Abstract
Background
The number of randomized trials of agents targeting oncogene-addicted tumors has surged in the past 10 years. Using a meta-analysis, we explored whether improvements in objective response rate (ORR) in comparative trials using targeted agents could serve as a potential surrogate endpoint for improvements in progression-free survival (PFS) or overall survival (OS) in populations with oncogene-addicted cancer.
Patients and methods
Using commercial text mining software I2E, we searched ClinicalTrials.gov and MEDLINE databases for randomized, phase III trials based on prospectively defined criteria, including (i) use of agents targeting EGFR activating mutations, ALK rearrangements, BRAF V600E or V600K mutations, and BCR-ABL fusion protein; (ii) molecularly enriched trial population or subpopulation; (iii) control arm only randomized to chemo/cytotoxic therapy. Correlative analyses were performed using ORR, OS, and PFS data from trials that met these criteria.
Results
A total of 62 trials were identified; 15 met all of the prespecified criteria. The ORR effect size (both the difference in ORR between arms and the log odds ratio) and log PFS hazard ratio were strongly correlated: –0.78 (P = 0.0007) for the ORR difference model; –0.74 (P = 0.0017) for the log odds ratio model. ORR effect size was positively correlated with the log OS hazard ratio, but more weakly: –0.67 (P = 0.013) for the ORR difference model and –0.58 (P = 0.036) for the log odds ratio model. Analysis of the treatment effects between OS and PFS found no correlation.
Conclusions
These analyses identified a strong correlation between treatment effects on ORR and PFS in randomized clinical trials investigating agents targeting oncogene-driven cancers. A weaker correlation was observed between ORR and OS. These meta-analysis results support the use of a high ORR forming the basis of an initial regulatory approval in biomarker-driven studies.
Key words: meta-analysis, targeted therapy, correlation, objective response rate, progression-free survival, overall survival
Highlights
-
•
Meta-analysis of trials comparing an agent targeting a primary oncogenic driver versus nontargeted therapy.
-
•
Correlative analysis showed that ORR effect size and log PFS HR were strongly correlated.
-
•
ORR effect size was positively correlated with the log OS HR ratio, but more weakly.
-
•
Analysis of the treatment effects between OS and PFS found no correlation.
-
•
Results support the use of a high ORR forming the basis of an initial regulatory approval in biomarker-driven studies.
Introduction
Over the past two decades, advances in drug development and sequencing technologies have greatly expanded the field of genomically driven personalized medicine. Large-scale cancer genomic research efforts have enabled the systematic identification of a growing number of genomic alterations directly involved in cancer growth and proliferation. Oncology research efforts have reflected the growing interest in personalized medicine, driven by the robust clinical outcomes observed with selectively targeted therapies when compared with nonselective treatment options such as chemotherapy.1, 2, 3, 4, 5, 6
Regular regulatory approval can be granted based on clinically meaningful improvement in patient symptoms, function, or progression-free survival (PFS) in a randomized, controlled phase III study. However, overall survival (OS) continues to be the regulatory gold standard as it is the most widely accepted efficacy endpoint for comparative studies and the one least affected by researcher bias. As the interpretability of OS may be confounded by multiple factors such as postprogression follow-up, treatment crossover, and subsequent therapies, the use of PFS has been adopted as a valid and clinically meaningful primary endpoint in late-phase cancer research. More recently, trials testing targeted therapeutic agents have been utilizing objective response rate (ORR) as the primary trial endpoint in single-arm studies. A high ORR, as a surrogate endpoint in oncology, is thought to reasonably predict treatment-related clinical benefit since spontaneous tumor regression is extremely rare in the absence of a therapeutic intervention.7
The validity of using clinical response rates as a surrogate endpoint for PFS or OS was previously explored through a meta-analysis of 14 advanced non-small-cell lung cancer (NSCLC) clinical trials, 3 of which focused on targeted therapy.7 The analyses presented herein build on this work by focusing exclusively on trials of targeted agents. Specifically, we perform a meta-analysis of comparative trials using targeted agents to examine whether improvements in ORR could serve as a surrogate endpoint for improvements in PFS or OS in populations with oncogene addicted cancers.
