Abstract
Research into pollinators in managed landscapes has recently combined approaches of pollination ecology and landscape ecology, because key stressors are likely to interact across wide areas. While laboratory and field experiments are valuable for furthering understanding, studies are required to investigate the interacting drivers of pollinator health and diversity across a broader range of landscapes and a wider array of taxa. Here, we use a network of 96 study landscapes in six topographically diverse regions of Britain, to test the combined importance of honeybee density, insecticide loadings, floral resource availability and habitat diversity to pollinator communities. We also explore the interactions between these drivers and the cover and proximity of semi-natural habitat. We found that among our four drivers, only honeybee density was positively related to wild pollinator abundance and diversity, and the positive association between abundance and floral resources depended on insecticide loadings and habitat diversity. By contrast, our exploratory models including habitat composition metrics revealed a complex suite of interactive effects. These results demonstrate that improving pollinator community composition and health is unlikely to be achieved with general resource enhancements only. Rather, local land-use context should be considered in fine-tuning pollinator management and conservation.
This article is part of the theme issue ‘Natural processes influencing pollinator health: from chemistry to landscapes’.
Keywords: agriculture, bumblebees, competition, hoverflies, landuse, solitary bees
1. Introduction
The health of insect pollinator populations and communities has become a topic of global importance in recent decades, not least because of widely reported declines [1,2] and the reliance of ecosystems on pollination services [3]. Pollinators are under pressure from multiple interacting stressors [4], with clear physiological and behavioural implications of management practices such as insecticide application [5,6], honeybee hive placement [7,8] and floral resource enhancement [9]. Laboratory and semi-field studies of these impacts often focus on individual species such as the managed honeybee or key bumblebee species. Yet attention is beginning to turn towards the importance of combining the approaches of pollination ecology and landscape ecology [3,10], particularly as the key stressors of pollinator decline are likely to interact across wide areas [11]. In this study, we examine the combination and interaction of landscape factors that are likely to affect pollinator populations and communities across the widely varying UK countryside.
Our understanding of pollinator health has advanced significantly over the past decades by studies conducted on small groups of species or at small scales. For example, exposure to toxic pesticides can directly affect health, indirectly impact performance via foraging and reproduction [12,13], and also impair immune responses to pathogens [4]. High densities of managed honeybees may be a stressor for wild bee populations under some conditions [7], due to competition for floral resources [14] or due to the increased risk of pathogen spill over [15]. Similarly, poor nutrition due to low-quality floral resource provision can increase the incidence of disease in honeybees [16], and pathogens are more likely to be spread in landscapes with low flower diversity [15,17]. However, we are also discovering some interactions between these and other important drivers, such as the moderating influence of diverse floral resources and semi-natural habitat (SNH) on the effects of agricultural chemicals on insect development [18,19]. The stressors to this diverse guild of insects are manifold and interactive [4], yet we lack comprehensive, standardized field studies to fully demonstrate how these factors influence the wider pollinator community [3,20].
Research into landscape-scale drivers of pollination populations and communities has grown significantly in the last two decades [3,10,11,20–22], to the extent that landscape-scale pollinator conservation is strongly encouraged by governments [3]. However, further research is needed to synthesize the impacts of widely diverging land management practices across topographically diverse countries and to determine context-specific recommendations. Recent research has made great strides in identifying the important interactive effects of habitat type, landscape configuration and other drivers, but studies are often only focused on particular crop types [23,24], certain habitat types [25,26], or limited species groups [27,28]. However, we require further studies to examine the scenarios in which landscape composition and configuration are important in mitigating the impacts of drivers such as habitat loss and fragmentation [29–33]. This information would be of considerable use when identifying landscape features to be prioritized for safeguarding pollinator communities (e.g. [34]).
In this study, we use a network of landscape study sites representing the full land-use gradients in six regions of Great Britain. Our site selection protocol was designed to test the combined importance of four well-documented landscape drivers of pollinator community health (honeybee density, insecticide loadings, floral resource availability and habitat diversity [35]). We surveyed the study sites for a wide range of pollinating insects for 2 years and aimed to understand how these land-use factors are linked to pollinator density and diversity, which we use as proxies for community health. We predicted that, in line with previous work, managed honeybee densities and insecticide application would have negative impacts on wild pollinator community composition across the country [7,36]. Conversely, we predict floral resources and habitat diversity to have positive impacts due to their importance in enhancing pollinator health at individual, population and community levels [10,26,37]. In addition, as resource provision has the potential to offset the negative effects of intensive agriculture [10,15,18], we expected to find similar interactions between our drivers. Furthermore, during field surveys, we observed that the configuration of wide-ranging habitat types are likely to play important roles in pollinator community composition in British landscapes, as also shown elsewhere [38,39]. Therefore, we also explore the potential for these land-use factors to enhance our positive drivers and mitigate negative ones [3].
2. Methods
(a) . Pollinator health
In this study, we use measures of pollinator community composition, including abundance and diversity, as proxies for community health. There are limitations to this approach because community health is typically measured across several years, requires historical baseline data and/or involves direct measurements of fitness (e.g. longevity, reproductive success) [40]. However, such data are difficult to collect over multiple landscapes and for entire communities. We therefore use more convenient diversity and abundance measures as indicators of community health, as it is reasonable to expect that landscapes with many populations able to optimally use resources to improve fitness, are likely to result in diverse and abundant communities. This is not always true (e.g. [41]), but wild bee abundance and diversity have been correlated with pollinator success in some systems (e.g. [42]).
(b) . Study site selection
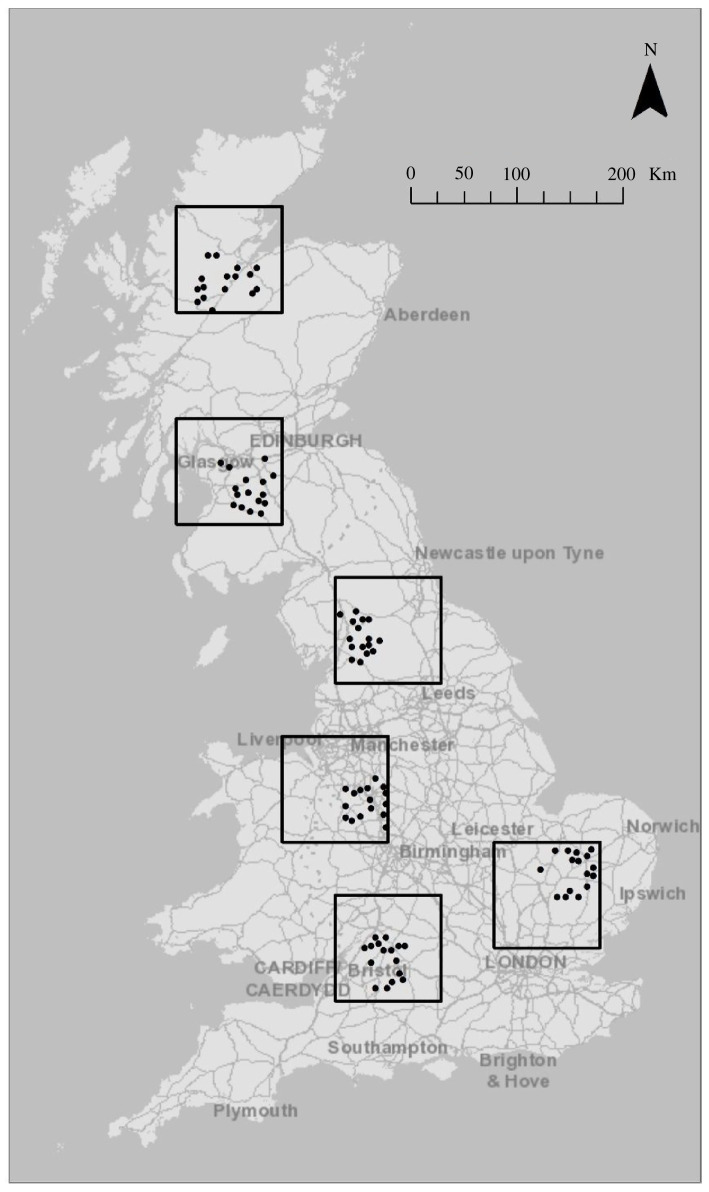
A detailed account of the selection of our study regions and sites is published elsewhere [35], but we will provide a brief overview here. We first selected six 100 × 100 km ‘focal regions’ to represent the vegetation and bioclimatic gradients of Great Britain. All possible combinations of six 100 km grid squares covering the country were measured in terms of the proportional area of all broad habitat types (using the 2007 Land Cover Map; [43]). The process was repeated for the Institute of Terrestrial Ecology land classes (a stratification of all British 1 km squares allowing for representative, unbiased sampling given topography, climate and human infrastructure; [44]), and the six-region combination that provided the closest representation of Britain in both respects was selected (figure 1).
