Abstract
Ventriculomegaly induced by the abnormal accumulation of cerebrospinal fluid (CSF) leads to hydrocephalus, which is accompanied by neuroinflammation and mitochondrial oxidative stress. The mitochondrial stress activates mitochondrial unfolded protein response (UPRmt), which is essential for mitochondrial protein homeostasis. However, the association of inflammatory response and UPRmt in the pathogenesis of hydrocephalus is still unclear. To assess their relevance in the pathogenesis of hydrocephalus, we established a kaolin-induced hydrocephalus model in 8-week-old male C57BL/6J mice and evaluated it over time. We found that kaolin-injected mice showed prominent ventricular dilation, motor behavior defects at the 3-day, followed by the activation of microglia and UPRmt in the motor cortex at the 5-day. In addition, PARP-1/NF-κB signaling and apoptotic cell death appeared at the 5-day. Taken together, our findings demonstrate that activation of microglia and UPRmt occurs after hydrocephalic ventricular expansion and behavioral abnormal-ities which could be lead to apoptotic neuronal cell death, providing a new perspective on the pathogenic mechanism of hydrocephalus.
Keywords: Hydrocephalus, Microglia, Neuroinflammation, UPRmt
INTRODUCTION
Hydrocephalus is a common neurological disorder caused by abnormalities in cerebrospinal fluid (CSF) circulation and absorption, which results in the accumulation of CSF in the ventricular system and the dilation of ventricles (1). Increased CSF volume in ventricles generates shear stress, which leads to deformation of the ventricles and cortical thinning (2, 3). The dysregulation of the neuronal activity in the motor cortex is responsible for gait disturbances in patients with idiopathic normal pressure hydrocephalus (iNPH) (4). And, iNPH patient’s motor function can be recovered by CSF drainage, which is related to enhanced activity of frontal motor areas (5). Although the ventriculomegaly correlated with motor deficits in kaolin-induced hydrocephalus rats, the underlying mechanisms are not yet clear (6).
Neuroinflammation-related biomarkers in CSF are increasingly being used to diagnose patients with hydrocephalus (7). Neuroinflammation and brain injury within the white matter of the corpus callosum, accompanied by the increased pro-inflammatory factors such as interleukin-6 (IL-6) and interleukin-1β (IL-1β) has been shown in the neonatal hydrocephalus model (8). The production of IL-6 and interleukin-8 (IL-8) were up-regulated in idiopathic hydrocephalus patients (9). Moreover, interleukin-10 (IL-10) and interleukin-33 (IL-33) in CSF can be used to monitor the hydrocephalus progression and the effectiveness of shunt surgery (10). In neuroinflammation, glial cells produce pro-inflammatory factors, such as tumor necrosis factor-α (TNF-α) and IL-6, that promote neuroinflammation and secondary brain damage (11). The increased expression of TNF-α is associated with periventricular white matter lesions and demyelination in patients with normal pressure hydrocephalus (NPH) (12). The neuroinflammatory changes caused by ventriculomegaly could be a trigger for neuronal damage in the brain, which eventually leads to behavioral and cognitive problems in iNPH (13). Nevertheless, little evidence has been provided in support of the relevance of neuroinflammation in hydrocephalus-related ventricular dilation and behavioral abnormalities.
Mitochondria have been considered to be responsible for stress adaptation response against external insult such as inflammation, oxidative stress that could be attenuated disease progression (14). The protective pathway that enhances stress resilience through the strengthening of mitochondrial function includes mitochondrial unfolded protein response (UPRmt) (15). To maintain the integrity of mitochondrial structure and function, UPRmt leads to the increase of the mitochondrial molecular chaperones and proteases expression such as heat shock protein 60 (HSP60), mitochondrial protease Lon protease (LONP1), and caseinolytic peptidase P (CLPP) (16). These molecules promote the recovery of the mitochondrial network to ensure optimal cellular function (17). Importantly, activated UPRmt has been reported as a pathological feature of neurological diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS) (18). Nevertheless, little evidence has been provided in support of the UPRmt is involved in hydrocephalus. We hypothesized that gliosis and the UPRmt are involved in the pathological process of hydrocephalus. Here, to test this hypothesis, we observed a kaolin-induced hydrocephalus mouse model over time, based on the well-established kaolin injection model described in rats (19). The elucidating for the involvement of UPRmt and gliosis in kaolin-injected mice would contribute to demonstrating a novel opinion on the pathogenesis of hydrocephalus.
