10 ± 0.64 |
| GLU (mmol L−1) | 8.56 ± 0.94 | 12.17 ± 1.25b | 10.37 ± 1.07 | 9.40 ± 2.74 |
| CHOL (mmol L−1) | 1.83 ± 0.11 | 1.92 ± 0.16 | 1.93 ± 0.30 | 1.70 ± 0.20 |
P < 0.05 SG vs. CG.
P < 0.01 SG vs. CG.
P < 0.05 SGG vs. SG.
Histopathological observations
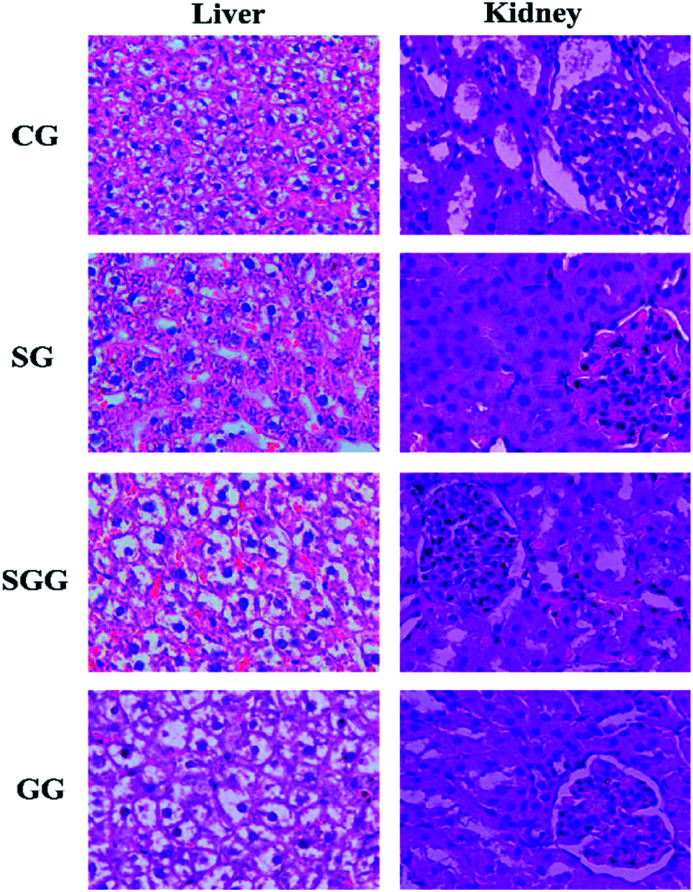
Histopathological examination is the most intuitive way to evaluate the organ damage. Collected liver samples and kidney samples were used for HE staining to observe liver injury and kidney injury induced by Semen Strychni as well as the protective effect of licorice (Fig. 1). The liver tissue of the CG and the GG showed complete hepatic lobule structure and normal hepatocyte plasma membrane, but in the Semen Strychni group, liver tissue exhibited extensive degeneration, necrosis and inflammatory cell infiltration. Treatment with licorice reduced the degree of liver injury markedly.
Fig. 1. Photomicrographs (×400) of HE-stained liver and kidney sections obtained from the CG group; SG group; SGG group and GG group.
With regard to kidney sections, as shown in Fig. 1, the kidney in the CG showed no signs of obvious abnormality and damage. In contrast, there were significant changes in the SG. Glomerular and renal tubules showed degeneration, together with extensive edema of renal tissue. Compared with the SG, all above symptoms were improved after the licorice treatment, but still with a slight degree of edema. These results implied that licorice could greatly alleviate liver damage and kidney damage caused by Semen Strychni.
Method validation
The established method was validated by determining stability, precision, and repeatability. The results of validation were presented as two different forms by referring to the published literature,19 including score plots of PCA for QC samples (Fig. S1(B1–B3 and D1–D3) in ESI†) and the plots of distribution of %RSD for metabolites' areas in QC samples (Fig. S1(A1–A3 and C1–C3) in ESI†).
The stability in autosampler was evaluated by injecting a same QC sample at 0, 2, 4, 8 and 12 h, respectively. The results showed that all QC samples were within two times of the SD in score plots. Furthermore, the distributions of %RSD for QC samples indicated that 87.45% (positive mode) and 95.27% (negative mode) of sum of responses had a %RSD of <20%. A random sample was continuously injected for five times, which was used to investigate the precision. The results showed that all QC samples were within two times of the SD in score plots, and the distributions of %RSD for QC samples indicated that 92.60% (positive mode) and 95.27% (negative mode) of sum of responses had a %RSD of <20%. Five aliquots of a random sample were used to evaluate the repeatability. The results showed that all QC samples were within two times of the SD in score plots, and the distributions of %RSD for QC samples indicated that 81.36% (positive mode) and 94.48% (negative mode) of sum of responses had a %RSD of <20%. All the above results confirmed that the stability, precision and repeatability of this method were satisfactory and can be applicable to the subsequent metabolomics analysis.
