Abstract
Background
Synovial sarcoma (SS) occurs in both adult and pediatric patients. The primary aim of this study is to describe the outcomes, prognostic factors, and treatment of patients with metastatic SS within a nationwide cohort.
Patients and methods
All pediatric and adult patients with metastatic SS are registered in the French Sarcoma Group database. Data were collected from the national database https://conticabase.sarcomabcb.org/ up to March 2020. Descriptive and comparative analyses were conducted using SAS 9.4 and Stata Special Edition 16.1 software.
Results
Between January 1981 and December 2019, 417 patients with metastatic SS from 17 French sarcoma centers were included, including 64 (15.3%) under the age of 26 years. Median age was 42.5 years (range 9-87 years). The metastases were synchronous (cohort 1) or metachronous (cohort 2) in 18.9% (N = 79) and 81.1% (N = 338) patients, respectively. Median overall survival (OS) from the date of metastasis was 22.3 months (95% confidence interval 19.7-24.1 months). First-line chemotherapy without ifosfamide and/or doxorubicin was unfavorable for progression-free survival and OS (P < 0.001). Concerning cohort 1, young age, surgery of the primary tumor, and single metastatic site were independent favorable prognostic factors for OS. In cohort 2, surgery within an expert French Sarcoma Group center, absence of chemotherapy in the perioperative setting, the lungs as a single metastatic site, time to first metastasis >12 months, local therapy, and ifosfamide in the first metastatic line were independent favorable prognostic factors.
Conclusions
The outcome of patients with metastatic SS is influenced by local treatment, management in reference centers, and cytotoxic treatments given in the perioperative and metastatic setting.
Key words: metastatic synovial sarcoma
Highlights
-
•
METASYN is the largest retrospective study on metastatic adult and pediatric SS.
-
•
This study confirms that surgery remains the mainstay for improving OS in reference centers.
-
•
METASYN emphasizes the importance of focal treatment of metastases for OS.
-
•
This study offers real-life results in a metastatic setting and is a useful support for developing new strategies.
Introduction
Synovial sarcoma (SS) is a malignant mesenchymal tumor characterized by a specific t(X; 18) (p11.2; q11.2) chromosomal translocation with a high risk of metastases that can even occur late, up to 5 years.1, 2, 3, 4 SS represents <3% of cases of soft tissue sarcoma (STS), with an incidence of 1.674/million/year according to recent epidemiological data from the NetSarc+ network in 2021, but is the most common non-rhabdomyosarcoma in young patients.5, 6, 7, 8 Its second characteristic is a prognosis usually described as worse for adult versus pediatric patients, possibly because of differences in additional somatic genetic rearrangements.9, 10, 11 In terms of prognosis, there are still questions regarding age and the specificities of SS,6,12, 13, 14, 15 such as perioperative treatment. In a metastatic context, ifosfamide and Adriamycin regimens remain the backbone of treatment, but new emerging treatments for SS include regorafenib and T-cell therapy based on NY-ESO-1 and MAGE-A4 in selected subgroups of patients.16, 17, 18
In this context, characterizing the natural history of SS is important, and requires larger series than those previously reported. The objective of ‘METASYN’, a retrospective study by the French Sarcoma Group (FSG), was to describe the management and outcomes of adult and pediatric patients with metastatic SS and included in the Conticabase database.
Material and methods
Conticabase
The Conticanet database and tumor bank contains anonymized information describing tumors, treatment, and follow-up, as well as tumor sample availability and molecular biology analyses for mesenchymal tumors. The data have been collected thanks to the Conticabase https://conticabase.sarcomabcb.org/ set up in 2005.
Patients
Clinical data were collected from the Conticabase (https://conticabase.sarcomabcb.org) and not from the NetSarc database, which was set up later. The study was first approved by the FSG, then declared to the Commission Nationale de l’Informatique et des Libertés (CNIL) on 18 July 2018, number 2203224, and approved by the ethics committee in Angers on 4 February 2020 (approval number 2020/10). All living patients received an information letter. Clinical data for the 417 patients were updated. Between July 1980 and April 2019, the sex, histology, grade, depth, size, location, treatment, relapse, and survival of the 417 adults, children, and adolescents and young adults (AYA, 15-25 years old) with treated metastatic SS were collected.
Two cohorts of patients were created in ‘METASYN’: cohort 1 for patients with synchronous metastatic SS, and cohort 2 for patients with metachronous metastatic SS. For the first cohort, the date of diagnosis corresponded to the diagnosis of the metastasis, whereas for the second, the date of diagnosis of the metastasis came after that of the initial diagnosis (range 1-478, median 20 months).
Statistical analysis
All data were anonymized and analyzed retrospectively. Qualitative factors were described by the frequency of their respective modalities and compared using Pearson’s chi-square test (or Fisher’s exact test). Continuous factors were described by their mean ± standard deviation (or median—interquartile—range) and compared using Student’s t-test (or the Mann–Whitney/Wilcoxon test). The dose intensity of ifosfamide was calculated as the total dose received and the number of courses. Overall survival (OS) was defined as the time from the date of treatment of the first metastatic relapse to the date of death. Progression-free survival (PFS) was defined as the time from the start of the reporting periods to the date of progression (or the date of the next line or the date of death). All survivals were described using Kaplan–Meier curves. Log-rank tests and univariate Cox proportional-hazards analyses were carried out to identify prognostic factors in each cohort. At the final step, and for each cohort separately, the confounding factors were taken into account to assess the independent prognostic impact on OS and PFS of the parameters studied. Variables with a P value <0.20 at the univariate step were introduced into a semi-parametric multivariate Cox model to calculate adjusted hazard ratios (HR) with their 95% confidence interval (CI). The validity of the final model for proportional hazards was tested. All tests were carried out in a bilateral formulation and the significance limit was set at 5%. All analyses were carried out using SAS 9.4 (SAS Institute Inc., Cary, NC) and Stata Special Edition 16.1 (StataCorp, College Station, TX) software.
Results
Patient and tumor characteristics
The Conticabase collected data from 417 patients from 17 FSG centers. NetSarc is the French clinical reference network for soft tissue and visceral sarcomas, implemented in 2010 and approved by the French National Cancer Institute (INCa) in 2014 (28 centers). New patients from other centers, now called ‘NetSarc+ centers’, could not be included retrospectively. The Conticabase included 1127 patients (56 children, 1071 adults) with SS, of whom 417 (9 children, 408 adults) had metastatic disease between 15 July 1980 and 24 April 2019. Sixty-four (15.3%) children and AYA were identified, of whom nine patients were younger than 18 years old. The median age at metastasis diagnosis was 42.5 years (range: 9-87 years). Patient characteristics are presented in Table 1. Metastases were synchronous for 79/417 (18.9%) patients (cohort 1) and metachronous for 338/417 (81.1%) patients (cohort 2). The primary tumor was located in the limbs, thorax, and pelvis for, respectively, 242/416 (58.2%), 74/416 (17.8%), and 46/416 (11.1%) patients. The lungs were the first location for metastases in 284/373 (76.1%) patients, followed by the lymph nodes 22/373 (5.9%) and pleura 19/373 (5.1%). Mean primary tumor size was 126 ± 50.4 and 79.7 ± 41.5 mm in cohorts 1 and 2, respectively (P < 0.0001). No other differences in the tumors were noted between the two cohorts in terms of patient characteristics. Notably, there was no significant difference in age. In cohort 2, relapses occurred 1, 2, 5, and 10 years after the initial diagnosis for 71.7%, 41.7%, 14.3%, and 4.5% of patients, respectively (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100402). Median follow-up was 6.9 (4.7-11.3) years after diagnosis of the metastasis for the entire cohort.