Methods
Selection criteria
Using the commercial text mining software I2E (Linguamatics), the clinicaltrials.gov and MEDLINE databases were searched for randomized, phase III clinical trials that were investigating targeted therapy. Search terms included terms derived from these key criteria: (i) the trial tested an agent targeting EGFR activating mutations, ALK rearrangements, BRAF V600E or V600K mutations, or BCR-ABL fusion protein; (ii) the trial population (or a ≥20 patient subgroup of the population) was molecularly enriched; (iii) the control arm(s) did not include targeted agents directed toward the molecularly enriched population; (iv) the study was a randomized phase III trial reporting ORR and PFS; and (v) trial results were published from 2000 to present in English.
Outcome measures
ORR was defined as the proportion of patients who exhibited a complete or partial response as defined by the response criteria used in the study. All studies included in this meta-analysis used RECIST version 1.1, except one study that used RECIST8 and one study for which formal response criteria were not listed.9 PFS was defined as the time from random assignment to progression or death from any cause. Patients who had not progressed at data cut-off were censored at the date of the final disease assessment. All trials used PFS as the primary efficacy outcome except one study that used OS.10 OS was defined as the period from randomization to death from any cause. Patients who were alive on the day of data cut-off were censored at the last day of follow-up. All analyses used the intent-to-treat population.
Statistical analyses
ORR, OS, and PFS data were manually extracted from the ClinicalTrials.gov site for each trial meeting the criteria. Data absent from the ClinicalTrials.gov site were obtained from the associated reference. Weighted linear regression models were used to investigate the association between treatment effects on ORR, PFS, and OS. Weighted Pearson correlation analyses (with associated P values) were performed under logarithmic transformation (except for the ORR difference), with weights equal to the precision (back-solved from reported 95% confidence intervals) associated with the log PFS hazard ratios (for the ORR/PFS analyses) and the log OS hazard ratios (for the ORR/OS and PFS/OS analyses). Logistic regression models were used to derive ORR odds ratios where not directly reported in the source reference. Hazard ratios estimated from Cox proportional hazards regression models were used to express the treatment effects on PFS and OS. An odds ratio of >1 (experimental versus control) and a hazard ratio of <1 (experimental versus control) signified the experimental group resulted in a favorable result over the control group.
Results
A schematic of the search strategy is shown in Figure 1. Database mining initially identified 61 publications meeting the search criteria. Thirteen publications were removed as duplicate reports of the same trial; thus 48 unique trials were reviewed. Further review excluded 17 publications because the trial included the targeted therapy in both treatment arms. An additional two trials were removed because the therapies were ineligible. Of the remaining 29 trials, after review of the study results as reported in ClinicalTrials.gov or the primary manuscript, 13 additional trials were excluded due to missing ORR or PFS results (n = 3) or an ineligible (unselected) population, that is, one that was not selected for the driver alteration of interest (n = 10). One other trial was excluded due to inadequate sample size. Fifteen trials met all of the inclusion criteria and were included in the meta-analysis.5, 6,8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20
Figure 1.
Flow chart detailing trial selection for meta-analysis.
The schematic details the steps taken to identify phase III clinical trials that met the predefined criteria to be included in the meta-analysis.
ORR, objective response rate; PFS, progression-free survival.
aOne of the 62 trials (IRIS trial of imatinib) was not identified by the search criteria due to the use of hematologic response rather than objective (RECIST) response. This trial was added to the list of trials identified by the search. Later analysis found it to have little impact on numeric results or conclusions.
Results from the 15 trials were published from 2003 to 2020. Data from each of the trials are summarized in Table 1. Thirteen of the 15 trials tested a treatment of NSCLC; one each studied melanoma and chronic myeloid leukemia. Population size, treatment therapy, ORR, median PFS, median OS were extracted for each treatment arm, as well as the PFS hazard ratio and OS hazard ratio comparing the arms, where available. When subgroup results for the appropriate biomarker population (associated with the target of the experimental regimen) were available for studies that enrolled more broadly, the subgroup results were used in lieu of the intent-to-treat results.