LC-MS analysis of metabolomic profiling and identification of biomarkers
The typical total ion chromatograms (TICs) obtained from the positive and negative modes in urine samples were shown in Fig. 2. The chromatographic peaks of each group were well separated under positive and negative mode and the response degree of metabolites was also different among groups.
Fig. 2. Typical total ion chromatograms (TICs) of the four groups in positive (A) and negative (B) mode.
The QC samples were applied to monitor the stability of the analytical system. After UV-scaling, the three-dimensional data matrixes of QC samples and real samples were analyzed by PCA model. The PCA score plots of QC samples showed all QC samples were within two times of the SD, which suggested that the analytical system was stable, and the acquired experimental data was reliable (Fig. S2 in ESI†).
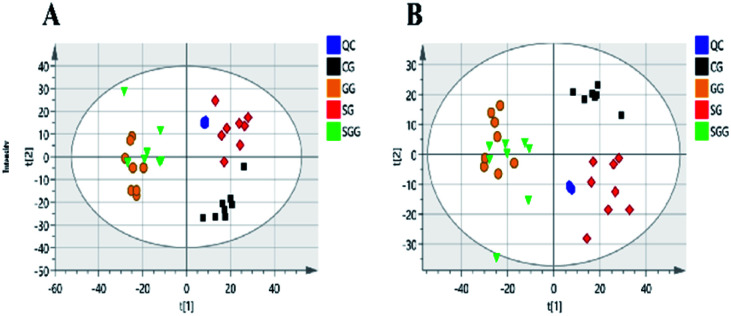
The main significance of the PCA model is to examine whether the separation tendency between groups are obvious from the sample distribution of PCA scores plots. Each point in the score plots represents one sample, and the position of sample in space is determined by the differences contained in the metabolites. In this study, the PCA scores plot (Fig. 3) showed all the QC samples were clustered and the three experimental groups (the CG, SG and SGG) were totally separated from each other, indicating the metabolic differences in urine could be distinguished by administrations.
Fig. 3. PCA score plots of all samples in positive (A) and negative (B) mode.
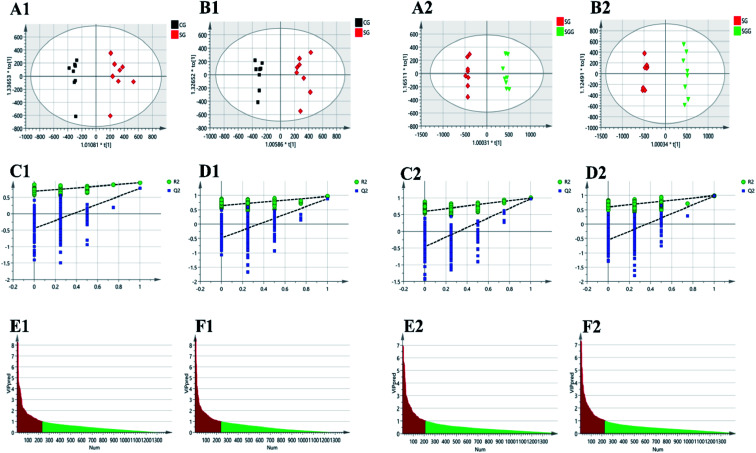
Supervised OPLS-DA was carried out to further select the variables that were responsible for classification. Firstly, we screened the variables related to Semen Strychni-induced acute toxicity by establishing the OPLS-DA model of the CG and the SG. A completely separation between the CG and the SG was shown in Fig. 4(A1 and B1), implying that Semen Strychni administration could cause the disturbance of metabolic profile. The model parameters of R2Y values in positive and negative modes were 0.943 and 0.967, and Q2 values were 0.773 and 0.886, respectively, which indicated the well predictability and validity of the model. Furthermore, the 200 permutation tests were conducted to verify whether the model was overfitted. According to the validation criteria described in the previous study that R2 and Q2 values were lower than the original ones and the intercept of Q2 was below zero,16 our model was reliable without overfitting (Fig. 4(C1 and D1)). Variables with VIP values >1.0 were chosen, eventually 234 and 240 variables were remained in positive and negative mode, respectively (Fig. 4(E1 and F1)). For screening variables related to the detoxification effect of licorice, the OPLS-DA model of SG and SGG was developed. OPLS-DA score plots (Fig. 4(A2 and B2)) showed a good separation between the SG and the SGG, which suggested that licorice could regulate the changes induced by Semen Strychni. The R2Y values in positive and negative modes were 0.994 and 0.993, and Q2 values were 0.969 and 0.98, respectively. Moreover, the result of permutation test also showed the model was fitted and had a high degree of reliability (Fig. 4(C2 and D2)). Eventually, 211 variables in positive mode alone with 225 variables in negative mode were selected for later analysis (Fig. 4(E2 and F2)).
