Abstract
Background
Adrenocortical carcinoma (ACC) is a rare and aggressive malignancy with a poor prognosis. No efficacious treatment options are currently available for patients with advanced metastatic disease with disease progression to standard etoposide, doxorubicin, cisplatin and mitotane (EDP-M) therapy. We assessed the activity and tolerability of cabazitaxel as a second/third-line approach in metastatic ACC.
Patients and methods
Patients included in this single-center, phase II study (ClinicalTrials.gov identifier NCT03257891) had disease progression to a cisplatin-containing regimen (such as EDP) plus mitotane, plus/minus a further chemotherapy line. Cabazitaxel was administered intravenously at 25 mg/m2 on day 1 of a 21-day cycle, for a maximum of six cycles. The primary endpoint was a disease control rate after 4 months.
Results
From March 2018 to September 2019, 25 eligible patients were enrolled. A disease control rate after 4 months was obtained in six patients (24%). No patients attained a disease response according to RECIST 1.1, 9 patients (36%) had stable disease and 16 patients (64%) progressive disease. Median progression-free survival and overall survival were 1.5 months (range 0.3-7 months) and 6 months (range 1-22.2 months), respectively. Cabazitaxel therapy was well tolerated and only three (12%) patients developed grade 3 toxicity which were nausea in one patient (4%) and anemia in two patients (8%).
Conclusions
Cabazitaxel has a manageable toxicity profile but is poorly active as second/third-line treatment in advanced ACC patients. These results do not support further evaluation of cabazitaxel in this setting.
Key words: adrenocortical cancer, advanced, pretreated, cabazitaxel
Highlights
-
•
Cabazitaxel is well tolerated but poorly active as second/third-line treatment in patients with advanced ACC.
-
•
Combining RECIST and Choi criteria could be of value in the assessment of disease response to chemotherapy in ACC patients.
-
•
Older age, elevated cortisol and LDH blood level correlate with a greater risk of death in multivariate analysis.
Introduction
Adrenocortical carcinoma (ACC) is a rare and aggressive tumor with an incidence of 0.7-2 new cases per million populations per year. Early diagnosis followed by radical surgical resection are the only options that can give ACC patients a chance of cure. Adjuvant therapy with mitotane can be prescribed in selected cases.1, 2, 3, 4 The standard systemic treatment of advanced/metastatic ACC patients, not eligible for surgery, is mitotane, which is administered either alone or in combination with etoposide, doxorubicin and cisplatin (EDP-M regimen).5, 6, 7 Although some pathological responses have been observed, the efficacy of EDP-M is limited and most initially responding patients are destined to relapse and die of disease.6,8 Other cytotoxic therapies, administered to patients with disease progression to EDP-M, did not show remarkable activity.9, 10, 11 Molecular target therapies, attempted up to now, appeared unefficacious,12 while immunotherapy with modern immune checkpoint inhibitors has shown some promising, results;13 however, additional studies are needed, and strategies to overcome mechanisms of primary immune resistance of ACC should be implemented.14,15 Due to their demonstrated efficacy in the management of several malignancies, taxanes have also been tested in ACC.16 A preclinical study has shown an interesting antineoplastic effect of paclitaxel against ACC cells.17 Published clinical trials, however, did not confirm the efficacy of either docetaxel in combination with cisplatin in a first-line setting or paclitaxel in combination with sorafenib in ACC patients with disease progression to EDP-M.18,19 The multidrug resistant 1 (MDR1) gene, which encodes for the expression of p-glycoprotein (Pgp), an energy-dependent transporter protein that can shuttle cytotoxic drugs out of the cell, is a major cause of chemotherapy failure in cancer treatment, including taxanes. High levels of expression of MDR1/Pgp are found in both the normal adrenal gland and adrenocortical cancer.20,21 Cabazitaxel is a novel tubulin-binding taxane that differs from other taxanes because of its poor affinity for Pgp.22 This drug has demonstrated antitumour activity in tumor cell models resistant to paclitaxel and docetaxel.23 The results of the phase III TROPIC study24 showed a clear superiority of cabazitaxel over mitoxantrone in terms of survival in metastatic castration-resistant prostate cancer patients with disease progression to docetaxel. The efficacy of cabazitaxel in MDR1 expressing tumors provides a rationale for testing this drug in the treatment of ACC patients. In a recently published in vitro study, our group has demonstrated that cabazitaxel is active in reducing ACC cell viability, both in ACC cell lines and in ACC primary cell cultures, and its cytotoxic effect is not modified by pharmacologically targeting MDR1/Pgp.25 On the basis of the abovementioned rationale and our preclinical data, we designed and conducted the cabACC trial, a phase II, prospective, nonrandomized, open label, single-arm study aiming to test the activity of cabazitaxel in advanced ACC patients with disease progression to EDP-M.
Patients and methods
Study design and participants
The cabACC trial (ClinicalTrials.gov identifier: NCT03257891, Eudract number: 2017-001591-35) is a prospective, nonrandomized, open label, single-arm, phase II study in which cabazitaxel was administered as second/third-line therapy in advanced patients with ACC, consecutively recruited at the Medical Oncology Unit of the ASST-Spedali Civili, University of Brescia, Italy. To be enrolled in this trial, the patients had to meet the following inclusion criteria: pathological diagnosis of ACC; locally advanced or metastatic disease not suitable for surgery (stage III-IV); radiologically measurable disease according to RECIST criteria; age 18 years or older; Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0-2; life expectancy of at least 3 months; adequate baseline end organ function including absolute neutrophil count >1500/ml, platelets >100 000/ml, hemoglobin >9.0 g/dl (without transfusion within 7 days); total bilirubin <1.5 upper limit of normal (ULN); aspartate aminotransferase/alanine aminotransferase (AST/ALT) ≤1.5 × ULN or AST/ALT ≤5 × ULN if liver function abnormalities are due to the underlying malignancy; serum creatinine <1.5 ULN; effective contraception in premenopausal female and male patients; written informed consent and ability to comply with the protocol procedures. Mitotane should have been stopped at least 1 month before the study entry, but it could be continued in patients with cortisol-secreting ACC.
Main exclusion criteria were the following: history of prior malignancy, except for cured nonmelanoma skin cancer and cured in situ cervical carcinoma; active clinically serious infections; symptomatic metastatic brain or meningeal tumors; seizure disorder requiring medication; decompensated heart failure (ejection fraction >45%); myocardial infarction or revascularization procedure during the last 6 months; unstable angina pectoris; uncontrolled cardiac arrhythmia; hypertension not controlled by medications; pregnant or breast-feeding condition; any other severe acute or chronic medical or psychiatric condition, or laboratory abnormality that would have imparted, in the judgment of the investigator, excess risk associated with study participation or study drug administration, or which, in the judgment of the investigator, would have made the patient inappropriate for entry into this study.