Figure 1.
Map of the six 100 × 100 km study regions (black squares) selected to represent Britain in terms of broad habitats, topography and climate. The black dots depict the 16 study sites chosen within each region.
Within each of the six regions, 16 study sites measuring 2 × 2 km were selected along four gradients: (i) honeybee densities, estimated from Beebase (www.nationalbeeunit.com) database information on local colony densities and weighted by distance using data on honeybee foraging distances; (ii) insecticide loadings (including the loadings derived from insecticidal properties of fungicides and herbicides), measured as a summed honeybee hazard score, estimated from areas of 36 crop groups and insecticides usage data from the Pesticide Usage Survey; (iii) floral resource availability (kilograms of sugar from nectar per hectare per year), estimated by combining flowering species cover (insect-pollinated species including trees and bushes) per unit cover of each habitat from the Countryside Survey 2007 [45] and models of per-flower nectar quantity parameterized using field measurements of nectar production [46]; and (iv) habitat diversity values, calculated as Shannon diversity indices using habitat cover data from the 2007 Land Cover Map (LCM) [43]. Full details of these estimates can be found in the electronic supplementary material.
To select the 16 sites in each region, we first scored all possible 2500 grid squares of each region along the four gradients, standardized the values and applied a selection algorithm to find the 16 sites that maximized the difference between high and low values of the four drivers and the orthogonality between them. For full details on the field site choice, see Gillespie et al. [35]. The final 16 sites chosen for each region were thus considered to represent every combination of relatively high and low values for each of the four gradients. The values of the gradients of the final sites were subject to validation over the 2-year survey period [35] and validated scores are used as predictor variables in this study. Insecticide loadings were adjusted first with ground referencing habitat and crop types. This resulted in a large number of our sites, particularly in Scotland and northern England, having insecticide loadings corrected to zero, because arable fields detected by the LCM 2007 were often reseeded grassland. Loadings for sites with confirmed conventional crops were further validated via questionnaires provided to some landowners (where land ownership could be identified). For sites with confirmed chemical applications, the correlation between estimated and validated loadings was rs = 0.67 [35]. Floral resource availability was validated through flower species surveys collected during the study period and statistically modelled nectar availability (see electronic supplementary material for details; correlation with estimated values: rs = 0.28), and habitat diversity was validated by field surveyors confirming or correcting maps of broad habitat classes (correlation with estimated values: rs = 0.77) [35]. However, we were unable to improve on our original estimates of the honeybee density variable, as honeybees are poorly represented in pan trap samples ([47]; see below), and so the original modelled estimates of this gradient were retained.
We selected sites based on their values at the ‘tetrad’ 2 × 2 km scale because this is the finest scale at which most datasets are available, and due to the relatively high mobility of many European pollinators [48]. However, as many solitary bees tend to forage across much smaller scales, we also calculated floral resource availability and habitat diversity for a central ‘inner’ square (667 × 667 m) at each site (where pollinator collection was conducted; see below). We then tested whether these inner square variables were preferable predictors of pollinator responses to tetrad level variables (see Data analysis below).
(c) . Pollinator collection
In each region, a team of two surveyors was employed each year to collect pollinator specimens following a standardized protocol. Pollinators were trapped using pan traps consisting of three bowls painted yellow, white and blue with UV-reflecting paint [47], and attached to a wooden stake at the height of the vegetation. These traps are ‘activity-based’ with the colours acting as an attractant to foraging insects. It is possible that local flowering plant context affects the effectiveness of such a method. We attempted to control for this possibility by measuring floral resources in the area surrounding each trap (1 m radius), but the variable was not significant in statistical modelling (not shown) and was omitted from the final analysis. Furthermore, previous testing has found pan traps to sample pollinator communities more efficiently than observational methods [47]. Five pan traps were assigned to each site, and they were placed within a central square (667 × 667 m) of each 2 × 2 km site, and using the following criteria: away from potential disturbance by livestock and humans, in unshaded, open habitats, and approximately equidistant and at least 100 m from each other.
Each time a trap was set up, the bowls were half filled with water and a drop of unscented detergent was added to break the surface tension. Whenever possible, the traps were placed out when forecasts predicted clear, dry conditions and left in place for 24 h before the bowls were removed and the insect material transferred to plastic bags for later mounting. The traps were sampled three times (round 1: May, round 2: June–July, round 3: August–September; see electronic supplementary material, table S1 for precise dates), randomizing the order of survey sites each time. Due to the geographic spread of the field sites across the region, it was usually only possible to set up pan traps in four sites at a time, although in some regions with limited access to sites (e.g. Inverness-shire), fewer sites were sampled in a day. Collected insect material was pinned and mounted during the summer of collection, and specimens were identified to species by Hymettus Ltd. Taxonomic resources included Stubbs & Falk [49] for hoverflies, and test keys that formed the basis of Else & Edwards [50] for bees and wasps.
(d) . Floral resource diversity, habitat composition and habitat configuration
In order to explore the mitigating effect of landscape context on responses to the four key drivers, we derived three further variables. First, floral resource diversity was calculated using the flower species and nectar data used to validate the floral resource availability driver. We first estimated each flowering species' nectar provision in µg per m2 for each of 28 broad habitat types (the broad habitat sub-classes listed in the electronic supplementary material, table S6, plus the linear features: hedgerows, water edges, stone walls and fence lines). We then scaled this up to the landscape scale, by multiplying the values by the area of each habitat type for each site. These values were summed for each species to derive their contribution to the site-level floral resource availability. We used these contributions to calculate the Shannon diversity index of floral resources for site (see electronic supplementary materials for full details). We preferred this measure to a flower species diversity index, because it emphasizes the richness and evenness of nectar sources [46].
Second, habitat composition was defined as the percentage cover of SNH in each site and was derived from validated land cover data described above. We included all habitat types not classed as arable, improved grassland, urban and open water in this calculation. Therefore, our measure of SNH comprises all aspects of forest (including conifer plantations), and all types of rough, low-productivity grassland. Across Britain, these types of habitat may be subject to varying levels of management, but in comparison to arable and improved grassland, this can be considered low intensity. Further, while conifer plantations are not typically useful foraging habitats for wild pollinators, in many of our sites, particularly in Scotland and Eastern England, the large areas of conifer are managed as nature reserves and recreational areas and may represent useful nesting habitat, structural diversity and corridors for movement. We also selected this measure because it provided a broad gradient of data across all six regions, and because the use of separate habitat class percentage covers as individual variables led to problems with collinearity and residual heterogeneity.
Third, habitat configuration was estimated as an index of habitat proximity following a method described by Carrié et al. [39]. On each site, 100 m buffers contoured around patch boundaries were created for each SNH patch, including linear features (features found at field margins such as hedgerows, water features and fence lines). Subsequently, the area of overlapping buffer zones was calculated and divided by the total buffer area to represent habitat proximity. Therefore, high values of this index are likely to represent landscapes with many closely located patches of SNH, and low values may represent sites dominated by intensively managed land types or by large patches of a single SNH type. Spatial calculations were conducted using QGIS (v. 3.10.3 [51]).
