Abstract
Background
The phase I GATTO study (NCT03360734) explored the feasibility, tolerability and preliminary activity of combining gatipotuzumab, a novel humanized monoclonal antibody binding to the tumor-associated epitope of mucin 1 (TA-MUC1) and an anti-epidermal growth factor receptor (anti-EGFR) antibody in refractory solid tumors.
Patients and methods
Initially the study enrolled primary phase (PP) patients with EGFR-positive metastatic solid tumors, for whom no standard treatment was available. Patients received gatipotuzumab administered at 1400 mg every 2 weeks, 6 weeks after the start of the glyco-optimized anti-EGFR antibody tomuzotuximab at 1200 mg every 2 weeks. As this regimen was proven safe, enrollment continued in an expansion phase (EP) of patients with refractory metastatic colorectal cancer, non-small-cell lung cancer, head and neck cancer and breast cancer. Tomuzotuximab and gatipotuzumab were given at the same doses and gatipotuzumab treatment started 1 week after the first dose of the anti-EGFR antibody. Additionally, investigators could use a commercial anti-EGFR antibody in place of tomuzotuximab.
Results
A total of 52 patients were enrolled, 20 in the PP and 32 in the EP. The combined treatment was well tolerated and no dose-limiting toxicity was observed in the whole study, nor related serious adverse event or death. Preliminary activity of the combination was observed, with one and four RECIST partial responses in the PP and EP, all in colorectal cancer patients. The trial was accompanied by a comprehensive translational research program for identification of biomarkers, including soluble TA-MUC1 (sTA-MUC1) in serum. In the EP, patients with baseline sTA-MUC1 levels above the median appeared to have improved progression-free survival and overall survival.
Conclusions
Combination of a TA-MUC1-targeting antibody and an EGFR-targeting antibody is safe and feasible. Interesting antitumor activity was observed in heavily pretreated patients. Future studies should test this combination together with chemotherapy and explore the potential of sTA-MUC1 as a companion biomarker for further development of the combination.
Key words: phase I, monoclonal antibody, EGFR, TA-MUC1, colorectal cancer, lung cancer
Highlights
-
•
Gatipotuzumab is a humanized antibody targeting TA-MUC1 that can be safely combined with anti-EGFR antibodies.
-
•
The combination of gatipotuzumab and an anti-EGFR antibody shows promising activity in refractory metastatic cancers patients.
-
•
Pretreatment levels of TA-MUC1 may be a useful biomarker to identify patients more likely to benefit.
-
•
The favorable safety profile warrants further assessment of the two antibodies also in combination with chemotherapy.
Introduction
Mucin 1 (MUC1 also known as episialin, PEM, H23Ag, EMA, CA15-3 and MCA) is a transmembrane protein with a heavily glycosylated extracellular domain normally expressed in glandular or luminal epithelial cells in a variety of organs; in healthy tissues, the extended negatively charged sugar branches of MUC1 protect the underlying epithelia by creating an anti-adhesive physical barrier preventing pathogenic colonization. Aberrantly glycosylated tumor-associated MUC1 (TA-MUC1) is overexpressed in most human epithelial cancers and has gained attention as an oncogenic molecule. TA-MUC1-expressing tumor cells become poorly adherent and metastatic. The barrier function of MUC1 also protects tumor cells from death by the host immune system and a variety of cytotoxic drugs normally used in cancer chemotherapies.1 Consequently, high-level MUC1 expression by tumors is frequently associated with a poor prognosis.2 The