Abstract
Background and Aim:
The primary aim was to validate the Pancreatitis Activity Scoring System (PASS) in a multicenter prospectively ascertained acute pancreatitis (AP) cohort. Second, we investigated the association of early PASS trajectories with disease severity and length of hospital stay (LOS).
Methods:
Data were prospectively collected through the APPRENTICE consortium (2015–2018). AP severity was categorized based on revised Atlanta classification. Delta PASS (ΔPASS) was calculated by subtracting activity score from baseline value. PASS trajectories were compared between severity subsets. Subsequently, the cohort was subdivided into three LOS subgroups as short (S-LOS): 2–3 days; intermediate (I-LOS): 3–7 days; and long (L-LOS): ≥7 days. The generalized estimating equations model was implemented to compare PASS trajectories.
Results:
There were 434 subjects analyzed including 322 (74%) mild, 86 (20%) moderately severe, and 26 (6%) severe AP. Severe AP subjects had the highest activity levels and the slowest rate of decline in activity (P = 0.039). Focusing on mild AP, L-LOS subjects (34%) had 28 points per day slower decline; whereas, S-LOS group (13%) showed 34 points per day sharper decrease compared with I-LOS (53%; P < 0.001). We noticed an outlier subset with a median admission-PASS of 466 compared with 140 in the rest. Morphine equivalent dose constituted 80% of the total PASS in the outliers (median morphine equivalent dose score = 392), compared with only 25% in normal-range subjects (score = 33, P value < 0.001).
Conclusions:
This study highlighted that PASS can quantify AP activity. Significant differences in PASS trajectories were found both in revised Atlanta classification severity and LOS groups, which can be harnessed in AP monitoring/management (ClincialTrials.gov number, NCT03075618).
Keywords: Acute pancreatitis, Disease activity, Length of hospital stay, Pancreas
Introduction
Acute pancreatitis (AP) is one of the most frequent gastrointestinal causes of hospitalization in the USA.1 Incidence of disease has been increasing steadily over recent decades, while mortality rate dropped.2 AP encompasses a diverse spectrum, which in the majority of patients is a mild self-limited disease.3 However, the disease still carries a 10–20% risk of a severe course characterized by persistent organ failure and 10–30% mortality rate.4–6 During inpatient care, AP may demonstrate highly variable clinical manifestations, which impose a clinical challenge for the managing team.7 Even in the absence of local and systemic complications, patients may experience a protracted hospital stay due to persistent symptoms, such as abdominal pain and/or oral intolerance.8,9 This notion further stresses the importance of establishing tools for monitoring disease activity specifically in the mild AP stratum.
Activity indices are useful, applicable tools with clinical implications, which can assist clinical management as well as research studies assessing treatment modalities. Studies on other complex syndromes, such as Crohn’s disease, rheumatoid arthritis, and systemic vasculitis have established the benefit of activity indices for continuous assessment, guiding treatment, and monitoring disease status.10–12 Moreover, activity indices have been regularly implemented in clinical trials to assess responses to therapeutic agents.13,14 Due to the variable course of AP, dynamic assessment of disease is an essential step in delivering high-quality care. This approach warrants a metric, which can capture the fluctuating manifestations of disease.
Recently, the Pancreatitis Activity Scoring System (PASS) was introduced as a dynamic measure to quantify AP activity.15 Unlike severity scores, which classify AP based on the outcomes, PASS captures the progressive nature of the disease via its dynamic manifestations.16,17 Initial PASS development was based on a Delphi procedure and has subsequently been assessed in another cohort from the region of central/southern California.15 Validation of PASS amongst a diverse cohort and practice patterns facilitated in a multicenter fashion would be required to establish its generalizability. In addition, an elaborate analysis is required to understand the significance of sequential PASS measurements.
The Acute Pancreatitis Patients Registry to Examine Novel Therapies in Clinical Experience (APPRENTICE) is a multicenter collaboration for harmonized prospective recruitment of AP patients.18 Such a prospective cohort provides an opportunity to assess the applicability and generalizability of the PASS index.
The aims of this study were to (i) assess the performance of PASS as a disease activity index in a large AP cohort enrolled from various sites across the USA, (ii) evaluate the rate of PASS changes during the course of AP in the RAC subgroups, and (iii) determine the clinical significance of early PASS trajectories and their association with LOS.
Methods
The APPRENTICE is a prospective multicenter consortium launched in 2015 and coordinated at the University of Pittsburgh Medical Center. The study protocol was conducted following the ethical committee regulations and approved by institutional review boards (IRB) at each site. The University of Pittsburgh’s IRB approved the study protocol and acted as an umbrella IRB for subsites (PRO15040389). Data management was conducted in accordance with the data use agreements signed between all the participating centers. The study was registered in clinicaltrials.gov (NCT03075618), and the detailed methodology has been published.18
Data were collected from sites in North America. Enrollment was conducted between August 2015 and January 2018. AP diagnosis was based on presence of ≥2 of the following features: characteristic epigastric pain, serum lipase >3 times the upper limit of normal, and/or characteristic radiological findings. Adult AP patients (≥18 years) within 7 days from pain onset were deemed eligible. Patients with chronic pancreatitis, organ transplantation, or pancreatic cancer were excluded.18 De-identified data were entered into the Research Electronic Data Capture (REDCap) tool.19
Data collected within the first 12 h of hospitalization were assigned to the admission time point. Subsequently, study time points were assigned data from the preceding 12 h in the following pattern: 24-h (12–24-h events), 48-h (36–48-h events), 72-h time points (60–72-h events), and day 7 (156–168-hour events). The discharge time point reflected the 12 h leading to patient’s discharge. PASS consists of five separately weighted parameters15: organ failure (×100 per organ), oral intolerance (×40), systemic inflammatory response syndrome (SIRS) (×25 per criterion), morphine equivalent dose or MED (×5), and pain score (×5).
