Abstract
In this work we have designed and developed a low cost and simple instrument to purify air in an enclosure. The device sucks up the air in the enclosed area, kills the microorganisms and let clean air flow out. A combination of an ultra violet light and an electric field are used to kill the microorganisms in air. Three electric field chambers (radial, parallel, perpendicular) are used to clean air more effectively. Stainless steel meshes were used to increase the density of the electric fields. The outer covers were made with plastic and wood. The instrument was tested against an evaporated bacterial solution (Staphylococcus aureus) by letting it flow through the instrument and measuring the bacterial concentration of the output air. The results showed the instrument is extremely effective even when tested against high bacterial concentrations. The instrument is extremely useful to clean air in closed rooms such as in hospitals, schools, etc. and prevent the spread of airborne diseases.
Keywords: Air purifier, High electric field, Ultra violet lamp, Microorganisms, Bacteria
Specifications table
| Hardware name | UV assisted multi-electric field air purifier |
|---|---|
| Subject area |
|
| Hardware type |
|
| Closest commercial analog | LG Signature 3-Speed (Covers: 316 Sq.-ft) Non-HEPA and UV White Air Purifier |
| Open source license | CERN Open Hardware License v2 |
| Cost of hardware | 120 $ |
Hardware in context
Harmful microorganisms suspended in air or attached to surfaces are a major threat to human health. Before the deadly out-break of the corona virus (COVID-19) in 2020 [1], [2], [3] there have been several such cases reported in the past such as, the “severe acute respiratory syndrome” (SARS) in 2003 and H1N1 in 2010 [4], [5], [6]. The COVID-19 virus was the deadliest due to several reasons. It could spread from a human or an animal to another when they are in close range (<2m) by respiratory droplets via speaking/coughing/sneezing…etc. The viruses can enter the recipient's lung via the mouth or nose. Also these respiratory droplets can stay on surfaces and air for several hours and could get in contact with an uninfected person and enter via moth/nose/eye soon as they touch them [7].
The chemical method is the most commonly used to eliminate harmful microorganisms [8], [9]. There are several major drawbacks when using chemicals in this manner. Chemicals may kill microorganisms selectively; they take time to kill microorganisms completely (in some cases up to 1 h). The unreacted chemical compounds could pollute the environment and the cleaning process is normally limited to the surfaces, less effective when used against airborne microorganisms [10], [11]. Given these issues related to using chemical disinfectors to clean air, researchers have been looking at alternative methods.
Effective and low cost air purifiers have the potential to reduce the exposure of humans to virus-laden aerosols in any type of indoor environments such as hospitals, schools, trains, etc. Almost in all air purifiers filters are used and need to be replaced and disposed as medical waste or disinfect thoroughly to prevent secondary contamination. As a result, they cost more and consume more energy [12]. Most currently available air purifiers have high-efficiency particulate air filters (HEPA) for particles filtration [13]. Several other air purifying methods were also reported in the recent past. For example, ·OH Fog-drops based on an electric-field discharge plasma, which can kill microorganisms in air quickly [14], [15], [16]; Ultra violet (UV) technology based of air sterilization [17]; Air rapid heating rapid cooling sterilization [18] …etc. Useful information on such methods can be found in a review paper by Choi et al. in 2021 [19]. Using an electric field to clean air have been a known topic in the past, yet has been mostly focused on removing particles such as dust, soot, etc. [20], [21]. Most of such systems use fibrous filtration although, there is an intrinsic conflict between filtration efficiency, low air resistance, and long service life. For decades, scientists have been struggling to come up with novel filter materials to overcome such issues [22], [23], [24], [25], [26].
To overcome most of the issues associated with air purification, we have designed and developed a simple, novel low cost air purifier which use a combination of UV light with a direct high voltage electric field to kill microorganisms in air. The electric field was designed as a strong multi directional electric field to kill microorganisms more effectively. The prototype of the instrument has been built and proven to be highly effective against high bacterial concentrations.