(e) . Data analysis
We pooled the insect pollinator data from the five pan traps at each site and across the three rounds and analysed data from both years in the same models (i.e. each site was represented by 2 years of sampling data). We used the sampled pollinators to estimate pollinator abundance, species richness and the inverse Simpson diversity index (1/D). We derived these measures for the ‘full’ wild pollinator community (all hoverflies, wasps and bees except honeybees), as well as separately for bumblebees (Bombus spp.), solitary bees (including cleptoparasitic species) and hoverflies (Diptera: Syrphidae). These community response measures were then analysed in two ways, using a confirmatory approach to test our original hypotheses, and an exploratory approach to assess possible mediating roles of habitat cover and proximity. For the confirmatory models, we fitted generalized linear mixed models (GLMM) to each response with the four drivers (honeybees, insecticides, habitat diversity and floral resource availability) and all two-way interactions as fixed explanatory variables. Higher order interactions were excluded for clarity and to avoid complex interpretation. To improve model convergence, insecticides and floral resource availability were log-transformed, and all drivers were scaled and centred. We tested whether the ‘inner’ scale variables for floral resource availability and habitat diversity were better predictors than those at the tetrad scale using the Akaike information criterion, and by comparing residual diagnostic plots. For all response variables, the choice of variables made negligible difference to model fit (ΔAIC < 2), and we proceeded with the tetrad scale variables for consistency. The exploratory approach followed the same procedure, but included the scaled and centred variables SNH, habitat proximity and floral resource diversity, and all two-way interactions between them and the four main drivers.
All data analyses were performed in the R programming environment (v. 4.1.0 [52]). Mixed models were fit using the glmmTMB package [53], and in all cases, a fixed factor for sampling year (2012/13) and a fixed integer variable for the number of pan trap bowls successfully collected (out of a total of 45) were included as covariates to account for differences between years and for the effect of trap bowls being disturbed by animals or passers-by, respectively. Random intercepts for ‘site’ (n = 96) nested within ‘region’ (n = 6) were also specified. There was no collinearity between the explanatory variables, which was checked using variance inflation factors with the performance package [54]. The error distribution for each response variable was determined using residual diagnostic plots and tests applied using the residual simulation methods of the DHARMa package [55]. In most cases for count data (abundance and species richness), the negative binomial distribution with quadratic parameterization (‘nbinom2’ family) provided the best fit to the data, although in some cases (bumblebee and hoverfly species richness), the Poisson distribution provided a better fit. The gamma distribution with a log link was used to model the inverse Simpson diversity index. All model (simulated) residuals were inspected visually for assumptions of linear modelling (normality and homoscedasticity). Model residuals were also tested for spatial and temporal autocorrelation, and the random structure adequately accounted for the clustering and repeated nature of the sampling. Following model validation, 95% confidence intervals were calculated for all model estimates using the confint function of the glmmTMB package, which computes Wald intervals by default. Significant interactions (those with confidence intervals not including zero) were plotted with simple slopes, where the predicted effect of one interacting variable is plotted for several fixed values of the second interacting variable. In most cases, we chose to keep the second interacting variable constant at the first, second and third quantile values. The exception was for insecticide loadings. Our sites were relatively evenly distributed between those with and without insecticide loadings. We therefore kept this variable constant at zero and at the median of those sites with insecticide loadings.
3. Results
In total, we collected 20 236 insect pollinators representing 294 species, with a greater number of individuals and species collected in 2012 (table 1). Most bee individuals and species were captured in the two southernmost regions (Cambridgeshire and Wiltshire), and a high number of hoverflies were caught in the ‘middle’ regions of Ayrshire, Yorkshire and Staffordshire. The northernmost region, Inverness-shire, had the lowest numbers of individuals and species across all groups.
Table 1.
Captures of pollinator individuals (and species numbers) across the six focal regions and for the three pollinator groups. Individuals identified only to genus were removed from the dataset when calculating species numbers.
| all pollinators |
bumblebees |
solitary bees |
hoverflies |
|||||
|---|---|---|---|---|---|---|---|---|
| 2012 | 2013 | 2012 | 2013 | 2012 | 2013 | 2012 | 2013 | |
| Suffolk/Cambridgeshire | 1830 (142) | 1224 (116) | 474 (10) | 420 (9) | 884 (66) | 331 (45) | 342 (35) | 424 (43) |
| Gloucestershire/Wiltshire | 1568 (126) | 967 (94) | 154 (9) | 195 (11) | 515 (59) | 240 (34) | 845 (39) | 516 (41) |
| Staffordshire | 2402 (85) | 1636 (89) | 147 (10) | 427 (11) | 87 (23) | 133 (26) | 2159 (46) | 1048 (41) |
| Yorkshire | 1144 (50) | 658 (57) | 65 (7) | 78 (9) | 21 (8) | 69 (13) | 1055 (32) | 505 (32) |
| Ayrshire/Renfrewshire | 4961 (78) | 2885 (71) | 198 (12) | 308 (10) | 24 (10) | 31 (6) | 4731 (52) | 2496 (49) |
| Inverness-shire | 664 (60) | 297 (45) | 172 (9) | 114 (9) | 37 (9) | 18 (6) | 440 (37) | 149 (23) |
| total | 12 569 (240) | 7667 (205) | 1210 (17) | 1542 (16) | 1568 (86) | 822 (67) | 9572 (89) | 5138 (84) |
(a) . Confirmatory analysis
The four target drivers as main effects in our GLMMs did not significantly affect the abundance of total pollinators, or of bumblebees or hoverflies when considered separately (electronic supplementary material, table S8), but there was a positive association between managed honeybee density and solitary bee abundance. This relationship was also present for both total pollinator and solitary bee richness and diversity (electronic supplementary material, tables S9 and S10).
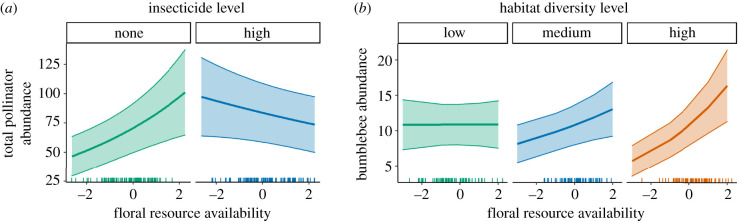
We only found two significant interactions between the focal drivers. First, the effect of floral resource availability on total pollinator abundance depended on insecticide loadings in the surrounding landscape, with the positive influence of floral resource availability most pronounced when loadings were absent, and the association apparently reversed at high loadings, although with high uncertainty (electronic supplementary material, table S8; figure 2a). Second, the association between floral resource availability and bumblebee abundance depended on habitat diversity, suggesting that floral resources were more beneficial to bumblebees in landscapes with diverse habitats (electronic supplementary material, table S8; figure 2b).
Figure 2.
Interaction graphs for (a) the abundance of total insect pollinators plotted against floral resource availability when insecticides are absent, and when insecticides are ‘high’ (median insecticides for sites with non-zero values), and (b) bumblebee abundance for three levels of habitat diversity at the 1st, 2nd and 3rd quartile. Regression lines show the predicted abundance from the GLMM (in counts) when all other predictors are held constant at mean values. Shaded areas are ±1 s.e. See electronic supplementary material, table S8 for full model results.