Organ failure including respiratory, cardiovascular, or renal dysfunction was defined according to modified Marshal score ≥2.20 Oral intolerance was defined as worsening abdominal pain and/or vomiting following the resumption of oral feeding diet SIRS score was defined as the cumulative score assigned to each of the following four variables: heart rate (>90 beats per minute), respiratory rate >20/minute or PCO2 < mmHg, core temperature (<36 or >38), and white blood cell count (<4000/mm3 or >12 000/mm3).21 MED score was calculated by converting opiate analgesics dose to the intravenous MED. Abdominal pain was based on numeric visual analogue scale ranging from 0 (no pain) to 10 (worst pain). PASS was calculated based on the worst or extreme findings at each time point.
Severity of AP was classified according to revised Atlanta classification (RAC).17 Subjects were subdivided based on the total length of hospital stay (LOS) into three subgroups: short (S-LOS): 2–3 days; intermediate (I-LOS): 3–7 days; and long (L-LOS): ≥7 days.
Statistical methodology
Patients were considered to have missing SIRS score if ≥3 elements were not recorded. Patients missing all three of SIRS, MED, and pain score were excluded. For subjects with >2 SIRS components available and within normal range, cumulative score was considered to be “zero.” For those with >2 SIRS components available and at least another PASS point, pain and/or MED score data were imputed from patients with similar severity levels.
Median and interquartile range (IQR) were reported for continuous variables, while totals and proportions for categorical variables. For all subgroups, PASS score for each time point was calculated. To examine differences in PASS trajectories over time, a variable named delta PASS (ΔPASS) was generated, which reflected change in the score compared with the baseline PASS at admission. The generalized estimating equations (GEE) model was implemented to compare PASS trajectories. The main analysis of this study was focused on PASS slopes with ΔPASS being the key variable. This outcome can only be calculated when serial PASS values are available. Therefore, per our analysis plan, we did not include subjects with <2 days of hospital stay, in whom the PASS trajectory cannot be assessed. The subgroup with LOS < 2 days consisted of only 26 subjects (5.7%).
The AP cohort was subdivided based on RAC and based on LOS. Moreover, we did an exclusive analysis comparing three LOS subgroups within the mild RAC cohort. When comparing the three RAC groups via GEE model, moderately severe served as the reference group, and I-LOS was the reference group amongst LOS subsets. In addition to trajectory analyses, we performed a cross-sectional comparison of PASS between subgroups at admission and discharge using the Kruskal–Wallis test.
We also identified a subgroup within our study population with an outstanding activity level on admission. Outlier PASS values were defined as those with score >[(1.5 × IQR) + 75th percentile].22 Raw and percent contribution to overall PASS score for each of the components as well as patient characteristics were compared between outliers and normal-range subgroups. Within the normal-range and the outlier groups, PASS was regressed on MED score, and the percent of variation in PASS attributed to the MED score was calculated as model sums of squares divided by total sums of squares.
Data management was performed in R version 3.4.1 (Vienna, Austria),23 and statistical analysis was performed in STATA version 15.1 (College Station, TX, USA).24 All tests were two sided, and a P value < 0.05 was considered significant.
Results
A total of 460 AP patients from North American sites were enrolled. Twenty-six subjects were excluded due to missing data or death. PASS was analyzed in 434 subjects. The studied population were collected from University of Pittsburgh Medical Center (110 subjects [25%]), Eastern Maine (76 [18%]), Cleveland Clinic (74 [17%]), Johns Hopkins (64 [15%]), Indiana University (55 [12%]), Allegheny General Hospital (31 [7%]), Kaiser Permanente (13 [3%]), and Medical University of Southern Carolina (11 [3%]).
The median age of AP population was 52 years, 50% were male, and 77% were Caucasian. The most prevalent AP etiology was biliary (34%), and median LOS was 5 days (range, 2–70). The baseline characteristics of the study cohort are summarized in Table 1. With respect to the length of hospitalization, 57 subjects (13%) had S-LOS, 232 (53%) I-LOS, and 145 (34%) were in L-LOS subgroup. Overall, the median admission PASS index was 142 (105–204). Subsequently, PASS showed a consistent decline: at 24 h (132 [85–203]) and 48 h (107 [40–175]). Discharge PASS was calculable in 151 subjects. Sixty cases (40%) were discharged with a PASS score of zero. Overall, the median discharge PASS was 15 (IQR, 15–45).
Table 1.
Characteristics of the overall study population
| Variable | Value (n = 434) |
|---|---|
| Age, median (IQR) | 52 (37–65) |
| Male, N (%) | 218 (50.2) |
| Race/ethnicity, N (%) | |
| Hispanic/Latino | 18 (4.2) |
| Asian Indian | 6 (1.4) |
| Black/African American | 66 (15.2) |
| White/Caucasian | 335 (77.2) |
| Other | 9 (2.1) |
| BMI, median (IQR) | 28.5 (23.7–34.5) |
| Smoking (active smokers), N (%) | 86 (19.8) |
| Alcohol (active drinkers), N (%) | 64 (14.8) |
| Positive SIRS (≥2) on admission | 124 (28.6) |
| Transferred, N (%) | 148 (34.1) |
| Index acute pancreatitis N (%) | 272 (62.7%) |
| Etiology, N (%) | |
| Biliary | 147 (33.9) |
| Alcoholic | 84 (19.4) |
| Post-endoscopic retrograde | 78 (18.0) |
| cholangiopancreatography | |
| Idiopathic | 71 (16.4) |
| HTG | 26 (6.0) |
| Other | 28 (6.5) |
| Length of stay (median, IQR) | 5 (3–8) |
| RAC, N (%) | |
| Mild | 322 (74.2) |
| Moderate | 86 (19.8) |
| Severe | 26 (6.0) |
BMI, body mass index; HTG, hypertriglyceridemia; IQR, interquartile range; RAC, revised Atlanta classification; SIRS, systemic inflammatory response syndrome.
Comparison of PASS trajectories between RAC subsets.
