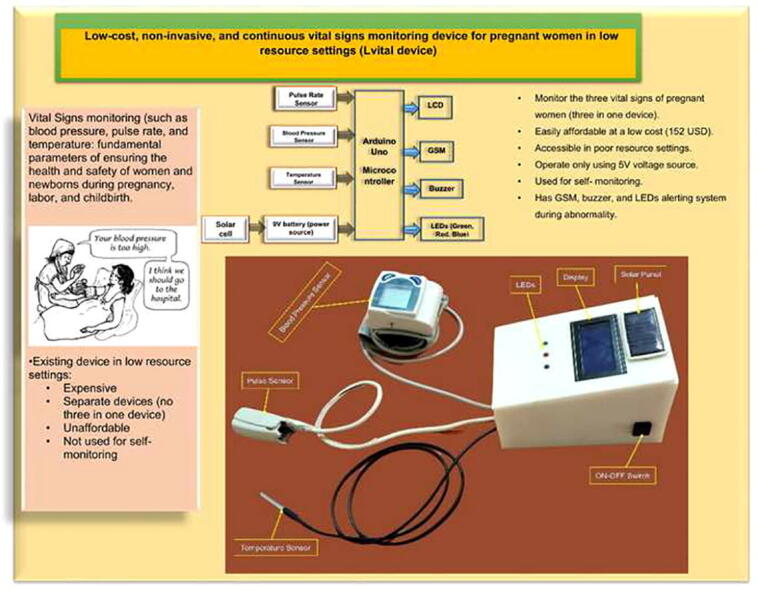
Graphical abstract
Keywords: Vital signs, Cuffless, Blood Pressure, Pulse rate, Temperature, 3D print
Abstract
Around 800 women die each day from complications of pregnancy and childbirth in the world. Vital Signs monitoring (such as blood pressure, pulse rate, and temperature) are among fundamental parameters of ensuring the health and safety of women and newborns during pregnancy, labor, and childbirth. Approximately, 10% of women experience hypertension (greater than140/90) during pregnancy. High blood pressure during pregnancy can cause extra stress on the heart and kidneys and can increase the risk of heart disease. Therefore, early recognition of abnormal vital signs, which are induced due to pregnancy can allow for timely identification of clinical deterioration. Currently used technologies are expensive and complex design with implementation challenges in low-resource settings where maternal morbidity and mortality is higher. Thus, considering the above need, here a hardware device has been designed and developed, which is a low cost, and portable for pregnant women’s vital signs (with cuff-less blood pressure, heart rate, and body temperature) monitoring device. The developed device would have a remarkable benefit of monitoring the maternal vital signs especially for those in low resource settings, where there is a high paucity of vital signs monitoring devices.
Specifications table
| Hardware name | Low cost, non-invasive, continuous monitoring device of vital signs of women during pregnancy in low resource settings (Lvital device) |
|---|---|
| Subject area |
|
| Hardware type |
|
| Closest commercial analog | Digital Blood Pressure/or Sphygmomanometer, pulse-oximeter or stethoscope, and simple thermometer |
| Open source license | GNU General Public License (GPL) v3.0 |
| Cost of hardware | 152 USD |
| Source file repository | https://doi.org/10.17605/OSF.IO/H6XKQ |
Hardware in context
Monitoring of vital signs of pregnant women is essential since she is at risk of developing many complications related to the pregnancy or the fetus [1], [2]. The statistics on pregnancy and its outcomes are staggering: an estimated 250000–280000 women die during childbirth each year. Unfortunately, many women receive little or no care during or before pregnancy [3]. Some of these complications are hypertensive disorder, gestational diabetes, and cardiac disorders [4]. Maternal and neonatal mortality remains a major issue in the majority of low and middle-income countries, particularly in rural areas. [5], [4]. There are various separated gold standard vital signs measuring devices that are currently used by pregnant women at various hospitals and health centers. Among these, a sphygmomanometer, which is a cuff-based monitoring device, is used for measuring blood pressure. On the other side, a stethoscope is used for measuring the heart sound, and a simple non-continuous thermometer is helped for measuring the temperature of the body [1], [6]. However, the aforementioned separate devices had their own drawbacks. Among these, the cuff-based sphygmomanometer is unable to continuously monitor the blood pressure, and it makes discomfort for the women, as it is cuff-based. The stethoscope is not a digital-based and continuous heart rate monitoring device. In addition to this, the woman herself can't measure her parameter solely at home. In developing countries, population growth is not fully offset by the increase in available health resources. Despite technological advances, the majority of the population still lacks sufficient medical facilities and resources. This is harsh especially the case for low-income people living in rural or remote areas. Cheap and reliable healthcare monitoring for people living in such areas, enabling rapid monitoring of the basic vital signs of large number of pregnant women and making this data available to physicians around the world [7].
So far, Zhang. Q et al proposed a highly wearable cuff‑less blood pressure and heart rate monitoring with single‑arm electrocardiogram and photoplethysmograph signals. Their system used a single-arm blood pressure monitoring system, which allows for the placement of the PPG sensor and ECG electrodes all on the left upper arm, to enable long-term daily applications with critical wearability and comfort requirements. [8]. A.R. Thelkar, and K. Dulecha proposed a blood pressure, heartbeat, and body temperature Measurement device by using GSM and a low-cost microcontroller with a health care Announcement. The proposed system uses the GPRS mobile communication system to check for patient abnormalities within the results of simulated biomedical parameters. The device would immediately notice normal or abnormal changes in these parameter measurements from current measurements for early medical assistance from a close relative or physician. In the absence of medical care or assistance, the system sends GPRS messages directly to the family, doctor, or caregiver's mobile phone. In this way, the system was developed to function as a two-way communication system that allows nurses/doctors to send SMS at any time to know the patient's status [9]. In [7] V.V. Garbhapu, and S. Gopalan innovative and efficient biomedical devices have been developed that can simultaneously and quickly monitor the vital signs of large numbers of people and send the information wirelessly to doctors or medical facilities around the world. The device was represented by a hub-and-spoke model, where the spokes are sensor nodes and consist of the microcontroller MSP430G2553 and the wireless transceiver nRF24L01 (IEEE 802.15.4). The hub consists of a Raspberry Pi 3 with the same transceiver. The data received by the hub can be sent to the doctor via the Raspberry Pi 3′s built-in IEEE 802.11 (WiFi protocol). However, the device needs a WiFi or Ethernet connection and to be connected to any sensor node. T. Shaown et al. [10], proposed a low-cost health monitoring system: a smart technological device for elderly people. This study presented a successful method for modified health support, and it has been implemented as a prototype. They proposed a reliable IoT-based health monitoring system for elderly people. The system incorporated a variety of sensors, such as EKG sensors, pulse oximeter sensors, and temperature sensors, to collect data from the patient's body and address them to the cloud for planning and testing. Moreover, they used video surveillance cameras to continuously monitor the patient's movements. If a patient's body were found to be abnormal, a pre-programmed emergency medical assistance email would be sent to a certified doctor or individual to investigate the patient's critical condition and be prescribed to resolve a serious health problem. In [11] B. Moatamed et al. proposed a low-cost scalable and potentially ubiquitous system for indoor remote health monitoring using low energy Bluetooth beacons and a smartwatch. The proposed system consists of two major components: proximity-based sensors (Beacons) and Bluetooth-enabled smartwatches. The system draws sensor fusion and activity classification by employing posture detection and indoor localization, in concert. Supplementing a dataset most amenable to technology with limited additional factors such as mood, self-efficacy, and salient feedback empowers the system to help better understand patients’ daily storylines.
Sakphrom. S et al. proposed an intelligent Medical System with Low-Cost Wearable Monitoring Devices to Measure Basic Vital Signals of Admitted Patients. Their paper describes the development of a low-cost Wireless Body Sensor Network (WBSN) for vital sign monitoring, which includes a low-cost smart wristwatch with an ESP-32 microcontroller and three sensors: heart rate (HR), blood pressure (BP), and body temperature (BT), as well as an Internet of Things (IoT) platform [12]. Adeline Boatin et.al presented the results of Verified functionality and acceptance of wireless fetal monitoring technology in pregnant women. The experiment was tested in an inpatient labor unit with full-term singleton pregnancies [13] S.Subalakshmi et.al introduces a monitoring framework to screen physiological changes in pregnant women. The attached sensors on the patient's body detect the uterine constriction, heart rate, blood pressure, etc. It will recognize the unusual conditions and send an alert to the doctor [14]. In [15] K. Elango, and K. Muniandi was proposed a low-cost wearable remote healthcare monitoring system. The system includes fall, heart rate, and temperature detections system for the patient. By viewing the collected data remotely, healthcare professionals can monitor the patient's health. This portable health monitoring system was created with easily available sensors and it has been designed to be inexpensive.
Nevertheless, all of the designed blood pressure monitoring were cuff-based, and some of them were not consider the low resource settings infrastructure for its operation. Our hardware device diagnoses three vital signs at once, provides non-invasive and continuous and cuffless blood pressure, heart rate, and temperature monitoring capability. It is easily portable; home-based monitoring and low-cost system. It saves diagnosing time and provides GSM service to send data (status of the women’s vital signs) to parents and nurses. The developed hardware is operated with a 9 V battery and is easily maintainable as enabling it to operate in low resource settings. Early warning systems are recommended for monitoring the condition of hospitalized pregnant and postnatal women to allow for the early detection and management of clinical deterioration [16].
Hardware description
Approximately 820 women die in pregnancy and childbirth every day worldwide, with 99% of these occurring in low-resource settings [17]. Hypertensive disorders complicate 10% of pregnancies and pre-eclampsia affects 2–8% [4]. In addition to this, heart disease and stroke caused more than 1 in 3 deaths (34%) [18]. Cardiovascular disease is one of the leading causes of death in many countries, accounting for over 15 million deaths worldwide. The gap between the primary side effects of heart disease and the need for reparative treatment involves a myriad of patients and can be fatal. One of the key findings of the epidemiological evidence is that using resources for early detection and treatment of heart disease is more likely to reduce heat-related mortality than improve treatment after hospitalization [9]. On the other hand, the temperature variation that occurred during the pregnancy is the other factor [6]. Therefore, they need to be monitored and controlled carefully. A high-risk pregnancy can lead both mother and fetus to death [19]. Wearable sensors, smart textiles, and other mobile health (mHealth) innovations present exciting new opportunities to enhance the diagnosis, clinical monitoring, and management of pregnancy health outside of traditional care settings [20]. However, currently, it is not easy to afford medical devices in a low-resource setting to monitor pregnant women’s vital signs adequately, and continuously. The existing devices are not easily affordable in rural areas of Ethiopia due to cost, and lack of electric power. Moreover, it is difficult to get a device, which integrates different vital parameters in one compartment. Therefore, our hardware device plays a significant role in regularly monitoring pregnancy -induced vital signs change especially in low-resource settings where the problem is a high burden.
