Abstract
Background
Faecal incontinence is a distressing disorder with high social stigma. Not all people with faecal incontinence can be cured with conservative or surgical treatment and they may need to rely on containment products, such as anal plugs.
Objectives
To assess the performance of different types of anal plugs for containment of faecal incontinence.
Search methods
We searched the Cochrane Incontinence Group Specialised Register, which contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE In‐Process, ClinicalTrials.gov, World Health Organization (WHO) ICTRP and handsearching of journals and conference proceedings (searched 26 May 2015). Reference lists of identified trials were searched and plug manufacturers were contacted for trials. No language or other limitations were imposed.
Selection criteria
Types of studies: this review was limited to randomised and quasi‐randomised controlled trials (including crossovers) of anal plug use for the management of faecal incontinence.
Types of participants: children and adults with faecal incontinence.
Types of interventions: any type of anal plug. Comparison interventions might include no treatment, conservative (physical) treatments, nutritional interventions, surgery, pads and other types or sizes of plugs.
Data collection and analysis
Two reviewers independently assessed methodological quality and extracted data from the included trials. Authors of all included trials were contacted for clarification concerning methodological issues.
Main results
Four studies with a total of 136 participants were included. Two studies compared the use of plugs versus no plugs, one study compared two sizes of the same brand of plug, and one study compared two brands of plugs. In all included studies there was considerable dropout (in total 48 (35%) dropped out before the end of the study) for varying reasons. Data presented are thus subject to potential bias. 'Pseudo‐continence' was, however, achieved by some of those who continued to use plugs, at least in the short‐term. In a comparison of two different types of plug, plug loss was less often reported and overall satisfaction was greater during use of polyurethane plugs than polyvinyl‐alcohol plugs.
Authors' conclusions
The available data were limited and incomplete, and not all pre‐specified outcomes could be evaluated. Consequently, only tentative conclusions are possible. The available data suggest that anal plugs can be difficult to tolerate. However, if they are tolerated they can be helpful in preventing incontinence. Plugs could then be useful in a selected group of people either as a substitute for other forms of management or as an adjuvant treatment option. Plugs come in different designs and sizes; the review showed that the selection of the type of plug can impact on its performance.
Plain language summary
Plugs for preventing the loss of stool in patients with faecal incontinence
Faecal incontinence is defined as the involuntary passage of faecal material through the anal canal and is a common and embarrassing problem. Different treatments exist, including dietary measures, drugs, specialized physiotherapy of the pelvic floor, and surgery. However, not all patients can be cured. These patients might be helped by using anal plugs. Different types of anal plugs are known, all aiming to block the loss of stool to control their incontinence. The aim of this review was to assess the performance of different types of anal plugs for containment of faecal incontinence.
Four studies with a total of 136 participants were included. Two studies compared the use of plugs versus no plugs. The involuntary loss of stool was effectively blocked (pseudo‐continence) in six (38%) participants who continued to use the plugs, at least in the short‐term. One study compared two sizes of the same brand of plug; due to the high dropout in this study and the incomplete data, no results concerning this comparison are available. In one study a comparison of two different brands of plug was made. Loss of plug was reported by 7 patients (30%) with a polyurethane (PU) plug and by 15 patients (65%) with the polyvinyl‐alcohol (PVA) plug. Overall satisfaction, defined as patients' opinion that the plug was good to very good, was reported more often for the PU plug (n = 17) than for the PVA plug (n = 8).
In all included studies there was considerable dropout; in total 48 participants (35%) dropped out before the end of the study for varying reasons. Data presented are thus subject to potential bias, and only tentative conclusions are possible. The available data suggest that anal plugs can be difficult to tolerate. However, if they are tolerated they can be helpful in preventing incontinence. Plugs could then be useful in a selected group of people either as a substitute for other forms of management or as an adjuvant treatment option. Plugs come in different designs and sizes; the review showed that the selection of the type of plug can impact on its performance.
Background
Faecal incontinence is defined as the involuntary passage of faecal material through the anal canal (Soffer 2000). The reported prevalence values range from 1.4% in the general population (defined as soiling of underwear, outer clothing, furnishing, or bedding several times a month or more often) (Perry 2002); to 46% in institutionalised elderly people (defined as at least one incontinent episode per week) (Borrie 1992). It is possible that the real prevalence is even higher than reported as faecal incontinence is associated with high social stigma and people are reluctant to seek help for this disorder because of embarrassment (Jorge 1993; Mavrantonis 1998).
The causes for faecal incontinence are diverse. In most cases a combination of factors leads to incontinence. Frequently cited causes are injuries during childbirth and prior anorectal surgery (Kamm 1998; Toglia 1998). But many other causes have been described, including loose stool, intestinal hurry, and neurological disease or injury.
Treatments available range from conservative therapy, such as dietary recommendations and anti‐diarrhoeal medication, to surgical treatment by either sphincter repair, dynamic graciloplasty, artificial anal sphincter implantation, or sacral nerve stimulation (Jorge 1993; Matzel 2003). Nowadays, the most common treatments are pelvic floor muscle training ‐ with or without biofeedback ‐ and anterior anal sphincter repair (Kamm 1998). The reported success rates with these forms of treatment vary, but it is recognized that none of the treatments will resolve the incontinence problems in all patients. Cochrane reviews are available that cover some of these treatments: these include drug treatment (Omar 2013); electrical stimulation, sphincter exercises and biofeedback (Hosker 2007; Norton 2012); sacral nerve stimulation (Mowatt 2007); surgery (Brown 2013); and management of faecal incontinence and constipation in adults with central neurological diseases (Coggrave 2014).
