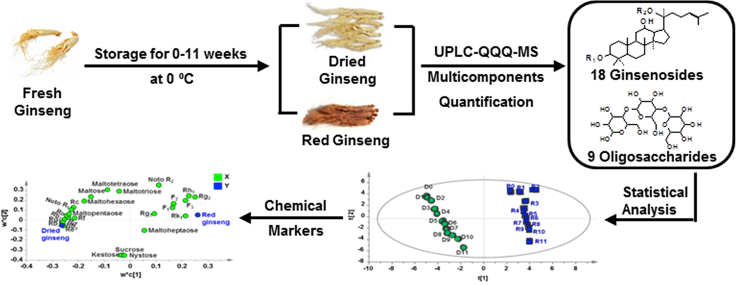
Abstract
Background
Dried and red ginseng are well-known types of processed ginseng and are widely used as healthy food. The dried and red ginseng quality may vary with the storage period of raw ginseng. Therefore, herein, the effect of the storage period of fresh ginseng on processed ginseng quality was evaluated through multicomponent quantification with statistical analysis.
Methods
A method based on ultrahigh performance liquid chromatography coupled to triple quadrupole mass spectrometry in multiple-reaction monitoring mode (UPLC-MRM-MS) was developed for quantitation of ginsenosides and oligosaccharides in dried and red ginseng. Principal component analysis and partial least squares discriminant analysis were conducted to evaluate the dynamic distributions of ginsenosides and oligosaccharides after different storage periods.
Results
Eighteen PPD, PPT and OLE ginsenosides and nine reducing and nonreducing oligosaccharides were identified and quantified. With storage period extension, the ginsenoside content in the processed ginseng increased slightly in the first 2 weeks and decreased gradually in the following 9 weeks. The content of reducing oligosaccharides decreased continuously as storage time extending, while that of the nonreducing oligosaccharides increased. Chemical conversions occurred during storage, based on which potential chemical markers for the storage period evaluation of fresh ginseng were screened.
Conclusion
According to ginsenoside and oligosaccharide distributions, it was found that the optimal storage period was 2 weeks and that the storage period of fresh ginseng should not exceed 4 weeks at 0 °C. This study provides deep insights into the quality control of processed ginseng and comprehensive factors for storage of raw ginseng.
Keywords: ginseng, multicomponents, statistical analysis, storage period, UPLC-MRM-MS
Graphical abstract
1. Introduction
Ginseng is the dried root and rhizome of Panax gingseng Meyer. After harvesting, fresh ginseng is dried and steamed to produce dried and red ginseng, respectively [1]. Dried and red ginseng are two of the most important processed products of ginseng that are commercially available and are commonly used in clinical applications. Ginsenosides exhibit high antioxidation, antifatigue, antitumor, antidepression, and antidiabetics activities and enhance immunity [[2], [3], [4], [5], [6], [7]]. Therefore, the type and content of ginsenosides are commonly utilized as quality markers for the analysis of ginseng [8,9]. As we all known, there are many other chemical constituents of ginseng that induce important bioactivities. Among them, oligosaccharides have gained considerable attention in healthcare in recent years. Ginseng oligosaccharides exhibit immunomodulatory, antitumor, antioxidation, antidiabetic, and other activities [[10], [11], [12]]. Oligosaccharides also play a synergistic regulatory role in imparting other pharmacological activities of ginseng. Therefore, the accurate identification and quantification of the oligosaccharides in ginseng are necessary for quality, safety, and efficacy control.
Storage has an important effect on the quality of Chinese medicine. Considering increasing market demand for ginseng, significant attention has been paid to its preservation and storage, which are some of the most important parameters that affect ginseng quality. After harvesting, fresh ginseng can be preserved by storing it at low temperature storage and/or waiting for drying or steaming. During storage, some of the active components in fresh ginseng may undergo changes that are closely associated with storage period and environment [13,14]. However, there are no reports on the effects of storage periods of fresh ginseng on the quality of its processed products. To this end, herein, the effects of storage period of fresh ginseng on the contents of main ginsenosides and oligosaccharides in dried and red ginseng are investigated.
Recent studies have characterized ginsenosides through HPLC and HPLC-MS [9,[15], [16], [17]]. Because of the development of HPLC-MS with high sensitivity and resolution, precision of quantitation of TCM has been significantly improved. Many modern techniques have been applied for oligosaccharides detection, including GC, LC, IR, NMR, MS, and combined derivatization [18,19]. However, the quantitative analysis of oligosaccharides is challenging. Methylation is an important technique for the characterization of saccharides [20]. Application of solid-phase methylation to derivatize oligosaccharides could reduce their polarity and enhance the response signal in MS. The improvement of sensitivity and resolution of such methods can facilitate the accurate structural identification and quantification of oligosaccharides.
