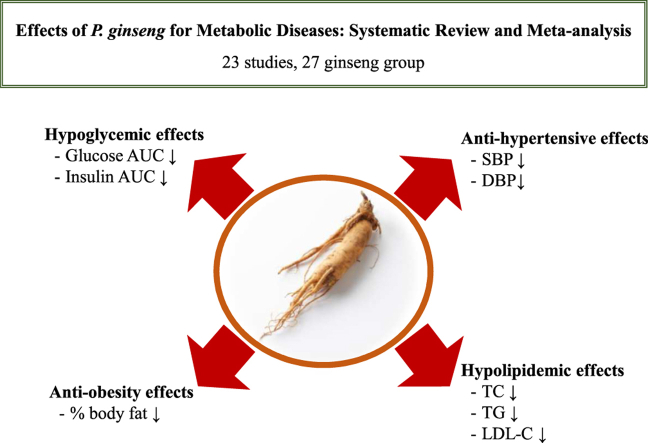
Abstract
Panax ginseng is a medicinal plant is a material with various pharmacological activities and research suggests that it is particularly effective in representative metabolic diseases such as hyperglycemia, hypertension, and hyperlipidemia. Therefore, in this study, systematic review and meta-analysis were performed to investigate the comprehensive effect of P. ginseng on metabolic parameters representing these metabolic diseases. A total of 23 papers were collected for inclusion in the study, from which 27 datasets were collected. The investigational products included P. ginseng and Korean Red ginseng. Across the included studies, the dose ranged from 200 mg to 8 g and the supplementation period lasted from four to 24 weeks. The study subjects varied from healthy adults to those with diabetes, hypertension, obesity, and/or hyperlipidemia. As a result of the analysis, the levels of glucose and insulin area under the curves, % body fat, systolic and diastolic blood pressures, total cholesterol, triglycerides, and low-density lipoprotein cholesterol were significantly reduced in the P. ginseng group as compared with in the placebo group. In conclusion, P. ginseng supplementation may act as an adjuvant to prevent the development of metabolic diseases by improving markers related to blood glucose, blood pressure, and blood lipids.
Keywords: Panax ginseng, Blood glucose, Body fat, Blood pressure, Blood lipids
Graphical abstract
1. Introduction
The development of modern society has led to a convenient and affluent lifestyle for many that, combined with excessive nutrition, fast food consumption, reduced physical activity, and excessive stress, has become a major cause of various metabolic diseases. Typical metabolic diseases include diabetes, hypertension, and hyperlipidemia, which lead to serious conditions such as cardiovascular disease, atherosclerosis, cerebrovascular disease, and cancer, increasing mortality rates. Since metabolic diseases are usually caused by lifestyle, multiple diseases can develop simultaneously as age increases. In particular, metabolic syndrome is a combination of risk factors for various metabolic diseases showing abnormal levels. When various metabolic diseases are present, the incidence and mortality of severe diseases are remarkably increased [[1], [2], [3]]. The main cause of metabolic syndrome and metabolic diseases is insulin resistance [1,4,5]. Therefore, managing obesity, the main cause of insulin resistance, is as important as monitoring blood glucose, blood pressure, and blood lipid levels. The progression of various metabolic diseases, including metabolic syndrome, toward the onset of associated conditions can be mitigated by improving lifestyle habits. Therefore, many people are beginning to pay ample attention to correcting their eating habits and lifestyle to prevent diseases; in this vein, consumer demand for functional foods that support health also continues to increase.
Among the various functional foods, products made from ginseng are some of the most commonly consumed globally. Ginseng is a perennial plant of the Araliaceae family and a medicinal crop that has long been widely used in Asia. Ginseng includes various species such as Panax ginseng, Panax quinquefolium, Panax notoginseng, and Panax japonicas. Of these, P. ginseng, mainly grown in Korea and China, accounts for the largest proportion of global ginseng production [6]. The main component of P. ginseng is ginsenoside, one of the saponin groups; to date, more than about 100 kinds of ginsenosides have been reported [7,8]. To maximize the pharmacological activities of ginseng by increasing the bioavailability of ginsenoside, red ginseng, black ginseng, and fermented ginseng, which have undergone steaming and fermentation processes, are also widely consumed [[9], [10], [11]]. The outstanding pharmacological activities of P. ginseng have been reported by many papers and various reviews, systematic reviews, and meta-analyses have been conducted with the accumulation of numerous data. Most of these studies were on cancer [[12], [13], [14], [15]], diabetes [[16], [17], [18], [19]], cardiovascular disease [[20], [21], [22], [23], [24]], and neurodegenerative diseases [[25], [26], [27], [28], [29]]. Regarding metabolic diseases, review articles on blood glucose [[16], [17], [18], [19]], blood lipids [30], and obesity [31,32] have been published, while systematic reviews and meta-analyses of randomized controlled trials (RCTs) have only covered blood glucose [18] and blood lipids [30] to date. In particular, in the case of the blood glucose study, only Korean Red ginseng was included as the investigational product, while the study population was limited to those patients with type 2 diabetes [18]. In addition, until now, no investigation has comprehensively and systemically reviewed the markers of P. ginseng related to metabolic diseases such as blood glucose, blood pressure, body fat, and blood lipids.
Given the growing aging society with a high probability of being exposed to multiple diseases at the same time and since the risk of metabolic disease is constantly increasing, it is important to reveal the multitarget efficacy of functional ingredients on metabolic diseases. Therefore, in this study, we analyzed the clinical effects of P. ginseng on metabolic parameters representing various metabolic diseases that are increasingly crucial factors to comprehend in terms of prevention and treatment. For this, a systematic review and meta-analysis were conducted by selecting RCTs that measured the effects of P. ginseng on metabolic parameters in various study populations.
2. Methods
2.1. Study registration
The study protocol (CRD42020208191) was registered in the PROSPERO database (https://www.crd.york.ac.uk/PROSPERO/).
2.2. Criteria for considering studies
The studies included in this systematic review and meta-analysis were RCTs of P. ginseng on metabolic parameters. Studies with an intervention period lasting longer than four weeks were selected and there were no restrictions on the characteristics of the study subjects. Studies incorporating P. ginseng as a part of a complex intervention were excluded from this investigation.
2.3. Outcome measures
The effects of P. ginseng supplementation on metabolic parameters were evaluated by focusing on the following items: glucose [glucose (fasting, 2-h postprandial, area under the curve (AUC)), insulin (fasting, 2-h postprandial, AUC), and hemoglobin A1c (HbA1c)], blood pressure [systolic blood pressure (SBP) and diastolic blood pressure (DBP)], body fat [body weight, body mass index (BMI), % body fat, and waist circumference (WC)], and lipid levels [total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C)].
2.4. Search methods
The present study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. PubMed/Medline, the Web of Science, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) were chosen as databases for literature searches and were reviewed for eligible studies published until September 2020. Key search terms included the following: [(Korean ginseng) OR (Panax ginseng Meyer) OR (red ginseng)] AND [(glucose) OR (blood sugar) OR (glucose intolerance) OR (insulin resistance) OR (insulin sensitivity) OR (hyperglycemia) OR (hyperinsulinemia) OR (diabet∗) OR (T2DM) OR (lipid) OR (lipide∗) OR (cholesterol) OR (triglyce∗) OR (hypertension) OR (blood pressure) OR (obes∗) OR (overweight) OR (body weight) OR (body mass index) OR (body fat) OR (waist circumference) OR (waist-hip ratio)] AND [(clinical trial) OR (clinical study) OR (human study) OR (intervention)]. To locate additional studies, all reference lists of the selected articles were reviewed (Supplementary Table 1).
2.5. Selection of studies and data extraction
For the selection of eligible studies for inclusion, two reviewers independently screened the search results, the first focusing on titles and/or abstracts and the second focusing on the full text. Any disagreements were resolved by consensus; when a consensus was not reached, a decision was made involving a third reviewer. Two reviewers independently extracted the following data using a standardized data extraction format, with inconsistent results solved through confirmation and discussion of the original paper: first author, publication year, study design, subjects’ characteristics, types of interventions, and results of outcomes. Multiple supplement groups within one study were considered as individual data.
2.6. Assessment of the risk of bias
Two reviewers independently assessed the risk of bias among the included studies using the Cochrane risk of bias tool, which assesses the risk of bias in studies according to random sequence generation, allocation concealment, blinding of the subjects and personnel, blinding of the outcomes assessment, incomplete outcomes data, selective reporting, and other biases.
2.7. Statistical analyses
A meta-analysis was performed using Review Manager version 5.4 (Cochrane Collaboration, London, England) and R software version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria). The units of all evaluation markers were properly standardized. We used the mean change and standard deviation (SD) values of the markers to investigate the effect size of the collected data. For studies that presented only standard error (SE), SE was converted into SD by multiplying the square root of the sample size.
Pooled data was analyzed using a fixed-effects model and the data were expressed as weighted mean difference (WMD) with 95 % confidence interval (CI) values for continuous outcomes. Moreover, subgroup analysis was performed based on clinical conditions of the subjects. The I2 statistic test was used to estimate the percentage of heterogeneity between studies; heterogeneity was confirmed if the I2 value was 50 % or more and, if heterogeneity was confirmed, data was analyzed by applying a random-effects model. A sensitivity analysis was conducted to estimate the effects of omission for each study. Publication bias was evaluated using funnel plot and Egger's weighted regression test, and when it was judged to be statistically significant, the effect was adjusted using the “trim and fill” method [33]. A p-value of less than 0.05 was considered to be statistically significant.