cytoplasmic MUC1 domain is known to interact with proteins with kinase activity [Protein Kinase C Delta (PKCd), Glycogen synthase kinase-3 beta (GSK3b), epidermal growth factor receptor (EGFR) and SRC Proto-Oncogene (c-Src)] and without kinase activity (Tumor protein p53, estrogen receptor alpha (ERa), beta-catenin) which are involved in different signaling pathways. Based on available evidence, TA-MUC1 plays a critical role in creating the conditions necessary for cancer development, through its interaction with several intracellular signaling pathways3, 4, 5 and its ability to regulate EGFR stability and its cellular localization.6,7
The EGFR pathway is often hyperactivated in multiple cancer types, and an association of MUC1 with EGFR has been demonstrated in several preclinical models with evidence of cross-talk between EGFR-related and MUC1-related signaling pathways7,8; for instance, EGFR inhibitors were shown to increase MUC1 shedding into plasma.8
Gatipotuzumab is a glyco-engineered humanized monoclonal antibody which binds TA-MUC1 on the surface of tumor cells, activating the immune system to induce antibody-dependent cellular cytotoxicity (ADCC) and phagocytosis against TA-MUC1-expressing tumor cells.9 A multicenter phase I study found that infusion-related reactions (IRRs) were the most common treatment-emergent adverse events (TEAE), mainly of mild severity and occurring at the first infusion.10 Efficacy data suggested that trough levels of at least 30 μg/ml were needed to achieve clinical benefit with gatipotuzumab. Tomuzotuximab is an improved second-generation anti-EGFR antibody that specifically binds to EGFR and acts as a competitive antagonist at the EGFR ligand binding site.11 Tomuzotuximab is designed to fully retain the antigen-binding properties of cetuximab, but is modified by fully human glycosylation and glycosylation optimization, thus improving its ADCC-mediated antitumor efficacy and preventing allergic reactions observed with cetuximab.12,13 In the clinical studies, tomuzotuximab infusions caused IRRs with higher frequency and severity than gatipotuzumab, mainly in the initial treatment cycles.
Here we report results from a single-arm phase Ib study and expansion phase (EP) designed to evaluate the safety, pharmacokinetics (PK) and preliminary activity of gatipotuzumab plus anti-EGFR antibodies (including tomuzotuximab and panitumumab) in patients with advanced solid tumors, for whom no standard treatment was available.
Material and methods
Study design and treatment
The GATTO study (NCT03360734) was a single-arm phase Ib basket trial divided into a primary phase (PP) including 20 patients and an EP including 30 patients, aiming to assess the safety and activity of combined therapy with gatipotuzumab and anti-EGFR antibodies.
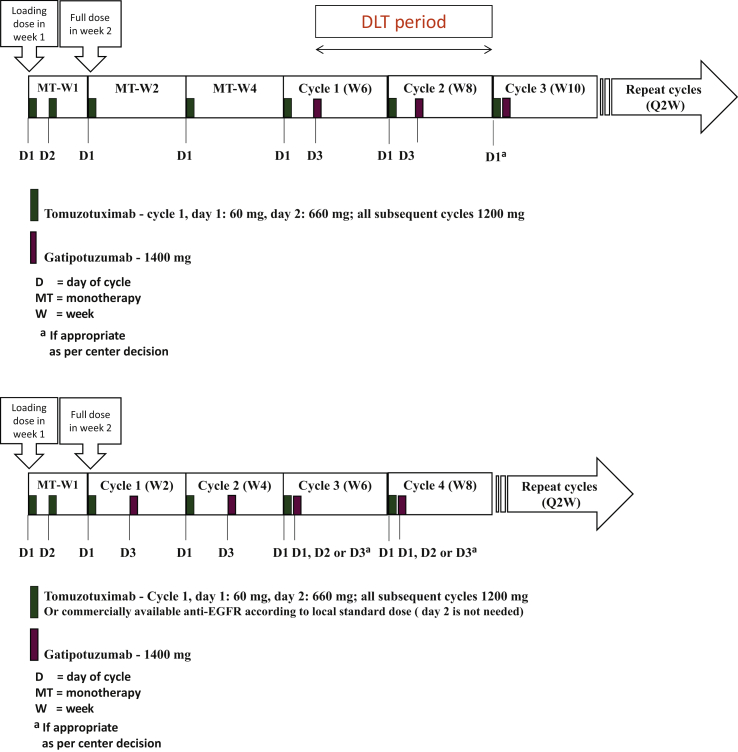
Tomuzotuximab was administered first, since this antibody induces more frequent and severe IRRs, the occurrences of which tend to decrease after repeated administrations. A dose of 1200 mg every 2 weeks (Q2W) tomuzotuximab was given starting from the second week of administration, preceded by a dose of 720 mg tomuzotuximab in the first week of administration. This initial dose, administered over 2 days, had the dual scope of reducing the severity of IRRs and acting as a loading dose before the Q2W scheduling of subsequent tomuzotuximab administrations. In the PP, gatipotuzumab was given at a dose of 1400 mg Q2W starting from week 6 (Figure 1). The first six patients in the PP were enrolled into a safety run-in phase and the number of dose-limiting toxicities (DLTs) were evaluated, in order to de-escalate the doses if needed.