Fig. 4. OPLS-DA for screening of poisoning markers and detoxification markers by comparing CG and SG, SG and SGG, respectively. (A1, A2) and (B1, B2) are the OPLS-DA score plots in positive and negative modes, respectively. (C1, C2) and (D1, D2) represents the results of OPLS-DA model validation in positive and negative modes, respectively. (E1, E2) and (F1, F2) are the OPLS-DA VIP plots in positive and negative mode, respectively.
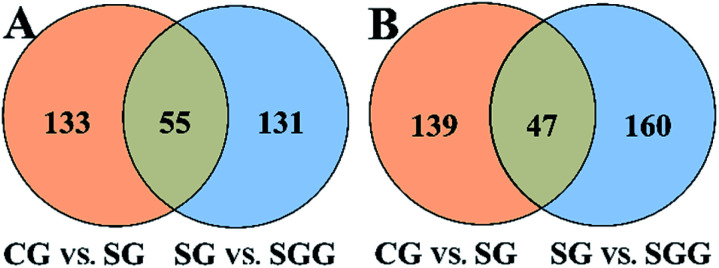
All the selected variables were further tested by Student's t-test and intersection analysis. In this way, 55 and 47 candidate biomarkers that not only were significantly changed upon Semen Strychni administration but also were reversed by licorice treatment were obtained in positive and negative modes, respectively (Fig. 5). Among those candidate biomarkers, 19 endogenous metabolites and 4 exogenous compounds from Semen Strychni were identified, and none of the above 23 compounds have negative confidence intervals on the VIP column plot (Fig. S3 in ESI†). All the detailed information was listed in Tables 2 and 3.
Fig. 5. Venn diagrams of biomarkers in positive (A) and negative (B) mode.
Identification of potential endogenous biomarkers.
| Number | RT (min) | m/z | Formula | Ion | Error (ppm) | FCb | RSD% | Identification | |
|---|---|---|---|---|---|---|---|---|---|
| SG vs. CG | SGG vs. SG | ||||||||
| 1 | 1.97 | 115.0031 | C4H4O4 | [M − H]− | 4.97 | 1.35a | 0.54a | 2.68 | Fumaric acid |
| 2 | 11.32 | 127.0764 | C7H12O2 | [M − H]− | 0.67 | 1.79a | 0.37a | 2.08 | Pantolactone |
| 3 | 3.52 | 148.0401 | C8H7NO2 | [M − H]− | 1.4 | 1.31a | 0.73a | 11.01 | 5,6-Dihydroxyindole |
| 4 | 5.65 | 160.0406 | C9H7NO2 | [M − H]− | −1.24 | 1.29a | 0.69a | 4.02 | Indole-3-carboxylic acid |
| 5 | 1.71 | 166.0176 | C4H9NO4S | [M − H]− | 2.44 | 1.76a | 1.45a | 0.74 | N-Acetyltaurine |
| 6 | 1.58 | 167.0208 | C5H4N4O3 | [M − H]− | 1.32 | 1.88a | 1.62a | 1.75 | Uric acid |
| 7 | 1.97 | 175.0239 | C6H8O6 | [M − H]− | 5.09 | 1.34a | 0.54a | 3.30 | d-Glucurono-6,3-lactone |
| 8 | 37.97 | 242.1758 | C13H25NO3 | [M − H]− | 1.61 | 1.66a | 0.60a | 2.93 | N-Undecanoylglycine |
| 9 | 1.97 | 254.9813 | C6H8O9S | [M − H]− | 0.19 | 1.30a | 0.56a | 1.17 | Ascorbic acid-2-sulfate |
| 10 | 2.39 | 263.0231 | C9H12O7S | [M − H]− | −0.03 | 1.35a | 0.64a | 4.01 | 3-Methoxy-4-hydroxyphenylglycol sulfate |
| 11 | 16.76 | 267.1600 | C15H24O4 | [M − H]− | 0.46 | 1.87a | 0.55a | 2.27 | Prostaglandin lactone-diol |
| 12 | 1.63 | 300.0394 | C8H15NO9S | [M − H]− | −0.28 | 2.32a | 0.57a | 4.83 | N-Acetylglucosamine 6-sulfate |
| 13 | 1.42 | 104.1072 | C5H14NO | [M + H]+ | −2.81 | 2.07a | 0.51a | 1.40 | Choline |
| 14 | 1.74 | 130.0862 | C6H11NO2 | [M + H]+ | 0.23 | 0.57a | 2.59a | 5.14 | N4-Acetylaminobutanal |
| 15 | 1.45 | 132.0772 | C4H9N3O2 | [M + H]+ | −3.56 | 0.51a | 0.37a | 1.68 | Creatine |
| 16 | 3.05 | 151.0614 | C6H6N4O | [M + H]+ | −0.39 | 1.55a | 0.63a | 1.21 | 1-Methylhypoxanthine |
| 17 | 8.51 | 185.1285 | C9H16N2O2 | [M + H]+ | −0.6 | 1.66a | 0.71a | 3.46 | N-(3-Acetamidopropyl)pyrrolidin-2-one |
| 18 | 2.93 | 229.1549 | C11H20N2O3 | [M + H]+ | −0.9 | 1.40a | 0.60a | 3.81 | Leucylproline |
| 19 | 30.27 | 792.5961 | C46H82NO7P | [M + H]+ | 6.81 | 1.41a | 0.58a | 5.29 | PC(P-18:0/20:5(5Z,8Z,11Z,14Z,17Z)) |
P < 0.05 SG vs. CG or SGG vs. SG.