Table 1.
Reported results of phase III trials included in the meta-analysis
| Trial ID (NCT no.) | Experimental arm therapy | Control arm therapy | Cancer target | Oncogene target | Line of therapy | Experimental Arm |
Control Arm |
PFS HR | OS HR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | ORR | mPFS (months) | mOS (months) | N | ORR | mPFS (months) | mOS (months) | ||||||||
| 009496505 | Afatinib | Pemetrexed/cisplatin | NSCLC | EGFR | 1 | 230 | 0.565 | 11.17 | 28.16 | 115 | 0.226 | 6.90 | 28.22 | 0.576 | 0.880 |
| 0112139311 | Afatinib | Gemcitabine/cisplatin | NSCLC | EGFR | 1 | 242 | 0.678 | 11.01 | 23.10 | 122 | 0.230 | 5.59 | 23.46 | 0.281 | 0.904 |
| 0260434212 | Alectinib | Pemetrexed or docetaxel | NSCLC | ALK | 3 | 79 | 0.506 | 10.9 | 27.8 | 40 | 0.025 | 1.4 | NE | 0.20 | NE |
| 0115414013 | Crizotinib | Pemetrexed/platinum | NSCLC | ALK | 1 | 172 | 0.744 | 10.9 | NE | 171 | 0.450 | 7.0 | 47.5 | 0.454 | 0.76 |
| 0163900114 | Crizotinib | Pemetrexed/platinum | NSCLC | ALK | 1 | 104 | 0.875 | 11.1 | 28.5 | 103 | 0.456 | 6.8 | 27.7 | 0.402 | 0.897 |
| 009328936 | Crizotinib | Pemetrexed or docetaxel | NSCLC | ALK | 2 | 173 | 0.653 | 7.7 | 21.7 | 174 | 0.195 | 3.0 | 21.9 | 0.487 | 0.854 |
| 0122788915 | Dabrafenib | Dacarbazine | Melanoma | BRAF | 1 | 187 | 0.599 | 6.9 | 20.0 | 63 | 0.238 | 2.7 | 15.6 | 0.40 | 0.82 |
| 01000025a10 | Dacomitinib | Placebo | NSCLC | EGFR | 2-4 | 114 | 0.114 | 3.52 | 7.23 | 68 | 0.015 | 0.95 | 7.52 | 0.48 | 0.98 |
| 0134296516 | Erlotinib | Gemcitabine/cisplatin | NSCLC | EGFR | 1 | 110 | 0.627 | 11.0 | 26.3 | 107 | 0.336 | 5.5 | 25.5 | 0.34 | 0.91 |
| 00883779a17 | Erlotinib/gemcitabine/platinum | Placebo/gemcitabine/platinum | NSCLC | EGFR | 1 | 49 | 0.84 | 16.8 | 30.3 | 48 | 0.15 | 6.9 | 20.6 | 0.25 | 0.72 |
| 00322452a8 | Gefitinib | Carboplatin/paclitaxel | NSCLC | EGFR | 1 | 132 | 0.712 | NR | NR | 129 | 0.473 | NR | NR | 0.48 | 0.78 |
| 0154417918 | Gefitinib/pemetrexed/cisplatin | Placebo/pemetrexed/cisplatin | NSCLC | EGFR | 2 | 133 | 0.316 | 5.4 | 14.8 | 132 | 0.341 | 5.4 | 17.2 | 0.86 | 1.62 |
| IRISb9 | Imatinib | Interferon/cytarabine | CML | BCR-ABL | 1 | 553 | 0.953 | NR | NR | 553 | 0.555 | NR | NR | 0.378 | NR |
| 0215198119 | Osimertinib | Pemetrexed/platinum | NSCLC | EGFR T790M | 2 | 279 | 0.706 | 10.1 | 26.8 | 140 | 0.314 | 4.4 | 22.5 | 0.30 | 0.87 |
| 0295974920 | Osimertinib | Docetaxel/bevacizumab | NSCLC | EGFR T790M | 3 | 74 | 0.616 | 10.2 | 15.65 | 73 | 0.083 | 2.95 | 15.29 | 0.23 | 0.79 |
Note: Values are provided in this table as reported on ClinicalTrials.gov or in the reference, with no rounding.