The disease stage according to modified European Network for the Study of Adrenal Tumors (mENSAT) classification,26 tumor Grade, Resection status, Age of patients and presence of Symptoms at ACC diagnosis (accordigly to the GRAS parameters),27,28 resection status of the primary tumor, and previous ACC-correlated medical and surgical treatments were collected.
This investigator-driven study was approved by the Ethical Review Board of ASST-Spedali Civili in Brescia (Protocol number: 2729) and was designed and conducted in accordance with Good Clinical Practice and the Declaration of Helsinki. Written consent was obtained from each patient.
Treatment administered
Cabazitaxel was administered intravenously in 1 h at the dose of 25 mg/m2 every 21 days. The drug was provided by Sanofi-Aventis S.p.A, (Milano, Italy). Treatment was continued until disease progression, unacceptable toxicity, investigator’s decision, or consent withdrawal. Premedications and antiemetic prophylaxis were recommended as per institutional guidelines.
All patients received a prophylactic subcutaneous injection of long-acting granulocyte colony-stimulating factor (G-CSF) 48 h after each cabazitaxel administration.
Cabazitaxel treatment was delayed in cases of grade 3-4 neutropenia, persisting for >7 days and/or without recovery on day 21. Cabazitaxel dose reduction of 20% was allowed in case of dose-limiting toxicity defined as: grade 4 neutropenia lasting >5 days; grade 3/4 febrile neutropenia; grade 3 thrombocytopenia and non-hematologic toxicity >grade 2, excluding nausea, vomiting, fatigue, and alopecia. Reduced cabazitaxel dose was not re-escalated.
Adverse events were monitored throughout the study and reported using National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.03.Physical examination, PS, routine laboratory tests, endocrine work-up, and mitotane plasma levels were evaluated at baseline and during cabazitaxel treatment.
Disease re-staging by computed tomography (CT) scan and/or magnetic resonance imaging was carried out every 8 weeks for the first 4 months and every 12 weeks afterwards until disease progression or patient drop-out from the study.
In case of documented disease progression, the study was interrupted and the patients were followed for survival.
The study was conducted in a single center and all radiological images were evaluated centrally by an experienced radiologist (R.A.) who carried out three-dimensional semiautomatic segmentation of target lesions at CT carried out at baseline and after four chemotherapy cycles, using a dedicated software (Multi-Modality Tumor Tracking, Philips IntelliSpace Portal, version 10; Philips Healthcare, Best, The Netherlands). Disease response was assessed according to RECIST 1.1 and Choi criteria as described in the preceding text.29
Outcomes
The primary aim was to assess cabazitaxel activity in terms of progression-free survival (PFS) at 4 months, as measured by the proportion of non-progressing patients after 4 months of treatment using the RECIST 1.1 criteria.
Secondary aims were the impact of cabazitaxel treatment on other activity and efficacy endpoints, such as the objective response rate, evaluated by RECIST and Choi (in which a response is based on decrease in tumor size ≥10% or decrease in tumor density ≥15% on CT); hormone response in patients with secreting ACC; PFS, defined as the time from patient enrollment in the trial to disease progression or death from any cause; overall survival (OS), defined as the time from patient enrollment in the trial to death due to any cause; quality of life, assessed by the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30; and toxicity, assessed by NCI-CTCAE version 4.03 criteria. As mitotane notoriously interferes with the metabolism of several drugs,30 an ancillary study was conducted to assess the pharmacokinetic profile of cabazitaxel in relation to serum mitotane levels.
Cabazitaxel quantification by high-performance liquid chromatography–tandem mass spectrometry in patient plasma
Sample preparation and liquid chromatography-tandem mass spectrometry (LC-MS/MS) was carried out according to de Bruijn et al.30 with minor modifications. Ultra performance LC was carried out using the DionexTM UltiMateTM 3000 system (Thermo Scientific, Milan, Italy) equipped with an LPG-3400SD quaternary analytical pump, a WPS-3000SL analytical autosampler, and a TCC-3000SD thermostatted column compartment. An isocratic mobile phase was used with 50% of each phase and a runtime of 20 min. The cabazitaxel blood concentration was assessed during the first cycle infusion at baseline, and after 30 min, 55 min, 24 h, 48 h, and 96 h. Mass transitions of m/z were optimized for cabazitaxel (858→555) and the internal standard 2H6-cabazitaxel (864→561). Collision-induced dissociation with Multi-stage Mass Spectrometry (CID-MSn) experiments were carried out on an electrospray ionization tandem mass spectrometer (LCQ Fleet Ion Trap MSn, Thermo Scientific), set as reported in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100422. The calibration curves were obtained using 100 μl of negative control plasma. The residues were resuspended at the final concentrations of 5 ng/ml-500 ng/ml cabazitaxel and of 5-250 mg/ml 2H6-cabazitaxel.
Sample size and statistical analysis
The primary endpoint of the study was to estimate PFS at a fixed time point of 4 months. PFS rate was evaluated in all the patients registered in the study, according to the intent-to-treat principle. The size of this trial was determined using the optimal two-stage phase II study design by Simon.31 Accordingly, the sample size was assessed in order to refuse a PFS rate of 15% (p0) after 4 months and to provide a statistical power of 80% in assessing the activity of the regimen asPFS rate after 4 months of 40%. The upper limit for the first-stage drug rejection was one non-progressing patient after 4 months out of the first consecutive seven. The upper limit of the second-stage rejection was six non-progressing patients after 4 months out of 25 consecutively enrolled patients. Descriptive statistics were carried out through means and range for continuous variables or frequencies and percentages for categorical variables. The Kolmogorov–Smirnov and Shapiro–Wilk tests were used to assess whether continuous variables were normally distributed. The Student’s t-test or the corresponding non-parametric Mann–Whitney U test was used, when appropriate, for group comparisons. Correlations between variables were assessed with the Pearson test.
Survival curves were calculated with the Kaplan–Meier method and compared with the log-rank test. The Cox proportional hazard model was used to assess the hazard ratio (HR) of progression and death both in univariate and multivariate analyses. All tests were two-sided and P < 0.05 was considered statistically significant. Statistical analysis was carried out using SPSS (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.) and R [R Core Team (2019). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria]. We considered results as statistically significant only for P value <0.05.
Results
Patient characteristics
From March 2018 to September 2019, 25 consecutive patients with advanced ACC, meeting the eligibility criteria, entered the study. The characteristics of the enrolled patients are summarized in Table 1. Median age was 50 years (20-69), female/male ratio was 2.6 (18/7) and 15 patients (60%) had hormone hypersecreting ACC at diagnosis.