FC: fold change. Fold change with a value >1 indicates increase, while a value >1 indicates decrease.
Identification of exogenous compounds.
| Number | RT (min) | m/z | Formula | Ion | Error (ppm) | SG vs. CG | SGG vs. SG | Identification |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.41 | 375.1297 | C16H24O10 | [M − H]− | −0.004 | ↑a | ↓a | Loganic acid |
| 2 | 15.97 | 335.1754 | C21H23N2O2 | [M + H]+ | 0.16 | ↑a | ↓a | Strychnine |
| 3 | 12.53 | 351.1708 | C21H23N2O3 | [M + H]+ | −1.42 | ↑a | ↓a | Hydroxystrychnine |
| 4 | 18.14 | 395.1958 | C23H27N2O4 | [M + H]+ | 1.95 | ↑a | ↓a | Brucine |
P < 0.05 SG vs. CG or SGG vs. SG. ↓ indicates decrease, ↑ indicates increase.
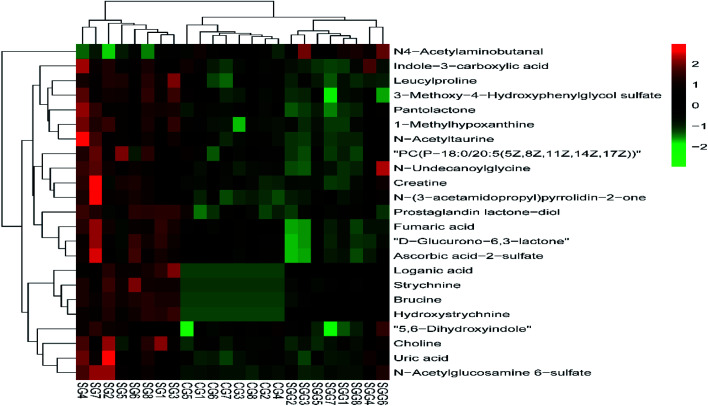
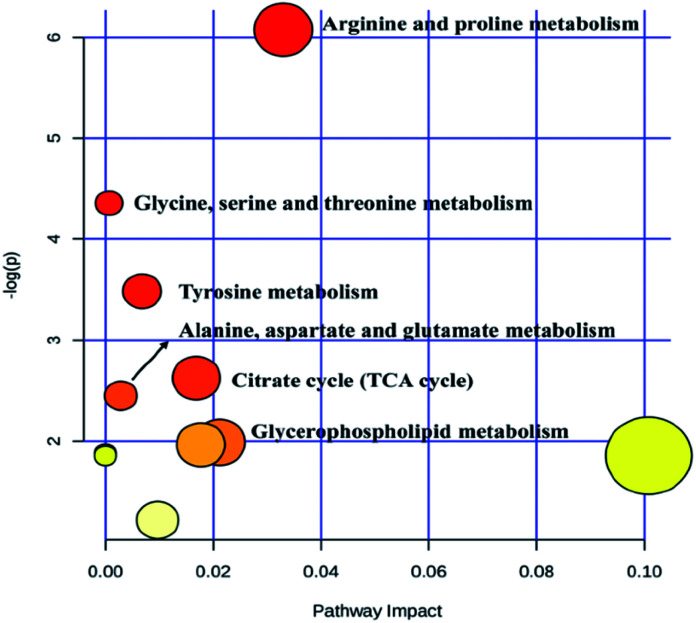
Heat map, representing the results of hierarchical clustering analysis, can provided an intuitive visualization of the differences in expression patterns of metabolites. The results have shown that the expression pattern of the same compound was obviously different between the CG and the SG. The expression pattern in the SGG had an opposite tendency compared to the SG (Fig. 6). Meanwhile, 19 endogenous differential metabolites were input into the MetaboAnalyst database in order to search for possible metabolic pathways. Fig. 7 clearly showed that the alterations were involved in arginine and proline metabolism; glycerophospholipid metabolism; TCA cycle; tyrosine metabolism; glycine, serine and threonine metabolism; alanine, aspartate and glutamate metabolism.