Figure 1.
Treatment scheme in the 20 patients of the primary phase (upper diagram) and 30 patients of the expansion phase (lower diagram).
D, Day of cycle; EGFR, epidermal growth factor receptor; MT, monotherapy; Q2W, every 2 weeks; W, week.
After the safe treatment of 20 patients in the PP of the study and the absence of DLT, an additional 30 patients were enrolled in the EP with the combination of the two antibodies already started in the second week of treatment (Figure 1). Use of a commercially available anti-EGFR antibody instead of tomuzotuximab in combination with gatipotuzumab was allowed at the investigator’s choice in the EP. As these antibodies are not glyco-engineered like tomuzotuximab, their combination with gatipotuzumab was not expected to cause more toxicity, with improved convenience for patients and treating centers, as these antibodies do not require the split of the first infusion over 2 days.
Patients
The 20 patients in the PP had refractory solid tumors with positive EGFR immunohistochemistry (IHC) expression (≥25% of tumor cells) as assessed by a local laboratory, and were allocated to five cohorts: non-small-cell lung cancer (NSCLC), gastrointestinal cancer, breast cancer (BC), gynecological cancers and miscellaneous tumors (limited to 20% of the total sample size). Detailed inclusion criteria are reported in Supplementary Tables S1 and S2, available at https://doi.org/10.1016/j.esmoop.2022.100447.
In the EP, EGFR expression was not an inclusion criterion as the focus was shifted to refractory colorectal cancer (CRC) and other tumors with known anti-EGFR treatment sensitivity, as a surrogate for RAS wild type status at the time of prior treatment lines. These 30 additional patients were allocated to four cohorts: (i) refractory metastatic CRC (mCRC), who failed prior treatment with standard chemotherapeutics and both anti-vascular endothelial growth factor and anti-EGFR antibodies; (ii) recurrent and/or metastatic head and neck cancers who failed prior treatment with a checkpoint inhibitor and at least one line of chemotherapy as appropriate; (iii) refractory metastatic NSCLC who failed all standard treatment options; and (iv) refractory metastatic BC who failed all standard treatment options.
Endpoints and assessment
The safety and tolerability of the combination were the study primary endpoints. Secondary endpoints included activity parameters measured by RECIST 1.1 criteria, overall survival (OS), immunogenicity and PK.
Exploratory endpoints included a broad translational research (TR) program aimed to explore the relationship to antitumor activity and safety outcomes of several pharmacodynamic parameters, including soluble TA-MUC1 (sTA-MUC1), CA15-3, CA125 and EGFR (sEGFR) in serum analyzed by validated enzyme-linked immunosorbent assay or electrochemiluminescence immunoassay (ECLIA) assays and expression of the tumor-specific TA-MUC1 and EGFR in the tumor tissue samples analyzed by IHC. The serum biomarkers were analyzed before and after tomuzotuximab and gatipotuzumab infusions in all patients and for all cancer subtypes. The concentrations of these biomarkers were also assessed in relation to patient responder status. Analyses included measurements of absolute and relative changes from baseline for each biomarker investigated. For the central PK assessments, serum samples were taken and analyzed for tomuzotuximab concentrations immediately before (considered as Cmin) and directly after its infusion (considered as Cmax) from the first to the ninth infusion (PP) or seventh infusion (EP), 48 h after the start of the second infusion (only PP), immediately before and directly after the first two infusions of gatipotuzumab, and at the safety follow-up 28 days after the last infusion of tomuzotuximab. Gatipotuzumab concentrations were assessed in serum samples taken immediately before and directly after each infusion of gatipotuzumab from the first to the sixth infusion, 168 h (7 days) after the start of the third infusion, and at the safety follow-up visit. Antibody concentrations were analyzed by validated ECLIA-based assays.