Hardware description
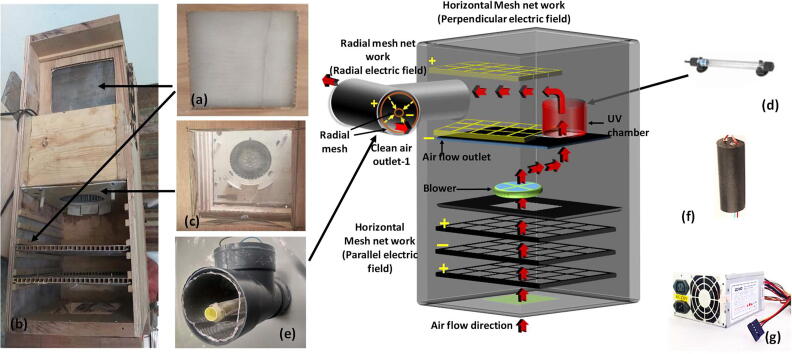
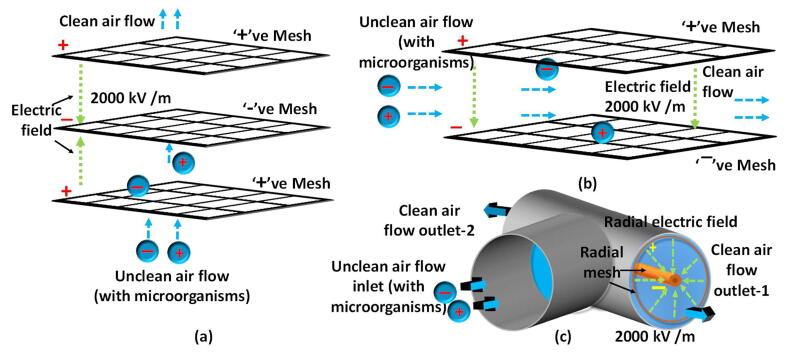
This work describes the design of an air cleaning device which uses the combination of a UV light and an electric field to clean air by killing all harmful microorganisms. The instrument uses two methods (UV, High electric field ∼2000 kV m−1) to kill any microorganisms present in the air. The high electric field was divided in to three main parts to kill the microorganisms more efficiently. A blower was used to suck the air from the environment. The instrument is designed to send the incoming air flow through each chamber in the following order: parallel electric field, UV chamber, perpendicular electric field, and a radial electric field (Fig. 1). The parallel and perpendicular electric fields were created using two mesh networks (Fig. 2a and b). The radial electric field was created using two cylindrical type mesh networks inside a polyvinyl chloride (PVC) tube (Fig. 2c). The meshes have many wire crossing points which have a high charge density. This increases the probability of attracting any charged microorganisms. The distance between two meshes was maintained at a level which make sure there are no sparks created. Absence of sparks are important to avoid ozone production inside the machine.
Fig. 1.
The main components of the E-Field/UV air purifier with the flow diagram.
Fig. 2.
The three air cleaning electric chambers. a) The parallel electric field (The air flow is parallel to the electric field), b) The perpendicular electric field (The air flow is perpendicular to the electric field), c) The radial electric field (The air flow is radial to the electric field).
Most of the existing machines with air cleaning components use a UV light. The high electric field is more efficient in killing microorganisms and hence the combination of UV light and electric field leads to an ideal air cleaner. By using a powerful blower or by moving the instrument it can be applied to clean air in larger indoor areas. The low cost (120 $) of the instrument makes it even more attractive compared to the available commercial products in the market (1700 $) and the development process is extremely simple and can be achieved under minimum lab facilities.
-
•
The E-Field/UV air purifier could be used to kill viruses in air and would be ideal for use in closed rooms.
-
•
The E-Field/UV air purifier technology can be applied to any air conditioner machine.
-
•
In biomedical research the resistivity of any microorganism can be tested using E-Field/UV air purifier.
The calculated air flow rate of the instrument was 0.188 m3 s−1. The residence time in each chamber is as follows, Parallel chamber 0.042 s, Perpendicular chamber 0.042 s, Radial chamber 0.0229 s. We used an anemometer (AM 4201) to measure the air speed when calculating above parameters.
