Abstract
Objectives
This mixed-method study aimed to understand the effectiveness of linkage to biopsy and treatment in women with a high-risk mammography result (Breast Imaging Reporting and Data System, BI-RADS 4 and 5) in the national telemammography programme and to explore women’s experiences during this process.
Setting
Quantitative component: we collected and linked health data from the telemammography reading centre, the national public health insurance, the national centre for disease control and the national referral cancer centre. Qualitative component: we interviewed participants from different regions of the country representing diverse social and geographical backgrounds.
Participants
Quantitative: women who underwent telemammography between July 2017 and September 2018 and had high-risk results (BI-RADS 4–5) were collected. Qualitative: women with a high-risk telemammography result, healthcare providers and administrators.
Outcomes measures
Quantitative: we determined biopsy and treatment linkage rates and delays. Qualitative: we explored barriers and facilitators for obtaining a biopsy and initiating treatment.
Results
Of 126 women with high-risk results, 48.4% had documentation of biopsy and 37.5% experienced a delay of >45 days to biopsy. Of 51 women diagnosed with breast cancer, 86.4% had evidence of treatment initiation, but 69.2% initiated treatment >45 days after biopsy. Travelling to major cities for care, administrative factors and breast cancer misconceptions, among other factors, impeded timely, continuous care for breast cancer. A multidisciplinary and culturally tailored patient education facilitated understanding of the disease and prompt decision making about subsequent medical care.
Conclusions
Strengthened breast cancer care capacity outside the capital city, standardised referral pathways, ensured financial support for travel expenses, and enhanced patient education are required to secure linkage to the breast cancer care continuum. Robust information systems are needed to track patients and to evaluate the programme’s performance.
Keywords: breast tumours, breast imaging, gynaecological oncology
Strengths and limitations of this study.
This is among the very limited studies evaluating linkages to care after high-risk mammography results in a middle-income country.
This study collected users’ perspectives from different geographical settings of Peru.
This study is an exhaustive evaluation that used both quantitative and qualitive research methods.
The lack of integration of the health information systems in the Ministry of Health may have caused underestimation of the percentage of women who obtained care.
The follow-up time for women who obtained a high-risk telemammography result was heterogeneous.
Introduction
Breast cancer is the most commonly diagnosed cancer and the leading cause of cancer-related deaths among the female population worldwide.1 To date, mammography screening is the only early detection method that has been proven to reduce breast cancer mortality.2 Pooled results from randomised trials in the USA, Canada and Europe, show a 19% reduction in breast cancer mortality associated with mammography screening.3 Currently, the World Health Organization (WHO) supports organised, population-based mammography screening as an essential tool for the control of breast cancer.2
For mammography screening to reduce breast cancer mortality, timely diagnosis and effective treatment must follow.4 Cancer diagnosis and treatment are complex and requires coordination across multiple medical specialists, as well as adequate healthcare facilities and equipment.5 Thus, patients in low-income and middle-income countries (LMICs), where less than 5% of the necessary resources for cancer diagnosis and treatment are available,6 may face great difficulties securing care. Suboptimal diagnosis and treatment rates and delays could undermine the effectiveness of a screening programme in reducing breast cancer mortality.
In 2017, the Peru Ministry of Health (MOH) launched a free telemedicine-based mammography programme targeting women living outside of the major metropolitan area of Lima and receiving government-subsidised health insurance. The programme aimed to circumvent the lack of radiologists in the provinces by digitally transferring mammography images to Lima, the nation’s capital, for review. We examined rates of and time to biopsy and breast cancer treatment initiation after a high-risk telemammography result among women participating in this national programme and sought to understand women’s experiences seeking diagnostic and treatment services.
Methods
Study setting
In Peru, individuals living in poverty receive government-subsidised insurance, known as the Comprehensive Health Insurance (SIS). In 2012, breast cancer care (diagnosis, treatment and palliative services) was added to the SIS health package7; however, most services remain centralised in Lima, where they are provided mainly by the National Institute of Neoplastic Diseases (INEN).8 Outside Lima, two regional cancer institutes and some general hospitals offer cancer services on a varied and limited basis. When services are not available at one of the general hospitals, patients are referred to the regional cancer institute or to INEN.
Peru’s MOH telemammography programme is the primary mammography provider among SIS recipients and as of September 2018, 14 hospitals in 11 regions participated in the programme. At these hospitals, the cancer programme staff conduct mammography testing, result reporting, and referrals. Asymptomatic women aged 50–69 years old are invited for screening through routine clinical visits or community outreach activities. Symptomatic women may be referred for a diagnostic mammogram. Digital images are transferred securely via the internet to a reading centre in Lima, where trained radiologists provide a result within a few days. Following international guidelines, individuals with a Breast Imaging Reporting and Data System (BI-RADS) result of 4 or 5 are supposed to be referred for biopsy.9 If cancer is diagnosed, treatment is planned, including referrals, as needed.
Study design
We conducted a mixed-methods study with a concurrent design.10 We described the frequency and time required for biopsy and treatment initiation, and qualitatively explored the factors impeding and facilitating care.
Study population
Quantitative component
We conducted a retrospective review of data collected from all women aged >18 years with SIS insurance, who had a telemammography through the MOH programme between July 2017 and September 2018 and obtained a high-risk result.
Qualitative component
We used purposeful sampling to identify and interview 32 key stakeholders comprised of women with a high-risk telemammography result, healthcare providers (local cancer programme nurses and midwives, and physicians from the hospital oncology services), local programme coordinators, and policymakers of the MOH. We included women known to have experienced barriers to obtaining care and women who obtained care more easily; all of them had undergone a mammography through the telemammography programme. Potential women participants were first identified by the cancer programme staff and provided a brief explanation of the research. After a first verbal acceptance, the research team visited them at their homes to formally invite them to participate. To ensure we had perspectives from different parts of the country, informants from diverse geographical areas of the country were selected.
