Abstract
As negative regulators of cytokine signaling pathways, suppressors of cytokine signaling (SOCS) proteins have been reported to possess both pro-tumor and anti-tumor functions. Our recent studies have demonstrated suppressive effects of SOCS1 on epithelial to mesenchymal signaling in colorectal cancer cells in response to fractionated ionizing radiation or oxidative stress. The objective of the present study was to determine the radiosensitizing action of SOCS1 as an anti-tumor mechanism in color-ectal cancer cell model. In HCT116 cells exposed to ionizing radiation, SOCS1 over-expression shifted cell cycle arrest from G2/M to G1 and promoted radiation-induced apoptosis in a p53-dependent manner with down-regulation of cyclin B and up-regulation of p21. On the other hand, SOCS1 knock-down resulted in a reduced apoptosis with a decrease in G1 arrest. The regulatory action of SOCS1 on the radiation response was mediated by inhibition of radiation-induced Jak3/STAT3 and Erk activities, thereby blocking G1 to S transition. Radiation-induced early ROS signal was responsible for the activation of Jak3/Erk/STAT3 that led to cell survival response. Our data col-lectively indicate that SOCS1 can promote radiosensitivity of colorectal cancer cells by counteracting ROS-mediated survival signal, thereby blocking cell cycle progression from G1 to S. The resulting increase in G1 arrest with p53 activation then contributes to the promotion of apoptotic response upon radiation. Thus, induction of SOCS1 expression may increase therapeutic efficacy of radiation in tumors with low SOCS1 levels.
Keywords: Cell cycle modulation, Cell survival signaling, Radiation stress, Reactive oxygen species, Suppressors of cytokine signaling1
INTRODUCTION
Initially identified as negative regulators of cytokine signal transduction, suppressors of cytokine signaling (SOCS) have been widely studied for their anti-inflammatory and growth-inhibitory actions in immune scenarios and malignant conditions (1). Earlier studies have shown that SOCS genes are silenced in various tumors by promoter DNA methylation, the reversal of which will result in the suppression of tumor cell growth (2-4). These findings have triggered investigations on the anti-tumor function of SOCS in diverse systems and revealed differential actions of SOCS in isoform- and cell type-specific manners (5-8). In several prostate cancer cell lines, SOCS1 and SOCS3 can act as negative growth regulators of IL-6 and androgen, respectively (6, 7). SOCS1 and SOCS3 both can promote Fas-induced apoptosis of leukemic T cells through down-regulation of cell survival pathways by NF-κB and Bfl-1 (5). More recently, we and others have demonstrated the negative regulation of epithelial to mesenchymal transition (EMT) by SOCS1 in colon cancer cell lines (9-11).
On the contrary, anti-apoptotic and pro-tumor effects of SOCS have been also reported (12-14). In Jurkat T cells, TNF alpha-induced or ROS-mediated apoptosis can be inhibited by SOCS1 over-expression through attenuation of Jak1 with protection of protein tyrosine phosphatases (12). There is a positive correlation of cell growth or resistance to cell death with SOCS1 expression in colon cancer cells (13). Silencing SOCS1 can inhibit subcutaneous tumor growth of melanoma cells and metastatic development in the lung (14).
With these differential effects of SOCS in diverse tumor cell systems reported, the ability of SOCS to affect tumor sensitivity to therapeutic modality such as ionizing radiation (IR) has been of interest. In this respect, SOCS3 has exhibited opposite effects on radiosensitivity of cells in glioblastoma multiforme (GBM) and non-small cell lung carcinoma (NSCLC) by activating Erk/MAPK and blocking entry into S phase, respectively (15, 16). Sitko et al. (17) have observed that SOCS3-deficient MEF cells are accumulated in the G2/M phase without undergoing G1 phase arrest upon irradiation. G1 arrest was resumed by re-introducing SOCS3 gene accompanied by p21 up-regulation in the absence of p53 activation (17). Unlike the case of SOCS3, much less is known for the effect of SOCS1 on radiation response through the regulation of cell cycle, apoptosis, and survival affecting tumor growth.