3. Results
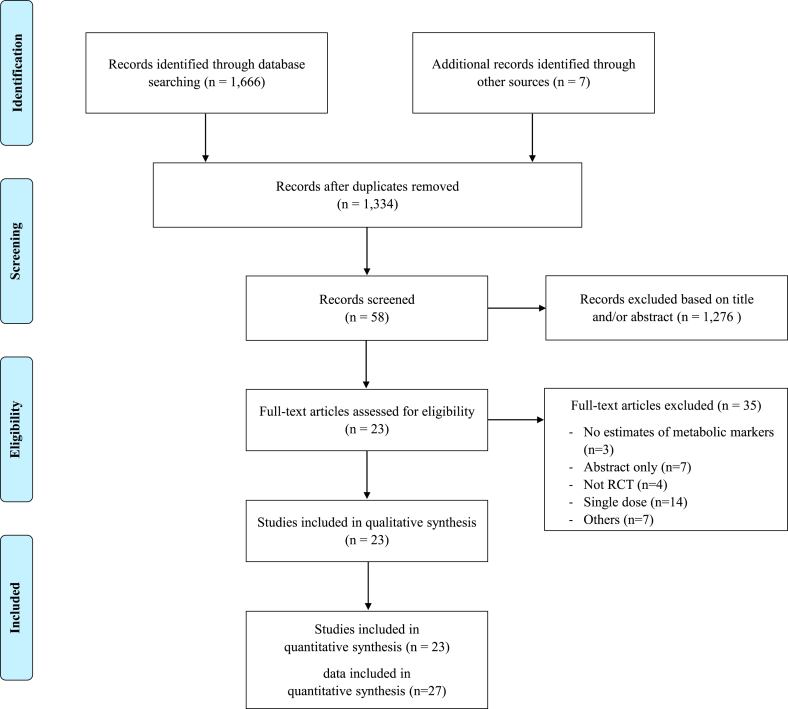
As a result of searching the literature databases to select RCTs that evaluated the effects of P. ginseng on metabolic parameters, a total of 1334 studies were collected after excluding duplicates. Of these, 1276 papers were further excluded following the title and/or abstract review and 35 papers were additionally following the full-text review; finally, 23 articles were included in this systematic review and meta-analysis (Fig. 1). A total of 27 P. ginseng group datasets were included given the approval for the inclusion of multiple supplement groups from a single study.
Fig. 1.
Flow diagram of the included studies.
3.1. Study description
Eligible studies’ characteristics are detailed in Table 1. The selected 23 studies were all RCTs, including 20 that were parallel-design studies and three that were crossover-design studies. The characteristics of the subjects of each study are as follows: two studies included healthy subjects [34,35]; 11 studies included impaired glucose tolerance, or diabetic subjects [[36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]]; two studies included prehypertensive or hypertensive subjects [47,48]; three studies included overweight or obese subjects [[49], [50], [51]]; one study included hyperlipidemic subjects [52]; three studies included subjects with metabolic syndrome [[53], [54], [55]]; and one study included postmenopausal subjects [56]. Of the 23 studies, five used P. ginseng [35,39,43,46,52], 17 used Korean Red Ginseng [34,36,38,[40], [41], [42],44,45,[47], [48], [49], [50], [51],[53], [54], [55], [56]], and one used P. ginseng berry [37] as investigational products, respectively. All studies measured metabolic disease–related markers such as blood glucose, blood pressure, body fat, and blood lipid levels. The mean dosage of the investigational products was 2.85 ± 2.00 g (range: 200 mg–8 g), and the mean study period was 10.78 ± 5.18 weeks (range: 4–24 weeks).
Table 1.
Characteristics of included studies
| No. | First author (Year), Location | Study design | Types of interventions | Sample size | Duration (weeks) | Efficacy evaluation (metabolic marker) |
|---|---|---|---|---|---|---|
|
Healthy subjects | ||||||
| 1 | Kim (2012), South Korea [34] | RCT, parallel | RG 300 mg or RG 600 mg or placebo | 69 (23/group) | 8 | BMI, SBP, DBP |
| 2 |
Reay (2009), U.K. [35] |
RCT, cross-over |
Study 1: G115 200 mg or placebo Study 2: Cheong Kwan Jang 200 mg or placebo |
Study 1: 23 Study 2: 14 |
8 |
HbA1c, FG, FI |
|
FGT or IGT or type 2 diabetic subjects | ||||||
| 3 | Bang (2014), South Korea [36] |
RCT, parallel | RG 5 g or placebo | 60 (30/group) | 12 | BMI, SBP, DBP, TG, TC, HDL-C, LDL-C, FG, PG, glucose AUC, HbA1c, FI, PI, insulin AUC |
| 4 | Choi (2018), South Korea [37] |
RCT, parallel | Ginseng berry 1 g or placebo | 72 (34 in the ginseng berry group, 38 in the placebo group) | 12 | FG, PG, FI, PI, TG, HDL-C, LDL-C, TC, HbA1c |
| 5 | Kim (2011), South Korea [38] |
RCT, parallel | RG 780 mg or placebo | 46 (23/group) | 12 | FG, FI, HbA1c, TC, TG, HDL-C, LDL-C, weight, BMI, WC, SBP, DBP |
| 6 | Ma (2008), Hong Kong [39] |
RCT, crossover | Ginseng 2.214 g or placebo | 20 (10/group) | 4 | FG, PG, FI, PI, glucose AUC, insulin AUC |
| 7 | Oh (2014), South Korea [40] |
RCT, parallel | Fermented RG 2.7 g or placebo | 42 (21/group) | 4 | FG, PG, glucose AUC, FI, PI, TC, HDL-C, LDL-C, TG |
| 8 | Park (2020), South Korea [41] |
RCT, parallel | RG 3 g or placebo | 70 (35/group) | 24 | BMI, SBP, DBP, HbA1c, FG, FI |
| 9 | Park (2020), South Korea [42] |
RCT, parallel | RG 3 g or placebo | 70 (35/group) | 24 | BMI, SBP, DBP, HbA1c, FG, PG, FI, PI, TC, TG, HDL-C, LDL-C |
| 10 | Park 2014, South Korea [43] |
RCT, parallel | Ginseng 960 mg or placebo | 23 (12 in the ginseng group, 11 in the placebo group) | 8 | FG, PG, glucose AUC, FI, PI, insulin AUC |
| 11 | Reeds (2011), U.S. [44] |
RCT, parallel | RG 3 g for 2 wks and then 8 g for 2 wks, ginsenoside Re 250 mg for 2 wks and then 500 mg for 2 wks, or placebo | 15 (5/group) | 4 | Weight, BMI, %BF, FG, glucose AUC, FI, insulin AUC, HbA1c, TC, TG, HDL-C, LDL-C |
| 12 | Vuksan (2008), Canada [45] |