Where incontinence persists despite active treatment there may be no option other than containment. Brazzelli 2002 have reviewed the use of pads for the containment of anal and urinary incontinence. There is also a Cochrane review covering this topic (Fader 2008). Problems when using pads for faecal incontinence are that the odour from the anal leakage is difficult to control and extensive use of pads can result in skin condition problems. A possible way to avoid these problems is the use of an anal plug (sometimes called 'tampon'): a device specially developed for containing faecal incontinence.
Different types of anal plugs are known, all aiming to block the loss of stool. They were first used in patients suffering from faecal incontinence due to major neurological problems, such as caused by spina bifida (Norton 2001a). Nowadays, plugs are also sometimes used by patients with faecal incontinence who do not have an underlying neurological condition.
At this point it is unclear how effective anal plugs are in controlling stool loss in patients with faecal incontinence (with or without neurological impairments) and whether some types of anal plugs are more effective than others. This review aims to bring together in a systematic way the best available evidence to address these issues.
Objectives
To assess the performance of different types of anal plugs for containment of faecal incontinence.
The following comparisons were considered: 1. anal plugs versus no plugs; 2. one type of anal plug versus another; 3. anal plugs versus any other treatment.
Methods
Criteria for considering studies for this review
Types of studies
This review was limited to randomised and quasi‐randomised controlled trials (including crossovers) of anal plug use for the management of faecal incontinence.
Types of participants
All patients (children and adults) with faecal incontinence.
Types of interventions
Studies investigating the relative performance of anal plugs. Potential comparison interventions include no treatment, conservative (physical) treatments, nutritional interventions, surgery, pads, and other types or sizes of plugs.
Types of outcome measures
1. Patient symptoms:
frequency of incontinence of stool or flatus (diary or self‐report);
degree of incontinence (e.g. stool weight);
incontinence score;
episodes of anal urgency.
2. Physical measures:
achievement of pseudo‐continence (continence only while wearing a plug);
wearing time and frequency of use;
leakage rate;
odour control.
3. Patient satisfaction:
satisfaction with incontinence‐controlling capacity;
tolerability of plug (including persistence in using the plug);
comfort of plug in use;
comfort of plug removal/ease of removal;
feeling of cleanness.
4. Health status measures:
impact of incontinence on health status, social life, and quality of life.
5. Costs
6. Other outcomes:
non pre‐specified outcomes later judged important when performing the review.
Search methods for identification of studies
Electronic searches
We formulated a comprehensive and exhaustive search strategy in an attempt to identify all relevant studies regardless of language or publication status (published, unpublished, in press, and in progress).
This review has drawn on the search strategy developed for the Cochrane Incontinence Group. Relevant trials were identified from the Group's Specialised Register of Trials, which is described under the Group's module in the Cochrane Library. The Register contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and MEDLINE In‐Process, ClinicalTrials.gov, WHO ICTRP and handsearching of journals and conference proceedings.
The Incontinence Group Specialised Register was searched using the Group's own keyword system. The search terms used are given in Appendix 1. The date of the most recent search of the register for this review: 26 May 2015.
For the first published version of this review the authors performed additional searches which are detailed in Appendix 2.
Searching other resources
Additionally all reference lists of identified trials were searched. We contacted two manufacturers that marketed plugs to ask for details of unpublished or ongoing trials. We did not impose any language or other limitations on the searches.
Data collection and analysis
Study selection
Two reviewers assessed the title and abstract of references identified by the search strategy. The full reports of all potentially eligible randomised and quasi‐randomised controlled trials were then obtained for further assessment of eligibility. Any disagreements were resolved by discussion. Studies were only included if they were randomised or quasi‐randomised trials.
Methodological quality assessment
The quality of eligible trials was assessed independently by the two reviewers using a pre‐defined quality assessment form (see details under the Incontinence Group in The Cochrane Library). Reviewers were not blind to author, institution or journal. Disagreements between reviewers were resolved by discussion. Studies were not excluded from the review on the basis of methodological quality.
Data abstraction
Relevant data regarding inclusion criteria (study design, participants, interventions and outcomes), quality criteria (randomisation and blinding), and results were extracted independently by the two reviewers using a data abstraction form adapted from the form designed by the Dutch Cochrane Centre. In cases where insufficient data were reported authors were contacted for further information (such as method of randomisation, statistical methods).
Data analysis
Data were analysed using the MetaView statistical software in Review Manager 4.2.5 (RevMan 2003). For dichotomous variables, risk ratios (RR) and 95% confidence intervals (CI) were derived for each outcome. It was not possible to combine data from the included studies as outcomes, and type of comparisons varied. We instead present a qualitative synthesis of the results of the primary studies.
Results
Description of studies
The search strategy identified 13 potentially eligible studies. When full citations were obtained nine studies could not be included: seven were patient series, one was a case study, and one study was excluded as we understood from the author that this paper did not report a randomised trial.