CML, chronic myeloid leukemia; HR, hazard ratio; ID, identification; mOS, median overall survival; mPFS, median progression-free survival; NCT, National Clinical Trial; NE, non-estimable; NR, not reported at the time of the analysis; NSCLC, non-small-cell lung cancer; ORR, objective response rate; OS, overall survival; PFS, progression-free survival.
Data derived from a trial subgroup.
No NCT number.
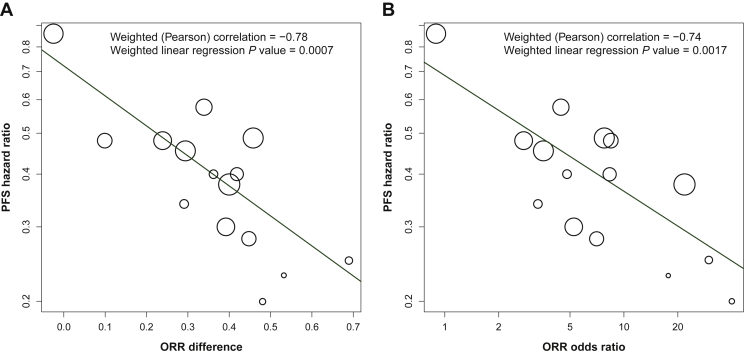
Among the 15 trials, the ORR ranged from 0.015 to 0.555 (1.5%-55.5%) for the control arm and from 0.114 to 0.953 (11.4%-95.3%) for the experimental arm. The PFS hazard ratio ranged from 0.20 to 0.86. The relationship between ORR and PFS was studied by plotting the PFS hazard ratio (y-axis) against two common transformations of the ORR results: the ORR effect size, that is, the difference between the ORR of the two treatment arms (Figure 2A) and the natural logarithm of the ORR odds ratio (Figure 2B). To reflect differences in sample size, the size of each circle was made proportional to the precision associated with the estimate of the log PFS hazard ratio. A strong linear relationship between ORR effect size and associated PFS effect size was apparent in the data. To quantify the strength of this relationship, linear regression models were fit, and weighted Pearson correlation coefficients calculated. The weighted Pearson correlation between the ORR difference and the log PFS hazard ratio was equal to –0.78. Similarly, the weighted correlation between the log ORR odds ratio and the log PFS hazard ratio was –0.74, demonstrating the robustness of the correlation. For both ORR transformations, the slope parameter in the linear regression was strongly statistically significant; the two-sided P value for the ORR difference model was 0.0007 and the corresponding P value for the ORR log odds ratio model was 0.0017.
Figure 2.
Scatter plot of the relationship of the PFS hazard ratio with ORR difference and ORR odds ratio.
For each of 15 trials, (A) the difference of the ORR between the two treatment arms was plotted against the log of the PFS HR, and (B) the log of the ORR odds ratio was plotted against the log of the PFS HR. Each circle represents the ORR/PFS effect sizes for an individual clinical trial. For both plots, the relative size of each circle is based on the standard error associated with the reported hazard ratio. The weighted least squares regression lines (solid blue lines) are plotted in both panels.
ORR, objective response rate; PFS, progression-free survival.