Herein, a method based on UPLC-QQQ-MS was developed and validated to evaluate main ginsenosides and oligosaccharides in dried ginseng and steam-processed red ginseng. The MRM mode in MS detection allows accurate quantification of components in complex extracts. The main 18 ginsenosides and 9 oligosaccharides constituents were identified by combining retention time and m/z data. The contents of PPD, PPT, and OLE ginsenosides and those of reducing and nonreducing oligosaccharides were calculated and compared. Based on multicomponent quantification assessment through statistical analysis, the effect of storage period on composition of ginsenosides and oligosaccharides was charified. Each type of ginsenoside conversion and oligosaccharide transformation that occurred during storage was investigated, and their possible mechanisms were discussed. A multivariable statistical analysis approach, PLS-DA, was applied for evaluation of distribution of ginsenosides and oligosaccharides in dried and red ginseng after different storage periods, and the contributions of ginsenoside and oligosaccharide markers to storage period were discovered. This study provides a scientific basis for the selection of an appropriate storage period of fresh ginseng before drying and steaming processes for practical production. Moreover, the results presented herein can be useful for the utilization of ginseng as medicinal resources.
2. Materials and methods
2.1. Chemicals and reagents
Acetonitrile (HPLC grade) was purchased from Fisher Scientific. Formic acid (HPLC grade) and methanol, ethanol, acetic acid, DMSO, CH3I, NaOH, and CH2Cl2 (analytical grade) were purchased from Sigma-Aldrich. Ultrapure water (18 MΩ/cm) was made with a Milli-Q water system.
Reference ginsenosides Rb1, Rc, Rb2, Rb3, Rd, Rg3, F2, Rk1, Re, Noto R1, Rg1, Rf, Noto R2, Rg2, F3, F1, Rh1 and Ro (Standard Technical). Reference maltose, maltotriose, maltotetraose, maltopentaose, maltohexaose, maltoheptaose, sucrose, kestose, and nystose (Aladdin)
2.2. Ginseng samples
Four-year-old fresh ginseng was collected from Fusong, Jilin Province, and identified by Professor Shumin Wang of Changchun University of Traditional Chinese Medicine. Fresh intact ginseng was stored in a refrigerated warehouse with temperature adjusted to 0 °C. For 0-11 weeks of storage, the same amount of fresh ginseng was taken for processing on a weekly basis. Dried ginseng was placed in a cool and ventilated place until it was dried completely. Red ginseng was steamed at 100 °C for 4h. Dried and red ginseng were powdered and passed through a 60 mesh sieve.
2.3. Reference and sample solution preparation
Eighteen ginsenoside references were individually dissolved with 80% methanol-water to 1 mg/mL and were then combined for preparing stock solutions and were further diluted to achieve desired concentrations for quantification analysis. The ginsenoside extraction procedure was optimized according to previous studies [9]; 1 g of dried or red ginseng powder was extracted with 20 mL of 80% methanol-water by ultrasonication for 30 min at room temperature. The extraction was repeated 3 times. The extractants were combined and concentrated under reduced pressure and redissolved in 1 mL of 80% methanol-water.
Nine oligosaccharide references were accurately weighed and dissolved with DMSO to 1 mg/mL individually. The sample extraction was based on a previously reported method published by our group [21], and solid-phase methylation method was modified for further improvement. Ten grams of dried and red ginseng powders were extracted with 200 mL of water by stirring continuously at 70 °C for 5 h. The extraction was repeated 3 times. Next, the supernatants were combined and concentrated under reduced pressure to 100 mL. The concentration was precipitated with 80% ethanol solution and made to stand for 12 h at 4 °C. The solution was filtered and concentrated to 25 mL. Next, 1 mL of the oligosaccharide extracts was taken for freeze drying and redissolved in 1 mL of DMSO. The time for complete solid-phase methylation was shortened, and the amount of a single methylation sample was increased, which was more rapid and effective than the original method. A long methylation column (150 mm × 2.1 mm) was packed with NaOH powder and washed with DMSO before methylation reaction. Each oligosaccharide reference or extracted DMSO solution 900 μL was mixed with 100 μL of CH3I and was pumped into solid-phase methylation column at an optimized flowrate of 200 μL/min. Then, methylated products were washed out with 4 mL of DMSO, and 5 mL of 5% acetic acid was added to stop methylation; this was followed by three cycles of 10 mL CH2Cl2 extraction. Combined CH2Cl2 extractions and evaporated to dryness in a 45 °C water bath. Residues were dissolved in methanol to 10 mL.