Concerning RAC severity, 322 subjects (74%) developed mild AP, 86 cases (20%) moderately severe, and 26 subjects (6%) experienced severe AP. Admission PASS was significantly different between mild (135, [100–187]), moderately-severe (168, [132–232]), and severe RAC subsets (231, [165–265], P value = 0.0001). At each time point, the severe subgroup had the highest, whereas the mild subjects had the lowest index (Fig. 1).
Figure 1.
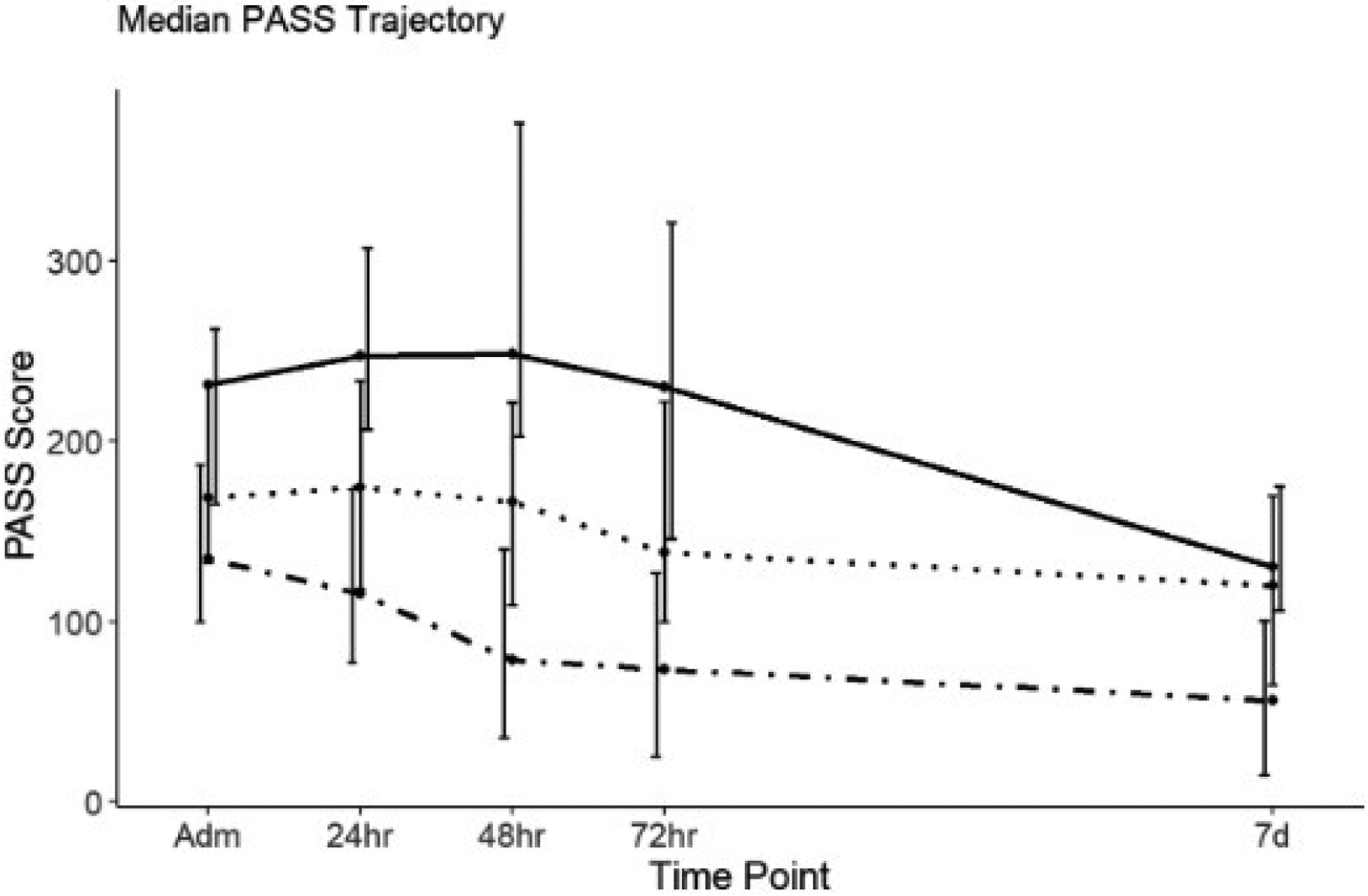
Pancreatitis Activity Scoring System (PASS) trajectories amongst three severity subsets of acute pancreatitis cohort categorized based on revised Atlanta classification. Revised Atlanta classification (RAC): , Mild;
, Mild;  , Moderate;
, Moderate;  , Severe.
, Severe.
The early PASS slopes showed a significant difference between the RAC subgroups. Mild subjects had the sharpest decline within the first 24 (19 points per day, P < 0.0001) and 48 h (17 points per day, P = 0.0031) compared with the moderately severe subgroup. On the contrary, severe subgroup demonstrated the slowest decline amongst the three RAC subgroups. Severe subjects had 49 points per day slower decrease at 24 h (P value < 0.0001) and 38 points per day slower decrease at 48-h time point (P value < 0.0001) compared with the reference group.
Comparison of trajectories between LOS subsets.
The three LOS groups exhibited a down-trending PASS pattern throughout study time points (Fig. 2). Admission PASS was significantly different between S-LOS (120, [100–158]), I-LOS (142, [102–203]), and L-LOS subsets (160, [122–230], P value = 0.008). At each time point, the L-LOS subgroup had the highest, whereas the S-LOS subjects had the lowest index (Table 2).
Figure 2.