We have utilized colorectal cancer (CRC) cells constructed for over-expression or knock-down of SOCS1 to investigate its anti-tumor action through the regulation of radiation response. In the present work we have found that SOCS1 promotes radiosensitivity of HCT116 CRC cells to apoptosis by counteracting radiation signals for survival mediated by Jak/Erk and STAT3 and increasing p53 activity. This is accompanied by a shift of cell cycle arrest from G2/M to G1. Importantly, we have noted that radiation-induced early ROS generation is responsible for the survival signal to induce cell cycle progression, which is subject to counter-regulation by SOCS1. Such radiosensitizing actions of SOCS1 might be useful for developing anti-tumor regimens to overcome resistance to radiation therapy, particularly for tumors with low levels of SOCS1 expression.
RESULTS
SOCS1 promotes gamma irradiation-induced apoptosis of colorectal cancer cells with increases of DNA damage and p53 activation
Previous studies have demonstrated the apoptosis-regulatory function of SOCS can differ depending on the apoptosis-inducing stimuli and target cell systems employed (5-10). In order to study the role of SOCS in IR-induced apoptosis, we utilized HCT116 colorectal cancer (CRC) cells The HCT116 cell line was chosen as the target cell system upon screening a panel of colorectal cancer cell lines since it showed moderate expression levels of SOCS1, making it suitable for both over-expression and knock-down for SOCS1 (10).
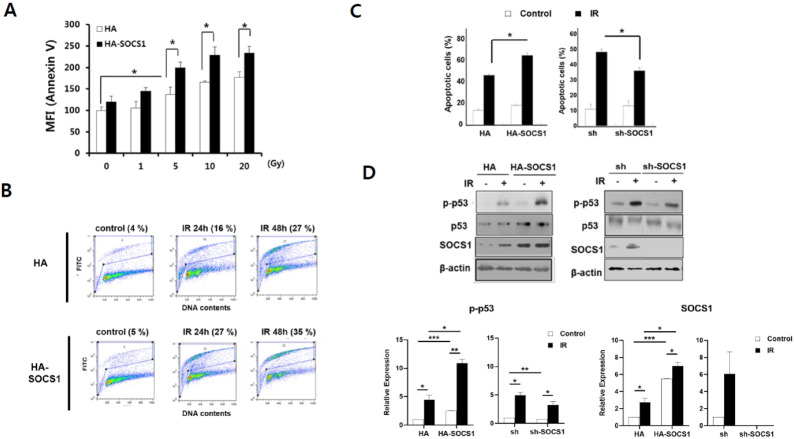
HCT116 cells bearing wild-type functional p53 gene (18), denoted as HCT116/p53wt, were exposed to varying doses of γ-IR. Apoptosis was then measured. A modest induction of apoptosis was observed at 24 h after radiation in a dose-dependent manner (130-160% increase in mean fluorescence intensity (MFI) over control upon exposure to 5-20 Gy, Fig. 1A). Compared to mock cells, SOCS1-transduced cells exhibited generally enhanced apoptosis upon IR treatment. Radiation-induced DNA damage that led to apoptosis was then assessed using TUNEL assays. Results indicated that DNA cleavage was progressively increased by 48 h in parental HCT116/p53 wt cells, which was further enhanced in SOCS1-transduced cells (Fig. 1B). Accord-ingly, apoptosis determined at 48 h revealed 3-fold increase in Annexin-V positive populations, which was further enhanced in SOCS1 over-expressing cells (Fig. 1C). Apoptosis was signi-ficantly increased (45% increase) in SOCS1-transduced cells but significantly decreased in SOCS1 knock-down cells (40% decrease) as compared to that in respective mock cells (Fig. 1C), showing the pro-apoptotic effect of SOCS1. As a mediator of apoptotic response, p-p53 level representing p53 activation was increased by γ-IR. It was further enhanced in SOCS1 over-expressing cells as compared to mock cells (Fig. 1D). On the other hand, p-p53 level appeared to be reduced in SOCS1-ablated cells. The regulation profile of apoptosis resembled that of p53 activation in SOCS1-overexpressing and SOCS1-ablated cells, indicating that apoptosis promotion by SOCS1 was associated with p53 activation (Fig. 1C, D).
Fig. 1.