Statistical analysis
No predefined statistical hypothesis was made, with all statistical analysis being descriptive in nature. All safety analyses were carried out on the safety population and were repeated for the combined treatment population. All analyses were done for the first batch of 20 patients, for the second batch of 30 patients and for the total of 50 patients.
Results
Safety
By the time of the final analysis in January 2021, 52 patients were enrolled and 50 received at least one dose of both gatipotuzumab and anti-EGFR antibodies (Table 1). In both study phases, at the explored doses and with different treatment schedules and different anti-EGFR antibody, no DLTs were observed, nor was there any need to de-escalate doses.
Table 1.
Participating patients by cancer subtype in the primary and expansion cohorts
| Cancer subtype, n (%) | Primary cohort (n = 20) | Expansion cohort (n = 30) | All patients (N = 50) |
|---|---|---|---|
| Colorectal cancer | 6 (30.0) | 19 (63.3) | 25 (50.0) |
| Gastrointestinal other | 5 (25.0) | 0 (0.0) | 5 (10.0) |
| Pancreas cancer | 4 (20.0) | 0 (0.0) | 4 (8.0) |
| Non-small-cell lung cancer | 0 (0.0) | 5 (16.7) | 5 (10.0) |
| Head and neck and salivary gland tumors | 3 (15.0) | 4 (13.3) | 7 (14.0) |
| Breast cancer | 0 (0.0) | 2 (6.7) | 2 (4.0) |
| Gynecological cancers | 1 (5.0) | 0 (0.0) | 1 (2.0) |
| Other | 1 (5.0) | 0 (0.0) | 1 (2.0) |
In the PP, all 20 patients experienced 186 TEAEs overall. The vast majority of reported TEAEs were mild to moderate (83.9%), whereas 26 events (14.0%) were severe, 3 (1.6%) were assessed as life-threatening and 1 (0.5%) was fatal. One mCRC patient had a fatal TEAE of pneumonia, unrelated to tomuzotuximab or gatipotuzumab. Nine TEAEs in five patients were reported as serious, none related to treatment drugs. Of the 186 reported TEAEs, 90 (48.4%) were considered related to treatment, all to tomuzotuximab, including 16 IRR events observed in eight patients. Twenty-two TEAEs were also considered related to gatipotuzumab.
In the EP, the investigators had the choice to use a commercially available anti-EGFR in the place of tomuzotuximab, and this was the case for nine CRC patients who received panitumumab in combination with gatipotuzumab. All 30 patients experienced 256 TEAEs overall, the vast majority being mild to moderate (95.7%), whereas 10 events (3.9%) were severe and 1 (0.4%) was assessed as life-threatening; no fatal TEAE occurred. Five TEAEs in five patients were reported as serious, none related to treatment drugs. Of the 256 reported TEAEs, 115 (44.9%) were considered related to treatment; of these, 104 (40.6%) TEAEs were considered related to tomuzotuximab or panitumumab, including 35 IRR events reported in 10 patients treated with tomuzotuximab, and 31 (12.1%) TEAEs were considered related to gatipotuzumab.
Related adverse effects were mainly IRRs expected with tomuzotuximab, and skin and metabolic toxicity commonly linked to anti-EGFR treatment. Tomuzotuximab-related IRRs were manageable and did not require treatment discontinuation. IRRs mainly occurred at first dosing in the PP of the study, whereas in the EP, some IRRs were observed also in later cycles, probably in relation to the earlier start of gatipotuzumab, but none had noteworthy severity or required treatment modifications. In the PP, eight patients experienced overall 16 events of IRRs, with only one event of chills being reported as severe. In the EP, 10 patients experienced 40 events of IRRs, all of mild or moderate intensity. More information about TEAEs can be found in Supplementary Tables S1 and S2, available at https://doi.org/10.1016/j.esmoop.2022.100447.