Table 1.
Patients characteristics
| Patient characteristics | Number | (%), [range] |
|---|---|---|
| Patients enrolled | 25 | |
| Median age at cabACC start, years | 50 | [20-69] |
| Male | 7 | (28) |
| Death | 25 | (100) |
| Clinical presentation at ACC diagnosis | ||
| Hormone hypersecretion | 15 | (60) |
| Mass compression | 6 | (24) |
| Incidentaloma | 4 | (16) |
| Hormone hypersecretion at ACC diagnosis | ||
| Clinically relevant | 13 | (52) |
| Cortisol with other hormones | 12 | (48) |
| Cortisol alone | 4 | (16) |
| Cortisol + aldosterone | 3 | (12) |
| Cortisol + androgens | 1 | (4) |
| Androgens + aldosterone + cortisol | 2 | (8) |
| Cortisol + androgens + estrogens | 2 | (8) |
| Androgens alone | 2 | (8) |
| Estrogens alone | 0 | (0) |
| Androgens + aldosterone | 1 | (4) |
| Primary surgery | 23 | (92) |
| R0 | 20 | 20 (80) |
| Median RFS after R0 surgery (months) | 8.4 | [0.9-74.3] |
| Mitotane adjuvant | 18 | (72) |
| Weiss score, median 7 [2-8] | ||
| ≥7 | 11 | (44) |
| Ki67, median 20 [5-50] | ||
| ≥20 | 20 | (80) |
| GRAS score | ||
| 1 | 6 | (24) |
| 2 | 15 | (60) |
| 3 | 4 | (16) |
| ENSAT stage at ACC diagnosis | ||
| I-II | 11 | (44) |
| III-IV | 14 | (56) |
| Synchronous metastasis at ACC diagnosis | ||
| Liver metastasis | 5 | (20) |
| Lung metastasis | 7 | (28) |
| More than 2 sites | 2 | (8) |
| First-line chemotherapy treatment | ||
| EDP | 23 | (92) |
| Cisplatin alone | 2 | (8) |
| Best response after first line | ||
| PR | 7 | (28) |
| PD | 9 | (36) |
| SD | 9 | (36) |
| PFSm | 6 | (1-16) |
| Types of second chemotherapy line | ||
| Cisplatin based | 2 | (8) |
| Gemcitabine-capecitabine | 7 | (28) |
| Taxane | 2 | (8) |
| Types of third chemotherapy line | ||
| Cisplatin based | 3 | (12) |
| Gemcitabine-capecitabine | 1 | (4) |
| Carboplatin | 1 | (4) |
| Temozolomide | 1 | (4) |
| Chemotherapy lines before cabACC, median 2 [1-3] | ||
| 1 Line | 25 | (100) |
| 2 Lines | 11 | (44) |
| 3 Lines | 6 | (24) |
| cabACC description | ||
| Number of cabACC cycles, median 2 | ||
| 6 Cycles | 5 | (20) |
| 5 Cycles | 3 | (12) |
| 4 Cycles | 4 | (16) |
| 3 Cycles | 2 | (8) |
| 2 Cycles | 6 | (24) |
| 1 Cycle | 5 | (20) |
| mENSAT stage at cabACC start | ||
| IVA | 9 | (36) |
| IVB | 11 | (44) |
| IVC | 5 | (20) |
| Metastasis at cabACC start | ||
| Liver metastasis | 17 | (68) |
| Lung metastasis | 19 | (76) |
| More than 2 sites | 16 | (64) |
| Hormone hypersecretion symptoms at cabACC start | ||
| Hypertension | 1 | (4) |
| Cortisol-related | 1 | (4) |
| BMI at cabACC, median 24 | ||
| <24 | 13 | 52 |
| >24 | 12 | 48 |
| Mitotane concentration during cabACC (until first cycle) | ||
| <14 mg/l | 18 | (72) |
| ≥14 mg/l | 7 | (28) |
| Charlson’s Comorbidity Index (CCI) at cabACC start | ||
| Score 6 | 19 | (76) |
| Score 7 | 4 | (16) |
| Score 8 | 1 | (4) |
| Score 9 | 1 | (4) |
| ECOG PS at cabACC start | ||
| 1 | 7 | (28) |
| 2 | 6 | (24) |
| Albumin at cabACC start | ||
| Low level (<3.22 g/l) | 17 | (68) |
| Hemoglobin (Hb) at cabACC start | ||
| Low level | 18 | (72) |
| NLR, median 1.9 [0.9-0.7] | ||
| ≥1.9 | 14 | (46) |
| ≥5 | 4 | (16) |
| dNLR, median 1.6 [0.6-5.6] | ||
| ≥1.6 | 13 | (52) |
| ≥3 | 3 | (12) |
| LDH | ||
| Upper limits of normal | 12 | (48) |
| ALP | ||
| Upper limits of normal | 15 | (60) |
ACC, adrenocortical carcinoma; ALP, alkaline phosphatase; BMI, body mass index; dNLR, derived neutrophil-to-lymphocyte ratio; ECOG PS, Eastern Cooperative Oncology Group performance status; EDP, etoposide, doxorubicin, cisplatin; GRAS, grade, resection status, age, symptoms; LDH, lactate dehydrogenase; mENSAT, modified European Network for the Study of Adrenal Tumors; PD, progressive disease; PFSm, median progression-free survival; PR, partial response; RFS, relapse free survival; SD, stable disease.
Twenty-three patients (92%) underwent primary surgery as the first treatment and 20 of them (80%) obtained a complete resection (R0). Adjuvant mitotane was administered in 18 patients (72%).
Twenty patients (80%) had a Ki67 value >20% at diagnosis. Six patients were classified as having a GRAS score of 1, 15 (60%) GRAS 2 (intermediate) and 4 (16%) GRAS 3 (pejorative). Median Weiss score was 8 (range 3-9). Median disease-free survival of the surgical treated patients was 8.4 months (range 0.9-74.3). The first-line treatment of metastatic disease was mitotane associated with either cisplatin alone in 2 (8%) patients, or EDP in 23 (92%) patients.
Seven patients (28%) had received a second chemotherapy line (consisting of gemcitabine plus capecitabine or platinum-based chemotherapy) and six patients (24%) a third chemotherapy line (consisting of cisplatin or carboplatin, temozolomide, gemcitabine plus capecitabine), respectively.
At baseline, 18 patients (72%) presented an ECOG PS ≤1. A rechallenge with cisplatin or carboplatin was adopted in patients who achieved a sustained response to first-line EDP (>6 months). Rechallenge with these drugs was considered as further-line therapy.
Seven patients (28%) had mitotane blood levels within the therapeutic range (≥14 mg/l) at the time of the study entry.