RESULTS
Ventricular enlargement and neurobehavioral defects appear at the 3-day after kaolin injection
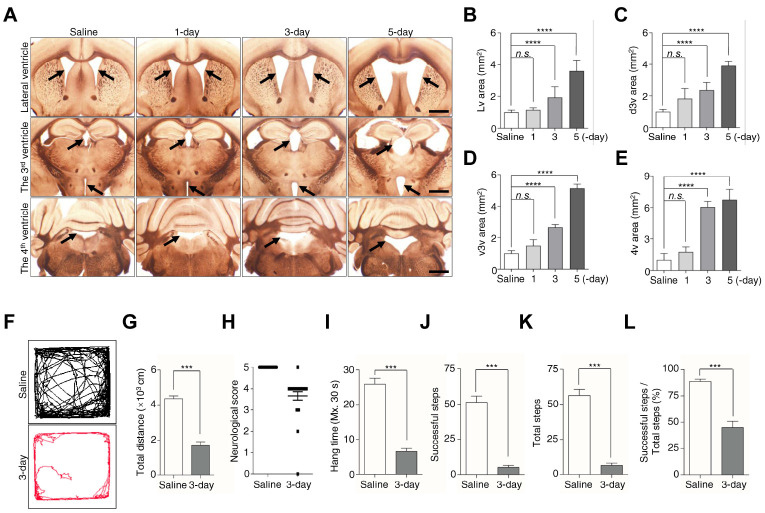
The pathological symptoms of hydrocephalus begin with ventricular dilation (20). Injection of kaolin into the cisterna magna resulted in disruption of CSF flow and an increase in ventricular size (21). To determine whether kaolin-injected mice developed hydrocephalus, we measured the size of the lateral ventricle (Lv), the dorsal part of the 3rd ventricle (d3v), the ventral part of the 3rd ventricle (v3v), and the 4th ventricle (4v), in the saline-treated group and 1-, 3-, 5-day kaolin-treated groups. There were no significant changes in the sizes of these ventricles in the 1-day group, but obvious ventricular enlargement in the 3-day and 5-day group, compared with the saline group (Fig. 1A-E). The size of the Lv, d3v, v3v, and 4v increased over 2-fold in the 3-day group, and more than 3-fold in the 5-day group, compared with the saline group (Fig. 1A-E). These results indicate that kaolin induces ventricular expansion starting at the 3-day, and continue expansion at the 5-day after injection.
Fig. 1.
Kaolin-induced hydrocephalus mice show ventricular enlargement and motor disturbances. (A) Coronal sections showed ventricles saline and 1, 3, 5-day after kaolin injection. (B-E) Bar plots were showed the Lv, d3v, v3v, and 4v areas. Black arrowheads indicate the ventricles, in the 3v figures, the arrowheads represent the d3v (top) and the v3v (bottom). (F, G) Movement activity was measured for 10 min in the open-field test. (H) The neurological function was scored by a 5-point paradigm and plotted. (I-L) Bar plots showed the results were calculated for 30 s in a horizontal grid test. Bregma in Lv (+0.14 mm), in 3v (−1.12 mm to −1.46 mm), in 4v (−5.88 mm). Ventricles size (n = 6), behavior test (n (saline) = 16, n (3-day) = 24 for the neurological score, n (3-day) = 23 for the open-field test and horizontal grid test, **P < 0.01, ***P < 0.001; n.s., not significant). Scale bar: A: 200 μm.
The behavioral symptoms of hydrocephalus are associated with ventricular dilatation, such as shuffling gait (22). As we found a significant change of ventricular dilatation from the 3-day after kaolin injection, we assessed the behavior test in the 3-day group. We initially used the open-field test to measure the mice’s distance traveled for 10 min (23). We found saline-treated mice moved normally in the apparatus, but kaolin-treated mice showed difficulty walking and traveled distance 60% shorter than the saline group (Fig. 1F, G).
To verify whether the hypokinetic movement in kaolin mice is correlated with neurologic dysfunction, we evaluated the differences in neurobehavioral function between saline and 3-day groups, according to the modified neurological score (24). In the 3-day group, 75% of the mice showed decreased scavenging and scatter reflexes, indicative of neurological dysfunction (Fig. 1H). We next performed a horizontal grid test to examine the muscle strength and motor ability of kaolin mice, by analyzing hang time, successful steps, and total steps (25). Compared with saline mice, kaolin mice showed that the average hang time was reduced by 75% (Fig. 1I), the number of successful steps and total steps was decreased by ∼90% (Fig. 1J, K), and the percentage of successful steps was approximately halved (Fig. 1L). Taken together, these results showed that locomotor ability and muscular strength prominently are reduced with ventricular enlargement at the 3-day.