Table 1.
Characteristics of patients with metastatic synovial sarcoma and treatment of the primary tumor and metastatic disease in cohort 1 (synchronous metastatic disease) and cohort 2 (metachronous metastatic disease)
| Patient characteristics | Global | Cohort 1 | Cohort 2 | P |
|---|---|---|---|---|
| Sex, n (%) | n = 417 | n = 79 | n = 338 | 0.2660 (K) |
| Male | 230 (55.2) | 48 (60.8) | 182 (53.8) | |
| Female | 187 (44.8) | 31 (39.2) | 156 (46.2) | |
| Age at metastasis diagnosis, years, n (%) | n = 417 | n = 79 | n = 338 | 0.2476 (S) |
| Mean ± SD | 42.5 ± 15.1 | 40.7 ± 16.4 | 42.9 ± 14.8 | |
| ≤25 | 64 (15.3) | 17 (21.5) | 47 (13.9) | 0.0910 (K) |
| >25 | 353 (84.7) | 62 (78.5) | 291 (86.1) | |
| Years of metastasis diagnosis, n (%) | n = 417 | n = 79 | n = 338 | 0.8435 (K) |
| <2000 | 118 (28.3) | 23 (29.1) | 95 (28.1) | |
| 2000-2010 | 177 (42.4) | 35 (44.3) | 142 (42.0) | |
| >2010 | 122 (29.3) | 21 (26.6) | 101 (29.9) | |
| Tumor site, n (%) | n = 416 | n = 79 | n = 337 | 0.2378 (F) |
| Limb | 242 (58.2) | 48 (60.8) | 194 (57.6) | |
| Thorax | 74 (17.8) | 16 (20.3) | 58 (17.2) | |
| Pelvis | 46 (11.1) | 11 (13.9) | 35 (10.4) | |
| Abdomen | 25 (6.0) | 2 (2.5) | 23 (6.8) | |
| Head and neck | 23 (5.5) | 1 (1.3) | 22 (6.5) | |
| Trunk wall | 6 (1.4) | 1 (1.3) | 5 (1.5) | |
| Size, cm, n (%) | n = 371 | n = 70 | n = 301 | <0.0001 (W) |
| Mean ± SD | 88.5 ± 46.9 | 126 ± 50.4 | 79.7 ± 41.5 | |
| <5 | 64 (17.3) | 2 (2.9) | 62 (20.6) | <0.0001 (F) |
| ≥5 | 306 (82.5) | 68 (97.1) | 238 (79.1) | |
| Single metastatic site, n (%) | n = 373 | n = 66 | n = 307 | 0.0401 (F) |
| Lung | 284 (76.1) | 55 (83.3) | 229 (74.6) | |
| Lymph node | 22 (5.9) | 0 | 22 (7.2) | |
| Pleura | 19 (5.1) | 3 (4.6) | 16 (5.2) | |
| Bone | 16 (4.3) | 3 (4.6) | 13 (4.2) | |
| Peritoneum | 11 (2.9) | 0 | 11 (3.6) | |
| Liver | 6 (1.6) | 3 (4.6) | 3 (1.0) | |
| Other | 15 (4) | 2 (3) | 13 (4.2) |
Number of patients with available data (n), Chi-square test (K), Fisher exact test (F).
SD, standard deviation.
Treatment
The treatment of the primary tumor is described in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100402.
General pattern: initial surgery was carried out outside of one of the FSG’s expert centers for 200/368 (54.3%) of patients. The R0 resection rate was 148/368 (40.2%) for all patients. The R0 rate was 64.1% when surgery was carried out within the network versus 51.3% outside the network (Fisher’s exact P = 0.049).
In cohort 1, initial surgery was carried out in 46/79 (58.2%) patients. The R0 rate was 17/46 (37%). In the perioperative setting, radiotherapy was delivered in 33/79 (41.8%).
In cohort 2, initial surgery was carried out in 322/368 (95.3%) patients. The R0 rate was 131/322 (40.7%). In the perioperative setting, radiotherapy was delivered in 227/338 (67.2%) of patients. Some 216/338 (63.9%) patients received perioperative chemotherapy. This chemotherapy was administered in the neoadjuvant setting, adjuvant setting, or both for 71/338 (21%), 103/338 (30.5%), and 26/338 (7.7%) patients respectively. A total of 85/216 (39.4%) of patients received ifosfamide-based chemotherapy.
The treatment of the metastatic disease is described in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100402. Ninety-nine patients (31.5%) were included in a clinical trial.
First-line treatment
At the first metastatic relapse, 202/417 (48.4%) patients received local treatment of the metastases, with no statistical difference between cohorts. This local treatment included surgery, radiotherapy, and thermal ablation for 157/417 (77.7%), 91/417 (45%), and 34/417 (16.9%) of patients, respectively. Focal treatment was offered mostly to patients with an SS diagnosed 20 years ago: for 63/87 (72.4%), 79/131 (60.3%), and 60/115 (52.2%) patients before 2000, between 2000 and 2010, and after 2010, respectively (P = 0.004). Focal treatment was offered more frequently in cases of late relapse, with a median time of 19.4 months versus 12 months for patients without focal treatment (P < 0.001). Patients mostly had only one metastatic site 186/202 (92.1%) versus 103/131 (78.6%) (P = 0.001), and the lung was the single metastatic site (P = 0.002), Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2022.100402.
As the systemic first-line treatment of metastases, 314/417 (75.3%) patients received chemotherapy, with a median number of 3 lines (range 1-10 lines): 314/417 (75.3%), 244/417 (58.5%), 170/417 (40.8%), and 110/417 (26.4%) patients received 1, 2, 3, and >3 chemotherapy lines. The most frequently administered agent was ifosfamide, given to 216/314 (68.8%) of these metastatic SS patients. A total of 98 patients did not receive ifosfamide; 51 (52%) had already received it in the (neo)adjuvant setting or local relapses.
In cohort 1, ifosfamide was administered with doxorubicin to 29/59 (49.2%) patients. In cohort 2, ifosfamide was administered with doxorubicin to 71/255 (27.8%) patients. Ifosfamide rechallenge was carried out in 30/133 (22.5%) patients in cohort 2.
Further lines of treatment
Local treatment of metastases was carried out in cases of second and third metastatic relapse for 88/417 (21.1%) and 42/417 (10.1%) patients, with no statistical difference between cohorts. As the second line, 27 patients underwent polychemotherapy, including anthracycline and/or ifosfamide, 18 had another combination of polychemotherapy, 151 had monotherapy: 8 received doxorubicin, 43 received ifosfamide, 39 trabectedin, 24 a tyrosine kinase inhibitor (TKI), and 37 another systemic therapy.
Response to treatment
As the first line for metastases, chemotherapy led to an objective response rate (ORR) of 94/240 (39.2%) for the entire cohort. There was no statistically significant difference between cohorts: the ORR for cohorts 1 and 2 was 50% versus 36.8%, respectively (P = 0.094).
In further metastatic lines, chemotherapy led to an ORR of 44/196 (22.4%), 25/120 (20.8%), 11/79 (13.9%), and 5/38 (13.2%) in the second, third, fourth, and fifth lines, respectively, for the entire cohort. In cohort 1, the ORR was 27.3% and 21.9% in the second and third lines. In cohort 2, the ORR was 21.1% and 20.5% in second and third line. There was no statistically significant difference between cohorts. The ORR according to agent and line of treatment is recorded in Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2022.100402. The ORR was better for multiregimen chemotherapy and monochemotherapy with ifosfamide.