By the same procedure, the extraction of each sample was repeated 5 times and filtered through 0.22 μm microfilter before UPLC-MS analysis.
2.4. Ginsenoside and oligosaccharide determination
UPLC system (Ultimate 3000, Dionex) was coupled with TSQ Endura triple quadrupole mass spectrometer (Thermo Fisher Scientific Inc.) equipped with an ESI ion source. Ginsenosides and oligosaccharides were separated using Thermo Scientific Syncronis C18 column (100 mm × 2.1 mm, 1.7 μm) at 35 °C. The mobile phase consisted of 0.1% formic acid aqueous solution (A) and 0.1% formic acid acetonitrile solution (B). The flow rate was 200 μL/min, and injection volume was 5 μL. For analysis in MRM scan mode at normal scan rate, isolation width was 1.0 Da and mass scale was calibrated as standard procedure.
The gradient elution program of ginsenosides was set: 0-5 min 25% B, 5-25 min 25% - 100% B. Under negative ion mode, ion transfer and vaporizer temperatures were set to 320 °C and 290 °C. The spray voltage −2.5 kV and capillary voltage −20 V. The sheath and auxiliary gas flow rates were 40 and 10 arb.
For oligosaccharides, gradient elution program was set: 0-2 min 10% - 60% B, 2-10 min 60% B. In positive ion mode, ion transfer and vaporizer temperatures were set to 340 °C and 300 °C. The spray voltage 3.5 kV and capillary voltage 40 V. The sheath and auxiliary gas flow rates were 40 and 12 arb.
2.5. Multivariate statistical analysis
UPLC-MRM-MS data of ginsenosides and oligosaccharides were processed using Xcalibur software (Thermo Fisher Scientific Inc.). The mean contents of 20 ginsenosides and 9 oligosaccharides were imported into SPSS software 19.0 and SIMCA-P software 13.0 for multivariate statistical analysis.
3. Results and discussion
3.1. Determination of ginsenosides and oligosaccharides by UPLC-MRM-MS
First, each ginsenoside and methylated oligosaccharides solution was injected directly into mass spectrometer by syringe pump at 5 μL/min to optimize experimental parameters automatically and manually. The negative ion mode was chosen for ginsenosides because of higher signal intensity of [M-H]- ion. The positive ion mode was in higher relative intensity for methylated oligosaccharides. The pairs of quantitative and qualitative ions in MRM mode were selected by automatic tuning, and the collision energies were obtained. After direct MS optimization, UPLC was coupled to mass spectrometer. Combined references were analyzed for determination of their retention times.
The quantification was carried out by UPLC-MRM-MS and the method was validated (Table S1). The linearity was investigated by calculating correlation coefficients (r) of regression curves. The regression equation and linear range of references were obtained, which all showed a good linear relationship with r > 0.999. The precision and repeatability were determined after 5 detection cycles in a single day and 3 detection cycles on 3 consecutive days; the relative standard deviations (RSD) were found to be < 2.5%. The limit of detection (LOD) and quantification (LOQ) were measured at signal-to-noise ratios (S/N) of 3 and 10. The LOD and LOQ values demonstrated that the developed method was sensitive for quantifying ginsenosides and oligosaccharides in ginseng extracts. The recoveries were determined by spiking known amounts of each reference at 3 different levels into extracts. The average recoveries confirmed high accuracy of the proposed UPLC-MRM-MS method.
3.2. Influence of storage period of fresh ginseng on the distribution of ginsenosides in dried and red ginseng
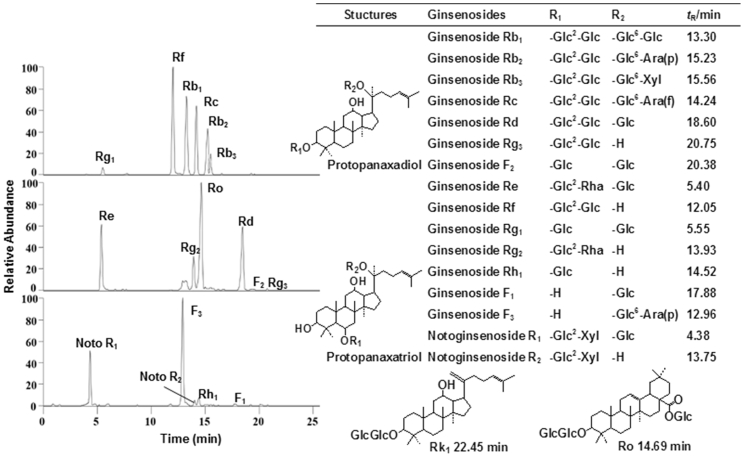
The 18 ginsenosides in ginseng samples subjected to different storage periods were analyzed by developed UPLC-MRM-MS method; extracted ion chromatograms (EIC) of quantitative ion pairs were shown in Fig. 1. The presence of 18 ginsenosides was confirmed by observation of set ion-pairs and retention time comparison with references. The peak areas integrations of quantitative ion pairs were applied for ginsenoside quantification. The contents were calculated by regression equation; the mean values and standard deviations were listed in Table S2.