Thirteen of the 15 trials used for this meta-analysis also had published OS data. Median OS values (months) and the OS hazard ratio were recorded in Table 1 for each of the trials. To examine the relationship between the treatment effect on ORR and OS, the difference between the ORR of the two treatment arms was plotted against log OS hazard ratio (Figure 3A). Like the corresponding PFS data, the resultant scatter plot showed a positive correlation. The weighted Pearson correlation was –0.67, and the weighted linear regression P value was 0.013. Likewise, log OS HR correlated with the ORR odds ratio (Figure 3B). The weighted Pearson correlation was –0.58, and the weighted linear regression P value was 0.036. It is important to note, however, that study 01 544 17918 (a second-line NSCLC trial which evaluated the addition of gefitinib to chemotherapy versus chemotherapy alone) carries high leverage within the correlative analyses of ORR/OS. Indeed, removal of this study (which appears in the upper-left corner of both Figure 3 plots) from the ORR/OS analyses results in a substantial decrease in the magnitude of correlation and a loss of statistical significance (weighted correlation between log OS HR and ORR difference is –0.37, P = 0.2355; weighted correlation between log OS HR and log odds ratio is –0.07, P = 0.8247). By contrast, removal of this study from the ORR/PFS analyses does not result in a loss of statistical significance for the correlative analyses of ORR/PFS significance (weighted correlation between log PFS HR and ORR difference is –0.58, P = 0.0292; weighted correlation between log PFS HR and log odds ratio is –0.53, P = 0.0497).
Figure 3.
Scatter plot of the relationship of the OS hazard ratio with ORR difference and ORR odds ratio.
For the 13 trials with OS data, (A) the difference of the ORR between the two treatment arms was plotted against the log of the OS hazard ratio, and (B) the log of the ORR odds ratio was plotted against the log of the OS hazard ratio. Each circle represents the ORR/OS effect sizes for an individual clinical trial. For both plots, the relative size of each circle is based on the precision associated with the reported PFS hazard ratio. The weighted least squares regression lines (solid blue lines) are plotted in both panels.
ORR, objective response rate; OS, overall survival.
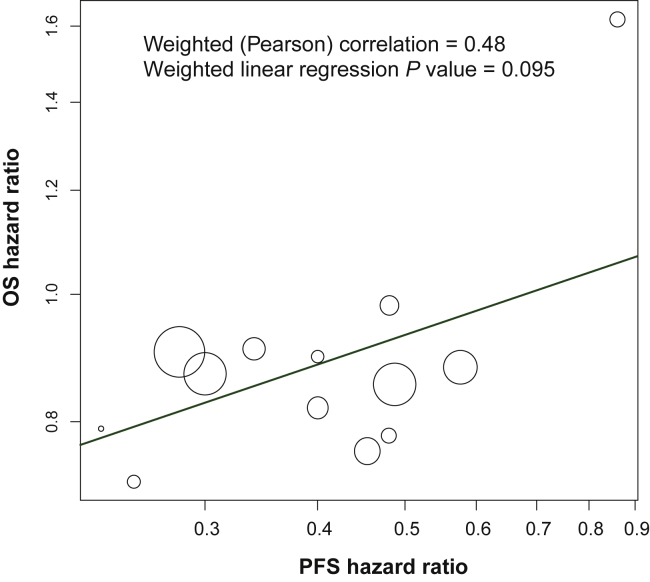
To explore the relationship between PFS and OS, a scatter plot was constructed of PFS HR versus OS HR displayed on the log scale (Figure 4). The weighted Pearson correlation was 0.48 with a weighted linear regression P value of 0.095, indicating no statistically significant correlation between the two efficacy measures, although a possible trend in that direction.
Figure 4.
Scatter plot of the relationship of the PFS hazard ratio with OS hazard ratio.
The PFS hazard ratio was plotted against the OS hazard ratio for the 13 trials with both PFS and OS data. The relative size of each circle is based on the precision associated with the reported OS hazard ratios. The weighted least squares regression line (solid blue line) is shown.
OS, overall survival; PFS, progression-free survival.