(b) . Exploratory analysis
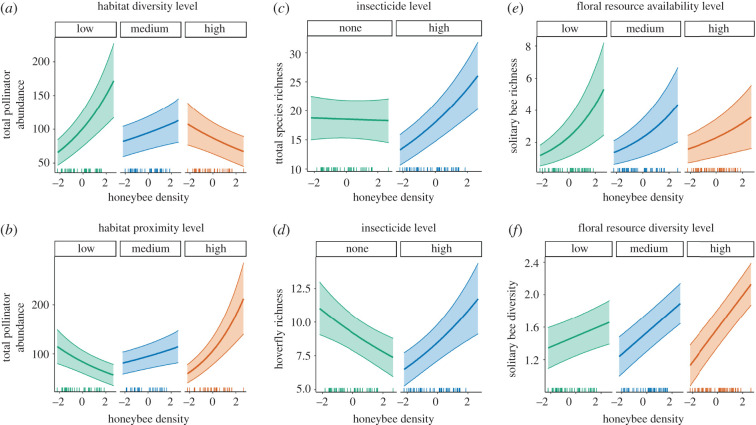
The exploratory models revealed several consistent interactions between focal drivers and additional variables. Honeybee density was found to interact with habitat diversity for the abundance of all pollinators (figure 3a) and solitary bees separately (electronic supplementary material, figure S2 and table S11), as well as the richness of all pollinators, solitary bees and hoverflies (electronic supplementary material, figure S2 and table S12). The positive association between these responses and honeybee density occurred at low to medium habitat diversity, and the opposite occurred in more diverse landscapes (as illustrated by figure 3a). A similar interaction was found between honeybee density and SNH proximity for some of the same responses (total pollinator abundance: figure 3b; electronic supplementary material, table S11; solitary bee abundance: electronic supplementary material, figure S2 and table S11; total richness and hoverfly richness: electronic supplementary material, figure S3 and table S12; hoverfly diversity: electronic supplementary material, figure S4 and table S13). For example, the simple slopes of this model term suggests that more abundant and diverse pollinator communities occur at high honeybee densities and when SNH patches are close together, but there may be a negative relationship with honeybee densities in landscapes with low habitat proximity (figure 3b).
Figure 3.
Interaction graphs of the significant interactive effects of landscape drivers on (a,b) the abundance of total insect pollinators, (c) total species richness, (d) hoverfly richness, (e) solitary bee richness, and (f) solitary bee diversity. In graphs (c) and (d), insecticide loadings are either absent (none) or ‘high’ (median insecticide loadings for sites with non-zero values). In all other graphs, the second predictor level is held constant at the first, second and third quartiles. Regression lines show the predicted abundance, richness or diversity from the GLMM when all other predictors are held constant at mean values. Shaded areas are ±1 s.e. See electronic supplementary material, tables S11–S13 for interaction confidence intervals.
Honeybee density was also found to interact with insecticide loadings for total and hoverfly species richness (figure 3c,d; electronic supplementary material, table S12), and with floral resource availability for solitary bee richness (figure 3e; electronic supplementary material, table S12) and solitary bee diversity models (figure 3f; electronic supplementary material, table S13). At high insecticide loadings, there was a positive association between honeybee density and both total and hoverfly richness, but the opposite pattern for hoverfly richness in the absence of insecticides. In addition, honeybee densities were more strongly positively associated with solitary bee richness at lower levels of floral resource availability, and with solitary bee diversity at higher levels of floral resource diversity.
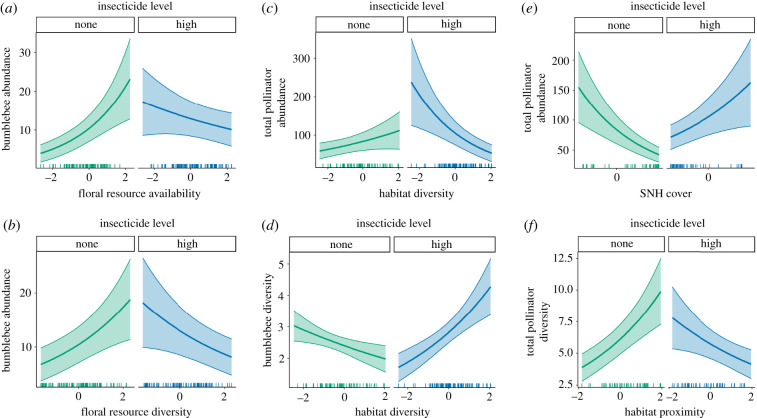
Insecticides also interacted with floral resources, habitat diversity and the amount of SNH in the landscape, and these were mainly found for total pollinator and bumblebee abundance (electronic supplementary material, figure S2 and table S11; figure 4a) and bumblebee diversity (electronic supplementary material, figure S4 and table S13). First, total pollinator and bumblebee abundance were positively associated with floral resource availability when insecticides were absent, and show weak negative relationship when insecticides were present (figure 4a). A similar pattern is also shown for the interaction between insecticide loadings and floral resource diversity (figure 4b).
Figure 4.
Interaction plots of the significant interactive effects of landscape drivers on (a,b) bumblebee abundance of total insect pollinators, (c,e) abundance of total insect pollinators, (d) bumblebee diversity and (f) total species diversity. In all graphs, insecticide loadings are held constant at either absent (none) or ‘high’ (median insecticide loadings for sites with non-zero values). Regression lines show the predicted abundance or diversity from the GLMM when all other predictors are held constant at mean values. Shaded areas are ±1 s.e. See electronic supplementary material, tables S11–S13 for interaction confidence intervals.
There was a contrasting interaction between insecticides and habitat diversity for total abundance and bumblebee diversity. Habitat diversity appears to be negatively associated with total pollinator abundance in the presence of insecticides, but positively associated when they were absent (figure 3c; electronic supplementary material, table S11). Conversely, habitat diversity was positively related to bumblebee diversity in landscapes where insecticides were applied, and negatively related in the absence of insecticides (figure 3d; electronic supplementary material, table S13).
A similar contrasting pattern was found in relation to SNH variables. The abundance of all pollinators and hoverflies were positively related to SNH cover when insecticides were present in the landscape, but negatively related in untreated landscapes (figure 3e; electronic supplementary material figure S2 and table S11). The interaction between insecticides and habitat proximity showed the opposite pattern for total pollinator diversity (figure 3f; electronic supplementary material, table S13). Landscapes with no insecticide applications and SNH patches in close proximity were associated with high species diversity. However, relatively high diversity was also related to high insecticides and low connection between habitat patches.
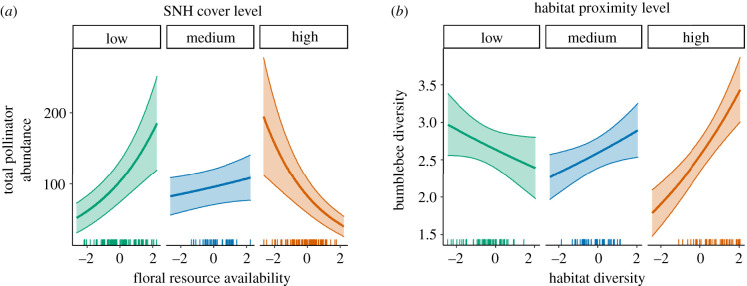
In addition to the interactions detailed above, floral resource availability interacted with SNH cover for a number of abundance and richness responses. These patterns were all similar, indicating that SNH availability promoted total abundance (figure 5a; electronic supplementary material, table S11) and richness (electronic supplementary material, figure S3 and table S12), bumblebee abundance (electronic supplementary material, figure S2 and table S11) and the abundance and richness of hoverflies when floral resources were scarce (electronic supplementary material, figures S2 and S3, and tables S11 and S12). Similarly, floral resource availability was important to these responses when SNH cover was low. Surprisingly, a combination of both high SNH cover and high floral resource availability lead to some of the lowest numbers of predicted species. Finally, in addition to the interactions involving habitat diversity above, bumblebee diversity was predicted to be highest when both habitat diversity and proximity were high (figure 5b; electronic supplementary material, table S13).
Figure 5.
Interaction plots of selected significant interactive effects of landscape drivers on (a) abundance of total insect pollinators, and (b) bumble diversity. In both graphs, the second predictor level is held constant at the first, second and third quartiles. Regression lines show the predicted abundance or diversity from the GLMM when all other predictors are held constant at mean values. Shaded areas are ±1 s.e. See electronic supplementary material, tables S11 and S13 for interaction confidence intervals.