PFS
Median PFS (mPFS) in a first-line metastatic setting was 6 months [95% confidence interval (CI) 5.26-7.36 months] for the entire cohort, with no statistical difference between cohorts. Ifosfamide combined with doxorubicin or alone provided better PFS than other regimens (Figure 1) (log-rank P value <0.001): 8.5 months (95% CI 7-10.6 months) and 7.7 months (95% CI 5.4-10.4 months), respectively, versus 4 months (95% CI 2.2-7.9 months) for doxorubicin as the single agent. Trabectedin and a TKI yielded an mPFS of 2.6 months (95% CI 1.3-4.1 months) and 4.1 months (95% CI 2.7-5.0 months), respectively (Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2022.100402).
Figure 1.
(A) Progression-freesurvival and (B) overall survival according to first metastatic chemotherapy line.
For subsequent treatment lines in metastatic settings, mPFS was 4.1 months (95% CI 3.45-4.70 months), 2.8 months (95% CI 2.27-3.12 months), and 2 months (95% CI 1.81-2.29 months) in the second, third, and beyond lines, respectively, for the entire cohort. Focusing on agent, in the second line, mPFS was 4.4 months (95% CI 2.4-6.0 months) for ifosfamide combined with doxorubicin, and 6.3 months (95% CI 4.2-8.0 months) for ifosfamide alone versus 2.1 months (95% CI 0.8-6.5 months) for doxorubicin as the single agent. Trabectedin and TKI yielded a mPFS of 4.3 months (95% CI 1.4-8.9 months) and 4.4 months (95% CI 3.3-5.9 months) in the second line, respectively.
OS and prognostic factors
The 5-year OS rate after the date of diagnosis of the metastasis was 14.8% (95% CI 11.1% to 18.9%) and median OS was 22.3 months (95% CI 19.7-24.1 months) with no difference between cohorts (log-rank P value = 0.69) and similar OS over time [<2000; (2000-2010); >2010] (log-rank P value = 0.62).
In the univariate analysis, favorable prognostic factors for cohort 1 were younger age as a continuous variable, surgery of the primary tumor, R0 margins versus R2 for the resection of the primary tumor, and the lungs as a single metastatic site (Table 2). In cohort 2, for patients who underwent surgery (322/338) (95.3%), favorable prognostic factors included were primary surgery within an expert FSG center, absence of chemotherapy in the perioperative setting, the lung as a single metastatic site, time to first metastasis >12 months, local treatment of the metastasis as the first line, and ifosfamide- and doxorubicin-based chemotherapy as the first metastatic treatment (Table 3).
Table 2.
Prognostic factors for overall survival in cohort 1 (synchronous metastatic disease): univariate and multivariate analyses (n = 79)
| Characteristics | Univariate analysis |
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P> z | Hazard ratio | 95% CI | P > z | |||
| Age at diagnosis (continuous variable) | 1.02 | 1.01 | 1.04 | <0.01 | 1.02 | 1.01 | 1.04 | <0.01 |
| Age ≤25 years versus > 25 years old | 1.62 | 0.85 | 3.07 | 0.14 | ||||
| Sex (male versus female) | 0.89 | 0.53 | 1.50 | 0.66 | ||||
| Year of metastasis diagnosis: | ||||||||
| 2000-2010 versus <2000 | 1.51 | 0.79 | 2.89 | 0.21 | ||||
| >2010 versus <2000 | 1.62 | 0.81 | 3.22 | 0.17 | ||||
| Tumor size of primary tumor <50 mm or >50 mm | 2.16 | 0.29 | 15.9 | 0.45 | ||||
| Surgery of the primary tumor yes versus no | 0.53 | 0.31 | 0.90 | 0.02 | 0.58 | 0.34 | 0.99 | 0.04 |
| Surgeon in network versus extra network (n = 42)a | 0.64 | 0.31 | 1.33 | 0.23 | ||||
| Margin of primary tumor (n = 33)b | ||||||||
| R1 versus R0 | 1.84 | 0.74 | 4.59 | 0.19 | ||||
| R2 versus R0 | 4.19 | 1.05 | 16.7 | 0.04 | ||||
| Radiotherapy in perioperative setting versus no | 1.04 | 0.49 | 2.18 | 0.93 | ||||
| Metastatic site: | ||||||||
| Other single versus lung single | 1.74 | 0.81 | 3.76 | 0.15 | 1.90 | 0.88 | 4.15 | 0.10 |
| Multiple versus lung single | 3.59 | 1.82 | 7.08 | <0.01 | 3.89 | 1.93 | 7.83 | <0.01 |
| Local treatment at first metastatic line | 0.64 | 0.37 | 1.12 | 0.12 | ||||
| Ifosfamide at first metastatic line versus no | 0.64 | 0.35 | 1.20 | 0.14 | ||||
| Doxorubicin at first metastatic line versus no | 1.15 | 0.61 | 2.20 | 0.66 | ||||
Characteristics that are clinically significant are highlighted in bold. Only age at diagnosis and variables that had a P value of significance <0.10 in the univariate analysis were introduced in the semi-parametric multivariate Cox model.
CI, confidence interval.
Surgery in network versus extra network was not introduced in multivariate analysis due to missing data.
Margin of primary tumor was not introduced in multivariate analysis due to missing data.
Table 3.
Prognostic factors for overall survival in cohort 2 with surgery (metachronous metastatic disease): univariate and multivariate analyses (n = 322)
| Characteristics | Univariate analysis |
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P> |z| | Hazard ratio | 95% CI | P> |z| | |||
| Age at metastatic diagnosis (continuous variable) | 1.01 | 0.99 | 1.01 | 0.62 | 1.01 | 0.99 | 1.01 | 0.12 |
| Age ≤25 years versus >25 years old | 1.11 | 0.78 | 1.58 | 0.55 | ||||
| Sex (male versus female) | 1.16 | 0.90 | 1.49 | 0.24 | ||||
| Year of metastasis diagnosis: | ||||||||
| 2000-2010 versus <2000 | 0.80 | 0.60 | 1.06 | 0.12 | ||||
| >2010 versus <2000 | 0.82 | 0.59 | 1.14 | 0.23 | ||||
| Tumor size of primary tumor <50 mm or >50 mm | 1.08 | 0.78 | 1.50 | 0.63 | ||||
| Surgery in network versus extra network | 0.67 | 0.52 | 0.88 | <0.01 | 0.57 | 0.41 | 0.78 | <0.01 |
| Margin of primary tumor (n = 219)a | ||||||||
| R1 versus R0 | 1.28 | 0.92 | 1.76 | 0.14 | ||||
| R2 versus R0 | 1.69 | 0.98 | 2.92 | 0.06 | ||||
| Radiotherapy in perioperative setting versus no | 0.81 | 0.20 | 3.34 | 0.77 | ||||
| Chemotherapy in perioperative setting versus no | 1.26 | 0.98 | 1.63 | 0.07 | 1.56 | 1.08 | 2.26 | 0.02 |
| Metastatic site: | ||||||||
| Other single versus lung single | 1.58 | 1.13 | 2.22 | <0.01 | 1.67 | 1.16 | 2.41 | <0.01 |
| Multiple versus lung single | 3.44 | 2.18 | 5.43 | <0.01 | 2.17 | 1.32 | 3.57 | <0.01 |
| Time to first metastasis >12 months versus <12 months | 0.45 | 0.34 | 0.60 | <0.01 | 0.36 | 0.25 | 0.52 | <0.01 |
| Local treatment at first metastatic line | 0.34 | 0.25 | 0.45 | <0.01 | 0.40 | 0.29 | 0.57 | <0.01 |
| Ifosfamide at first metastatic line versus no (n = 236) | 0.64 | 0.48 | 0.84 | <0.01 | 0.69 | 0.51 | 0.93 | 0.02 |
| Doxorubicin at first metastatic line versus no (n = 236) | 0.68 | 0.52 | 0.90 | 0.006 | 0.75 | 0.52 | 1.08 | 0.12 |
Characteristics that are clinically significant are highlighted in bold.