Thus in total, four studies met our inclusion criteria (Bond 2005; Norton 2001b; Pfrommer 2000; Van Winckel 2005). Two of these studies were derived from the Specialised Trials Register of the incontinence group (Bond 2005; Pfrommer 2000). One was derived by the additional searches performed by one of the authors (Norton 2001b). The final trial was obtained by contacting an anal plug manufacturer (Van Winckel 2005).
The reports of Bond 2005 and Van Winckel 2005, two of the included trials, had not been published at the time of finishing the original version of the review (Deutekom 2005) . We received permission from the authors to use their data in our review. For the 2010 update of the review (2010, Issue 1 search updated but no new citation) one of the trials had been published but did not add any extra data to that provided by the author for the original version of this review (Van Winckel 2005). For the 2012 update the published journal article for Bond 2005 was available but again this did not add any extra data to that provided by the author for the original version of this review. The flow of literature through the assessment process is shown in Figure 1.
1.

PRISMA study flow diagram.
The total number of participants across the trials was 136. For a detailed description of individual studies please refer to the table of Characteristics of included studies.
Design
Three studies used a randomised crossover designs (Norton 2001b; Pfrommer 2000; Van Winckel 2005); and one was a parallel group randomised controlled trial (Bond 2005).
Sample size
Sample sizes were 16 (Van Winckel 2005); 34 (Norton 2001b); 38 (Pfrommer 2000); and 48 (Bond 2005).
Diagnosis
All studies included patients with faecal incontinence. One study included patients who were partially continent or incontinent following imperforate anus repair (Pfrommer 2000). One study included children who had faecal incontinence due to a high type imperforate anus and children with spina bifida (Van Winckel 2005). One study included children (older than 4 years) and young adults (16 to 45 years) who were incontinent due to congenital or acquired neurogenic disorders (Bond 2005); and one included adult outpatients after failure of previous treatment (Norton 2001b).
Location/setting
One trial was carried out in Scotland and participants were identified primarily by hospital specialists from Paediatric Surgery or Gastroenterology in Aberdeen, Inverness and Glasgow (Bond 2005). One trial was carried out in Germany in a hospital for Paediatric Surgery (Pfrommer 2000); one in Belgium at the departments of Paediatrics and Urology in an academic medical centre (Van Winckel 2005); and one in England in a specialist colorectal hospital where patients received an individual instruction with a nurse specialist (Norton 2001b).
Interventions
The four identified trials made the following comparisons:
1. anal plug versus no plug (Bond 2005; Van Winckel 2005);
2‐I. one type of anal plug versus another: comparison of two sizes of the same type of plug (polyurethane anal plug) (Norton 2001b);
2‐II. one type of anal plug versus another: comparison of two different types of plugs (polyurethane anal plug versus polyvinyl‐alcohol plug) (Pfrommer 2000).
Length of treatment
Three trials lasted between four and six weeks (Norton 2001b; Pfrommer 2000; Van Winckel 2005). One trial lasted one year (Bond 2005).
Outcomes
Common reported outcomes were frequency of incontinent episodes (effectiveness of treatment), satisfaction and tolerance.
Risk of bias in included studies
Potential for selection bias at trial entry
In all crossover trials the order of the intervention was randomised (Norton 2001b; Pfrommer 2000; Van Winckel 2005). In none of these studies were details provided concerning the methods used for randomisation and concealment. In the parallel group randomised controlled trial the participants were randomly allocated to the intervention or control group (Bond 2005). Randomisation was performed using pre‐determined codes.
Potential for bias at time of treatment or outcome assessment
As the studies included in this review investigated anal plugs it is difficult to blind patients and staff to intervention. In two studies the use of plugs was compared to a control intervention in which patients did not receive any treatment (Bond 2005; Van Winckel 2005): blinding was impossible. In the remaining two randomised crossover studies two types or two sizes of plugs were compared (Norton 2001b; Pfrommer 2000). Neither study reported any blinding.
Potential for bias in trial analysis
In three studies the number and reasons for patient dropouts were clearly described (Norton 2001b; Pfrommer 2000; Van Winckel 2005).
In one study 23 of the 34 (68%) patients did not start or dropped out (Norton 2001b). Reasons why patients dropped out were: they disliked the idea and did not start the study (n = 4); they failed to attend the clinic (n = 2); they dropped out because of discomfort after trying the first plug (n = 8); and they dropped out after trying one size of plug, refusing to try the second one (n = 9).
In one study 15 of the 38 patients (39%) dropped out before the end of the study (Pfrommer 2000). Two patients liked the first tested product and ended participation; six patients found the smallest available size of the products (tested first) to be too big; two patients reported discomfort; one patient constantly lost one of the products; and four patients failed to complete the protocol for non‐plug related reasons.
In one study 4 of the 16 patients dropped out (25%) (Van Winckel 2005). Reported reasons for this were discomfort and pain (n = 2); and losing the plug (n = 2).
In one study 6 patients dropped out from the 48 included, but did not report any reasons for this (Bond 2005). All those who dropped out were in the intervention group.
In only one of the studies was an intention‐to‐treat analysis performed (Van Winckel 2005). In none of the trials was there a description whether data analysis was performed blindly.