SOCS1 promotes radiation-induced apoptosis of HCT116 cells involving increased DNA damage and p53 activation. HCT116/p53wt cells stably transfected with HA and HA-SOCS1 constructs were exposed to indicated doses of γ-IR. Cells were harvested at 24 h and apoptotic cells were measured by Annexin-V staining (A). Cells were treated with γ-IR at 5 Gy (B-D) and analyzed for DNA damage by performing Terminal deoxynucleotide transferase dUTP nick end label-ling (TUNEL) assays (B). Apoptosis was determined at 48 h upon receiving 5 Gy (C). Western blot was performed for p-p53, p53, and SOCS1 with densitometric analysis of protein levels (D).
Modulation of γ-IR-induced cell cycle arrest by SOCS1 from G2/M to G1
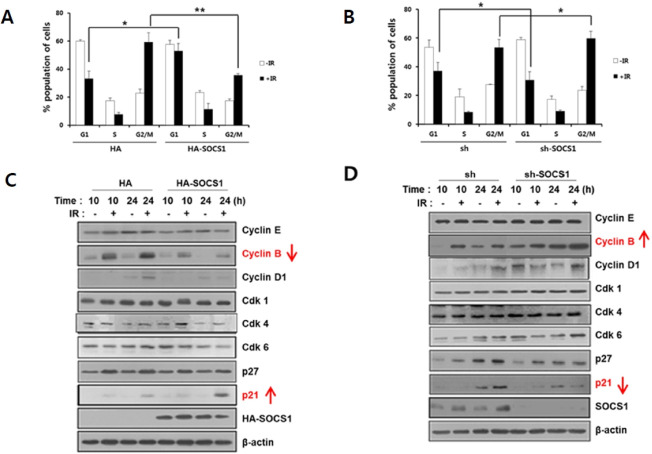
To investigate the underlying mechanism for SOCS1-mediated promotion of IR-induced apoptosis, the effect of SOCS1 on radiation-induced cell cycle changes was analyzed. Upon expo-sure to γ-IR at 5 Gy, HCT116/p53wt cells exhibited a prominent G2/M phase arrest with decreases of G1 and S phase cells. It was noted that SOCS1 over-expressing cells upon radiation displayed a significant increase in G1 arrest and a suppression in G2/M arrest as compared to mock cells (Fig. 2A). In contrast, SOCS1 knock-down caused a decrease in G1 arrest and an increase in G2/M arrest (Fig. 2B). Such effect was correlated with changes in expression levels of p21 and cyclin B. Over-expression of SOCS1 induced up-regulation of p21 and down-regulation of cyclin B at 24 h after γ-IR (Fig. 2C), whereas SOCS1 knock-down caused the opposite regulation (Fig. 2D). These data strongly indicate that SOCS1 can regulate γ-IR-induced cell cycle changes, leading to the promotion of G1 arrest and subsequent steps to p53-mediated pro-apoptotic response. Instead, G2/M population was reduced with a decrease in cyclin B, a G2/M cyclin in HA-SOCS1 cells.
Fig. 2.
Modulation of radiation-induced cell cycle arrest by SOCS1, reducing IR-induced G2/M arrest and promoting G1 arrest. Cell cycle analysis of HCT116/p53 wt cells stably transfected with HA and HA-SOCS1 or sh and shSOCS1 were performed at 24 h post irradiation of 5 Gy by flow cytometry using the Cellquest program as described in the text (A, B). Cells were subjected to immunoblotting to analyze expression levels of cell cycle marker proteins (C, D: Supplementary Fig. 6A).
We noted that SOCS1 expression was substantially induced within 1-4 h post irradiation, suggesting a potential regulatory role of SOCS1 during radiation-induced response in these cells (Supplementary Fig. 1A, D). Unlike SOCS1, SOCS3 over-expression had no effect on cell cycle regulation or apoptosis induced by γ-IR in these cells, consistent with the lack of SOCS3 induction by γ-IR (Supplementary Fig. 1). We also noted that p53-null HCT116 cells did not show notable changes in cell cycle or apoptotic response upon SOCS1 over-expression, suggesting a p53-dependent apoptosis-promoting effect of SOCS1 (Supplementary Fig. 2), which might be associated with p21-mediated G1 arrest. The radiosensitizing effect of SOCS1 on apoptosis was also observed in RKO, another human colon cancer cell line bearing wt p53 (Supplementary Fig. 3), but not in Jurkat T cells with p53 mutation (Supplementary Fig. 3C). In fact, an opposite regulatory effect of SOCS1 causing suppression of γ-IR-induced apoptosis in Jurkat T cells was associated with an increase in cells at G2/M arrest (Supplementary Fig. 3D).