Fig. 1.
The structures and UPLC-MRM-MS chromatograms of ginsenosides.
In dried ginseng, the contents of Noto R2 and Ro were higher after 1 week storage, after which they gradually decreased. The total contents of Rg1 and Re in dried ginseng during storage period of 0-11 weeks were all higher than 3 mg/g; at 2 and 3 weeks of storage, the highest content was observed. The content of ginsenoside Rb1 was ≥2 mg/g during storage period of 0-11 weeks; the highest content was observed after storage period of 0, 1 and 2 weeks. As storage period was extended beyond 2 weeks, the content of ginsenoside Rb1 gradually decreased. The contents of Rb2, Rb3, Rd, Rg3, F2, Rc, Noto R1, Rf, Rg2, F3, Rh1 and F1 were higher after 2-3 weeks of storage; their contents after 3-4 weeks of storage were similar to those after 0-1 week and then decreased gradually. Among the 18 ginsenosides, Rk1 was not detected.
In red ginseng, total contents of Rg1 and Re were ≥ 2.5 mg/g after first 6 weeks of storage. After fresh ginseng was steamed to red ginseng, the content of Rb1 was ≥ 2 mg/g after 0-5 weeks of storage. Among the 18 ginsenosides, the rare ginsenoside Rk1 was uniquely detected in red ginseng, and its content was higher after 2-3 weeks of storage. The contents of Noto R2 and Ro were higher at the first week of storage and then decreased gradually, which was consistent with those in dried ginseng. The contents of Rb1, Rb2, Rb3, Rd, Rg3, F2, Re, Rc, Noto R1, Rg2, F3, Rh1 and F1 were higher after 2-3 weeks of storage; their contents after 3-6 weeks storage were similar to those after 0-1 week of storage and then decreased gradually. The contents of Rg1 and Rf in red ginseng decreased with increasing storage period.
There was a significant difference in the content of ginsenosides between dried and red ginsengs. The contents of ginsenosides Rb1, Rb2, Rd, Re, Ro, Rb3, Rg1, Noto R1, Rc, Rf were higher in dried ginseng, while those of Noto R2, F3, Rk1, Rg3, F1, Rg2, Rh1, and F2 with small molecular weights, were higher in red ginseng because of chemical transformation of ginsenosides during the steaming process. The results showed that higher ginsenosides contents were mainly observed in dried and red ginsengs processed from fresh ginseng stored for 0-2 weeks; therefore, 2 weeks was considered to be the optimal storage period.
Considering their aglycone structures, ginsenosides can be divided into 3 types: PPD, PPT, and OLE ginsenosides [22], as presented in Fig. 1. Different aglycone structures lead to considerable differences in pharmacological activities of ginsenosides. PPD ginsenosides exhibit many pharmacological effects such as immunity enhancement, antitumor and antifatigue activities are better than PPT ginsenosides [[23], [24], [25]]. PPT ginsenosides play a stronger role in increasing synaptic plasticity, improving cerebral artery occlusion and improving memory [[26], [27], [28]]. Among the 18 ginsenosides, Rb1, Rc, Rb2, Rb3, Rd, Rg3, F2, and Rk1 were found to be PPD type, Noto R1, Rg1, Re, Rf, Noto R2, Rg2, F3, F1, Rh1 were of PPT type, and Ro was an OLE type. The content changes of different types of ginsenosides after different storage periods of fresh ginseng were also summarized and compared. The total contents of PPD and PPT ginsenosides in dried ginseng reached a maximum after 2 weeks of storage, and then gradually decreased with extension of storage period. There was no significant difference between total contents of PPD and PPT ginsenosides in dried ginseng after 3-4 weeks and 0-1 weeks of storage. The highest total contents of PPD ginsenosides in red ginseng were observed after 2 weeks of storage. The PPT ginsenoside content of red ginseng was the highest when fresh ginseng was stored for 1 week. The results showed that the storage period of fresh ginseng should not exceed 4 weeks for drying and steaming processes. In order to induce a stronger effect, it is better to store fresh ginseng at 4 °C for 2 weeks before processing. The average contents of PPD and PPT ginsenosides in red ginseng were lower than those in dried ginseng, which was also indicated that ginsenosides had been transformed during steaming.