Key procedures
Quantitative component
Data sources: Telemammography results and basic demographic information were obtained from the telemammography reading centre in Lima (Villa El Salvador Hospital). Because there was no national database for tracking patients along the breast cancer continuum of care, person-data on biopsy and treatment were extracted from three independent data sources using the national identification number of each subject: SIS electronic databases, the National Cancer Surveillance registry of Peru’s Centers for Disease Control and Prevention (CDC), and INEN medical electronic and paper records. Access to these data sources was requested to the corresponding institutions. These data sources include diagnostic procedures, biopsy results and treatments. Data from SIS, CDC and INEN were available through 31 December 2018; 1 November 2019 and 15 January 2020; respectively. Thus, each woman was followed for a minimum of 90 days and a maximum of 470 days following mammography (figure 1).
Figure 1.
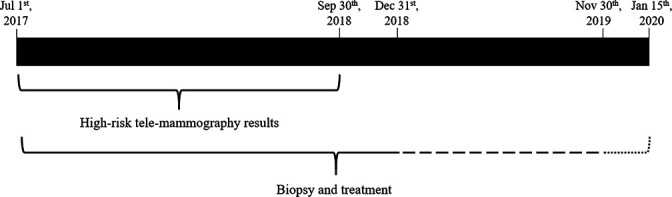
Availability of biopsy and treatment information from the three study data sources. Solid lines: Data available for SIS, CDC and INEN. Dashed line: data available for CDC and INEN. Dotted line: data available for INEN. SIS, Comprehensive Health Insurance (the government-subsidised insurance); CDC, Peru’s Centers for Disease Control and Prevention; INEN, National Institute of Neoplastic Diseases.
Outcomes: A high-risk telemammography result was defined as a BI-RADS result of 4 or 5.11 The biopsy rate was defined as the proportion of women with a high-risk telemammography result who had evidence of a breast biopsy documented in the available data sources. The treatment initiation rate was defined as the proportion of women with confirmed breast cancer who had evidence of initiating chemotherapy, surgery, radiation or hormonal therapy.
We calculated the time to biopsy and treatment initiation among those who secured these services. Adapting definitions from a consensus statement,12 we defined the diagnosis interval as the time from telemammography result to biopsy result, the treatment interval as the time from biopsy result to treatment initiation, and the health system interval as the time from telemammography result to treatment initiation. For each interval, we calculated the proportion of women that experienced delays. Because delays >90 days from breast cancer symptom discovery to treatment initiation correlate with advanced stage at diagnosis and worse survival,13 14 we defined a health system delay as a health system interval >90 days, and diagnosis and treatment delays as >45 days. We calculated the frequency of women with suboptimal care, defined as the presence of biopsy or treatment delay or the absence of biopsy or treatment despite the indication.
Qualitative component
Data collection: We conducted individual, in-depth interviews using semistructured interview guides to explore the barriers and facilitators to biopsy or treatment initiation. For women with a high-risk telemammography, topics included the experience of pursuing and following referral for care; strategies for overcoming difficulties in seeking care and recommendations for improvement. Interviews with healthcare providers and administrators (programme coordinators and policymakers) explored how breast cancer care is administered and delivered; programme strengths and weaknesses; and recommendations for improvement. The first author (RAE) conducted face-to-face interviews in Spanish (the local language and RAE’s native language). Interviews lasted approximately 50 min and were audiorecorded and transcribed verbatim.
Data analysis
Quantitative component
Data were cleaned thoroughly by RAE using Stata V.14 and supervised by MFF. We reported descriptive statistics and analysed data using Stata V.14. We examined time intervals to biopsy and treatment both as continuous variables and also as binary variables to identify the proportion of women experiencing delays in care.
Qualitative component
We conducted content analysis on the transcripts uploaded to Dedoose.15 A subset of interviews was open coded using short descriptive labels from which the first codebook draft was constructed. The draft codebook was piloted in a separate subset of interviews; codes were added, eliminated or merged to create the final version used to code the dataset. The coded data were inductively analysed to identify key themes related to the barriers and facilitators for obtaining a biopsy or initiating treatment. Using an iterative approach, the draft themes were revised, resulting in a set of final themes. Illustrative quotes for each theme were extracted and translated into English.
Results
Quantitative findings
Biopsy and treatment initiation rates and delays
From 1 July 2017 to 30 September 2018, 6899 telemammography tests were conducted through the MOH services. Of these, 147 (2.1%) women had a high-risk mammography result (72.8% with BI-RADS 4 and 27.2% with BI-RADS 5). After the exclusion of 21 individuals with data discrepancies or who did not meet the inclusion criteria (figure 2), 126 women were included for analysis (71.4% with BI-RADS 4 and 28.6% with BI-RADS 5). Their mean age was 53.3 years (SD: 11.3).
Figure 2.
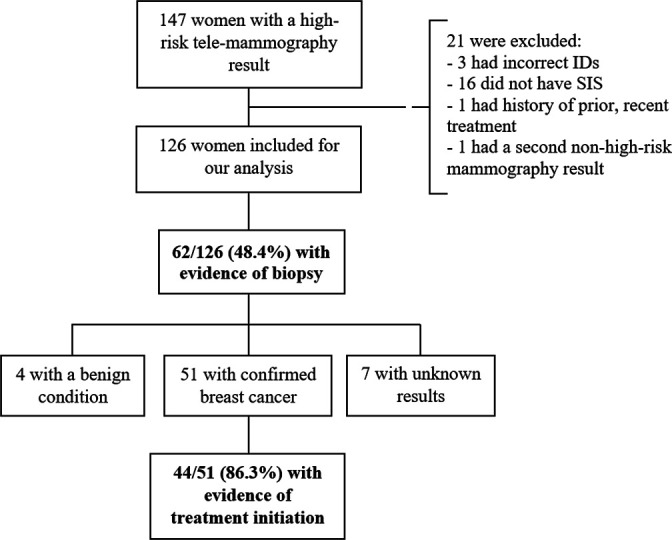
Flowchart of biopsy and treatment initiation rates among women with a high -risk telemammography result. ID, National Identification Number; SIS, Comprehensive Health Insurance (the government-subsidised insurance).