Only age at metastatic diagnosis and variables that had a P value of significance <0.10 in the univariate analysis were introduced in the semi-parametric multivariate Cox model.
CI, confidence interval.
Margin of primary tumor was not introduced in multivariate analysis due to missing data.
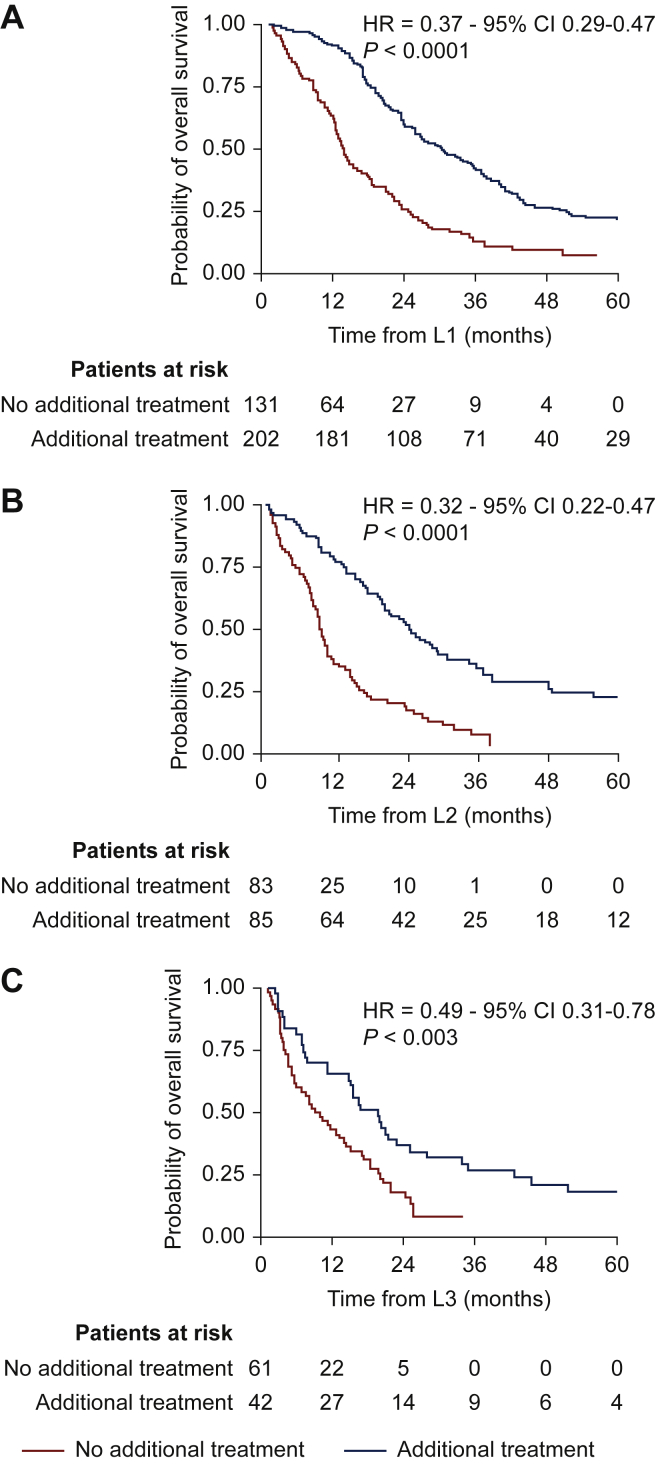
In the multivariate analysis, younger age, surgery of the primary tumor, and a single metastatic site remained independent favorable prognostic factors for OS in cohort 1 (Table 2). For cohort 2, primary surgery within an expert FSG center, absence of chemotherapy in the perioperative setting, the lung as a single metastatic site, time to first metastasis >12 months, local therapy as the first-line metastatic treatment, and ifosfamide in the first metastatic line were independent favorable prognostic factors (Table 3, Figure 1) (log-rank P value <0.001). The dose intensity of ifosfamide did not translate into improved OS [<9000 mg/m2, (log-rank P value = 0.059); 9000-12 000 mg/m2, (log-rank P value = 0.07); 12 000-14 000 mg/m2, (log-rank P value = 0.19); >14 000 mg/m2, (log-rank P value = 0.23)]. Even in advanced disease, in the second- or third-line treatment, additional focal treatment of the metastases provided better survival: the mPFS was 24.3 months versus 8.9 months (HR = 0.32, P < 0.001) in second-line and 19.5 months versus 7.7 months (HR = 0.49, P = 0.003) in third-line (Figure 2).
Figure 2.
Overall survival for (A) first-line, (B) second-line, and (C) third-line treatments of metastases depending on additional focal treatment.
CI, confidence interval; HR, hazard ratio.
Discussion
To our knowledge, the specific outcome of metastatic SS is not extensively reported in the literature. METASYN reports the FSG experience with one of the largest retrospective studies on metastatic adult and pediatric SS.
For prognostic factors, tumor size, usually observed in series focused on localized SS,19, 20, 21, 22 disappeared in favor of other parameters23: few metastases, location in the lungs, and occurrence beyond 1year were significantly linked to better OS in this study, in accordance with the literature.19, 20, 21,24,25 Age as a prognostic factor is a point of debate, as proven in localized SS.7,12,20,23,26,27 This has been less clearly established in the metastatic setting due to both the low number of studies and the contradictory results that have been observed.14,19,21,26 In the current study, young age as a continuous variable remained a favorable prognostic factor for synchronous metastatic patients. Beyond age, the genomic profiles of adult and pediatric SS patients backed up the hypothesis of heterogeneity for SS: Complexity Index in Sarcomas (CINSARC) and genomic index show that the adult tumor genome is more frequently rearranged.9,11
Concerning routine clinical practice, METASYN shows that surgery of the primary tumor with R0 margins and carried out in a reference center confers a significant advantage for OS, as well as for patients with de novo metastases in multivariate analyses. In the field of oncology, primary tumor surgery in the de novo metastatic setting is often debated. The most recent trials on clear-cell renal carcinoma and prostate cancer have led to new guidelines.28,29 For sarcoma, the literature insists on surgery for local control rather than for survival.30 Furthermore, the complexity of the surgery has not made such trials possible. The ‘METASYN’ results support R0 surgery of the primary tumor being offered in the metastatic setting, especially in young patients with oligometastases, provided that the surgery is reasonable.21
For metastases, the value of focal treatment is also debated, with limited evidence-based medicine. Twenty years ago, the Royal Marsden Hospital (RMH) experiment revealed no impact of metastasis surgery on survival.14 Nevertheless, by identifying metastases earlier with an efficient computed tomography scan, focal treatments could be proposed for less advanced disease and probably contribute to this trend. METASYN demonstrates better OS for metastasis surgery in multivariate analysis in accordance with studies dedicated to SS.19,26,31 Offering focal treatment to selected patients with lung oligometastases, even for synchronous metastases or after several lines of treatment, is a fair option as late relapse is not unusual in SS.13,15,25,31
Perioperative chemotherapy is not standard for STS, and European Society for Medical Oncology (ESMO) guidelines do not systematically recommend adjuvant chemotherapy as standard treatment (leaving it to the multidisciplinary tumor board to decide on a case per case basis in view of insufficient evidence).32, 33, 34, 35, 36, 37, 38 For SS, chemotherapy is a debated moot point too.19,20,39,40 There are not enough prospective studies for this rare histotype, and the most representative is the ISG-STS 1001 trial: no superiority for histotype-tailored chemotherapy was reported, but caution is needed when interpreting the results: standard treatment made possible better PFS and OS for certain patients, with a high risk of metastasis.37,41
METASYN shows that chemotherapy in a (neo)adjuvant setting is associated with worse survival in multivariate analyses. For patients treated with ifosfamide as the first line for the metastases, mPFS was similar whether or not they had received ifosfamide in a (neo)adjuvant setting (P = 0.80). The retrospective nature of this study obviously makes it prone to bias, here related to the aggressiveness of the disease. Histological data with monophasic or biphasic subtypes or fusion partners (SS1, SS2) were not available. As a result of all this, absolutely no conclusions can be drawn.