3.3. Effect of storage period of fresh ginseng on the distribution of oligosaccharides in dried and red ginseng
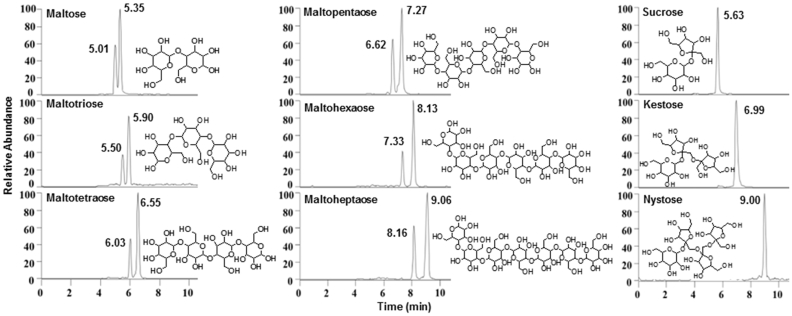
The presence of these 9 methylated oligosaccharides was confirmed by comparing their molecular weights with m/z of their methylated products. As examples, maltotetraose and nystose were considered. The molecular weight of maltotetraose is 666.58, and there are 14 OH groups in its structure. In UPLC-MRM-MS detection, m/z 885.43 ion was shown as [M+Na]+, indicating that 14 OH groups of maltotetraose were completely methylated. Maltotetraose is a reducing oligosaccharide with an anomeric hydroxyl group at its reducing end. Therefore, a pair of isomeric products was obtained after solid-phase methylation, resulting in end group isomerization. As shown in Fig. 2, the retention times of methylated maltotetraose products pair were at 6.03 and 6.55 min. Moreover, for the ion pairs shown, 14 OH groups in structure of nystose were also completely methylated. However, as nystose is a non-reducing oligosaccharide without an anomeric hydroxyl, no isomerization of end groups occurred after methylation. The methylated product presented only 1 peak in EIC of nystose. It can be seen that 9 oligosaccharides were completely methylated after solid-phase methylation. Maltose, maltotriose, maltotetraose, maltopentaose, maltohexaose, and maltoheptaose are reducing oligosaccharides, and their methylated products were a pair of isomers. Moreover, sucrose, fructotriose, and tetrasaccharide are non-reducing oligosaccharides; therefore, their methylated products were a single compound.
Fig. 2.
The structures and UPLC-MRM-MS chromatograms of methylated oligosaccharides.
The oligosaccharide extracts of dried and red ginseng were methylated and analyzed by UPLC-MRM-MS. Comparing retention times and m/z information of each peak with those of references, it can be seen that methylated products of maltose, maltotriose, maltotetraose, maltopentaose, maltohexaose, maltoheptaose, sucrose, kestose and nystose were all present in dried and red ginseng. The contents of 9 oligosaccharides in dried and red ginsengs produced from fresh ginseng after different storage periods were calculated by standard curve. Because methylated reducing oligosaccharides will produce 2 isomers, the total content of 2 isomers is used for calculation. Through comparative analysis of oligosaccharides in dried and red ginseng, the contents of several oligosaccharides in dried ginseng were found to be significantly higher than those in red ginseng, which indicated that some oligosaccharides in red ginseng were lost during steaming process. Oligosaccharides may be hydrolyzed during steaming and, some of them could be consumed during Maillard reaction in steaming process, which will eventually lead to low contents of oligosaccharides in red ginseng.
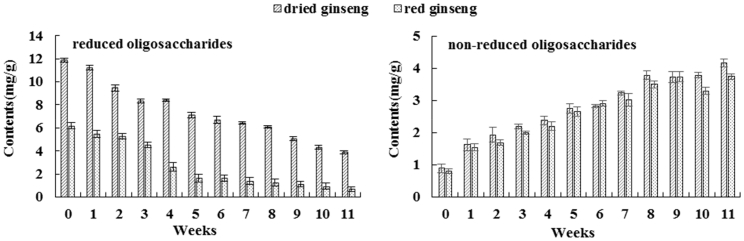
The total contents of reducing and nonreducing oligosaccharides detected in dried and red ginseng after different storage periods were compared. SPSS software was used to analyze distributions of reducing and nonreducing oligosaccharides and is represented by a box diagram, as shown in Fig. 3. The total contents of reducing oligosaccharides in dried ginseng were significantly higher than those in red ginseng after 0-11 weeks of storage. The total contents of nonreducing oligosaccharides in dried ginseng were slightly higher than those in red ginseng. The total contents of 6 reducing oligosaccharides in dried and red ginseng were reduced as storage period of fresh ginseng was extended. This could be due to the conversion of reducing oligosaccharides to monosaccharides during storage. Moreover, 3 nonreducing oligosaccharides showed increasing contents, as the polysaccharides were gradually converted to nonreducing oligosaccharides upon storage period extension, which also decrease. These results indicated that storage period of fresh ginseng affected the distribution of oligosaccharides in its dried and steamed processed products.