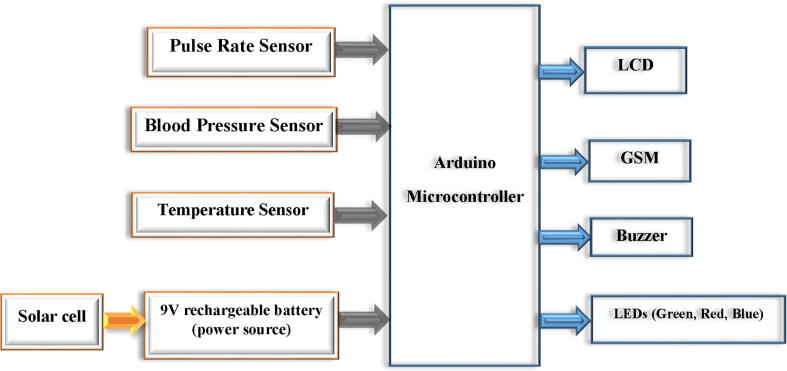
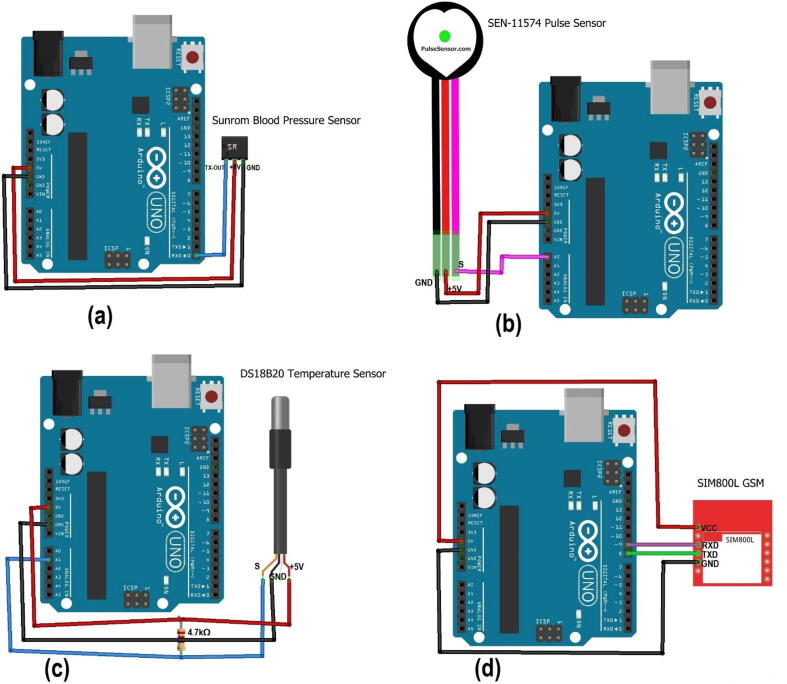
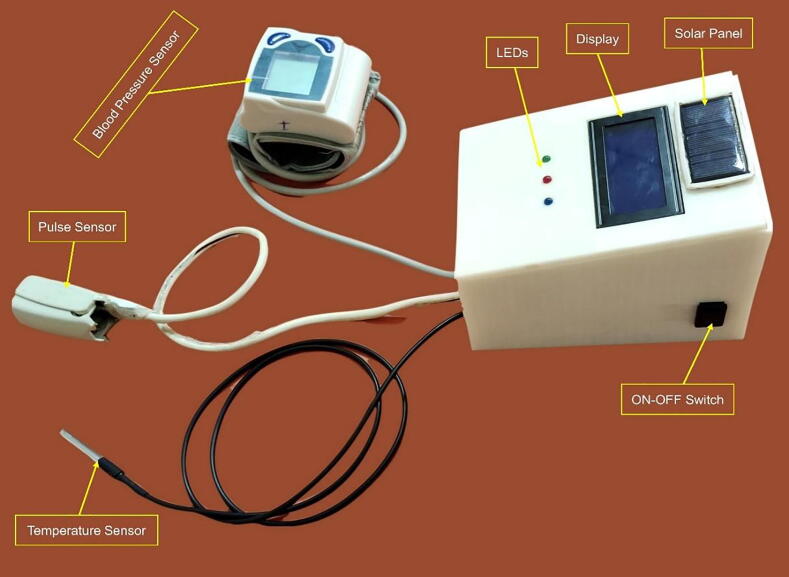
The blood pressure sensor was designed to be attached to the wrist of women. The heart rate and temperature sensors were attached to the finger, and armpit of the woman respectively. The proposed design is based on non-invasive near-infrared transmission spectroscopy optical photometry sensor. The non-invasive and cuffless sensor systems allow continuous measurement of the blood pressure and heart rate which is based on a near-infrared measurement method and Pulse Transit Time (PTT) method respectively [21]. The developed algorithm is pre-programmed in the Arduino microcontroller and integrated into the hardware device. The microcontroller processes the entire algorithm and converts the analog readings obtained from the sensors to digital values to be displayed on LCD. When the system reads an abnormal value (based on the pre-programmed on the microcontroller), it displayed the value and interprets the condition as well as makes an alarm to notify the caregivers/parents. Moreover, the GSM system enabled to sending of a short text to notify them whenever there are abnormal readings. The developed system has three light-emitting diodes (LED) to indicate normality, or abnormality of the measured parameter. Fig. 1 depicts the general block diagram and electrical circuit diagram of the device. Generally, it has the following important significant improvement upon standard practices.
Fig. 1.
The general block diagram of the device.
Effective monitoring device for hypertension/preeclampsia, and heart rate and temperature deviation.
It is a cuffless blood pressure monitoring device: the cuff-less diagnostic approach enables the hardware device to be very comfortable, and painless than the existing one.
Cost-effective: Most of the currently existing devices found in low resource settings of Ethiopia were come in the form of donations or purchased with an expensive amount of foreign currency. However, our device can be affordable at a low cost since it is an indigenous product. The prototype cost of the device is estimated to be less than 152 USD.
Power utilization: our device operates only using 5 V voltage. However, since the recommended input voltage for the Arduino Uno microcontroller is from 7 V to 12 V voltage, we have used a 9 V battery for our microcontroller as input voltage. Nevertheless, the system draws only 5 V to operate its function. In addition to this, our device incorporated a solar empowered mechanism, which helps to recharge the battery and make the system electrical power independent. This would help the device to be easily accessible for low-resource setting areas.
Electrical components
We were designed a microcontroller heart rate, temperature, and blood pressure monitoring device which helps in effective and early monitoring of vital signs of pregnant women. The design is low cost, affordable, simple to use, effective, and maintainable, it highly solves the problem stated and is highly needed by the end-users of the device. The design will incorporate the following different materials:
Arduino Uno R3: a microcontroller used to control the sensors.
Pulse sensors: used to sense and measure the heart rate in the beat per minute.
Temperature sensors: used to sense and measure the temperature in degree centigrade.
Blood Pressure sensor: used to sense and measure the blood pressure in mmHg.
GSM: used to send a short text to physicians or nurses/parents during an emergency.
LCD: to display the vital signs/ body parameter with their respective unit.
Buzzer: to make a sound during abnormality.
Green LED: indicates normal values of the parameter.
Red LED: it will blink during abnormal cases.
Blue LED: show the heart beating status.
9 V rechargeable battery and 9 V solar cell: used as a source of power.
The following Fig. 1 shows the general block diagram of the device.
Mechanical components
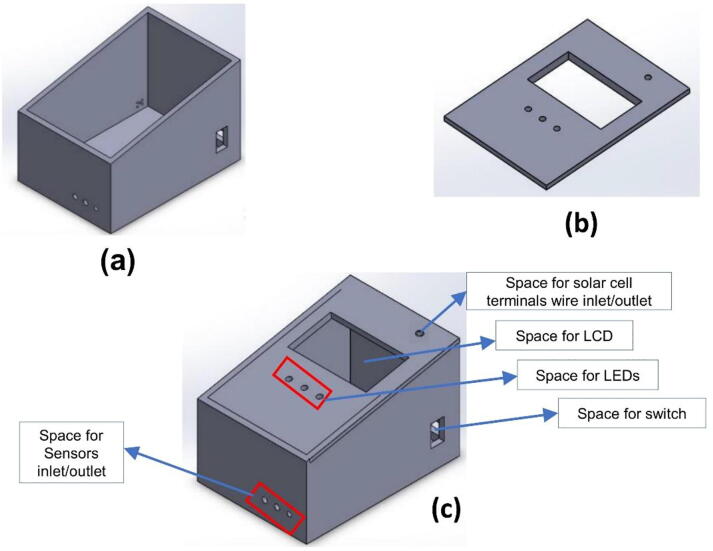
The 3D printed plastic box was used to cover the developed circuit design in one compartment. The box was first designed using Solid Work software. The 3D design has an inclined box shape in which the height at the front side is a little bit longer than the back side. The box holds, all the circuit design together including the microcontroller and its connection with all components (Fig. 2a. It has a hole for LCD (Fig. 2 b), an ON-OFF switch, USB connection, and sensors insertion in different places of the main box (see Fig. 2c. The volume of the box is 1875 cm3. You can find the 3D STL designed file at https://doi.org/10.17605/OSF.IO/H6XKQ
Fig. 2.
a) The main 3D designed box where the microcontroller and other circuit was placed, b) The cover lid, c) The final assembled 3D design.
Arduino Uno software
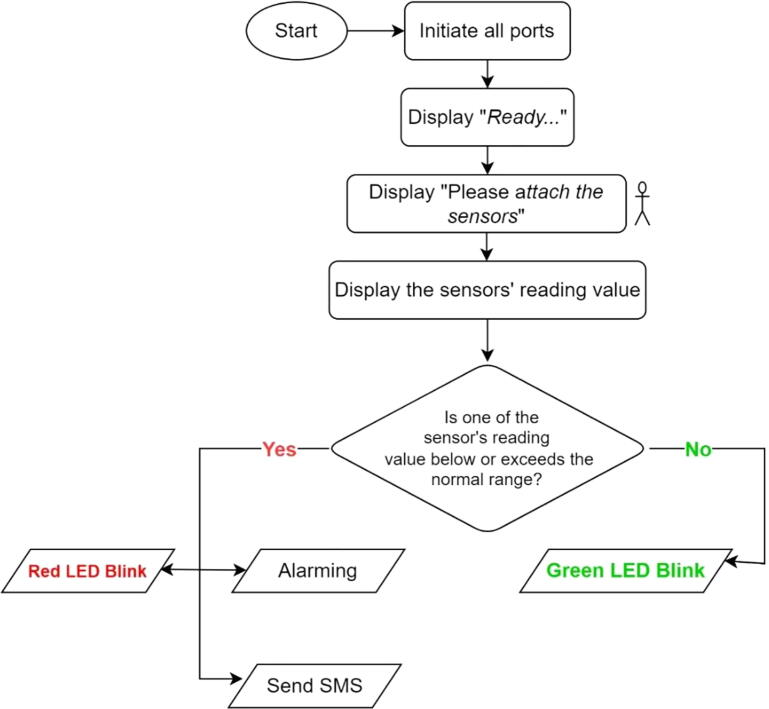
We have used Arduino IDE software to develop the script. Users can create changes here if the need for customization arises. The Arduino code is operated as follows. As shown in Fig. 3, when the device is started to operate, first it initiates all necessary ports (pins) including both analog and digital pins. Then, the device order the user to be ready for the next action. To do this, the device displays the “ Ready…” text on the LCD. During this time the user needs to be ready for attaching all sensors to the correct site. After a few seconds, the Lvital device order the user to attach the sensors to the appropriate site of the body. To do this, the machine displays the “ Please attach the sensors“ text on the LCD. After waiting a minute, the device started to display the reading values from the sensors on the LCD. Now, after waiting a minute, if any of the reading values among the three sensors were exceeded or lowered below the normal range, the system would make an alarm, red LED would blink and it sends a short text message to the caregiver phone number. However, if the reading values are within the normal range only the green LED would be a blink. When these parameters are normal the device would not send a text to the caregiver.