Discussion
The meta-analysis results presented here demonstrate a correlation between ORR and PFS effects in randomized clinical trials investigating the comparative efficacy between targeted agents and nontargeted treatment options in molecularly enriched patient populations. The potential selection bias from including in the meta-analysis phase III studies that had already been deemed to be promising (typically based on high response rates in earlier trials) is not expected to be consequential in this setting: scenarios to which the results of this paper are applicable are cases of early-phase studies in which a strong ORR treatment effect is observed, or in which the ORR clearly exceeds a threshold expected under standard of care. Therefore the range of response rate outcomes that one would like to translate to a corresponding range of effects on PFS or OS is confined already to a set of promising outcomes. The linear relationship observed when the ORR effect size (either the difference between ORR of the two treatment arms or the ORR odds ratio) was plotted against the PFS hazard ratio indicates that the magnitude of the treatment effect on ORR was proportional to the treatment effect on PFS. These results may support the use of ORR as an initial efficacy endpoint in trials assessing targeted therapeutic agents. Moreover, a better understanding of the relationship between ORR and PFS could be informative in the planning and design of future trials in a molecularly enriched cancer patient setting by enabling prediction of a potential treatment effect size expected for PFS. For instance, this prediction could be based on an inferred ORR effect calculated as the difference between the response rate observed from an early-phase single-arm study and the putative response rate from an appropriate historical control cohort. Two key limitations of such an approach are (i) the typical challenges inherent in cross-trial comparisons, and (ii) the fact that the only available historical control data may be derived from an unselected population, one not reflecting the genomic alteration of interest. In addition, the studies analyzed were not a systematically chosen set. For feasibility purposes, a select number of oncogenic driver-kinase inhibitor pairs were chosen based on substantial activity (high ORR and durable PFS), maturity of follow-up (e.g. drivers discovered earlier than others like ROS1/RET/NTRK were included here), and relative frequency (i.e. the 1% events like ROS1 were not included).
As a clinical trial endpoint, ORR directly measures the antitumor activity of the intervention, because spontaneous tumor regression is infrequent. The correlation of ORR with PFS, an established trial endpoint, is of interest because ORR can be assessed very rapidly. Targeted agents will typically achieve a tumor response in molecularly selected patients within 2-3 months because the treatment targets the driver of an oncogene-addicted cancer. For example, the LIBRETTO-001 trial that assessed efficacy of selpercatinib in patients with metastatic RET fusion-positive NSCLC recorded a median time to response of 1.8 months—timing that corresponded to the first radiological assessment at 8 weeks.21
Investigation of the correlation between ORR and OS effects also identified a positive association, but it was weaker and less robust to potential outlying observations. In addition, there was no significant association between treatment effects on OS and PFS, seemingly in accord with the weak ORR/OS association. Other reported analyses have also failed to observe a positive correlation between ORR and OS.7,22,23 For example, a meta-analysis of 14 trials (dated 2003-2013) of new treatments for NSCLC by Blumenthal and colleagues7 noted that the association between the ORR odds ratio and OS HR was weak (R2 = 0.09, 95% CI 0-0.33).7 By contrast, there was a strong correlation between the ORR odds ratio and PFS HR in the same analysis: R2 = 0.89 (95% CI 0.80-0.98). The authors reasoned that the lack of correlation between ORR and OS might be due to confounding factors such as treatment crossover following progression, number of subsequent therapies, long postprogression survival in the first-line setting, and noncancer deaths. Interestingly, the trials upon which our OS meta-analysis were based included 10 of 13 trials with a high degree of crossover (up to 85%) and 8 of 13 trials that tested front-line treatments. Perhaps because our analysis focused exclusively on targeted therapies tested in molecularly enriched populations with corresponding large effect sizes relative to the control, detection of a weak, but positive correlation between ORR and OS efficacy endpoints was possible despite the confounding factors.