Antitumor activity
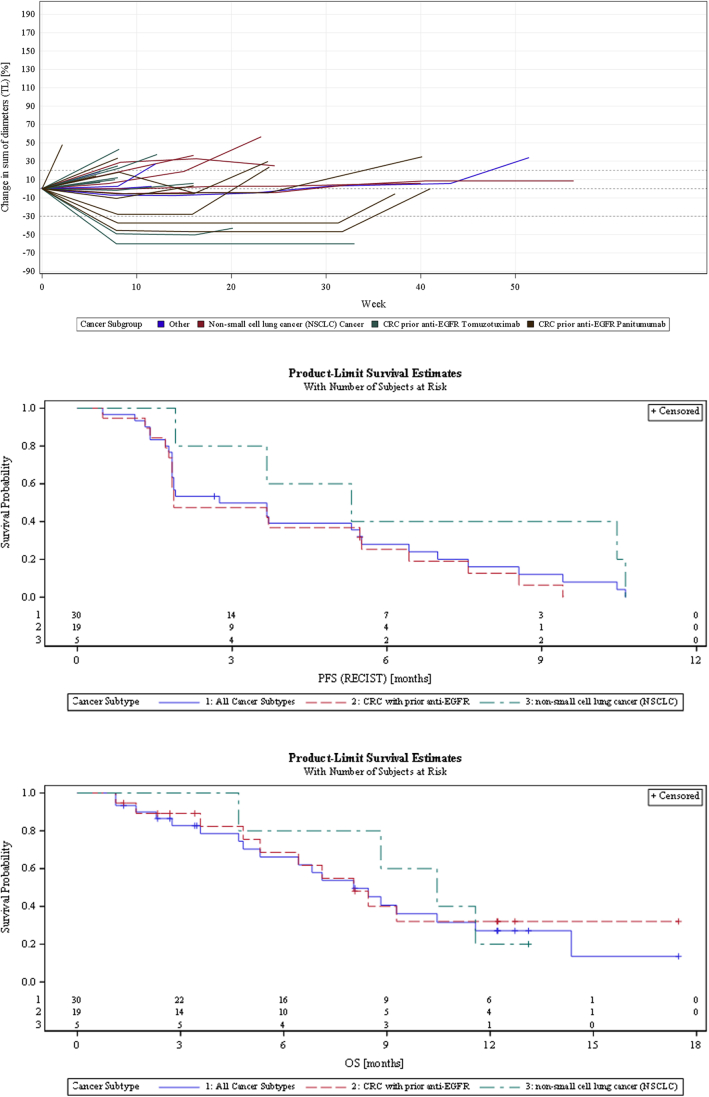
Median progression-free survival (PFS) in the 20 PP patients was 2.0 months [95% confidence interval (CI) 1.7-3.2 months] and median OS was 11.1 months (95% CI 4.1-18.7 months), as expected in late-stage metastatic cancer patients with no standard treatment options. Two responses were observed in mCRC patients who had received prior anti-EGFR treatment, only one confirmed by RECIST 1.1 criteria. Despite the rather small size of this subgroup of patients, these results were considered encouraging and warranting further exploration in the EP of the study. In the 30 patients participating in the EP, median PFS was 2.8 months (95% CI 1.8-5.5 months) and median OS was 8.0 months (95% CI 4.8-11.6 months) (Figure 2). OS in this phase of the study was to some extent affected by early censoring in five (16.7%) patients who withdrew their informed consent shortly after their disease progression and could no longer be followed.
Figure 2.
Spider plots and progression-free survival and overall survival Kaplan–Meier curves for the 30 expansion phase patients.
CRC, colorectal cancer; EGFR, epidermal growth factor receptor; OS, overall survival; PFS, progression-free survival; TL, target lesion.
Four confirmed RECIST 1.1 responses were observed out of 19 mCRC patients, two each in patients treated with either tomuzotuximab or panitumumab. PFS and duration of response (DOR) were numerically superior in mCRC patients treated with panitumumab [PFS 5.5 months (95% CI 0.5-8.6 months) and DOR 7.2 months (95% CI 6.7-7.6 months)] compared with those who received tomuzotuximab [PFS 1.9 months (95% CI 1.4-3.7 months) and DOR 3.8 months (95% CI 1.9-5.8 months)]. More information about activity observed in CRC patients can be found in Supplementary Figures S1 and S2, Tables S3 and S4, available at https://doi.org/10.1016/j.esmoop.2022.100447. No responses were observed in the five NSCLC patients, but median PFS was 5.3 months (95% CI 1.9-10.6 months) and median OS was 10.5 months (95% CI 4.7 months to not calculable).