Fig. 3.
The general flow chart of the Arduino firmware (Arduino code).
Design files summary
| Design file name | File type | Open source license | Location of the file |
|---|---|---|---|
| D1 | Solid Work (STL, SLDPRT and DXF) files | GNU General Public License (GPL) 3.0 | Folder: ‘STL files for 3D printing components’https://doi.org/10.17605/OSF.IO/H6XKQ |
| D2 | Figures (images) | GNU General Public License (GPL) 3.0 | Folder: ‘Electrical components connection’https://doi.org/10.17605/OSF.IO/H6XKQ |
| D3 | Figures (images) | GNU General Public License (GPL) 3.0 | Folder: ‘Mechanical (box design) and Final Prototype’https://doi.org/10.17605/OSF.IO/H6XKQ |
| D4 | Zip file (.ino format file) | GNU General Public License (GPL) 3.0 | https://doi.org/10.17605/OSF.IO/H6XKQ |
From the above table, D1 is the 3D design STL, SLDPRT, and DXF files, which include both the case (main box) and the coverlid STL file that has been developed using solid work software. D2 represents the electrical components connection file and it includes the general wiring/schematic diagram of the system. Moreover, it displays each sensor, and LCD, LEDs, and GSM connection procedure with the microcontroller. D3 is enumerated for the box design and final prototype file, which includes the integrated 3D design file in the figure, and the final developed real prototype. In addition to this, the D4 is represented for the Arduino code, and how to uploading of the Arduino code to the board instruction. All the software libraries link required for running the components (including sim800L, OneWire, PulseSensorPlayground.h, and DallasTemperature.h libraries) is provided in the comments section of Arduino code itself.
Bill of materials summary
| Designator | Component | Number | Cost per unit in USD | Total cost in USD | Source of materials | Material type |
|---|---|---|---|---|---|---|
| U1 | Arduino Uno R3, ATmega328P | 1 | 11.67 | 11.67 | https://bit.ly/3AqZyke | Electronics |
| U2 | GSM, SIM800L | 1 | 4.25 | 4.25 | https://bit.ly/3nNWX0g | Electronics |
| U3 | Blood Pressure Sensor, Sunrom | 1 | 40.83 | 40.83 | https://bit.ly/3nJGSsJ | Electronics |
| U4 | Pulse Sensor - SEN-11574 | 1 | 24.95 | 24.95 | https://bit.ly/3lBJjL3 | Electronics |
| U5 | Vktech DS18b20 Waterproof Temperature Sensor | 1 | 14.49 | 14.49 | https://amzn.to/2Xys1Gc | Electronics |
| U6 | Standard LCD, 16x4 | 1 | 17.95 | 17.95 | https://bit.ly/3lH4Yl0 | Electronics |
| U7 | Passive buzzer | 1 | 1 | 1 | https://amzn.to/2Xxxr4j | Electronics |
| U8 | Jumper Wire | 1pack | 2.69 | 2.69 | https://amzn.to/3hNmbIv | Electronics |
| U9 | LED,(Red, green, yellow) | 1pack | 8.99 | 8.99 | https://amzn.to/3EB8Rkq | Electronics |
| U10 | Resistor (470 Ω) | 4 | 0.01 | 0.04 | https://amzn.to/3Aoxn5r | Electronics |
| U11 | Stripboard | 1pack | 1 | 1 | https://redirect.is/9ii8zhz | Electronics |
| U12 | ON-OFF Switch | 1 | 0.5 | 0.5 | https://redirect.is/86wv5ou | Electronics |
| U13 | 9 V mini solar panel | 1 | 5.45 | 5.45 | https://bit.ly/3D9BCCQ | Electronics |
| U14 | 9 V NiMH rechargeable battery | 1 | 7.72 | 7.72 | https://bit.ly/3sYryLm | Electronics |
| U15 | 3D Plastic box(15 cm _ 12 cm _ 7 cm) | 1 | 10 | 10 | Local Store | Plastic |
Build instructions
3-D print casing
The main box and lid case using a 3-D design with infill 30% and layer 0.2 mm has been printed. We recommend using PLA + 3D printing filament with a diameter of 1.75 mm. Fig. 4 below shows the result of the 3-D print of the box case from the top view. The 3D case design was purposefully built more robust as a prototype and could be made smaller if the internal circuit is made on PCB.
Fig. 4.

The 3D printed main box.
Connect LCD, LEDs, and buzzer to Arduino Uno
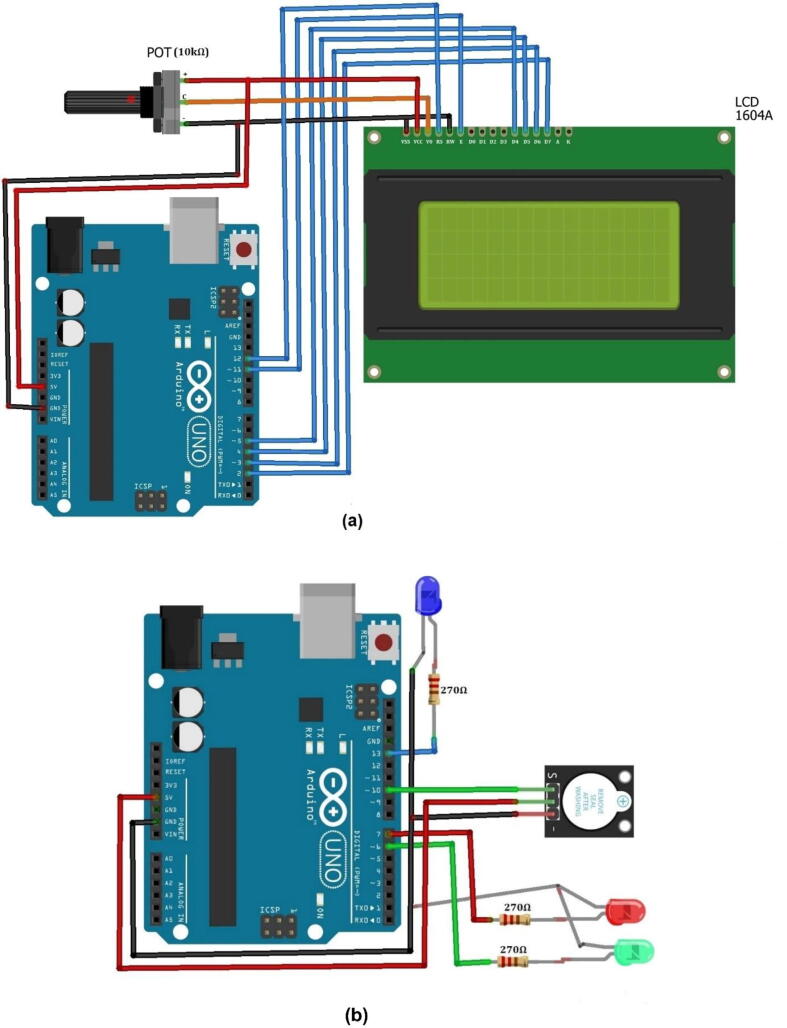
The next step was connecting the LCD, LEDs, and buzzer with Arduino Uno (see Fig. 5a & b. We were used a 16x4 dimension LCD. As shown in Fig. 5a the LCD’s VSS (GND) pin is connected to the GND pin of the Arduino microcontroller and one end (usually the negatively labeled end) of the 10 k potentiometer. Pin 2 (Vcc) is connected with the 5 V, Vcc pin of Arduino and the positive end of the 10 k potentiometer. The third pin of LCD, Vo (the contrast control pin) is connected with the middle pin of the 10 k potentiometer. The 10 k potentiometer is used to control the contrast of LCD manually. The RS (the register selector) and E (the enabler) pins of LCD are connected with the 11th and 12th pins of Arduino respectively. The LCD pins from D4 to D7 are connected with pins 5, 4, 3, 2 of Arduino respectively. On the other hand, from Fig. 5b, the anode (terminal + ) of green, red, and blue LEDs are connected to 270 Ω resistors solely and the other terminal of the resistors were connected to the 6th, 7th, and 13th pins of Arduino respectively. The cathode (terminal -) of LEDs was connected to the GND pin of the Arduino. Here, the ballast resistors (270 Ω) were used to limit the current through the LEDs and prevent excess current that can burn out them. Moreover, the signal (S) pin, the anode terminal, and the cathode terminal of the buzzer sensor were connected with the 10th, the + 5 V, and the GND pins of Arduino respectively. All these connections were declared in the code accurately.
Fig. 5.
Shows the connection of a) LCD, b) LEDs and buzzer with Arduino, and c) LCD and LEDs with coverlid.
Integration of the three sensors and GSM with Arduino
As we have tried to mention in this paper, three sensors (blood pressure sensor, pulse rate sensor, and D218B20 digital temperature sensors) were used to construct the prototype. We used sunrom type of blood pressure sensor. It is a blood pressure sensor with serial output for external projects of embedded circuit processing and displaying. It has three pins with signal, power, and ground output. It can sense the systolic, diastolic, and pulse rate of the human body. It comes with a compact design, which can be easily fitted over a wrist like a watch. It is easy to use wrist style and eliminates pumping. The measured data could be displayed either on the compact sensor display itself or/and on the external 16x4 size LCD of our device. Nevertheless, even if it includes a pulse rate sensor inside the component and can able measure the pulse rate along with the systolic and systolic blood pressure, it would not continuously measure the pulse rate. Thus, in our device, we used the SEN-11574 pulse sensor for the continuous measuring of pulse rate. The open-source Arduino code and more explanations of the sunroom blood pressure sensor are provided at [22]. The sunrom type of blood pressure sensor has three pins (the data; TX-OUT: transmitting, power (+5V), and ground pin (GND)) pins. Thus, the TX-OUT pin, +5V, and GND pins of the sensor were connected to the Rx (0), +5V, and GND pins of Arduino Uno respectively (see Fig. 6a. On the other hand, as shown in Fig. 6b, the SEN-11574 pulse sensor pins of signal (S, pink color), power (+5V, red color), and ground (black color) were connected to the Analog pin (A0), +5V, and GND pins of Arduino Uno respectively. In addition to this, the D218B20 digital temperature has three pins. The power and ground pins of the sensor were connected to their respective pins on the Arduino Uno pins. However, the signal pin of the sensor (the yellow color wire) was connected to pin D2 of the Arduino Uno, and the power pin of the Arduino with 4,7kΩ resistor in between connections (see Fig. 6c. We used a 4.7kΩ ohm resistor between VDD (power) and Signal of the waterproof temperature sensor (DS18B20). When the device does not have its own power supply, it uses the power going through the pull-up resistor-thus with this resistance (4.7 k) it is powered properly. In addition to this, the Tx and Rx pins of GSM were connected to pins 8 (as Rx), and 9 (as Tx) of Arduino respectively and it has been also declared in the code accordingly (see Fig. 6d.