4. Discussion
In this study, we have used the most comprehensive nationwide network of study sites to explore the multiple, interacting drivers of insect pollinator communities in Great Britain. We found that four landscape-scale factors considered important to pollinators could not provide simple explanations for abundance, richness or diversity patterns, except for an unexpected positive relationship between honeybee density and both total pollinators and solitary bees. While some factors combined to explain total and bumblebee abundance and richness in our confirmatory models, we revealed a complex set of responses when incorporating landscape composition and proximity variables in our exploratory models, supporting previous work suggesting that improving pollinator population and community health requires an understanding of local and regional land-use factors [7,10,31]. We should note that our project was not designed to test a priori hypotheses about these interactions, and we recommend that further studies seek to confirm these findings. Caution should also be used when interpreting the results involving two of our four drivers: honeybee density is the one variable that we could not validate with collected data, and the insecticide loadings variable did not consist of as wide a range of values within regions as we would have hoped [35].
(a) . Managed honeybee density
We expected the estimated density of managed honeybees to have a negative relationship with most functional groups of wild pollinators via either competition for food or the transmission of pathogens [7]. As a simple effect, there were only positive associations of honeybee density on total richness and diversity, and on all three solitary bee responses. These may be geographical artefacts, however, because solitary bee abundance and diversity generally decreased with latitude, and our knowledge of honeybee densities was likely to be more accurate in the southern regions. It is also possible that the scale of our study sites were inappropriate for solitary bees, although finer scale measures of floral resource availability and habitat diversity did not improve our models. Nevertheless, the exploratory models also suggest that the positive relationship with solitary bee diversity was strongest in landscapes with high floral resource availability and diversity. We expected honeybees to compete with wild pollinators mainly for floral resources, since honeybee nesting is provided by beekeepers. However, competition is thought to be context dependent and the majority of reported negative impacts of honeybees are from territories where the species is not native [7,26]. Apis mellifera is native to the UK [56], which could contribute to the ability of honeybees and wild pollinators to coexist in suitable locations when resources are abundant [7,42]. The placement of honeybee colonies in resource-rich environments with coincidental healthy wild pollinator communities seems an unlikely explanation, given that very few beekeepers in the UK move their honeybee colonies, instead tending to keep bees close to where they live.
We also found context dependence in the association, with only weak negative relationships with abundance in landscapes with low habitat proximity (dominated by arable, grassland or large single patches of SNH) and high habitat diversity. Sites in our study with this combination of landscape properties are those with many small isolated patches of SNH, which may provide nesting sites for bees, but require them to forage far into the agricultural matrix where floral resources may be scarce. Similar patterns have been reported in Sweden, where competition between honeybees and wild bees and hoverflies increased with crop field size [57], or when the amount of semi-natural grassland in the surrounding landscape was low [58]. By contrast, sites in our study with low habitat diversity but high proximity to SNH, which seem to promote coexistence between managed and wild pollinators, are those with several large patches dominated by a single use such as moorland, rough grassland or even improved grassland, and divided by linear features (e.g. hedgerows, ditches and fence lines). These sites may be ideal situations with abundant resources for both managed honeybee hives and pollinator communities, and we suggest focused research on these large habitat types. Interestingly, there remain significant gaps in our understanding of how honeybees can influence population-level responses of plant communities, such as plant abundance or distribution [7], perhaps masking wider benefits to wild pollinators.
(b) . Insecticide loadings
This variable was less well distributed among the regions because virtually no insecticides were applied in the three northernmost landscapes, but large amounts were applied in the two southernmost regions [35]. Nevertheless, in landscapes where insecticides were not applied, we found positive effects of other drivers such as floral resource availability, resource diversity and habitat proximity. Interestingly, floral resource availability and diversity appeared to have a negative association with pollinators in the presence of insecticides. We interpret this as an increased exposure to insecticide in the presence of abundant and diverse food resources, such as in chemically treated mass-flowering crops [59], or because forage plants in adjacent uncultivated habitats can be sources of insecticide exposure for pollinators via drift or soil pathways [36,60,61]. While field microcosm experiments suggest that diverse forage sources provided as alternatives to mass-flowering crops should offset the negative impacts of insecticides [18], we did not find evidence of this at the landscape scale.
There was also a positive relationship between SNH and total pollinator abundance, and between bumblebee diversity and habitat diversity in the presence of insecticides, in line with previous findings [19,62]. These studies suggest that higher amounts of SNH in the landscape support pollinator communities by providing a refuge from intensive agricultural practices such as chemical applications [19], although pesticide residues have been found in sites with up to 89% semi-natural grassland in the surroundings [63]. It is not clear why SNH cover had a negative impact in the absence of insecticide application, but may be due to our inclusion of conifer plantations in SNH. Untreated sites with high SNH are likely to be those in the northern regions with high covers of conifer and moorland, and these sites typically had low pollinator catches. Exclusion of conifer plantations from our SNH variable would have resulted in these sites scoring very low on the SNH scale, perhaps nullifying the interaction effects shown here. In any case, as our study design was limited in detecting within-region relationships between insecticides and pollinators [35], future work on this scale should base landscape selection on ground-truthed chemical application data. For example, while the chemical application data we used was of a high standard, our reliance on the LCM 2007 to select sites with ‘high’ estimated insecticides prevented this gradient from reflecting the full range of loadings in Britain. Further studies could also focus on the indirect effect of herbicides via floral resources.
(c) . Floral resource availability
As well as a positive relationship with pollinator abundance and diversity in the absence of insecticides, floral resources were important to bumblebee abundance in landscapes with high habitat diversity. This is unsurprising as a diversity of habitats provides a range of nesting resources for bumblebees [26], and a correspondingly high level of continuous food supply is required to support healthy colonies [64]. As central place foragers, bees are more likely to forage efficiently when flowering plants are abundant within a short distance of the nest [65]. A more surprising result is that we did not find the same synergistic interaction for more groups. This is perhaps because our scale of study was not appropriate for solitary bees with shorter foraging ranges, for example.
In contrast with the above pattern, we found that floral resources were important in landscapes with low cover of SNH. This supports theories of floral provision in agricultural landscapes, where small patches of nesting resources, such as SNH, should be interspersed with rich floral resources to benefit pollinators [20,31]. In our landscapes, the combination of low SNH cover and high floral resource availability corresponds to sites with high arable cover including mass-flowering crops or with a high cover of improved grassland with flower-rich field boundaries. Bumblebees may be particularly attracted to mass-flowering areas over SNH [38,65], and other bees and hoverflies may benefit from the connectivity effect provided by floral resources in field margins [20]. Conversely, the apparent negative relationship between pollinators and SNH under high floral resources occurs in sites with large areas of heathland and rough grazing. In such wide, open places, pollinators may concentrate around patches of flowers rather than disperse [66] and are likely only attracted to our pan trap bowls when resources are low. Alternatively, floral resources may be relatively homogeneous at these sites resulting in low abundances of pollinating insects [30].
(d) . Habitat diversity
We expected habitat diversity in general to have positive associations with the diversity of the pollinator community, as a greater array of habitat cover types provide a range of alternative nesting substrates and niches [26]. However, as we have shown, this can be mediated by landscape context such as local honeybee densities and insecticide loadings. Furthermore, in our landscapes, low habitat diversity can correspond to large covers of intensive land uses such as arable or improved grassland, or conversely to a dominance of SNH such as heathland or low-intensity habitat such as a coniferous forest. When other habitat variables were included in models, habitat diversity showed the expected positive relationship with bumblebee diversity when habitat proximity was also high. This supports findings that the provision of habitat patches per se is not always sufficient to promote all aspects of pollinator community abundance and diversity, but that habitat patches should be connected or at least within foraging range of a variety of functional groups [20,31].