We found evidence of biopsy in 62/126 (48.4%) women (figure 2). Of these, biopsy result dates were available in 48, of whom 18 (37.5%) experienced a diagnosis delay (median diagnosis interval=39.5 days) (table 1). Among the 62 women with evidence of biopsy, 49 had data on where the procedure was performed, and in 32 (65.3%) it took place in a different region from where they lived.
Table 1.
Time intervals and delays between mammography, biopsy and treatment initiation among 126 women with a high-risk telemammography result who obtained this care
| Time interval | Median days (IQR; range) | Delay, n (%) |
| From telemammography result to biopsy result (n=48, N=62) | 39.5 (25.5–65; 7–263) | 18 (37.5) |
| From biopsy result to treatment initiation (n=39, N=44) | 65.3 (32–118; 8–416) | 27 (69.2) |
| From telemammography result to treatment initiation (n=44, N=44) | 109.5 (69.5–168; 10–442) | 29 (65.9) |
n=number of women with dates available; N=total number of women who completed the corresponding step.
Of the 62 women who had a biopsy (67.7% with BI-RADS 4 and 32.3% with BI-RADS 5), 51 were diagnosed with breast cancer, 4 had a benign condition and 7 did not have a result in their medical record. Of the 35 women who had BI-RADS 4 mammography results and had a known biopsy result, 85% were found to have breast cancer, while among the 20 women with BI-RADS 5 mammography results and a known biopsy result, 100% had breast cancer. Of those diagnosed with breast cancer, we found evidence that 44/51 (86.3%) initiated treatment. Of these, the dates of the biopsy results and treatment initiation were available in 39, of whom 27 (69.2%) experienced a treatment delay (median treatment interval=65.3 days) (table 1). Among the 44 women with evidence of treatment, data about the treatment hospital were missing for one individual; of the 43 remaining, 35 (81.4%) initiated treatment in a different region than where she lived. Health system delays were observed in 29/44 (65.9%) women (median health system interval=109.5 days) (table 1). Excluding 14 individuals with missing dates, 104/112 (92.4%) women received suboptimal care.
Qualitative findings
Study population
We interviewed 32 people: 13 women with a high-risk telemammography result, 13 healthcare providers, 3 programme coordinators and 3 policymakers. See table 2 for details on the geographic areas were informants belonged to.
Table 2.
Characteristics of the 32 in-depth interview participants
| Provenance | Patients | Providers | Programme coordinators | Policymakers |
| Lima (capital) | – | 4 | – | 3 |
| Coast (North) | 3 | 1 | 1 | – |
| Highlands (Centre) | 3 | 2 | 1 | – |
| Highlands (South) | 4 | 4 | – | – |
| Rainforest (East) | 3 | 2 | 1 | – |
Findings
Undergoing biopsy and initiating breast cancer treatment was impeded by several factors clustering around three primary themes: (A) the toll of getting care in major cities following referrals, (B) patients’ misconceptions and access to information and (C) administrative and operational barriers. Some of these factors primarily affected the diagnosis interval, others influenced mainly the treatment interval, while others impacted both intervals. This relationship is illustrated in figure 3. Although scarce, a few facilitators were identified and are detailed in a fourth theme, (D) facilitators.
Figure 3.
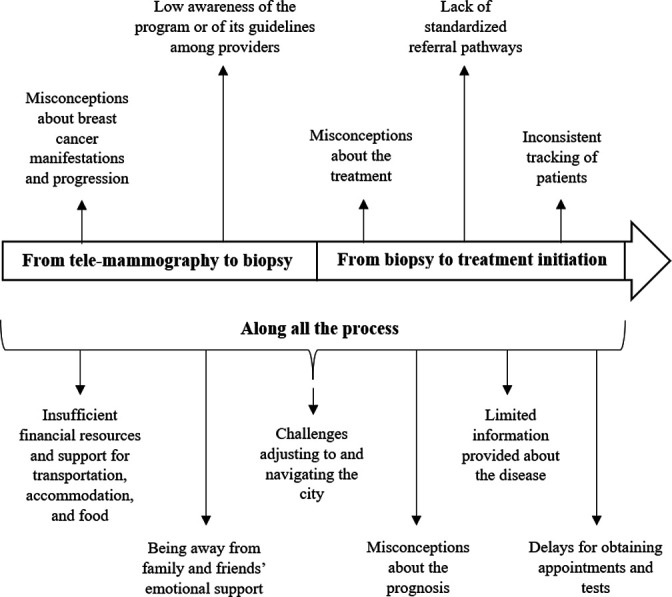
Barriers for obtaining a biopsy and initiating treatment and related affected intervals after obtaining a high-risk telemammography result.
Theme A: the toll of getting care in major cities following referrals
Insufficient financial resources and support for transportation, accommodation and food
Referral to a hospital in a major city at some point during follow-up was inevitable for almost all patients living outside of Lima. Informants agreed that most women could not afford the transportation, housing and food expenses associated with residence outside of their hometowns. Patients mentioned that they did not receive any subsidy from SIS for these expenses. Providers and administrators perceived these constraints as preventing patients from receiving care. (table 3, quote #1)
Table 3.