Fig. 6.
Integration of (a) blood pressure sensor, (b) pulse rate sensor, (c) temperature sensor, and (d) GSM with Arduino.
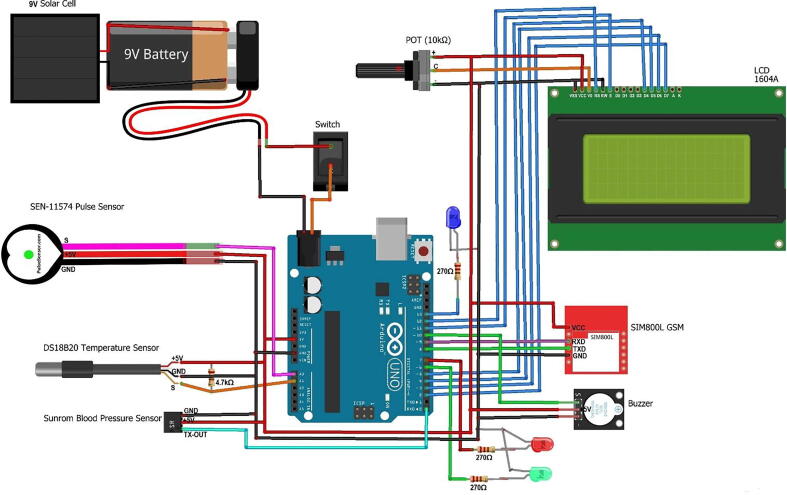
The following Fig. 7 shows the general wiring/schematic diagram shows all the aforementioned partially connected components along with the 9 V solar panel, which helps to charge the 9 V battery. According to the solar hub idea mentioned in [23], whenever we select a solar panel that fits a battery, we have to consider the voltage of that battery and always the voltage of the battery and the voltage of the panel should be the same. Therefore, we used 9 V solar panel to charge a 9 V battery. Because 9 V battery needs 9 V voltage to charge the battery. However, if your solar panel gives a higher voltage than your battery needs, it would damage your battery instantly. So, in such a case, you have to use a voltage controller to lower the voltage to the aforesaid acre and connect it to the battery.
Fig. 7.
The general wiring/schematic diagram of the hardware.
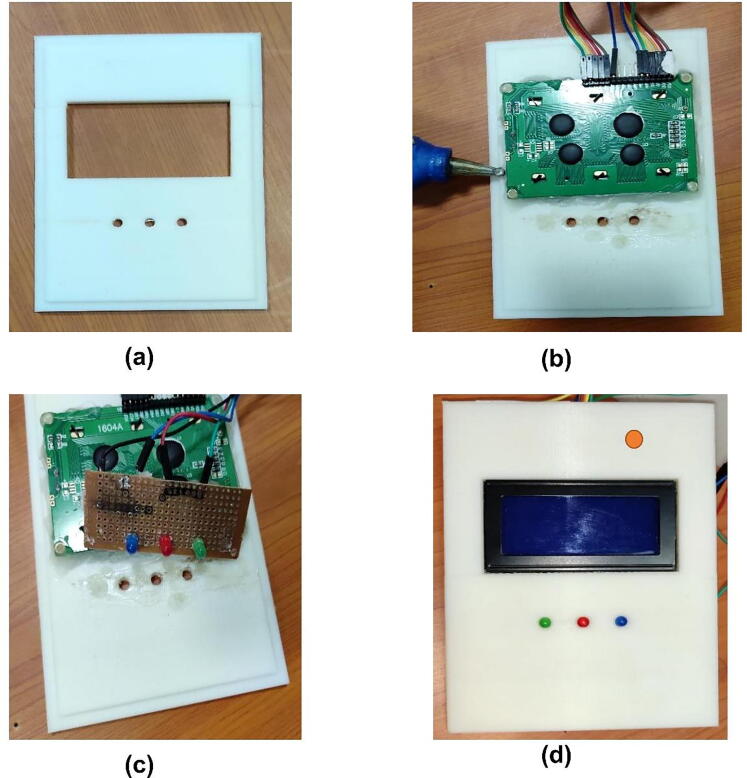
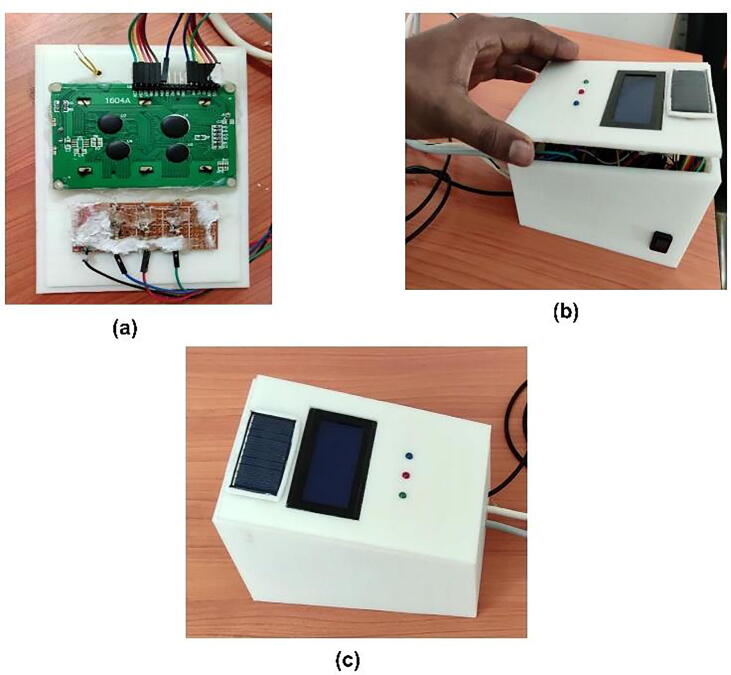
Mounting LCD 16x4 and LED in the upper case (lid)
The 16x4 LCD which was connected to the Arduino board using jumper wires was finally mounted into the lid of the developed prototype. Fig. 8a shows the backside of the lid/cover with space for mounting LCD and the three LEDs. After mounting the LCD it has been stickied using glue (see Fig. 8b. Before mounting the LEDs to the lid, it has been mounted on the stripboard (see Fig. 8c to bring them common ground and finally mounted on the lid. Fig. 8d shows the LCD and LEDs mounted in the upper case (lid).
Fig. 8.
LCD and LEDs mounting in the upper case. (a) the lid with LCD and LEDs space, (b) attaching the LCD with lid using glue. (c) mounting the LEDs on stripboard and lid, (d) front side of the lid after mounting LCD and LEDs.
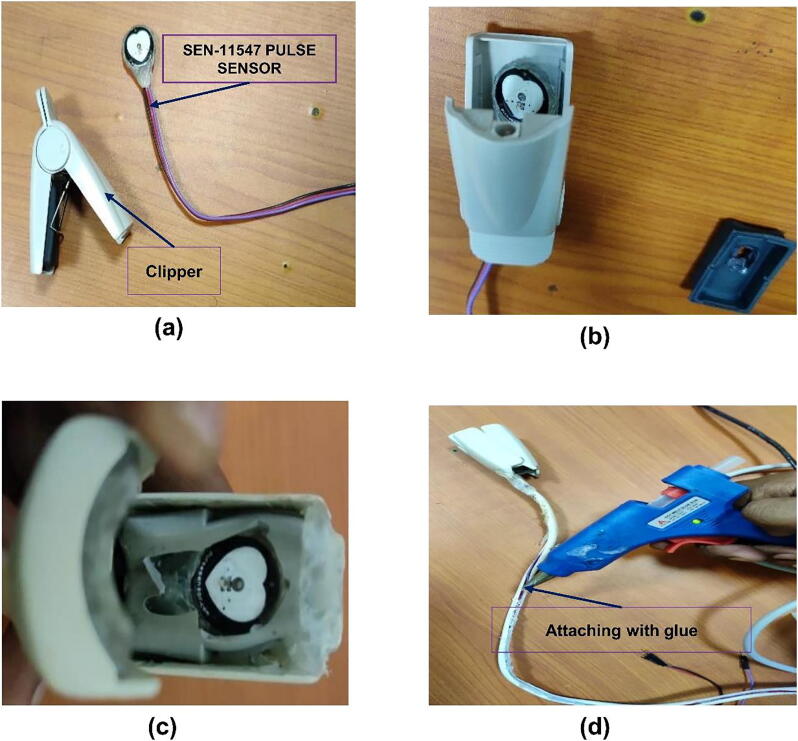
Connect pulse sensor SEN 11,574 with the plastic enclosure
For esthetics purposes, instead of leaving the SEN-11574 pulse sensor as it is, we enclose the sensor part /tip part into the clipper taken from a non-functional pulse-oximeter finger clipper to make it more esthetic in the final prototype (see Fig. 9a & b. Fig. 9a, b & c shows the bare sensor and the clipper, the sensor inside the clipper, and the final sensor attached with the clipper using glue respectively. We used glue to attach the sensor with the clipper. In the same fashion, we insulated the wires (the three wires of the sensor) using plastic coating taken from the same non-functional pulse oximeter and attached it with glue as shown in Fig. 9d.
Fig. 9.
Shows steps to the pulse sensor SEN-11574 with the plastic enclosure. (a) clipper with the sensor, (b) putting the sensor inside the clipper, (c) attaching the sensor with glue inside the clipper, (d) attaching the plastic cover with glue.
Fixing the solar panel to the hardware
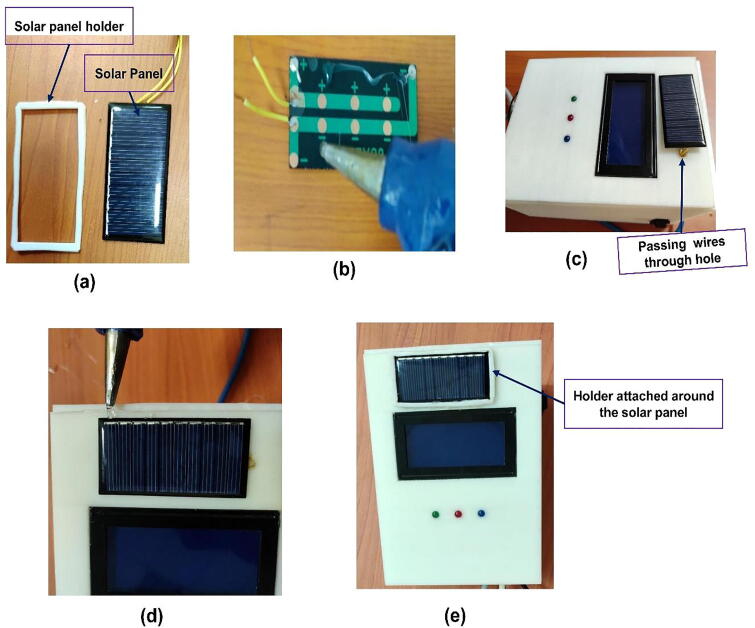
The solar panel has been fixed on the top of the lip/case cover just above the LCD (see Fig. 10e. Since the wire from the positive and negative terminals of the solar need to be connected to the respective terminals of the battery, a small hole has been made to insert the wires from the solar panel (Fig. 10c to the rechargeable battery inside the case. We used glue at the backside of the panel to attach it to the lid(cover case) of the prototype (Fig. 10b. Moreover, a holder made from plastic has been used around the solar panel to fix the solar panel in its position (see Fig. 10a, d, & e.