RCT, crossover | RG 6 g or placebo | 39 | 12 | HbA1c, FG, PG, glucose AUC, FI, PI, insulin AUC |
| 13 |
Yoon (2012), South Korea [46] |
RCT, parallel |
Ginseng 1.5 g, 2 g, 3 g, or placebo |
72 (18/group) |
8 |
FG, PG, HbA1c |
|
Prehypertensive or hypertensive subjects | ||||||
| 14 | Cha (2016), South Korea [47] |
RCT, parallel | RG 5 g or placebo | 70 (35/group) | 12 | BMI, SBP, DBP, TC, TG, HDL-C, LDL-C |
| 15 |
Rhee (2011), South Korea [48] |
RCT, parallel |
RG 3 g or placebo |
80 (40/group) |
12 |
FG, TC, LDL-C, TG, HDL-C, SBP, DBP |
|
Overweight or obese subjects | ||||||
| 16 | Cho (2013), South Korea [49] |
RCT, parallel | RG 6 g or placebo | 68 (34/group) | 12 | BMI, %BF, FG, FI, TC, LDL-C, HDL-C, TG |
| 17 | Kim (2002), South Korea [50] |
RCT, parallel | RG, exercise, exercise and RG, or placebo | 28(7/group) | 12 | Weight, %BF, TC, HDL-C, LDL-C, TG |
| 18 |
Kim (2002), South Korea [51] |
RCT, parallel |
Exercise, RG, exercise + RG, or placebo |
28(7/group) |
12 |
TC, TG, HDL-C, LDL-C, weight, %BF |
|
Hyperlipidemic subjects | ||||||
| 19 |
Delui (2013), Iran [52] |
RCT, parallel |
Ginseng 500 mg or placebo |
40 (20/group) |
8 |
TC, TG, LDL-C, HDL-C, FG |
|
Metabolic syndrome | ||||||
| 20 | Jung (2016), South Korea [53] |
RCT, parallel | RG 3 g or placebo | 72 (36/group) | 4 | BMI, SBP, DBP, FG, TC, TG, HDL-C, FI |
| 21 | Park (2012), South Korea [54] |
RCT, parallel | RG 3 g or placebo | 60 (30/group) | 12 | WC, SBP, DBP, TC, HDL-C, TG, FG, FI |
| 22 |
Shim (2012), South Korea [55] |
RCT, parallel |
RG 4.5 g or placebo |
60 (29 in the KRG group, 31 in the placebo group) |
12 |
SBP |
|
Postmenopausal subjects | ||||||
| 23 | Kim (2012), South Korea [56] | RCT, parallel | RG 3 g or placebo | 72 (36/group) | 12 | TC, LDL-C, HDL-C, TG |
AUC, area under the curve; FG, fasting glucose; FI, fasting insulin; %BF, percent body fat; PG, postprandial glucose; PI, postprandial insulin; TC, total cholesterol; TG, triglyceride; WC, waist circumference
3.2. Changes in blood glucose–related markers
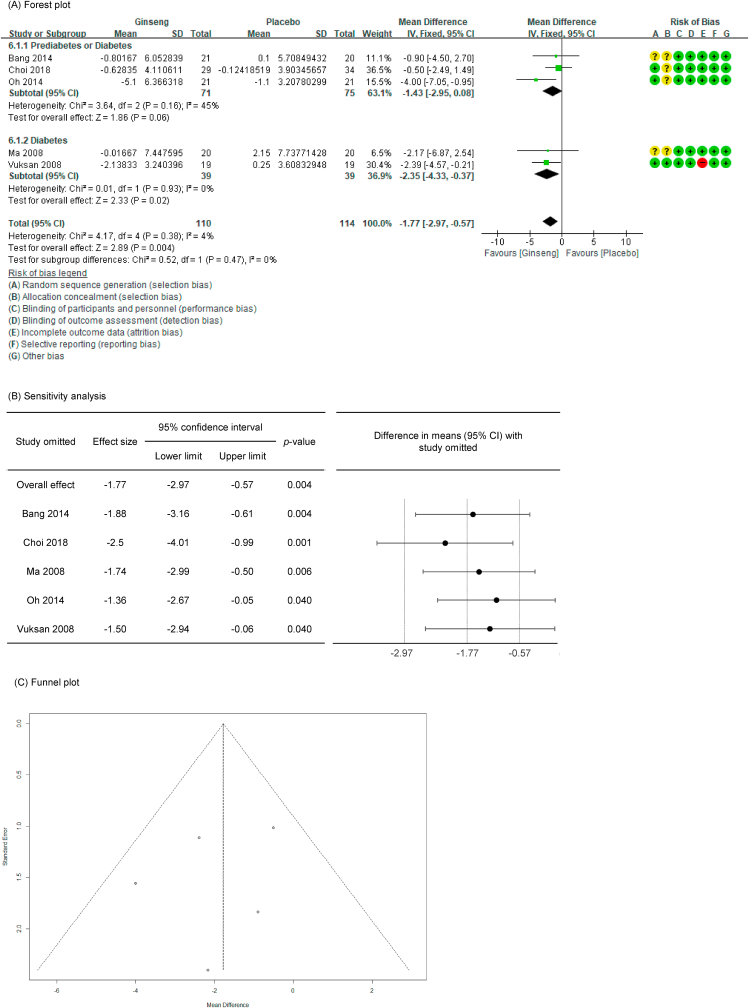
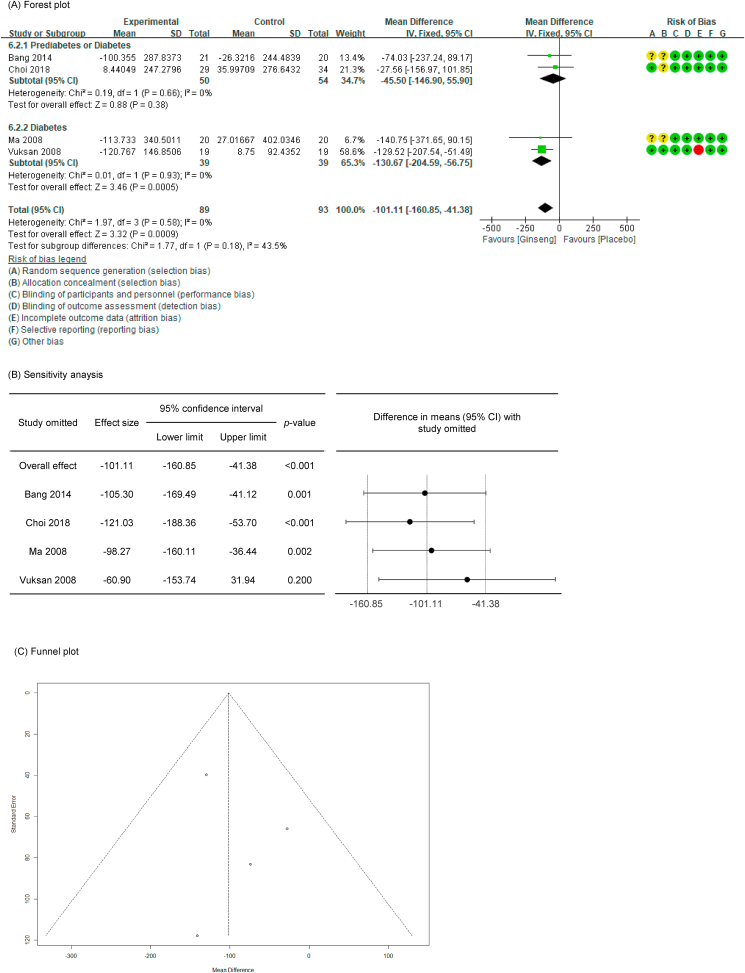
Of the 27 P. ginseng group datasets, a total of 20 datasets were measured for blood glucose–related markers, with the resultant details as follows: 19 included fasting glucose (FG, n = 872) [[35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48],52,54], 18 included fasting insulin (FI, n = 750) [[35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47],53,54], nine included 2-h postprandial glucose (PG, n = 375) [36,37,39,40,42,43,46], six included 2-h postprandial insulin (PI, n = 267) [36,37,39,40,42,43], five included glucose AUC (n = 224) [36,37,39,40,45], four included insulin AUC (n = 182) [36,37,39,45], and 12 included HbA1c (n = 476) [[36], [37], [38],[41], [42], [43],46,54].
In all cases, the heterogeneity was judged to be low (I2 values of 13 %, 0 %, 0 %, 23 %, 4 %, 0 %, and 18 % for FG, FI, 2-h PG, 2-h PI, glucose AUC, glucose insulin, and HbA1c, respectively), so it was analyzed using a fixed-effects model. Upon analyzing the effects size of P. ginseng supplementation, as compared with the placebo, it was revealed that the glucose AUC was decreased by 1.77 mmol/L∗hr (95 % CI: −2.97 to −0.57) and the insulin AUC was decreased by 101.11 pmol/L∗hr (95 % CI: −160.85 to −41.38), showing a statistically significant difference (p = 0.0004 and p = 0.0009). However, there was no statistically significant difference in FG, FI, 2-h PG, 2-h PI, or HbA1c between the P. ginseng and placebo supplementation groups (data not shown). In subgroup analysis, the effects of P. ginseng supplementation on glucose AUC and insulin AUC were stronger in diabetic subjects than in prediabetic subjects (Fig. 2, Fig. 3A). Glucose AUC was robust in the sensitivity analysis but insulin AUC decreased by 8.77 when one study was omitted, resulting in a loss of significance (p = 0.200) (Fig. 2, Fig. 3B) [45]. Funnel plot and Egger's test revealed that there were no publication bias for glucose AUC and insulin AUC. Funnel plots of glucose AUC and insulin AUC are shown in Fig. 2, Fig. 3C.
Fig. 2.
Forest plot, sensitivity analysis, and publication bias of changes in glucose AUC: (A) forest plot, (B) sensitivity analysis, (C) publication bias.