PK
Sampling and analysis of the tomuzotuximab and gatipotuzumab concentrations was mainly designed to use the data in a population PK assessment. Looking at the tomuzotuximab concentrations before and after the first infusions with tomuzotuximab, when most of the IRRs were observed, there were no notable differences between patients who experienced IRRs (n = 18) or not (n = 23). Also, there were no notable differences in tomuzotuximab or gatipotuzumab concentrations between responders and non-responders. Comparing the Cmin and Cmax concentrations of tomuzotuximab before and after the tomuzotuximab infusion of cycle 1 (in the absence of gatipotuzumab) with that of cycle 2, 3, 4 and 5 (in the presence of gatipotuzumab), no indications were seen that the infusions with gatipotuzumab had a significant impact on the PK characteristics of tomuzotuximab.
Pharmacodynamic parameters and immunogenicity
Following infusion of gatipotuzumab, the sTA-MUC1 levels dropped and remained suppressed throughout gatipotuzumab treatment. During the PP of the study (in which gatipotuzumab was administered 6 weeks after the start of treatment with tomuzotuximab), a slight increase in sTA-MUC1 levels was observed in a majority of patients during treatment with tomuzotuximab alone (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2022.100447). No such increase was observed for EP patients who received gatipotuzumab earlier, 1 week after the start of tomuzotuximab treatment.
Regarding the extensive TR program, the only parameter reported below in detail is sTA-MUC1, as data hint at a possible predictive role that was not observed with all other investigated soluble and histological parameters. By analyzing PFS and OS values according to the overall median baseline value of serum TA-MUC1, sTA-MUC1 levels above the median behaved differently in the two phases of the study, as summarized in Tables 2 and 3. For patients enrolled in the PP, higher baseline sTA-MUC1 values seemed a negative factor, particularly for OS, as also described in the literature.2 This did not seem to be the case in EP patients, in whom numerically better results were observed with baseline sTA-MUC1 values above the median 61.42 U/ml. These results were probably only marginally affected by the early censoring for OS in five (16.7%) patients in the EP, as three of them had baseline values below the median and two above. No TA-MUC1 correlation with efficacy was found on histological samples. This difference can be explained by the different methodologies and by the fact that histological samples often predated the start of study treatment by several months, as opposed to sTA-MUC1.
Table 2.
Summary of PFS outcomes based on sTA-MUC1 baseline values
| Study phase/subgroup (patients n) | Median PFS (95% CI), months | PFSR at 3 months, % (95% CI) | PFSR at 6 months, % (95% CI) |
|---|---|---|---|
| Primary phase (n = 20) | 2.0 (1.7-3.2) | 30.0 (12.3-50.1) | 15.0 (3.7-33.5) |
| sTA-MUC1 result at baseline <61.42 U/ml (n = 9) | 1.8 (1.3-9.2) | 33.3 (7.8-62.3) | 22.2 (3.4-51.3) |
| sTA-MUC1 result at baseline ≥61.42 U/ml (n = 11) | 2.0 (1.4-3.2) | 27.3 (6.5-53.9) | 9.1 (0.5-33.3) |
| Extension phase (n = 30) | 2.8 (1.8-5.5) | 49.8 (31.0-66.0) | 28.0 (13.2-45.0) |
| sTA-MUC1 result at baseline <61.42 U/ml (n = 16) | 1.9 (1.4-3.7) | 37.5 (15.4-59.8) | 15.0 (2.6-37.4) |
| sTA-MUC1 result at baseline ≥61.42 U/ml (n = 14) | 5.4 (1.8-7.0) | 64.3 (34.3-83.3) | 41.7 (16.4-65.4) |
| mCRC with prior anti-EGFR (n = 19) | 1.9 (1.8-5.5) | 47.4 (24.4-67.3) | 25.3 (8.6-46.2) |
| sTA-MUC1 result at baseline <61.42 U/ml (n = 10) | 1.8 (0.5-3.7) | 30.0 (7.1-57.8) | 10.0 (0.6-35.8) |
| sTA-MUC1 result at baseline ≥61.42 U/ml (n = 9) | 5.5 (1.4-7.6) | 66.7 (28.2-87.8) | 41.7 (10.9-70.8) |
CI, confidence interval; EGFR, epidermal growth factor; mCRC, metastatic colorectal cancer; PFS, progression-free survival; PFSR, progression-free survival rate; sTA-MUC1, soluble tumor-associated epitope of mucin 1.