SOCS1 targets γ-IR-induced activation of Jak3, Erk, and STAT3 to induce G1 arrest and suppress cell cycle progression
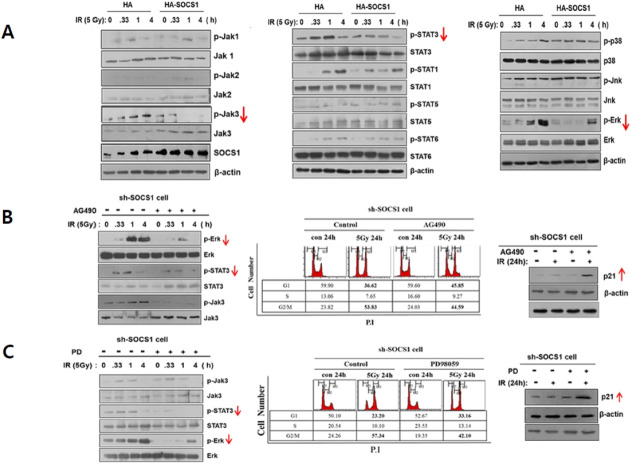
Prior to undergoing apoptosis upon receiving radiation, tumor cells may activate survival signaling pathways to cope with radiation stress. In fact, HCT116 cells exposed to γ-IR at an apoptosis-inducing dose of 5 Gy exhibited early activation of Jak/STAT and MAPK family members including Jak1/Jak3, STAT1/STAT3, and Erk/p38 within 15 min to 1 h (Fig. 3A). To identify signaling pathways affected by SOCS1 during the radiation response, effects of SOCS1 over-expression or knock-down on these factors were examined. Results showed that Jak3, STAT3, and Erk activities induced by irradiation were generally down-regulated in SOCS1 over-expressing cells (Fig. 3A), while these were up-regulated in SOCS1-ablated cells at basal or IR-induced levels (Supplementary Fig. 4). Other Jak/STAT and MAPK family members examined were either not notably activated upon γ-IR (e.g., Jak2, STAT5, STAT6) or activated upon γ-IR treatment but not regulated by SOCS1 (e.g., Jak1, STAT1, p38, Jnk). These results suggest that Jak3/STAT3 and Erk could be potential targets of SOCS1 action in γ-IR-induced stress response in HCT116 cells, which might lead to cell cycle arrest at G1 phase and subsequent apoptosis. To explore the signaling mechanism of SOCS1 action, we examined effects of specific inhibitors for Jak/STAT and Erk pathways on the IR-induced response regulated by SOCS1.
Fig. 3.
Radiation-induced early activation of Jak3/STAT3 and Erk is suppressed by SOCS1, and the inhibition of Jak3/STAT3 and Erk in shSOCS1 cells leads to G1 arrest restoration. Analysis of Jak/STAT and MAPK activation kinetics induced by γ-IR. HCT116/p53 wt cells stably transfected with HA and HA-SOCS1 were exposed to γ-IR at 5 Gy and harvested at indicated time points. Cell lysates were prepared to analyze Jak/STAT and MAPK activation status by immunblotting (A). sh and shSOCS1-transfected cells were analyzed for Jak/STAT and MAPK activation status and cell cycle changes upon irradiation with or without pre-treatment with inhibitors of Jak (AG490) or Erk (PD). Effects of AG490 and PD on on Jak/STAT and Erk were examined (B, C: Supplementary Fig. 6B).
Elevated levels of both phospho-Erk and phospho-STAT3 in shSOCS1 cells were down-regulated in the presence of AG490, a Jak inhibitor (Supplementary Fig. 3B and 4). However, levels of phospho-STAT3, but not phospho-Jak3, were suppressed upon treatment with an MEK/Erk inhibitor PD98059 (Fig. 3C). This indicates that Jak3 can control Erk and subsequently regulate STAT3 activities during the radiation response as targets of SOCS1. In addition, it was noted that the reduction in G1 arrest in SOCS1-ablated cells was substantially restored by treatment with AG490 and PD98059 with up-regulated p21 expression. These data indicate that suppression of pro-survival pathways by SOCS1 can interfere with cell cycle progression from G1, which can act in concert with the promotion of p53-dependent pro-apoptotic response under radiation.