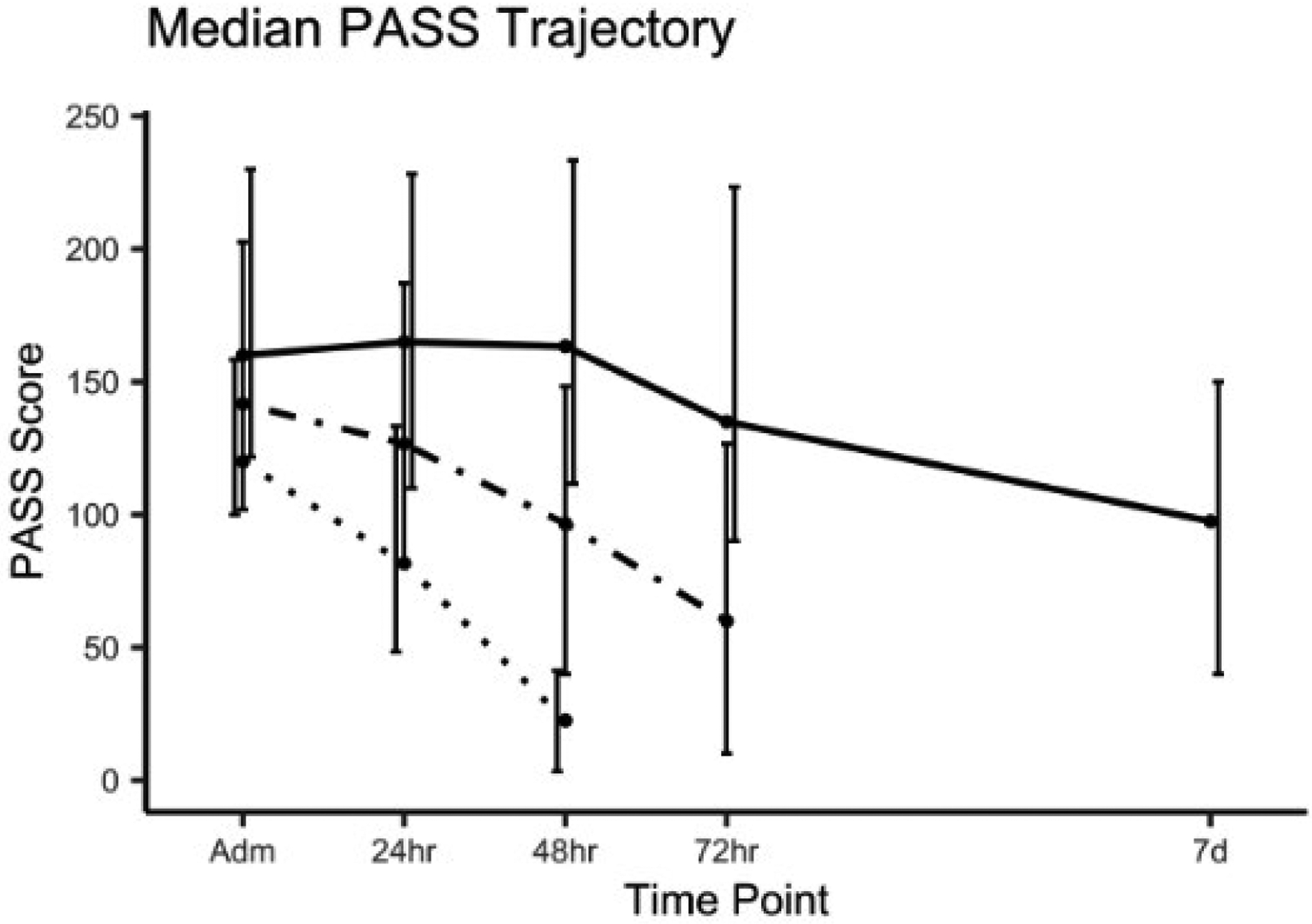
Pancreatitis Activity Scoring System (PASS) trajectories amongst three length of stay (LOS) subsets of acute pancreatitis cohort: short LOS (S-LOS), intermediate LOS (I-LOS), and long LOS (L-LOS). LOS cohort:  , S-LOS;
, S-LOS; , I-LOS;
, I-LOS; , L-LOS.
, L-LOS.
Table 2.
Serial PASS in three length of stay (LOS) subgroups: short (S-LOS). intermediate (I-LOS), and long (L-LOS)
| Study time points | ||||||
|---|---|---|---|---|---|---|
| Length of stay subgroup | N (%) | Admission median (IQR) | 24 h median (IQR) | 48 h median (IQR) | 72 h median (IQR) | 7 days median (IQR) |
| S-LOS (48–72 h) | 57 (13.1%) | 120 (100–158) | 82 (48–133) | 23 (3–41) | — | — |
| I-LOS (72 h-7 days) | 232 (53.4%) | 142 (102–203) | 127 (82–187) | 97 (40–148) | 60 (10–127) | — |
| L-LOS (7 day+) | 145 (33.4%) | 160 (122–230) | 165 (110–228) | 163 (112–233) | 135 (90–223) | 98 (40–150) |
IQR, interquartile range.
Early PASS slopes were significantly different between LOS subgroups. L-LOS subjects had 23 points per day slower decline in activity index while S-LOS subgroup had 36 points per day sharper decrease in PASS compared with I-LOS as the reference group (P value < 0.001). The detailed comparison of PASS trajectories is tabulated in Table 3. Activity level at discharge did not show significant difference (S-LOS: 23 [3–41], I-LOS: 0 [0–45], L-LOS: 26 [0–75], P value = 0.09).
Table 3.
Generalized estimating equations: comparison of PASS and modified PASS trajectories between short (S-LOS), intermediate (I-LOS), and long (L-LOS) subsets
| Variable | Estimate (95% confidence interval) | P value |
|---|---|---|
| Difference in PASS rate of change | ||
| S-LOS | 36 (2, 71) | — |
| I-LOS (reference) | Reference | <0.001 |
| L-LOS | −23 (−36, −9) | — |
| ΔPASS 24 h | ||
| S-LOS | 17 (5, 28) | 0.005 |
| I-LOS (reference) | Reference | — |
| L-LOS | −24 (−32, 15) | <0.001 |
| ΔPASS 48 h | ||
| S-LOS | 53 (18, 88)> | 0.003 |
| I-LOS (reference) | Reference | — |
| L-LOS | −46 (−61,−31) | <0.001 |
| ΔPASS 72 h | ||
| S-LOS | — | — |
| I-LOS (reference) | Reference | <0.001 |
| L-LOS | −69 (−96, −42) | — |
PASS, Pancreatitis Activity Scoring System.