According to the mENSAT classification,26 9 patients (36%) had a stage IVA disease, 11 (44%) stage IVB and 5 (20%) stage IVC. With regards to routine clinical chemistry parameters with common prognostic significance: albumin levels below the normal cut-off were observed in 10 patients (40%), 12 (48%) patients had ULN lactate dehydrogenase (LDH) levels and 15 (60%) patients ULN of alkaline phosphatase levels.
At the cabACC start, four patients (16%) had a neutrophil-to-lymphocyte ratio (NLR) ≥5 and three patients (12%) had a derived NLR ≥3, the cut-offs most often indicated in the literature.
Treatment administered and patient outcome
A total of 80 cabazitaxel cycles were administered. The patients received a median number of two cycles (range 1-6). A total of 5 patients (20%) completed the treatment plan (six cycles), 3 patients (12%) received five cycles, 4 patients (16%) four cycles and the remaining 13 patients (52%) received three cycles or less (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100422).
Cabazitaxel treatment was interrupted due to radiological disease progression in 11 patients (44%), eligibility for local treatment of the residual disease in 2 patients (i.e. redo surgery 1 patient and liver embolization 1 patient) after four and six cycles, respectively (8%) and clinical deterioration because of the disease progression in 12 patients (48%).
Disease control after 4 months was obtained in six patients (24%). No patients attained a disease response according to RECIST 1.1, 9 patients (36%) had stable disease and 16 patients (64%) progressive disease (PD).
A total of 18 patients (72%) were fully assessable for Choi response criteria: a partial response was observed in 10 patients (55.5%), 7 patients (39%) had stable disease and 1 patient (5.5%) had PD.
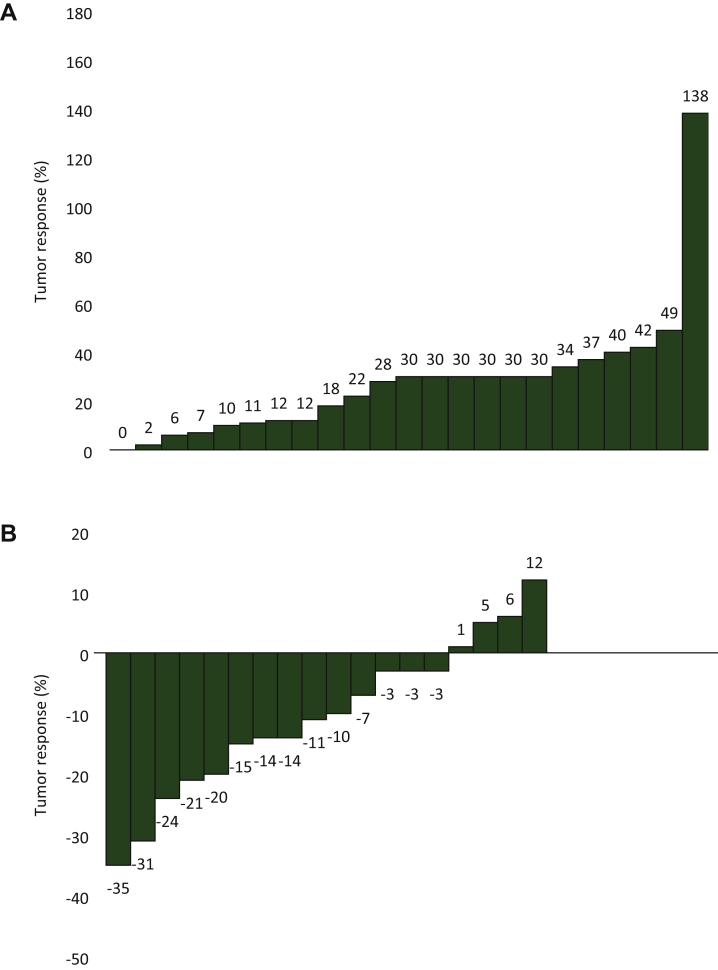
The waterfall diagrams of percentage change in tumor variation after treatment at first disease assessment, according to RECIST 1.1 and Choi criteria, are depicted in Figure 1. Noteworthy, no patient experienced tumor shrinkage of any magnitude; in all patients there was an increase in the size of the target neoplastic lesions.
Figure 1.
(A) Tumor response according to RECIST. (B) Tumor response according to Choi.
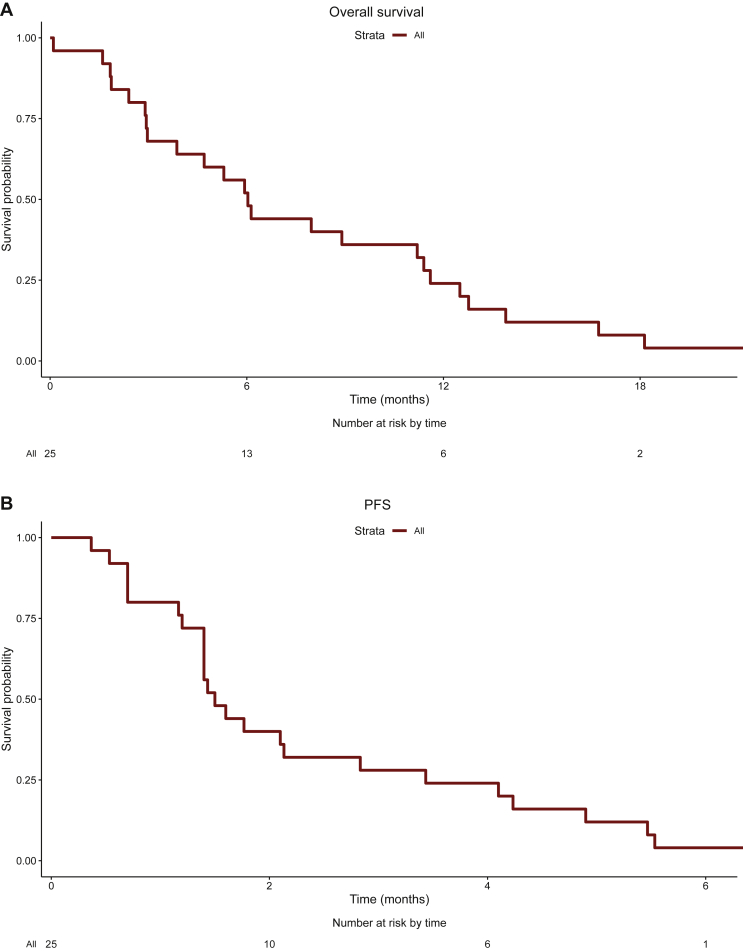
Median PFS and OS in the whole series were 1.5 month (range 0.3-7 months) and 6.0 months (range 1-22.2 months), respectively (Figure 2). Disease response according to Choi was associated with a significant PFS prolongation (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2022.100422).