5. Conclusion
Our results are difficult to distil into simple, generalizable statements. We found rather few simple effects of the often-cited key drivers of pollinator community composition and distribution across highly variable topographic areas. This suggests that such variables do not generalize well across regions that are characterized by their land use, climate and management. While we have not directly measured pollinator fitness, we infer from these results that improving pollinator community health at the landscape scale is also unlikely to have a quick or general fix. When it comes to conservation or restoration of pollinator communities, our study supports other studies that call for taxon- and context-specific decisions to be made [7,32,67]. Furthermore, unlike other studies that find no effect of SNH on pollinator communities [29,68,69], we find support for studies that include landscape composition and configuration variables as interactive terms in models [38,70]. As the reality of interacting landscape drivers and their effects on pollinator community composition and health is likely to be even more complex than what we have been able to test, we further recommend that better policy and practice decisions are likely to be reached by taking multi-driver, multi-taxa approaches.
Despite the complexity of our results, some key messages are clear. First, pollinator community health, if it is indeed correlated with abundant and diverse pollinator assemblages, is likely to be enhanced by increasing the availability and diversity of floral resources, but the landscape context in terms of insecticide loadings, habitat diversity and habitat proximity should be considered in their selection and placement. Second, in intensively managed landscapes, floral resources can be important when SNH cover or proximity is low, and habitat diversity and configuration can also play important, though complex roles. Third, while other studies have found that beneficial resources can offset negative influences, we did not find consistent evidence of this. Thus, instead of simply relying on boosting pollinator resources to rectify otherwise unhealthy management practices, we recommend that pollinator conservation should be fine-tuned in relation to local land-use context. Finally, we re-iterate that many of our findings were revealed from exploratory data analysis, and we did not have sufficient data for cross-validation. We therefore further recommend future landscape-scale research confirming the importance of habitat context to the drivers of pollinator communities.
Acknowledgements
We would like to extend thanks to our network of farmers, landowners and land managers who provided access to their land to survey pollinators and to our field assistants for conducting the fieldwork: Nicole Dunn, Hayley Wiswell, Ewan Munro, Katy Donald, Jessica Heikkinen, Katherine White, Clare Pemberton, Paul Webb, Mark Tilzey, Sara Iversen, Robin Curtis, Paul Hill, Sam Bacon, Paul Wilson, Bex Cartwright, John Fitzgerald, Patrick Hancock, Mel Stone, Robert Day, James McGill and Tracie Evans. We also thank the taxonomists for identifying insect material: Megan McKercher, George Else, Kirsty Robertson, A Ricarte, L Truslove, M Smith, M Kayser and Z Nedeljković.
Data accessibility
We include data used in the paper as an electronic supplementary material table.
The data are provided in the electronic supplementary material [71].
Authors' contributions
M.A.G.: data curation, formal analysis, methodology, visualization, writing—original draft and writing—review and editing; M.B.: data curation, formal analysis, methodology, validation, writing—original draft and writing—review and editing; K.B.: conceptualization, funding acquisition, methodology, project administration, writing—original draft and writing—review and editing; N.B.: conceptualization, data curation, funding acquisition, methodology, project administration, writing—original draft and writing—review and editing; G.B.: conceptualization, data curation, funding acquisition, methodology, project administration, supervision, writing—original draft and writing—review and editing; A.C.: data curation, formal analysis, methodology, writing—original draft and writing—review and editing; N.D.: data curation, methodology, writing—original draft and writing—review and editing; R.E.: data curation, methodology, writing—original draft and writing—review and editing; J.M.: conceptualization, funding acquisition, methodology, project administration, supervision, writing—original draft and writing—review and editing; R.D.M.: conceptualization, data curation, formal analysis, funding acquisition, methodology, writing—original draft and writing—review and editing; E.M.: methodology, writing—original draft and writing—review and editing; M.M.: formal analysis, methodology, writing—original draft and writing—review and editing; S.P.: conceptualization, funding acquisition, methodology, project administration, supervision, writing—original draft and writing—review and editing; S.R.: data curation, funding acquisition, methodology, supervision, writing—original draft and writing—review and editing; C.R.: data curation, formal analysis, methodology, writing—original draft and writing—review and editing; D.S.: methodology, writing—original draft and writing—review and editing; S.S.: conceptualization, formal analysis, funding acquisition, methodology, writing—original draft and writing—review and editing; C.W.: data curation, methodology, writing—original draft and writing—review and editing; W.E.K.: conceptualization, formal analysis, funding acquisition, methodology, project administration, supervision, writing—original draft and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This research was supported by the UK Insect Pollinator Initiative project ‘AgriLand: Linking agriculture and land-use change to pollinator populations', funded under the Living with Environmental Change programme, a collaboration between Biotechnology and Biological Sciences Research Council (BBSRC), the Wellcome Trust, Scottish Government, Department of Environment, Food and Rural Affairs (DEFRA) and Natural Environment Research Council (NERC): grant no. BB/H014934/1.
References
- 1.Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJB, Collen B. 2014. Defaunation in the Anthropocene. Science 345, 401-406. ( 10.1126/science.1251817) [DOI] [PubMed] [Google Scholar]
- 2.Zattara EE, Aizen MA. 2021. Worldwide occurrence records suggest a global decline in bee species richness. One Earth 4, 114-123. ( 10.1016/j.oneear.2020.12.005) [DOI] [Google Scholar]
- 3.Betts MG, Hadley AS, Kormann U. 2019. The landscape ecology of pollination. Landsc. Ecol. 34, 961-966. ( 10.1007/s10980-019-00845-4) [DOI] [Google Scholar]
- 4.Goulson D, Nicholls E, Botías C, Rotheray E. 2015. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1255957. ( 10.1126/science.1255957) [DOI] [PubMed] [Google Scholar]
- 5.Crall JD, et al. 2018. Neonicotinoid exposure disrupts bumblebee nest behavior, social networks, and thermoregulation. Science 362, 683. ( 10.1126/science.aat1598) [DOI] [PubMed] [Google Scholar]
- 6.Arce AN, Rodrigues AR, Yu JJ, Colgan TJ, Wurm Y, Gill RJ. 2018. Foraging bumblebees acquire a preference for neonicotinoid-treated food with prolonged exposure. Proc. R. Soc. B 285, 20180655. ( 10.1098/rspb.2018.0655) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallinger RE, Gaines-Day HR, Gratton C. 2017. Do managed bees have negative effects on wild bees?: a systematic review of the literature. PLoS ONE 12, e0189268. ( 10.1371/journal.pone.0189268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paini DR. 2004. Impact of the introduced honey bee (Apis mellifera) (Hymenoptera: Apidae) on native bees: a review. Austral Ecol. 29, 399-407. ( 10.1111/j.1442-9993.2004.01376.x) [DOI] [Google Scholar]