In this context, the FSG’s decision is to carry on working to better define the role of adjuvant chemotherapy, and a further step is ongoing with a new prognostic tool, the CINSARC signature,9,10 incorporated into several trials in the NetSarc network in an adjuvant setting (CHIC-STS01, CIRSARC).42, 43, 44, 45, 46 For the first results in high-risk STS patients treated with preoperative chemotherapy with radiochemotherapy, CINSARC did not correlate with different disease-free survival and OS. While this may well be due to a failure of this specific gene signature in this specific patient population, an alternative hypothesis is that preoperative chemotherapy may improve the prognosis of higher-risk patients.47
In the metastatic setting, METASYN shows that ifosfamide-based chemotherapy is still the backbone of treatment, with the well-known chemosensitivity of SS,14,48, 49, 50, 51, 52, 53 and yields superior ORR, PFS, and OS even without dose dense intensity. In METASYN, no link between dose dense intensity and survival was observed. Nevertheless, in this retrospective study, only the initial schedule was observed and not any potential dose reduction. As proposed in the clinical practice guidelines,36 the METASYN results support using multiagent chemotherapy with ifosfamide for selected patients with symptoms and oligometastatic disease as part of a multidisciplinary approach.33,54 METASYN confirms the chemosensitivity of SS beyond the first line, as already demonstrated with a rechallenge of both ifosfamide and other agents.14,15,48 Trabectedin is used in more than a quarter of responders as the third line according to series that reported objective responses and high rates of stable disease (30%-50%), even for heavily pretreated patients, with OS ranging between 9 and 13 months. Trabectedin should be an option for certain patients with SS, even if better results are obtained in L-sarcomas.50,55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65 TKIs for SS appear to be another major treatment in METASYN, with mPFS equal to 4.4 months. Since 2009, TKIs have demonstrated activity with pazopanib with a 49% 3-month PFS rate,66 and later on in PALETTE with better PFS for leiomyosarcomas and SS, and with regorafenib in REGOSARC with an mPFS of 5.6 months.16,67 Finally, new agents have emerged thanks to active research and identification of immunogenic antigens (NY-ESO-1 and MAGE-A4) frequently expressed on SS.68,69 After the initial disappointment with anti-programmed cell death protein 1 immunotherapy, 11% and 0% partial responses in the SARC 028 and ALLIANCE A091401 trials, respectively, promising data have been reported recently.70, 71, 72 A phase I trial by the Memorial Sloan Kettering Cancer Center (MSKCC) with letetresgene, an NY-ESO-1-specific T-cell receptor T-cell therapy, reported significant results: 50% ORR, mPFS of 15 months, and median OS of 24 months for heavily pretreated patients.17 The MD Anderson Cancer Center carried out another phase I trial with ADP-A2M4 T-cell therapy for MAGE-A4+ SS, obtaining impressive results: 44% ORR and mPFS of 20 weeks.17,18,72 These trials are currently in progress.
In the METASYN study, 34/339 (10%) patients were alive after 5 years. The clinical parameters for longer survival included metachronous metastases (P = 0.057), younger patients (P = 0.039), few metastases (P = 0.02), and lung location (P = 0.004). Treatment parameters significantly linked to this longer survival were: surgery of the primary tumor (P = 0.021), surgery in a NetSarc center (P = 0.036), and focal treatment of metastases (P < 0.001).
The fact that patient records are kept over a long period of time, in our case almost 40 years, is always questionable because of classification and treatment changes over time, although this does not apply to SS, and for a rare histotype it is relatively common.14,20,26,27 The positive effect of time is that it is possible to make comparisons across periods in real life. First of all, METASYN reports the same clinical characteristics across various periods and countries: synchronous metastases for a quarter of the patients, limbs as the main site,14,20,21,73 primary tumor size of >5 cm,14,20,21,73 especially in patients with synchronous metastases,20, 21, 22,74 and the lungs as the usual metastatic site.14,20,21 The second criticism is that the FSG database includes data for all patients but because the size of the cohorts was too small between children and adults, the differences in clinical presentation could not be shown. In published series, mostly focused on patients with localized SS, no difference has been observed, even if a trend has been identified in the literature with a higher proportion of locations in the thigh, large invasive tumors, and an increased risk of synchronous metastases for AYA in comparison to children.7,26,27 Third, and because of the retrospective nature of METASYN, caution is needed when evaluating the response rate as there was probably not always a RECIST reference or a centralized radiological review. Nevertheless, these results provide a trend for agents and that is useful in SS in real life, with ifosfamide and secondly TKIs and trabectedin as benchmarks for new agents. So far, few studies have provided a response rate for one sarcoma subtype in several lines outside of trials.15,16 The same comment can be made with regard to PFS, again with caution in the interpretation. Nevertheless, the mPFS in first-line treatment with ifosfamide, 6-7 months, seems close to the mPFS in other series.15,75 Finally, METASYN provides median OS and 5-year OS of 22 months and 14.8%, which is quite similar to previous series with median OS between 15 and 22 months.13, 14, 15,49,65,75 This helps us feel confident with these results. With a long recruitment period, METASYN shows no improvement across periods and emphasizes the unmet need for new agents in SS. Survival was better than the OS in the EORTC trials15; perhaps administering several lines with new agents, even ‘off-label’, contributed to a survival advantage in METASYN. This is quite interesting, and once again similar to previous studies. It demonstrates that a survival advantage through the choice of polychemotherapy as the first line had to be stated for medical practice in the specific cases mentioned above.15,65,75 The lack of power in the latest EORTC trial in the first line, A versus AI, may explain the different conclusion.54 Monotherapy should nevertheless always be considered in palliative sarcoma cases with very poor prognosis as a means of improving quality of life.54
Conclusion
This new study by the Conticabase network confirms that surgery is the mainstay treatment in reference centers for improving OS. METASYN emphasizes the importance for OS of focal treatment of metastases. (Neo)adjuvant treatment has undoubtedly been a never-ending moot point and patients need to be enrolled in the current clinical trial based on the CINSARC signature. Finally, this study offers real-life results in a metastatic setting and is a useful support for developing promising new strategies.
Acknowledgements
GSF-GETO, Net-SArc and RRePS.
Funding
None declared.