Microglia activates at the 5-day after kaolin injection
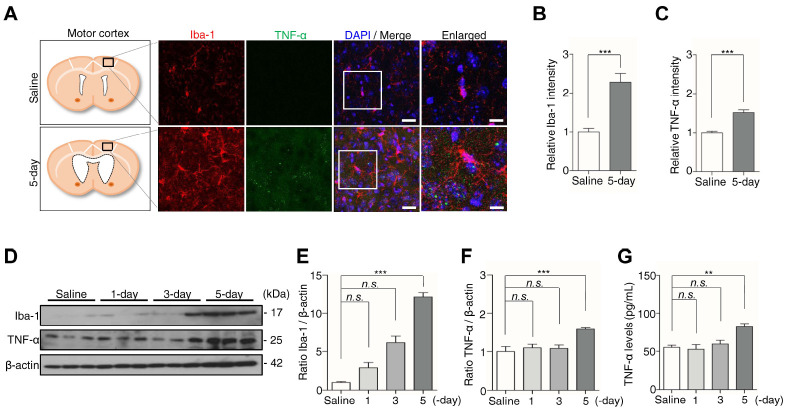
In the kaolin-induced hydrocephalic rats, neuroinflammation and microglial reaction followed by ventricular enlargement (26). Moreover, the expression of the inflammatory factor TNF-α is increased in the CSF of NPH patients (12). To investigate whether microglia and TNF-α increase in our kaolin-induced hydrocephalic mice, we monitored the expression of the microglia marker Iba-1, and inflammatory factor, TNF-α in the motor cortex. Microglia in the kaolin group exhibited an altered morphology, characterized by an enlarged cell body and a “bushier” appearance, accompanied by the expression of TNF-α up-regulated in the 5-day group, compared with the saline group (Fig. 2A). Immunofluorescence staining showed that the expression of Iba-1 increased 2.3-fold, with TNF-α staining intensity increased by 1.5-fold in the 5-day group, compared with the saline group (Fig. 2B, C).
Fig. 2.
Kaolin-induced hydrocephalus mice activate microglia at the 5-day. (A) The motor cortex was stained for Iba-1 (red) and TNF-α (green). (B, C) The immunofluorescence intensity of Iba-1 and TNF-α was quantified. (D) The expression of Iba-1 and TNF-α in the motor cortex was analyzed by western blotting. (E, F) The intensity value of Iba-1 and TNF-α was shown. (G) The TNF-α level was analyzed by ELISA. Western blotting (n = 3, from three independent samples performed twice independently), immunofluorescence and ELISA (n = 6, **P < 0.01, ***P < 0.001; n.s., not significant). Scale bar: A: 20 μm, the enlarged images: 10 μm.
In addition, Iba-1 protein levels also increased ∼12-fold, accompanied by a 1.6-fold increase in TNF-α in the 5-day group, compared with the saline group, whereas Iba-1 and TNF-α protein levels in the 1- and 3-day groups showed no significant changes compared with the saline group (Fig. 2D-F). Consistently, TNF-α levels in brain tissue lysates, determined by ELISA, increased 1.5-fold in the 5-day group, compared with the saline group (Fig. 2G). These results suggest that inflammatory response occurs after the enlargement of the ventricle in the kaolin-induced hydrocephalic mice.
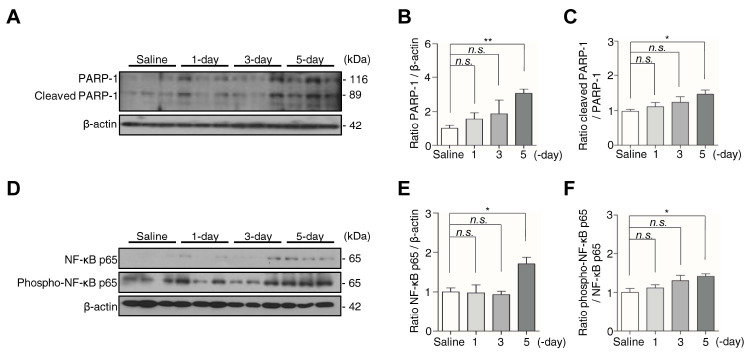
Apoptotic neuronal cell death occurs in the motor cortex at the 5-day after kaolin injection
Microglia promote the release of inflammatory cytokine TNF-α, resulting in progressive neuronal cell death (27). Furthermore, nuclear factor kappa B (NF-κB) is coactivated with poly (ADP- ribose) polymerase-1 (PARP-1), which participates in cell death in microgliosis (28). Therefore, to examine neuronal cell death under the condition of increased TNF-α in kaolin-injected mice, we assessed the expression of PARP-1 and cleaved PARP-1, as well as NF-κB p65 and phospho-NF-κB p65, the hub subunit of NF-κB, in the motor cortex (29). This analysis showed that compared with the saline group, mice in the 5-day group showed a 3.1-fold increase in PARP-1 expression, a 1.5-fold increase in cleaved PARP-1 (Fig. 3A-C), a 1.7-fold increase in NF-κB p65, and 1.4-fold increase phospho-NF-κB p65 (Fig. 3D-F). By contrast, none of these proteins exhibited a change in expression in 1-day or 3-day groups. Using TUNEL staining and Nissl staining, we observed the number of neuronal cells decreased in the 5-day group compared with the saline group (Supplementary Fig. 1). Collectively, these results suggest that coactivated PARP-1 and NF-κB are involved in the neuronal apoptosis that occurred at 5-day after kaolin injection.