Fig. 6. Heat map of differential metabolites.
Fig. 7. Metabolic pathway of potential biomarkers.
Discussion
Although Semen Strychni is a valuable TCM, fatal toxicity limits its clinical use and the mechanism of toxicity has not been completely clarified to date. Clinical cases and studies have shown that Semen Strychni can cause hepatotoxicity and nephrotoxicity.5,20 And in TCM prescription, Semen Strychni was often used in combination with licorice to reduce toxicity. The protective roles of licorice in renal injury induced by Semen Strychni was also found in the previous study.8,21 In addition, licorice preparations such as magnesium isoglycyrrhizinate injection and glycyrrhizic acid enteric-coated capsules are commonly used clinically as hepatoprotective drugs. Therefore, it is very promising that the injury caused by Semen Strychni can be alleviated by the treatment of licorice. Based on the above information, the liver and kidney were selected to evaluate the acute toxicity caused by Semen Strychni and the protective effect of licorice. In this study, the rat model of the detoxification effect of licorice against Semen Strychni-induced acute toxicity was developed successfully, which was manifested by the results of biochemical assay and histopathological examination. ALT and AST, as conventional indexes for liver function evaluation, are in dynamic equilibrium under normal physiological conditions, but when liver injury occurs, increased membrane permeability leads to the release of ALT and AST into the blood. However, the ALT and AST levels were not elevated in the poisoned rats. Combined with pathological observation, this may be due to the strong hepatotoxicity of Strychni Semen, resulting in massive hepatocyte necrosis. There were not enough liver cells to release ALT and AST, so the ALT and AST levels in the blood were not elevated. The above also indicated that ALT and AST, as markers to reflect acute hepatotoxicity caused by Strychni Semen, were unsatisfactory. Thus, a metabolomics method was needed to discover more sensitive markers and provided a holistic view to explicate the toxicity mechanism of Semen Strychni as well as the therapeutic effect of licorice.
On the one hand, 4 exogenous compounds from Semen Strychni were identified in this study, including strychnine, brucine, loganic acid and hydroxystrychnine. Strychnine and brucine, the main strychnos alkaloids, are responsible for the toxic properties of Semen Strychni. Loganic acid is an organic acid isolated from the Semen Strychni. Hydroxystrychnine is a phase I metabolite formed by strychnine hydroxylation. Documents demonstrated that CYP3A4 played a crucial role in the metabolism of strychnos alkaloids22 and it can be induced by licorice.6 In the present study, the levels of strychnine, brucine and hydroxystrychnine were significantly decreased in the SGG in comparison with the SG, suggesting that licorice may accelerate the phase I metabolism of strychnine and brucine through inducing CYP3A4. It was also reported that hydroxystrychnine can be further metabolized into phase II metabolite via glucuronidation.23 Therefore, we can infer that licorice may accelerate the phase II metabolism as well, converting toxic compounds into phase II metabolites which are more polar and less toxic, then excreting in urine. Disappointedly, no glucuronide conjugates of strychnine or brucine were detected in this study.
On the other hand, this research found 19 endogenous biomarkers. The alterations of small metabolites in urine reflected the turbulence of the whole organism's internal environment due to the intervention of Semen Strychni. At the same time, licorice was able to regulate these disordered metabolites to a certain extent. More importantly, deep insights into the acute toxicity of Semen Strychni and the detoxification effect of licorice could be provided from pathways regarding defined biomarkers. Detailed explanation will be discussed later.