Role of ROS signal in γ-IR-induced cell survival pathways which is counteracted by SOCS1
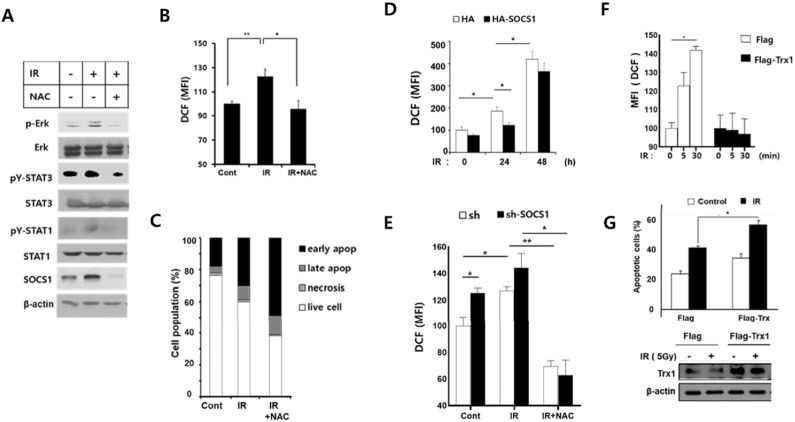
Our earlier studies have demonstrated that SOCS1 can counter-act ROS signals and regulate cell survival and apoptosis under oxidative stress (10, 12). We have also shown that exposure to hydrogen peroxide or low dose γ-IR given by fractionized ionizing radiation can induce ROS-mediated activation of EMT signaling and that SOCS1 can down-regulate ROS levels to suppress EMT response involving Src/Akt/ Erk in HCT116/p53wt cells (10, 11). Having observed early activations of Jak3, Erk, and STAT3 upon receiving apoptosis-inducing dose of γ-IR and their suppressions by SOCS1, we examined the role of ROS signal during IR-induced stress response and the potential role of SOCS1 in ROS regulation. When HCT116 cells were exposed to γ-IR (5 Gy), Erk and STAT3 showed modest activation along with induction of SOCS1 in 30 min, all of which were down-regulated by a pretreatment with N-acetyl cysteine (NAC), an antioxidant (Fig. 4A). Indeed, NAC-sensitive ROS generation was observed under this condition (Fig. 4B). NAC treatment not only suppressed early activation of Erk and STAT3, but also induced a significant increase in apoptotic cell pop-ulations by 24 h, suggesting a role of ROS in mediating cell survival pathways (Fig. 4C). Importantly, under apoptosis condition, ROS levels were suppressed in SOCS1-transfected cells, while early ROS generation were increased in SOCS1 knock- down cells (Fig. 4D, E). Upon thioredoxin (Trx1) over-expression, cells displayed impaired response to γ-IR for early ROS generation and increased apoptotic response by 24 h (Fig. 4F, G). These results suggest that the ROS signal is responsible for the early phase activation of survival signaling pathways triggered by γ-IR and that SOCS1 down-regulation of ROS levels may interfere with this process, contributing to increased apoptotic response to radiation.
Fig. 4.
SOCS1 counter-regulates reactive oxygen species (ROS) responsible for the survival signal during radiation response to promote apoptosis. HCT116/p53 wt cells were irradiated with γ-IR at 5 Gy with or without NAC treatment (1 h pretreatment at 1 mM). Cells were analyzed for signaling mediators by immunoblotting (A) and ROS generation (B) at 30 min. Cell death was measured at 24 h post irradiation by propidium iodide and Annexin-V staining (C). The γ-IR-induced ROS generation was assessed in cells with SOCS1 knock-down at 10 min and with SOCS1 over-expression at indicated time points (D, E). HCT116/p53 wt cells transfected with Flag or Flag-Trx1 were irradiated at 5 Gy and analyzed for ROS generation, apoptotic response and Trx1 expression at 24 h (F, G).
DISCUSSION
Radiation therapy is a major anti-cancer regimen. Ionizing radiation is known to exert tumor killing effects by causing DNA damage with double-strand breaks. IR induces mitotic arrest directly and by generating high levels of ROS indirectly which leads to DNA damage (19). In this regard, resistance to radiation therapy is often correlated with low ROS-inducing potential of tumor cells. In fact, sublethal doses of ionizing radiation can generate low ROS levels which may trigger survival signaling pathways (19). Thus, to increase tumor sensitivity to radiation, not only the activation of DNA damage leading to apoptosis, but also the suppression of cell survival signaling through ROS regulation should be considered.