The meta-analysis detailed here focused exclusively on assessing ORR (difference and odds ratio) as a surrogate endpoint for PFS and OS, based on randomized phase III trials investigating targeted agents in corresponding molecularly enriched populations. The evolution and phase III trial testing of targeted therapeutics led to our meta-analysis identifying 15 trials, of which 13 focused on lung cancer. A greater number of trials and ones representing a wider range of cancer types would have provided a more robust analysis. However, we believe it is biologically plausible to extrapolate from these meta-analysis results to other trials investigating targeted agents and selected populations. In particular, ORR and PFS are both tumor-based endpoints, so it is reasonable to believe that the high ORR associated with successful targeted agents would be associated with protracted PFS. The meta-analysis by Blumenthal and colleagues led to a similar conclusion.7
As additional targeted agents are developed, a high ORR identified in an early-phase single-arm trial could form the basis of an initial regulatory approval, especially when there are limited patient numbers with the driver alteration of interest. Because ORR can be assessed in single-arm trials and then compared with historic controls, ORR has been used as a surrogate endpoint for accelerated approvals, with the requirement for later confirmation of clinical benefits in randomized trials. Our findings further warrant the use of ORR as an early surrogate for PFS in biomarker-driven studies.
Acknowledgements
Eli Lilly and Company funded this analysis and the medical writing assistance, provided by Mary Dugan Wood.
Funding
Eli Lilly and Company (no grant number).
Disclosure
BJS reports personal fees from Loxo Oncology, during the conduct of the study; personal fees from AstraZeneca, Novartis, Roche/Genentech, Bristol Myers Squibb, Merck, Gritstone Oncology, Amgen, Sanofi/Regeneron, and grants and personal fees from Pfizer, all outside the submitted work. HHL reports personal fees for Advisory Board participation from Bayer, Boehringer Ingelheim, Celgene, and Eli Lilly; personal fees for Advisory Board and Speaker’s Bureau participation and research funding from Novartis; personal fees for Speaker’s Bureau participation from Guardant Health and Eisai; travel support and research funding from Merck; research funding from Mundipharma, and travel support from Pfizer. YS reports personal fees from Eli Lilly, Roche, AstraZeneca, Takeda, Pfizer, and Merck. ZMT, PF, BKL, and AS report employment and stock ownership with Eli Lilly. JW reports Advisory Board and lecture fees from AbbVie, Amgen, AstraZeneca, Blueprint, Bristol Myers Squibb, Boehringer Ingelheim, Chugai, Ignyta, Janssen, Eli Lilly, Loxo Oncology, Merck, Novartis, Pfizer, Roche, and Takeda; and research support to the institution from Bristol Myers Squibb, Johnson and Johnson, Novartis, and Pfizer. JC-HY reports honoraria for speeches and Advisory Boards from Boehringer Ingelheim, Eli Lilly, Bayer, Roche/Genentech, Chugai, Merck, Pfizer, Novartis, Bristol Myers Squibb, and Ono Pharmaceutical; and honoraria for Advisory Boards from AstraZeneca, Astellas, Merck Serono, Celgene, Merrimack, Yuhan Pharmaceuticals, Daiichi Sankyo, Hansoh, Takeda, and Blueprint Medicines. AD reports honoraria from Advisory Board participation from Ignyta/Genentech/Roche, Loxo Oncology/Bayer/Eli Lilly, Takeda/Ariad/Millenium, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Helsinn, BeiGene, BerGenBio, Hengrui Therapeutics, Exelixis, Tyra Biosciences, Verastem, MORE Health, AbbVie, 14ner/Elevation Oncology, Remedica Ltd., ArcherDX, Monopteros, Novartis, EMD Serono, Melendi, Liberum, Repare therapeutics; associated research support paid to the institution by Pfizer, Exelixis, GlaxoSmithKline, Teva, Taiho, PharmaMar; CME honoraria from Medscape, OncLive, PeerVoice, Physicians Education Resources, Targeted Oncology, Research to Practice, Axis, PeerView Institute, Paradigm Medical Communications, WebMD, MJH Life Sciences; royalties from Wolters Kluwer; and other support from Merck, Puma, Merus, and Boehringer Ingelheim.