Effects of interventions
Three randomised crossover trials and one randomised controlled trial were included in this review. As the reported outcome measures varied amongst trials, a quantitative synthesis of the results was not feasible. In the summary tables 'N' denotes the total number of patients and 'n' denotes the number of patients who had the outcome. Unfortunately the data from the randomised crossover studies were not presented in a form suitable for inclusion in the formal analysis.
Comparison 1: anal plugs versus no plugs
Two of the included studies compared the use of anal plugs with standard treatment (Bond 2005; Van Winckel 2005). In both studies patients were allowed to choose their preferred size of plug. In both trials a choice could be made between small or larger Coloplast plugs.
Patient symptoms
Pseudo‐continence was reported in six out of 16 patients in the treatment period in the crossover study, and in none of the 16 during the control period (Analysis 1.7). Patients achieving pseudo‐continence were reported to show greater satisfaction with treatment during plug use (no further data provided by the author) than when not using a plug (Van Winckel 2005). Three of the 16 patients (two with anal atresia and one with spina bifida) continued using the plug after the study. Neither stool frequency nor stool consistency was affected by use of the plug (no further data provided by the author). In the parallel group trial, clinically derived condition‐specific measures (such as protection, rash/skin problems, and unpleasant odour) tended to favour the plugs group for all patients (adults and children) (Analysis 1.4), although no difference was statistically significant, confidence intervals were wide, and dropout rates were considerable (Bond 2005).
1.7. Analysis.
Comparison 1 Anal plugs versus no plugs, Outcome 7 Achievement of pseudo‐continence.
| Achievement of pseudo‐continence | ||
|---|---|---|
| Study | Anal plug period | Control period |
| Van Winckel 2005 | 6/12 | 0/12 |
1.4. Analysis.

Comparison 1 Anal plugs versus no plugs, Outcome 4 Condition‐specific measures of faecal incontinence improved.
Patient satisfaction
In the randomised crossover trial four patients did not complete the treatment period due to discomfort and pain (n = 2; anal atresia) and losing of the plug (n = 2; spina bifida) (Van Winckel 2005). The plug was thus not tolerated in four out of 16 patients. All patients tolerated the control period (Analysis 1.8).
1.8. Analysis.
Comparison 1 Anal plugs versus no plugs, Outcome 8 Intolerance of intervention.
| Intolerance of intervention | ||
|---|---|---|
| Study | Anal plug period | Control period |
| Van Winckel 2005 | 4/16 | 0/16 |
Health status measures
Bond 2005 reported data for adults on changes in general health (3/14 versus 0/5; risk ratio (RR) was 2.63 (95% confidence interval (CI) 0.16 to 43.63) (Analysis 1.1)); bodily pain (6/15 versus 3/5; (RR 0.67, 95% CI 0.26 to 1.72) (Analysis 1.2)); and various measures of well being (derived from SF‐36) (Analysis 1.3). Confidence intervals were all wide, reflecting the small numbers studied.
1.1. Analysis.

Comparison 1 Anal plugs versus no plugs, Outcome 1 General health improved ‐ adults.
1.2. Analysis.

Comparison 1 Anal plugs versus no plugs, Outcome 2 Bodily pain improved ‐ adults.
1.3. Analysis.
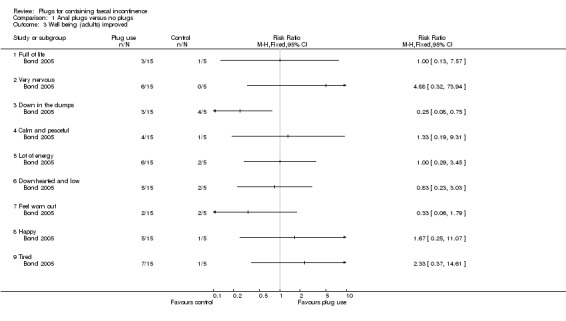
Comparison 1 Anal plugs versus no plugs, Outcome 3 Well being (adults) improved.
Costs
Little or no evidence was obtained that the plug led to significant reductions in the overall costs of care (Bond 2005).
No data were available for the other pre‐specified outcomes (see Table of comparisons).
Comparison 2‐I: one type of anal plug versus another (comparison of two sizes)
One study compared two sizes of a plug in a randomised crossover design (Norton 2001b). Due to the high dropout in this study and the incomplete data, no results concerning the comparison are available.
Comparison 2‐II: one type of anal plug versus another (comparison of two types)
One study compared two types of plugs in a randomised crossover design: polyurethane anal plug (Conveen, Coloplast) (PU plug) versus EFF‐EFF polyvinyl‐alcohol plug (Med. SSE‐System) (PVA plug) (Pfrommer 2000).
Patient symptoms
The absence of soiling episodes was reported in 15 (65%) patients when using the PU plug and by 14 (60%) patients when using the PVA plug (Analysis 2.1)
2.1. Analysis.
Comparison 2 One type of anal plug versus another type, Outcome 1 Plug effectiveness: number of people with no soiling.
| Plug effectiveness: number of people with no soiling | ||
|---|---|---|
| Study | PU plug | PVA plug |
| Pfrommer 2000 | 15/23 | 14/23 |
Patient satisfaction
Feelings of security were reported by 16 patients (69%) while using the PU plug and by 10 patients (43%) when using the PVA plug (Analysis 2.2). Loss of plug was reported by 7 patients (30%) with the PU plug and by 15 patient 65% with the PVA plug (Analysis 2.3). Inconvenience was reported by 9 patients (39%) when using the PU plug and by 16 (69%) patients when using the PVA plug (Analysis 2.4). Overall satisfaction, defined as patients' opinion that the plug was good to very good, was reported more often for the PU plug (n = 17) than for the PVA plug (n = 8) (Analysis 2.5). 14 patients preferred the PU plug, 5 patients preferred the PVA plug, and 4 patients reported no preference.
