Abstract
Purpose of Review
To examine the prevalence rates of ultra-processed food addiction across different weight classes and offer guidelines for diagnosis and treatment. Clinicians are provided with practical considerations in the assessment of ultra-processed food addiction beyond the use of validated instruments.
Recent Findings
The weighted mean prevalence of ultra-processed food addiction is approximately 20% worldwide and varies widely based on the sample. At first glance, there appears a linear relationship between ultra-processed food addiction and BMI class. Further investigation indicates a J-shaped curve with heightened prevalence among the underweight. These findings highlight the need to assess for additional factors that may increase objective or subjective food addiction symptoms including eating disorders, dietary restraint, and other mental health diagnoses.
Summary
While clinical considerations across different weight classes vary, overemphasis on weight status may detract from the clinical utility of the ultra-processed food addiction construct. Considering weight status in conjunction with other psychiatric symptoms helps to better understand the various biopsychosocial mechanisms that influence eating behavior and can inform individualized treatment strategies.
Keywords: Food addiction, BMI, Eating disorders, Dietary restraint, Weight stigma, Trauma
Introduction
The phenomenon of food addiction (FA) has been mentioned in over 2,500 published articles to date [1•]. Most research studies have operationalized FA using the Yale Food Addiction Scale (YFAS) [2] and more recently YFAS 2.0 [3] based on DSM-5 criteria for substance use disorders (SUDs). FA can be understood as hedonic eating after homeostatic requirements have been met [4, 5] with continued consumption despite negative consequences [6]. Paradoxically, FA symptoms have been reported among underweight individuals who may not be meeting their energy needs [7•], necessitating a closer examination of the FA construct through the lens of eating disorder (ED) psychopathology and related symptoms.
The purpose of this review is to describe the reported prevalence rates of FA across different weight classes and to provide clinical guidelines for diagnosis and treatment, with particular emphasis on the underweight category. The aim is to offer clinicians practical guidelines in the assessment and treatment of FA beyond the use of validated instruments.
There is growing trend toward use of the term “ultra-processed food addiction” (UPFA) emphasizing noteworthy differences from minimally processed foods in their addictive potential [8–12]. Given that the YFAS specifically asks about the consumption of ultra-processed foods with added sugars, salts, and fats, the terms FA and UPFA have been used interchangeably. The UPFA nomenclature may advance the utility and specificity of the FA construct, given that nearly 60% of the calories consumed in the USA come from ultra-processed foods [13]. Henceforth, the term UPFA will be used to describe the phenomenon of food addiction.
While clinical considerations across different weight classes may vary, this review argues that overemphasis on weight status may detract from the clinical utility of the UPFA construct. Weight (and BMI) is often analyzed as an outcome which is influenced by an array of biological, psychological, social, and behavioral causes whereas addictions are defined (and diagnosed) solely by behavioral criteria. Thus, consideration of UPFA as a behavioral health disorder (rather than weight disorder) may provide more insight into clinical treatment strategies.
Several studies have failed to show significant correlations between UPFA and BMI [14–18]; therefore, efforts to address UPFA clinically should not be synonymous with weight loss. Rather, UPFA has been associated with reduced quality of life [19] through mechanisms such as emotion dysregulation [20–22] which can be targeted. Furthermore, UPFA has been associated with altered psychosocial functioning more than with metabolic parameters [18]. Meanwhile, considering one’s weight status in the clinical evaluation of UPFA might be useful to better understand the various biopsychosocial mechanisms that influence eating behavior (including the subjective experience of it).
Ultra-processed Food Addiction Prevalence
The most recent systematic review and meta-analysis of 272 studies using the YFAS, YFAS 2.0, and derivative scales reported a weighted mean prevalence of UPFA diagnoses at 20% worldwide (95% CI: 18–21%) [1•]. The prevalence of UPFA varies widely based on the sample under study. Clinical samples have higher prevalence than non-clinical samples [1•]. A nationally representative sample from the USA reported UPFA prevalence at 15% [7•], which closely mirrors prevalence rates of other substance-related behaviors in the USA [23]. While the prevalence of UPFA is generally higher in women than men [24–26], these findings are not consistent [7•].
UPFA prevalence estimates are higher in adults compared to children and adolescents (15%; 95% CI: 11–19%) [27], suggesting that symptoms develop over time, much like other addictions. Increased exposure to ultra-processed foods may contribute to early recruitment of brain regions associated with food consumption and choice [9]. A blunted reward experience might increase overconsumption behavior in attempt to reattain the expected reward [28]. Reward-related susceptibility to addictive processes may also be exacerbated by various forms of stress, trauma, and adversity often associated with low socioeconomic status and unhealthful food environments [29].
Recent studies have shown that the COVID-19 pandemic increased the incidence rate of UPFA [30] and associated weight gain from eating behavior attributable to circumstances such as isolation [31]. It has also been documented that COVID-19 led to a surge in EDs [32]. Directionality between UPFA and ED symptoms during the pandemic (and before) remains unclear. A major shortcoming in research linking UPFA to EDs is a failure to disentangle the temporal sequence of disorder onset. This limitation may make the interpretation of rising UPFA prevalence “noisy” because many ED symptoms can increase YFAS scores [33•]. Similarly, UPFA can precede ED behaviors, generating a chicken versus egg conundrum for researchers and clinicians.
Ultra-processed Food Addiction and Eating Disorders
Clinical samples (such as those with EDs) have higher UPFA prevalence than community-based or representative samples [34]. Specifically, individuals with bulimia nervosa (BN) have the highest prevalence of UPFA (48–95%), followed by binge eating disorder (BED; 55–80%), and anorexia nervosa (AN; 44–70%) [1•, 35•, 36]. Most studies investigating the association between AN and UPFA found that UPFA symptoms are higher among those with AN binge-purge type (AN-BP) compared to the restrictive subtype (AN-R) [37, 38, 39•, 40].
An understanding of the heterogeneity in UPFA presentations necessitates assessment for ED psychopathology, particularly the role of restrained eating and other compensatory behaviors as a cause and/or consequence of UPFA symptoms [33•]. The symptoms of UPFA in under-to-normal weight ranges may represent individuals exhibiting compensatory behaviors (e.g., purging in BN or AN-BP, or over-exercising) which keeps their BMI stable (or decreasing) despite the presence of addiction-like eating (e.g., bingeing, loss of control) commonly associated with weight gain [41•]. It has been suggested that weight control behavior in individuals with BN may dampen reported associations between addiction-like eating and BMI [42, 43].
There is a growing trend toward viewing UPFA and EDs from a transdiagnostic perspective [44–46] particularly in relation to food cravings [47]. The clinical considerations discussed herein are designed to improve our understanding of the UPFA construct in individuals with and without EDs. Equally important, UPFA is frequently seen in subjects without EDs (or other psychiatric disorders) in the general population, which suggests that UPFA is a distinct entity from EDs and other psychiatric illness [48].
Ultra-processed Food Addiction and Body Mass Index
Without a nuanced understanding of how UPFA symptoms present clinically in the under and normal weight categories, a linear relationship between UPFA and BMI might be assumed. Figure 1 is adapted from the recent Praxedes et al. meta-analysis [1•] and represents the broadest possible relationship between UPFA and BMI. The pooled prevalence among individuals considered normal weight was 17% (95% CI: 16–19%); among overweight was 24% (95% CI: 19–29%); and among those considered obese was 28% (95% CI: 24–32%). Not enough UPFA studies among the underweight class were available for pooled analysis. At first glance, the relationship between UPFA and BMI appears linear.
Fig. 1.
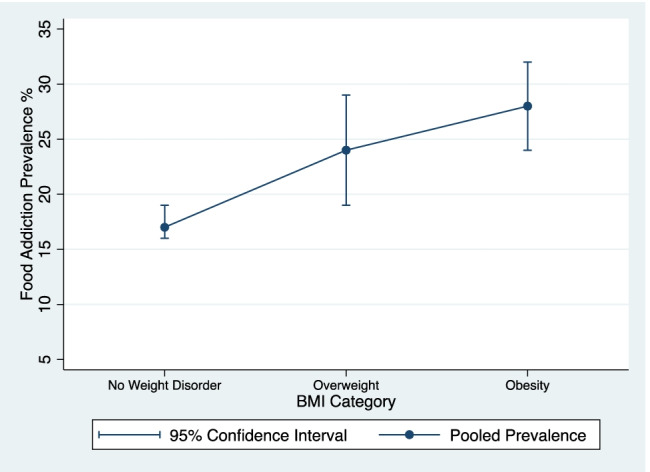
The broadest view of the relationship between BMI and ultra-processed food addiction prevalence from meta-analytic data reported by Praxedes et al. [1•]
Other investigations of UPFA prevalence including individuals with EDs generates J-shaped curves, with the underweight category demonstrating sharply higher UPFA prevalence (12–46%) [7•, 41•, 49•]. A representative USA sample indicated that the prevalence of UPFA is paradoxically highest in the underweight category (46%) [7•]. A representative German sample indicated UPFA prevalence among underweight (15%) as higher than class I obesity (12%) but lower than class II (21%) [49•]. Not surprisingly, prevalence rates differ widely according to sample demographics. It is also possible that food insecurity may increase UPFA symptoms, which may be relevant across BMI classes [50, 51]. Key findings from several recent studies show that obesity cannot be explained solely by UPFA [52–54].