Fig. 3.
Kaolin-induced hydrocephalus mice show neuronal apoptosis associated with PARP-1 and NF-κB up-regulated at the 5-day. (A) The protein expression of PARP-1 and cleaved PARP-1 in the motor cortex were analyzed by western blotting. (B, C) The intensity value of PARP-1 and cleaved PARP-1 was shown. (D) The expression of NF-κB p65 and phospho-NF-κB p65 in the motor cortex were analyzed by western blotting. (E, F) The intensity value of NF-κB p65 and phospho-NF-κB p65 was shown. PARP-1 and NF-κB p65 proteins levels were normalized to β-actin, cleaved PARP-1 protein level was normalized to PARP-1, and phospho-NF-κB p65 protein level was normalized to NF-κB p65. (n = 3, from three independent samples performed twice independently, *P < 0.05, **P < 0.01; n.s., not significant).
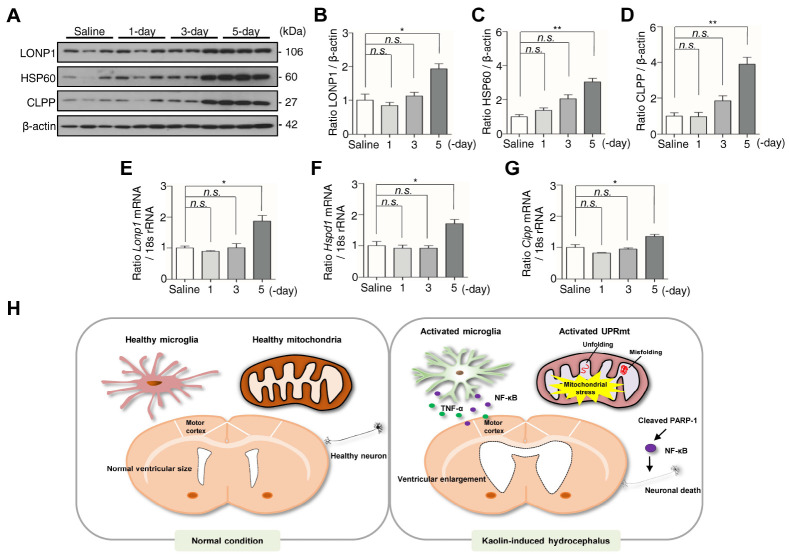
UPRmt activates at the 5-day after kaolin injection
Both neuroinflammation and mitochondrial dysfunction are crucial pathomechanisms in neurological diseases (30). During mitochondrial dysfunction, cells activate several defense mechanisms that serve to maintain optimal cellular function, in particular, UPRmt (15). To determine whether the UPRmt is activated in kaolin-injected mice, we assessed the expression of mitochondrial molecular chaperones and proteases LONP1, HSP60, and CLPP (17). The expression of three proteins was significantly increased in the 5-day group compared with the saline group, with LONP1 increasing 1.9-fold (Fig. 4A, B), HSP60 increasing 3-fold (Fig. 4A, C), and CLPP increasing 3.9-fold (Fig. 4A, D). Consistent with the protein results, the transcriptional level of Lonp1, Hspd1, and Clpp roughly increased 2-fold in the 5-day group compared with the saline group (Fig. 4E-G). Taken together, our results demonstrate that activation of microglia and UPRmt in the motor cortex are following hydrocephalic ventricular expansion and behavioral abnormalities, that could contribute the apoptotic neuronal cell death.
Fig. 4.
Kaolin-induced hydrocephalus mice activate UPRmt at the 5-day. (A) The expression of LONP1, HSP60, and CLPP in the motor cortex was analyzed by western blotting. (B-D) The intensity value of LONP1, HSP60, and CLPP was shown. (E-G) The expression of Lonp1, Hspd1, and Clpp in the motor cortex was analyzed by qPCR. Western blotting (n = 3, from three independent samples performed twice independently), qPCR n = 6 (*P < 0.05, **P < 0.01; n.s., not significant). (H) The schematic represents the presence of microglia and UPRmt in the motor cortex are following hydrocephalic ventricular expansion and behavioral abnormalities.
DISCUSSION
Hydrocephalic patients show behavioral abnormalities with abnormal accumulation of CSF in ventricles (1). In addition, inflammatory cytokines such as TNF-α, IL-1β, IL-6 were suggested as the biomarkers of NPH patients because the cytokines increased in CSF of NPH patients (31, 32). However, the association between neuroinflammatory response and behavior symptoms as well as the mechanism underlying the ventricular enlargement that may induce behavior defects are still unclear. Kaolin (aluminum silicate) has been used to generate hydrocephalus by direct cisterna magna injection in animal models (33). The kaolin-induced hydrocephalus is a well-established animal hydrocephalus model, to study the pathogenesis of hydrocephalus (34). Kaolin was localized to the fourth ventricle by the cisterna magna injection, the obstructive hydrocephalus was expected to develop within seven days after induction (19). Many researchers have been reported that ventricular dilation, behavioral defects, and neuroinflammation in the kaolin-induced hydrocephalus model (35-37). However, the mitochondria-related pathogenesis in the kaolin-induced hydrocephalus model is not fully understood. In the present study, we sequentially investigated the symptoms of the kaolin-induced hydrocephalus mice over time after the injection of kaolin. We observed enlarged ventricle and behavior defects at the 3-day after that time, microglia activated and TNF-α level increased at the 5-day after kaolin injection. We also demonstrated that UPRmt were induced at the 5-day when inflammatory response occurred with apoptotic neuronal cell death in the kaolin-induced hydrocephalus mice.