Although SOCS1 has emerged as a potent tumor suppressor in various cancers, its mechanism of action in the regulation of tumor sensitivity to radiation remains unclear. Thus, the present study was conducted to investigate radiosensitizing effects of SOCS1 using a colorectal cancer model. Our data indicated that while γ-IR induced ROS mediating Jak/STAT3 and Erk activation for cell survival signaling, SOCS1 counteracted these pathways by down-regulating ROS. This result is in line with our recent studies showing that SOCS1 can also suppress the invasion and EMT induced by hydrogen peroxide and fractionated ionizing radiation via ROS regulation in colorectal cancer cells (10, 11).
Induction of thioredoxin (Trx1) has been found to be responsible for SOCS1-mediated ROS down-regulation in diverse cell types including colon cancer cells (10, 11, 20). Thus, we examined the role of Trx1 in cell survival vs. apoptotic response induced by radiation. Indeed, Trx1 transfection caused a blockade in the early increase of ROS level and promoted the apoptotic response induced by radiation, supporting the role of ROS signal in SOCS1-mediated cell survival under radiation stress (Fig. 4F, G).
SOCS1 and SOCS3 share common structural features such as SH2 domain, Jak kinase inhibitory domain, and SOCS-box motif. However, regulatory effects of SOCS1 and SOCS3 on radiation-induced DNA damage response and apoptosis seem to differ depending on cell types (15, 16). We have observed that while SOCS1 exhibited a pro-apoptotic function in p53 wt/HCT116 CRC cells with cell cycle shift to G1, SOCS3 had no effects on the apoptosis or cell cycle changes induced by γ-IR (Supplementary Fig. 1). In p53 wt-bearing RKO CRC cells, however, both SOCS1 and SOCS3 promoted apoptotic response (Supplementary Fig. 3A, B).
As we observed p53 activation in HCT116 CRC cells, p53-dependent p21 induction and inhibition of cell cycle progression from G1 to S phase by SOCS1-mediated attenuation of Jak3/Erk/STAT3 might have resulted in accumulation of cells in G1 arrest when cells became apoptotic in time. This result was in a good agreement with the role of Jak3/STAT3 inhibition in the induction of cell cycle arrest and apoptosis in colon carcinoma cells (21). STAT3 has been implicated in the promotion of cell survival and cell cycle progression as well as in the stimulation of tumor metastasis through MMP7 induction (22, 23). Thus, SOCS1-mediated JAK/STAT3 pathway inhibition might have contributed to overall anti-tumor and anti-metastatic effects of SOCS1 on colon cancers. Together, these results support the anti-invasive and anti-EMT role of SOCS1 through Jak/STAT inhibition in colon cancer models (10, 23).
The induction of G1 arrest and pro-apoptotic function of SOCS1 appeared to be p53-dependent as p53-null cells were unresponsive to SOCS1 over-expression (Supplementary Fig. 2). In addition, Jurkat (p53 mt) cells showed anti-apoptotic response with increased G2/M arrest upon SOCS1 over-expression (Supplementary Fig. 3C, D). In this regard, the lack of significant apoptosis noted in HCT116/p53 null cells seemed to correlate with the increased G2/M arrest upon radiation as compared to p53 wt cells (Supplementary Fig. 2, 24). As the ability of SOCS1 to activate p53 through the interaction with ATM has been suggested (25), it is likely that SOCS1 induced by γ-IR participates in DNA damage response in a positive feed-back loop to modulate cell cycle changes and to increase radiosensitivity for apoptosis. In summary, data of the present study suggest that radiosensitizing anti-tumor action of SOCS1 is exerted though suppression of ROS-mediated survival signaling leading to cell cycle arrest at G1 and promotion of apoptotic response in colon cancer cells (Supplementary Fig. 5). As the emergence of radioresistance in diverse tumor cells poses difficulty in radiation therapy, SOCS1 might be considered as a radiosensitizing agent not only through modulation of DNA damage response and cell cycle arrest, but also through regulation of γ-IR-induced ROS and associated survival signaling pathways in relevant tumor models.