Data from the German sample [49•] are presented for conceptual purposes in Fig. 2. The prevalence rates in Fig. 2 are not intended for direct comparison to the meta-analytic findings in Fig. 1, but rather to illustrate how a more fine-grained analysis of BMI reveals information that could be overlooked. A non-linear relationship suggests that UPFA is not the only contributor to weight gain and may reflect a distinct phenotype of problematic eating that is not synonymous with obesity [49•]. It is also possible that self-reported UPFA symptoms in the lowest weight class are relics of dieting and caloric deprivation that mimic behavioral indicators of addiction. Examples include eating more than intended due to a violation of dietary rules, or cravings that reflect a homeostatic drive for food from an undernourished state [7•].
Fig. 2.
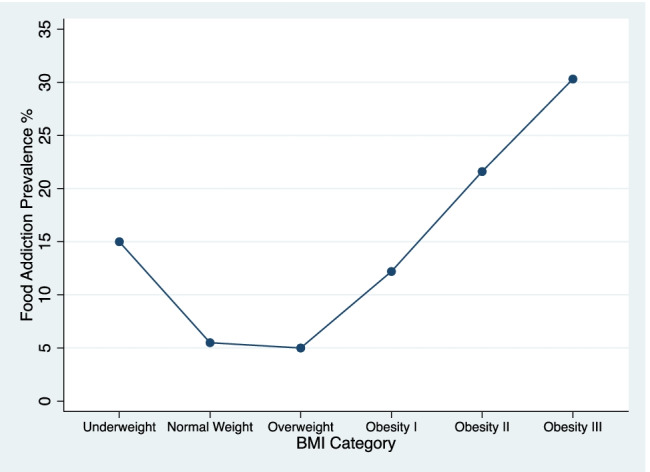
A closer view of the J-shaped relationship between BMI and ultra-processed food addiction prevalence from a nationally representative German sample reported by Hauck et al. [49•]
In the next section, the literature exploring the non-linear relationship between UPFA and BMI categories are reviewed. Furthermore, mental health considerations are included to assist in developing treatment strategies when positive UPFA screens occur in different BMI categories. Recommendations prioritize ED recovery and are designed to reflect weight-inclusive approaches to managing health [55•]. An argument will be made that UPFA appears primarily related to eating pathology and only secondarily related to body weight [52].
Ultra-processed Food Addiction and Dietary Restraint
This section reviews often overlooked clinical symptoms among individuals with UPFA who also exhibit high levels of dietary restraint (DR). While binge eating and UPFA are not synonymous, research linking DR to binge eating will be used to conceptualize relationships between UPFA and DR. It is also plausible that dieters may not subjectively binge but rather display other characteristics of addiction-like eating such as social impairment, avoidance, and emotional distress, all of which could increase UPFA severity indicators.
Published data linking UPFA and DR are mixed. Cross-sectional data have linked UPFA scores with higher levels of DR [56] whereas the validation studies of the YFAS and YFAS 2.0 showed no significant correlations between UPFA and DR [3, 57]. Among adolescents in a weight loss program, UPFA was not related to eating restraint [58]. Other investigations of adolescents have shown positive correlations between UPFA and DR [59]. Less is known about the temporal relationship between DR and UPFA symptoms, requiring longitudinal investigations in samples with and without current (or prior) EDs.
While it is well known that efforts to restrain eating often contribute to binge eating, other models suggest that UPFA leads to weight gain and then progress to DR [60]. This pattern is frequently observed among other addictive disorders. For example, repeated alcohol exposure can lead to problematic use which could in turn lead a person to restrict their alcohol use to mitigate these problems. The return to alcohol (i.e., relapse) closely resembles the way some return to highly palatable foods following a period of abstinence [46].
Early research among women with BN revealed that the first binge preceded diet onset in 9–37% of cases [61, 62]. In a cohort study of young women dieting, only 3.5% developed an ED of clinical severity during the 2-year follow-up [63]. A recent review found no experimental support for the DR theories of EDs, suggesting that DR may activate vulnerability factors leading to ED symptoms but only among those already vulnerable [64]. As previously stated, DR may be a consequence of weight gain and the perception of overeating. Therefore, divergent models are needed to better understand directionality between UPFA and DR [60].
Food cravings have been linked to dieting [65], and efforts to restrain eating predicted future overeating during COVID-19 through negative emotions [66]. Data on twins has shown that frequent attempts to lose weight reflect future susceptibility to weight gain [67], but the precise mechanisms remain unclear. A randomized controlled trial using internet- and app-based intervention demonstrated that targeting DR led to greater reductions in binge-eating eating episodes compared with controls [68]. Taken together, it appears that DR may be one contributor to UPFA symptoms such as binge eating and therefore might be a useful target for clinical interventions among individuals meeting criteria for UPFA, especially those with comorbid EDs.
Other research suggests food reward tends to decrease during weight management interventions [69]. A study using caloric restriction for at least 12 weeks reduced food cravings, supporting a deconditioning model [70]. Various dimensions of food cravings are differentially related to outcomes in dieting [65]. Short-term selective food deprivation increases cravings for avoided foods while long-term energy restriction results in craving reductions, indicating a reduction of previously acquired conditioned responses [71].
In summary, DR has been viewed as both the problem and the solution to rising BMIs [72]. Efforts to lose weight or restrict food can make eating pathology worse [73–75]. It is unclear whether efforts to eat differently (emphasizing quality rather than quantity) should be classified as disordered behavior [29] including Orthorexia Nervosa [76], but likely depends on an individual’s psychiatric profile [33•]. Individuals who meet criteria for UPFA and engage in DR may experience varying effects on their body weight, partially depending on whether the restraint is part of a recovery plan, mistargeted (i.e., excessive), inadequately supported, or part of a restrictive ED [29], which can be driven by body dissatisfaction.
Ultra-processed Food Addiction and Body Image Disturbance
Body image disturbances are known correlates of DR and EDs and are related to quality-of-life measures [77]. Susceptibility to attentional biases toward appearance-related information might be one vulnerability factor in the prolonged persistence of body image disturbances in daily life [78]. Cohort data found that the adolescent drive for thinness continues into adulthood and may predict compulsive eating behavior (reward-based eating) and greater BMI independent of childhood weight [79]. Body image disturbance may persist over the life course and should be evaluated when treating all forms of disordered eating behavior.
Body image disturbance has been independently associated with UPFA symptoms, suggesting that it may be one important factor in the development and/or maintenance of UPFA [80]. While it is likely to be a consequence of UPFA, assessment of body image through validated instruments or by clinical evaluation may be important for developing treatment interventions. For example, if body dissatisfaction drives DR which in turn contributes to UPFA symptoms, targeting body image disturbances might reduce dieting and improve addiction-like eating [81]. While the number of young people with elevated BMIs continues to grow, so has the percentage of youths experiencing body dissatisfaction and weight stigma [82], necessitating incorporation of this discourse into public health campaigns.
Weight Stigma
Recent conceptual models have suggested that the experience of weight stigma may drive DR and contribute to UPFA symptoms [29]. Among adolescents, UPFA significantly mediated the association between weight-related self-stigma and binge eating [83]. In a longitudinal study of young adults, fear of being stigmatized predicted worsening UPFA symptoms over time [84]. These studies suggest that the experience of weight stigma contributes to UPFA among some individuals. Other research suggests that understanding UPFA broadly reduces weight stigma by minimizing the blame narrative associated with “personal responsibility” [85, 86]. Several authors have proposed that targeting weight stigma at the societal level should be a public health goal [87]. Targeting the internalization of weight bias can be beneficial among those with UPFA, regardless of their weight status.
Anti-obesity messaging may put vulnerable individuals at risk for EDs [88]; a highly undesirable outcome. Meanwhile, evidence-based treatments for obesity can reduce disordered eating behaviors without increasing internalized weight stigma [89]. Divergent views in the field may arise from a failure to separate advocacy from evidence, as well as training/discipline bias. Efforts to find a common ground between the prevention of obesity and EDs are greatly needed [64]. But given that UPFA is not synonymous with obesity, it is imperative that researchers and clinicians understand the potential relationship between weight stigma and UPFA independent of BMI.