Figure 2.
(A) Overall survival. (B) Progression-free survival.
At disease progression, further chemotherapy was administered in 17 patients (68%); in particular, 14 (56%) patients received one line whereas 3 (12%) patients received two lines. Cytotoxic treatment administered consisted of gemcitabine-containing regimens (seven patients), temozolomide (two patients), metronomic capecitabine (two patients), metronomic cyclophosphamide (three patients), cisplatin rechallenge (two patients) and cabozantinib (one patient).
Prognostic factors
Two sets of variables were evaluated as potential prognostic factors in this ACC series: traditional prognostic clinicopathological variables (including ENSAT stage, Ki67, resection status, hormonal hypersecretion) assessed at initial diagnosis of ACC and clinical characteristics evaluated at trial registration (including ECOG PS, Charlson’s Comorbidity Index, pattern of metastases distribution, laboratory abnormalities). As reported in Table 2, Table 2b, no variable was significantly associated with PFS, whereas older age (HR 3.30, 95% CI 1.33-8.23), cortisol hypersecretion (HR 2.69, 95% CI 1.14-6.34), ULN LDH serum levels (HR 4.42, 95% CI 1.60-12.21), lower albumin values (HR 10.20, 95% CI 2.24-46.41) and NLR ≥5 (HR 6.20, 95% CI 1.70-22.69) were correlated with a greater risk of death in univariate analysis and age (HR 4.65, 95% CI 1.65-13.09), elevated cortisol levels (HR 3.45, 95% CI 1.35-8.79) and ULN LDH (HR 5.12, 95% CI 1.70-15.37) values maintained their prognostic role in multivariate analysis.
Table 2.
Endpoints PFS
| PFS | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P | HR | 95% CI | P |
| Age at diagnosis, years | ||||||
| >45 | 1.75 | 0.77-3.97 | 0.182 | |||
| Sex | ||||||
| Male | 1.12 | 0.72-1.74 | 0.624 | |||
| ENSAT stage at diagnosis | ||||||
| III-IV | 1.06 | 0.47-2.41 | 0.882 | |||
| Hormone hypersecretion | ||||||
| Yes | 1.27 | 0.57-2.87 | 0.560 | |||
| Proliferation index (Ki67) | 0.99 | 0.96-1.03 | 0.626 | |||
| Surgery of primary tumor | ||||||
| No | 0.78 | 0.18-3.37 | 0.739 | |||
| ECOG performance status at cabACC start | ||||||
| ≥1 | 0.78 | 0.31-1.94 | 0.594 | |||
| Charlson’s comorbidity index at cabACC start | ||||||
| >6 | 0.95 | 0.37-2.41 | 0.907 | |||
| Metastases lung at cabACC start | ||||||
| Yes | 0.65 | 0.24-1.74 | 0.651 | |||
| Metastases liver at cabACC start | ||||||
| Yes | 1.41 | 0.59-3.41 | 0.440 | |||
| ≥2 Metastatic sites at cabACC start | ||||||
| Yes | 1.14 | 0.50-2.60 | 0.765 | |||
| Prior chemotherapy lines (before cabACC) | ||||||
| Less than 2 lines | 2.17 | 0.89-5.28 | 0.089 | |||
| High LDH at cabACC start | ||||||
| Upper limit of normal | 1.30 | 0.58-2.91 | 0.526 | |||
| Albumin at cabACC start | ||||||
| Under limit of normal | 2.15 | 0.87-5.28 | 0.096 | |||
| Neutrophil-to-lymphocyte ratio (NLR) at cabACC start | ||||||
| ≥5 | 1.34 | 0.45-4.01 | 0.599 | |||
| Hemoglobin (Hb) at cabACC start | ||||||
| <12/13 g/dl (female/male) | 1.14 | 0.47-2.77 | 0.777 | |||
| BMI | ||||||
| < Median(23.4) | 1.01 | 0.43-2.37 | 0.980 | |||
| Mitotane blood level | ||||||
| ≥14 mg/l | 1.86 | 0.74-7.71 | 0.186 | |||
Table 2b.
Endopoints OS
| OS |
Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P | HR | 95% CI | P |
| Age at diagnosis | ||||||
| >45 | 3.30 | 1.33-8.23 | 0.010 | 4.65 | 1.65-13.09 | 0.004 |
| Sex | ||||||
| Male | 1.09 | 0.44-2.69 | 0.848 | |||
| ENSAT stage at diagnosis | ||||||
| III-IV | 1.54 | 0.64-3.72 | 0.339 | |||
| Hormone hypersecretion | ||||||
| Yes | 2.69 | 1.14-6.34 | 0.024 | 3.45 | 1.35-8.79 | 0.009 |
| Proliferation index (Ki67) | 1.02 | 0.99-1.06 | 0.254 | |||
| Surgery of primary tumor | ||||||
| No | 1.94 | 0.44-8.63 | 0.384 | |||
| ECOG performance status at cabACC start | ||||||
| ≥1 | 1.11 | 0.43-2.86 | 0.825 | |||
| Charlson’s comorbidity index at cabACC start | ||||||
| >6 | 1.68 | 0.64-4.44 | 0.293 | |||
| Metastases lung at cabACC start | ||||||
| Yes | 1.28 | 0.51-3.26 | 0.600 | |||
| Metastases liver at cabACC start | ||||||
| Yes | 2.07 | 0.80-5.31 | 0.132 | |||
| ≥2 metastatic sites at cabACC start | ||||||
| Yes | 1.33 | 0.58-3.05 | 0.504 | |||
| Prior chemotherapy lines (before cabACC) | ||||||
| Less than 2 lines | 1.03 | 0.46-2.32 | 0.939 | |||
| LDH at cabACC start | ||||||
| Upper limit of normal | 4.42 | 1.60-12.21 | 0.004 | 5.12 | 1.70-15.37 | 0.004 |
| Albumin at cabACC start | ||||||
| Under limit of normal | 10.20 | 2.24-46.41 | 0.003 | |||
| Neutrophil-to-lymphocyte ratio (NLR) at cabACC start | ||||||
| ≥5 | 6.20 | 1.70-22.69 | 0.006 | |||
| Hemoglobin (Hb) at cabACC start | ||||||
| <12/13 g/dl (female/male) | 1.60 | 0.66-3.89 | 0.302 | |||
| BMI | ||||||
| < Median(23.4) | 1.13 | 0.50-2.55 | 0.772 | |||
| Mitotane blood level | ||||||
| ≥14 mg/l | 1.21 | 0.47-3.07 | 0.686 | |||
BMI, body mass index; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio, LDH, lactate dehydrogenase; ENSAT, European Network for the Study of Adrenal Tumors OS, overall survival; PFS, progression-free survival.