Fig. 3.
Forest plot, sensitivity analysis, and publication bias of changes in insulin AUC: (A) forest plot, (B) sensitivity analysis, (C) publication bias.
3.3. Changes in blood pressure–related markers
Of the 27 P. ginseng group datasets, a total of 15 datasets were measured for blood pressure–related markers, with the resultant details as follows: 15 included SBP (n = 702) [34,36,38,41,42,[45], [46], [47], [48],[53], [54], [55]] and 14 included diastolic DBP (n = 621) [34,36,38,41,42,[45], [46], [47], [48],53,54].
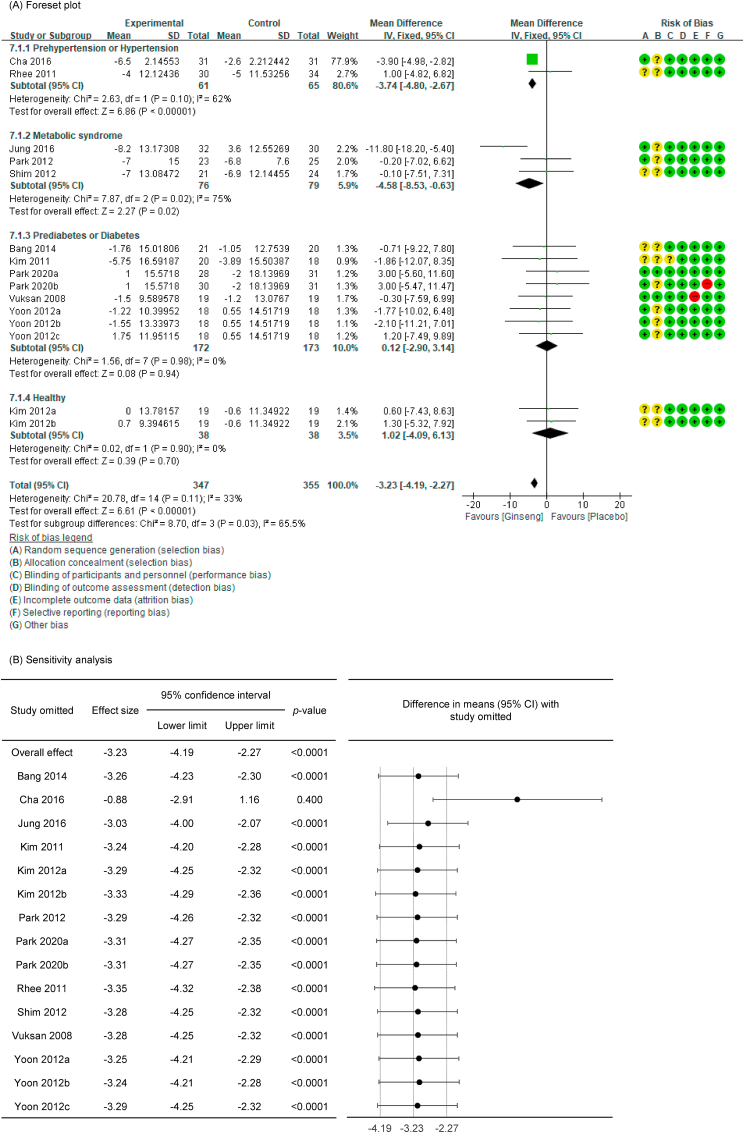
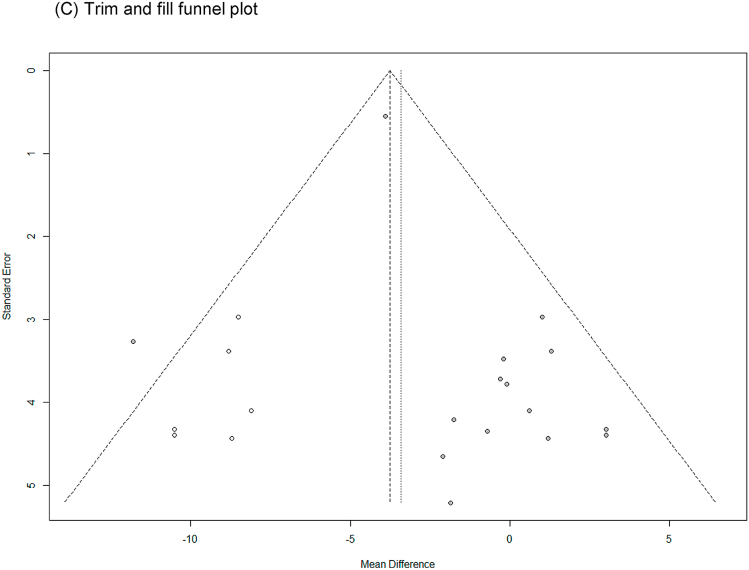
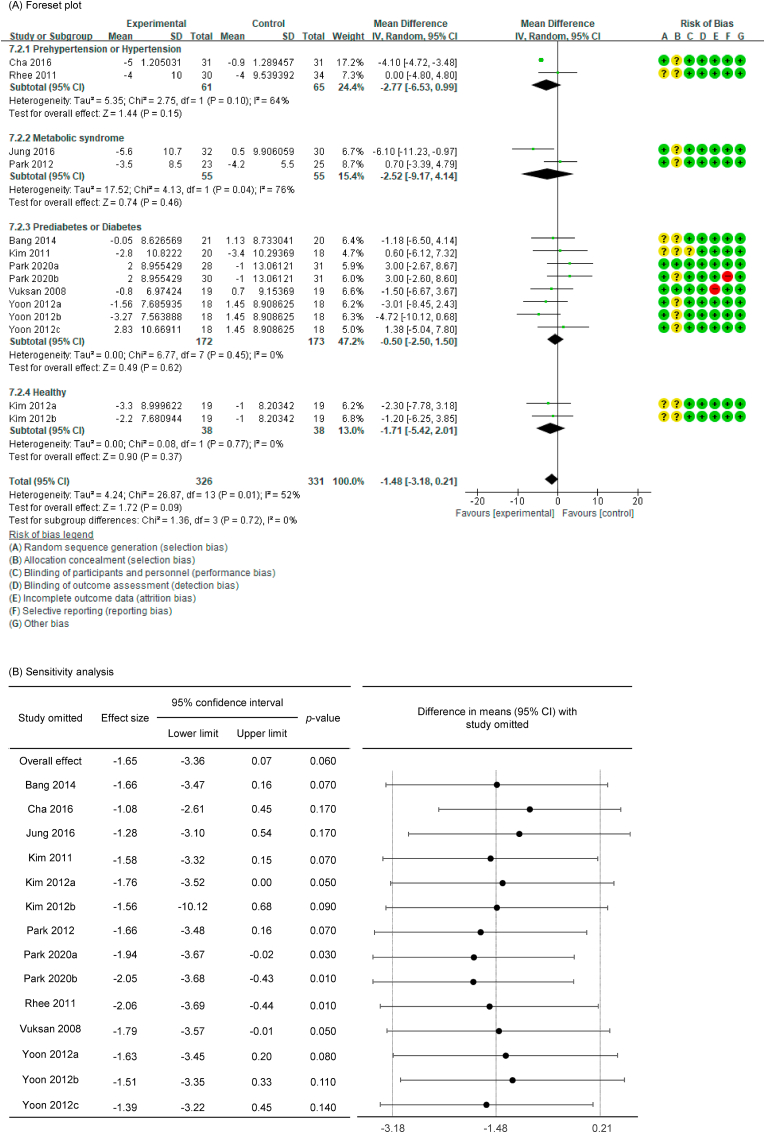
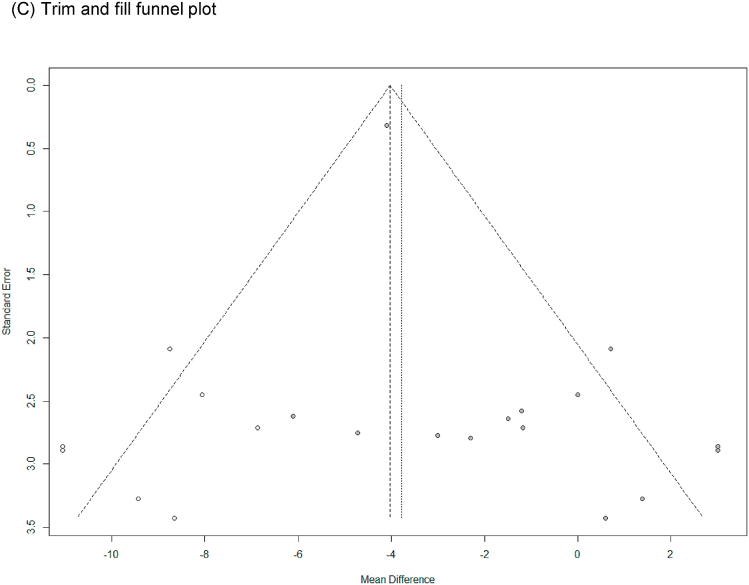
SBP with low heterogeneity was analyzed using a fixed-effects model (I2 = 33 %), and DBP with high heterogeneity was analyzed using a random-effects model (I2 = 51 %). Upon analyzing the effects size of P. ginseng supplementation, as compared with the placebo, it was revealed that the SBP was decreased by 3.23 mmHg (95 % CI: −4.19 to −2.27), showing a statistically significant difference (p < 0.00001). In subgroup analysis, the effects of P. ginseng supplementation on SBP was stronger in prehypertension, hypertension, and metabolic syndrome subjects (Fig. 4A). Meanwhile, the DBP decreased by 1.48 mmHg (95 % CI: −3.18 to 0.21) in the P. ginseng supplementation group as compared with in the placebo group, but this result was not statistically significant (p = 0.09) (Fig. 5A). As a result of the sensitivity analysis, SBP decreased by 0.88 when one study omitted, resulting in a loss of significance (p = 0.400) (Fig. 4B) [47]. DBP obtained significance by decreasing by −1.94, −2.05, and −2.06 when the three studies were omitted (p = 0.03, p = 0.01, p = 0.01) (Fig. 5B) [41,42,48]. Egger's test revealed that there were publication bias for SBP and DBP (p = 0.021, p = 0.002). Six studies had to be trimmed and filled by trim and fill analysis to adjust the publication bias of SBP. As a result, the effect size increased and the direction of effect did not changed (MD = −3.76, 95 % CI: −4.67, −2.84). In DBP, seven studies had to be trimmed and filled, resulting in an increase in effect size and no change in effect direction (MD: −3.783, 95 % CI: −5.380, −2.187). Trim and fill funnel plots of SBP and DBP are shown in Fig. 4, Fig. 5C.
Fig. 4.
Forest plot, sensitivity analysis, and publication bias of changes in SBP: (A) forest plot, (B) sensitivity analysis, (C) trim and fill publication bias.
Fig. 5.
Forest plot, sensitivity analysis, and publication bias of changes in DBP: (A) forest plot, (B) sensitivity analysis, (C) trim and fill publication bias.
3.4. Changes in body fat–related markers
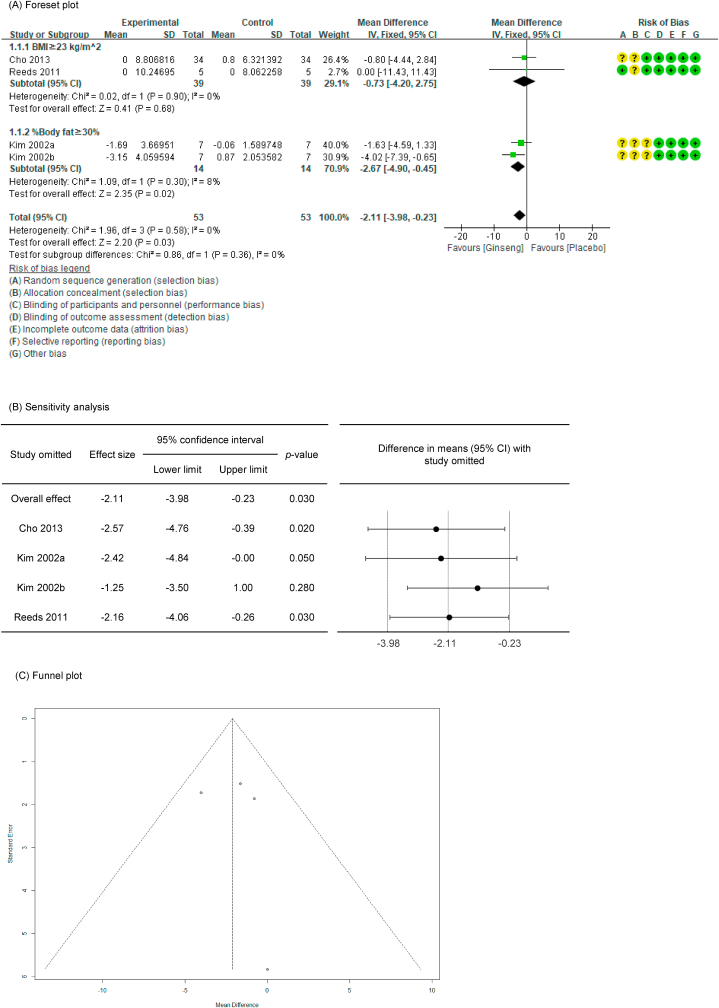
Of the 27 P. ginseng group datasets, a total of 16 datasets were measured for body fat–related markers, with the resultant details as follows: four included body weight (n = 76) [38,44,50,51], 12 included BMI (n = 491) [34,36,38,41,42,44,46,47,52,56], four included % body fat (n = 106) [44,[49], [50], [51]], and five included WC (n = 194) [38,46,54].
In all cases, the heterogeneity was judged to be low (all I2 values were 0%), so it was analyzed using a fixed-effects model. Upon analyzing the effects size of P. ginseng supplementation, as compared with the placebo, it was revealed that the % body fat was decreased by 2.11 % (95 % CI: −3.98 to −0.23), showing a statistically significant difference (p = 0.03). Conversely, there were no statistically significant differences in body weight, BMI, or WC between the P. ginseng and placebo supplementation groups (data not shown). In subgroup analysis, the effect on % body fat was stronger in studies that enrolled obese subjects based on % body fat rather than BMI (Fig. 4) (Fig. 6A). As a result of the sensitivity analysis, % body fat decreased by 1.25 when one study omitted, resulting in a loss of significance (p = 0.28) (Fig. 6B) [51]. Funnel plot and Egger's test manifested that there was no publication bias. The funnel plot of % body fat are shown in Fig. 6C.
Fig. 6.
Forest plot, sensitivity analysis, and publication bias of changes in % body fat: (A) forest plot, (B) sensitivity analysis, (C) publication bias.