Fig. 10.
Mounting the solar panel on the hardware. (a) the solar panel with holder, (b) putting glue at the backside of the solar panel (c) attaching the panel on the top of the lid. (d) putting glue to attach the panel holder, (d) holder attached around the solar panel to strengthen the solar panel in its position.
Closing the main case with upper case (lid)
The lid has been designed to be directly fit into the lower case by itself around the edge without using another attachment (Fig. 11a. Therefore, by taking the backside of the lid (Fig. 11a, we easily mounted it on the lower case (Fig. 11b. As shown in Fig. 11c the lid was directly fixed to close on the lower case.
Fig. 11.
Steps to close the lower case with the upper case (lid). (a) backside of the lid, (b)mounting the backside of the lid to the lower case, (c) lid fixed to the lower case.
Fig. 12 shows the final integrated prototype. It includes all the 3 sensors, the 16x4 LCD, and the microcontroller Arduino Uno along with all the circuit connections covered within the 3D printed box.
Fig. 12.
The final concept proof developed prototype of the device.
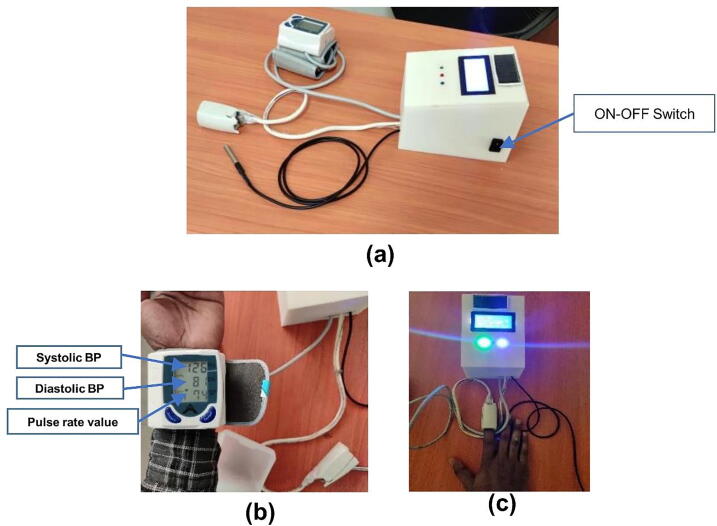
Operation instructions
To operate our device just follow the following steps. First, switch ON the device from its OFF state (see the ON-OFF switch in Fig. 13a. This helps the microcontroller to draw 9 V voltage from the DC battery integrated with the microcontroller. Next, attach the sensors to the appropriate place of the body (blood pressure on the wrist, pulse rate to the finger, and temperature sensor to the armpit (see Fig. 13b&c). the person needs to sit upright, and stable position while the sensors are attached to their body. Since the device has no hazardous effect on the human body, anyone can test the performance of the concept-proof device by measuring their body parameter. Then wait for a few seconds, and watch the value on the display. However, since the device is not yet validated by the authorized organization, it is highly forbidden to use for clinical purposes and the testing measured value was not used for clinical diagnosis.
Fig. 13.
Operation and testing of the device. (a) shows ON the device, (b) measuring blood pressure, (c) measuring the continuous pulse rate and temperature.
Validation and characterization
Blood pressure, pulse rate, and temperature measurement validation
For validation, 20 volunteers were participated to test their blood pressure, pulse rate, and body temperature from the sensor attached to the wrist, point finger, and armpit sites respectively. We compared our device measurement value with their respective reference device. We have used a sphygmomanometer, pulse oximeter, and thermometer as a reference device to measure blood pressure, pulse rate, and temperature to compare with our developed device respectively.
Above Table 1shows the one-time/punctual measurement of 20 volunteers. From the table, we have determined the accurate measurement value of the prototype device compared with the measurement results of a device with a sphygmomanometer, pulse oximeter, and thermometer to measure blood pressure, pulse rate, and body temperature respectively. The comparison is shown as a percentage error of all the three parameters calculated using the following Equations 1–3 [10].
| (1) |
| (2) |
| (3) |
Table 1.
Comparison of our device performance level with gold standard methods.
| Participant number | Blood pressure testing | Pulse rate testing | Temperature testing | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Systolic blood pressure measurement | Diastolic blood pressure measurement | Using pulse oximeter device | Using Lvital device | % Error | Using thermometer | Using our device | %Error | |||||
| Using mercury sphygmomanometer (in mmHg) | Using Lvital device | % Error | Using sphygmomanometer | UsingLvital device | % Error | |||||||
| 1 | 110 | 110 | 0 | 70 | 68 | 2.86 | 68 | 69 | 1.43 | 37 | 36.8 | 0.54 |
| 2 | 125 | 126 | 0.8 | 80 | 81 | 1.25 | 72 | 74 | 1.37 | 36.7 | 36.6 | 0.27 |
| 3 | 105 | 105 | 1 | 60 | 61 | 1.67 | 70.1 | 68 | 0.00 | 37.1 | 37 | 0.27 |
| 4 | 120 | 118 | 1.81 | 80 | 78 | 2.50 | 71 | 70 | 1.43 | 36.9 | 36.7 | 0.54 |
| 5 | 110 | 110 | 0.85 | 64 | 63 | 1.56 | 72 | 71 | 1.41 | 37.2 | 37 | 0.54 |
| 6 | 125 | 123 | 0 | 79 | 78 | 1.27 | 69 | 70 | 1.45 | 36.9 | 36.8 | 0.27 |
| 7 | 110 | 110 | 2.5 | 70 | 72 | 2.86 | 72 | 70 | 0.00 | 37.4 | 37.1 | 0.80 |
| 8 | 120 | 120 | 0 | 75 | 75 | 0.00 | 72 | 73 | 1.45 | 37.2 | 37 | 0.54 |
| 9 | 100 | 102 | 2 | 65 | 67 | 3.08 | 73 | 74 | 1.39 | 36.9 | 36.8 | 0.27 |
| 10 | 105 | 106 | 2.72 | 75 | 74 | 1.33 | 74 | 76 | 2.94 | 36.88 | 36.85 | 0.08 |
| 11 | 100 | 103 | 3 | 60 | 61 | 1.67 | 69 | 70 | 1.45 | 37 | 37.3 | 0.81 |
| 12 | 120 | 120 | 0 | 75 | 73 | 2.67 | 70 | 70 | 0.00 | 36.9 | 37 | 0.27 |
| 13 | 115 | 118 | 2.60 | 70 | 72 | 2.86 | 71 | 71 | 0.00 | 36.8 | 36.7 | 0.27 |
| 14 | 123 | 120 | 2.43 | 80 | 78 | 2.50 | 76 | 75 | 1.32 | 37.3 | 37.1 | 0.54 |
| 15 | 110 | 111 | 0.90 | 75 | 75 | 0.00 | 70 | 69 | 1.43 | 37 | 36.95 | 0.14 |
| 16 | 120 | 120 | 0 | 80 | 80 | 0.00 | 68 | 67 | 1.47 | 36.77 | 36.78 | 0.03 |
| 17 | 110 | 110 | 0 | 64 | 64 | 0.00 | 76 | 74 | 2.63 | 36.9 | 37 | 0.27 |
| 18 | 115 | 112 | 2.60 | 70 | 68 | 2.86 | 72 | 73 | 1.39 | 37 | 37.2 | 0.54 |
| 19 | 120 | 120 | 0 | 80 | 80 | 0.00 | 71 | 72 | 1.41 | 36.88 | 37 | 0.33 |
| 20 | 125 | 123 | 1.6 | 85 | 82 | 3.53 | 74 | 73 | 1.35 | 37.1 | 37.2 | 0.27 |
| Average % error | 0.82 | 1.72 | 1.54 | 0.476 | ||||||||
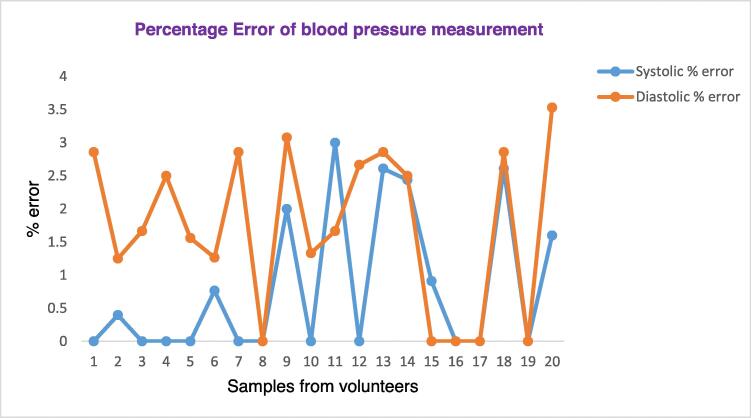
Fig. 14 below shows the plot of the calculated systolic and diastolic percentage error obtained by comparing our device with the gold-standard device, a sphygmomanometer. As depicted in the Figure, among the 20 samples, in 10 of the systolic measurements our device (Lvital device) shows the same measurement with the gold standard, sphygmomanometer. Moreover, the minimum error calculated was 0.5 with a maximum of 3. Nevertheless, the systolic measurement of our device is accurate with an average error factor of 0.82%. On the other hand, the diastolic measurement shows less accurate as compared with the systolic value. The proportion of calculating error factor of the diastolic measurement is 1.72%.
Fig. 14.
Percentage Error factor of blood pressure measurements.