Bold indicates statistically significant P-values.
Treatment toxicity
Patients were evaluated after each cycle with both clinical examination and blood chemistry (complete blood count, liver and renal function) (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100422).
As depicted in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100422, the most frequent toxicities were: asthenia, which was grade 1 or 2 in 22 patients (88%), hematological toxicity, i.e. anemia grade 1/2 in 14 patients (56%), grade 3 in one patient (4%) and neutropenia grade 1/2 in two patients (8%), leukopenia in one patient (4%) and thrombocytopenia in one patient (4%). None of patients had to reduce or delay cabazitaxel administration due to toxicity.
Cabazitaxel pharmacokinetics results
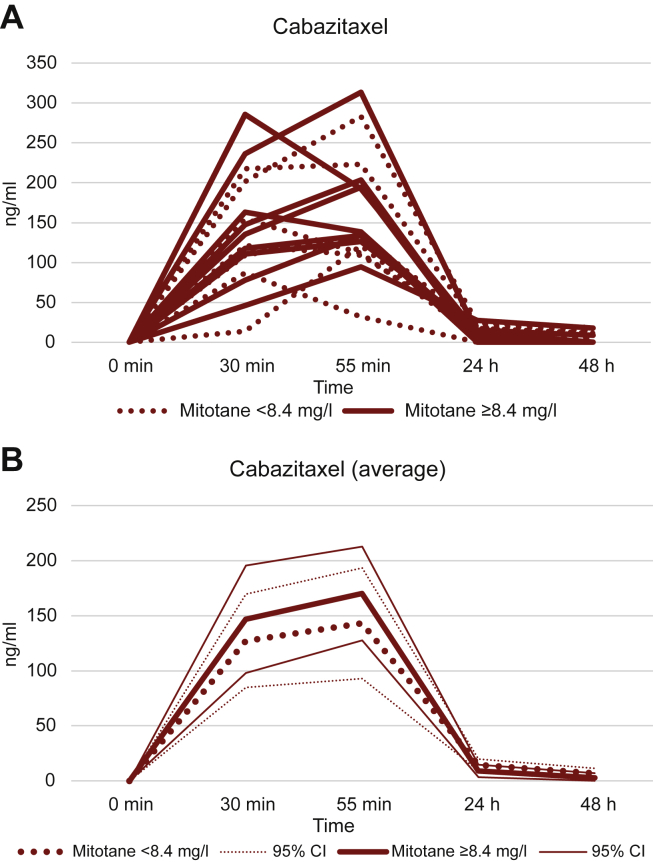
Cabazitaxel pharmacokinetics data were obtained from 18 patients: mean plasma concentrations (± standard deviation) 30 min after the infusion start and 5 min before infusion end were 136.87 ng/ml (±66.94) and 158.15 ng/ml (±67.91 ng/ml), respectively. Cabazitaxel plasma concentration decreased rapidly in the subsequent days; in particular, at 24 h and 48 h post-infusion we could detect cabazitaxel only in 14 and 7 patients, respectively. At 96 h post-infusion no cabazitaxel was found in plasma. A non-significant inverse relationship between serum cabazitaxel and serum mitotane levels was observed at 24 h (Pearson’s r −0.46, P = 0.066) and 48 h (Pearson’s r −0.43, P = 0.084), however, no difference in cabazitaxel concentration changes over time was observed according to mitotane levels above and below the median (Figure 3).
Figure 3.
Variation of cabazitaxel concentration according to mitotane. Dashed line: mitotane blood concentration under median; continuous line: mitotane blood concentration above median. CI, confidence interval.
Discussion
The management of patients with metastatic adrenocortical cancer is challenging.32 No chemotherapeutic regimen has been demonstrated to be substantially efficacious in patients with failure to EDP-M therapy. The combination of capecitabine and gemcitabine and single agent temozolomide are the only cytotoxic therapies that have demonstrated some activities in this setting. Despite few cases showing exceptional benefits,33,34 however, disease responses to these treatments were rarely observed and were of short duration.
Although more than half of ACCs have one or more potentially actionable genomic alterations, results of next-generation sequencing or other methods to assess ACC for personalized approaches revealed that the vast majority of DNA alterations detected are not druggable by molecular target agents currently available in the treatment of solid tumors results.35,36
In the current single-institution, nonrandomized phase II study, cabazitaxel showed a disease control rate after 4 months of 24%. Therefore, formally we could not conclude that this drug is not efficacious in the management of chemotherapy pretreated advanced ACC patients, according to the predefined limits that we have fixed to calculate the sample size of this trial.
No disease responses were obtained, however, and the waterfall diagrams revealed that no patients even attained a tumor shrinkage considering the RECIST response criteria.
Noteworthy, when the activity was assessed according to Choi criteria, disease response was observed in 55% of patients; this is consistent with a previous experience suggesting a possible role of these criteria, usually used to evaluate the activity of molecular target agents,37,38 in the assessment of response to chemotherapy in ACC patients.29 The assessment of response according to Choi, however, was a secondary endpoint of this study, so these results should be considered hypothesis generating. The association of the Choi response with improved disease-free survival supports the rationale of testing these criteria in future studies. Unfortunately, the patients enrolled in this trial did not benefit from the administration of cabazitaxel, the median PFS was only 1.5 months and the very short PFS observed is consistent with a poor efficacy of cabazitaxel in this patient setting. Therefore the cabACC study population was an inappropriate setting for the purpose of exploring the validity of any response criteria. Indeed, the prognosis of the patients enrolled in this study was poor; all of them died with a median OS of 6 months. PFS and OS results in this study were inferior to those observed in published trials testing other chemotherapy regimens as a second-line approach in ACC.9, 10, 11,34,39 Although patient selection could have accounted for the OS differences observed, PFS is mainly influenced by treatment efficacy. The observed PFS of 1.5 months in this study is similar to the PFS of 44 and 46 days obtained in patients treated with placebo or linsitinib, respectively, in the GALACTICC study, a randomized clinical trial that failed to obtain an advantage of an insulin growth factor receptor inhibitor over placebo as a second-line approach in ACC patients.40
Overall, the treatment was well tolerated; the use of prophylactic granulocyte growth factors has limited the severity of neutropenia which is the most frequent cabazitaxel toxicity.24 Recent studies evaluating cabazitaxel in pretreated patients with prostate cancer, non-small-cell lung cancer, breast and head and neck carcinoma, have reported consistently higher rates of severe (grade 3-4) neutropenia, despite primary prophylaxis with granulocyte colony-stimulating factor.18,19
Younger age and frequent hormonal hypersecretion may have contributed to the improved hematological tolerability of cabazitaxel in our series of ACC patients.