Disclosure
JYB: research support and honoraria from Novartis, GlaxoSmithKline, Bayer, Roche, Deciphera, Ignyta, Merck Sharp & Dohme (MSD), Bristol Myers Squibb (BMS), Pharmamar. OM: declares financial interests from Bayer, Blueprint Medicines, BMS, Eli Lilly, Ipsen, MSD, Pfizer, Roche, Servier, Bayer. All other authors have declared no conflicts of interest. JYB holds grants from NetSarc (INCA and DGOS) and RREPS (INCA & DGOS), RESOS (INCA & DGOS), LYRICAN (INCA-DGOS-INSERM 12563, Association DAM’s, Eurosarc (FP7-278742), Fondation ARC, Infosarcome, InterSARC (INCA), LabEx DEvweCAN (ANR-10-LABX0061), PIA Institut Convergence François Rabelais PLAsCAN (PLASCAN, 17-CONV-0002), La Ligue de L’Ain contre le Cancer, La Ligue contre le Cancer, EURACAN (EC 739521), and RHU4 DEPGYN (ANR-18-RHUS-0009).
Supplementary data
References
- 1.Fletcher C.D.M. The evolving classification of soft tissue tumours: an update based on the new WHO classification. Histopathology. 2006;48(1):3–12. doi: 10.1111/j.1365-2559.2005.02284.x. [DOI] [PubMed] [Google Scholar]
- 2.Ladanyi M. Fusions of the SYT and SSX genes in synovial sarcoma. Oncogene. 2001;20(40):5755–5762. doi: 10.1038/sj.onc.1204601. [DOI] [PubMed] [Google Scholar]
- 3.Krieg A.H., Hefti F., Speth B.M., et al. Synovial sarcomas usually metastasize after >5 years: a multicenter retrospective analysis with minimum follow-up of 10 years for survivors. Ann Oncol. 2011;22(2):458–467. doi: 10.1093/annonc/mdq394. [DOI] [PubMed] [Google Scholar]
- 4.Coindre J.M., Terrier P., Guillou L., et al. Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer. 2001;91(10):1914–1926. doi: 10.1002/1097-0142(20010515)91:10<1914::aid-cncr1214>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 5.de Pinieux G., Karanian M., Le Loarer F., et al. Nationwide incidence of sarcomas and connective tissue tumors of intermediate malignancy over four years using an expert pathology review network. PLoS One. 2021;16(2) doi: 10.1371/journal.pone.0246958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrari A., Sultan I., Huang T.T., et al. Soft tissue sarcoma across the age spectrum: a population-based study from the surveillance epidemiology and end results database: Soft Tissue Sarcomas Across Age Groups. Pediatr Blood Cancer. 2011;57(6) doi: 10.1002/pbc.23252. 943-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sultan I., Rodriguez-Galindo C., Saab R., Yasir S., Casanova M., Ferrari A. Comparing children and adults with synovial sarcoma in the Surveillance, Epidemiology, and End Results program, 1983 to 2005: an analysis of 1268 patients. Cancer. 2009;115(15):3537–3547. doi: 10.1002/cncr.24424. [DOI] [PubMed] [Google Scholar]
- 8.Ducimetière F., Lurkin A., Ranchère-Vince D., et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One. 2011;6(8) doi: 10.1371/journal.pone.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagarde P., Przybyl J., Brulard C., et al. Chromosome instability accounts for reverse metastatic outcomes of pediatric and adult synovial sarcomas. J Clin Oncol. 2013;31(5):608–615. doi: 10.1200/JCO.2012.46.0147. [DOI] [PubMed] [Google Scholar]
- 10.Chibon F., Lagarde P., Salas S., et al. Validated prediction of clinical outcome in sarcomas and multiple types of cancer on the basis of a gene expression signature related to genome complexity. Nat Med. 2010;16(7):781–787. doi: 10.1038/nm.2174. [DOI] [PubMed] [Google Scholar]
- 11.Vlenterie M., Hillebrandt-Roeffen M.H.S., Flucke U.E., et al. Next generation sequencing in synovial sarcoma reveals novel gene mutations. Oncotarget. 2015;6(33):34680–34690. doi: 10.18632/oncotarget.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brennan B., Stiller C., Grimer R., Dennis N., Broggio J., Francis M. Outcome and the effect of age and socioeconomic status in 1318 patients with synovial sarcoma in the English National Cancer Registry: 1985–2009. Clin Sarcoma Res. 2016;6(1):18. doi: 10.1186/s13569-016-0058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Italiano A., Mathoulin-Pelissier S., Cesne A.L., et al. Trends in survival for patients with metastatic soft-tissue sarcoma. Cancer. 2011;117(5):1049–1054. doi: 10.1002/cncr.25538. [DOI] [PubMed] [Google Scholar]
- 14.Spurrell E.L., Fisher C., Thomas J.M., Judson I.R. Prognostic factors in advanced synovial sarcoma: an analysis of 104 patients treated at the Royal Marsden Hospital. Ann Oncol. 2005;16(3):437–444. doi: 10.1093/annonc/mdi082. [DOI] [PubMed] [Google Scholar]
- 15.Vlenterie M., Litière S., Rizzo E., et al. Outcome of chemotherapy in advanced synovial sarcoma patients: review of 15 clinical trials from the European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group; setting a new landmark for studies in this entity. Eur J Cancer. 2016;58:62–72. doi: 10.1016/j.ejca.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Mir O., Brodowicz T., Italiano A., et al. Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016;17(12):1732–1742. doi: 10.1016/S1470-2045(16)30507-1. [DOI] [PubMed] [Google Scholar]
- 17.D’Angelo S., Demetri G., Tine B.V., et al. 298 Final analysis of the phase 1 trial of NY-ESO-1-specific T-cell receptor (TCR) T-cell therapy (letetresgene autoleucel; GSK3377794) in patients with advanced synovial sarcoma (SS) J Immunother Cancer. 2020;8(suppl 3) https://jitc.bmj.com/content/8/Suppl_3/A325 Available at. [Google Scholar]
- 18.Durable Responses with ADP-A2M4 in Synovial Sarcoma with Confirmed Responses in 44% of Patients and Disease Control Rate of 94% Presented at CTOS [Internet]. GlobeNewswire News Room. 2020. http://www.globenewswire.com/news-release/2020/11/19/2130181/0/en/Durable-Responses-with-ADP-A2M4-in-Synovial-Sarcoma-with-Confirmed-Responses-in-44-of-Patients-and-Disease-Control-Rate-of-94-Presented-at-CTOS.html Available at.