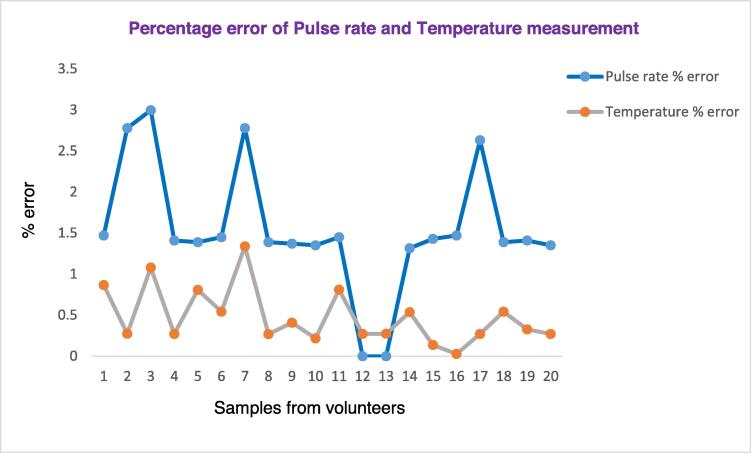
Fig. 15 below shows the plot of the pulse rate and body temperature measurements percentage error obtained by comparing our device with the gold-standard device, pulse oximeter, and thermometer respectively. As shown in Fig. 15, among the 20 samples from volunteers, the temperature measurement shows a good accuracy than the pulse rate. In all measurements, the percentage error of the temperature is low, with an average error factor of 0.476%. This shows our temperature-measuring sensor has less error. Moreover, the pulse rate sensor is accurate with an error factor of 1.54%.
Fig. 15.
Percentage error factor of pulse rate and temperature measurement.
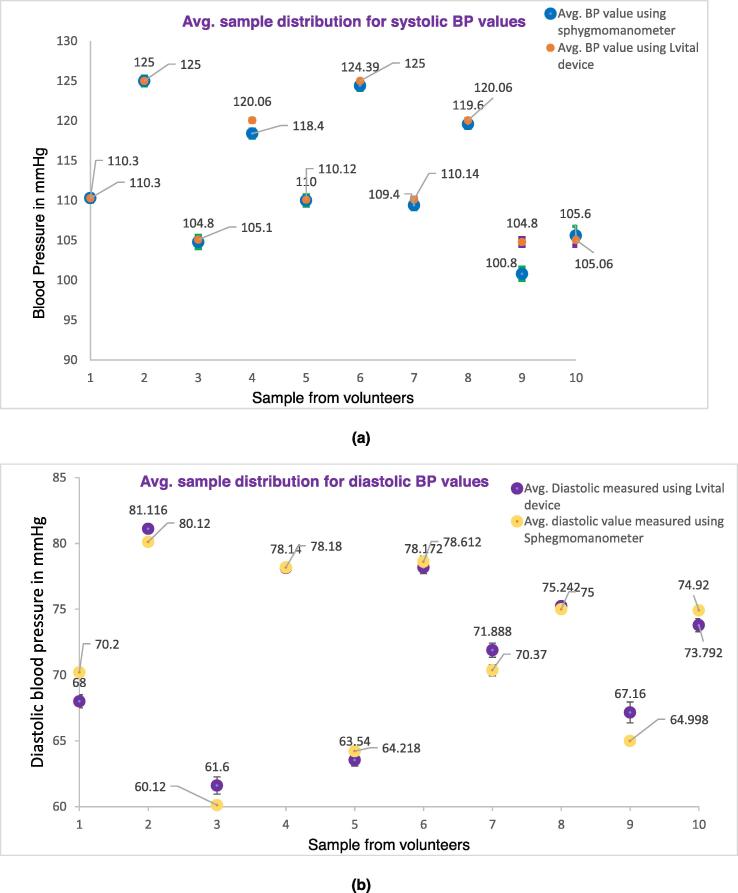
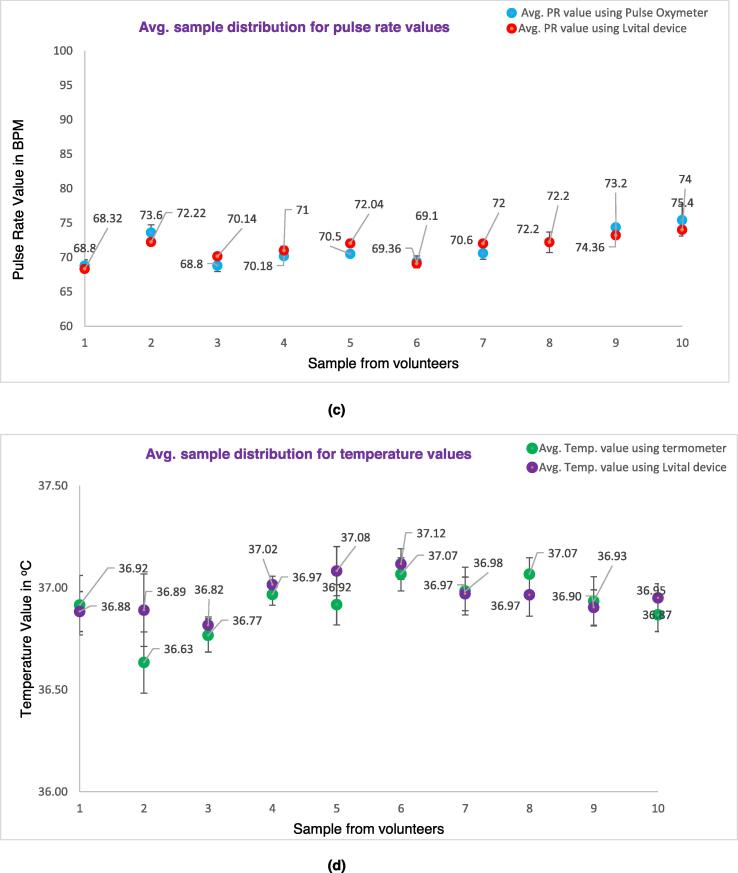
The following Fig. 16 shows the error bar distribution of an average of 6 samples (made with three operators) recorded for blood pressure (both systolic and diastolic value) (see Fig. 16 a & b), pulse rate (see Fig. 16c, and body temperature (see Fig. 16d and compared with a sphygmomanometer, pulse oximeter, and thermometer respectively. To do this the mean and standard deviation of each measurement were compared with their respective gold standard device. The detailed calculation of the measurement is provided as supplementary material in the excel file. The result shows that the measured value of our device is not far from the gold standard measurement of their respective devices. From the repeated sample measurement, the systolic, diastolic, pulse rate, and temperature have 96.4%, 93.5%, 97.43%, and 98.5% accuracy as compared with the gold standard/reference devices respectively.
Fig. 16.
Distribution of the average samples recorded by the Lvital device and the comparator device, of the respective parameter. a) Systolic blood pressure measurement, b) diastolic blood pressure measurement, c) Pulse rate measurement, d) Body temperature measurement.
In addition to this, the following Table 2 below shows the repeatability, reproducibility, combined R&R, product variation, and total variation estimates along with the percentage of the total variation and the decision made based on the range and average method of measurement system analysis. The detailed calculations of each parameter are found in the supplementary material in excel form.
Table 2.
Shows the reproducibility of the device along with other parameters
| Source | Estimates | % of total variation | Decision | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Systolic | Diastolic | PR | Temp. | Systolic | Diastolic | PR | Temp | Systolic | Diastolic | PR | Temp | |
| Repeatability | 3.79 | 2.86 | 1.83 | 0.26 | 9.68 | 9.01 | 13.92 | 24.53 | Acceptable | Acceptable | Acceptable | Acceptable with condition. |
| Reproducibility | 1.54 | 0.99 | 1.38 | 0.087 | 3.93 | 3.12 | 10.49 | 8.21 | Acceptable | Acceptable | Acceptable | Acceptable |
| Combined R&R | 4.1 | 3.03 | 2.29 | 0.28 | 10.5 | 9.54 | 17.41 | 26.42 | Acceptable | Acceptable | Acceptablewithin a condition | AcceptAble within condition |
| Product variation | 38.96 | 31.59 | 12.95 | 1.02 | 99.49 | 99.50 | 98.48 | 96.23 | No criteria given | – | – | – |
| Total Variation | 39.17 | 31.74 | 13.15 | 1.06 | ||||||||
The estimates are calculated using the range and average method of the measurement system and were calculated as percentages of the total variation. These proportions are compared to the decision criteria mentioned by the range and average method in [24]. As shown from Table 2 above, the repeatability of systolic, and diastolic was 9.68, 9.01 respectively, and according to the range and average standard; these values were below the acceptable limit of 10%. Moreover, the repeatability of pulse rate and temperature measurement was 13.92% and 24.53% and these values are between 10% and 30% of repeatability, which are acceptable for certain considerations. According to the standard, it is acceptable depending on the gage settings, measurement method followed by the operators, calibration of the device, etc. The Reproducibility of systolic, diastolic, pulse rate, and temperature measurement is 3.93%, 3.12%, 10.49%, and 8.21%. According to the range and average standard, these values were below the acceptable limit of 10%, with only the pulse rate being in the marginal of the limit. Therefore, these variabilities for the measurement system are satisfactory and adequate. Moreover, the combined R&R is between 10% and 30 % range and they are acceptable depending on the measurement settings, measurement method followed by the operators, calibration. Generally, from the measurement, we conclude that our device, Lvital, is reproducible enough and it is adequate according to range and average standard.
Green and red LED validation
According to [25], the normal vital signs change with age, sex, weight, exercise capability, and overall health of pregnant women. Normal vital sign ranges for the average healthy adult women while resting are, 90/60 mmHg to 120/80 mmHg, 60 to 100 beats per minute, and 36.5 °C to 37.3 °C for blood pressure, pulse rate, and body temperature respectively. Thus, in our device algorithm, red LED, and active buzzer turned ON when the blood pressure is greater than 120/90 mmHg and less than 90/60 mmHg (this include the elevated, stage 1, and stage 2 hypertension), heart rate is above 100 BPM, and less than 60 BPM. Moreover, it would continue in an active state if the temperature value is above 37.4%, and less than 36.3 °C (in the armpit measurement). In addition to this, during this abnormal case, the GSM would be triggered to send short SMS to show the sign of abnormality to the physician or their parents. On the other hand, a green LED is turned on to show the normal measurement range. The blue LED is always turned on whenever there is pulse beat signal is sensed from the person.
Power consumption and battery life validation
The power characterization of Lvital device was conducted with voltage and current measurement resolution of 0.01 V and 0.001 A, respectively using USB Meter UM24 (RuiDeng Tech., China). The maximum current consumption during LED, buzzer, and GSM turn on (when the sensors detect people at their respective site) is 130 mA. On the other hand, the minimum current consumption during LED, buzzer, and GSM turn off (when the sensor does not detect people) is 50 mA. The voltage is stable at around 4.5 V to 5.00 V. To measure the power consumption of our device, the total current is assumed by 30% for the maximum current and 70% for the minimum current. The power consumption of Lvital device is calculated as follows using Equations 4–6 from [26].
| (4) |
where W is power consumption, V is voltage, is the maximum current, and is the minimum current. The energy of the one nickel-metal hydride battery was calculated by 80% of the total energy.
| (5) |
| (6) |
where is battery lifetime. From the calculation, our device will work without charging up to . On the other hand, the Lvital device can continue operating while the hardware is charging because of the solar cell used. The solar panel we used has 9 V voltage, 2 W power, 222 mA current capacity. The charging time would depend on the AMPERES (amp) and VOLTAGE of the battery, and WATT (W) of the solar panel [23]. First, we should know how much power will be consumed to fully charge a battery. We can get the power in electricity by multiplyingvolts (v) fromamperes (amp). So we can get the power required to fully charge a battery by simply multiplying the voltage value of the battery and the amperes of the battery.