Comparison of trajectories between LOS subsets in the mild AP group.
Because 74% of patients were classified as mild AP, we elected to further analyze PASS within the mild stratum based on the LOS. The three LOS subgroups demonstrated down-trending activity trajectories throughout study time points (Fig. 3). S-LOS mild subset had lower admission PASS (120, [100–158]), compared with I-LOS (138, [99–198]), and L-LOS (140, [98–188]); however, this difference was not statistically significant (P value = 0.067). Table 4 shows the median scores at each time point.
Figure 3.
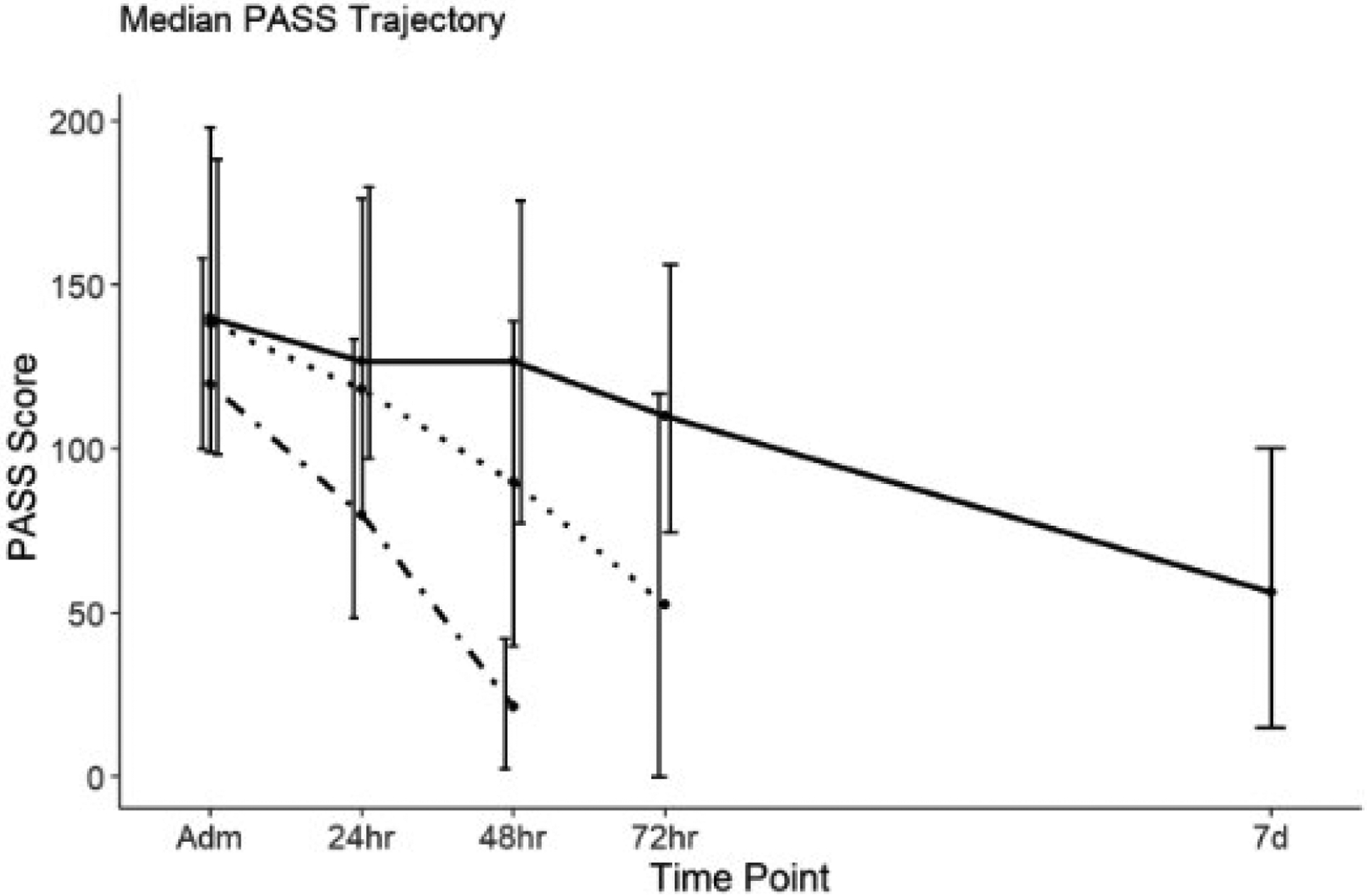
Pancreatitis Activity Scoring System (PASS) trajectories amongst three length of stay (LOS) subsets of mild acute pancreatitis cohort: short LOS (S-LOS), intermediate LOS (I-LOS), and long LOS (L-LOS). LOS:  , S-LOS;
, S-LOS;  , I-LOS;
, I-LOS;  , L-LOS.
, L-LOS.
Table 4.
Serial Pancreatitis Activity Scoring System in mild acute pancreatitis: median Pancreatitis Activity Scoring System in three length of stay (LOS) subgroups: short (S-LOS), intermediate (I-LOS), and long (L-LOS)
| Length of stay subgroup in mild stratum | Study time points | |||||
|---|---|---|---|---|---|---|
| N (%) | Admission median (IQR) | 24 h median (IQR) | 48 h median (IQR) | 72 h median (IQR) | 7 days median (IQR) | |
| S-LOS (48–72 h) | 56 (17.4%) | 120 (100–158.3) | 80 (48.3–133.6) | 21.3 (2.5–42.2) | — | — |
| I-LOS (72 h-7 days) | 199 (61.8%) | 138.3 (99.2–198.3) | 118.3 (80.0–176.5) | 90.0 (40.0–139.2) | 52.5 (0.0–117.1) | — |
| L-LOS (7 day+) | 67 (20.8%) | 140.0 (98.3–188.3) | 126.7 (97.1–180.0) | 126.7 (77.5–175.8) | 110.0 (74.6–156.2) | 56.2 (15.0–100.4) |
IQR, interquartile range.