MATERIALS AND METHODS
Cell culture and gene transfection
Cell culture conditions and details for gene transfection to obtain stable cell lines are described in Supplemental materials.
Radiation treatments of cells
Cells were irradiated with 2-20 Gy at room temperature with a 137Cs γ-source irradiator at a dose rate of 5.66 Gy/min using an IBL 437 type H irradiator (CIS Biointernational, Nice, France) (11).
Apoptosis measurement and cell cycle analysis
Methods used for the analysis of apoptotic populations and cell cycle were provided in Supplemental materials.
Western blot and densitometric analysis
Immunoblotting with respective antibodies as well as the densitomertic analysis are described in Supplemental materials.
Terminal deoxynucleotide transferase dUTP nick end labelling (TUNEL) assays
Methods used for TUNEL assays were provided in Supplemental materials.
ROS measurement
Intracellular ROS levels were determined as described previously (11).
Statistical analysis
For statistical analysis, the experiments were performed at least in three independent sets. The values are presented as means ± SE. Statistical significance was determined by a Student’s t-test. A value of *P < 0.05, **P < 0.01 and ***P < 0.001 was considered statistically significant.
SUPPLEMENTARY MATERIALS
ACKNOWLEDGEMENTS
This study was supported in part by NRF grants #20100018682, #2013M2B2A9A03051275, #2015003291, #2016911262, #2018 R1A2B6002201, and 2022R1H1A2003471. H Jeong was also supported in part by 2017 Global Ph.D Fellowship.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signaling and immune regulation. Nat Rev Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 2.Sutherland KD, Lindeman GJ, Choong DY, et al. Differential hypermethylation of SOCS genes in ovarian and breast carcinomas. Oncogene. 2004;23:7726–7733. doi: 10.1038/sj.onc.1207787. [DOI] [PubMed] [Google Scholar]
- 3.Yoshikawa H, Matsubara K, Qian GS, et al. SOCS-1, a negative regulator of the JAK/STAT pathway, is silenced by methylation in human hepatocellular carcinoma and shows growth-suppression activity. Nat Genet. 2001;28:29–35. doi: 10.1038/ng0501-29. [DOI] [PubMed] [Google Scholar]
- 4.Oshimo Y, Kuraoka K, Nakayama H, et al. Epigenetic inactivation of SOCS-1 by CpG island hypermethylation in human gastric carcinoma. Int J Cancer. 2004;112:1003–1009. doi: 10.1002/ijc.20521. [DOI] [PubMed] [Google Scholar]
- 5.Oh J, Kim SH, Ahn S, Lee CE. Suppressors of cytokine signaling promote Fas-induced apoptosis through down-regulation of NF-κB and mitochondrial Bfl-1 in leukemic T cells. J Immunol. 2012;189:5561–5571. doi: 10.4049/jimmunol.1103415. [DOI] [PubMed] [Google Scholar]
- 6.Neuwirt H, Puhr M, Santer FR, et al. Suppressor of cytokine signaling (SOCS)-1 is expressed in human prostate cancer and exerts growth-inhibitory function through down-regulation of cyclins and cyclin-dependent kinases. Am J Pathol. 2009;174:31921–31930. doi: 10.2353/ajpath.2009.080751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neuwirt H, Puhr M, Cavarretta IT, et al. Suppressor of cytokine signalling-3 is up-regulated by androgen in prostate cancer cell lines and inhibits androgen-mediated proliferation and secretion. Endocr Relat Cancer. 2007;14:1007–1019. doi: 10.1677/ERC-07-0172. [DOI] [PubMed] [Google Scholar]
- 8.Scutti JA, Matsuo AL, Pereira FV, et al. Role of SOCS-1 gene on melanoma cell growth and tumor development. Transl Oncol. 2011;4:101–109. doi: 10.1593/tlo.10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David M, Naudin C, Letourneur M, et al. Suppressor of cytokine signaling 1 modulates invasion and metastatic potential of colorectal cancer cells. Mol Oncol. 2014;8:942–955. doi: 10.1016/j.molonc.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung SH, Kim SM, Lee CE. Mechanism of suppressors of cytokine signaling inhibition of epithelial-mesenchymal transition signaling through ROS regulation in colon cancer cells: suppression of Src leading to thioredoxin up-regulation. Oncotarget. 2016;7:62559–62571. doi: 10.18632/oncotarget.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S, Kim SH, Lee CE. SOCS1 represses fractionated ionizing radiation-induced EMT signaling pathways through the counter-regulation of ROS scavenging and ROS-generating systems. Cell Physiol Biochem. 2020;54:1026–1040. doi: 10.33594/000000285.6fe17f5d379645a7a11cb4183d75ef5d [DOI] [PubMed] [Google Scholar]