Ultra-processed Food Addiction and Other Mental Health Disorders
A detailed discussion of the associations between relevant mental disorders and UPFA is beyond the scope of this review. Nevertheless, some of the associations between select mental health disorders and UPFA will be summarized to aid clinicians treating UPFA. An 8-step assessment process to discern UPFA from DR using psychiatric symptoms has recently been published [33•]. This process has not been validated but may be useful as a roadmap for conceptualizing UPFA treatment in the context of EDs (emphasizing DR as a proxy for restrictive behaviors associated with EDs).
Substance Use Disorders
Research linking UPFA to SUDs are mixed. There is some evidence of elevated UPFA prevalence in those with SUDs [90] as well as cross-addiction between food and substances of abuse [91] but these associations were not statistically significant in a recent meta-analysis [92]. Nevertheless, UPFA has been associated with a family history of addiction [20] and brain reward dysfunction [11]. Based on clinical experience, many individuals with severe UPFA do not cross-addict into other substances, partly because highly palatable foods are always available and therefore remain the substance of choice (or no choice).
Impulsivity strongly correlates with problematic substance use [93, 94] and is consistently associated with UPFA [95]. While it has been suggested that a history of SUD might help with clinical implications of UPFA [33•, 46], impulsivity might be a more informative concept. Higher distress-driven impulsivity has been associated with more addiction-like eating behaviors among participants classified as cognitively inflexible [96]. Thus, the presence of any current or previous SUD may be a proxy measure for impulsive behavior when treating UPFA. A family history of SUD should be considered in the assessment [46].
In patients with both UPFA and SUD, targeted treatments that reduce impulsivity and increase self-directedness may improve treatment outcomes [97]. While this may be difficult, evidence of UPFA channeled by trait levels of impulsivity may help explain why addictive-like behaviors can be recalcitrant to change [21]. The associations between UPFA and impulsivity have been shown to associate with more frequent weight fluctuations and higher BMIs [98]. This information may be helpful to clinicians working with disordered eating by reducing stigma surrounding eating pathology, advancing trauma-informed care for addictive disorders [99].
Trauma and Post-traumatic Stress Disorder (PTSD)
Trauma, particularly early in life, has been associated with increased BMI over the lifespan and UPFA may be one mediating mechanism [43, 100]. The risk for UPFA increases following exposure to early life psychological, sexual, and physical abuse [19, 101]. In fact, all forms of trauma increase risk for UPFA, likely driven by PTSD symptoms [102]. The association between early life adversity and UPFA might be the result of multiple pathways of biological embedding [43], such as reward processing [103, 104] which can heighten risk for SUDs [105] and impulsivity [106].
Associations between early life trauma and EDs are well-described [107, 108] but efforts to investigate the role of UPFA as a mediator, moderator, or outcome are limited. UPFA, as well as emotional eating, have been shown to mediate the relationship between psychological distress and BMI [109]. Based on the available evidence, it appears important to look for signs of trauma and PTSD when designing interventions for UPFA. Considerations include the mechanisms of biological embedding that may impact eating behavior as well as the possibility that some nutrition interventions (e.g., excessive DR) can be triggering for some individuals, particularly those with food-related trauma (e.g., parents who imposed dieting and body shaming [110]).
Depressive Symptoms
In a systematic review and meta-analysis, the weighted mean correlation between UPFA and depression was 0.46 (95% CI: 0.36–0.55) [92]. This relationship is bidirectional and may be important for psychoeducation in those with UPFA. Ultra-processed food consumption increases the risk of developing depression [111, 112] while “Mediterranean-Style” eating patterns have been associated with reductions in these symptoms [113]. Thus, when depressive symptoms are present, patients with UPFA should be counseled to eat in ways known to reduce these symptoms [114].
There is a U-shaped curve between BMI and depression, where the highest rates occur in underweight and obese categories [115]. It is plausible that associations between underweight and depression stem from undernutrition, although this is not well documented. From clinical experience, counseling efforts focused on mental health outcomes such as depressive symptoms rather than weight are welcomed by most patients (once recent data on weight science have been discussed).
Clinical Considerations of Ultra-processed Food Addiction Across Weight Classes
In this final section, clinical considerations for UPFA are broken down by weight classes, with emphasis on the under and normal weight categories. Differentiation by weight status does not imply that UPFA treatment should be determined by BMI alone, but rather that BMI might influence intervention priorities. It is important to distinguish UPFA from restrictive EDs before UPFA-related treatment is implemented.
Recommendations are generated from available evidence and the clinical experience of the author which have not been validated. Systematic reviews of interventions for UPFA have recently been published [116, 117]. Ketogenic diets used in the treatment of binge eating and UPFA [118, 119] are controversial and are not discussed here but may have utility in select cases.
Underweight
The heightened prevalence of UPFA among those in the underweight BMI category and its treatment has not been well described. When other comorbid conditions have been identified, it is useful to explore the items on the YFAS that specifically contribute to elevated UPFA scores. An interview-style assessment is helpful to ascertain if these individuals engage in objective or subjective overeating [7•]. Other questions such as changing social, occupational, or recreational activities, or continuing to eat despite physical or psychological problems may reflect subjective experiences, especially when considering ultra-processed foods that often elicit moral judgments about these foods [34].
The six clinical considerations below are derived from an expanded 8-step process designed to help separate overlapping signs and symptoms of UPFA and DR [33•] but did not consider differences across BMI categories. When UPFA is detected among the underweight class:
Rule out food insecurity or other forms of socioeconomic disadvantage or deprivation that might be elevating UPFA symptoms.
Assess for the presence of AN, particularly AN-BP. If present, the current evidence-based treatment includes weight restoration [120]. Obsessive–compulsive disorders and anxiety frequently occur when AN is present [121, 122]. In patients with AN-R, a high YFAS score often reflects fear of overeating, bingeing, or loss of control rather than the actual behavior [123]. This concept is supported by evidence of AN patients reporting greater UPFA symptomatology while being able to successfully regulate their food cravings [124]. Recent research has suggested there are two distinct phenotypes among those with co-occurring AN and UPFA: (1) restriction that drives perceived or actual UPFA symptoms; and (2) genuine UPFA that is premorbid to AN, where a heightened reward response to ultra-processed foods leads to restriction or fasting [39•]. If the latter is present, nutrition interventions aimed at reduced exposure to ultra-processed foods while simultaneously pursuing weight restoration may be clinically appropriate. It may also be useful to discuss the pros and cons of engaging in a 12-Step program for compulsive eating, as these groups can be attractive to those feeling misunderstood by clinicians but can reinforce restrictive ED symptoms in some cases [125].
It is important to assess for DR and other compensatory behaviors (e.g., excessive exercise) in the absence of clinically significant ED that may be driving weight loss. If these behaviors are driven by body dissatisfaction, therapeutic interventions aimed at promoting positive body image can be helpful [126]. When present, it is important to address restrained eating and the relentless pursuit of thinness as risk factors for EDs and potential predictors of compulsive eating and rebound weight gain later in life [79]. Investigating weight history is informative to better understand metabolic and psychological concerns associated with weight-cycling [127]. After weight restoration, the goal is weight stability. Outcomes are improved when a registered dietitian nutritionist specialist is involved [128].
It is important to assess for the presence of current SUD which may drive malnutrition [129]. When present, evidence-informed recommendations should address the specific substances used [130] while abstinence or harm-reduction strategies are being pursued.
Determine if trauma symptoms or trauma history is present and provide general psychoeducation that links adversity to food and body issues as one part of an established evidence-based trauma treatment [131]. Overlooking trauma may contribute to poor treatment outcomes because unresolved adversity may be considered a fundamental cause of all downstream psychopathology [132].
Evaluate for depressive symptoms and if present, consider the possibility that undernutrition, body image disturbance, or pathological restrained eating is contributing to this outcome and target all three potential vulnerability factors. In some cases, anti-depressant medications may be indicated.
Normal Weight
The normal weight class has the lowest UPFA prevalence, but there are important clinical considerations which aim to distinguish contributions from EDs such as BN and BED. Much like the underweight category, investigating the specific YFAS questions which lead to a positive UPFA screen might help clarify subjective versus objective overeating. Once this assessment has been made, continue to the steps outlined for the overweight/obese class. Treatment for UPFA in the normal weight class includes:
Investigate BN and BED and address associated behaviors such as bingeing, purging, and restricting with the goal of reducing or eliminating these behaviors. Abstinence from purging is an important goal but may take time, particularly in outpatient settings. Identifying “trigger foods” with the goal of abstinence is controversial among ED professionals. It can be helpful to determine the order of UPFA or ED manifestation. If the ED came first, traditional ED treatment which incorporates these foods might be the safest course. One of the goals of normalizing these foods is to disrupt the cognitive distortion of good versus bad foods. If UPFA appeared first, consider excluding specific foods that contribute to bingeing, but only after other clinical considerations have been identified.