The GEE model detected a significant difference in PASS trajectories between the three LOS subgroups within the mild AP cohort (Table 5). L-LOS subjects had 28 points per day slower decline in activity index compared with I-LOS as the reference group (P value < 0.0001). Contrarily, S-LOS group had 34 points per day sharper decrease in PASS (P value < 0.0001). Moreover, L-LOS subjects had the lowest ΔPASS at 24-h (P value = 0.007), 48-h (P value < 0.0001), and at 72-h time point (P value < 0.0001) compared with I-LOS subset. Figure 3 exhibits the PASS trajectories in Mild AP.
Table 5.
Generalized estimating equations in mild acute pancreatitis stratum: comparison of PASS trajectories between short (S-LOS), intermediate (I-LOS), and long (L-LOS) subsets
| Estimate (95% confidence interval) | P value | |
|---|---|---|
| Difference in PASS rate of change | ||
| S-LOS | 34.5 (5.3, 63.6) | <0.0001 |
| I-LOS | Reference | |
| L-LOS | −28.4 (41.9, −14.8) | |
| ΔPASS 24 h | ||
| S-LOS | 14.6 (5.2, 24.0) | 0.0025 |
| I-LOS | Reference | — |
| L-LOS | −12.1 (−20.8, −3.3) | 0.0069 |
| ΔPASS 48 h | ||
| S-LOS | 49.1 (19.9, 78.3) | 0.0011 |
| I-LOS | Reference | — |
| L-LOS | −40.5 (−55.8,−25.1) | <0.0001 |
| ΔPASS 72 h | ||
| S-LOS | — | — |
| I-LOS | Reference | — |
| L-LOS | −68.8 (−96.4, 41.3) | <0.0001 |
PASS, Pancreatitis Activity Scoring System.
Outliers.
Based on admission PASS, 24 subjects were identified as outliers (exceeding the cut-off score of 355) with a median index of 487 compared with 140 in normal-range subjects. Median scores related to each of the PASS components were compared between outliers and normal-range stratum. Median MED score showed a pronounced difference (382 in outliers vs 33 in normal cases, P value < 0.001). Comparing the proportional distribution of PASS elements, MED contributed 80% to the score in the outliers, while only 25% in normal-range subjects. Furthermore, 97% of the variation in admission PASS was explained by MED score in the outliers; whereas only 67% in the normal-range subjects. (Fig. 4).
Figure 4.
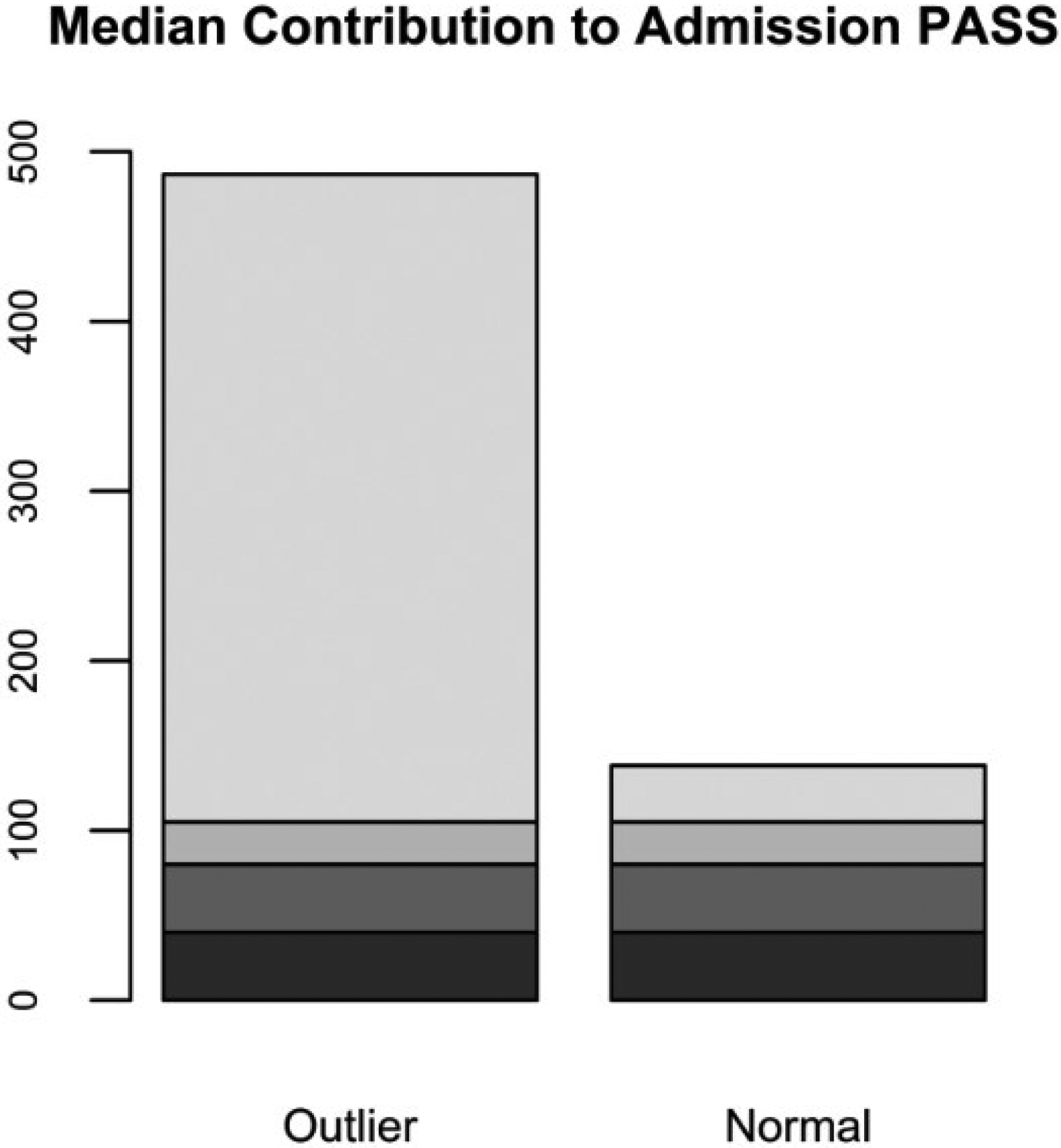
Median contribution of each component to admission Pancreatitis Activity Scoring System (PASS) in normal-range versus outlier subset. MED, morphine equivalent dose; SIRS, systemic inflammatory response syndrome.  , Pain;
, Pain;  , Oral intolerance;
, Oral intolerance;  , Organ failure;
, Organ failure;  , SIRS;
, SIRS;  , MED.