Table 3.
Summary of OS outcomes based on sTA-MUC1 baseline values
| Study phase/subgroup (patients n) | Median OS (95% CI), months | OSR at 6 months, % (95% CI) | OSR at 12 months, % (95% CI) |
|---|---|---|---|
| Primary phase (n = 20) | 11.1 (4.1-18.7) | 67.7 (41.6-84.0) | 41.4 (18.0-63.4) |
| sTA-MUC1 result at baseline <61.42 U/ml (n = 9) | 17.3 (2.6-NC) | 77.8 (36.5-93.9) | 66.7 (28.2-87.8) |
| sTA-MUC1 result at baseline ≥61.42 U/ml (n = 11) | 11.1 (2.8-11.4) | 58.3 (23.0-82.1) | 14.6 (0.8-46.6) |
| Extension phase (n = 30) | 8.0 (4.8-11.6) | 66.1 (44.7-80.9) | 27.1 (11.3-45.7) |
| sTA-MUC1 result at baseline <61.42 U/ml (n = 16) | 4.8 (2.3-8.8) | 49.4 (21.5-72.3) | 24.7 (6.1-49.7) |
| sTA-MUC1 result at baseline ≥61.42 U/ml (n = 14) | 9.3 (5.3-14.4) | 83.9 (49.4-95.7) | 28.8 (7.0-55.7) |
| mCRC with prior anti-EGFR (n = 19) | 8.0 (4.8-NC) | 68.6 (39.7-85.7) | 32.0 (10.6-56.1) |
| sTA-MUC1 result at baseline <61.42 U/ml (n = 10) | 4.8 (1.1-NC) | 47.3 (11.7-77.0) | 31.5 (4.7-64.6) |
| sTA-MUC1 result at baseline ≥61.42 U/ml (n = 9) | 8.5 (5.3-NC) | 87.5 (38.7-98.1) | 31.3 (4.8-64.1) |
CI, confidence interval; EGFR, epidermal growth factor receptor; mCRC, metastatic colorectal cancer; NC, not calculated; OS, overall survival; OSR, overall survival rate; sTA-MUC1, soluble tumor-associated epitope of mucin 1.
The anti-drug antibody (ADA) assay results for gatipotuzumab and tomuzotuximab showed that none of the patients in the combined treatment population were positive for antibodies against tomuzotuximab. In the case of gatipotuzumab, four patients in both the PP (three patients) and the EP of the study (one patient) were ADA-positive, mostly at time points before the treatment with gatipotuzumab was started. Only one patient became positive for gatipotuzumab ADA after the start of treatment with gatipotuzumab at the follow-up visit 28 days after the last infusion. Of the patients who were positive for gatipotuzumab ADA, only one PP patient showed some response (SD), whereas the others were all non-responders.
Discussion
The study results proved the combination of anti-EGFR and anti-TA-MUC1 feasible and with interesting activity in refractory patients without standard treatment options. The safety profile of gatipotuzumab and tomuzotuximab was consistent with the known safety profile of these drugs to date from prior studies. Tomuzotuximab-related IRRs were manageable, not requiring treatment discontinuation and mainly observed at first dosing in the PP of the study. Gatipotuzumab confirmed a favorable safety profile when administered in combination with either tomuzotuximab or panitumumab, also in the EP when treatment was started in close proximity to anti-EGFR treatment. Concerning the RECIST responses observed in mCRC patients, in this study RAS mutation status was not prospectively assessed. Using liquid biopsy results to select patients without RAS and Raf mutations may actually allow to identify patients more likely to benefit from this treatment. Though tomuzotuximab is a glyco-optimized antibody, the promising activity results in patients who received gatipotuzumab and panitumumab, an immunoglobulin G2 antibody with inactive Fc fragment, seem to exclude a relevant role for ADCC in this late clinical setting, thus supporting a synergistic effect from dual targeting likely exploitable when combining gatipotuzumab with any approved anti-EGFR antibody. It is also interesting to see that these results concur frequently with studies reporting on combined chemotherapy and anti-EGFR treatment in similar patient populations,14 even though our results should not be overstated due to the small sample size of the mCRC subgroups. Of note, before study completion the sponsor planned to amend the study and enroll 12 additional patients to receive the triplet of gatipotuzumab, panitumumab and irinotecan, but due to the SARS-CoV-2 pandemic, the amended protocol never came into effect and no patients were recruited.