Same as #3 for the underweight class. Discussion of weight stigma in society and internalized weight bias can be helpful. A common goal is to avoid the relentless pursuit of thinness by stressing consumption of an appropriate amount of food from all macronutrient categories at meals and throughout the day.
The clinician should assess for comorbidities such as SUDs which may be contributing to addiction-like eating. If present, use substance-specific protocols [130] while pursuing abstinence or harm-reduction strategies. The temporal sequence between disorders of food and other substances should be investigated. If UPFA symptoms clearly preceded other substance use, this may favor moving toward abstinence from addictive foods, after other clinical considerations have been made.
Same as #5 for the underweight class.
Same as #6 for the underweight class with additional consideration that excessive reward activation may contribute to this outcome, aiming to reduce addiction-like eating gradually over time.
Overweight/Obesity
All weight categories above normal have been merged to emphasize that UPFA recovery can be pursued independent of weight status. Clinicians and patients may assume that weight loss is the key outcome of UPFA recovery, but this assumption detracts from important quality of life outcomes which should be prioritized. The likelihood of transitioning from an obese classification to normal weight over a 9-year period is less than 1% [133]. For this reason, other goals such as reducing disordered eating, other addictive behaviors, trauma symptoms, and depressive symptoms should be prioritized. When patients lose weight during UPFA treatment, it is often the result of better nutrition and lifestyle habits, as well as improved stress management. One way to deemphasize weight in UPFA treatment is to avoid weighing people throughout treatment, as well as discourage them from weighing themselves.
If UPFA is detected among the overweight/obese class, the steps outlined for normal weight should be pursued first. Once these considerations have been made, UPFA may exist as a separate entity from an ED or other diagnosis. When UPFA has become the primary target for recovery:
Create a meal strategy that is consistent in nutrients with positive sensory experiences with food. Avoiding hunger and gaps in nutrients are important in creating a consistent (yet varied) experience with food to reduce hedonic overeating [134•]. For example, three meals plus one or two snacks containing all the macronutrients at meals. Deficiencies in fiber, protein, and fat might contribute to hunger which could increase the risk for hedonic eating. Meals and snacks consumed throughout the day should include food that is hot, cold, crunchy, creamy, savory, and sweet (i.e., fruit). Over-restricting caloric intake is not recommended, as the goal is to reduce symptoms of UPFA [134•]. Cravings should diminish over time and emphasizing patience is critical.
Switching the goal from weight loss to improved mental health quality of life can reduce the perception of weight stigma. Addressing internalized weight bias might reduce feelings of stress and adversity that in turn make improvements in eating more accessible [29]. Treatment professionals should discuss the issue of fat shaming in society [135].
Move toward a low glycemic carbohydrate diet (e.g., beans and high-fiber whole grains) given that high glycemic carbohydrates (e.g., refined grains and added sugars) can interact with mesolimbic dopamine systems that heighten food cravings and may lead to loss of control [45, 136]. Identifying individual trigger foods that should be avoided can be helpful. Usually, these foods are a combination of high fat and refined carbohydrates [12]. Foods to avoid can be modified throughout the treatment and recovery process using trial-and-error as well as exposure-based therapies.
Emphasize cooking meals at home rather than relying on purchasing prepared food. Not only does this improve the ingredients used in preparation but can foster a connection with food (and future-directed thinking) that reduces impulsive food choices (author anecdote). While improving the home food environment can be helpful, many individuals live with family members who consume their trigger foods, making recovery difficult. However, recovery can be enhanced by pursuing steps five through seven below.
Target impulsivity and habitual patterns of eating by focusing on the neurobehavioral correlates of self-regulation through practices such as mindfulness-based techniques [137, 138•]. Interventions targeted at improving executive function including episodic future thinking, meditation, and exercise can be helpful to support recovery of dysfunctional reward-related processes [139].
Provide evidenced-based treatments for improving emotional regulation [140–142].
Encourage resilience through social support. The perception of lack of social support has been associated with UPFA [143]. Targeting positive social connections is beneficial for other forms of addiction recovery and should not be overlooked in disorders of eating [45]. 12-Step programs such as Overeaters Anonymous is one well-established example [125]. The addiction framework may reduce perceptions of personal failure [138•] which may be ameliorated through engagement with a like-minded community.
Conclusions
It is critical to understand the social, psychological, cognitive, behavioral, and physiological factors in the UPFA construct [5]. This includes EDs, DR, body image disturbance, weight stigma, and the presence of other psychiatric comorbidities including SUDs, trauma/PTSD, depressive symptoms, and others. Providers should screen for associated conditions before designing clinical interventions for UPFA. Clinicians should address their own conscious or subconscious biases often when working in mental and behavioral health settings.
Individuals with UPFA require additional psychosocial support along with lifestyle modification. If not executed through an ED-informed and weight-neutral lens, interventions for UPFA can be dangerous, increasing the risk for clinically significant EDs among vulnerable groups. In addition to considering the presence of other comorbidities, investigating which individual questions on the YFAS contribute to a positive screen can be informative. The temporal sequence of disorder onset can also help with case conceptualization but is only one of many puzzle pieces. It may take several sessions including some trial-and-error and discussion of the pros versus cons of different approaches before treatment trajectories become clear.
If a genuine UPFA exists, the steps outlined in this review can be used for interventions that include abstinence or harm reduction, with the goal of improving mental health quality of life rather than weight modification. While weight loss frequently occurs in UPFA-informed interventions, providers should emphasize weight-neutral language and weight-inclusive approaches to reduce weight stigma and provide body-positive affirming care. One goal of UPFA treatment should be to end weight-cycling and create consistency with food. Stratifying by BMI class can aid in prioritizing treatment goals but should not supersede other clinical considerations. Clinical trials are needed to improve treatment outcomes for UPFA and would benefit from collaboration (rather than competition) from ED professionals. Cross-disciplinary efforts from all biopsychosocial domains will be critical to advance the field of food addiction.
Compliance with Ethical Standards
Conflict of Interest
The author declares no competing interests.