Barriers and facilitators for obtaining a biopsy and initiating treatment after a high-risk telemammography result
| Theme | Subtheme | Excerpts |
| A. The toll of getting care in major cities following referrals | Insufficient financial resources and support for transportation, accommodation and food |
Quote #1: They have to assume the expenses; they have to. So, often, because of the little money that they have, they don't go [to the city].(midwife, highlands) |
|
Quote #2: There are some shelters here where patients can stay, but they have limited access for a group of patients; first the pediatric patients and then the rest.(physician, Lima) | ||
|
Quote #3: We sold some animals. On the farm, we had sheep, cattle, and we sold everything, even the land we had to sell, to save her. If we hadn't made those efforts, my wife wouldn't be alive now. We did it to save her.(patient’s husband, highlands) | ||
| Being away from family and friends' emotional support |
Quote #4 Interviewer: How important do you think family support is during this time [before having the biopsy]? Interviewee: Well, you are desperate, you feel like dying, but they talk to you, they talk with you. They give you support, psychological support. It’s as if they were saying, ‘Mom, you are not alone; you are with me’.(patient, highlands) |
|
|
Quote #5 Interviewee: If they come from the provinces, they come alone. They can't come with all their family. Or they come to the first consult with a relative, and then they say things like ‘well, he is my husband, but he has to go back to my town to take care of my children. And they leave. That’s the reality of the people who come from the provinces outside of Lima. Interviewer: How does it affect care? Interviewee: It affects care because the patient must think twice before continuing care. Either she abandons it or comes irregularly.(physician, Lima) | ||
| Challenges adjusting to and navigating the city |
Quote #6 The cultural shock [of going to the city] is very strong. They feel overwhelmed; sometimes so overwhelmed that they prefer to leave care and go back to their towns.(physician, highlands) |
|
| B. Patients’ misconceptions and access to information | Misconceptions about breast cancer manifestations and progression |
Quote #7 I did not give it too much importance because I did not have any pain. I thought that maybe they were wrong. I didn't give it importance, so I didn't do anything.(patient, highlands) |
|
Quote #8 Patients say that [having a biopsy] is worse, because when they prick you or take a piece of your breast that’s when the cancer awakens. And that’s why they don’t want to have the biopsy.(nurse, highlands) | ||
| Misconceptions about the treatment |
Quote #9 When you tell someone she has breast cancer, the first thing they think of is that it is “daño” (a sort of witchcraft), so they go first to the shamans and later, if they continue feeling sick, they come back.(program coordinator, rainforest) |
|
| Misconceptions about the prognosis |
Quote #10 Many times, I've heard that when you have cancer you have it until the end. You just have to wait for your death. Once you have it there is no cure.(patient, highlands) |
|
| Limited information provided about the disease |
Quote #11 I would have liked for them to explain it to me more thoroughly, perhaps that way I would have gone, it would have encouraged me. Because sometimes, when they explained to you well, you are conscientious and go. But if they give you a test result that only says get another test because the first test wasn't normal, you don't give it adequate importance.(…)They didn't say anything more than giving you a number, where I should go, and all of that.(patient, highlands). |
|
| C. Administrative and operational barriers | Delays for obtaining appointments and tests |
Quote #12 Interviewer: What happened the day that you went to the hospital? How did it go? Interviewee: I went very early, very early, but the line was already long, and as I needed to work, I got fatigued and didn't go back. So, I haven't done the test. Nothing. I left it there.(patient, highlands) |
|
Quote #13 For these tests, they have to come one day and for these others another day. And that’s how the time passes by.(…). So sometimes when they are told to do one more test they say ‘Miss, I've been there, three months have passed, and I haven't started treatment yet’.(nurse, highlands) | ||
| Low awareness of the programme or of its guidelines among providers |
Quote #14 We had a patient with BI-RADS 4 who needed a biopsy, but the closest hospital didn't have biopsy services. So, we coordinated to refer her to a regional cancer center. After a lot of insistence, they could transfer her to the regional cancer center, and it happens that when she arrives at the facility, they ordered a repeat mammography.(policymaker, Lima) |
|
| Lack of standardised referral pathways |
Quote #15 Interviewer: Did they ask you if you wanted to go to [region X] instead of Lima? Interviewee: No, they didn't say anything. If I had known that in [region X] they had chemotherapy, I wouldn't have gone to Lima, because I didn't have enough money or someone to help me. If I knew they had it here, I would have stayed.(patient, coast) |
|
| Inconsistent tracking of patients |
Quote #16 Interviewer: What type of follow-up do you do here? Interviewee: Once they have a biopsy in the [local] hospital, and it comes back positive, they call the patient or her primary care center to inform her of the result. They talk with the patient to see what’s best: to send her to [the regional hospital] or Lima(…) Interviewer: And what happens once they are referred? Interviewee: We don't do further follow-up. I'd be lying if I say we do. We don't do more follow-up.(program coordinator, coast) |
|
|
Quote #17 The systems are divorced; they are not integrated. So, you are taking mammography tests but there is not a structure that integrates the screening with the treatment or with the diagnosis.(policymaker, Lima) | ||
| D. Facilitators | Having family or a friend living in the city |
Quote #18 They say: ‘I don't worry much about the stay, Miss, I have family there.’ The majority that wants to go to [a major city] is because they have family there.(midwife, coast) |
| Collaborative and family inclusive explanation |
Quote #19 The psychologist has helped me a lot(…)The psychologist is part of your disease, [the psychologist] cheers you up. It is not only the doctor who helps, the psychologist too. [The psychologist] talks with you in a particular way and makes you understand.(patient, highlands) |
|
|
Quote #20 Interviewer: How do you convince them [to obtain a biopsy)? Interviewee: Taking your time and explaining kindly. Sometimes the patient accepts (undergoing biopsy), but the relative doesn't, so you need to explain it all to the family, too.(…)You need to explain to every one of them because in their way of living, all the family influences, and then they accept.(physician, highlands) | ||
| Facilitated appointments |
Quote #21 Interviewer: How do patients from other regions get care here? Interviewee: They just come and get an appointment. Here in the oncology department, we have a system that we called 'unlimited appointments.' We give an appointment to everyone who arrives before 9:00 a.m. Interviewer: What day is the appointment? Interviewee: For the same day. So, they don't have to come back another day.(physician, highlands) |
Interviewees highlighted the need to find external sources of financial support. Sometimes families organised fundraising activities. Other times, non-profit organisations, churches or local municipalities provided financial support for transportation or living expenses; however, interviewees agreed that these resources were limited due to restricted budgets and prioritisation of other vulnerable populations such as paediatric patients (table 3, quote #2). Some times, the economic burden of these expenses forced families to take out loans and/or sell assets (table 3, quote #3).