Fig. 3.
The reduced and non-reduced oligosaccharides contents changes via different storage of fresh ginseng.
3.4. Statistical analysis of multicomponents in processed products from fresh ginseng after different storage periods
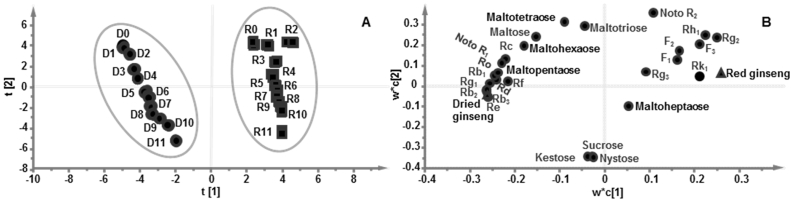
Based on contents of target ginsenosides and oligosaccharides in dried and red ginseng prepared from fresh ginseng after different storage periods, the contents of ginsenosides and oligosaccharides were taken as statistical variables simultaneously. PCA was carried out to investigate the differences of all samples. The first and second principal components represented 95% of the variable information, including most of sample information [29]. PLS-DA was applied to further evaluate contribution of target ginsenosides and oligosaccharides to the differences of dried and red ginseng after different storage periods. The PLS-DA score plot was shown in Fig. 4A, the 0-11 weeks dried ginsengs were gathered in one group, whereas the 0-11 weeks red ginseng were gathered in the other group, indicating that the target ginsenosides and oligosaccharides significantly varied. The influence of storage period on ginsenoside and oligosaccharide distributions was presented. The contribution of each target ginsenosides and oligosaccharides to the differences of storage periods was also calculated; VIP value exceeding 1.0 was chosen as a differential marker. In the loading plot (Fig. 4B), a spot further from origin represented a greater contribution of components for discrimination. Therefore, ginsenosides Re, Rg1, Rg2, Rb1, Rb2, and Rb3 were obtained among 18 ginsenosides, and maltose, maltohexaose and maltopentaose were obtained among 9 oligosaccharides. These results indicated that storage period of fresh ginseng had a certain effect on its processed products dried and red ginseng.
Fig. 4.
PLS-DA score chart (A) and loading contribution diagram (B) of ginsenosides and oligosaccharides in dried (D0-11) and red (R0-11) ginsengs from 0-11 weeks storage of fresh ginseng.
4. Conclusions
In this study, considering storage period of fresh ginseng as a variable and the contents of ginsenosides and oligosaccharides as an index, the change trend of contents of ginsenosides and oligosaccharides in dried and red ginseng with the extension of storage period of fresh ginseng was investigated. UPLC-MRM-MS was conducted to determine ginsenoside and oligosaccharide contents. Solid-phase methylation was performed to improve signal of oligosaccharides for their quantitation by UPLC-MRM-MS. Based on statistical assessment of multicomponents, 2 weeks was found to be the optimal storage period and the storage period of fresh ginseng should not exceed 4 weeks at 0 °C before drying and steaming processes. Therefore, in order to ensure efficacy, the storage period of fresh ginseng should not be too long. Ginsenosides Re, Rg1, Rg2, Rb1, Rb2, Rb3 and maltose, maltohexaose and maltopentaose were screened as potential chemical markers for evaluation of storage period of fresh ginseng. This study provided a scientific basis for the selection of an appropriate storage period of fresh ginseng before drying and steaming processes. The holistic quality control of dried and red ginseng was conducted by determining both ginsenoside and oligosaccharide distributions, which can be utilized as comprehensive factors for establishing storage procedure of fresh ginseng. These results are of great significance for the complete utilization of ginseng as medicinal resources.