References
- 1.Lindeman N.I., Cagle P.T., Aisner D.L., et al. Updated testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors (2018) Arch Pathol Lab Med. 2018;142:321–346. doi: 10.5858/arpa.2017-0388-CP. [DOI] [PubMed] [Google Scholar]
- 2.Subbiah V., Cote G.J. Advances in targeting RET-dependent cancers. Cancer Discov. 2020;10:498–505. doi: 10.1158/2159-8290.CD-19-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim E.S., Hirsh V., Mok T., et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372:1809–1818. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 4.Rosell R., Carcereny E., Gervais R., et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 5.Sequist L.V., Yang J.C., Yamamoto N., et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 6.Shaw A.T., Kim D.W., Nakagawa K., et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. New Engl J Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 7.Blumenthal G.M., Karuri S.W., Zhang H., et al. Overall response rate, progression-free survival, and overall survival with targeted and standard therapies in advanced non–small-cell lung cancer: US Food and Drug Administration trial-level and patient-level analyses. J Clin Oncol. 2015;33:1008–1014. doi: 10.1200/JCO.2014.59.0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mok T.S., Wu Y.L., Thongprasert S., et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. New Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien S.G., Guilhot F., Larson R.A., et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. New Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 10.Ellis P.M., Shepherd F.A., Millward M., et al. Dacomitinib compared with placebo in pretreated patients with advanced or metastatic non-small-cell lung cancer (NCIC CTG BR. 26): a double-blind, randomised, phase 3 trial. Lancet Oncol. 2014;15:1379–1388. doi: 10.1016/S1470-2045(14)70472-3. [DOI] [PubMed] [Google Scholar]
- 11.Wu Y.L., Zhou C., Hu C.P., et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–222. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 12.Novello S., Mazieres J., Oh I.J., et al. Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Ann Oncol. 2018;29:1409–1416. doi: 10.1093/annonc/mdy121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solomon B.J., Mok T., Kim D.W., et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 14.Wu Y.L., Lu S., Lu Y., et al. Results of PROFILE 1029, a phase III comparison of first-line crizotinib versus chemotherapy in East Asian patients with ALK-positive advanced non-small cell lung cancer. J Thorac Oncol. 2018;13:1539–1548. doi: 10.1016/j.jtho.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Hauschild A., Grob J.J., Demidov L.V., et al. An update on BREAK-3, a phase III, randomized trial: dabrafenib (DAB) versus dacarbazine (DTIC) in patients with BRAF V600E-positive mutation metastatic melanoma (MM) J Clin Oncol. 2013;31(15 suppl):9013. [Google Scholar]
- 16.Wu Y.L., Zhou C., Liam C.K., et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26:1883–1889. doi: 10.1093/annonc/mdv270. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y.L., Lee J.S., Thongprasert S., et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol. 2013;14:777–786. doi: 10.1016/S1470-2045(13)70254-7. [DOI] [PubMed] [Google Scholar]
- 18.Soria J.C., Wu Y.L., Nakagawa K., et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol. 2015;16:990–998. doi: 10.1016/S1470-2045(15)00121-7. [DOI] [PubMed] [Google Scholar]
- 19.Papadimitrakopoulou V.A., Mok T.S., Han J.Y., et al. Osimertinib versus platinum-pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann Oncol. 2020;31:1536–1544. doi: 10.1016/j.annonc.2020.08.2100. [DOI] [PubMed] [Google Scholar]
- 20.Nie K., Zhang Z., Zhang C., et al. Osimertinib compared docetaxel-bevacizumab as third-line treatment in EGFR T790M mutated non-small-cell lung cancer. Lung Cancer. 2018;121:5–11. doi: 10.1016/j.lungcan.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Drilon A., Oxnard G.R., Tan S.W.D., et al. Efficacy of selpercatinib in RET fusion-positive non-small cell lung cancer. New Engl J Med. 2020;383:813–824. doi: 10.1056/NEJMoa2005653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiteni F., Westeel V., Pivot X., Borg C., Vernerey D., Bonnetain F. Endpoints in cancer clinical trials. J Visc Surg. 2014;151:17–22. doi: 10.1016/j.jviscsurg.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Buyse M., Sargent D.J., Grothey A., Matheson A., De Gramont A. Biomarkers and surrogate end points—the challenge of statistical validation. Nature Rev Clin Oncol. 2010;7:309–317. doi: 10.1038/nrclinonc.2010.43. [DOI] [PubMed] [Google Scholar]