Being away from family and friends’ emotional support
Close relatives and friends were a vital source of motivation and emotional support as patients sought breast cancer care. Patients noted that the presence of loved ones transmitted confidence. Interviewees acknowledged that this accompaniment was essential, especially around the time of diagnosis (table 3, quote #4).
When patients left their hometowns to reside in the cities, this support was frequently diminished. Patients described how the cost of travel and competing responsibilities prevented loved ones from accompanying them. Providers and administrators referred that the weakened support network put patients at risk of withdrawing from care (table 3, quote #5).
Challenges adjusting to and navigating the city
For some patients, residence in a metropolitan area represented a major cultural change and logistical challenges. Informants described how many patients pursuing care in the cities were accustomed to country life. Living in and navigating a new city, at times in a different language, was perceived by providers and programme coordinators as a ‘cultural shock’ for patients which interfered in their care (table 3, quote #6).
Theme B: patients’ misconceptions and access to information
Misconceptions about breast cancer manifestations and progression
Some misconceptions about how breast cancer manifests and progresses contributed to delays in pursuing a biopsy. For example, a high-risk mammography result was recognised as serious by some patients but denied by others in the absence of symptoms, preventing them from seeking further care (table 3, quote #7). Other patients felt that touching or manipulating the breast ‘awakens’ the disease, preferring to ‘let it rest’ instead of obtaining a biopsy (table 3, quote #8).
Misconceptions about treatment
Providers reported that women looked for therapies with herbs and shamans as their first treatment option. They felt that this caused disengagement from facility-based healthcare with women returning only when no improvement was seen with this traditional treatment, at which point symptoms had often worsened (table 3, quote #9).
Misconceptions about the prognosis
Prior experiences with breast cancer led some women to perceive the disease as a non-curable condition. Whether because they had heard or seen others’ fatal experiences with breast cancer, many women expressed feeling that the ultimate outcome of breast cancer was certain death (table 3, quote #10). This conception of breast cancer made some women question the utility of treatment, creating delays for accepting care.
Limited information provided about breast cancer
Many patients noted the limited information about mammography findings, breast cancer treatment and prognosis communicated to them by the clinical team. Instead, they felt that communication was focused on conveying information about the next administrative steps. As some referred, a better explanation would have led to making good choices earlier (table 3, quote #11).
Theme C: administrative and operational barriers
Delays in obtaining appointments and tests
Informants relayed difficulties in obtaining appointments. For example, in ‘first come, first served’ medical services, many had to arrive at the facility very early in the morning and wait in long lines without the guarantee of an appointment that day. Some women expressed frustration with this process, noting that it led them to discontinue seeking care (table 3, quote #12).
When appointments could be booked in advance, they were often scheduled for several weeks later, with test results delayed up to a month or more. One nurse described a patient’s onerous experience trying to complete the tests requested (table 3, quote #13).
Limited awareness of the programme among providers
Not all physicians reported awareness of the MOH telemammography programme. Those unfamiliar with the programme doubted the validity of tele-mammography results (thinking that they were reported by untrained radiologists) and usually ordered a second mammography at their hospital (table 3, quote #14). In other cases, the cancer programme nurses and midwives wanted to ‘double check’ each high-risk telemammography result so they would order a breast ultrasound before referring for biopsy, contrary to national guidelines. These extra procedures contributed to delays and the administrative burdens on the patient.
Lack of standardised referral pathways
There is no formal standardised referral pathway for high-risk telemammography results. The providers’ choice of referral hospital, particularly for treatment, was usually based on his/her perceptions of available services or quality of care. As noted by most informants, INEN was often the hospital of choice. Policy-makers agreed that this approach did not take advantage of the resources available at closer regional hospitals. One woman’s comment illustrated how this system failed to account for patients’ convenience (table 3, quote #15).
Inconsistent tracking of patients
The follow-up of women did not occur uniformly along the continuum of care. While the cancer programme nurses and midwives closely followed patients who received care in the local hospital, programme coordinators agreed that patient tracking stopped once referred to hospitals in other regions (table 3, quote #16).
The programmatic follow-up tool, created by the MOH to strengthen tracking activities, was not used consistently by all healthcare providers and coordinators and scarcely monitored by the MOH officials. In addition, policymakers reported that tracking of patients through health information systems would not be possible due to a ‘divorce’ between the MOH’s and hospitals’ digital data systems (table 3, quote #17).
Theme D: facilitators
Having family or a friend living in the city
Interviewees expressed that having a relative or a close friend living in the city where patients were referred facilitated access to care. When patients could stay with friends or family, it alleviated much of the financial hardship (table 3, quote #18). Also, patients felt secure in knowing that someone could help them navigate the city or take care of them once treatments started.