Footnotes
This article is part of Topical Collection on Food Addiction
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Praxedes DRS, Silva-Júnior AE, Macena ML, Oliveira AD, Cardoso KS, Nunes LO, Monteiro MB, Melo ISV, Gearhardt AN, Bueno NB. Prevalence of food addiction determined by the Yale Food Addiction Scale and associated factors: a systematic review with meta-analysis. Eur Eat Disord Rev. 2021 doi: 10.1002/erv.2878. [DOI] [PubMed] [Google Scholar]
- 2.Gearhardt A, Corbin W, Brownell K. Preliminary validation of the Yale Food Addiction Scale. Appetite. 2009 doi: 10.1016/j.appet.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Gearhardt A, Corbin W, Brownell K. Development of the Yale Food Addiction Scale Version 2.0. Psychol Addict Behav. 2016 doi: 10.1037/adb0000136. [DOI] [PubMed] [Google Scholar]
- 4.Lowe MR, Butryn ML. Hedonic hunger: a new dimension of appetite? Physiol Behav. 2007;91:432–439. doi: 10.1016/j.physbeh.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Lerma-Cabrera J, Carvajal F, Lopez-Legarrea P. Food addiction as a new piece of the obesity framework. Nutr J. 2016;15:5. doi: 10.1186/s12937-016-0124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiss DA, Avena N, Rada P. Sugar addiction: from evolution to revolution. Front Psych. 2018;9:545. doi: 10.3389/fpsyt.2018.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulte EM, Gearhardt AN. Associations of food addiction in a sample recruited to be nationally representative of the United States. Eur Eat Disord Rev. 2018 doi: 10.1002/erv.2575. [DOI] [PubMed] [Google Scholar]
- 8.Lustig RH. Ultraprocessed food: addictive, toxic, and ready for regulation. Nutrients. 2020;12:3401. doi: 10.3390/nu12113401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Contreras-Rodriguez O, Solanas M, Escorihuela RM. Dissecting ultra-processed foods and drinks: do they have a potential to impact the brain? Rev Endocr Metabolic Disord. 2022;1–21. [DOI] [PubMed]
- 10.Schulte EM, Avena NM, Gearhardt AN. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS ONE. 2015;10:e0117959. doi: 10.1371/journal.pone.0117959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon EL, Ariel-Donges AH, Bauman V, Merlo LJ. What is the evidence for “food addiction?”. A systematic review Nutrients. 2018;10:477. doi: 10.3390/nu10040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pursey KM, Skinner J, Leary M, Burrows T. The relationship between addictive eating and dietary intake: a systematic review. Nutrients. 2021;14:164. doi: 10.3390/nu14010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baraldi LG, Steele EM, Canella DS, Monteiro CA. Consumption of ultra-processed foods and associated sociodemographic factors in the USA between 2007 and 2012: evidence from a nationally representative cross-sectional study. BMJ Open. 2018;8:e020574. doi: 10.1136/bmjopen-2017-020574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berenson AB, Laz TH, Pohlmeier AM, Rahman M, Cunningham KA. Prevalence of food addiction among low-income reproductive-aged women. J Women’s Heal. 2015;24:740–744. doi: 10.1089/jwh.2014.5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis C, Levitan RD, Kaplan AS, Kennedy JL, Carter JC. Food cravings, appetite, and snack-food consumption in response to a psychomotor stimulant drug: the moderating effect of “food-addiction”. Front Psychol. 2014;5:403. doi: 10.3389/fpsyg.2014.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ceccarini M, Manzoni GM, Castelnuovo G, Molinari E. An evaluation of the Italian version of the Yale Food Addiction Scale in obese adult inpatients engaged in a 1-month-weight-loss treatment. J Med Food. 2015;18:1281–1287. doi: 10.1089/jmf.2014.0188. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed AY, Sayed AM. Prevalence of food addiction and its relationship to body mass index. Egypt J Medical Hum Genet. 2017;18:257–260. doi: 10.1016/j.ejmhg.2016.10.002. [DOI] [Google Scholar]
- 18.Kiyici S, Koca N, Sigirli D, Aslan BB, Guclu M, Kisakol G. Food addiction correlates with psychosocial functioning more than metabolic parameters in patients with obesity. Metab Syndr Relat D. 2020 doi: 10.1089/met.2019.0108. [DOI] [PubMed] [Google Scholar]
- 19.Nunes-Neto PR, Köhler CA, Schuch FB, et al. Food addiction: prevalence, psychopathological correlates and associations with quality of life in a large sample. J Psychiatr Res. 2018;96:145–152. doi: 10.1016/j.jpsychires.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Wenzel KR, Weinstock J, McGrath AB. The clinical significance of food addiction. J Addict Med. 2020;1. [DOI] [PubMed]
- 21.Mantilla EF, Clinton D, Monell E, Levallius J, Birgegård A. Impulsivity and compulsivity as parallel mediators of emotion dysregulation in eating‐related addictive‐like behaviors, alcohol use, and compulsive exercise. Brain Behav. 2021;e2458. [DOI] [PMC free article] [PubMed]
- 22.Schulte EM, Grilo CM, Gearhardt AN. Shared and unique mechanisms underlying binge eating disorder and addictive disorders. Clin Psychol Rev. 2016 doi: 10.1016/j.cpr.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.SAMHSA . Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. Rockville: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2019. [Google Scholar]
- 24.Pedram P, Wadden D, Amini P, et al. Food addiction: its prevalence and significant association with obesity in the general population. PLoS ONE. 2013;8:e74832. doi: 10.1371/journal.pone.0074832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pursey KM, Stanwell P, Gearhardt AN, Collins CE, Burrows TL. The prevalence of food addiction as assessed by the Yale Food Addiction Scale: a systematic review. Nutrients. 2014;6:4552–4590. doi: 10.3390/nu6104552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Şengör G, Gezer C. Food addiction and its relationship with disordered eating behaviours and obesity. Eat Weight Disord Ewd. 2019;24:1031–1039. doi: 10.1007/s40519-019-00662-3. [DOI] [PubMed] [Google Scholar]
- 27.Yekaninejad MS, Badrooj N, Vosoughi F, Lin C, Potenza MN, Pakpour AH. Prevalence of food addiction in children and adolescents: a systematic review and meta-analysis. Obes Rev. 2021 doi: 10.1111/obr.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volkow ND, Wang G-J, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obes Rev. 2012;14:2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiss DA, Avena N, Gold M. Food addiction and psychosocial adversity: biological embedding, contextual factors, and public health implications. Nutrients. 2020;12:3521. doi: 10.3390/nu12113521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borisenkov MF, Popov SV, Smirnov VV, et al. Association between food addiction and time perspective during COVID-19 isolation. Eat Weight Disord - Stud Anorexia Bulimia Obes. 2022;1–7. [DOI] [PMC free article] [PubMed]
- 31.Schulte EM, Kral TVE, Allison KC. A cross-sectional examination of reported changes to weight, eating, and activity behaviors during the COVID-19 pandemic among United States adults with food addiction. Appetite. 2021;105740. [DOI] [PMC free article] [PubMed]
- 32.Miniati M, Marzetti F, Palagini L, Marazziti D, Orrù G, Conversano C, Gemignani A. Eating disorders spectrum during the COVID pandemic: a systematic review. Front Psychol. 2021;12:663376. doi: 10.3389/fpsyg.2021.663376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiss D, Brewerton T. Separating the signal from the noise: how psychiatric diagnoses can help discern food addiction from dietary restraint. Nutrients. 2020;12:2937. doi: 10.3390/nu12102937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliveira J, Colombarolli MS, Cordás TA. Prevalence and correlates of food addiction: systematic review of studies with the YFAS 2.0. Obes Res Clin Pract. 2021 doi: 10.1016/j.orcp.2021.03.014. [DOI] [PubMed] [Google Scholar]
- 35.• Meule A, Gearhardt AN. Ten years of the Yale Food Addiction Scale: a Review of version 2.0. Curr Addict Rep. 2019;1–11. This review incorporates data on eating disorders into prevalence estimates for food addiction.
- 36.Fauconnier M, Rousselet M, Brunault P, Thiabaud E, Lambert S, Rocher B, Challet-Bouju G, Grall-Bronnec M. Food addiction among female patients seeking treatment for an eating disorder: prevalence and associated factors. Nutrients. 2020;12:1897. doi: 10.3390/nu12061897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Speranza M, Revah-Levy A, Giquel L, Loas G, Venisse J, Jeammet P, Corcos M. An investigation of Goodman’s addictive disorder criteria in eating disorders. Eur Eat Disord Rev. 2012;20:182–189. doi: 10.1002/erv.1140. [DOI] [PubMed] [Google Scholar]
- 38.Granero R, Hilker I, Agüera Z, et al. Food addiction in a Spanish sample of eating disorders: DSM-5 diagnostic subtype differentiation and validation data. Eur Eat Disord Rev. 2014;22:389–396. doi: 10.1002/erv.2311. [DOI] [PubMed] [Google Scholar]
- 39.• Tran H, Poinsot P, Guillaume S, Delaunay D, Bernetiere M, Bégin C, Fourneret P, Peretti N, Iceta S. Food addiction as a proxy for anorexia nervosa severity: new data based on the Yale Food Addiction Scale 2.0. Psychiat Res. 2020;293:113472. This article describes important considerations in the assessment of food addiction among those with anorexia nervosa. [DOI] [PubMed]
- 40.Carlson L, Steward T, Agüera Z, et al. Associations of food addiction and nonsuicidal self-injury among women with an eating disorder: a common strategy for regulating emotions? Eur Eat Disord Rev. 2018;26:629–637. doi: 10.1002/erv.2646. [DOI] [PubMed] [Google Scholar]