Acknowledgements
This work was supported by the National Key Research and Development Program of Ministry of Science and Technology (2017YFC1702105), Science and Technology Development Plan Project of Jilin Province (20200201096JC).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2021.06.007.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Gyo I., Geun A.N., Seok B.B., Woo L.M., Won P.H., Hwa J.K., Goo C.B., Kyun H.C., Kyu P.C., Seong K.Y. In situ analysis of chemical components induced by steaming between fresh ginseng, steamed ginseng, and red ginseng. J Ginseng Res. 2017;41:361–369. doi: 10.1016/j.jgr.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Z.Q. Chemical insights into ginseng as a resource for natural antioxidants. Chem Rev. 2012;112:3329–3355. doi: 10.1021/cr100174k. [DOI] [PubMed] [Google Scholar]
- 3.Zhen G., Zhang L., Du Y.N., Yu R.B., Liu X.M., Cao F.R., Chang Q., Deng X.W., Xia M., He H. De novo assembly and comparative analysis of root transcriptomes from different varieties of Panax ginseng C. A. Meyer grown in different environments. Sci China Life Sci. 2015;58:1099–1111. doi: 10.1007/s11427-015-4961-x. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y.Y., Zhang Y.Q., Song W., Zhang Y., Dong X., Tan M.Q. Ginsenoside Rh2 improves the cisplatin anti-tumor effect in lung adenocarcinoma A549 cells via superoxide and PD-L1. Anti-Cancer Agent Me. 2020;20:495–503. doi: 10.2174/1871520619666191209091230. [DOI] [PubMed] [Google Scholar]
- 5.Fan W.X., Huang Y.L., Zheng H., Li S.Q., Li Z.H., Yuan L., Cheng X., He C.S., Sun J.F. Ginsenosides for the treatment of metabolic syndrome and cardiovascular diseases: pharmacology and mechanisms. Biomed Pharmacother. 2020;132:110915. doi: 10.1016/j.biopha.2020.110915. [DOI] [PubMed] [Google Scholar]
- 6.Lee D.K., Park S., Long N.P., Min J.E., Kim H.M., Yang E., Lee S.J., Lim J., Kwon S.W. Research quality-based multivariate modeling for comparison of the pharmacological effects of black and red ginseng. Nutrients. 2020;12:2590. doi: 10.3390/nu12092590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang S., Min H. Ginseng, the 'immunity boost': the effects of Panax ginseng on immune system. J Ginseng Res. 2012;36:354–368. doi: 10.5142/jgr.2012.36.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J.B., Li M.J., Chen L.X., Wang Y.F., Li S.S., Zhang Y.W., Zhang L., Song M.G., Liu C., Hua M., et al. Effects of processing method on the pharmacokinetics and tissue distribution of orally administered ginseng. J Ginseng Res. 2018;42:27–34. doi: 10.1016/j.jgr.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiu Y., Li X., Sun X.L., Xiao D., Miao R., Zhao H.X., Liu S.Y. Simultaneous determination and difference evaluation of 14 ginsenosides in Panax ginseng roots cultivated in different areas and ages by high-performance liquid chromatography coupled with triple quadrupole mass spectrometer in the multiple reaction–monitoring mode combined with multivariate statistical analysis. J Ginseng Res. 2019;43:508–516. doi: 10.1016/j.jgr.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seo J.Y., Lee C.W., Choi D.J., Lee J., Lee J.Y., Park Y.I. Ginseng marc-derived low-molecular weight oligosaccharide inhibits the growth of skin melanoma cells via activation of RAW264.7 cells. Int Immunopharmacol. 2015;29:344–353. doi: 10.1016/j.intimp.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 11.Jiao L.L., Zhang X.Y., Li B., Liu Z., Wang M.Z., Liu S.Y. Anti-tumour and immunomodulatory activities of oligosaccharides isolated from Panax ginseng C.A.Meyer. Int J Biol Macromol. 2014;65:229–233. doi: 10.1016/j.ijbiomac.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 12.Jiao L.L., Li B., Wang M.Z., Liu Z., Zhang X.Y., Liu S.Y. Antioxidant activities of the oligosaccharides from the roots, flowers and leaves of Panax ginseng C. A. Meyer. Carbohyd Polym. 2014;106:293–298. doi: 10.1016/j.carbpol.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 13.Chung I.M., Kim J.W., Seguin P., Jun Y.M., Kim S.H. Ginsenosides and phenolics in fresh and processed Korean ginseng (Panax ginseng C.A. Meyer): effects of cultivation location, year, and storage period. Food Chem. 2012;130:73–83. [Google Scholar]