3.5. Changes in blood lipid–related markers
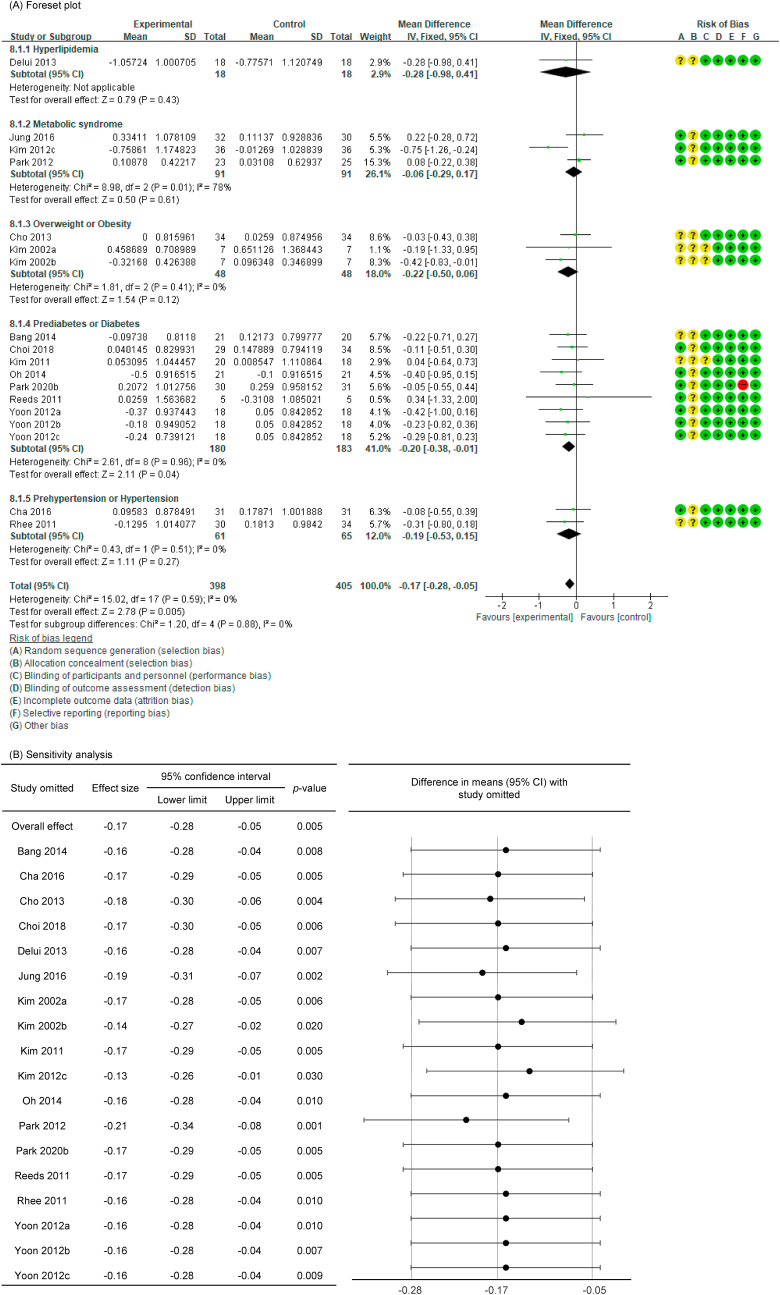
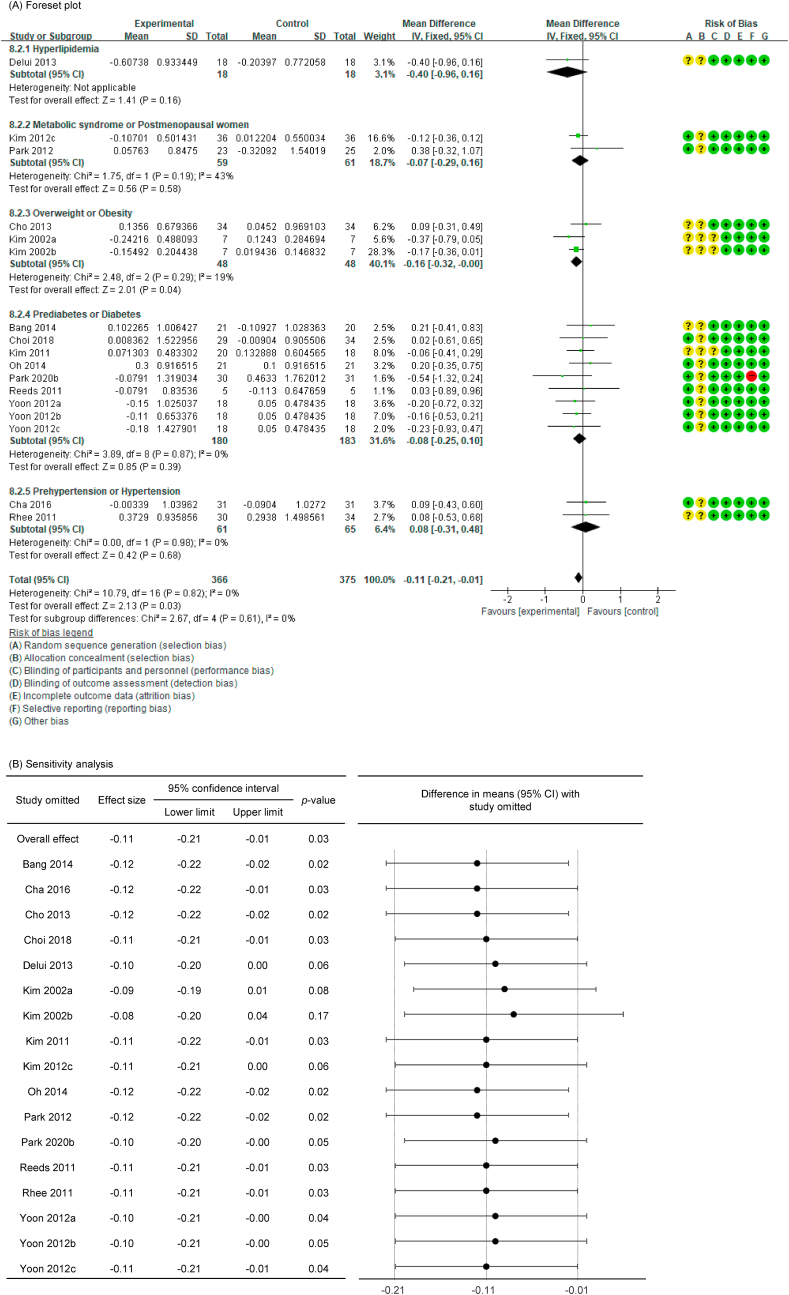
Of the 27 P. ginseng group datasets, a total of 18 datasets were assessed for blood lipid–related markers, with the resultant details as follows: 18 included TC (n = 803) [[36], [37], [38],40,42,44,[46], [47], [48], [49], [50], [51], [52], [53], [54],56], 17 included TG (n = 741) [[36], [37], [38],40,42,44,[46], [47], [48], [49], [50], [51], [52],54,56], 16 included HDL-C (n = 753) [[36], [37], [38],40,42,44,46,47,49,50,[52], [53], [54],56], and 15 included LDL-C (n = 657) [[36], [37], [38],44,[46], [47], [48], [49], [50], [51], [52],54,56].
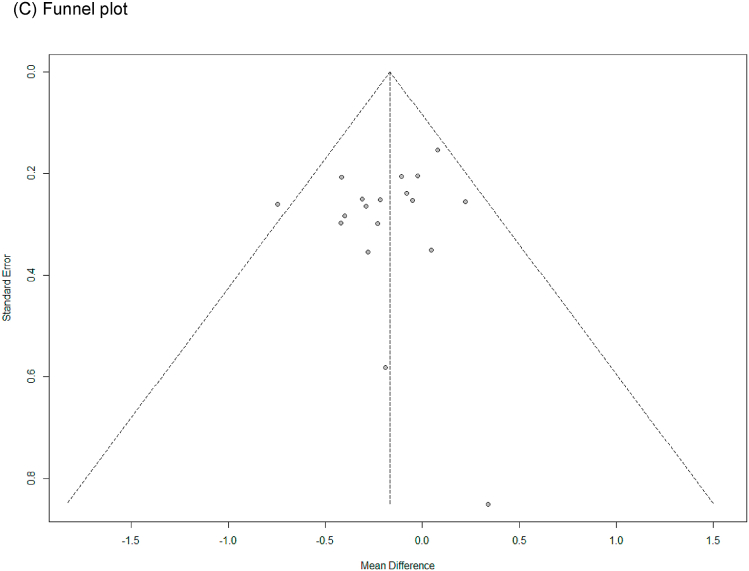
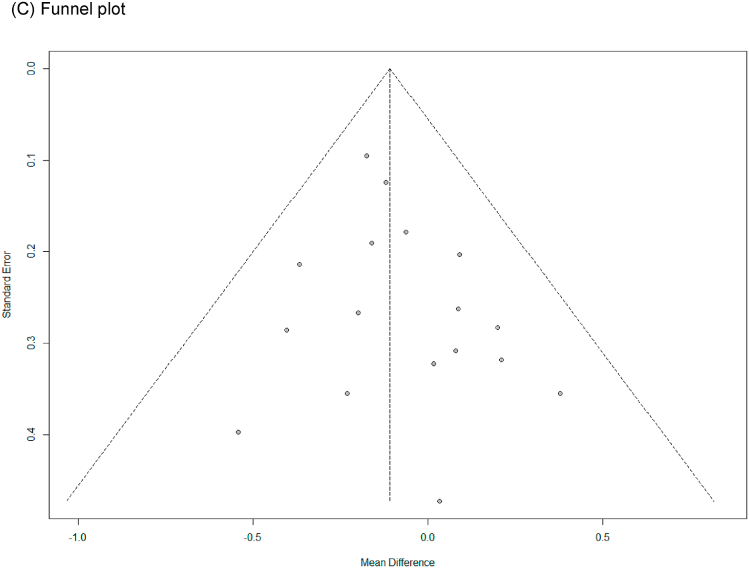
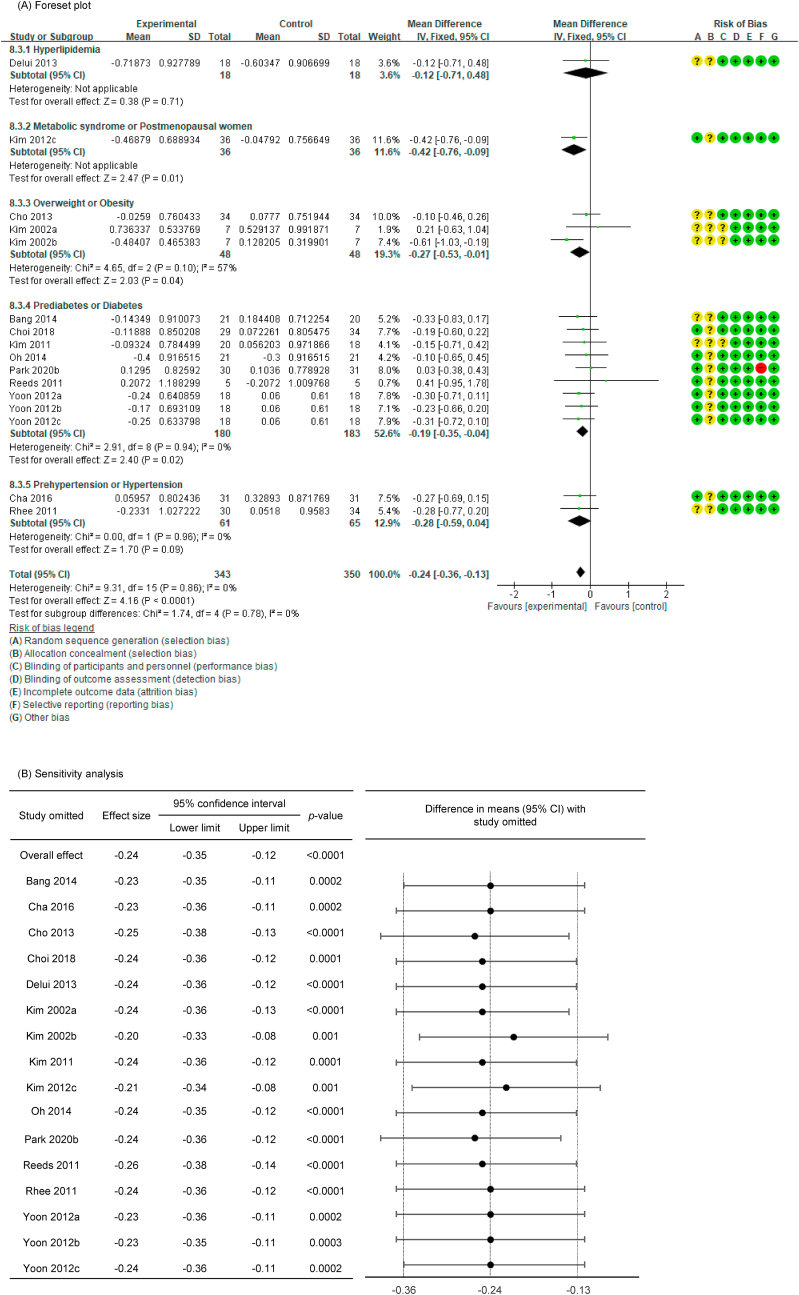
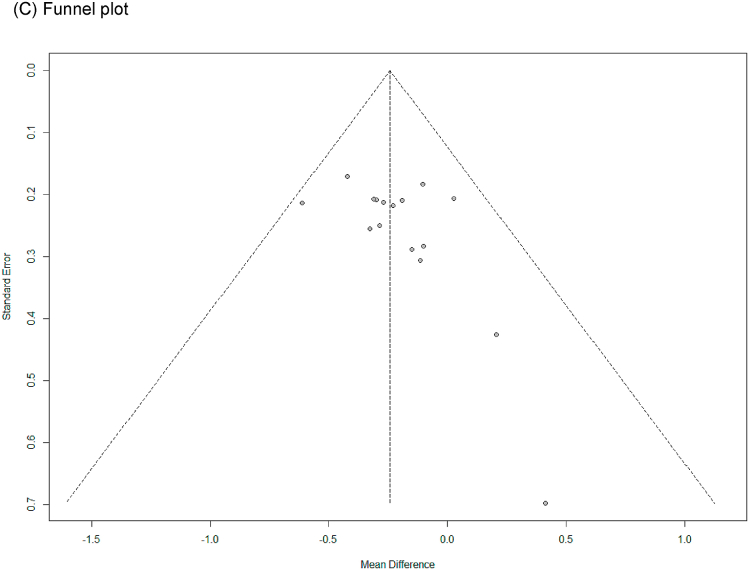
In all cases, the heterogeneity was judged to be low (all I2 values were 0%), so the data were analyzed using a fixed-effects model. Upon analyzing the effect size of P. ginseng supplementation, as compared with the placebo, it was revealed that the TC was decreased by 0.17 mmol/L (95 % CI: −0.28 to −0.05), the TG was decreased by 0.11 mmol/L (95 % CI: −0.21 to −0.01), and the LDL-C was decreased by 0.24 mmol/L (95 % CI: −0.36 to −0.13), showing a statistically significant difference (p = 0.005, p = 0.030, and p < 0.0001). There was no statistically significant difference in HDL-C between the P. ginseng and placebo supplementation groups (data not shown). In subgroup analysis, the effect of P. ginseng supplementation on TC was stronger in prediabetic or diabetic subjects. The effect on TG was more significant overweight or obese subjects, and LDL-C was more significant in metabolic syndrome or postmenopausal women; overweight or obese subjects; prediabetic or diabetic subjects (Fig. 7, Fig. 8, Fig. 9A). Total cholesterol and LDL cholesterol were robust in the sensitivity analysis but TG decreased by 0.10, 0.09, 0.08, and 0.11 when the four studies were omitted, resulting in a loss of significance (p = 0.06, p = 0.08, p = 0.17, p = 0.06) (Fig. 7, Fig. 8, Fig. 9B) [[50], [51], [52],56]. Funnel plot and Egger's test manifested that there were no publication bias for TC, TG, and LDL-C. Funnel plots of TC, TG, and LDL-C are shown in Fig. 7, Fig. 8, Fig. 9C.
Fig. 7.
Forest plot, sensitivity analysis, and publication bias of changes in TC: (A) forest plot, (B) sensitivity analysis, (C) publication bias.
Fig. 8.
Forest plot, sensitivity analysis, and publication bias of changes in TG: (A) forest plot, (B) sensitivity analysis, (C) publication bias.
Fig. 9.
Forest plot, sensitivity analysis, and publication bias of changes in LDL-C: (A) forest plot, (B) sensitivity analysis, (C) publication bias.
4. Discussion
The present systematic review and meta-analysis were conducted to verify the effects of P. ginseng supplementation on metabolic disease–related markers. For this, 23 RCT papers were collected and the changes in markers related to blood glucose, blood pressure, body fat, and blood lipids, which are major markers of metabolic diseases, were compared with those following placebo treatment using data from 27 ginseng supplement groups. As a result of this analysis, it was found that glucose AUC, insulin AUC, SBP, DBP, % body fat, TC, TG, and LDL-C were significantly decreased by P. ginseng supplementation.
As previously reported, there were no significant changes in FG, PG, or HbA1c among the blood glucose–related markers [18]. FG, PG, and HbA1c have traditionally been used as key markers to determine whether blood glucose is well-regulated, but there are limitations in providing accurate information about blood glucose responses and changes after meals by presenting values only at a specific point in time [57]. On the other hand, glucose and insulin AUC have the advantage of being able to determine whether blood glucose and insulin are normally regulated by observing the pattern of changes in blood glucose and insulin for a certain period of time, not a specific time point after oral glucose tolerance test (OGTT); these parameters have therefore been considered key measurements in efforts to determine glucose intolerance in recent years [58]. It has already been found in preclinical studies that ginseng administration improves insulin sensitivity by enhancing insulin signaling [59,60]. Therefore, as our results have shown, significant decreases in glucose and insulin AUC can be an important basis for improving glucose intolerance by ginseng supplementation. In particular, as a result of subgroup analysis, it was found that the effect was stronger in diabetic patients than in prediabetic subjects. However, it is a concern that the range of study types and the size of the overall study population are still relatively limited, and since the importance of AUC measurement is increasingly emerging, clinical studies on this subject should be continued in the future.