Among those endogenous metabolites, creatine, N4-acetylaminobutanal, leucylproline, 5,6-dihydroxyindole, indole-3-carboxylic acid and N-undecanoylglycine are associated with amino acid metabolism. Creatine is a nitrogenous organic acid synthesized by glycine, arginine and methionine. As an energy buffer for systems, it plays a central role throughout the body. According to the previous study, creatine strengthened cellular energetics via creatine/phosphocreatine system.24 When the amount of ATP is not enough to sustain the body's energy requirements, phosphocreatine can reversibly transfer its N-phosphoryl group to adenosine diphosphate (ADP), thereby increasing ATP levels. Similarly, the accumulated creatine can also be phosphorylated by ATP to regenerate phosphocreatine.25 In the present study, increased creatine level was found in the SG, which suggested the normal function of the creatine/phosphocreatine system was disrupted in Semen Strychni-treated rats. This effect was probably caused due to the disorder of energy metabolism induced by Semen Strychni. In contrast, licorice treatment returned the creatine level to normal, indicating licorice had the ability to regulate dysfunction of the creatine/phosphocreatine system and improve energy homeostasis. N4-Acetylaminobutanal is a product that participates in arginine and proline metabolism.26 In our research, N4-acetylaminobutanal was down-regulated in the SG compared to the CG, meaning the arginine and proline metabolism were turbulent. However, the level of N4-acetylaminobutanal was significantly increased by licorice treatment, which indicated arginine and proline metabolism could be regulated by licorice. Leucylproline is a dipeptide composed of leucine and proline. Although most dipeptides are only transient intermediates that will enter into specific amino acid degradation pathway for further proteolysis, they are known to have cellular signaling roles.27 Therefore, the alteration of leucylproline may be a reflection of protein metabolism disorders. Just like leucylproline, the changes in 5,6-dihydroxyindole (metabolite of tyrosine), indole-3-carboxylic acid (metabolite of tryptophan) and N-undecanoylglycine (metabolite of glycine) also showed amino acid metabolism were affected.
The TCA cycle is an important metabolism hub connecting the three nutrients (carbohydrates, lipids and amino acids), and plays a crucial role in aerobic metabolism of the body and energy production.26 Fumaric acid is an important intermediate in the TCA cycle. In the present investigation, it was observed that the content of fumaric acid was increased in the SG, which suggested the TCA cycle was probably inhibited, subsequently resulting in insufficient energy supply. In accordance with our guess, literatures have shown that the disorder of brain energy metabolism occurred after Semen Strychni extract treatment and as a result, the levels of ATP, ADP and AMP significantly decreased.28 Based on our results and the published reports, one of the possible mechanisms of Semen Strychni induced-acute toxicity might be explained as the destruction of energy metabolism caused by TCA cycle inhibition. While licorice could reverse the up-regulated fumaric acid, meaning that the improvement of energy metabolism may account for the detoxification effect of licorice.
Choline and phosphatidylcholine (PC) (a phosphorylated metabolites of choline) are necessary building blocks of biomembrane and are vital for normal function of all cells. Document demonstrated that choline metabolism played an important role in sustaining normal liver function.29 In the present study, the increased levels of choline and PC in the SG likely implied that the hepatotoxicity of Semen Strychni were induced by the abnormal choline metabolism and glycerophospholipid metabolism. d-Glucurono-6,3-lactone, a metabolite of glucose in the liver, has been used to treat drug-induced liver injury. The high level of d-glucurono-6,3-lactone in the SG may be an indicator that the body was trying to protect itself from the hepatotoxicity caused by Semen Strychni through increasing the conversion of glucose to d-glucurono-6,3-lactone. Moreover, this pathway relevant to liver damage has never been reported in metabolomics research.
Evidence have shown that Semen Strychni could enhance oxidative stress.4 Uric acid is the final oxidation product of purine metabolism and can provide an antioxidant defense.30 Similar to uric acid, ascorbic acid-2-sulfate could also protect the body from oxidative stress injury. Uric acid and ascorbic acid-2-sulfate increased in the SG may reflect the body self-protection as a result of anti-oxidative characteristics. Consistent with our results, the significantly up-regulated uric acid was observed in HEK 293t cell lysates after Semen Strychni administration.11 Hence, uric acid might be a promising biomarker for the diagnosis of nephrotoxicity. Meanwhile, the elevated level of uric acid and ascorbic acid-2-sulfate were restored by licorice treatment, which might be based on the anti-oxidative ability of licorice. Licorice likely eased the burden of oxidative stress.
Taurine, one of the most abundant amino acids, have various functions related to cell development, nutrition and survival.31 It also had anti-oxidative and anti-apoptotic activities. Previous study has already been proposed that taurine might be considered a potential indicator of renal toxicity.32N-Acetyltaurine, an acetylation product of taurine, which was found to increase in urinary concentration after the consumption of ethanol.33 Accordingly, the up-regulated N-acetyltaurine level in response to Semen Strychni exposure may be partially due to the enhancement of taurine acetylation. Nevertheless, no significant changes in taurine level were found in our experiment. In brief, N-acetyltaurine may serve as an indirect marker of renal toxicity.