2.2. Analysis.
Comparison 2 One type of anal plug versus another type, Outcome 2 Feeling of security.
| Feeling of security | ||
|---|---|---|
| Study | PU plug | PVA plug |
| Pfrommer 2000 | 16/23 | 10/23 |
2.3. Analysis.
Comparison 2 One type of anal plug versus another type, Outcome 3 Loss of plug.
| Loss of plug | ||
|---|---|---|
| Study | PU plug | PVA plug |
| Pfrommer 2000 | 7/23 | 15/23 |
2.4. Analysis.
Comparison 2 One type of anal plug versus another type, Outcome 4 Inconvenience.
| Inconvenience | ||
|---|---|---|
| Study | PU plug | PVA plug |
| Pfrommer 2000 | 9/23 | 16/23 |
2.5. Analysis.
Comparison 2 One type of anal plug versus another type, Outcome 5 Overall satisfaction.
| Overall satisfaction | ||
|---|---|---|
| Study | PU plug | PVA plug |
| Pfrommer 2000 | 17/23 | 8/23 |
No data were available for the other pre‐specified outcomes.
Comparison 3: anal plug versus any other treatment
No eligible trials were found.
Discussion
This review of anal plugs for the containment of faecal incontinence was limited by the small quantity of eligible studies and participants and the fact that combining data was either impossible or inappropriate. Only one parallel group randomised controlled trial that compared the use of plugs with no intervention could be included (Bond 2005). The other three included trials were randomised crossover studies. These also reported dropouts and are further limited by not allowing longer‐term acceptability rates to be assessed. One such trial compared plug use versus no intervention (Van Winckel 2005). The other two studies compared two types of plugs. Two sizes of the same plug were investigated in one study (Norton 2001b); and two brands of plugs were compared in the other study (Pfrommer 2000).
Reported outcome measures varied. Two studies reported patient symptoms (Pfrommer 2000; Van Winckel 2005); one study reported physical measures (Bond 2005); two studies reported patient satisfaction (Pfrommer 2000; Van Winckel 2005); and one study reported the outcome of health status measures and costs (Bond 2005). There are other variables that may influence the successful wearing of anal plugs, such as leakage rate, skin problems, odour, wearing time, frequency of use, age, social environment and patient characteristics. Unfortunately there were insufficient data in the included studies to take these factors into consideration in our review.
Participant groups also varied between the studies. The participants in two studies suffered from faecal incontinence due to congenital diseases (Van Winckel 2005; Pfrommer 2000). These patients are a minority in the total population of patients with faecal incontinence.
Due to the diversity in comparisons, outcome measures, and type of participants, we were not able to perform a quantitative synthesis of the data but described the data per comparison. This does not allow us to state firm and precise conclusions and emphasizes the need for further research.
The methodological quality of the four included trials was generally poor. Inclusion and exclusion criteria were given in two studies (Bond 2005; Van Winckel 2005); and one study described only inclusion criteria (Pfrommer 2000). However, none of the studies provided outcomes related to either severity or frequency of faecal incontinence.
Concealment of allocation was performed in only one study (Bond 2005). Due to the nature of the intervention of the studies it appeared to be impossible to blind the patients or outcome assessors. Only one trial reported that the researcher who was responsible for the inclusion of patients was securely blinded to the randomisation process (Bond 2005).
Incompleteness of follow‐up occurred in most trials, caused by selective withdrawal of patients, to a large extent related to intolerance or dissatisfaction with the intervention. An intention‐to‐treat analysis could be performed in only one trial (Van Winckel 2005). In the study comparing two sizes of anal plugs the high rate of dropout meant that it was not possible to extract data (Norton 2001b). However, it did not appear that there was a difference in dropout rates between studies or patient groups. Most trials studied small patient groups, limiting the power to detect differences between groups.
One trial excluded data from patients who reported that they did not have difficulties with these particular outcomes before or after the intervention (Bond 2005). Thus, Bond 2005 only reported comparisons between the intervention group and control group when the outcome troubled the patient and it was reported as the same, improved or worse. The results presented in this review included data from all the patients. Consequently, our results differ from those published by the author.
Authors' conclusions
Implications for practice.
This review has focused on the performance of anal plugs for containment of faecal incontinence. The available data were limited and incomplete and not all pre‐specified outcomes could be evaluated. Consequently, we can only draw tentative conclusions.
The available data suggest that anal plugs can be difficult to tolerate. For those who do persist, the limited evidence available suggests that plugs may be helpful in alleviating the problems caused by incontinence. In a minority of people with faecal incontinence, plugs could be useful either as a substitute for other forms of management or as an adjuvant treatment option. Plugs come in different designs and sizes; the review showed that the selection of the type of plug can impact on its performance. Specifically, a polyurethane plug performed better than a polyvinyl‐alcohol plug in one trial.
Implications for research.