Design files summary
| Design file name | File type | Open source license | Location of the file |
|---|---|---|---|
| Fig. 1 | Power point figure | CERN Open Hardware License v2 | Available with the article as a figure and a separate editable power point file is attached |
| Fig. 2 | Power point figure | CERN Open Hardware License v2 | Available with the article as a figure and a separate editable power point file is attached |
Bill of materials summary
| Designator/Image | Component | Number | Cost per unit -currency | Total cost – currency | Source of materials | Material type |
|---|---|---|---|---|---|---|
 |
High voltage generator | 1 | LKR 3000 (15 $) | LKR 3000 (15 $) | – | Non-specific |
 |
UV lamp (9 W) | 1 | LKR 4000 (20 $) | LKR 4000 (20 $) | – | Non-specific |
 |
Stainless steel mesh (10×10 cm) | 6 | LKR 700 (3.5 $) | LKR 4200 (21 $) | – | Metal |
 |
Silicone glue | 1 | LKR 400 (2 $) | LKR 400 (2 $) | – | Organic |
 |
Fan Blower | 1 | LKR 5000 (25 $) | LKR 5000 (25 $) | – | Non-specific |
 |
PVC T-socket (Diameter 10 cm) | 1 | LKR 500 (2.5 $) | LKR 500 (2.5 $) | – | Organic |
 |
Second hand PC Power supply unit | 1 | LKR 1500 (7.5 $) | LKR 1500 (7.5 $) | – | Non-specific |
 |
PVC conduit pipe | 1 | LKR 20 (0.1 $) | LKR 20 (0.1 $) | – | Organic |
| Switch/Wires/Wooden parts/Stickers/Nuts and bolts | 1 | LKR 2000 (10 $) | LKR 2000 (10 $) | – | Non-specific |
Build instructions
The following instructions will guide any user to fully assemble the proposed E-Field/UV air purifier in this article.
Step-01: The mesh frames and networks to use in the high electric field chambers (Parallel, Perpendicular) were completed by fixing a wooden frame around each stainless steel mesh (Fig. 1a).
Step-02: The frames were fixed inside the main wooden frame to make the parallel and perpendicular electric fields (Fig. 1b). The edges and connections were made air tight using silicone glue. The gap between meshes in electric fields was fixed at around 3 cm.
Step-03: Outer enclosure and chambers were constructed using wooden frames (Fig. 1b). Each chamber was separated using wooden sheets and silicone glue was applied to the joints to avoid air leaks.
Step-04: The blower was fixed in the middle of the chamber (Fig. 1c).
Step-05: UV light was fixed in the chamber in between blower and parallel electric field chamber.
The high voltage generator was fixed in the chamber (Fig. 1d).
Step-06: The radial electric field chamber was created by creating two cylinders with a stainless steel meshes and inserting them in a PVC tube. Small diameter cylinder (‘- ‘ve terminal) was wound around a PVC conduit pipe (diameter 1.5 cm) and inserted in the middle of the tube with larger diameter (10 cm) and the large diameter cylinder (‘+’ve tube) was inserted closer to the wall of the tube (Fig. 1e).
Step-07: The high voltage generator was inserted and connected to all three electric field chambers and the power supply was inserted and connected to the generator.
Step-08: Finally, an insulating sticker was used to cover the outer body of the instrument.
The insulation is extremely important since a high electric field is present inside the instrument.
Operation instructions
Operation of the instrument is simple and straight forward. Once the power is switched on, the instrument will start to suck in unpurified air from the environment and will pump out clean air.
As the instrument contains a high electric field, care should be taken to keep it away from any wet conditions. If the instrument is damaged it should not be used until fixed. While the instrument is in operation, no attempts should be made to adjust any internal components.
Validation and characterization
Methodology
Staphylococcus aureus ATCC strain 25,923 was streaked on blood agar and incubated at 37 °C for 24 hrs. A bacterial suspension (neat) was made by dissolving 10 colonies of bacteria in 10 mL of phosphate-buffered saline (PBS). A 10-fold serial dilution was made using PBS as the diluent. The neat and the first 3 dilutions (10−1, 10−2, and 10−3) were used for the experiment. The aerobic plate count (APC) of the neat was determined as previously described [27].