Studies have shown that Semen Strychni has a strong toxicity to the central nervous system and can affect the metabolism of neurotransmitters.34 Norepinephrine (NE), an important neurotransmitter in the central nervous system, regulates many physiological activities in the body. 3-Methoxy-4-hydroxyphenylglycol sulfate, a sulfate metabolite of NE, may partially reflect the changes of central NE. In the present work, excessive excretion of 3-methoxy-4-hydroxyphenylglycol sulfate in the SG, suggesting that the central NE metabolism was enhanced, thus leading to the dysfunction of the central nervous system. After licorice treatment, the level of 3-methoxy-4-hydroxyphenylglycol sulfate was significantly down-regulated, which implied that the detoxification effect of licorice against neurotoxicity induced by Semen Strychni is probably via regulation of neurotransmitter metabolism.
Conclusions
In this study, a HPLC-Q-TOF/MS based urinary metabolomics approach combined with multivariate statistical analysis was employed to explore the Semen Strychni-induced acute toxicity and the detoxification effect of licorice. In total, 19 endogenous differential metabolites from urine samples were screened and identified as putative biomarkers, which provided options for the next step of the verification of markers. These significantly altered metabolites indicated that amino acid metabolism, TCA cycle, glycerophospholipid metabolism, oxidative stress and energy metabolism were perturbed in the poisoned rats, whereas licorice was able to reverse these alterations, which implied that the detoxification role of licorice may rely on restoring endogenous metabolic disorders caused by Semen Strychni. Meanwhile, the down-regulated strychnine, brucine, hydroxystrychnine and loganic acid were observed in detoxification group. Thus, accelerating the metabolism of Semen Strychni might be interpreted as another possible detoxification mechanism of licorice. Overall, these results demonstrated that the metabolomics strategy could be a useful tool for toxicity assessment and detoxification mechanism investigation of TCM.
The present study supplied an integral understanding in the detoxification effect of licorice on Semen Strychni-induced acute toxicity, thereby benefitting to the clinical application of Semen Strychni. However, the specificity and sensitivity of most markers need to be further confirmed before they can be incorporated into daily practice. The biological metabolic network is extremely complex and the various pathways are interactively related, which makes it difficult to make an accurate toxicity evaluation based on a single marker. Therefore, verifying new markers and finding the best combination of markers may be the focus of future research.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was financially supported by the National Nature Science Foundation of China (No. 81974533) and the Fundamental Research Funds for the Central Universities of Central South University (No. 502211910).
Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra08568e
References
- Yin W. Wang T. Yin F. Cai B. J. Ethnopharmacol. 2003;88:205–214. doi: 10.1016/s0378-8741(03)00224-1. [DOI] [PubMed] [Google Scholar]
- Singh A. Saharan V. Ram V. Bhandari A. Bhati R. J. Ayurveda Integr. Med. 2012;3:80–84. doi: 10.4103/0975-9476.96523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraswati S. Agrawal S. S. Cancer Lett. 2013;332:83–93. doi: 10.1016/j.canlet.2013.01.012. [DOI] [PubMed] [Google Scholar]
- Li S. Chu Y. Zhang R. Sun L. Chen X. Nutrients. 2018;10:514. doi: 10.3390/nu10040514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y. F. Liu S. F. Chen X. D. Feng M. R. Song F. Y. Gao X. X. J. Ethnopharmacol. 2018;210:242–253. doi: 10.1016/j.jep.2017.06.027. [DOI] [PubMed] [Google Scholar]
- Tai T. Huang X. Su Y. Su Y. Ji J. Jiang Z. Zhang L. J. Ethnopharmacol. 2014;152:358–363. doi: 10.1016/j.jep.2014.01.026. [DOI] [PubMed] [Google Scholar]
- Li N. Zhou T. Wu F. Wang R. Zhao Q. Zhang J. Yang B. Ma B. Expert Opin. Drug Metab. Toxicol. 2019;15:167–177. doi: 10.1080/17425255.2019.1563595. [DOI] [PubMed] [Google Scholar]