Reactive microglia serve as a potential pathogenic mechanism for neonatal hydrocephalus (38). Activation of microglia in the white matter is related to ventricular dilatation (6). After the change to reactive microglia by ventriculomegaly, it starts to release the inflammatory mediator TNF-α, which can provide a positive feedback loop to spread the inflammatory reaction around the microenvironment which is called gliosis (39). TNF-α was correlated with the severity of congenital hydrocephalic mice (12, 40, 41). Similarly, the level of TNF-α in CSF is positively correlated with the severity of NPH patients, and drainage surgery can not only improve the clinical symp-toms of hydrocephalus but also completely reduce the secretion of TNF-α (12). The presence of TNF-α could explain the re-active gliosis is closely associated with the severity of ventricular dilation in hydrocephalic rats (36). Consistent with our findings, inflammatory responses result from ventricle enlargement in the hydrocephalic brain. Although we investigated microglia activation by observing morphological changes of microglia and increase of Iba-1 expression, we can not find a profound change of astrocyte within 5-days in the kaolin-induced hydrocephalic mice (Supplementary Fig. 2), unlike in the hydrocephalic rat model (36). Astrocyte alteration needs to be in further investigation in the hydrocephalus mice over time.
In neurodegenerative diseases, glia-mediated neuroinflammation aggravates neuronal degeneration and increases neuronal cell death (42-44). PARP-1 acts as the coactivator of NF-κB, which plays a crucial role in inflammatory disorders (45). Furthermore, PARP-1 promotes DNA repair, cleaved PARP-1 initiates the apoptotic cell death pathway (46). In a mouse model of traumatic brain injury (TBI), PARP-1 induces neuronal cell death through microglial activation (47). These reports indicate that neuronal cell death is related to microglial activation by the PARP-1/NF-κB signaling pathway. Consistent with these results, the expression of PARP-1, cleaved PARP-1, and NF-κB is increased at the 5-day after kaolin injection, suggesting the pre-sence of apoptotic neurons in kaolin-treated mice. Although we labeled broken DNA strands in the motor cortex using TUNEL staining, which is a well-known assay of neuronal apoptosis, axonal damage-associated molecules, such as neurofilament light (NFL) and total-tau (T-tau) in kaolin mice need to be further investigated (48).
Mitochondrial oxidative stress is the common feature of chro-nic neurodegenerative diseases (49, 50). Mitochondrial oxidative phosphorylation (OXPHOS) dysfunction produces reactive oxygen species (ROS), which mediates neuronal dysfunction and aggravates perinatal hydrocephalus (14, 51). During mitochondrial and cellular dysfunction, mitochondrial stress responses intervene to rebuild correct protein and maintain cellular homeostasis, UPRmt. This pathway mediates the adaptative responses against microenvironmental stimuli (52). In the case of Surf1 (−/−) mice which showed reduced cytochrome c oxidase (CcO) activity, UPRmt might contribute to the enhancement of stress adaptation response by increased expression of UPRmt components CLPP, HSP60, and LONP1 (53). We also found that LONP1, HSP60, and CLPP protein expression and mRNA level increased at the 5-day after kaolin injection when inflammatory response upregulated. Taken together, we demonstrated that inflammatory response and UPRmt activation simultaneously occurred with neuronal cell death after ventricular enlargement. Although we assumed that UPRmt progresses neuronal cell death by observing apoptotic neuronal cells, the role of UPRmt that may induce or alleviate apoptosis remains unclear and require further investigation.
In conclusion, kaolin-induced hydrocephalus mice showed prominent dilation of ventricles and motor behavior defects at the 3-day. The inflammatory response such as microglia activation and increase of TNF-α occurred with PARP-1/NF-κB signaling and UPRmt upregulation at the 5-day. By demonstrating that activated microglia and UPRmt are following hydrocephalic ventricular expansion and behavioral abnormalities, our findings provide new insights into the pathogenic mechanism of hydrocephalus (Fig. 4H).
MATERIALS AND METHODS
Materials and methods are available in the supplemental material.