The main cause of various metabolic diseases, including metabolic syndrome, is insulin resistance, which is derived from obesity due to excess body fat [1,2,4]. Obesity is judged by weight, BMI, WC, and % body fat. Because weight and BMI are also affected by muscle mass, the use of WC or % body fat is considered more appropriate [[61], [62], [63]]. In this study, it was confirmed that the % body fat was decreased from the pooled data of three clinical trials that enrolled subjects who were overweight or obesity (BMI ≥23 kg/m^2 or %body fat ≥30 %). Although significant effects could not be confirmed among other markers, a significant decrease in % body fat was confirmed in a small sample size. Moreover, in subgroup analysis, the effect of reducing % body fat was stronger when obese subjects were selected based on % body fat rather than BMI. It is already known through several studies that P. ginseng and ginsenoside inhibit adipogenesis and lipid accumulation in adipocytes [64,65]. Therefore, if well-designed clinical studies that established appropriate inclusion criteria to determine obesity are sufficiently accumulated, significant results could be expected for other markers as well.
In addition, P. ginsesng has been proven to reduce blood lipids in various clinical studies and, in this study, as in a previously reported meta-analysis, TC, TG, and LDL-C were decreased [30]. Subgroup analysis of subjects with hyperlipidemia showed no significant reductions in TC, TG, and LDL-C. However since only one study selected hyperlipidemic subjects, it is insufficient to evaluate the effect. On the other hand, as a result of analyzing subjects with metabolic diseases such as metabolic syndrome, menopause, obesity, and diabetes into subgroups, blood lipids were significantly decreased. Many studies have shown that obesity, diabetes, and menopause are highly correlated with hyperlipidemia [[66], [67], [68]]. Therefore, although hypolipidemic effect of P. ginseng was confirmed through this study, a clearer effect can be expected if more studies are conducted on subjects with dyslipidemia in the future.
In this study, blood pressure was also significantly improved in the P. ginseng supplement group. In preclinical studies, ginseng administration decreased the blood pressure through the activation of endothelial nitric oxide synthase and the release of nitric oxide [69,70]. Similarly, SBP and DBP were decreased in this study, which analyzed various subject groups ranging from healthy individuals to hypertensive patients. Subgroup analysis showed a greater effect in subjects with prehypertension or hypertension and metabolic syndrome. Therefore, it was confirmed once again that the selection of appropriate study subjects is important.
A comprehensive analysis showed that the major markers of metabolic syndrome and metabolic diseases were significantly improved when P. ginseng products were consumed for a long period lasting four weeks or longer. Based on this result, it can be expected that the intake of P. ginseng can play a sufficient role as an adjuvant for the prevention and improvement of metabolic diseases. To our knowledge, this is the first systematic review and meta-analysis to simultaneously evaluate the effects of P. ginseng on several markers related to metabolic diseases. However, the study subjects surveyed in this study were varied, ranging from healthy individuals to menopausal women and patients with obesity, diabetes, hypertension, and hyperlipidemia, so, while it is good to generalize these results, there was a limit to obtaining more specific results. Therefore, more clinical trials on metabolic syndrome should be accumulated in the future for further investigation.
5. Conclusions
A systematic review and meta-analysis were conducted by collecting studies that evaluated changes in metabolic disease–related markers driven by the long-term use of P. ginseng in various study populations. Significant changes were found in markers related to blood glucose, insulin resistance, blood pressure, and blood lipids. Based on these findings, supplementation with P. ginseng could be adopted as adjuvant therapy for diabetes, hypertension, and hyperlipidemia. Through this study, P. ginseng supplementation has established an academic basis that it can be used as adjuvant therapy for diabetes, hypertension, and hyperlipidemia.
Declaration of competing interest
All contributing authors declare no conflicts of interest exist.
Acknowledgments
This study was supported by the Main Research Program from the Korea Food Research Institute (KFRI), funded by the Ministry of Science, ICT and Future Planning (E0210601-01).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2021.10.002.