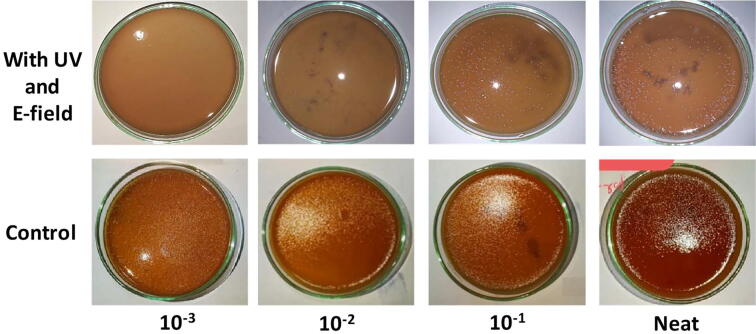
Each of the bacterial suspensions was sprayed into the inlet of the instrument. A single spray delivered 0.5 µL of the bacterial suspension into the device. The flow of air coming through the outlet following a single spray was captured to a blood agar plate by holding the plate against the airflow for 2 min. One at a time a single spray of each suspension was subjected to four conditions: without the activity of UV radiation and electric field (control), with the activity of UV radiation only, with the activity of electric field only, and with the activity of UV radiation and the electric field. To remove any residual effect from the previously applied UV radiation or the electric field, the device was turned off for 2 min between consecutive sprays. Triplicate of plates were used to assess the effect of each combination, the suspension, and the condition. After capturing the bacteria in the airflow coming through the outlet, plates were incubated at 37 °C for 18 hrs. Colony counts were obtained to determine the capacity of the device in reducing the bacterial load. Minitab statistical software version 18 (Minitab Pty Ltd, Sydney, Australia) was used to perform statistical analysis. Comparison of colony-forming units (CFU) between conditions was done using paired T-test. A P-value < 0.05 was considered statistically significant.
Results and discussion
As expected the device substantially reduced the bacteria in the airflow. The aerobic plate count of the neat was 6.64×108 CFU per milliliter. The two conditions; the control and the activity of UV radiation only, yielded CFU that are impractical to count. It is convincing that the activity of UV radiation used in this device itself was inadequate in reducing bacterial counts to a notable level. However, following the application of the electric field only an obvious reduction in CFU was observed compared to that of the control or treatment with UV radiation only. The activity of UV radiation and electric field together significantly (P = 0.04) reduced CFU compared to that of the activity of the electric field only (Table 1).
Table 1.
Mean bacterial colony counts observed at the end of incubation at 37 °C for 18 hrs.
| Dilution | Mean bacterial colony counts |
|||
|---|---|---|---|---|
| without the activity of UV radiation and electric field | with the activity of UV radiation only | with the activity of electric field only | with the activity of UV radiation and electric field | |
| Neat | TNC* | TNC | 224 | 210 |
| 10−1 | TNC | TNC | 161 | 140 |
| 10−2 | TNC | TNC | 90 | 75 |
| 10−3 | TNC | TNC | 78 | 10 |
TNC: Too numerous to count.
The species of bacteria chosen for this experiment, Staphylococcus aureus, is usually found in air as a contaminant [28]. The bacterial load given to the device in this experiment is substantially above the number of bacteria normally found in the contaminated air [29]. Active air sampling in an enclosed environment with the use of an air sampler would be the ideal approach to assess the effect of the device but such an experiment will fail to determine the capacity of the device in reducing bacterial counts [30]. From the experiment conducted herein, it is evident that the electric field used in this device substantially reduces the counts of bacteria in the airflow and an augmented effect can be achieved by combining the effect of UV radiation with the voltage (Fig. 3).
Fig. 3.
The bacterial growth variation for air with different bacterial concentrations, with and without electric field and UV light.
As the experiment have shown clearly that the instrument is successful in removing bacterial microorganisms in air. Also the usage of the multidirectional electric field has proven to be effective than any normal unidirectional electric field. The design of the instrument is simple and the manufacturing cost is low. Due to the high electric field caution should be taken when repairing the instrument in case of a damage. We strongly believe it will be successful against viruses such as COVID-19 as well. Unfortunately, we still do not have access to test the instrument against viruses/COVID-19. Hence the virus test is going to be the most prioritize future work/research with this instrument. Apart from that the instrument can be used as a research instrument to study how microorganisms behave in various types of electric fields.
CRediT authorship contribution statement
D.N.P. Ruwan Jayakantha: Conceptualization, Methodology. H.M.N. Bandara: Conceptualization, Writing – original draft. Nadeesha M. Gunawardana: Conceptualization, Methodology. R.P.V. Jayantha Rajapakse: Supervision, Writing – review & editing. Dulari S. Thilakarathne: Conceptualization, Writing – original draft, Formal analysis. Elisabetta Comini: Supervision, Writing – original draft, Writing – review & editing. Nanda Gunawardhana: Supervision, Formal analysis, Writing – review & editing. S.M.M.L. Karunarathne: Conceptualization, Writing – original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Biographies

Ruwan Jayakantha received his B.Sc and M.Phil. degrees in 2010 and 2016 from University of Peradeniya, Sri Lanka. He is currently working as a research assistant in the Sri Lanka Technological Campus. He is also doing a Ph.D. at Brescia University. His research interests include, Electronics, Mechanics, Nano Technology and Computational Physics. He has received several patents for designing instruments such as, Pressure analyzer for measuring breathability, Specific surface area analyzer for powder materials.