SUPPLEMENTARY MATERIALS
ACKNOWLEDGEMENTS
This research was supported by the Ministry of Science, ICT (grant number NRF-2017R1A5A2015385, 2019M3E5D1A020 68575, 2019R1F1A1059586).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Kahle KT, Kulkarni AV, Limbrick DD, Jr, Warf BC. Hydrocephalus in children. Lancet. 2016;387:788–799. doi: 10.1016/S0140-6736(15)60694-8. [DOI] [PubMed] [Google Scholar]
- 2.Levine DN. Intracranial pressure and ventricular expansion in hydrocephalus: have we been asking the wrong question? J Neurol Sci. 2008;269:1–11. doi: 10.1016/j.jns.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Ferris CF, Cai X, Qiao J, et al. Life without a brain: Neuroradiological and behavioral evidence of neuroplasticity necessary to sustain brain function in the face of severe hydrocephalus. Sci Rep. 2019;9:16479. doi: 10.1038/s41598-019-53042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chistyakov AV, Hafner H, Sinai A, Kaplan B, Zaaroor M. Motor cortex disinhibition in normal-pressure hydrocephalus. J Neurosurg. 2012;116:453–459. doi: 10.3171/2011.9.JNS11678. [DOI] [PubMed] [Google Scholar]
- 5.Lenfeldt N, Larsson A, Nyberg L, et al. Idiopathic normal pressure hydrocephalus: increased supplementary motor activity accounts for improvement after CSF drainage. Brain. 2008;131:2904–2912. doi: 10.1093/brain/awn232. [DOI] [PubMed] [Google Scholar]
- 6.Olopade FE, Shokunbi MT, Sirén AL. The relationship between ventricular dilatation, neuropathological and neurobehavioural changes in hydrocephalic rats. Fluids Barriers CNS. 2012;9:19. doi: 10.1186/2045-8118-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris CA, Morales DM, Arshad R, McAllister JP, 2nd, Limbrick DD., Jr Cerebrospinal fluid biomarkers of neuroinflammation in children with hydrocephalus and shunt malfunction. Fluids Barriers CNS. 2021;18:4. doi: 10.1186/s12987-021-00237-4.63dd31d05c724b05a8a6d088e4924eb3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goulding DS, Vogel RC, Pandya CD, et al. Neonatal hydrocephalus leads to white matter neuroinflammation and injury in the corpus callosum of Ccdc39 hydrocephalic mice. J Neurosurg Pediatr. 2020;25:476–483. doi: 10.3171/2019.12.PEDS19625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czubowicz K, Głowacki M, Fersten E, Kozłowska E, Strosznajder RP, Czernicki Z. Levels of selected pro- and anti-inflammatory cytokines in cerebrospinal fluid in patients with hydrocephalus. Folia Neuropathol. 2017;55:301–307. doi: 10.5114/fn.2017.72389. [DOI] [PubMed] [Google Scholar]
- 10.Sosvorova L, Mohapl M, Vcelak J, Hill M, Vitku J, Hampl R. The impact of selected cytokines in the follow-up of normal pressure hydrocephalus. Physiol Res. 2015;64:S283–S290. doi: 10.33549/physiolres.933069. [DOI] [PubMed] [Google Scholar]
- 11.Gaire BP, Choi JW. Critical roles of lysophospholipid receptors in activation of neuroglia and their neuroinflammatory responses. Int J Mol Sci. 2021;22:7864. doi: 10.3390/ijms22157864.cbc3f1c088a84566a49e516d32b9f407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarkowski E, Tullberg M, Fredman P, Wikkelsö C. Normal pressure hydrocephalus triggers intrathecal production of TNF-alpha. Neurobiol Aging. 2003;24:707–714. doi: 10.1016/S0197-4580(02)00187-2. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Zhang Y, Hu F, Ding J, Wang X. Pathogenesis and pathophysiology of idiopathic normal pressure hydrocephalus. CNS Neurosci Ther. 2020;26:1230–1240. doi: 10.1111/cns.13526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Zhou Z, Min W. Mitochondria, oxidative stress and innate immunity. Front Physiol. 2018;9:1487. doi: 10.3389/fphys.2018.01487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melber A, Haynes CM. UPR(mt) regulation and output: a stress response mediated by mitochondrial-nuclear communication. Cell Res. 2018;28:281–295. doi: 10.1038/cr.2018.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorrentino V, Menzies KJ, Auwerx J. Repairing mitochondrial dysfunction in disease. Annu Rev Pharmacol Toxicol. 2018;58:353–389. doi: 10.1146/annurev-pharmtox-010716-104908. [DOI] [PubMed] [Google Scholar]
- 17.Shpilka T, Haynes CM. The mitochondrial UPR: mechanisms, physiological functions and implications in ageing. Nat Rev Mol Cell Biol. 2018;19:109–120. doi: 10.1038/nrm.2017.110. [DOI] [PubMed] [Google Scholar]