- 9.Woodcock BA, Savage J, Bullock JM, Nowakowski M, Orr R, Tallowin JRB, Pywell RF. 2014. Enhancing floral resources for pollinators in productive agricultural grasslands. Biol. Conserv. 171, 44-51. ( 10.1016/j.biocon.2014.01.023) [DOI] [Google Scholar]
- 10.Alaux C, et al. 2017. A 'landscape physiology' approach for assessing bee health highlights the benefits of floral landscape enrichment and semi-natural habitats. Sci. Rep. 7, 10. ( 10.1038/srep40568) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanbergen AJ, et al. 2013. Threats to an ecosystem service: pressures on pollinators. Front. Ecol. Environ. 11, 251-259. ( 10.1890/120126) [DOI] [Google Scholar]
- 12.Whitehorn PR, O'Connor S, Wackers FL, Goulson D. 2012. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336, 351-352. ( 10.1126/science.1215025) [DOI] [PubMed] [Google Scholar]
- 13.Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. 2010. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345-353. ( 10.1016/j.tree.2010.01.007) [DOI] [PubMed] [Google Scholar]
- 14.Elbgami T, Kunin WE, Hughes WOH, Biesmeijer JC. 2014. The effect of proximity to a honeybee apiary on bumblebee colony fitness, development, and performance. Apidologie 45, 504-513. ( 10.1007/s13592-013-0265-y) [DOI] [Google Scholar]
- 15.McNeil DJ, McCormick E, Heimann AC, Kammerer M, Douglas MR, Goslee SC, Grozinger CM, Hines HM. 2020. Bumble bees in landscapes with abundant floral resources have lower pathogen loads. Sci. Rep. 10, 12. ( 10.1038/s41598-020-78119-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolezal AG, Toth AL. 2018. Feedbacks between nutrition and disease in honey bee health. Curr. Opin. Insect Sci. 26, 114-119. ( 10.1016/j.cois.2018.02.006) [DOI] [PubMed] [Google Scholar]
- 17.McArt SH, Koch H, Irwin RE, Adler LS. 2014. Arranging the bouquet of disease: floral traits and the transmission of plant and animal pathogens. Ecol. Lett. 17, 624-636. ( 10.1111/ele.12257) [DOI] [PubMed] [Google Scholar]
- 18.Klaus F, Tscharntke T, Bischoff G, Grass I. 2021. Floral resource diversification promotes solitary bee reproduction and may offset insecticide effects: evidence from a semi-field experiment. Ecol. Lett. 24, 668-675. ( 10.1111/ele.13683) [DOI] [PubMed] [Google Scholar]
- 19.Park MG, Blitzer EJ, Gibbs J, Losey JE, Danforth BN. 2015. Negative effects of pesticides on wild bee communities can be buffered by landscape context. Proc. R. Soc. B 282, 20150299. ( 10.1098/rspb.2015.0299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hadley AS, Betts MG. 2012. The effects of landscape fragmentation on pollination dynamics: absence of evidence not evidence of absence. Biol. Rev. 87, 526-544. ( 10.1111/j.1469-185X.2011.00205.x) [DOI] [PubMed] [Google Scholar]
- 21.Martin EA, et al. 2019. The interplay of landscape composition and configuration: new pathways to manage functional biodiversity and agroecosystem services across Europe. Ecol. Lett. 22, 1083-1094. ( 10.1111/ele.13265) [DOI] [PubMed] [Google Scholar]
- 22.Kennedy CM, et al. 2013. A global quantitative synthesis of local and landscape effects on wild bee pollinators in agroecosystems. Ecol. Lett. 16, 584-599. ( 10.1111/ele.12082) [DOI] [PubMed] [Google Scholar]
- 23.Eeraerts M, Smagghe G, Meeus I. 2019. Pollinator diversity, floral resources and semi-natural habitat, instead of honey bees and intensive agriculture, enhance pollination service to sweet cherry. Agric. Ecosyst. Environ. 284, 7. ( 10.1016/j.agee.2019.106586) [DOI] [Google Scholar]
- 24.Proesmans W, Smagghe G, Meeus I, Bonte D, Verheyen K. 2019. The effect of mass-flowering orchards and semi-natural habitat on bumblebee colony performance. Landsc. Ecol. 34, 1033-1044. ( 10.1007/s10980-019-00836-5) [DOI] [Google Scholar]
- 25.Jones JA, Hutchinson R, Moldenke A, Pfeiffer V, Helderop E, Thomas E, Griffin J, Reinholtz A. 2019. Landscape patterns and diversity of meadow plants and flower-visitors in a mountain landscape. Landsc. Ecol. 34, 997-1014. ( 10.1007/s10980-018-0740-y) [DOI] [Google Scholar]
- 26.Ropars L, Affre L, Schurr L, Flacher F, Genoud D, Mutillod C, Geslin B. 2020. Land cover composition, local plant community composition and honeybee colony density affect wild bee species assemblages in a Mediterranean biodiversity hot-spot. Acta Oecol. 104, 103546. ( 10.1016/j.actao.2020.103546) [DOI] [Google Scholar]
- 27.Ekroos J, Jakobsson A, Wideen J, Herbertsson L, Rundlöf M, Smith HG. 2015. Effects of landscape composition and configuration on pollination in a native herb: a field experiment. Oecologia 179, 509-518. ( 10.1007/s00442-015-3370-y) [DOI] [PubMed] [Google Scholar]
- 28.Gervais A, Courtois È, Fournier V, Bélisle M. 2020. Landscape composition and local floral resources influence foraging behavior but not the size of Bombus impatiens Cresson (Hymenoptera: Apidae) workers. PLoS ONE 15, e0234498. ( 10.1371/journal.pone.0234498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hass AL, et al. 2018. Landscape configurational heterogeneity by small-scale agriculture, not crop diversity, maintains pollinators and plant reproduction in Western Europe. Proc. R. Soc. B 285, 20172242. ( 10.1098/rspb.2017.2242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breitbach N, Tillmann S, Schleuning M, Gruenewald C, Laube I, Steffan-Dewenter I, Boehning-Gaese K. 2012. Influence of habitat complexity and landscape configuration on pollination and seed-dispersal interactions of wild cherry trees. Oecologia 168, 425-437. ( 10.1007/s00442-011-2090-1) [DOI] [PubMed] [Google Scholar]
- 31.Brosi BJ, Armsworth PR, Daily GC. 2008. Optimal design of agricultural landscapes for pollination services. Conserv. Lett. 1, 27-36. ( 10.1111/j.1755-263X.2008.00004.x) [DOI] [Google Scholar]
- 32.Denning KR, Foster BL. 2018. Taxon-specific associations of tallgrass prairie flower visitors with site-scale forb communities and landscape composition and configuration. Biol. Conserv. 227, 74-81. ( 10.1016/j.biocon.2018.08.023) [DOI] [Google Scholar]
- 33.Holzschuh A, Steffan-Dewenter I, Tscharntke T. 2010. How do landscape composition and configuration, organic farming and fallow strips affect the diversity of bees, wasps and their parasitoids? J. Anim. Ecol. 79, 491-500. ( 10.1111/j.1365-2656.2009.01642.x) [DOI] [PubMed] [Google Scholar]
- 34.Cole LJ, et al. 2020. A critical analysis of the potential for EU Common Agricultural Policy measures to support wild pollinators on farmland. J. Appl. Ecol. 57, 681-694. ( 10.1111/1365-2664.13572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gillespie MAK, et al. 2017. A method for the objective selection of landscape-scale study regions and sites at the national level. Meth. Ecol. Evol. 8, 1468-1476. ( 10.1111/2041-210X.12779)) [DOI] [Google Scholar]
- 36.Botias C, Sanchez-Bayo F. 2018. The role of pesticides in pollinator declines. Ecosistemas 27, 34-41. ( 10.7818/ecos.1314) [DOI] [Google Scholar]
- 37.Holzschuh A, Steffan-Dewenter I, Kleijn D, Tscharntke T. 2007. Diversity of flower-visiting bees in cereal fields: effects of farming system, landscape composition and regional context. J. Appl. Ecol. 44, 41-49. ( 10.1111/j.1365-2664.2006.01259.x) [DOI] [Google Scholar]
- 38.Beyer N, Gabriel D, Kirsch F, Schulz-Kesting K, Dauber J, Westphal C. 2020. Functional groups of wild bees respond differently to faba bean Vicia faba L. cultivation at landscape scale. J. Appl. Ecol. 57, 2499-2508. ( 10.1111/1365-2664.13745) [DOI] [Google Scholar]