Dr. Nanda Gunawardhana is the Director of Research and International Relations at Sri Lanka Technological Campus (SLTC). Nanda obtained his Bachelor’s degree from University of Peradeniya, Sri Lanka and Ph.D from Baylor University, Texas, USA. He has completed two post-doctoral fellowships in Saga University and Fukuoka Industry and Science Foundation, Japan. He was awarded as the best young scientist in Sri Lanka in 2015 by National Science Foundation and TWAS, Italy and recipient of Prestigious Presidential awards for his scientific publications. He has published more than 100 papers and communications and delivered key-note speeches in many international conferences.

HMN Bandara received his B.Sc. from university of Peradeniya and Ph.D. from University of Aston. He is currently working in University of Peradeniya, Sri Lanka as an emeritus professor. His research areas include, Materials Chemistry, Polymer Chemistry, Nanocomposites, Nanotechnology, Solar Cells, Material Characterization, Polymers, Conducting Polymers and Dyes. He is an expert in instrument design and automation and has several patents.

Elisabetta Comini received her degree in physics at the University of Pisa in 1996 and Ph.D. degree in material science at the University of Brescia. Since 2016 she is working as a full professor at Brescia University. She has published more than 387 papers in leading scientific journals. EC is a researcher specialist in the growth of metal oxides, particularly nanowires, thin films and the measurement of their electronic, functional and structural properties. EC is the director of SENSOR laboratory (Brescia University, http://sensor.unibs.it) and is a co-founder of NASYS. Her Hirsch index (h-index) is 54 (Web of Science), 57 (Scopus), 64 (Google scholar).

Nadeesha Gunawardana received his M.Sc in NanoScience and NanoTechnology from Postgraduate Institute of Science University of Peradeniya in 2018. He has over five years of research experience. He served as a research assistant of projects supercapacitor and flow battery for four years, funded by CodeGen international. His research interests are focused on energy production and storage with aid of nano-materials. it includes synthesize graphene, graphene oxide, expanded graphite, and energy storage using supercapacitors and batteries.

Migara Karunarathne received his Ph.D from Sungkyunkwan university 2018. He is currently working in Sri Lanka Technological Campus as a senior lecturer. His research interests include study on the behaviour of liquid crystal materials and their applications in optics, Zinc Oxide nanoparticles based Dye sensitized solar cells, Waste management, Optical vortex arrays. Currently he is working on designing instruments and energy harvesting. His publications includes several subject areas including liquid crystal materials, dye solar cells and instrument design.

Prof. Jayanthe Rajapakse is a senior professor attached to Department of Veterinary Pathobiology, Faculty of Veterinary Medicine and Animal sciences, University of Peradeniya. He is a research expert in the field of veterinary science. He has published over 238 research articles which have been cited by 2753 researchers around the world. Prof. Rajapakse’s research primarily focuses on parasites of veterinary and medical importance. Other than that he has investigated zoonotic and vector borne pathogens in Sri Lanka. His works also include exploring pathogens of wildlife and therapeutic properties in herbal extracts. Prof. Rajapakse is an expert developing vaccines against parasites and he owns patents for developing anticancer therapeutics and antivenin for snake envenomation in Sri Lanka.

Dr. Dulari Thilakarathne completed her doctorate from University of Melbourne and currently works as an early career researcher attached to Department of Veterinary Pathobiology, Faculty of Veterinary Medicine and Animal sciences, University of Peradeniya. Her field of expertise is virology and her research interest include zoonotic and vector borne intracellular parasites.
Contributor Information
Elisabetta Comini, Email: Elisabetta.comini@unibs.it.
Nanda Gunawardhana, Email: nandag@sltc.ac.lk.
S.M.M.L. Karunarathne, Email: migarak@sltc.ac.lk, migara@gmail.com.