- 19.Stanelle E.J., Christison-Lagay E.R., Healey J.H., Singer S., Meyers P.A., La Quaglia M.P. Pediatric and adolescent synovial sarcoma: multivariate analysis of prognostic factors and survival outcomes. Ann Surg Oncol. 2013;20(1):73–79. doi: 10.1245/s10434-012-2587-9. [DOI] [PubMed] [Google Scholar]
- 20.Palmerini E., Staals E.L., Alberghini M., et al. Synovial sarcoma: retrospective analysis of 250 patients treated at a single institution. Cancer. 2009;115(13) doi: 10.1002/cncr.24370. 2988-2898. [DOI] [PubMed] [Google Scholar]
- 21.Scheer M., Dantonello T., Hallmen E., et al. Primary metastatic synovial sarcoma: experience of the CWS Study Group. Pediatr Blood Cancer. 2016;63(7):1198–1206. doi: 10.1002/pbc.25973. [DOI] [PubMed] [Google Scholar]
- 22.Spillane A.J., A’Hern R., Judson I.R., Fisher C., Thomas J.M. Synovial sarcoma: a clinicopathologic, staging, and prognostic assessment. J Clin Oncol. 2000;18(22):3794–3803. doi: 10.1200/JCO.2000.18.22.3794. [DOI] [PubMed] [Google Scholar]
- 23.Vlenterie M., Ho V.K.Y., Kaal S.E.J., Vlenterie R., Haas R., van der Graaf W.T.A. Age as an independent prognostic factor for survival of localised synovial sarcoma patients. Br J Cancer. 2015;113(11):1602–1606. doi: 10.1038/bjc.2015.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee K., Kang M.C., Lee H.W., et al. Pulmonary metastasectomy in adult patients with synovial sarcoma: a single-center experience. Korean J Thorac Cardiovasc Surg. 2016;49(6):451–455. doi: 10.5090/kjtcs.2016.49.6.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cariboni U., De Sanctis R., Giaretta M., et al. Survival outcome and prognostic factors after pulmonary metastasectomy in sarcoma patients: a 18-year experience at a single high-volume referral center. Am J Clin Oncol. 2019;42(1):6–11. doi: 10.1097/COC.0000000000000476. [DOI] [PubMed] [Google Scholar]
- 26.Smolle M.A., Parry M., Jeys L., Abudu S., Grimer R. Synovial sarcoma: do children do better? Eur J Surg Oncol. 2019;45(2):254–260. doi: 10.1016/j.ejso.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Scheer M., Blank B., Bauer S., et al. The Cooperative Weichteilsarkom Studiengruppe [CWS] Synovial sarcoma disease characteristics and primary tumor sites differ between patient age groups: a report of the Cooperative Weichteilsarkom Studiengruppe (CWS) J Cancer Res Clin Oncol. 2020;146(4):953–960. doi: 10.1007/s00432-019-03121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parker C.C., James N.D., Brawley C.D., et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353–2366. doi: 10.1016/S0140-6736(18)32486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Méjean A., Ravaud A., Thezenas S., et al. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N Engl J Med. 2018;379(5):417–427. doi: 10.1056/NEJMoa1803675. [DOI] [PubMed] [Google Scholar]
- 30.Bonvalot S., Levy A., Terrier P., et al. Primary extremity soft tissue sarcomas: does local control impact survival? Ann Surg Oncol. 2017;24(1):194–201. doi: 10.1245/s10434-016-5462-2. [DOI] [PubMed] [Google Scholar]
- 31.Blackmon S.H., Shah N., Roth J.A., et al. Resection of pulmonary and extrapulmonary sarcomatous metastases is associated with long-term survival. Ann Thorac Surg. 2009;88(3):877–885. doi: 10.1016/j.athoracsur.2009.04.144. [DOI] [PubMed] [Google Scholar]
- 32.Pervaiz N., Colterjohn N., Farrokhyar F., Tozer R., Figueredo A., Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113(3):573–581. doi: 10.1002/cncr.23592. [DOI] [PubMed] [Google Scholar]
- 33.Casali P.G., Abecassis N., Aro H.T., et al. Soft tissue and visceral sarcomas: ESMO–EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv51–iv67. doi: 10.1093/annonc/mdy096. [DOI] [PubMed] [Google Scholar]
- 34.Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma Meta-Analysis Collaboration. Lancet. 1997;350(9092):1647–1654. [PubMed] [Google Scholar]
- 35.Frustaci S., Gherlinzoni F., De Paoli A., et al. Adjuvant chemotherapy for adult soft tissue sarcomas of the extremities and girdles: results of the Italian randomized cooperative trial. J Clin Oncol. 2001;19(5):1238–1247. doi: 10.1200/JCO.2001.19.5.1238. [DOI] [PubMed] [Google Scholar]
- 36.Le Cesne A., Ouali M., Leahy M.G., et al. Doxorubicin-based adjuvant chemotherapy in soft tissue sarcoma: pooled analysis of two STBSG-EORTC phase III clinical trials. Ann Oncol. 2014;25(12):2425–2432. doi: 10.1093/annonc/mdu460. [DOI] [PubMed] [Google Scholar]
- 37.Gronchi A., Ferrari S., Quagliuolo V., et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 2017;18(6):812–822. doi: 10.1016/S1470-2045(17)30334-0. [DOI] [PubMed] [Google Scholar]
- 38.Eilber F.C., Brennan M.F., Eilber F.R., et al. Chemotherapy is associated with improved survival in adult patients with primary extremity synovial sarcoma. Ann Surg. 2007;246(1):105–113. doi: 10.1097/01.sla.0000262787.88639.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Italiano A., Penel N., Robin Y.-M., et al. Neo/adjuvant chemotherapy does not improve outcome in resected primary synovial sarcoma: a study of the French Sarcoma Group. Ann Oncol. 2009;20(3):425–430. doi: 10.1093/annonc/mdn678. [DOI] [PubMed] [Google Scholar]
- 40.Okcu M.F., Munsell M., Treuner J., et al. Synovial sarcoma of childhood and adolescence: a multicenter, multivariate analysis of outcome. J Clin Oncol. 2003;21(8):1602–1611. doi: 10.1200/JCO.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Pasquali S., Pizzamiglio S., Touati N., et al. The impact of chemotherapy on survival of patients with extremity and trunk wall soft tissue sarcoma: revisiting the results of the EORTC-STBSG 62931 randomised trial. Eur J Cancer. 2019;109:51–60. doi: 10.1016/j.ejca.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 42.Benefit of intensified peri-operative chemotherapy within high-risk CINSARC patients with resectable soft-tissue sarcomas - full text view - ClinicalTrials.gov [Internet] https://clinicaltrials.gov/ct2/show/NCT03805022 Available at.
- 43.Institut Claudius Regaud Interest of Peri Operative Chemotherapy in Patients with CINSARC High-risk Localized Grade 1 or 2 Soft Tissue Sarcoma. Clinicaltrials.gov; 2020 Nov. Report No.: NCT04307277. https://clinicaltrials.gov/ct2/show/NCT04307277 Available at.