Patterns and severity of the other toxicities were comparable with those observed by the drug in clinical trials involving other diseases.
None of the prognostic factors explored were significantly associated with PFS, while, age, cortisol hypersecretion and ULN LDH were significantly associated with poor survival. Cabazitaxel and Mitotane are metabolized by CYP3A4 hepatic enzyme. Mitotane stimulates the activity of CYP3A4A and has a very long half-life, therefore, despite no patient taking mitotane during cabazitaxel administration, a residual amount of drug was detectable in the blood of the enrolled patients and may potentially have interfered with the cabazitaxel pharmacokinetics.41 It should be noted, however, that plasma concentrations of cabazitaxel measured in our patients are consistent with the results of other published studies, in which the drug was administered with a similar dosing schedule.42,43
In conclusion, this phase II trial shows that cabazitaxel has a manageable toxicity profile but it is poorly active as second/third-line treatment in advanced ACC patients. These results do not support further evaluation of cabazitaxel in this setting.
Acknowledgments
Funding
This work was supported by Associazione Italiana Ricerca Cancro (AIRC) project IG14411 (Principal Investigator: AB), Fondazione Camillo Golgi, Brescia and F.I.R.M. onlus Foundation, Cremona (Italy) (no grant number). The drug cabazitaxel in the patients enrolled in the trial was provided by Sanofi Italy.
Disclosure
The authors have declared no conflicts of interest.
Supplementary data
References
- 1.Fassnacht M., Assie G., Baudin E., et al. on behalf of the ESMO Guidelines Committee Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO–EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Approved by the ESMO Guidelines Committee: June 2020. This publication supersedes the previously published version—Ann Oncol. 2012;23(suppl 7):vii131-vii138. Citation Data. Ann Oncol. 2020;31(11):1476–1490. doi: 10.1016/j.annonc.2020.08.2099. [DOI] [PubMed] [Google Scholar]
- 2.Fassnacht M., Dekkers O.M., Else T., et al. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2018;179:G1–G46. doi: 10.1530/EJE-18-0608. [DOI] [PubMed] [Google Scholar]
- 3.Berruti A., Grisanti S., Pulzer A., et al. Long-term outcomes of adjuvant mitotane therapy in patients with radically resected adrenocortical carcinoma. J Clin Endocrinol Metab. 2017;102:1358–1365. doi: 10.1210/jc.2016-2894. [DOI] [PubMed] [Google Scholar]
- 4.Terzolo M., Angeli A., Fassnacht M., et al. Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med. 2007;356(23):2372–2380. doi: 10.1056/NEJMoa063360. [DOI] [PubMed] [Google Scholar]
- 5.Berruti A., Terzolo M., Sperone P., et al. Etoposide, doxorubicin and cisplatin plus mitotane in the treatment of advanced adrenocortical carcinoma: a large prospective phase II trial. Endocr Relat Cancer. 2005;12(3):657–666. doi: 10.1677/erc.1.01025. [DOI] [PubMed] [Google Scholar]
- 6.Fassnacht M., Terzolo M., Allolio B., et al. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366:2189–2197. doi: 10.1056/NEJMoa1200966. [DOI] [PubMed] [Google Scholar]
- 7.Terzolo M., Daffara F., Ardito A., et al. Management of adrenal cancer: a 2013 update. J Endocrinol Invest. 2014;37:207–217. doi: 10.1007/s40618-013-0049-2. [DOI] [PubMed] [Google Scholar]
- 8.Laganà M., Grisanti S., Cosentini D., et al. Efficacy of the EDP-M scheme plus adjunctive surgery in the management of patients with advanced adrenocortical carcinoma: the Brescia experience. Cancers (Basel) 2020;12(4):41. doi: 10.3390/cancers12040941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cosentini D., Badalamenti G., Grisanti S., et al. Activity and safety of temozolomide in advanced adrenocortical carcinoma patients. Eur J Endocrinol. 2019;181:681–689. doi: 10.1530/EJE-19-0570. [DOI] [PubMed] [Google Scholar]
- 10.Henning J.E.K., Deutschbein T., Altieri B., et al. Gemcitabine-based chemotherapy in adrenocortical carcinoma: a multicenter study of efficacy and predictive factors. J Clin Endocrinol Metab. 2017;102:4323–4332. doi: 10.1210/jc.2017-01624. [DOI] [PubMed] [Google Scholar]
- 11.Grisanti S., Cosentini D., Laganà M., et al. Clinical prognostic factors in patients with metastatic adrenocortical carcinoma treated with second line gemcitabine plus capecitabine chemotherapy. Front Endocrinol (Lausanne) 2021;12:624102. doi: 10.3389/fendo.2021.624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grisanti S., Cosentini D., Laganà M., et al. Are we failing in treatment of adrenocortical carcinoma? Lights and shadows of molecular signatures. Curr Opin Endocr Metab Res. 2019;8:80–87. [Google Scholar]
- 13.Raj N., Zheng Y., Kelly V., et al. PD-1 blockade in advanced adrenocortical carcinoma. J Clin Oncol. 2020;38(1):71–80. doi: 10.1200/JCO.19.01586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cosentini D., Grisanti S., Dalla Volta A., et al. Immunotherapy failure in adrenocortical cancer: where next? Endocr Connect. 2018;7:E5–E8. doi: 10.1530/EC-18-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grisanti S., Cosentini D., Laganà M., et al. The long and winding road to effective immunotherapy in patients with adrenocortical carcinoma. Future Oncol. 2020;16(36):3017–3020. doi: 10.2217/fon-2020-0686. [DOI] [PubMed] [Google Scholar]
- 16.Mita A.C., Figlin R., Mita M.M. Cabazitaxel: more than a new taxane for metastatic castrateresistant prostate cancer? Clin Cancer Res. 2012;18(24):6574–6579. doi: 10.1158/1078-0432.CCR-12-1584. [DOI] [PubMed] [Google Scholar]
- 17.Fallo F., Pilon C., Barzon L., et al. Paclitaxel is an effective antiproliferative agent on the human NCI-H295 adrenocortical carcinoma cell line. Chemotherapy. 1998;44:129–134. doi: 10.1159/000007104. [DOI] [PubMed] [Google Scholar]
- 18.Urup T., Pawlak W.Z., Petersen P.M., Pappot H., Rørth M., Daugaard G. Treatment with docetaxel and cisplatin in advanced adrenocortical carcinoma, a phase II study. Br J Cancer. 2013;108(10):1994–1997. doi: 10.1038/bjc.2013.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berruti A., Sperone P., Ferrero A., et al. Phase II study of weekly paclitaxel and sorafenib as second/third-line therapy in patients with adrenocortical carcinoma. Eur J Endocrinol. 2012;166:451–458. doi: 10.1530/EJE-11-0918. [DOI] [PubMed] [Google Scholar]