- 41.Meule A. Food addiction and body-mass-index: a non-linear relationship. Med Hypotheses. 2012;79:508–511. doi: 10.1016/j.mehy.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 42.de Vries S, Meule A. Food addiction and bulimia nervosa: new data based on the Yale Food Addiction Scale 2.0. Eur Eat Disord Rev. 2016;24:518–522. doi: 10.1002/erv.2470. [DOI] [PubMed] [Google Scholar]
- 43.Wiss DA, Brewerton TD, Tomiyama AJ. Limitations of the protective measure theory in explaining the role of childhood sexual abuse in eating disorders, addictions, and obesity: an updated model with emphasis on biological embedding. Eat Weight Disord - Stud Anorexia Bulimia Obes. 2021;1–19. [DOI] [PubMed]
- 44.Fernandez-Aranda F, Karwautz A, Treasure J. Food addiction: a transdiagnostic construct of increasing interest. Eur Eat Disord Rev. 2018;26:536–540. doi: 10.1002/erv.2645. [DOI] [PubMed] [Google Scholar]
- 45.Treasure J, Leslie M, Chami R, Fernández-Aranda F. Are trans diagnostic models of eating disorders fit for purpose? A consideration of the evidence for food addiction. Eur Eat Disord Rev. 2018;26:83–91. doi: 10.1002/erv.2578. [DOI] [PubMed] [Google Scholar]
- 46.Wiss DA, Brewerton TD. Incorporating food addiction into disordered eating: the disordered eating food addiction nutrition guide (DEFANG) Eat Weight Disorders - Stud Anorexia Bulimia Obes. 2017;22:49–59. doi: 10.1007/s40519-016-0344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verzijl CL, Gius B, Schlauch R, Rancourt D. The transdiagnostic role of food craving: an application of substance use models. Appetite. 2021;170:105867. doi: 10.1016/j.appet.2021.105867. [DOI] [PubMed] [Google Scholar]
- 48.Giacomo E di, Aliberti F, Pescatore F, Santorelli M, Pessina R, Placenti V, Colmegna F, Clerici M. Disentangling binge eating disorder and food addiction: a systematic review and meta-analysis. Eat Weight Disord - Stud Anorexia Bulimia Obes. 2022;1–8. [DOI] [PMC free article] [PubMed]
- 49.Hauck C, Weiß A, Schulte EM, Meule A, Ellrott T. Prevalence of ‘food addiction’ as measured with the Yale Food Addiction Scale 2.0 in a representative German sample and its association with sex, age and weight categories. Obes Facts. 2017;10:12–24. doi: 10.1159/000456013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stinson EJ, Votruba SB, Venti C, Perez M, Krakoff J, Gluck ME. Food insecurity is associated with maladaptive eating behaviors and objectively measured overeating: food insecurity and overeating. Obesity. 2018;26:1841–1848. doi: 10.1002/oby.22305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hooper L, Mason SM, Telke S, Larson N, Neumark-Sztainer D. Experiencing household food insecurity during adolescence predicts disordered eating and elevated body mass index 8 years later. J Adolescent Health. 2022 doi: 10.1016/j.jadohealth.2021.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meule A. An addiction perspective on eating disorders and obesity. In: Hebebrand J, Herpetz-Dahlmann B, editors. Eating Disorders and Obesity in Children and Adolescents. Elsevier Inc; 2019. pp. 99–104. [Google Scholar]
- 53.Chao AM, Shaw JA, Pearl RL, Alamuddin N, Hopkins CM, Bakizada ZM, Berkowitz RI, Wadden TA. Prevalence and psychosocial correlates of food addiction in persons with obesity seeking weight reduction. Compr Psychiat. 2017;73:97–104. doi: 10.1016/j.comppsych.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vainik U, Misic B, Zeighami Y, Michaud A, Mõttus R, Dagher A. Obesity has limited behavioural overlap with addiction and psychiatric phenotypes. Nat Hum Behav. 2019;1–9. [DOI] [PubMed]
- 55.Hunger JM, Smith JP, Tomiyama AJ. An evidence-based rationale for adopting weight-inclusive health policy. Soc Iss Policy Rev. 2020;14:73–107. doi: 10.1111/sipr.12062. [DOI] [Google Scholar]
- 56.Brytek-Matera A, Obeid S, Akel M, Hallit S. How does food addiction relate to obesity? Patterns of psychological distress, eating behaviors and physical activity in a sample of Lebanese adults: The MATEO study. Int J Environ Res Pu. 2021;18:10979. doi: 10.3390/ijerph182010979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schulte EM, Gearhardt AN. Development of the modified Yale Food Addiction Scale version 2.0. Eur Eat Disord Rev. 2017 doi: 10.1002/erv.2515. [DOI] [PubMed] [Google Scholar]
- 58.Meule A, Hermann T, Kubler A. Food addiction in overweight and obese adolescents seeking weight-loss treatment. Eur Eat Disord Rev. 2015 doi: 10.1002/erv.2355. [DOI] [PubMed] [Google Scholar]
- 59.Schiestl ET, Gearhardt AN. Preliminary validation of the Yale Food Addiction Scale for children 2.0: a dimensional approach to scoring. Eur Eat Disord Rev. 2018;26:605–617. doi: 10.1002/erv.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wiss DA, Avena NM. Food addiction, binge eating, and the role of dietary restraint: converging evidence from animal and human studies. In: Berner LA, editor. Frank KW. Switzerland: Springer Nature; 2020. pp. 193–209. [Google Scholar]
- 61.Brewerton TD, Dansky BS, Kilpatrick DG, O’Neil PM. Which comes first in the pathogenesis of bulimia nervosa: dieting or bingeing? Int J Eat Disorder. 2000;28:259–264. doi: 10.1002/1098-108X(200011)28:3<259::AID-EAT2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 62.Mussell MP, Mitchell JE, Fenna CJ, Crosby RD, Miller JP, Hoberman HM. A comparison of onset of binge eating versus dieting in the development of bulimia nervosa. Int J Eat Disorder. 1997;21:353–360. doi: 10.1002/(SICI)1098-108X(1997)21:4<353::AID-EAT8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 63.Fairburn CG, Cooper Z, Doll HA, Davies BA. Identifying dieters who will develop an eating disorder: a prospective, population-based study. Am J Psychiat. 2005;162:2249–2255. doi: 10.1176/appi.ajp.162.12.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stewart TM, Martin CK, Williamson DA. The complicated relationship between dieting, dietary restraint, caloric restriction, and eating disorders: is a shift in public health messaging warranted? Int J Environ Res Pu. 2022;19:491. doi: 10.3390/ijerph19010491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meule A, Lutz A, Vögele C, Kübler A. Food cravings discriminate differentially between successful and unsuccessful dieters and non-dieters. Validation of the Food Cravings Questionnaires in German. Appetite. 2012;58:88–97. doi: 10.1016/j.appet.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 66.Cui Y, Liu X, Xiang G, Li Q, Xiao M, Chen H. The association of restrained eating and overeating during COVID-19: a cross-lagged model. Nutrients. 2021;13:4535. doi: 10.3390/nu13124535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pietiläinen KH, Saarni SE, Kaprio J, Rissanen A. Does dieting make you fat? A twin study. Int J Obesity. 2011;36:456–464. doi: 10.1038/ijo.2011.160. [DOI] [PubMed] [Google Scholar]
- 68.Linardon J, Messer M, Shatte A, Skvarc D, Rosato J, Rathgen A, Fuller-Tyszkiewicz M. Targeting dietary restraint to reduce binge eating: a randomised controlled trial of a blended internet- and smartphone app-based intervention. Psychol Med. 2021;1–11. [DOI] [PubMed]
- 69.Oustric P, Gibbons C, Beaulieu K, Blundell J, Finlayson G. Changes in food reward during weight management interventions – a systematic review. Obes Rev. 2018;19:1642–1658. doi: 10.1111/obr.12754. [DOI] [PubMed] [Google Scholar]
- 70.Kahathuduwa CN, Binks M, Martin CK, Dawson JA. Extended calorie restriction suppresses overall and specific food cravings: a systematic review and a meta-analysis. Obes Rev. 2017;18:1122–1135. doi: 10.1111/obr.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meule A. The psychology of food cravings: the role of food deprivation. Curr Nutr Rep. 2020;1–7. [DOI] [PMC free article] [PubMed]
- 72.Monnier L, Schlienger J-L, Colette C, Bonnet F. The obesity treatment dilemma: why dieting is both the answer and the problem? A mechanistic overview. Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 73.Andrés A, Saldaña C. Body dissatisfaction and dietary restraint influence binge eating behavior. Nutr Res. 2014;34:944–950. doi: 10.1016/j.nutres.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 74.Polivy J, Coleman J, Herman CP. The effect of deprivation on food cravings and eating behavior in restrained and unrestrained eaters. Int J Eat Disorder. 2005;38:301–309. doi: 10.1002/eat.20195. [DOI] [PubMed] [Google Scholar]
- 75.Herman CP, Mack D. Restrained and unrestrained eating. J Pers. 1975;43:647–660. doi: 10.1111/j.1467-6494.1975.tb00727.x. [DOI] [PubMed] [Google Scholar]
- 76.Novara C, Pardini S, Visioli F, Meda N. Orthorexia nervosa and dieting in a non-clinical sample: a prospective study. Eat Weight Disord - Stud Anorexia Bulimia Obes. 2022;1–13. [DOI] [PMC free article] [PubMed]
- 77.Nayir T, Uskun E, Yürekli MV, Devran H, Çelik A, Okyay RA. Does body image affect quality of life?: a population based study. PLoS ONE. 2016;11:e0163290. doi: 10.1371/journal.pone.0163290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fuller-Tyszkiewicz M, Vuong H, Linardon J, Krug I, Broadbent J, Rodgers RF. Body image in and out of the lab: correspondence between lab-based attentional bias data and body shape dissatisfaction experiences in daily life. Body Image. 2020;32:62–69. doi: 10.1016/j.bodyim.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 79.Laraia BA, Leung CW, Tomiyama AJ, Ritchie LD, Crawford PB, Epel ES. Drive for thinness in adolescents predicts greater adult BMI in the Growth and Health Study cohort over 20 years. Obesity. 2021;29:2126–2133. doi: 10.1002/oby.23285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Imperatori C, Innamorati M, Lamis DA, Farina B, Fabbricatore M, Contardi A. Body uneasiness is associated with food addiction symptoms: a cross-sectional study. Eur Eat Disord Rev. 2018 doi: 10.1002/erv.2640. [DOI] [PubMed] [Google Scholar]