Collaborative and family-inclusive approaches to care
Addressing patients’ concerns about breast cancer through a multidisciplinary approach was seen by providers as useful for improving the patient’s understanding of the disease and for making prompt medical decisions. Collaborative work among clinicians, psychologists and social workers facilitated communication around diagnosis and expectations for future care. Patients highlighted the benefit of receiving psychological support on diagnosis (table 3, quote #19). Providers emphasised that involving the family was necessary given its determinant role in health decision making (table 3, quote #20).
Facilitated appointments
Some hospitals and providers expedited appointments for their patients. In two hospitals, the medical appointments were scheduled within 1 day for patients coming from remote areas. In another, all patients arriving early were guaranteed to be seen that day. In other cases, providers coordinated appointments to reduce the administrative burden on the patients or leveraged their influence to secure a spot. These approaches, although not perfect, helped reduce appointment delays (table 3, quote #21).
Discussion
We evaluated linkages to breast cancer diagnostic and treatment services in the largest national telemammography programme in Peru. This adds to the very limited body of literature examining linkages to care among women undergoing breast cancer screening in a middle-income country. Identifying health system requirements for rapid breast cancer diagnosis is a priority for the WHO’s new Global Breast Cancer Initiative,16 and our findings contribute to understanding of important barriers and facilitators of timely diagnosis in Peru and similar settings. In women with a high-risk telemammography result among whom biopsy is indicated, we found evidence that biopsy was performed among fewer than half. Among women with breast cancer, we found evidence of treatment initiation in 86.3%. Delays in obtaining these services were common. Overall, the vast majority (92.4%) of women experienced suboptimal care (delayed care or no evidence of linkage to care). Our quantitative findings are complemented by qualitative evidence of substantial barriers to care. Through a mixed-methods design, we elucidated the ways in which diagnosis and treatment services for breast cancer were not easily accessible for women living in poverty throughout the country. These included travel barriers, administrative obstacles and patients’ misconceptions about breast cancer.
In our study, many women with breast cancer did not have evidence of biopsy or treatment, and centralisation of cancer services in Lima and a few other major cities likely contributes to delays and interruptions in care. Living outside Lima and/or in rural areas of the country has been shown to place individuals with cancer at higher risk of discontinuing care.17 18 Centralisation of cancer care facilities has also been found to disproportionately affect socioeconomically vulnerable populations and may contribute to persistent care disparities for breast cancer care in LMIC.19–21 In our study, although cancer services were offered free-of-charge, patients lacked the means for travelling to obtain those servicers. According to the National Cancer Control Plan, SIS should have subsidised the costs for transportation and for staying in the cities; however, this economic support was not received by any of the patients interviewed. A recent study on cervical cancer in Peru highlighted the same policy-implementation gap in women with cervical cancer.22 Given that 5 in 10 women in Peru live in poverty (<US$150 per month),23 our finding that insufficient economic resources for the expenses associated with centralised care in major cities (eg, transportation, accommodation and food) challenged care is not unexpected. Reducing inequalities for breast cancer care access in Peru must incorporate the existing free diagnostic and treatments services with decentralisation of these resources to bring them closer to those that need them.
Among women for whom we could confirm care, delays were common. Our finding that 65% of women experienced a health system delay is consistent with reports from other LMIC, where over 70% of patients start treatment three or more months after the first abnormal finding (a high-risk screening mammography or symptoms discovery).13 This finding is also supported by one local study reporting even longer health system delays (around 8 months), although under different circumstances and using different definitions.24 Long health system delays leads to advanced disease stage, a known risk factor for death from breast cancer.25 Efforts to decrease delays would be expected to increase breast cancer survival rates.
The observed 2% prevalence of BI-RADS 4 and 5 results found in our study is comparable to what would be expected for mammography screening.26–28 And, while the breakdown of BI-RADS 4 vs BI-RADS 5 among women with a BI-RADS result ≥4 tends to be variable,26 28 29 our finding that 73% and 27% of women had BI-RADS 4 and 5, respectively, was very similar to another Latin American study conducted in Brazil.29 In contrast, among women with biopsy results, the positivity rate found for women with BI-RADS 4 (89%), was higher than the expected. While at least 95% of biopsies of BI-RADS 5 results are typically positive, this statistic is much lower, around 20%–30%, for BI-RADS 4.30 31 While we cannot be certain, we do not believe this observation of our study is attributable to inadequate training, as radiologists from the telemammography programme were employed by the MOH and read the mammographs in compliance with MOH guidelines and standards. Likewise, most biopsies of our study were taken at INEN, a national referral centre for cancer, staffed with highly trained cancer pathologists. While the distribution of BI-RADS 4 vs BI-RADS 5 was comparable among women with evidence of biopsy (67.7% BI-RADS 4 and 32.3% BI-RADS 5) and withouth evidence of biopsy (75% BI-RADS 4 and 25% BI-RADS 5), if women with BI-RADS 4 and a negative biopsy were systematically screened out of the sample, this would explain our finding. However, we have no reason to believe this ocurred. This high positivity rate for malignancy among women with BI-RADS 4 results merits further research. This is especially true given that many women with BI-RADS 4 did not have record of biopsy and therefore may remained undiagnosed and untreated, supporting the need for a robust tracking information system.