Therefore, the power is calculated using Equation (7) as:
| (7) |
This means to fully charge a 9 V, 2A battery we need an 18 Watt-hours (18 W) power supply. As we have 9 V 2-Watt solar panel power supply it only supplies 2-Watt power per hour. So, it takes around 9 h for the solar panel to supply the total power battery required. In addition to this, we can calculate the charging hour using the current capacity of the charger using Equation (8) and its representation in Equation (9) as [27]:
| (8) |
| (9) |
Accordingly, with 2000mAh battery capacity and 222 mA solar panel output charging capacity the total battery charging time was,
s
This same as calculating the charging time using the power of the solar panel. However, in reality, each type of battery has its own discharging factor. Due to this their total charging hour would increase from the one calculated using the above equation. To calculate the real battery charging hour the above calculated hour was multiplied by the discharging factor of the battery. Since the discharging factor of the NiMH battery is 1.2, the total charging hour of the battery was approximated to become (9x1.2) 10.8 hrs (10 h and 48 min). However, for the energy of the one nickel-metal hydride battery which was calculated by 80% of the total energy that is (14.4 W), the total charging time is approximated to 7.2 h multiplied by 1.2 factor and is 8.64 h (8 h and 38.4 min).
GSM and alarming system validation
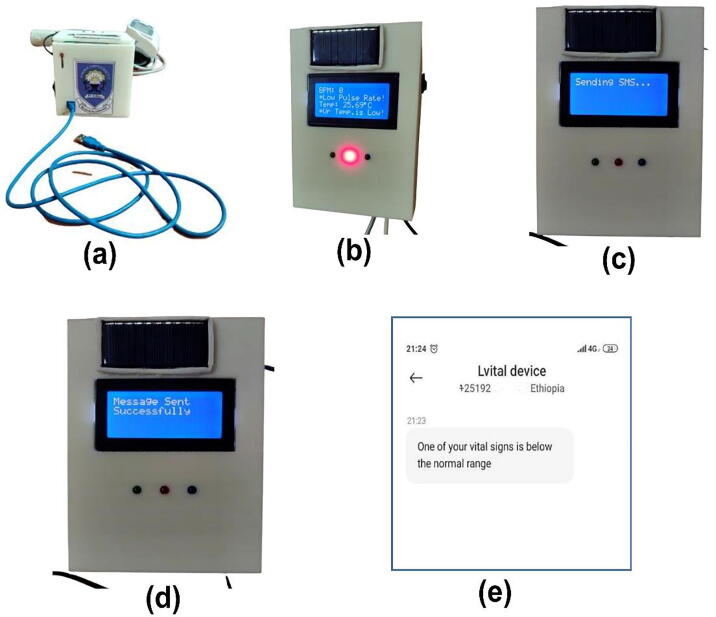
One of the main features included in Lvital device was the integration of the GSM modem and alarming system. Lvital device provides a system that is capable of providing real-time remote monitoring of the three vital signs with improvements of an alarm and SMS alert when they deviated from the normal range. This device is programmed such that it would sense and monitor the blood pressure, pulse rate beat, and temperature of the women triggered an alert by SMS messages sent to the mobile of the health personnel or caregiver and also buzz an alarm whenever the reading value has deviated from the normal reading. As it has been indicated in section 7.2 the normal vital sign ranges for the average healthy adult women while resting are, 90/60 mmHg to 120/80 mmHg, 60 to 100 beats per minute, and 36.5 °C to 37.3 °C for blood pressure, pulse rate, and body temperature respectively. Therefore, when any of the above vital sign readings were out of the range the Lvital device was triggered to send short SMS. When the blood pressure, pulse rate beat, and temperature reading were below 90 mmHg, 60 beats per minute, and 36.5 °C respectively, first the alarm was automatically triggered to make an alert. Then later on after a minute, the GSM modem triggered and sent a short text to the pre-printed caregiver's phone number with the content of “One of your vital signs is below the normal range” (see Fig. 17e. Moreover, when the reading value was exceeded the normal range the content of the message was changed to “One of your vital signs exceeds the normal range”. The system would not wait until all reading values are below or exceed the normal range, however, it would be triggered to send the text whenever only one vital sign reading has deviated from the acceptable range. This would help the women to get early monitoring care and treatment. When the device was triggered to send a short text, first a text “ Sending SMS…” (see Fig. 17c was displayed on the LCD. Then, after sending the SMS, it displayed “Message sent successfully” (see Fig. 17d. As shown in Fig. 17b this was validated by leaving the device without attaching the sensors to the volunteer's body so that the pulse sensor was read zero BPM (because we haven’t attached the sensor to the body), and the temperature sensor reads the room temperature (in which in our case was around 25.69 °C). As its first design, we developed the conceptual prototype and it would send SMS to the preprogrammed phone number and has no interface to accept phone numbers from end-users using a keypad or any other mechanism. Therefore, the correct phone number of the caregiver has to be preprogrammed first and the code needs to be uploaded to the system before starting to operate the device. That is why we leave a hole to connect the microcontroller and computer using USB (as shown in Fig. 17a so that whenever necessary a technical person would first insert the correct phone number in the code and upload it to the system before operation. However, in future work, we suggest including an interface to accept phone numbers from any user using a keypad, so that it would be easier to use for end-users.
Fig. 17.
The GSM Validation. (a) shows the front side of the prototype through which the USB is inserted and connected to the Arduino board to upload the developed Arduino script. (b) shows sample low temperature and pulse rate measurement without attaching the sensor to the human body (made testing purpose), (c) shows the GSM triggered to send SMS, (d) shows the SMS sent successfully to the caregiver phone number, (e) shows the content of the message received from the Lvital device.
Performance comparison
This project comes up with a vital sign (core physiological monitoring parameters specifically blood pressure, heart rate, and temperature) monitoring device for pregnant women. Generally, it has the following important a significant improvement upon standard practice.
Effective monitoring device for hypertension/preeclampsia, Heart problem, and temperature monitoring
It is a non-invasive and cuff-less blood pressure monitoring device: currently, in low resource settings, the cuff-based blood pressure measuring device (sphygmomanometer) is available. However, a sphygmomanometer is not comfortable for the person, and unable to continue monitoring her blood pressure. Therefore, our device is with a cuffless system, is very comfortable, and painless than the existing one.
Cost-effective: Most of the currently existing devices found in low-resource settings were come in the form of donations or purchased with an expensive amount of foreign currency. However, ours can be affordable at a low cost since it is an indigenous product. It is also difficult to get a device, which monitors all parameters together at a low cost. The production cost of our device is estimated to be less than 152 USD.
Power utilization: our device incorporated a solar empowered mechanism, which helps the device, electrical power independent. This will help for easy accessibility for rural areas of our country. Moreover, it only needs 9 V for its operation as compared to 220 V with the existing patient monitoring ting machine.
Integrate GSM technology to send short SMS service to notify the caregivers or parents during abnormal measured values.
The device takes less than a minute to display the measured value. Nevertheless, if the device has detected any abnormality, the GSM sends short text within two minutes.
Currently, new consumer health devices are being developed to easily monitor multiple physiological parameters regularly. These include different smart bands (in the form of a watch) commercially available to help to monitor different vital sign parameters including blood pressure, pulse rate, and body temperature [28]. Many of these vital signs measurement devices have yet to be formally studied in a clinical setting but have already spread widely throughout the consumer market [29]. Some of these devices investigated met accuracy guidelines for heart rate (HR) measurements, but they failed to meet the predefined accuracy guidelines for other vital sign measurements. The continued sale of consumer physiological monitors without prior validation and approval procedures is a public health concern. The results of their study indicate that some smart bands are not accurate enough to be used to monitor vital signs. For example, according to [29] for the BP measurements, the Everlast smartwatch did not correctly measure any (0%) of the hypertensive values for the values that were hypertensive when measured with the standard cuff. Although the watch met their predefined accuracy standard for heart rate (HR), it detected only 33% of the bradycardic HR values that were measured by the standard monitor. In addition to the accuracy problems, the watch failed to obtain any measurement at all for 32% of HR and 34% of systolic blood pressure (SBP) and diastolic blood pressure (DBP) measurements. The following Table 3shows the advantages and disadvantages of commercially available smart bands and our device, Lvital device [30].
Table 3.
Shows the comparative advantage and disadvantages of our device and commercially available low-cost smart band devices.
| Types of devices | Advantages | Disadvantages |
|---|---|---|
| Smart band devices (Watch based vital sign monitoring, Everlast TR10, BodiMetrics device, Patch monitors, etc.) |
|
|
| Our device (Lvital device) |
|
|
Most monitors used in hospitals today require the user to perform a calibration to a known standard before reliable clinical results are possible [31]. Regular vital sign monitoring devices calibration is highly recommended [32]. Calibration must be performed regularly to check its accuracy and precision and, if any errors are found, corrective measures have to be taken and adverse effects, if any, must be evaluated and documented. Even if there is no clear suggested calibration interval for all devices, we suggest for our device, Lvital device, to be checked in-house calibration within the daily interval and periodically on a quarterly based to ensure that the calibration remains within standard specification.
As future work, we suggest integrating Internet of Things (IoT) technology, so that it will be possible to send the measured data to the cloud, analyze using artificial intelligence, and notify the analyzed result to the healthcare personnel easily. In addition to this, we suggest integrating GPS to track the geo-reference (location) of the patient who is using the device to easily help the patient during an emergency. Moreover, to send SMS we suggest using the LoRaWAN module than the GSM module as its energy consumption could be lower, no phone subscription is required and it could be possible to generate SMS messages using the central node and deliver them to the healthcare personnel. In addition to this, we suggest including an interface that enables to acceptance of phone numbers from end-users using a keypad so that it would be easier to use for end-users.
Ethics statements
We have carried out our work according to The Code of Ethics of the World Medical Association (Declaration of Helsinki) and Uniform Requirements for manuscripts submitted to Biomedical Journals. Moreover, this project work was compiled with the Ethiopian National Ethics Review Guideline which was prepared by the FDRE Minister of Science, and Technology in September 2014, Fifth Edition. Moreover, during testing, relevant informed consent was obtained from those subjects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This project work was funded by Technology Transfer Office, with Technology Transfer Grant Award, through the University-Industry Linkage Office of Jimma Institute of Technology, which is supported by Jimma University with project No. UIL-TT/JiT/TT04/11.