- 12.Oh J, Hur MW, Lee CE. SOCS1 protects protein tyrosine phosphatases by thioredoxin up-regulation and attenuates JAKs to suppress ROS-mediated apoptosis. Oncogene. 2009;28:3145–3156. doi: 10.1038/onc.2009.169. [DOI] [PubMed] [Google Scholar]
- 13.Tobelaim WS, Beaurivage C, Champagne A, et al. Tumour-promoting role of SOCS1 in colorectal cancer cells. Sci Rep. 2015;5:14301. doi: 10.1038/srep14301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang FJ, Steeg PS, Price JE, et al. Molecular basis for the critical role of suppressor of cytokine signaling-1 in melanoma brain metastasis. Cancer Res. 2008;68:9634–9642. doi: 10.1158/0008-5472.CAN-08-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou H, Miki R, Eeva M, et al. Reciprocal regulation of SOCS1 and SOCS3 enhances resistance to ionizing radiation in glioblastoma multiforme. Clin Cancer Res. 2007;15:2344–2353. doi: 10.1158/1078-0432.CCR-06-2303. [DOI] [PubMed] [Google Scholar]
- 16.Lin YC, Lin CK, Tsai YH, et al. Adenovirus-mediated SOCS3 gene transfer inhibits the growth and enhances the radiosensitivity of human non-small cell lung cancer cells. Oncol Rep. 2010;24:1605–1612. doi: 10.3892/or_00001024. [DOI] [PubMed] [Google Scholar]
- 17.Sitko JC, Yeh B, Kim M, et al. SOCS3 regulates p21 expression and cell cycle arrest in response to DNA damage. Cell Signal. 2008;20:2221–2230. doi: 10.1016/j.cellsig.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Bodmer WF. Analysis of P53 mutations and their expression in 56 colorectal cancer cell lines. Proc Natl Acd Sci U S A. 2006;103:976–981. doi: 10.1073/pnas.0510146103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perillo B, Donato MD, Pezone A, et al. ROS in cancer therapy: the bright side of the moon. Exp Mol Med. 2020;52:192–302. doi: 10.1038/s12276-020-0384-2.3ff956a3ec744f26908fe41f22788e76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim G, Jeong H, Yoon H, et al. Anti-inflammatory mechanism of suppressors of cytokine signaling targets ROS via Nrf2/thioredoxin induction and inflammasone activation in macrophages. BMB Rep. 2020;53:640–645. doi: 10.5483/BMBRep.2020.53.12.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Q, Lai R, Chirieac LR, et al. Constitutive activation of JAK3/STAT3 in colon carcinoma tumors and cell lines: Inhibition of JAK3/STAT3 signaling induces apoptosis and cell cycle arrest of colon carcinoma cells. Am J Pathol. 2005;167:969–980. doi: 10.1016/S0002-9440(10)61187-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugase T, Takahashi T, Serada S, et al. SOCS1 gene therapy improves radiosensitivity and enhances irradiation-induced DNA damage in esophageal squamous cell carcinoma. Cancer Res. 2017;7:6975–6986. doi: 10.1158/0008-5472.CAN-17-1525. [DOI] [PubMed] [Google Scholar]
- 23.Xiong H, Zhang ZG, Tian XQ, et al. Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces tumor cell invasion in colorectal cancer cells. Neoplasia. 2008;10:287–297. doi: 10.1593/neo.07971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merritt AJ, Allen TD, Potten CS, Hickman JA. Apoptosis in small intestinal epithelial from p53-null mice: evidence for a delayed, p53-independent G2/M-associated cell death after gamma-irradiation. Oncogene. 1997;14:2759–2766. doi: 10.1038/sj.onc.1201126. [DOI] [PubMed] [Google Scholar]
- 25.Calabrese V, Mallette FA, Deschênes-Simard X, et al. SOCS1 links cytokine signaling to p53 and senescence. Mol Cell. 2009;11:754–767. doi: 10.1016/j.molcel.2009.09.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.