This review has illustrated how difficult it is to undertake rigorous evaluations of devices for incontinence. We were only able to extract data from three trials, of which two trials presented small patients groups. Crossover designs are attractive for chronic conditions like intractable faecal incontinence, but the trials reviewed showed high dropout rates (to the extent that in one trial no useful information was generated), and anyway do not allow longer‐term performance to be evaluated. Whether or not people persist with using a plug is likely to be a good indication of its value. However, the single parallel group randomised trial that could potentially address this issue illustrated the difficulties of using this design: recruitment proved difficult, and there were more dropouts in the group allocated plugs. This could, therefore, be a scenario where high‐quality observational studies could provide useful information. The strength of a randomised controlled trial, however, is that it allows a more reliable assessment of what would happen without the use of plugs; this would clearly be preferable if problems with compliance and retention in the study could be resolved.
There are lessons to be learnt for the design and conduct of future trials. Better reporting of study methods and detailed descriptions of interventions are necessities. Future studies should describe their procedure for blinding, especially in studies where it is quite difficult, if not impossible, to maintain true blinding. Patients will be aware of wearing a plug, and this may affect outcome. While tolerance is a key point in the willingness to use an anal plug, the reasons for patient withdrawal and dropout must be specified and the long‐term effects need to be evaluated. Both intention‐to‐treat analysis and per‐protocol analysis should be performed. Sensitivity analyses should explore sensible assumptions about those participants with missing data, for example due to dropout.
When evaluating different plugs, investigators should take into account costs and quality of life, as well as other, specific, advantages and disadvantages of plug use. To avoid bias from socially desired answers from participants, advantages and disadvantages of plug use should be elicited by an independent researcher.
What's new
| Date | Event | Description |
|---|---|---|
| 3 July 2015 | New search has been performed | A search of the Incontinence Review Group Specialised Trials Register was performed on 26th May 2015 but did not identify any new trial. |
| 3 July 2015 | New citation required but conclusions have not changed | A search of the Incontinence Review Group Specialised Trials Register was performed on 26th May 2015 but did not identify any new trial. |
History
Protocol first published: Issue 1, 2005 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 29 February 2012 | New search has been performed | A search of the Incontinence Review Group Specialised Trials Register was performed on 29th February 2012 but no new trials were found. |
| 29 February 2012 | New citation required but conclusions have not changed | A search of the Incontinence Review Group Specialised Trials Register was performed on 29th February 2012 but no new trials were found. |
| 16 July 2009 | New search has been performed | New search, no new trials. |
| 9 October 2008 | Amended | Converted to new review format. |
| 10 August 2006 | New search has been performed | A search of the Incontinence Group Specialised Trials Register was performed on 29 May 2006 but no new trials were found. For this update of the review one of the trials (Van Winckel 2005) has now been published but did not add any extra data to the unpublished data provided by the author for the original version of this review. |
| 25 May 2005 | New citation required and conclusions have changed | Substantive amendment. |
Acknowledgements
We are grateful to all members of the Cochrane Incontinence Group in Aberdeen (especially to June Cody and Sheila Wallace) for their assistance with the review. Thanks are also due to all authors and to suppliers of anal plugs for their cooperation. Finally we would like to thank Christine Norton for her useful comments on the protocol.
Appendices
Appendix 1. Cochrane Incontinence Group Specialised Register search terms
The Incontinence Group Specialised Register was searched using the Group's own keyword system. The search terms used were:
(design.rct* or design.cct*) AND ({INTVENT.MECH.DEVICE.ANAL.TAMPON} OR {INTVENT.MECH.device.pessaries.} OR {INTVENT.MECH.DEVICE.PLUG.} OR {INTVENT.MECH.DEVICE.PLUG.anal.} OR {INTVENT.MECH.DEVICE.VAGINAL.*} OR {INTVENT.MECH.DEVICES.} OR {INTVENT.MECH.devices.pessaries.} OR {INTVENT.MECH.})
(All searches were of the keyword field of Reference Manager 2012).
Date of the most recent search of the register for this review: 26 May 2015.
Appendix 2. Search methods for the first published version of this review
For the first version of this review (Deutekom 2005) the authors performed the following additional searches. All searches were carried out on 26 November 2004. The following electronic bibliographic databases were searched: MEDLINE (January 1966 to November 2004); CINAHL (January 1982 to November Week 3 2004); EMBASE (January 1996 to 2004 Week 47); INVERT (Dutch nursing database ‐ Index van de Nederlandstalige Verpleegkundige Tijdschriftliteratuur) (January 1993 to November 2004); and Web of Science (January 1988 to November 2004). The following search terms were used: 1.tampon* 2.plug* 3.incontinen* 4.stool* 5.faec* 6.fecal incontinence/ [mesh] 7.flatus [mesh] OR anal [mesh] 8.anus OR anal 9.(1 or 2) AND (3 or 4 or 5 or 6 or 7 or 8) Key: * = truncation symbol. Additionally all reference lists of identified trials were searched.
We contacted two manufacturers that marketed plugs to ask for details of unpublished or ongoing trials. We did not impose any language or other limitations on the searches.