Biographies

Kokeb Dese B.Sc. degree in Biomedical Engineering from Jimma University. M.Sc. degree in Biomedical Imaging from Jimma University. Lecturer at Jimma University, Institute of Technology, School of Biomedical Engineering since August 2016. Won different grant projects from Grand Challenges Ethiopia, and the Technology Transfer Office of Jimma University. Research interests include: AI-based cancer diagnosis, Optical imaging devices (Microscope), Ultrasound, MRI, X-RAY, CT-SCAN, PET, SPECT, and Mammogram imaging, Medical image processing and analysis, Biosignal processing and analysis, and Low cost biomedical device design using biosensors. Published works at https://orcid.org/0000-0003-3591-9570, Google Scholar: https://scholar.google.com/citations user=ciRef64AAAAJ&hl=en&oi=ao. Email address: kokebdese86@gmail.com or dese.gebremeskel@ju.edu.et

Gelan Ayana B.Sc. degree in Biomedical Engineering. M.Sc.degree in Biomedical Imaging. Currently, a Ph.D. candidate in Medical IT Convergence Engineering at Kumoh National Institute of Technology, Republic of Korea. Lecturer at Jimma University, Institute of Technology, School of Biomedical Engineering since August 2015. Won different grants from Grand Challenges Ethiopia (funded by Ministry of Health, Ethiopia) and from Jimma University. Research interests include: AI-based cancer diagnosis; Ultrasound, Mammogram, Microscope, CT, MRI, and Endoscopic imaging; Medical image analysis; Cancer immunology; Low-cost healthcare technology devices. Published works at https://orcid.org/0000-0002-5219-6098. Email address: gazboy100@gmail.com or gelan@kumoh.ac.kr

Dr. Gizeaddis Lamesgin Simegn Assistant Professor, School of Biomedical Engineering, Jimma Institute of Technology, Jimma University. Ph.D. degree in Biomedical Engineering from University of Cape Town, South Africa, 2019. M.Sc. degree in Biomedical Imaging from Addis Ababa University Institute of Technology, Addis Ababa University, 2015. B.Sc. degree in Electrical and Computer Engineering from Jimma University, 2010. Awarded different grants from Grand Challenges Ethiopia, and Ministry of innovation and Technology of Ethiopia. Research interests include medical image processing and analysis, Bio signal processing, MRI pulse sequence programming, MRI image reconstruction and analysis, Artificial Intellegence, Medical devices design e.t.c. Published works at ORCID:https://orcid.org/0000-0003-1333-4555, Google scholar: https://scholar.google.com/citations?user=22sczwwAAAAJ&hl=en. Email address: 1time.et@gmail.com or gizeaddis.lamesgin@ju.edu.et
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ohx.2022.e00276.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.B. Wassihun, B. Negese, H. Bedada, S. Bekele, A. Bante, T. Yeheyis, A. Abebe, D. uli, M. Mohammed, S. Gashawbez, E. Hussen, Knowledge of obstetric danger signs and associated factors: a study among mothers in Shashamane town, Oromia region, Ethiopia, Reprod. Health, 17(4) (2020). 10.1186/s12978-020-0853-z [DOI] [PMC free article] [PubMed]
- 2.Warri D., George A. Perceptions of pregnant women of reasons for late initiation of antenatal care: a qualitative interview study. BMC Pregnancy Childbirth. 2020;20(70) doi: 10.1186/s12884-020-2746-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lassi Z.S., Mansoor T., Salam R.A., Das J.K., Bhutta Z.A. Essential pre-pregnancy and pregnancy interventions for improved maternal, newborn and child health. Reprod. Health. 2014;11(2):S2. doi: 10.1186/1742-4755-11-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandsæter H.L., Horn J., Rich-Edwards J.W., Haugdahl H.S. Preeclampsia, gestational diabetes and later risk of cardiovascular disease: Women’s experiences and motivation for lifestyle changes explored in focus group interviews. BMC Pregnancy Childbirth. 2019;19:448. doi: 10.1186/s12884-019-2591-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crispín Milart P.H., Prieto-Egido I., Díaz Molina C.A., Martínez-Fernández A. Detection of high-risk pregnancies in low-resource settings: a case study in Guatemala. Reprod. Health. 2019;16:80. doi: 10.1186/s12978-019-0748-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rashid H., Kagami M., Ferdous F., Ma E., Terao T., Hayashi T., Wagatsuma Y. Temperature during pregnancy influences the fetal growth and birth size. Trop. Med. Health. 2017;45(1) doi: 10.1186/s41182-016-0041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garbhapu V.V., Gopalan S. IoT Based Low Cost Single Sensor Node Remote Health Monitoring System. Procedia Comput. Sci. 2017;113(4):408–415. doi: 10.1016/j.procs.2017.08.357. [DOI] [Google Scholar]
- 8.Zhang Q., Zhou D., Zeng X. Highly wearable cuff-less blood pressure and heart rate monitoring with single-arm electrocardiogram and photoplethysmogram signals. Biomed. Eng. Online. 2017;16(1):23. doi: 10.1186/s12938-017-0317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thelkar A.R., Dulecha K. Blood Pressure, Heart Beat and Body Temperature Measurement by Using GSM and Low-Cost Microcontroller with Health Care Announcement. J. Control Instrum. Eng. 2021;7(1):1–18. doi: 10.46610/JOCIE.2021.v07i01.001. [DOI] [Google Scholar]
- 10.T. Shaown, M. Shohrab Hossain, T.M. Mukur, Low-Cost Health Monitoring System: A Smart Technological Device for Elderly People. BT - Proceedings of Sixth International Congress on Information and Communication Technology, in: X.-S. Yang, S. Sherratt, N. Dey, A. Joshi (Eds.), Springer Singapore, Singapore, 2 (2021) 851–860.
- 11.B. Moatamed, Arjun, F. Shahmohammadi, R. Ramezani, A. Naeim, M. Sarrafzadeh, Low-cost indoor health monitoring system, in: 2016 IEEE 13th Int. Conf. Wearable Implant. Body Sens, Networks, 4 (2016) 159–164. 10.1109/BSN.2016.7516252
- 12.Sakphrom S., Limpiti T., Funsian K., Chandhaket S., Haiges R., Thinsurat K. Intelligent Medical System with Low-Cost Wearable Monitoring Devices to Measure Basic Vital Signals of Admitted Patients. Micromachines. 2021;12(8) doi: 10.3390/mi12080918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boatin Adeline A., Wylie Blair, Goldfarb Ilona, Azevedo Robin, Pittel Elena, Ng Courtney, Haberer Jessica, Frasch Martin Gerbert. Wireless fetal heart rate monitoring in inpatient full-term pregnant women: testing functionality and acceptability. PLoS One. 2015;10(1):e0117043. doi: 10.1371/journal.pone.011704310.1371/journal.pone.0117043.g00110.1371/journal.pone.0117043.g00210.1371/journal.pone.0117043.g00310.1371/journal.pone.0117043.g00410.1371/journal.pone.0117043.t00110.1371/journal.pone.0117043.t002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subalakshmi S., Murugaboopathi G., Senthilkumar D. Efficient EER-LEACH protocol for monitoring the activities of pregnant women using wearable body sensor network. Int. J. Eng. Technol. 2018;7(3.27):111–115. doi: 10.14419/ijet.v7i3.27.17666. [DOI] [Google Scholar]
- 15.K. Elango, K. Muniandi, A Low-Cost Wearable Remote Healthcare Monitoring System. Role Edge Anal. Sustain. Smart City Dev, 4(1) (2020) 219–242. https://doi.org/ 10.1002/9781119681328.ch11
- 16.Smith G.B., Isaacs R., Andrews L., Wee M.Y.K., van Teijlingen E., Bick D.E., Hundley V. Vital signs and other observations used to detect deterioration in pregnant women: an analysis of vital sign charts in consultant-led UK maternity units Obstetric vital signs charts. Int. J. Obstet. Anesth. 2017;30(1):44–51. doi: 10.1016/j.ijoa.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Vousden N., Nathan H.L., Shennan A.H. Innovations in vital signs measurement for the detection of hypertension and shock in pregnancy. Reprod. Health. 2018;15(1):92. doi: 10.1186/s12978-018-0533-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NCCDPHP, Heart Disease and Stroke. http://bitly.ws/hcQn , 2021 (accessed 10.11.21)
- 19.Pandit J.A., Lores E., Batlle D. Cuffless Blood Pressure Monitoring Promises and Challenges. Clin. J. Am. Soc. Nephrol. 2020;15(10):1–8. doi: 10.2215/CJN.03680320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.J. Runkle, M. Sugg, D. Boase, S.L. Galvin, C. C Coulson, Use of wearable sensors for pregnancy health and environmental monitoring: Descriptive findings from the perspective of patients and providers. Digit. Heal, 5 (2019) 2055207619828220. 10.1177/2055207619828220 [DOI] [PMC free article] [PubMed]
- 21.Fakhrulddin S.S., Gharghan S.K. An autonomous wireless health monitoring system based on heartbeat and accelerometer sensors. J. Sens. Actuator Networks. 2019;8(3):39. doi: 10.3390/jsan8030039. [DOI] [Google Scholar]
- 22.Sunrom Electronics and Technology, Blood Pressure Sensor - Serial output, Sunrom. https://bit.ly/30W2qJy , 2018 (accessed 5.11.21)
- 23.Solar Idea Hub, What size solar panel to charge a 9v battery? https://bit.ly/3JJASZE, 2021 (accessed 30.12.21)
- 24.B. McNeese, Acceptance Criteria for Measurement Systems Analysis (MSA), SPC Excel. https://bit.ly/2ZOVehS , 2018 (accessed 4.11.21)
- 25.Medlineplus, Vital Signs. https://bit.ly/3Io653Q , 2021 (accessed 1.12.21)
- 26.Abuzairi Tomy, Imaniati Sumantri Nur, Irfan Ahli, Maulana Mohamad Ridho. Infrared thermometer on the wall (iThermowall): An open source and 3-D print infrared thermometer for fever screening. HardwareX. 2021;9:e00168. doi: 10.1016/j.ohx.2020.e00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Converter To dot com, Rechargeable battery charging time vs. mA current calculator. https://bit.ly/3eS5oCo, 2021 (accessed 31.12.21)
- 28.Reeder B., David A. Health at hand: A systematic review of smart watch uses for health and wellness. J. Biomed. Inform. 2016;63:269–276. doi: 10.1016/j.jbi.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Hahnen C., Freeman C.G., Haldar N., Hamati J.N., Bard D.M., Murali V., Merli G.J., Joseph J.I., van Helmond N. Accuracy of Vital Signs Measurements by a Smartwatch and a Portable Health Device: Validation Study. JMIR Mhealth Uhealth. 2020;8(2) doi: 10.2196/16811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albers G.W., Bernstein R.A., Brachmann J., Camm J., Easton J.D., Fromm P., Goto S., Granger C.B., Hohnloser S.H., Hylek E., Jaffer A.K., Krieger D.W., Passman R., Pines J.M., Reed S.D., Rothwell P.M., Kowey P.R. Heart Rhythm Monitoring Strategies for Cryptogenic Stroke: 2015 Diagnostics and Monitoring Stroke Focus Group Report. J. Am. Heart Assoc. 2015;5(3) doi: 10.1161/JAHA.115.002944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.S. Edward, Guide to FDA Requirements and Importance of Medical Device Calibration, Med. Des. Briefs. https://bit.ly/3pje1uu , 2018 (accessed 25.11.21)
- 32.de Greeff A., Lorde I., Wilton A., Seed P., Coleman A.J., Shennan A.H. Calibration accuracy of hospital-based non-invasive blood pressure measuring devices. J. Hum. Hypertens. 2010;24(1):58–63. doi: 10.1038/jhh.2009.29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.