Our results raise several opportunities to improve the outcomes of the telemammography programme by facilitating follow-up care and decreasing delays for those with an abnormal mammography. Patient tracking could be improved by implementing a unified health information system that tracks patients across the continuum of care, allowing an accurate, full, and even real-time patient follow-up.32 33 Specifically for this breast cancer programme, the tracking system should be a digital platform that enables data entry at the care steps (mammography, result, and patient reporting; biopsy referral, biopsy, result and patient reporting; treatment referral, initiation and completion), at the different public healthcare facilities and even at private hospitals, enabling also the calculation of the time elapsed between steps. Appointment systems could be reconsidered to prioritise a patient-centred approach. Low compliance to guidelines among the MOH’s providers could be remedied by nationwide campaigns to build awareness of the programme, its processes, goals and achievements. Finally, our data suggest some misconceptions about breast cancer treatment and prognosis. The source of these misconceptions is likely multifactorial and includes an unawareness of the success of breast cancer treatment when diagnosed and treated promptly. Thus, multidisciplinary and culturally tailored patient education, incorporating family members or supporters as appropriate, and continued work to ensure access to effective diagnosis and treatment, may correct misconceptions about breast cancer that contributed to delays and discontinuation seeking care. Overall, a real comprehensive telemammography programme should not be seen as a separate breast cancer service but as part of the whole breast cancer continuum of care.
Evaluating breast cancer care using routinely collected data was challenging due to a lack of integration of health information systems of the different MOH components that managed and provided cancer care to the population subsidised by SIS. Although we used multiple national data sources to capture care access through different pathways, due to varying levels of follow-up and data completeness, we may have underestimated the proportion of women who obtained care. Thus, the quantitative results presented here were our best intent to disentangle the current health information puzzle existing in the public healthcare sector. Nonetheless, this study is a comprehensive evaluation that used both quantitative and qualitive research techniques to understand the situation in diverse geographical settings in Peru. Although particular challenges of very hard to reach women living in more remote areas may have note been explored, this study provides a close perspective of challenges in Peru, which may be broadly applicable to other middle-income countries with similar resource levels and health systems.
The benefit of mammography screening can only be realised if women with abnormal findings are successfully linked to high quality and timely diagnostic and treatment services. Our study underscores the need for strengthening the breast cancer diagnostic and treatment capacity of hospitals outside Lima to remove barriers and facilitate access to timely comprehensive breast cancer care. It also highlights the need for a strong patient education strategy and better dissemination of the information about the programme among providers nationwide. A unified health information system is needed to allow better tracking of patients after the mammography and along the breast cancer continuum of care. This information system should be part of an overall breast cancer data management system that facilitates programme monitoring, evaluation and research to guide appropriate and timely public health decisions and locally tailored policy development. All in all, ensuring timely linkage to diagnosis and treatment for women with an abnormal result in the telemammography programme will be critical to securing the screening programme’s success.
Supplementary Material
Acknowledgments
We are grateful to doctors Jose Cotrina, Willy Ramos, Mercedes Egues, Diego Venegas, Isabel Cotrina, Alcedo Jorges, Victor Palacios, Carmen Miyasato and Carlos Leon, as well as Lita Carrillo and Doris Vilca for their central role in data collection. We truly appreciate the contribution of all participants of the qualitative interviews.
Footnotes
Twitter: @mollyfranke1
LEP and JTG contributed equally.
Contributors: RAE, PJG and MFF conceptualised the study. RAE collected the data and wrote the first draft of the article. MFF, PJG, LEP and JTG helped to synthesise evidence, interpret results, and critically revise the manuscript. All authors approved the final draft. RAE secured funding for the study. RAE is the guarantor of this article.
Funding: This work was funded by Harvard Medical School’s Master of Medical Sciences in Global Health Delivery of the Department of Global Health and Social Medicine, Rockefeller Centre for Latin American Studies of Harvard, and Peru’s National Programme of Scholarships and Academic Credits (RJ-117-2017-MINEDU-VMGI-PRONABEC-OBPOST). The funding sources did not play any role in the study design; in the collection, analysis, and interpretation of the data; in writing of the report; nor in the decision to submit the paper for publication.
Competing interests: None declared.
Patient and public involvement statement: Patients were not involved in the development of the research question or in the design, recruitment or conduction of the study. Personnel from the Ministry of Health were involved in study design and recruitment. Results will be disseminated among the Ministry of Health staff.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request.
Ethics approval
The study protocol and instruments were approved by Institutional Review Boards from Harvard Medical School (IRB19-0589), Cayetano Heredia Peruvian University (#104252), INEN (INEN 20-02), and Villa El Salvador Hospital (#01744-2019). For the quantitative component, the informed consent requirement was waived. Participants in qualitative interviews provided written consent. Women with high-risk mammography results participating in the interviews received US$6 for time and transportation compensation.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Who position paper on mammography screening. Available: https://www.who.int/cancer/publications/mammography_screening/en/ [Accessed 06 Feb 2021].
- 3.Pace LE, Keating NL. A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA 2014;311:1327–35. 10.1001/jama.2014.1398 [DOI] [PubMed] [Google Scholar]
- 4.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 2005;353:1784–92. 10.1056/NEJMoa050518 [DOI] [PubMed] [Google Scholar]
- 5.Harford J, Azavedo E, Fischietto M, et al. Guideline implementation for breast healthcare in low- and middle-income countries: breast healthcare program resource allocation. Cancer 2008;113:2282–96. 10.1002/cncr.23841 [DOI] [PubMed] [Google Scholar]
- 6.Jones LA, Chilton JA, Hajek RA, et al. Between and within: international perspectives on cancer and health disparities. J Clin Oncol 2006;24:2204–8. 10.1200/JCO.2005.05.1813 [DOI] [PubMed] [Google Scholar]
- 7.Government of Peru . Supreme decree: Declaran de interés nacional La atención integral del cáncer Y mejoramiento del acceso a Los servicios oncológicos en El Perú Y dictan otras medidas, 2012. [Google Scholar]
- 8.Vidaurre T, Santos C, Gómez H, et al. The implementation of the plan Esperanza and response to the imPACT review. Lancet Oncol 2017;18:e595–606. 10.1016/S1470-2045(17)30598-3 [DOI] [PubMed] [Google Scholar]
- 9.American Cancer Society . Breast cancer early detection and diagnosis. Available: https://www.cancer.org/content/dam/CRC/PDF/Public/8579.00.pdf [Accessed 20 Jan 2020].