- Gu L. Wang X. Liu Z. Ju P. Zhang L. Zhang Y. Ma B. Bi K. Chen X. Food Chem. Toxicol. 2014;68:226–233. doi: 10.1016/j.fct.2014.03.028. [DOI] [PubMed] [Google Scholar]
- Zhang M. Deng Y. Wang C. Cai H. L. Wen J. Fang P. Zhang B. Li H. Yan M. Drug Test. Anal. 2018;10:262–271. doi: 10.1002/dta.2210. [DOI] [PubMed] [Google Scholar]
- Nicholson J. K. Lindon J. C. Holmes E. Xenobiotica. 1999;29:1181–1189. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- Gu L. Li S. Zhang R. Zhang Y. Wang X. Zhang K. Liu Z. Bi K. Chen X. RSC Adv. 2015;5:59591–59602. [Google Scholar]
- Wang Z. Zhang Y. Liu Q. Sun L. Lv M. Yu P. Chen X. Xenobiotica. 2019;49:216–226. doi: 10.1080/00498254.2018.1427905. [DOI] [PubMed] [Google Scholar]
- Gika H. G. Theodoridis G. A. Plumb R. S. Wilson I. D. J. Pharm. Biomed. Anal. 2014;87:12–25. doi: 10.1016/j.jpba.2013.06.032. [DOI] [PubMed] [Google Scholar]
- Cuykx M. Rodrigues R. M. Laukens K. Vanhaecke T. Covaci A. Arch. Toxicol. 2018;92:3007–3029. doi: 10.1007/s00204-018-2286-9. [DOI] [PubMed] [Google Scholar]
- Wei X. Yang B. Fan L. Zhang W. J. Jilin Agric. Univ. 2011;33:195–198. [Google Scholar]
- Gika H. G. Theodoridis G. A. Wingate J. E. Wilson I. D. J. Proteome Res. 2007;6:3291–3303. doi: 10.1021/pr070183p. [DOI] [PubMed] [Google Scholar]
- Trygg J. Holmes E. Lundstedt T. J. Proteome Res. 2007;6:469–479. doi: 10.1021/pr060594q. [DOI] [PubMed] [Google Scholar]
- Chong J. Soufan O. Li C. Caraus I. Li S. Bourque G. Wishart D. S. Xia J. Nucleic Acids Res. 2018;46:486–494. doi: 10.1093/nar/gky310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J. Yin P. Tan Y. Dong L. Hu C. Huang Q. Lu X. Wang H. Xu G. J. Proteome Res. 2014;13:3420–3431. doi: 10.1021/pr500390y. [DOI] [PubMed] [Google Scholar]
- Naik B. S. Chakrapani M. Malays. J. Pathol. 2009;31:67–69. [PubMed] [Google Scholar]
- Gu L. Wang X. Zhang Y. Jiang Y. Lu H. Bi K. Chen X. J. Sep. Sci. 2014;37:1058–1066. doi: 10.1002/jssc.201400053. [DOI] [PubMed] [Google Scholar]
- Li X. Wang K. Wei W. Liu Y. Gong L. Chem.-Biol. Interact. 2013;204:140–143. doi: 10.1016/j.cbi.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Liu X. Zheng S. Jiang Z. Liang C. Wang R. Zhou Z. Zhang Y. Yu Y. J. Sep. Sci. 2014;37:764–774. doi: 10.1002/jssc.201301055. [DOI] [PubMed] [Google Scholar]
- Marques E. P. Wyse A. T. S. Neurotoxic. Res. 2019;36:411–423. doi: 10.1007/s12640-019-00053-7. [DOI] [PubMed] [Google Scholar]
- de Graaf R. A. van Kranenburg A. Nicolay K. Biophys. J. 2000;78:1657–1664. doi: 10.1016/S0006-3495(00)76717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Sun W. Zheng J. Xu C. Wang X. Li T. Tang Y. Li Z. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2018;1100–1101:122–130. doi: 10.1016/j.jchromb.2018.10.005. [DOI] [PubMed] [Google Scholar]
- Chen Y. Duan J. Guo J. Shang E. Tang Y. Qian Y. Tao W. Liu P. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2016;1026:183–192. doi: 10.1016/j.jchromb.2015.08.024. [DOI] [PubMed] [Google Scholar]
- Hou C. Zhang R. Zhang K. Chen X. Metab. Brain Dis. 2017;32:2033–2044. doi: 10.1007/s11011-017-0082-5. [DOI] [PubMed] [Google Scholar]
- Corbin K. D. Zeisel S. H. Curr. Opin. Gastroenterol. 2012;28:159–165. doi: 10.1097/MOG.0b013e32834e7b4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames B. N. Cathcart R. Schwiers E. Hochstein P. Proc. Natl. Acad. Sci. U. S. A. 1981;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luginbühl M. König S. Schürch S. Weinmann W. Alcohol. 2017;65:11–18. doi: 10.1016/j.alcohol.2017.05.007. [DOI] [PubMed] [Google Scholar]
- Kim K. Um S. Y. Chung M. W. Jung S. C. Oh J. S. Kim S. H. Na H. S. Lee B. M. Choi K. H. Toxicol. Appl. Pharmacol. 2010;249:114–126. doi: 10.1016/j.taap.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Luginbühl M. Rutjens S. König S. Furrer J. Weinmann W. Anal. Bioanal. Chem. 2016;408:7529–7536. doi: 10.1007/s00216-016-9855-7. [DOI] [PubMed] [Google Scholar]
- Roy N. M. Arpie B. Lugo J. Linney E. Levin E. D. Cerutti D. Neurotoxicol. Teratol. 2012;34:587–591. doi: 10.1016/j.ntt.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.