- 39.Carrié, R., Andrieu E, Cunningham SA, Lentini PE, Loreau M, Ouin A. 2017. Relationships among ecological traits of wild bee communities along gradients of habitat amount and fragmentation. Ecography 40, 85-97. ( 10.1111/ecog.02632) [DOI] [Google Scholar]
- 40.López-Uribe MM, Ricigliano VA, Simone-Finstrom M. 2020. Defining pollinator health: a holistic approach based on ecological, genetic, and physiological factors. Annu. Rev. Anim. Biosci. 8, 269-294. ( 10.1146/annurev-animal-020518-115045) [DOI] [PubMed] [Google Scholar]
- 41.Westphal C, Steffan-Dewenter I, Tscharntke T. 2009. Mass flowering oilseed rape improves early colony growth but not sexual reproduction of bumblebees. J. Appl. Ecol. 46, 187-193. ( 10.1111/j.1365-2664.2008.01580.x) [DOI] [Google Scholar]
- 42.Evans E, Smart M, Cariveau D, Spivak M. 2018. Wild, native bees and managed honey bees benefit from similar agricultural land uses. Agricult. Ecosyst. Environ. 268, 162-170. ( 10.1016/j.agee.2018.09.014) [DOI] [Google Scholar]
- 43.Morton D, Rowland C, Wood C, Meek L, Marston C, Smith G, Wadsworth R, Simpson I. 2011. Final report for LCM2007: the new UK land cover map. Countryside Survey Technical Report No 11/07. Lancaster, UK: Centre for Ecology and Hydrology. [Google Scholar]
- 44.Bunce RGH, Barr CJ, Clarke RT, Howard DC, Lane AM. J. 1996. ITE Merlewood Land classification of Great Britain. J. Biogeogr. 23, 625-634. ( 10.1111/j.1365-2699.1996.tb00023.x) [DOI] [Google Scholar]
- 45.Carey PD, et al. 2008. Countryside survey: UK results from 2007. Lancaster, UK: Centre for Ecology & Hydrology. [Google Scholar]
- 46.Baude M, Kunin WE, Boatman ND, Conyers S, Davies N, Gillespie MAK, Morton RD, Smart SM, Memmott J. 2016. Historical nectar assessment reveals the fall and rise of floral resources in Britain. Nature 530, 85. ( 10.1038/nature16532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westphal C, et al. 2008. Measuring bee diversity in different European habitats and biogeographical regions. Ecol. Monogr. 78, 653-671. ( 10.1890/07-1292.1) [DOI] [Google Scholar]
- 48.Westphal C, Steffan-Dewenter I, Tscharntke T. 2006. Bumblebees experience landscapes at different spatial scales: possible implications for coexistence. Oecologia 149, 289-300. ( 10.1007/s00442-006-0448-6) [DOI] [PubMed] [Google Scholar]
- 49.Stubbs A, Falk S. 2002. British hoverflies: an illustrated identification guide, 2nd edn. Reading, UK: British Entomological and Natural History Society. [Google Scholar]
- 50.Else G, Edwards M. 2018. Handbook of the bees of the British Isles. London, UK: The Ray; Society. [Google Scholar]
- 51.Team QD. 2019. QGIS geographic information system. Open Source Geospatial Foundation Project. 3.10.3 ed.
- 52.Team RC. 2021. R: a language and environment for statistical computing. 4.1.0 ed. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 53.Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Maechler M, Bolker B.. 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378-400. ( 10.32614/RJ-2017-066) [DOI] [Google Scholar]
- 54.Lüdecke D, Ben Shachar M, Patil I, Waggoner P, Makowski D. 2021. performance: An R package for assessment, comparison and testing of statistical models. J. Open Source Software 6, 3139. ( 10.21105/joss.03139) [DOI]
- 55.Hartig F. 2020. DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.3.3.0. CRAN.
- 56.Carreck NL. 2008. Are honey bees (Apis mellifera L.) native to the British Isles? J. Apicult. Res. 47, 318-322. ( 10.1080/00218839.2008.11101482) [DOI] [Google Scholar]
- 57.Lindstrom SAM, Herbertsson L, Rundlof M, Bommarco R, Smith HG. 2016. Experimental evidence that honeybees depress wild insect densities in a flowering crop. Proc. R. Soc. B 283, 20161641. ( 10.1098/rspb.2016.1641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herbertsson L, Lindstrom SAM, Rundlof M, Bornmarco R, Smith HG. 2016. Competition between managed honeybees and wild bumblebees depends on landscape context. Basic Appl. Ecol. 17, 609-616. ( 10.1016/j.baae.2016.05.001) [DOI] [Google Scholar]
- 59.Centrella M, Russo L, Ramirez NM, Eitzer B, van Dyke M, Danforth B, Poveda K.. 2020. Diet diversity and pesticide risk mediate the negative effects of land use change on solitary bee offspring production. J. Appl. Ecol. 57, 1031-1042. ( 10.1111/1365-2664.13600) [DOI] [Google Scholar]
- 60.Rundlof M, et al. 2015. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 521, 77-U162. ( 10.1038/nature14420) [DOI] [PubMed] [Google Scholar]
- 61.Krupke CH, Hunt GJ, Eitzer BD, Andino G, Given K. 2012. Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS ONE 7, e29268. ( 10.1371/journal.pone.0029268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nicholson CC, Koh I, Richardson LL, Beauchemin A, Ricketts TH. 2017. Farm and landscape factors interact to affect the supply of pollination services. Agricult. Ecosyst. Environ. 250, 113-122. ( 10.1016/j.agee.2017.08.030) [DOI] [Google Scholar]
- 63.Hladik ML, Vandever M, Smalling KL. 2016. Exposure of native bees foraging in an agricultural landscape to current-use pesticides. Sci. Total Environ. 542, 469-477. ( 10.1016/j.scitotenv.2015.10.077) [DOI] [PubMed] [Google Scholar]
- 64.Jachula J, Denisow B, Wrzesien M. 2021. Habitat heterogeneity helps to mitigate pollinator nectar sugar deficit and discontinuity in an agricultural landscape. Sci. Total Environ. 782, 14. ( 10.1016/j.scitotenv.2021.146909) [DOI] [PubMed] [Google Scholar]
- 65.Walther-Hellwig K, Frankl R. 2000. Foraging habitats and foraging distances of bumblebees, Bombus spp. (Hym., Apidae), in an agricultural landscape. J. Appl. Entomol.—Z. Angewandte Entomol. 124, 299-306. ( 10.1046/j.1439-0418.2000.00484.x) [DOI] [Google Scholar]
- 66.Krimmer E, Martin EA, Krauss J, Holzschuh A, Steffan-Dewenter I. 2019. Size, age and surrounding semi-natural habitats modulate the effectiveness of flower-rich agri-environment schemes to promote pollinator visitation in crop fields. Agric. Ecosyst. Environ. 284, 8. ( 10.1016/j.agee.2019.106590) [DOI] [Google Scholar]
- 67.Bartual AM, et al. 2019. The potential of different semi-natural habitats to sustain pollinators and natural enemies in European agricultural landscapes. Agric. Ecosyst. Environ. 279, 43-52. ( 10.1016/j.agee.2019.04.009) [DOI] [Google Scholar]
- 68.Ekroos J, Kleijn D, Batáry P, Albrecht M, Báldi A, Blüthgen N, Knop E, Kovács-Hostyánszki A, Smith HG. 2020. High land-use intensity in grasslands constrains wild bee species richness in Europe. Biol. Conserv. 241, 108255. ( 10.1016/j.biocon.2019.108255) [DOI] [Google Scholar]
- 69.Struelens Q, Mina D, Dangles O. 2021. Combined effects of landscape composition and pesticide use on herbivore and pollinator functions in smallholder farms. CABI Agricult. Biosci. 2, 7. ( 10.1186/s43170-021-00027-w) [DOI] [Google Scholar]
- 70.Lane IG, Herron-Sweet CR, Portman ZM, Cariveau DP. 2020. Floral resource diversity drives bee community diversity in prairie restorations along an agricultural landscape gradient. J. Appl. Ecol. 57, 2010-2018. ( 10.1111/1365-2664.13694) [DOI] [Google Scholar]
- 71.Gillespie MAK, et al. 2022. Landscape-scale drivers of pollinator communities may depend on land-use configuration. FigShare. ( 10.6084/m9.figshare.c.5923117) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Gillespie MAK, et al. 2022. Landscape-scale drivers of pollinator communities may depend on land-use configuration. FigShare. ( 10.6084/m9.figshare.c.5923117) [DOI] [PMC free article] [PubMed]
Data Availability Statement
We include data used in the paper as an electronic supplementary material table.
The data are provided in the electronic supplementary material [71].