- 81.Hilker I, Sánchez I, Steward T, et al. Food addiction in bulimia nervosa: clinical correlates and association with response to a brief psychoeducational intervention. Eur Eat Disord Rev. 2016;24:482–488. doi: 10.1002/erv.2473. [DOI] [PubMed] [Google Scholar]
- 82.Richmond TK, Thurston IB, Sonneville KR. Weight-focused public health interventions—no benefit, some harm. Jama Pediatr. 2021 doi: 10.1001/jamapediatrics.2020.4777. [DOI] [PubMed] [Google Scholar]
- 83.Ahorsu DK, Lin C-Y, Imani V, Griffiths MD, Su J-A, Latner JD, Marshall RD, Pakpour AH. A prospective study on the link between weight-related self-stigma and binge eating: Role of food addiction and psychological distress. Int J Eat Disord. 2020 doi: 10.1002/eat.23219. [DOI] [PubMed] [Google Scholar]
- 84.Meadows A, Higgs S. Internalized weight stigma and the progression of food addiction over time. Body Image. 2020;34:67–71. doi: 10.1016/j.bodyim.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 85.Latner JD, Puhl RM, Murakami JM, O’Brien KS. Food addiction as a causal model of obesity. Effects on stigma, blame, and perceived psychopathology. Appetite. 2014;77:79–84. doi: 10.1016/j.appet.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 86.Obrien KS, Puhl RM, Latner JD, et al. The effect of a food addiction explanation model for weight control and obesity on weight stigma. Nutrients. 2020;12:294. doi: 10.3390/nu12020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Major B, Rathbone JA, Blodorn A, Hunger JM. The Countervailing Effects of Weight stigma on weight-loss motivation and perceived capacity for weight control. Personality Soc Psychol Bull. 2020;146167220903184 [DOI] [PubMed]
- 88.Mensinger JL, Cox SA, Henretty JR. Treatment outcomes and trajectories of change in patients attributing their eating disorder onset to anti-obesity messaging. Psychosom Med. 2021;83:777–786. doi: 10.1097/PSY.0000000000000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cardel MI, Newsome FA, Pearl RL, et al. Patient-centered care for obesity: how healthcare providers can treat obesity while actively addressing weight stigma and eating disorder risk. J Acad Nutr Diet. 2022 doi: 10.1016/j.jand.2022.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tinghino B, Lugoboni F, Amatulli A, et al. The FODRAT study (FOod addiction, DRugs, Alcohol and Tobacco): first data on food addiction prevalence among patients with addiction to drugs, tobacco and alcohol. Eat Weight Disord Ewd. 2020;1–7. [DOI] [PubMed]
- 91.Mies GW, Treur JL, Larsen JK, Halberstadt J, Pasman JA, Vink JM. The prevalence of food addiction in a large sample of adolescents and its association with addictive substances. Appetite. 2017;118:97–105. doi: 10.1016/j.appet.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 92.Burrows T, Kay-Lambkin F, Pursey K, Skinner J, Dayas C. Food addiction and associations with mental health symptoms: a systematic review with meta-analysis. J Hum Nutr Diet. 2018 doi: 10.1111/jhn.12532. [DOI] [PubMed] [Google Scholar]
- 93.Egervari G, Ciccocioppo R, Jentsch DJ, Hurd YL. Shaping vulnerability to addiction – the contribution of behavior, neural circuits and molecular mechanisms. Neurosci Biobehav Rev. 2018;85:117–125. doi: 10.1016/j.neubiorev.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jentsch DJ, Ashenhurst JR, Cervantes CM, Groman SM, James AS, Pennington ZT. Dissecting impulsivity and its relationships to drug addictions. Ann N Y Acad Sci. 2017;1327:1–26. doi: 10.1111/nyas.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maxwell AL, Gardiner E, Loxton NJ. Investigating the relationship between reward sensitivity, impulsivity, and food addiction: a systematic review. European Eat Disord Rev J Eat Disord Assoc. 2020 doi: 10.1002/erv.2732. [DOI] [PubMed] [Google Scholar]
- 96.Liu C, Rotaru K, Lee RSC, Tiego J, Suo C, Yücel M, Albertella L. Distress-driven impulsivity interacts with cognitive inflexibility to determine addiction-like eating. J Behav Addict. 2021 doi: 10.1556/2006.2021.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miranda-Olivos R, Agüera Z, Granero R, Vergeer RR, Dieguez C, Jiménez-Murcia S, Gearhardt AN, Fernández-Aranda F. Food addiction and lifetime alcohol and illicit drugs use in specific eating disorders. J Behav Addict. 2022 doi: 10.1556/2006.2021.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Güngör ES, Çelebi C, Akvardar Y. The relationship of food addiction with other eating pathologies and impulsivity: a case-control study. Frontiers Psychiatry. 2021;12:747474. doi: 10.3389/fpsyt.2021.747474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bartholow LAM, Huffman RT. The necessity of a trauma-informed paradigm in substance use disorder services. J Am Psychiat Nurses. 2021;107839032110364. [DOI] [PubMed]
- 100.Wiss DA, Brewerton TD. Adverse childhood experiences and adult obesity: a systematic review of plausible mechanisms and meta-analysis of cross-sectional studies. Physiol Behav. 2020;112964. [DOI] [PubMed]
- 101.Mason SM, Flint AJ, Field AE, Austin BS, Rich-Edwards JW. Abuse victimization in childhood or adolescence and risk of food addiction in adult women. Obesity. 2013;21:E775–E781. doi: 10.1002/oby.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mason SM, Flint AJ, Roberts AL, Agnew-Blais J, Koenen KC, Rich-Edwards JW. Posttraumatic stress disorder symptoms and food addiction in women by timing and type of trauma exposure. JAMA Psychiat. 2014;71:1271–1278. doi: 10.1001/jamapsychiatry.2014.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Novick AM, Levandowski ML, Laumann L, Philip NS, Price LH, Tyrka AR. The effects of early life stress on reward processing. J Psychiatr Res. 2018;101:80–103. doi: 10.1016/j.jpsychires.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Osadchiy V, Mayer EA, Bhatt R, et al. History of early life adversity is associated with increased food addiction and sex-specific alterations in reward network connectivity in obesity. Obes Sci Pract. 2019;5:416–436. doi: 10.1002/osp4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim S, Kwok S, Mayes LC, Potenza MN, Rutherford HJ, Strathearn L. Early adverse experience and substance addiction: dopamine, oxytocin, and glucocorticoid pathways. Ann N Y Acad Sci. 2017;1394:74–91. doi: 10.1111/nyas.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shin SH, McDonald S, Conley D. Profiles of adverse childhood experiences and impulsivity. Child Abuse Negl. 2018 doi: 10.1016/j.chiabu.2018.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dansky BS, Brewerton TD, Kilpatrick DG, O’Neil PM. The National Women’s Study: relationship of victimization and posttraumatic stress disorder to bulimia nervosa. Int J Eat Disorder. 1997;21:213–228. doi: 10.1002/(SICI)1098-108X(199704)21:3<213::AID-EAT2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 108.Brewerton TD, Perlman MM, Gavidia I, Suro G, Genet J, Bunnell DW. The association of traumatic events and posttraumatic stress disorder with greater eating disorder and comorbid symptom severity in residential eating disorder treatment centers. Int J Eat Disorder. 2020 doi: 10.1002/eat.23401. [DOI] [PubMed] [Google Scholar]
- 109.Bourdier L, Orri M, Carre A, Gearhardt AN, Romo L, Dantzer C, Berthoz S. Are emotionally driven and addictive-like eating behaviors the missing links between psychological distress and greater body weight? Appetite. 2018;120:536–546. doi: 10.1016/j.appet.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 110.Eisenberg ME, Berge JM, Fulkerson JA, Neumark-Sztainer D. Associations between hurtful weight-related comments by family and significant other and the development of disordered eating behaviors in young adults. J Behav Med. 2012;35:500–508. doi: 10.1007/s10865-011-9378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gómez-Donoso C, Sánchez-Villegas A, Martínez-González MA, Gea A, de Mendonça RD, Lahortiga-Ramos F, Bes-Rastrollo M. Ultra-processed food consumption and the incidence of depression in a Mediterranean cohort: the SUN Project. Eur J Nutr. 2019;59:1093–1103. doi: 10.1007/s00394-019-01970-1. [DOI] [PubMed] [Google Scholar]
- 112.Adjibade M, Julia C, Allès B, Touvier M, Lemogne C, Srour B, Hercberg S, Galan P, Assmann KE, Kesse-Guyot E. Prospective association between ultra-processed food consumption and incident depressive symptoms in the French NutriNet-Santé cohort. Bmc Med. 2019;17:78. doi: 10.1186/s12916-019-1312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lassale C, Batty GD, Baghdadli A, Jacka F, Sánchez-Villegas A, Kivimäki M, Akbaraly T. Healthy dietary indices and risk of depressive outcomes: a systematic review and meta-analysis of observational studies. Mol Psychiatr. 2019;24:965–986. doi: 10.1038/s41380-018-0237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Firth J, Marx W, Dash S, et al. The effects of dietary improvement on symptoms of depression and anxiety: a meta-analysis of randomized controlled trials. Psychosom Med. 2019;81:265–280. doi: 10.1097/PSY.0000000000000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Martin-Rodriguez E, Guillen-Grima F, Aubá E, Martí A, Brugos-Larumbe A. Relationship between body mass index and depression in women: a 7-year prospective cohort study. The APNA study. Eur Psychiat. 2016;32:55–60. doi: 10.1016/j.eurpsy.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 116.Cassin SE, Sijercic I, Montemarano V. Psychosocial interventions for food addiction: a systematic review. Curr Addict Reports. 2020;1–11.