, MED.
Patients with outlier admission scores were more commonly male (68% vs 51%), younger (45 vs 50 years), more frequently active drinkers (55% vs 37%), and active smokers (40% vs 22%, all P values < 0.01). Furthermore, AP in this subset was predominantly alcohol-induced (38% vs 20% in normal range) with biliary etiology being less common (25% vs 47%).
Discussion
In this prospective study, we validated the application of PASS as an AP activity instrument across several US sites from seven different states. Serial PASS measurements demonstrated a consistent decrease over the clinical course. Severe AP was associated with a higher activity index at each discrete time point. Subsequently, subjects were categorized based on the LOS, which is also a clinically and financially relevant outcome. Similar to RAC severity, the LOS subgroups exhibited significantly different PASS values and trajectories at discrete time points. We also utilized the LOS categories to differentiate the mild AP subjects, which represent the majority of AP cases, into subgroups. GEE analysis in the mild AP cohort, confirmed significantly different trajectories between the three LOS scenarios. This important finding of distinctive trends and differences in ΔPASS amongst the severity and LOS subgroups has not been demonstrated previously. This finding further underlines that serial PASS measurements during the early stage of AP can monitor disease progression and potentially guide management.
PASS score has been initially assessed in two studies originating from the region of central and southern California.15,25 Therefore, there is a need for additional independent large-scale surveys involving subjects from different regions to establish PASS applicability in a heterogeneous cohort distributed in various clinical settings. Our study analyzed prospectively enrolled data from eight sites across the USA. Furthermore, in contrast to previous publications, our focus was to analyze repeated PASS measurements using advanced statistical models. In other words, we utilized serial PASS measurements and analyzed score gradients instead of cross-sectional activity scores. As expected, patients with severe AP start on a higher activity level and maintain the superior activity levels compared with moderately severe or mild cases per RAC. The presence of organ failure and high score assigned to organ failure justifies the higher activity scores in these cases. We hypothesized that PASS slopes within the early phase of inpatient care will provide critical insight into the eventual clinical course of disease. We especially intended to test this hypothesis in the mild AP subset, which constitutes most clinical encounters. To this end, we utilized the GEE model to objectively correlate early PASS slopes with the risk of protracted hospital stay. The GEE model is a well-established statistical approach to analyze clustered data involving repeated measurements.26 This method compares the trajectories via analyzing the slopes of changes.27 Moreover, this GEE model is capable of accommodating missing data.28
PASS represents an activity index, is calculated in 12-h increments, and reflects evolving disease manifestations through the course of hospitalization. Thus, PASS enables physicians to quantify disease activity in a real-time dynamic fashion. This unique quality of PASS can be further exploited by an analytical approach targeting repeated measurements. By reviewing Figures 1–3, it is clear that PASS trajectory even within the first 24 h can provide the physician with helpful information about the expected LOS and potentially guide the clinical care. Based on its dynamic nature, subsequent PASS calculations at 48 h may further support the above projection when it continues to decline or alert the clinician about potential worsening clinical status and need for additional interventions when the index becomes higher. This notion is specifically important in the mild AP stratum, where clinical course is directly affected by the variable symptoms of pain and oral tolerance. Mild AP subclass constitutes the vast majority of AP cohort, and despite not developing local/systemic complications, some mild patients experience a protracted hospital stay. Based on our analysis in the mild AP population, different LOS subsets demonstrated significantly-different PASS slopes. These trends in the absence of local or systemic complications further stress the clinical relevance of serial PASS in the early stage of hospitalization.
Another important finding of the study was a similar discharge PASS between the LOS subgroups. The median discharge PASS score in our study was slightly lower than reported previously25; this difference may be explained by the limited number of subjects with calculable discharge PASS. Overall, this finding suggests that the PASS can guide clinicians on the timing of discharge from hospital.
Although our study confirmed the overall feasibility of PASS, it revealed a potential drawback in its current weighting structure. An outlier subgroup with outstandingly high PASS index was mapped. Component analysis revealed an inflated contribution of MED score in the composite PASS in this outlier group. The uneven contribution of MED is partly related to the high variability of opiate administration, but also to the design of PASS index. The opiate requirement was selected as one of the components qualified through consensus-based Delphi process.15 All other four components have defined ranges: organ failure (0–300), oral intolerance (0–40), SIRS (0–100), and pain (0–50). Contrarily, MED score has an unlimited quantity without a defined maximal upper limit value, which can potentially skew the composite PASS. The above suggest that an adjustment on the MED contribution may improve validity and simplify the integration of PASS into Electronic Medical Records as an automatic calculation.
Our study had a number of limitations to include unavailable discharge PASS in a portion of the cohort, incomplete narcotics history in some subjects as tramadol was not recorded, and lack of follow-up data. With regard to SIRS calculation, in subjects with three out of four SIRS variables being available and within normal ranges, an assumption was made that the fourth SIRS variable was within normal range. Furthermore, we noted a high prevalence of post-endoscopic retrograde cholangiopancreatography pancreatitis. This can be explained by the fact that the participating centers are tertiary referring sites with advanced endoscopy expertise.