- 14.Li Cy, Lau D.T.W., Dong T.T.X., Zhang J., Choi R.C.Y., Wu H.Q., Wang L.Y., Hong R.S., Li S.H., Song X., et al. Dual-index evaluation of character changes in Panax ginseng C. A. Mey stored in different conditions. J Agric Food Chem. 2013;61:6568–6573. doi: 10.1021/jf400456w. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L.N., Wang S.Y., Qu B.Q., Chi H.J., Quan Y.L., Wu X.H. Efficient separation determination of protopanaxatriol ginsenosides Rg1, Re, Rf, Rh1, Rg2 by HPLC. J Pharmaceut Biomed. 2019;170:48–53. doi: 10.1016/j.jpba.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 16.Guo Y.L., Wang Y., Zhao Y.L., Xu X.Y., Zhang H., Zhao C.B., Zheng M.Z., Liu S.Y., Wu Y.Z., Liu J.S. Chemical comparison of white ginseng before and after extrusion by UHPLC-Q-orbitrap-MS/MS and multivariate statistical analysis. J Anal Methods Chem. 2020;2020 doi: 10.1155/2020/4764219. Article ID 4764219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen W., Balan P., Popovich D.G. Ginsenosides analysis of New Zealand-grown forest Panax ginseng by LC-QTOF-MS/MS. J Ginseng Res. 2020;44:552–562. doi: 10.1016/j.jgr.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Y.F., Cai H.Q., Li W.Y., Xiu Y., Liu W.L., Chi H.Y., Shen H., Yang M.G., Pei J., Liu S.Y. A discrimination study of Asia ginseng and America ginseng by a comparison of ginsenosides, oligosaccharides and amino acids using a UPLC-MS method. J Liq Chrom Relat Tech. 2018;41:825–830. [Google Scholar]
- 19.Wan D.B., Jiao L.L., Yang H.M., Liu S.Y. Structural characterization and immunological activities of the water-soluble oligosaccharides isolated from the Panax ginseng roots. Planta. 2012;235:1289–1297. doi: 10.1007/s00425-011-1574-x. [DOI] [PubMed] [Google Scholar]
- 20.Kang P., Mechref Y., Novotny M.V. High-throughput solid-phase permethylation of glycans prior to mass spectrometry. Rapid Commun Mass Sp. 2008;22:721–734. doi: 10.1002/rcm.3395. [DOI] [PubMed] [Google Scholar]
- 21.Li L.L., Ma L., Guo Y.L., Liu W.L., Wang Y., Liu S.Y. Analysis of oligosaccharides from Panax ginseng by using solid-phase permethylation method combined with ultra-high-performance liquid chromatography-Q-Orbitrap/mass spectrometry. J Ginseng Res. 2019;8:1–9. doi: 10.1016/j.jgr.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin B.K., Kwon S.W., Park J.H. Chemical diversity of ginseng saponins from Panax ginseng. J Ginseng Res. 2015;39:287–298. doi: 10.1016/j.jgr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song C.Y., Zhang H.R., Guo Z.M., Yan J.Y., Jin G.W., Liang X.M. Determination of five protopanaxadiol ginsenosides in ginseng by solid-phase extraction-ultra performance liquid chromatography. Chin J Chromatogr. 2020;38:547–553. doi: 10.3724/SP.J.1123.2019.09003. [DOI] [PubMed] [Google Scholar]
- 24.Qian T.X., Jiang Z.H., Cai Z.W. High-performance liquid chromatography coupled with tandem mass spectrometry applied for metabolic study of ginsenoside Rb1 on rat. Anal Biochem. 2006;352:87–96. doi: 10.1016/j.ab.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 25.Qian T.X., Cai Z.W., Wong R.N.S., Jiang Z.H. Liquid chromatography/mass spectrometric analysis of rat samples for in vivo metabolism and pharmacokinetic studies of ginsenoside Rh2. Rapid Commun Mass Spectrom. 2005;19:3549–3554. doi: 10.1002/rcm.2232. [DOI] [PubMed] [Google Scholar]
- 26.Li G., Zhang N., Geng F., Liu G.L., Liu B., Lei X., Li G., Chen X. High-throughput metabolomics and ingenuity pathway approach reveals the pharmacological effect and targets of Ginsenoside Rg1 in Alzheimer's disease mice. Sci Rep. 2019;9:640–651. doi: 10.1038/s41598-019-43537-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L.M., Lu J.L., Zeng Y.Q., Guo Y.Q., Wu C., Zhao H.B., Zheng H., Jiao J.L. Improving Alzheimer's disease by altering gut microbiota in tree shrews with ginsenoside Rg1. FEMS Microbiol Lett. 2020;367 doi: 10.1093/femsle/fnaa011. fnaa011. [DOI] [PubMed] [Google Scholar]
- 28.Han J., Oh J.P., Yoo M., Cui C.H., Jeon B.M., Kim S.C., Han J.H. Minor ginsenoside F1 improves memory in APP/PS1 mice. Mol Brain. 2019;12:367–429. doi: 10.1186/s13041-019-0495-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang X., Wang N., Zhang N., Yue H., Liu S.Y. Influence of process on ginseng and American ginseng property by neurochemistry analysis. Chinese J Anal Chem. 2019;47:957–963. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.