- 117.Leary M, Pursey KM, Verdejo-Garcia A, Burrows TL. Current intervention treatments for food addiction: a systematic review. Behav Sci. 2021 doi: 10.3390/bs11060080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carmen M, Safer DL, Saslow LR, Kalayjian T, Mason AE, Westman EC, Dalai SS. Treating binge eating and food addiction symptoms with low-carbohydrate Ketogenic diets: a case series. J Eat Disord. 2020;8:2. doi: 10.1186/s40337-020-0278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rostanzo E, Marchetti M, Casini I, Aloisi AM. Very-low-calorie ketogenic diet: a potential treatment for binge eating and food addiction symptoms in women. A Pilot Study. Int J Environ Res Pu. 2021;18:12802. doi: 10.3390/ijerph182312802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Resmark G, Herpertz S, Herpertz-Dahlmann B, Zeeck A. Treatment of anorexia nervosa—new evidence-based guidelines. J Clin Medicine. 2019;8:153. doi: 10.3390/jcm8020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mandelli L, Draghetti S, Albert U, Ronchi DD, Atti A-R. Rates of comorbid obsessive-compulsive disorder in eating disorders: a meta-analysis of the literature. J Affect Disorders. 2020;277:12404. doi: 10.1016/j.jad.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 122.Yilmaz Z, Schaumberg K, Halvorsen M, et al. Predicting eating disorder and anxiety symptoms using disorder-specific and transdiagnostic polygenic scores for anorexia nervosa and obsessive-compulsive disorder. Psychol Med. 2022;1–15. [DOI] [PMC free article] [PubMed]
- 123.Albayrak Ö, Föcker M, Kliewer J, Esber S, Peters T, de Zwaan M, Hebebrand J. Eating-related psychopathology and food addiction in adolescent psychiatric inpatients. Eur Eat Disord Rev. 2017;25:214–220. doi: 10.1002/erv.2509. [DOI] [PubMed] [Google Scholar]
- 124.Mallorquí-Bagué N, Lozano-Madrid M, Testa G, et al. Clinical and neurophysiological correlates of emotion and food craving regulation in patients with anorexia nervosa. J Clin Med. 2020;9:960. doi: 10.3390/jcm9040960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bray B, Rodríguez-Martín BC, Wiss DA, Bray CE, Zwickey H. Overeaters anonymous: an overlooked intervention for binge eating disorder. Int J Environ Res Pu. 2021;18:7303. doi: 10.3390/ijerph18147303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guest E, Costa B, Williamson H, Meyrick J, Halliwell E, Harcourt D. The effectiveness of interventions aiming to promote positive body image in adults: a systematic review. Body Image. 2019;30:10–25. doi: 10.1016/j.bodyim.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 127.Brownell KD, Rodin J. Medical, metabolic, and psychological effects of weight cycling. Arch Intern Med. 1994;154:1325–1330. doi: 10.1001/archinte.1994.00420120035004. [DOI] [PubMed] [Google Scholar]
- 128.Jeffrey S, Heruc G. Balancing nutrition management and the role of dietitians in eating disorder treatment. J Eat Disord. 2020;8:64. doi: 10.1186/s40337-020-00344-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mahboub N, Rizk R, Karavetian M, Vries N de. Nutritional status and eating habits of people who use drugs and/or are undergoing treatment for recovery: a narrative review. Nutr Rev. 2020;nuaa095. [DOI] [PMC free article] [PubMed]
- 130.Wiss DA. The role of nutrition in addiction recovery: what we know and what we don’t. Elsevier. 2019 doi: 10.1016/b978-0-323-54856-4.00002-x. [DOI] [Google Scholar]
- 131.Watkins LE, Sprang KR, Rothbaum BO. Treating PTSD: a review of evidence-based psychotherapy interventions. Front Behav Neurosci. 2018;12:258. doi: 10.3389/fnbeh.2018.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, Jones L, Dunne MP. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health. 2017;2:e356–e366. doi: 10.1016/S2468-2667(17)30118-4. [DOI] [PubMed] [Google Scholar]
- 133.Fildes A, Charlton J, Rudisill C, Littlejohns P, Prevost AT, Gulliford MC. Probability of an obese person attaining normal body weight: cohort study using electronic health records. Am J Public Health. 2015;105:e54–e59. doi: 10.2105/AJPH.2015.302773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.• Wilcox CE. How to treat food addiction from a nutritional perspective: consideration of diet and abstinence. In: Food Addiction, Obesity, and Disorders of Overeating, An Evidence-Based Assessment and Clinical Guide. 2021;179–188. This chapter provides important consideration for the nutritional management of food addiction.
- 135.Brown-Bowers A, Ward A, Cormier N. Treating the binge or the (fat) body? Representations of fatness in a gold standard psychological treatment manual for binge eating disorder. Health. 2017;21:21–37. doi: 10.1177/1363459316674788. [DOI] [PubMed] [Google Scholar]
- 136.Leslie M, Lambert E, Treasure J. Towards a translational approach to food addiction: implications for bulimia nervosa. Curr Addict Reports. 2019;6:258–265. doi: 10.1007/s40429-019-00264-0. [DOI] [Google Scholar]
- 137.Imperatori C, Fabbricatore M, Vumbaca V, Innamorati M, Contardi A, Farina B. Food Addiction: definition, measurement and prevalence in healthy subjects and in patients with eating disorders. Riv Psichiatr. 2016;2:60–65. doi: 10.1708/2246.24196. [DOI] [PubMed] [Google Scholar]
- 138.Meule A. A Critical examination of the practical implications derived from the food addiction concept. Curr Obes Rep. 2019;8:11–17. doi: 10.1007/s13679-019-0326-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Basso JC, Satyal MK, Athamneh L, Bickel WK. Changes in temporal discounting, hedonic hunger, and food addiction during recovery from substance misuse. Appetite. 2021;105834. [DOI] [PMC free article] [PubMed]
- 140.Renna ME, Quintero JM, Fresco DM, Mennin DS. Emotion regulation therapy: a mechanism-targeted treatment for disorders of distress. Front Psychol. 2017;8:98. doi: 10.3389/fpsyg.2017.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dadomo H, Grecucci A, Giardini I, Ugolini E, Carmelita A, Panzeri M. Schema therapy for emotional dysregulation: theoretical implication and clinical applications. Front Psychol. 2016;7:1987. doi: 10.3389/fpsyg.2016.01987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Menefee DS, Ledoux T, Johnston CA. The importance of emotional regulation in mental health. Am J Lifestyle Med. 2022;16:28–31. doi: 10.1177/15598276211049771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li S, Schulte EM, Cui G, Li Z, Cheng Z, Xu H. Psychometric properties of the Chinese version of the modified Yale Food Addiction Scale version 2.0 (C-mYFAS 2.0): Prevalence of food addiction and relationship with resilience and social support. Eat Weight Disord - Stud Anorexia Bulimia Obes. 2022;27:273–284. doi: 10.1007/s40519-021-01174-9. [DOI] [PubMed] [Google Scholar]