Author contribution
Conception and design of the study: S. H. P., S. W. C., H. K. C., M. Y. C., J. T. H., and J. H. P.; acquisition of data or analysis and interpretation of data: S. H. P. and S. W. C.; drafting of the article or revising it critically for important intellectual content: S. H. P. and S. W. C.; and final approval of the version to be published: S. H. P., S. W. C., H. K. C., M. Y. C., J. T. H., and J. H. P.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Doyle S.L., Donohoe C.L., Lysaght J., Reynolds J.V. Visceral obesity, metabolic syndrome, insulin resistance and cancer. Proc Nutr Soc. 2012;71(1):181–189. doi: 10.1017/S002966511100320X. [DOI] [PubMed] [Google Scholar]
- 2.Hess P.L., Al-Khalidi H.R., Friedman D.J., Mulder H., Kucharska-Newton A., Rosamond W.R., Lopes R.D., Gersh B.J., Mark D.B., Curtis L.H., et al. The metabolic syndrome and risk of sudden cardiac death: the atherosclerosis risk in communities study. J Am Heart Assoc. 2017;6(8) doi: 10.1161/JAHA.117.006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tune J.D., Goodwill A.G., Sassoon D.J., Mather K.J. Cardiovascular consequences of metabolic syndrome. Transl Res. 2017;183:57–70. doi: 10.1016/j.trsl.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagry H.S., Raghavendran S., Carli F. Metabolic syndrome and insulin resistance: perioperative considerations. Anesthesiology. 2008;108(3):506–523. doi: 10.1097/ALN.0b013e3181649314. [DOI] [PubMed] [Google Scholar]
- 5.Gallagher E.J., LeRoith D., Karnieli E. The metabolic syndrome--from insulin resistance to obesity and diabetes. Endocrinol Metab Clin N Am. 2008;37(3):559–579. doi: 10.1016/j.ecl.2008.05.002. [vii] [DOI] [PubMed] [Google Scholar]
- 6.Baeg I.H., So S.H. The world ginseng market and the ginseng (Korea) J Ginseng Res. 2013;37(1):1–7. doi: 10.5142/jgr.2013.37.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong H.D., Choi S.Y., Kim Y.C., Cho C.W. Rapid determination of ginsenosides Rb1, Rf, and Rg1 in Korean ginseng using HPLC. J Ginseng Res. 2009;33(1):8–12. [Google Scholar]
- 8.Kim J.H., Yi Y.S., Kim M.Y., Cho J.Y. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J Ginseng Res. 2017;41(4):435–443. doi: 10.1016/j.jgr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee S.M., Bae B.S., Park H.W., Ahn N.G., Cho B.G., Cho Y.L., Kwak Y.S. Characterization of Korean red ginseng (Panax ginseng Meyer): history, preparation method, and chemical composition. J Ginseng Res. 2015;39(4):384–391. doi: 10.1016/j.jgr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metwaly A.M., Lianlian Z., Luqi H., Deqiang D. Black ginseng and its saponins: preparation, phytochemistry and pharmacological effects. Molecules. 2019;24(10) doi: 10.3390/molecules24101856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryu J.S., Lee H.J., Bae S.H., Kim S.Y., Park Y., Suh H.J., Jeong Y.H. The bioavailability of red ginseng extract fermented by Phellinus linteus. J Ginseng Res. 2013;37(1):108–116. doi: 10.5142/jgr.2013.37.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X., Chu S., Lin M., Gao Y., Liu Y., Yang S., Zhou X., Zhang Y., Hu Y., Wang H., et al. Anticancer property of ginsenoside Rh2 from ginseng. Eur J Med Chem. 2020;203 doi: 10.1016/j.ejmech.2020.112627. [DOI] [PubMed] [Google Scholar]
- 13.Ahuja A., Kim J.H., Kim J.H., Yi Y.S., Cho J.Y. Functional role of ginseng-derived compounds in cancer. J Ginseng Res. 2018;42(3):248–254. doi: 10.1016/j.jgr.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y.H., Kuruganti R., Gao Y. Recent advances in ginsenosides as potential therapeutics against breast cancer. Curr Top Med Chem. 2019;19(25):2334–2347. doi: 10.2174/1568026619666191018100848. [DOI] [PubMed] [Google Scholar]
- 15.Wang C.Z., Anderson S., Du W., He T.C., Yuan C.S. Red ginseng and cancer treatment. Chin J Nat Med. 2016;14(1):7–16. doi: 10.3724/SP.J.1009.2016.00007. [DOI] [PubMed] [Google Scholar]
- 16.Zhou P., Xie W., He S., Sun Y., Meng X., Sun G., Sun X. Ginsenoside Rb1 as an anti-diabetic agent and its underlying mechanism analysis. Cells. 2019;8(3) doi: 10.3390/cells8030204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng T.B., Yeung H.W. Hypoglycemic constituents of Panax ginseng. Gen Pharmacol. 1985;16(6):549–552. doi: 10.1016/0306-3623(85)90140-5. [DOI] [PubMed] [Google Scholar]
- 18.Kim S., Shin B.C., Lee M.S., Lee H., Ernst E. Red ginseng for type 2 diabetes mellitus: a systematic review of randomized controlled trials. Chin J Integr Med. 2011;17(12):937–944. doi: 10.1007/s11655-011-0937-2. [DOI] [PubMed] [Google Scholar]
- 19.Bai L., Gao J., Wei F., Zhao J., Wang D., Wei J. Therapeutic potential of ginsenosides as an adjuvant treatment for diabetes. Front Pharmacol. 2018;9 doi: 10.3389/fphar.2018.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irfan M., Kim M., Rhee M.H. Anti-platelet role of Korean ginseng and ginsenosides in cardiovascular diseases. J Ginseng Res. 2020;44(1):24–32. doi: 10.1016/j.jgr.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X. Cardiovascular protection by ginsenosides and their nitric oxide releasing action. Clin Exp Pharmacol Physiol. 1996;23(8):728–732. doi: 10.1111/j.1440-1681.1996.tb01767.x. [DOI] [PubMed] [Google Scholar]
- 22.Luo B.Y., Jiang J.L., Fang Y.F., Yang F., Yin M.D., Zhang B.C., Zhao R.R., Shao J.W. The effects of ginsenosides on platelet aggregation and vascular intima in the treatment of cardiovascular diseases: from molecular mechanisms to clinical applications. Pharmacol Res. 2020;159 doi: 10.1016/j.phrs.2020.105031. [DOI] [PubMed] [Google Scholar]
- 23.Kim J.H. Pharmacological and medical applications of Panax ginseng and ginsenosides: a review for use in cardiovascular diseases. J Ginseng Res. 2018;42(3):264–269. doi: 10.1016/j.jgr.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng S.D., Wu H.J., Wu D.L. Roles and mechanisms of ginseng in protecting heart. Chin J Integr Med. 2012;18(7):548–555. doi: 10.1007/s11655-012-1148-1. [DOI] [PubMed] [Google Scholar]
- 25.Kim K.H., Lee D., Lee H.L., Kim C.E., Jung K., Kang K.S. Beneficial effects of Panax ginseng for the treatment and prevention of neurodegenerative diseases: past findings and future directions. J Ginseng Res. 2018;42(3):239–247. doi: 10.1016/j.jgr.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou W., Wang Y., Zheng P., Cui R. Effects of ginseng on neurological disorders. Front Cell Neurosci. 2020;14:55. doi: 10.3389/fncel.2020.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang X., Li N., Pu Y., Zhang T., Wang B. Neuroprotective effects of ginseng phytochemicals: recent perspectives. Molecules. 2019;24(16) doi: 10.3390/molecules24162939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim H.J., Jung S.W., Kim S.Y., Cho I.H., Kim H.C., Rhim H., Kim M., Nah S.Y. Panax ginseng as an adjuvant treatment for Alzheimer's disease. J Ginseng Res. 2018;42(4):401–411. doi: 10.1016/j.jgr.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Razgonova M.P., Veselov V.V., Zakharenko A.M., Golokhvast K.S., Nosyrev A.E., Cravotto G., Tsatsakis A., Spandidos D.A. Panax ginseng components and the pathogenesis of Alzheimer's disease (Review) Mol Med Rep. 2019;19(4):2975–2998. doi: 10.3892/mmr.2019.9972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernandez-Garcia D., Granado-Serrano A.B., Martin-Gari M., Naudi A., Serrano J.C. Efficacy of Panax ginseng supplementation on blood lipid profile. A meta-analysis and systematic review of clinical randomized trials. J Ethnopharmacol. 2019;243 doi: 10.1016/j.jep.2019.112090. [DOI] [PubMed] [Google Scholar]
- 31.Park H.S., Cho J.H., Kim K.W., Chung W.S., Song M.Y. Effects of Panax ginseng on obesity in animal models: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2018;2018 doi: 10.1155/2018/2719794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L., Virgous C., Si H. Ginseng and obesity: observations and understanding in cultured cells, animals and humans. J Nutr Biochem. 2017;44:1–10. doi: 10.1016/j.jnutbio.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Duval S., Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 34.Kim J.Y., Park J.Y., Kang H.J., Kim O.Y., Lee J.H. Beneficial effects of Korean red ginseng on lymphocyte DNA damage, antioxidant enzyme activity, and LDL oxidation in healthy participants: a randomized, double-blind, placebo-controlled trial. Nutr J. 2012;11:47. doi: 10.1186/1475-2891-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reay J.L., Scholey A.B., Milne A., Fenwick J., Kennedy D.O. Panax ginseng has no effect on indices of glucose regulation following acute or chronic ingestion in healthy volunteers. Br J Nutr. 2009;101(11):1673–1678. doi: 10.1017/S0007114508123418. [DOI] [PubMed] [Google Scholar]
- 36.Bang H., Kwak J.H., Ahn H.Y., Shin D.Y., Lee J.H. Korean red ginseng improves glucose control in subjects with impaired fasting glucose, impaired glucose tolerance, or newly diagnosed type 2 diabetes mellitus. J Med Food. 2014;17(1):128–134. doi: 10.1089/jmf.2013.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi H.S., Kim S., Kim M.J., Kim M.S., Kim J., Park C.W., Seo D., Shin S.S., Oh S.W. Efficacy and safety of Panax ginseng berry extract on glycemic control: a 12-wk randomized, double-blind, and placebo-controlled clinical trial. J Ginseng Res. 2018;42(1):90–97. doi: 10.1016/j.jgr.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim H.O., Park M.J., Han J.S. Effects of fermented red ginseng supplementation on blood glucose and insulin resistance in type 2 diabetic patients. J Korean Soc Food Sci Nutr. 2011;40(5):696–703. [Google Scholar]