References
- 1.Cevik M., Bamford C.G.G., Ho A. COVID-19 pandemic—a focused review for clinicians. Clin Microbiol and Infec. 2020;26(7):842–847. doi: 10.1016/j.cmi.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.M. Nicola M.Z. Alsafi C. Sohrabi A. Kerwan A. Al-Jabir C. Iosifidis M. Agha R. Aghaf The socio-economic implications of the coronavirus pandemic (COVID-19): A review Int. J. Surg. 78 2020 185 193 https://dx.doi.org/10.1016%2Fj.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed]
- 3.K. Yuki M. Fujiogi S. Koutsogiannaki COVID-19 pathophysiology: A review J. Clin. Immunol. 215 2020 108427 https://dx.doi.org/10.1016%2Fj.clim.2020.108427. [DOI] [PMC free article] [PubMed]
- 4.Smith R.D. Responding to global infectious disease outbreaks: lessons from SARS on the role of risk perception, communication and management. Soc. Sci. Med. 2006;63(12):3113–3123. doi: 10.1016/j.socscimed.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.K.V. Holmes SARS coronavirus: a new challenge for prevention and therapy J. Clin. Investig. 111 11 2003 1605 1609 https://dx.doi.org/10.1172%2FJCI18819. [DOI] [PMC free article] [PubMed]
- 6.Kudo K., Manabe T. Clinical and sociological prospectives on pandemic (H1N1) 2009. Uirusu. 2010;60(1):9–15. doi: 10.2222/jsv.60.9. [DOI] [PubMed] [Google Scholar]
- 7.Jumlongkul A. Semi-outdoor filterless air purifier for smog and microbial protection with water purifier system. Environ. Res. 2021;197 doi: 10.1016/j.envres.2021.111149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leclercq L., Nardello-Rataj V. How to improve the chemical disinfection of contaminated surfaces by viruses, bacteria and fungus? Eur. J. Pharm. Sci. 2020;155 doi: 10.1016/j.ejps.2020.105559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasan T.H., Kadhum H.A., Alasedi K.K. The Using of Ethanol and Isopropyl Alcohol as a disinfectant. Int. J. Pharm. Res. 2021;13(1) [Google Scholar]
- 10.Raber E., Jin A., Noonan K., McGuire R., Kirvel R.D. Decontamination issues for chemical and biological warfare agents: how clean is clean enough? Int. J. Environ. Health Res. 2001;11(2):128–148. doi: 10.1080/09603120020047519. [DOI] [PubMed] [Google Scholar]
- 11.Raber E., McGuire R. Oxidative decontamination of chemical and biological warfare agents using L-Gel. J. Hazard. Mater. 2002;93(3):339–352. doi: 10.1016/s0304-3894(02)00051-1. [DOI] [PubMed] [Google Scholar]
- 12.Zhao B., Liu Y., Chen C. Air purifiers: A supplementary measure to remove airborne SARS-CoV-2. Build. Environ. 2020;177 doi: 10.1016/j.buildenv.2020.106918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C., Ji W., Zhao B. Size-dependent efficiencies of ultrafine particle removal of various filter media. Build. Environ. 2019;160 doi: 10.1016/j.buildenv.2019.106171. [DOI] [Google Scholar]
- 14.Zhang Z., Bai M., Yang X., Zhang N. Study on the Fast Killing of Harmful Microorganisms in Air Space Using ⋅OH Fogdrops Based on Strong Electric-Field Discharge Plasma. IEEE Trans Plasma Sci. 2011;39(8):1701–1705. doi: 10.1109/TPS.2011.2158454. [DOI] [Google Scholar]
- 15.R. Martínez Vimbert, M. Arano Loyo, D. Custodio Sánchez, J. Garcia Raurich, P.Monagas Asensio, Evidence of OH· radicals disinfecting indoor air and surfaces in a harmless for humans method., International Journal of Engineering Research & Science (IJOER). 6(4) (2020) 26-38. https://doi.org/10.5281/zenodo.3767882.