- 44.Orbach D., Mosseri V., Pissaloux D., et al. Genomic complexity in pediatric synovial sarcomas (Synobio study): the European pediatric soft tissue sarcoma group (EpSSG) experience. Cancer Med. 2018;7(4):1384–1393. doi: 10.1002/cam4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Italiano A. Is there value in molecular profiling of soft-tissue sarcoma? Curr Treat Options Oncol. 2018;19(12):78. doi: 10.1007/s11864-018-0589-y. [DOI] [PubMed] [Google Scholar]
- 46.Valentin T., Lesluyes T., Le Guellec S., Chibon F. Chemotherapy in localized soft tissue sarcoma: will we soon have to treat grade 1 tumors? Update on CINSARC performances. Ann Oncol. 2019;30(1):153–155. doi: 10.1093/annonc/mdy465. [DOI] [PubMed] [Google Scholar]
- 47.Pasquali S., Braglia L., Chibon F., et al. The prognostic value of CINSARC in a randomised trial comparing histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001) J Clin Oncol. 2020;38(15 Suppl) [Google Scholar]
- 48.Sleijfer S., Ouali M., van Glabbeke M., et al. Prognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: an exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG) Eur J Cancer. 2010;46(1):72–83. doi: 10.1016/j.ejca.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 49.Rosen G., Forscher C., Lowenbraun S., et al. Synovial sarcoma. Uniform response of metastases to high dose ifosfamide. Cancer. 1994;73(10):2506–2511. doi: 10.1002/1097-0142(19940515)73:10<2506::aid-cncr2820731009>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 50.Le Cesne A., Antoine E., Spielmann M., et al. High-dose ifosfamide: circumvention of resistance to standard-dose ifosfamide in advanced soft tissue sarcomas. J Clin Oncol. 1995;13(7):1600–1608. doi: 10.1200/JCO.1995.13.7.1600. [DOI] [PubMed] [Google Scholar]
- 51.Oosterom AT van, Mouridsen H.T., Nielsen O.S., et al. Results of randomised studies of the EORTC Soft Tissue and Bone Sarcoma Group (STBSG) with two different ifosfamide regimens in first- and second-line chemotherapy in advanced soft tissue sarcoma patients. Eur J Cancer. 2002;38(18):2397–2406. doi: 10.1016/s0959-8049(02)00491-4. [DOI] [PubMed] [Google Scholar]
- 52.Worden F.P., Taylor J.M.G., Biermann J.S., et al. Randomized phase II evaluation of 6 g/m2 of ifosfamide plus doxorubicin and granulocyte colony-stimulating factor (G-CSF) compared with 12 g/m2 of ifosfamide plus doxorubicin and G-CSF in the treatment of poor-prognosis soft tissue sarcoma. J Clin Oncol. 2005;23(1):105–112. doi: 10.1200/JCO.2005.05.108. [DOI] [PubMed] [Google Scholar]
- 53.Nielsen O.S., Judson I., van Hoesel Q., et al. Effect of high-dose ifosfamide in advanced soft tissue sarcomas. a multicentre phase II study of the EORTC soft tissue and bone sarcoma group. Eur J Cancer. 2000;36(1):61–67. doi: 10.1016/s0959-8049(99)00240-3. [DOI] [PubMed] [Google Scholar]
- 54.Judson I., Verweij J., Gelderblom H., et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15(4):415–423. doi: 10.1016/S1470-2045(14)70063-4. [DOI] [PubMed] [Google Scholar]
- 55.Kawai A., Araki N., Sugiura H., et al. Trabectedin monotherapy after standard chemotherapy versus best supportive care in patients with advanced, translocation-related sarcoma: a randomised, open-label, phase 2 study. Lancet Oncol. 2015;16(4):406–416. doi: 10.1016/S1470-2045(15)70098-7. [DOI] [PubMed] [Google Scholar]
- 56.Le Cesne A., Blay J.Y., Judson I., et al. Phase II study of ET-743 in advanced soft tissue sarcomas: a European Organisation for the Research and Treatment of Cancer (EORTC) soft tissue and bone sarcoma group trial. J Clin Oncol. 2005;23(3):576–584. doi: 10.1200/JCO.2005.01.180. [DOI] [PubMed] [Google Scholar]
- 57.Cesne A.L., Cresta S., Maki R.G., et al. A retrospective analysis of antitumour activity with trabectedin in translocation-related sarcomas. Eur J Cancer. 2012;48(16):3036–3044. doi: 10.1016/j.ejca.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 58.Le Cesne A., Ray-Coquard I., Duffaud F., et al. Trabectedin in patients with advanced soft tissue sarcoma: a retrospective national analysis of the French Sarcoma Group. Eur J Cancer. 2015;51(6):742–750. doi: 10.1016/j.ejca.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 59.Le Cesne A., Blay J.-Y., Domont J., Tresch-Bruneel E., Chevreau C., Bertucci F., et al. Interruption versus continuation of trabectedin in patients with soft-tissue sarcoma (T-DIS): a randomised phase 2 trial. Lancet Oncol. 2015;16(3):312–319. doi: 10.1016/S1470-2045(15)70031-8. [DOI] [PubMed] [Google Scholar]
- 60.Blay J.-Y., Italiano A., Ray-Coquard I., et al. Long-term outcome and effect of maintenance therapy in patients with advanced sarcoma treated with trabectedin: an analysis of 181 patients of the French ATU compassionate use program. BMC Cancer. 2013;13:64. doi: 10.1186/1471-2407-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Angarita F.A., Cannell A.J., Abdul Razak A.R., Dickson B.C., Blackstein M.E. Trabectedin for inoperable or recurrent soft tissue sarcoma in adult patients: a retrospective cohort study. BMC Cancer. 2016;16(1):30. doi: 10.1186/s12885-016-2054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buonadonna A., Benson C., Casanova J., et al. A noninterventional, multicenter, prospective phase IV study of trabectedin in patients with advanced soft tissue sarcoma. Anticancer Drugs. 2017;28(10):1157–1165. doi: 10.1097/CAD.0000000000000560. [DOI] [PubMed] [Google Scholar]
- 63.Araki N., Takahashi S., Sugiura H., et al. Retrospective inter- and intra-patient evaluation of trabectedin after best supportive care for patients with advanced translocation-related sarcoma after failure of standard chemotherapy. Eur J Cancer. 2016;56:122–130. doi: 10.1016/j.ejca.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 64.Sleijfer S., Ray-Coquard I., Papai Z., et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043) J Clin Oncol. 2009;27(19):3126–3132. doi: 10.1200/JCO.2008.21.3223. [DOI] [PubMed] [Google Scholar]
- 65.Sanfilippo R., et al. Trabectedin in advanced synovial sarcomas: a multicenter retrospective study from four European institutions and the Italian Rare Cancer Network. Anticancer Drugs. 2015;26:678–681. doi: 10.1097/CAD.0000000000000228. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van der Graaf W.T.A., Blay J.-Y., Chawla S.P., et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379(9829):1879–1886. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 67.Ramachandran I., Lowther D.E., Dryer-Minnerly R., et al. Systemic and local immunity following adoptive transfer of NY-ESO-1 SPEAR T cells in synovial sarcoma. J Immunother Cancer. 2019;7(1):276. doi: 10.1186/s40425-019-0762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Tine B.A., Butler M.O., Araujo D., et al. ADP-A2M4 (MAGE-A4) in patients with synovial sarcoma. Ann Oncol. 2019;30:v684–v685. [Google Scholar]
- 69.D’Angelo S.P., Mahoney M.R., Van Tine B.A., et al. Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 2018;19(3):416–426. doi: 10.1016/S1470-2045(18)30006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tawbi H.A., Burgess M., Bolejack V., et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017;18(11):1493–1501. doi: 10.1016/S1470-2045(17)30624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Toulmonde M., Penel N., Adam J., et al. Use of PD-1 targeting, macrophage infiltration, and IDO pathway activation in sarcomas. JAMA Oncol. 2018;4(1):93–97. doi: 10.1001/jamaoncol.2017.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.D’Angelo S.P., Melchiori L., Merchant M.S., et al. Antitumor activity associated with prolonged persistence of adoptively transferred NY-ESO-1 c259 T Cells in synovial sarcoma. Cancer Discov. 2018;8(8):944–957. doi: 10.1158/2159-8290.CD-17-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pollack S.M., Somaiah N., Araujo D.M., et al. Clinical outcomes of patients with advanced synovial sarcoma or myxoid/round cell liposarcoma treated at major cancer centers in the United States. Cancer Med. 2020;9(13):4593–4602. doi: 10.1002/cam4.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferrari A., Salvo G.L.D., Dall’Igna P., et al. Salvage rates and prognostic factors after relapse in children and adolescents with initially localised synovial sarcoma. Eur J Cancer. 2012;48(18):3448–3455. doi: 10.1016/j.ejca.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 75.Savina M., Le Cesne A., Blay J.-Y., et al. Patterns of care and outcomes of patients with METAstatic soft tissue SARComa in a real-life setting: the METASARC observational study. BMC Med. 2017;15(1):78. doi: 10.1186/s12916-017-0831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.