- 39.Ma S.W., Benzie I.F., Chu T.T., Fok B.S., Tomlinson B., Critchley L.A. Effect of Panax ginseng supplementation on biomarkers of glucose tolerance, antioxidant status and oxidative stress in type 2 diabetic subjects: results of a placebo-controlled human intervention trial. Diabetes Obes Metabol. 2008;10(11):1125–1127. doi: 10.1111/j.1463-1326.2008.00858.x. [DOI] [PubMed] [Google Scholar]
- 40.Oh M.R., Park S.H., Kim S.Y., Back H.I., Kim M.G., Jeon J.Y., Ha K.C., Na W.T., Cha Y.S., Park B.H., et al. Postprandial glucose-lowering effects of fermented red ginseng in subjects with impaired fasting glucose or type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. BMC Compl Alternative Med. 2014;14 doi: 10.1186/1472-6882-14-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park K., Ahn C.W., Kim Y., Nam J.S. The effect of Korean red ginseng on sarcopenia biomarkers in type 2 diabetes patients. Arch Gerontol Geriatr. 2020;90 doi: 10.1016/j.archger.2020.104108. [DOI] [PubMed] [Google Scholar]
- 42.Park K., Kim Y., Kim J., Kang S., Park J.S., Ahn C.W., Nam J.S. Supplementation with Korean red ginseng improves current perception threshold in Korean type 2 diabetes patients: a randomized, double-blind, placebo-controlled trial. J Diabetes Res. 2020;2020 doi: 10.1155/2020/5295328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park S.H., Oh M.R., Choi E.K., Kim M.G., Ha K.C., Lee S.K., Kim Y.G., Park B.H., Kim D.S., Chae S.W. An 8-wk, randomized, double-blind, placebo-controlled clinical trial for the antidiabetic effects of hydrolyzed ginseng extract. J Ginseng Res. 2014;38(4):239–243. doi: 10.1016/j.jgr.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reeds D.N., Patterson B.W., Okunade A., Holloszy J.O., Polonsky K.S., Klein S. Ginseng and ginsenoside Re do not improve beta-cell function or insulin sensitivity in overweight and obese subjects with impaired glucose tolerance or diabetes. Diabetes Care. 2011;34(5):1071–1076. doi: 10.2337/dc10-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vuksan V., Sung M.K., Sievenpiper J.L., Stavro P.M., Jenkins A.L., Di Buono M., Lee K.S., Leiter L.A., Nam K.Y., Arnason J.T., et al. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr Metabol Cardiovasc Dis. 2008;18(1):46–56. doi: 10.1016/j.numecd.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Yoon J.W., Kang S.M., Vassy J.L., Shin H., Lee Y.H., Ahn H.Y., Choi S.H., Park K.S., Jang H.C., Lim S. Efficacy and safety of ginsam, a vinegar extract from Panax ginseng, in type 2 diabetic patients: results of a double-blind, placebo-controlled study. J Diabetes Investig. 2012;3(3):309–317. doi: 10.1111/j.2040-1124.2011.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cha T.W., Kim M., Kim M., Chae J.S., Lee J.H. Blood pressure-lowering effect of Korean red ginseng associated with decreased circulating Lp-PLA2 activity and lysophosphatidylcholines and increased dihydrobiopterin level in prehypertensive subjects. Hypertens Res. 2016;39(6):449–456. doi: 10.1038/hr.2016.7. [DOI] [PubMed] [Google Scholar]
- 48.Rhee M.Y., Kim Y.S., Bae J.H., Nah D.Y., Kim Y.K., Lee M.M., Kim H.Y. Effect of Korean red ginseng on arterial stiffness in subjects with hypertension. J Alternative Compl Med. 2011;17(1):45–49. doi: 10.1089/acm.2010.0065. [DOI] [PubMed] [Google Scholar]
- 49.Cho Y.H., Ahn S.C., Lee S.Y., Jeong D.W., Choi E.J., Kim Y.J., Lee J.G., Lee Y.H., Shin B.C. Effect of Korean red ginseng on insulin sensitivity in non-diabetic healthy overweight and obese adults. Asia Pac J Clin Nutr. 2013;22(3):365–371. doi: 10.6133/apjcn.2013.22.3.04. [DOI] [PubMed] [Google Scholar]
- 50.Kim S.S., Kim J.D., Kim H., Shin M.S., Park C.K., Park M.H., Yang J.W. The effects of red ginseng product and combined exercise on blood lipids and body composition of obese women in their twenties. J Ginseng Res. 2002;26(2):59–66. [Google Scholar]
- 51.Kim S.S., Park H.Y., Byun Y.H., Hwang B.G., Lee J.H., Shim Y.J., Park C.K., Yang J.W. The effects on the blood lipid profiles and body fat by long term administration of red ginseng product. J Ginseng Res. 2002;26(2):67–73. [Google Scholar]
- 52.Delui M.H., Fatehi H., Manavifar M., Amini M., Ghayour-Mobarhan M., Zahedi M., Ferns G. The effects of Panax ginseng on lipid profile, pro-oxidant: antioxidant status and high-sensitivity C reactive protein levels in hyperlipidemic patients in Iran. Int J Prev Med. 2013;4(9):1045–1051. [PMC free article] [PubMed] [Google Scholar]
- 53.Jung D.H., Lee Y.J., Kim C.B., Kim J.Y., Shin S.H., Park J.K. Effects of ginseng on peripheral blood mitochondrial DNA copy number and hormones in men with metabolic syndrome: a randomized clinical and pilot study. Compl Ther Med. 2016;24:40–46. doi: 10.1016/j.ctim.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Park B.J., Lee Y.J., Lee H.R., Jung D.H., Na H.Y., Kim H.B., Shim J.Y. Effects of Korean red ginseng on cardiovascular risks in subjects with metabolic syndrome: a double-blind randomized controlled study. Korean J Fam Med. 2012;33(4):190–196. doi: 10.4082/kjfm.2012.33.4.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shim J.Y. ClinicalTrials.gov. 2012. Korean red ginseng and metabolic syndrome. [Google Scholar]
- 56.Kim S.Y., Seo S.K., Choi Y.M., Jeon Y.E., Lim K.J., Cho S., Choi Y.S., Lee B.S. Effects of red ginseng supplementation on menopausal symptoms and cardiovascular risk factors in postmenopausal women: a double-blind randomized controlled trial. Menopause. 2012;19(4):461–466. doi: 10.1097/gme.0b013e3182325e4b. [DOI] [PubMed] [Google Scholar]
- 57.Kuzuya T., Nakagawa S., Satoh J., Kanazawa Y., Iwamoto Y., Kobayashi M., Nanjo K., Sasaki A., Seino Y., Ito C., et al. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Res Clin Pract. 2002;55(1):65–85. doi: 10.1016/s0168-8227(01)00365-5. [DOI] [PubMed] [Google Scholar]
- 58.Sakaguchi K., Takeda K., Maeda M., Ogawa W., Sato T., Okada S., Ohnishi Y., Nakajima H., Kashiwagi A. Glucose area under the curve during oral glucose tolerance test as an index of glucose intolerance. Diabetol Int. 2016;7(1):53–58. doi: 10.1007/s13340-015-0212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee H.J., Lee Y.H., Park S.K., Kang E.S., Kim H.J., Lee Y.C., Choi C.S., Park S.E., Ahn C.W., Cha B.S., et al. Korean red ginseng (Panax ginseng) improves insulin sensitivity and attenuates the development of diabetes in Otsuka Long-Evans Tokushima fatty rats. Metabolism. 2009;58(8):1170–1177. doi: 10.1016/j.metabol.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 60.Lee S.H., Lee H.J., Lee Y.H., Lee B.W., Cha B.S., Kang E.S., Ahn C.W., Park J.S., Kim H.J., Lee E.Y., et al. Korean red ginseng (Panax ginseng) improves insulin sensitivity in high fat fed Sprague-Dawley rats. Phytother Res. 2012;26(1):142–147. doi: 10.1002/ptr.3610. [DOI] [PubMed] [Google Scholar]
- 61.Joseph L., Wasir J.S., Misra A., Vikram N.K., Goel K., Pandey R.M., Chandra M., Poddar P., Kondal D. Appropriate values of adiposity and lean body mass indices to detect cardiovascular risk factors in Asian Indians. Diabetes Technol Therapeut. 2011;13(9):899–906. doi: 10.1089/dia.2011.0014. [DOI] [PubMed] [Google Scholar]
- 62.Prentice A.M., Jebb S.A. Beyond body mass index. Obes Rev. 2001;2(3):141–147. doi: 10.1046/j.1467-789x.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 63.Pi-Sunyer F.X. Obesity: criteria and classification. Proc Nutr Soc. 2000;59(4):505–509. doi: 10.1017/s0029665100000732. [DOI] [PubMed] [Google Scholar]
- 64.Chen G., Li H., Zhao Y., Zhu H., Cai E., Gao Y., Liu S., Yang H., Zhang L. Saponins from stems and leaves of Panax ginseng prevent obesity via regulating thermogenesis, lipogenesis and lipolysis in high-fat diet-induced obese C57BL/6 mice. Food Chem Toxicol. 2017;106(Pt A):393–403. doi: 10.1016/j.fct.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 65.Shin S.S., Yoon M. Korean red ginseng (Panax ginseng) inhibits obesity and improves lipid metabolism in high fat diet-fed castrated mice. J Ethnopharmacol. 2018;210:80–87. doi: 10.1016/j.jep.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 66.Klop B., Elte J.W., Cabezas M.C. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5(4):1218–1240. doi: 10.3390/nu5041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Phan B.A., Toth P.P. Dyslipidemia in women: etiology and management. Int J Womens Health. 2014;6:185–194. doi: 10.2147/IJWH.S38133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O'Brien T., Nguyen T.T., Zimmerman B.R. Hyperlipidemia and diabetes mellitus. Mayo Clin Proc. 1998;73(10):969–976. doi: 10.4065/73.10.969. [DOI] [PubMed] [Google Scholar]
- 69.Hong S.Y., Kim J.Y., Ahn H.Y., Shin J.H., Kwon O. Panax ginseng extract rich in ginsenoside protopanaxatriol attenuates blood pressure elevation in spontaneously hypertensive rats by affecting the Akt-dependent phosphorylation of endothelial nitric oxide synthase. J Agric Food Chem. 2012;60(12):3086–3091. doi: 10.1021/jf204447y. [DOI] [PubMed] [Google Scholar]
- 70.Nagar H., Choi S., Jung S.B., Jeon B.H., Kim C.S. Rg3-enriched Korean Red Ginseng enhances blood pressure stability in spontaneously hypertensive rats. Integr Med Res. 2016;5(3):223–229. doi: 10.1016/j.imr.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.