- 16.Bai M., Zhang Z., Tian Y., Bai M. Rapidly eliminating pathogenic microorganisms in large air space using spraying •OH radicals. J Air Waste Manag Assoc. 2012;62(4):393–397. doi: 10.1080/10473289.2012.654896. [DOI] [PubMed] [Google Scholar]
- 17.J. Schulz, J, B. Endong, M. Clauss, J. Hartung, The potential of a new air cleaner to reduce airborne microorganisms in pig house air: preliminary results, Berl. Munch. Tierarztl. 126(3-4) (2013) 143-148. [PubMed]
- 18.Assaf J.C., Louka N. Novel air sterilization process for clean air production and microbial spread limitation using protection devices. OSFPREPRINTS. 2020 [Google Scholar]
- 19.H. Choi, P. Chatterjee, E. Lichtfouse, J.A. Martel, M. Hwang, C. Jinadatha, V.K. Sharma, Classical and alternative disinfection strategies to control the COVID-19 virus in healthcare facilities: a review, Environ. Chem. Lett. (2021) 1-7. https://dx.doi.org/10.1007%2Fs10311-021-01180-4. [DOI] [PMC free article] [PubMed]
- 20.Tian E., Mo J., Long Z., Luo H., Zhang Y. Experimental study of a compact electrostatically assisted air coarse filter for efficient particle removal: Synergistic particle charging and filter polarizing. Build Environ. 2018;135:153–161. doi: 10.1016/J.BUILDENV.2018.03.002. [DOI] [Google Scholar]
- 21.Tian E., Yu Q., Gao Y., Wang H., Wang C., Zhang Y., Li B., Zhu M., Mo J., Xu G., Li J. Ultralow Resistance Two-Stage Electrostatically Assisted Air Filtration by Polydopamine Coated PET Coarse Filter. Small. 2021:2102051. doi: 10.1002/smll.202102051. [DOI] [PubMed] [Google Scholar]
- 22.Liu C., Hsu P.C., Lee H.W., Ye M., Zheng G., Liu N., Li W., Cui Y. Transparent air filter for high-efficiency PM2.5 capture. Nat. Commun. 2015;6:6205. doi: 10.1038/ncomms7205. [DOI] [PubMed] [Google Scholar]
- 23.Wang S., Zhao X., Yin X., Yu J., Ding B. Electret Polyvinylidene Fluoride Nanofibers Hybridized by Polytetrafluoroethylene Nanoparticles for High-Efficiency Air Filtration. ACS Appl. Mater. Interfaces. 2016;8:23985. doi: 10.1021/acsami.6b08262. [DOI] [PubMed] [Google Scholar]
- 24.Zhang G.H., Zhu Q.H., Zhang L., Yong F., Zhang Z., Wang S.L., Wang Y., He L., Tao G.H. High-performance particulate matter including nanoscale particle removal by a self-powered air filter. Nat. Commun. 2020;11:1653. doi: 10.1038/s41467-020-15502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu H., Zhang S.C., Liu L.F., Yu J.Y., Ding B. High-Performance PM0.3 Air Filters Using Self-Polarized Electret Nanofiber/Nets. Adv. Funct. Mater. 2020;30:1909554. [Google Scholar]
- 26.Al Rai A., Stojanovska E., Fidan G., Yetgin E., Polat Y., Kilic A., Demir A., Yilmaz S. Structure and performance of electroblown PVDF-based nanofibrous electret filters. Polym. Eng. Sci. 2020;60:1186. doi: 10.1002/pen.25372. [DOI] [Google Scholar]
- 27.Maturin J.T. 8th edn. Silver Spring; Berlin: 1998. Peeler, Bacteriological Analytical Manual Chapter 3 Aerobic Plate Count, Food and Drug Administration (FDA), Bacteriological Analytical Manual Online. [Google Scholar]
- 28.Kunwar A., Tamrakar S., Poudel S., Sharma S., Parajuli P. Bacteriological Assessment of the Indoor Air of Different Hospitals of Kathmandu District. Int. J. Microbiol. 2019:1–9. doi: 10.1155/2019/5320807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nunes Z.G., Martins A.S., Altoe A.L.F., Nishikawa M.M., Leite M.O., Aguiar P.F., Fracalanzza S.E.L. Indoor air microbiological evaluation of offices, hospitals, industries, and shopping centers. Mem. Inst. Oswaldo Cruz. 2005;100(4):351–357. doi: 10.1590/S0074-02762005000400003. [DOI] [PubMed] [Google Scholar]
- 30.Konieczny P., Cegielska-Radziejewska R., Mroczek E., Dziedzic J. Analysis of Air Quality in Selected Areas of a Poultry Processing Plant with the Use of a Microbiological Air Sampler. Rev. Bras. Cienc. Avic. 2015;18(3):401–406. doi: 10.1590/1806-9061-2015-0156. [DOI] [Google Scholar]