- 20.Bates S.E., Shieh C.Y., Mickley L.A., et al. Mitotane enhances cytotoxicity of chemotherapy in cell lines expressing a multidrug resistance gene (mdr-1/P-glycoprotein) which is also expressed by adrenocortical carcinomas. J Clin Endocrinol Metab. 1991;73(1):18–29. doi: 10.1210/jcem-73-1-18. [DOI] [PubMed] [Google Scholar]
- 21.Villa R., Orlandi L., Berruti A., Dogliotti L., Zaffaroni N. Modulation of cytotoxic drug activity by mitotane and lonidamine in human adrenocortical carcinoma cells. Int J Oncol. 1999;14(1):133–138. doi: 10.3892/ijo.14.1.133. [DOI] [PubMed] [Google Scholar]
- 22.Paller C.J., Antonarakis E.S. Cabazitaxel: a novel second-line treatment for metastatic castration-resistant prostate cancer. Drug Des Devel Ther. 2011;5:117–124. doi: 10.2147/DDDT.S13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vrignaud P., Sémiond D., Lejeune P., et al. Preclinical antitumor activity of cabazitaxel, a semisynthetic taxane active in taxane-resistant tumors. Clin Cancer Res. 2013;19(11):2973–2983. doi: 10.1158/1078-0432.CCR-12-3146. [DOI] [PubMed] [Google Scholar]
- 24.de Bono J.S., Oudard S., Ozguroglu M., et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomized open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 25.Fragni M., Palma Lopez L.P., Rossini E., et al. In vitro cytotoxicity of cabazitaxel in adrenocortical carcinoma cell lines and human adrenocortical carcinoma primary cell cultures. Mol Cell Endocrinol. 2019;498:110585. doi: 10.1016/j.mce.2019.110585. [DOI] [PubMed] [Google Scholar]
- 26.Libé R., Borget I., Ronchi C.L., et al. Prognostic factors in stage III-IV adrenocortical carcinomas (ACC): an European Network for the Study of Adrenal Tumor (ENSAT) study. Ann Oncol. 2015;26(10):2119–2125. doi: 10.1093/annonc/mdv329. [DOI] [PubMed] [Google Scholar]
- 27.Baudin E. Adrenocortical carcinoma. Endocrine Tumor Board of Gustave Roussy. Endocrinol Metab Clin North Am. 2015;44(2):411–434. doi: 10.1016/j.ecl.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Elhassan Y.S., Altieri B., Berhane S., et al. S-GRAS score for prognostic classification of adrenocortical carcinoma: an international, multicenter ENSAT study. Eur J Endocrinol. 2021;186:25–36. doi: 10.1530/EJE-21-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ambrosini R., Balli M.C., Laganà M., et al. Adrenocortical carcinoma and CT assessment of therapy response: the value of combining multiple criteria. Cancers (Basel) 2020;12(6):1395. doi: 10.3390/cancers12061395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Bruijn P., de Graan A.J., Nieuweboer A., et al. Quantification of cabazitaxel in human plasma by liquid chromatography/triple-quadrupole mass spectrometry: a practical solution for non-specific binding. J Pharm Biomed Anal. 2012;59:117–122. doi: 10.1016/j.jpba.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 32.Berruti A., Libè R., Laganà M., et al. Morbidity and mortality of bone metastases in advanced adrenocortical carcinoma: a multicenter retrospective study. Eur J Endocrinol. 2019;180(5):311–320. doi: 10.1530/EJE-19-0026. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto A., Nakai Y., Oka T., et al. Advanced adrenocortical carcinoma successfully treated with gemcitabine plus capecitabine as second-line chemotherapy. IJU Case Rep. 2020;3(6):270–273. doi: 10.1002/iju5.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cosentini D., Turla A., Carminati O., et al. Case report: exceptional response to second line temozolomide therapy in a patient with metastatic adrenocortical carcinoma. Front Endocrinol (Lausanne) 2021;12:674039. doi: 10.3389/fendo.2021.674039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pozdeyev N., Fishbein L., Gay L.M., et al. Targeted genomic analysis of 364 adrenocortical carcinomas. Endocr Relat Cancer. 2021;28(10):671–681. doi: 10.1530/ERC-21-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garinet S., Nectoux J., Neou M., et al. Detection and monitoring of circulating tumor DNA in adrenocortical carcinoma. Endocr Relat Cancer. 2018;25(3):L13–L17. doi: 10.1530/ERC-17-0467. [DOI] [PubMed] [Google Scholar]
- 37.Benjamin R.S., Choi H., Macapinlac H.A., et al. We should desist using RECIST, at least in GIST. J Clin Oncol. 2007;25:1760–1764. doi: 10.1200/JCO.2006.07.3411. [DOI] [PubMed] [Google Scholar]
- 38.Faivre S., Zappa M., Vilgrain V., et al. Changes in tumor density in patients with advanced hepatocellular carcinoma treated with sunitinib. Clin Cancer Res. 2011;17:4504–4512. doi: 10.1158/1078-0432.CCR-10-1708. [DOI] [PubMed] [Google Scholar]
- 39.Sperone P., Ferrero A., Daffara F., et al. Gemcitabine plus metronomic 5-fluorouracil or capecitabine as a second-/third-line chemotherapy in advanced adrenocortical carcinoma: a multicenter phase II study. Endocr Relat Cancer. 2010;17:445–453. doi: 10.1677/ERC-09-0281. [DOI] [PubMed] [Google Scholar]
- 40.Fassnacht M., Berruti A., Baudin E., et al. Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet Oncol. 2015;16(4):426–435. doi: 10.1016/S1470-2045(15)70081-1. [DOI] [PubMed] [Google Scholar]
- 41.Ferron G., Dai Y., Semiond D., et al. Population pharmacokinetics of cabazitaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2013;71(3):681–692. doi: 10.1007/s00280-012-2058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mukai H., Takahashi S., Nozawa M., et al. Phase I dose-escalation and pharmacokinetic study (TED 11576) of cabazitaxel in Japanese patients with castration-resistant prostate cancer. Cancer Chemother Pharmacol. 2014;73(4):703–710. doi: 10.1007/s00280-014-2394-z. [DOI] [PubMed] [Google Scholar]
- 43.Azaro A., Rodón J., Machiels J.P., et al. A phase I pharmacokinetic and safety study of cabazitaxel in adult cancer patients with normal and impaired renal function. Cancer Chemother Pharmacol. 2016;78(6):1185–1197. doi: 10.1007/s00280-016-3175-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.