- 18.Shen Y, Ding M, Xie Z, et al. Activation of mitochondrial unfolded protein response in SHSY5Y expressing APP cells and APP/PS1 mice. Front Cell Neurosci. 2019;13:568. doi: 10.3389/fncel.2019.00568.0d2c5685cdba4a14a028933a628f0d0a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins P. Experimental obstructive hydrocephalus in the rat: a scanning electron microscopic study. Neuropathol Appl Neurobiol. 1979;5:457–468. doi: 10.1111/j.1365-2990.1979.tb00643.x. [DOI] [PubMed] [Google Scholar]
- 20.Schob S, Weiß A, Dieckow J, et al. Correlations of ventricular enlargement with rheologically active surfactant proteins in cerebrospinal fluid. Front Aging Neurosci. 2016;8:324. doi: 10.3389/fnagi.2016.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basati S, Desai B, Alaraj A, Charbel F, Linninger A. Cerebrospinal fluid volume measurements in hydrocephalic rats. J Neurosurg Pediatr. 2012;10:347–354. doi: 10.3171/2012.6.PEDS11457. [DOI] [PubMed] [Google Scholar]
- 22.Solana E, Poca MA, Sahuquillo J, Benejam B, Junqué C, Dronavalli M. Cognitive and motor improvement after retesting in normal-pressure hydrocephalus: a real change or merely a learning effect? J Neurosurg. 2010;112:399–409. doi: 10.3171/2009.4.JNS081664. [DOI] [PubMed] [Google Scholar]
- 23.Osmon KJ, Vyas M, Woodley E, Thompson P, Walia JS. Battery of behavioral tests assessing general locomotion, muscular strength, and coordination in mice. J Vis Exp. 2018;131:55491. doi: 10.3791/55491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bloch O, Auguste KI, Manley GT, Verkman AS. Accelerated progression of kaolin-induced hydrocephalus in aquaporin-4-deficient mice. J Cereb Blood Flow Metab. 2006;26:1527–1537. doi: 10.1038/sj.jcbfm.9600306. [DOI] [PubMed] [Google Scholar]
- 25.Kim ST, Son HJ, Choi JH, Ji IJ, Hwang O. Vertical grid test and modified horizontal grid test are sensitive methods for evaluating motor dysfunctions in the MPTP mouse model of Parkinson's disease. Brain Res. 2010;1306:176–183. doi: 10.1016/j.brainres.2009.09.103. [DOI] [PubMed] [Google Scholar]
- 26.Khan OH, Enno TL, Del Bigio MR. Brain damage in neonatal rats following kaolin induction of hydrocephalus. Exp Neurol. 2006;200:311–320. doi: 10.1016/j.expneurol.2006.02.113. [DOI] [PubMed] [Google Scholar]
- 27.Spagnuolo C, Moccia S, Russo GL. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur J Med Chem. 2018;153:105–115. doi: 10.1016/j.ejmech.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Gisslen T, Ennis K, Bhandari V, Rao R. Recurrent hypoinsulinemic hyperglycemia in neonatal rats increases PARP-1 and NF-κB expression and leads to microglial activation in the cerebral cortex. Pediatr Res. 2015;78:513–519. doi: 10.1038/pr.2015.136. [DOI] [PubMed] [Google Scholar]
- 29.Pan Z, Yang K, Wang H, et al. MFAP4 deficiency alleviates renal fibrosis through inhibition of NF-κB and TGF-β/Smad signaling pathways. FASEB J. 2020;34:14250–14263. doi: 10.1096/fj.202001026R. [DOI] [PubMed] [Google Scholar]
- 30.Harland M, Torres S, Liu J, Wang X. Neuronal mitochondria modulation of LPS-induced neuroinflammation. J Neurosci. 2020;40:1756–1765. doi: 10.1523/JNEUROSCI.2324-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sosvorova L, Kanceva R, Vcelak J, et al. The comparison of selected cerebrospinal fluid and serum cytokine levels in patients with multiple sclerosis and normal pressure hydrocephalus. Neuro Endocrinol Lett. 2015;36:564–571. [PubMed] [Google Scholar]
- 32.Park JC, Han SH, Mook-Jung I. Peripheral inflammatory biomarkers in Alzheimer's disease: a brief review. BMB Rep. 2020;53:10–19. doi: 10.5483/BMBRep.2020.53.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duru S, Oria M, Arevalo S, et al. Comparative study of intracisternal kaolin injection techniques to induce congenital hydrocephalus in fetal lamb. Childs Nerv Syst. 2019;35:843–849. doi: 10.1007/s00381-019-04096-1. [DOI] [PubMed] [Google Scholar]
- 34.Silverberg GD, Miller MC, Pascale CL, et al. Kaolin-induced chronic hydrocephalus accelerates amyloid deposition and vascular disease in transgenic rats expressing high levels of human APP. Fluids Barriers CNS. 2015;12:2. doi: 10.1186/2045-8118-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shim I, Ha Y, Chung JY, Lee HJ, Yang KH, Chang JW. Association of learning and memory impairments with changes in the septohippocampal cholinergic system in rats with kaolin-induced hydrocephalus. Neurosurgery. 2003;53:416–425. discussion 425. doi: 10.1227/01.NEU.0000073989.07810.D8. [DOI] [PubMed] [Google Scholar]