Data and analyses
Comparison 1. Anal plugs versus no plugs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 General health improved ‐ adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Bodily pain improved ‐ adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Well being (adults) improved | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Full of life | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Very nervous | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Down in the dumps | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Calm and peaceful | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Lot of energy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Downhearted and low | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Feel worn out | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Happy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 Tired | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Condition‐specific measures of faecal incontinence improved | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Protection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Rash/skin problems | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Unpleasant odour | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Staining/smearing | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Bowel movement in undergarments (last two weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Frequency of unpleasant odours | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Bowel movements in undergarments (on average day) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.8 Soiled/stained undergarment (on average day) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.9 Prevents staying away from home | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.10 Must avoid long journeys | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.11 Must always have a toilet nearby | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Stool frequency | Other data | No numeric data | ||
| 6 Costs | Other data | No numeric data | ||
| 7 Achievement of pseudo‐continence | Other data | No numeric data | ||
| 8 Intolerance of intervention | Other data | No numeric data |
1.5. Analysis.
Comparison 1 Anal plugs versus no plugs, Outcome 5 Stool frequency.
| Stool frequency | |
|---|---|
| Study | |
| Bond 2005 | No differences were observed between control and intervention group |
1.6. Analysis.
Comparison 1 Anal plugs versus no plugs, Outcome 6 Costs.
| Costs | |
|---|---|
| Study | |
| Bond 2005 | Little or no evidence that the plug led to significant reductions in the overall costs of care |
Comparison 2. One type of anal plug versus another type.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Plug effectiveness: number of people with no soiling | Other data | No numeric data | ||
| 2 Feeling of security | Other data | No numeric data | ||
| 3 Loss of plug | Other data | No numeric data | ||
| 4 Inconvenience | Other data | No numeric data | ||
| 5 Overall satisfaction | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bond 2005.
| Methods | Randomised controlled trial (2:1) | |
| Participants | Forty eight patients took part in the trial (28 children, age > 4 years; and 20 young adults, age 16 to 45 years) | |
| Interventions | 1. Polyurethane anal plug (Conveen, Coloplast) (two sizes) 2. no intervention | |
| Outcomes | ‐ Functional Status II‐R ‐SF‐36 ‐ a Patient‐Generated Index of Quality of Life (PGI) ‐ a Carer‐Generated Index (CGI) of Quality of Life ‐ the Dartmouth COOP Charts ‐ a condition‐ specific measure developed for the research ‐ qualitative data: advantages and disadvantages of the plug ‐ health service utilisation data ‐ costs data ‐ evaluation of education package |
|
| Notes | 31 (16 children and 15 adults) allocated plugs; 17 (12 children and 5 adults) allocated to no plugs. 6 participants did not complete the trial (5 in plug group and 1 in control group). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Norton 2001b.
| Methods | Randomised cross‐over trial | |
| Participants | Adult outpatients (n = 34) attending a specialist colorectal hospital after failure of previous treatment | |
| Interventions | 1. Polyurethane anal plug (Conveen, Coloplast) 37 mm diameter when open 2. Polyurethane anal plug (Conveen, Coloplast) 45 mm diameter when open | |
| Outcomes | ‐ comfort of inserting plug ‐ comfort of plug in use ‐ comfort of taking plug out ‐ capacity of controlling faecal leakage ‐ preference ‐ patient characteristics which predict when the plug will help the most |
|
| Notes | Of the 34 patients offered the plug, 4 refused as they disliked the idea, 2 failed to attend, 8 dropped out after trying first plug, because of discomfort and 9 dropped out after trying one size of plug, refusing to try the second size. 11 patients completed the protocol. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Pfrommer 2000.
| Methods | Randomised cross‐over trial | |
| Participants | 38 partially continent or incontinent patients following imperforate anus repair (age > 6 to 15) | |
| Interventions | 1. Polyurethane anal plug (Conveen, Coloplast) (size closed/open diameter of 14.5/38mm or 15.5/45mm; depending on anal canal diameter) 2. EFF‐EFF polyvinyl‐alcohol plug (Med. SSE‐System) (diameters ranging from 15mm to 38mm. Used size dependent on anal canal diameter) | |
| Outcomes | ‐ stool consistency ‐ awareness of repletion ‐ effectiveness of treatment ‐ feeling of security ‐ loss of plug ‐ inconvenience ‐ overall satisfaction | |
| Notes | 38 patients included. Drop‐out: ‐ 2 patients liked first tested product ‐ 6 patients found the smallest available size of the products tested first too big ‐ 2 patients reported discomfort ‐ 1 patient constantly lost one of the products ‐ 4 patients failed to complete the protocol for non‐plug related reasons | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Van Winckel 2005.
| Methods | Randomised cross‐over trial | |
| Participants | 7 patients (4 to 12 yrs; 3 girls and 4 boys) with high type of imperforate anus; and 9 patients with spina bifida (6 to 13 yrs, 2 girls and 7 boys) | |
| Interventions | 1. Polyurethane anal plug (Conveen, Coloplast) (size 12 or 13mm; depending on preference). 2. no intervention | |
| Outcomes | ‐ number of stools ‐ number of soiling episodes ‐ number of diapers or pads used ‐ number of plugs ‐ satisfaction | |
| Notes | 16 patients included. ‐ 2 (patients with imperforate anus) dropped‐out due to discomfort and pain ‐ 2 (patients with spina bifida) dropped out because of losing the plug | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Contributions of authors
The lead reviewer (MD) undertook additional literature searches and contacted authors and manufacturers for additional information when needed. The two reviewers (MD, AD) independently undertook the quality assessment of the trials and the data extraction. The lead reviewer entered the data. This was checked by the other reviewer (AD). Text until the discussion was drafted by the lead reviewer and checked by the other reviewer. Text starting with the discussion was checked by the lead reviewer and drafted by the other reviewer.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Incontinence.
Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bond 2005 {unpublished data only}
- Bond C. The anal plug: an evaluation of a novel management option for faecal incontinence. Final report to Chief Scientist Office, Scottish Executive Health Department, Edinburgh 2005. [sr‐incont6653]
- Bond C, Youngson G, MacPherson I, Garrett A, Bain N, Donald S, et al. Anal plugs for the management of fecal incontinence in children and adults: a randomized control trial. Journal of Clinical Gastroenterology 2007;41(1):45‐53. [sr‐incont22554] [DOI] [PubMed] [Google Scholar]
Norton 2001b {published data only}
- Norton C, Kamm MA. Anal plug for faecal incontinence. Colorectal Disease 2001;3(5):323‐7. [sr‐incont19091] [DOI] [PubMed] [Google Scholar]
Pfrommer 2000 {published data only}
- Pfrommer W, Holschneider AM, Loffler N, Schauff B, Ure BM. A new polyurethane anal plug in the treatment of incontinence after anal atresia repair. European Journal of Pediatric Surgery 2000;10(3):186‐90. [sr‐incont11013] [DOI] [PubMed] [Google Scholar]
Van Winckel 2005 {unpublished data only}
- Winckel M. Clinical evaluation of a new anal medical device to achieve faecal continence in spina bifida and anal atresia patients. personal communication 2005. [sr‐incont22025]
- Winckel M, Biervliet S, Laecke E, Hoebeke P. Is an anal plug useful in the treatment of fecal incontinence in children with spina bifida or anal atresia?. Journal of Urology 2006;176(1):342‐4. [sr‐incont22027] [DOI] [PubMed] [Google Scholar]
Additional references
Borrie 1992
- Borrie MJ, Davidson HA. Incontinence in institutions: costs and contributing factors. Canadian Medical Association Journal 1992;147(3):322‐8. [PMC free article] [PubMed] [Google Scholar]
Brazzelli 2002
- Brazzelli M, Shirran E, Vale L. Absorbent products for containing urinary and/or fecal incontinence in adults. Journal of Wound, Ostomy and Continence Nursing 2002;29(1):45‐54. [DOI] [PubMed] [Google Scholar]
Brown 2013
- Brown SR, Wadhawan H, Nelson RL. Surgery for faecal incontinence in adults. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD001757.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coggrave 2014
- Coggrave M, Norton C, Cody JD. Management of faecal incontinence and constipation in adults with central neurological diseases. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD002115.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fader 2008
- Fader M, Cottenden AM, Getliffe K. Absorbent products for moderate‐heavy urinary and/or faecal incontinence in women and men. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007408] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hosker 2007
- Hosker G, Cody JD, Norton CC. Electrical stimulation for faecal incontinence in adults. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD001310.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jorge 1993
- Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Diseases of the Colon & Rectum 1993;36(1):77‐97. [DOI] [PubMed] [Google Scholar]
Kamm 1998
- Kamm MA. Faecal incontinence. BMJ 1998;316(7130):528‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matzel 2003
- Matzel KE, Bittorf B, Stadelmaier U, Hohenberger W. Sacral nerve stimulation in the treatment of faecal incontinence. Chirurg 2003;74(1):26‐32. [DOI] [PubMed] [Google Scholar]
Mavrantonis 1998
- Mavrantonis C, Wexner SD. A clinical approach to fecal incontinence. Journal of Clinical Gastroenterology 1998;27(2):108‐21. [DOI] [PubMed] [Google Scholar]
Mowatt 2007
- Mowatt G, Glazener CMA, Jarrett M. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD004464.pub2] [DOI] [PubMed] [Google Scholar]
Norton 2001a
- Norton C, Kamm MA. Anal plug for faecal incontinence. Colorectal Disease 2001;3(5):323‐7. [DOI] [PubMed] [Google Scholar]
Norton 2012
- Norton C, Cody JD. Biofeedback and/or sphincter exercises for the treatment of faecal incontinence in adults. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD002111.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Omar 2013
- Omar MI, Alexander CE. Drug treatment for faecal incontinence in adults. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD002116.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perry 2002
- Perry S, Shaw C, McGrother C, Matthews RJ, Assassa RP, Dallosso H, et al. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut 2002;50(4):480‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reference Manager 2012
- Reference Manager Professional Edition Version 12. New York: Thomson Reuters 2012.
RevMan 2003 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 4.2.5. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2003.
Soffer 2000
- Soffer EE, Hull T. Fecal incontinence: a practical approach to evaluation and treatment. American Journal of Gastroenterology 2000;95:1873‐80. [DOI] [PubMed] [Google Scholar]
Toglia 1998
- Toglia MR. Pathophysiology of anorectal dysfunction. Obstetrics and Gynecology Clinics of North America 1998;25(4):771‐81. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Deutekom 2005
- Deutekom M, Dobben AC. Plugs for containing faecal incontinence. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD005086.pub2] [DOI] [PubMed] [Google Scholar]