On the other hand, this study had several strengths. Our cohort encompassed clinical settings from various geographical regions in the USA. APPRENTICE sites uniformly applied consistent inclusion/exclusion criteria and standardized questionnaires. APPRENTICE provided a robust platform for harmonized, prospective, consequential data collection coupled with centralized monitoring, which resulted in a large-data, prospective registry of a well-phenotyped AP cohort.
In summary, this study confirms the clinical relevance and feasibility of PASS as a novel disease activity measure in AP. Our study underlines the importance of early, serial calculations of PASS, especially in the mild stratum. PASS gradients are significantly associated with disease severity and the length of hospitalization. We also critically reviewed each of PASS components and detected a potential shortcoming in the PASS design related to the MED contribution. Collectively, these findings support the importance of PASS as an activity index in monitoring AP progression and potentially guiding management decisions.
Guarantor of the article:
Georgios Papachristou, MD, PhD.
References
- 1.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013; 144: 1252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spanier BW, Dijkgraaf MG, Bruno MJ. Epidemiology, aetiology and outcome of acute and chronic pancreatitis: an update. Best Pract. Res. Clin. Gastroenterol 2008; 22: 45–63. [DOI] [PubMed] [Google Scholar]
- 3.Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat. Rev. Gastroenterol. Hepatol 2019; 16: 479–96. [DOI] [PubMed] [Google Scholar]
- 4.Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010; 139: 813–20. [DOI] [PubMed] [Google Scholar]
- 5.Thandassery RB, Yadav TD, Dutta U, Appasani S, Singh K, Kochhar R. Dynamic nature of organ failure in severe acute pancreatitis: the impact of persistent and deteriorating organ failure. HPB (Oxford) 2013; 15: 523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitcomb DC. Clinical practice. Acute pancreatitis. N. Engl. J. Med 2006; 354: 2142–50. [DOI] [PubMed] [Google Scholar]
- 7.Vege SS, DiMagno MJ, Forsmark CE, Martel M, Barkun AN. Initial medical treatment of acute pancreatitis: American Gastroenterological Association Institute technical review. Gastroenterology 2018; 154: 1103–39. [DOI] [PubMed] [Google Scholar]
- 8.Singh H, Gougol A, Mounzer R et al. Which patients with mild acute pancreatitis require prolonged hospitalization? Clin. Transl. Gastroenterol 2017; 8: el29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francisco M, Valentin F, Cubiella J, Fernandez-Seara J. Factors related to length of hospital admission in mild interstitial acute pancreatitis. Rev. Esp. Enferm. Dig 2013; 105: 84–92. [DOI] [PubMed] [Google Scholar]
- 10.Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 1976; 70: 439–44. [PubMed] [Google Scholar]
- 11.van der Heijde DM, van’t Hof MA, Van Riel PL et al. Judging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity score. Ann. Rheum. Dis 1990; 49: 916–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luqmani RA, Bacon PA, Moots RJ et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM. 1994; 87: 671–8. [PubMed] [Google Scholar]
- 13.Monteleonc G, Neurath MF, Ardizzone S et al. Mongersen, an oral SMAD7 antisense oligonucleotide, and Crohn’s disease. N. Engl. J. Med 2015; 372: 1104–13. [DOI] [PubMed] [Google Scholar]
- 14.Papp K, Gordon K, Thaci D et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N. Engl. J. Med 2018; 379: 1313–21. [DOI] [PubMed] [Google Scholar]
- 15.Wu BU, Batech M, Quezada M et al. Dynamic measurement of disease activity in acute pancreatitis: the pancreatitis activity scoring system. Am. J. Gastroenterol 2017; 112: 1144–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dellinger EP, Forsmark CE, Layer P et al. Determinant-based classification of acute pancreatitis severity: an international multidisciplinary consultation. Ann. Surg 2012; 256: 875–80. [DOI] [PubMed] [Google Scholar]
- 17.Banks PA, Bollen TL, Dervenis C et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013; 62: 102–11. [DOI] [PubMed] [Google Scholar]
- 18.Papachristou GI, Machicado JD, Stevens T et al. Acute pancreatitis patient registry to examine novel therapies in clinical experience (APPRENTICE): an international, multicenter consortium for the study of acute pancreatitis. Ann. Gastroenterol 2017; 30: 106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform 2009; 42: 377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit. Care Med 1995; 23: 1638–52. [DOI] [PubMed] [Google Scholar]
- 21.Singh VK, Wu BU, Bollen TL et al. Early systemic inflammatory response syndrome is associated with severe acute pancreatitis. Clin. Gastroenterol. Hepatol 2009; 7: 1247–51. [DOI] [PubMed] [Google Scholar]
- 22.Rosner B Fundamentals of Biostatistics, 8th edn. Boston, MA: Cengage Learning, 2016. xix, 927 pages p. [Google Scholar]
- 23.Team RC. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2017. [Google Scholar]
- 24.StataCorp, ed. Stata Statistical Software, 15.1 edn. StataCorp LLC, 2017. [Google Scholar]
- 25.Buxbaum J, Quezada M, Chong B et al. The Pancreatitis Activity Scoring System predicts clinical outcomes in acute pancreatitis: findings from a prospective cohort study. Am. J. Gastroenterol 2018; 113: 755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Twisk JW. Longitudinal data analysis. A comparison between generalized estimating equations and random coefficient analysis. Eur. J. Epidemiol 2004; 19: 769–76. [DOI] [PubMed] [Google Scholar]
- 27.Jung SH, Ahn C. Sample size estimation for GEE method for comparing slopes in repeated measurements data. Stat. Med 2003; 22: 1305–15. [DOI] [PubMed] [Google Scholar]
- 28.Rochon J Application of GEE procedures for sample size calculations in repeated measures experiments. Stat. Med 1998; 17: 1643–58. [DOI] [PubMed] [Google Scholar]