- 36.Xu H, Zhang SL, Tan GW, et al. Reactive gliosis and neuroinflammation in rats with communicating hydrocephalus. Neuroscience. 2012;218:317–325. doi: 10.1016/j.neuroscience.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Olopade FE, Shokunbi MT, Azeez IA, Andrioli A, Scambi I, Bentivoglio M. Neuroinflammatory response in chronic hydrocephalus in juvenile rats. Neuroscience. 2019;419:14–22. doi: 10.1016/j.neuroscience.2019.08.049. [DOI] [PubMed] [Google Scholar]
- 38.Wu KY, Tang FL, Lee D, et al. Ependymal Vps35 promotes ependymal cell differentiation and survival, suppresses microglial activation, and prevents neonatal hydrocephalus. J Neurosci. 2020;40:3862–3879. doi: 10.1523/JNEUROSCI.1520-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brás JP, Bravo J, Freitas J, et al. TNF-alpha-induced microglia activation requires miR-342: impact on NF-kB signaling and neurotoxicity. Cell Death Dis. 2020;11:415. doi: 10.1038/s41419-020-2626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takase H, Chou SH, Hamanaka G, et al. Soluble vascular endothelial-cadherin in CSF after subarachnoid hemorrhage. Neurology. 2020;94:e1281–e1293. doi: 10.1212/WNL.0000000000008868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiménez AJ, Rodríguez-Pérez LM, Domínguez-Pinos MD, et al. Increased levels of tumour necrosis factor alpha (TNFα) but not transforming growth factor-beta 1 (TGFβ1) are associated with the severity of congenital hydrocephalus in the hyh mouse. Neuropathol Appl Neurobiol. 2014;40:911–932. doi: 10.1111/nan.12115. [DOI] [PubMed] [Google Scholar]
- 42.Joshi AU, Minhas PS, Liddelow SA, et al. Fragmented mitochondria released from microglia trigger A1 astrocytic response and propagate inflammatory neurodegeneration. Nat Neurosci. 2019;22:1635–1648. doi: 10.1038/s41593-019-0486-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 44.Kwak JH, Lee K. Forebrain glutamatergic neuron-specific Ctcf deletion induces reactive microgliosis and astrogliosis with neuronal loss in adult mouse hippocampus. BMB Rep. 2021;54:317–322. doi: 10.5483/BMBRep.2021.54.6.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hassa PO, Hottiger MO. The functional role of poly(ADP-ribose)polymerase 1 as novel coactivator of NF-kappaB in inflammatory disorders. Cell Mol Life Sci. 2002;59:1534–1553. doi: 10.1007/s00018-002-8527-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malhotra U, Zaidi AH, Kosovec JE, et al. Prognostic value and targeted inhibition of survivin expression in esophageal adenocarcinoma and cancer-adjacent squamous epithelium. PLoS One. 2013;8:e78343. doi: 10.1371/journal.pone.0078343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stoica BA, Loane DJ, Zhao Z, et al. PARP-1 inhibition attenuates neuronal loss, microglia activation and neurological deficits after traumatic brain injury. J Neurotrauma. 2014;31:758–772. doi: 10.1089/neu.2013.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niu LD, Xu W, Li JQ, et al. Genome-wide association study of cerebrospinal fluid neurofilament light levels in non-demented elders. Ann Transl Med. 2019;7:657. doi: 10.21037/atm.2019.10.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fischer R, Maier O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxid Med Cell Longev. 2015;2015:610813. doi: 10.1155/2015/610813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee Y, Park Y, Nam H, Lee JW, Yu SW. Translocator protein (TSPO): the new story of the old protein in neuroinflammation. BMB Rep. 2020;53:20–27. doi: 10.5483/BMBRep.2020.53.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Delavallée L, Mathiah N, Cabon L, et al. Mito-chondrial AIF loss causes metabolic reprogramming, caspase-independent cell death blockade, embryonic lethality, and perinatal hydrocephalus. Mol Metab. 2020;40:101027. doi: 10.1016/j.molmet.2020.101027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D'Amico D, Sorrentino V, Auwerx J. Cytosolic proteostasis networks of the mitochondrial stress response. Trends Biochem Sci. 2017;42:712–725. doi: 10.1016/j.tibs.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 53.Pharaoh G, Pulliam D, Hill S, Sataranatarajan K, Van Remmen H. Ablation of the mitochondrial complex IV assembly protein Surf1 leads to increased expression of the UPR(MT) and increased resistance to oxidative stress in primary cultures of fibroblasts. Redox Biol. 2016;8:430–438. doi: 10.1016/j.redox.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.