- 10.Creswell JW, Plano Clark VL. Designing and conducting mixed methods research. 3rd ed. Thousand Oaks, CA: SAGE Publications, 2017. [Google Scholar]
- 11.D'Orsi CJ, Sickles EA, Mendelson EB. ACR BI-RADS® atlas, breast imaging reporting and data system. 5th ed. Reston, VA: American College of Radiology, 2013. [Google Scholar]
- 12.Weller D, Vedsted P, Rubin G, et al. The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer 2012;106:1262–7. 10.1038/bjc.2012.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unger-Saldaña K. Challenges to the early diagnosis and treatment of breast cancer in developing countries. World J Clin Oncol 2014;5:465–77. 10.5306/wjco.v5.i3.465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richards MA, Westcombe AM, Love SB, et al. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet 1999;353:1119–26. 10.1016/S0140-6736(99)02143-1 [DOI] [PubMed] [Google Scholar]
- 15.Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005;15:1277–88. 10.1177/1049732305276687 [DOI] [PubMed] [Google Scholar]
- 16.Anderson BO, Ilbawi AM, Fidarova E, et al. The global breast cancer initiative: a strategic collaboration to strengthen health care for non-communicable diseases. Lancet Oncol 2021;22:578–81. 10.1016/S1470-2045(21)00071-1 [DOI] [PubMed] [Google Scholar]
- 17.Paz-Soldán VA, Bayer AM, Nussbaum L, et al. Structural barriers to screening for and treatment of cervical cancer in Peru. Reprod Health Matters 2012;20:49–58. 10.1016/S0968-8080(12)40680-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasquez L, Diaz R, Chavez S, et al. Factors associated with abandonment of therapy by children diagnosed with solid tumors in Peru. Pediatr Blood Cancer 2018;65:e27007. 10.1002/pbc.27007 [DOI] [PubMed] [Google Scholar]
- 19.Stitzenberg KB, Meropol NJ. Trends in centralization of cancer surgery. Ann Surg Oncol 2010;17:2824–31. 10.1245/s10434-010-1159-0 [DOI] [PubMed] [Google Scholar]
- 20.Lieberman-Cribbin W, Liu B, Leoncini E, et al. Temporal trends in centralization and racial disparities in utilization of high-volume hospitals for lung cancer surgery. Medicine 2017;96:e6573. 10.1097/MD.0000000000006573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinto JA, Pinillos L, Villarreal-Garza C, et al. Barriers in Latin America for the management of locally advanced breast cancer. Ecancermedicalscience 2019;13:897. 10.3332/ecancer.2019.897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nevin PE, Garcia PJ, Blas MM, et al. Inequities in cervical cancer care in Indigenous Peruvian women. Lancet Glob Health 2019;7:e556–7. 10.1016/S2214-109X(19)30044-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Instituto Peruano de Economía . La pobreza extrema en El Perú aumentó en El, 2019. Available: https://www.ipe.org.pe/portal/la-pobreza-extrema-en-el-peru-aumento-en-el-2019/ [Accessed 20 Nov 2020].
- 24.Romanoff A, Constant TH, Johnson KM, et al. Association of previous clinical breast examination with reduced delays and earlier-stage breast cancer diagnosis among women in Peru. JAMA Oncol 2017;3:1563–7. 10.1001/jamaoncol.2017.1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Unger-Saldaña K, Miranda A, Zarco-Espinosa G, et al. Health system delay and its effect on clinical stage of breast cancer: multicenter study. Cancer 2015;121:2198–206. 10.1002/cncr.29331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poplack SP, Tosteson AN, Grove MR, et al. Mammography in 53,803 women from the new Hampshire mammography network. Radiology 2000;217:832–40. 10.1148/radiology.217.3.r00dc33832 [DOI] [PubMed] [Google Scholar]
- 27.Eberl MM, Fox CH, Edge SB, et al. Bi-Rads classification for management of abnormal mammograms. J Am Board Fam Med 2006;19:161–4. 10.3122/jabfm.19.2.161 [DOI] [PubMed] [Google Scholar]
- 28.Sirous M, Shahnani PS, Sirous A. Investigation of frequency distribution of breast imaging reporting and data system (BIRADS) classification and epidemiological factors related to breast cancer in Iran: a 7-year study (2010-2016). Adv Biomed Res 2018;7:56. 10.4103/abr.abr_161_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milani V, Goldman SM, Finguerman F, et al. Presumed prevalence analysis on suspected and highly suspected breast cancer lesions in São Paulo using BIRADS criteria. Sao Paulo Med J 2007;125:210–4. 10.1590/S1516-31802007000400003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elezaby M, Li G, Bhargavan-Chatfield M, et al. ACR BI-RADS assessment category 4 subdivisions in diagnostic mammography: utilization and outcomes in the National mammography database. Radiology 2018;287:416–22. 10.1148/radiol.2017170770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orel SG, Kay N, Reynolds C, et al. Bi-Rads categorization as a predictor of malignancy. Radiology 1999;211:845–50. 10.1148/radiology.211.3.r99jn31845 [DOI] [PubMed] [Google Scholar]
- 32.Anttila A, Lönnberg S, Ponti A, et al. Towards better implementation of cancer screening in Europe through improved monitoring and evaluation and greater engagement of cancer registries. Eur J Cancer 2015;51:241–51. 10.1016/j.ejca.2014.10.022 [DOI] [PubMed] [Google Scholar]
- 33.Anand V, Sheley ME, Xu S, et al. Real time alert system: a disease management system Leveraging health information exchange. Online J Public Health Inform 2012;4:ojphi.v4i3.4303. 10.5210/ojphi.v4i3.4303 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request.