Finally, it is interesting to underscore that higher baseline sTA-MUC1 levels appeared to predict activity in EP patients but not in those enrolled in the PP, lending additional support to the complex interaction of TA-MUC1 with EGFR demonstrated in several preclinical models.7,9 Again, this finding should be interpreted with some caution, due to the small number of patients overall and in the histological subgroups where this was observed and some early censoring possibly affecting to some extent the OS analyses, but it certainly appears worthy of further exploration, especially when taking into account the difference in treatment schedule between the two study phases. Indeed, PP patients received only one dose of gatipotuzumab before the first computed tomography (CT) scan assessment, whereas those in the EP had received three doses of gatipotuzumab at the same assessment time point. Therefore, PP patients may be considered sort of negative controls not receiving enough combined treatment to change the natural history of heavily pretreated metastatic tumors, and thus higher sTA-MUC1 levels appear to be a negative prognostic factor for OS, as also reported in the literature. Instead, the more intense combined treatment received by EP patients before first CT scan assessment appears to reverse this effect, with patients with higher baseline sTA-MUC1 having no worse, and likely better, outcome with the antibody combination. An additional finding supporting the rationale for double targeting was observed during the PP of the study, in which an increase in sTA-MUC1 levels was observed in some patients following tomuzotuximab infusions before the start of gatipotuzumab administration at the third cycle.
Overall, the safety, activity and translational data suggest that the combination therapy of gatipotuzumab and tomuzotuximab or an approved anti-EGFR antibody is feasible and warranting further exploration, for instance combining this doublet with chemotherapy in future studies. sTA-MUC1 needs to be validated as a useful companion biomarker.
Acknowledgments
Funding
This work was supported by Glycotope GmbH (no grant number).
Disclosure
OS: advisory board participation, invited speaker or conference honoraria from: Merck Sarono, Merck Sharp & Dohme (MSD), Ipsen, AstraZeneca, Roche, Immunocore, CureVac, Glenmark and Bristol-Myers Squibb (BMS). FW: consultancy or advisory role for AbbVie, Amgen, ARIAD, Celgene, Jazz Pharmaceuticals, Novartis, MorphoSys and Pfizer; research funding from Amgen; patents, royalties or other intellectual property from a patent on immunotherapy in acute myeloid leukemia (AML) obtained together with Amgen; and travel support from Amgen, Daiichi Sankyo, Jazz Pharmaceuticals and Servier. DCG: advisory boards and travel expenses for conferences: Novartis, BMS, Astellas, Pfizer, Jannsen. MI: ESMO Research Fellowship sponsored by Roche; honoraria for serving as a speaker bureau for MSD. HB: employment with Glycotope GmbH. IAF: employment with Glycotope GmbH. RF: bureau, research collaboration and research support—AstraZeneca, GSK, MSD, Clovis, Roche. LD: consultant/advisor: AstraZeneca, GSK, MSD, Pharmamar, Clovis, Merck Serono, Novartis, Amgen, Eisai; promotional speaker: GSK, Clovis, AstraZeneca, Genmab, MSD; investigator/researcher: GSK, Clovis, MSD, AstraZeneca, Immonogen, Genmab, Corcept, Roche, Eisai; support for travel: AstraZeneca, GSK, Roche. KU: advisory boards, speaker bureau, trial support, research collaboration and research support; Amgen, AstraZeneca, BMS, Boehringer Ingelheim, Glycotope, Innate, Lilly, Medimmune, Merck Serono, MSD/Merck, Novartis, Pfizer, Roche/Genentech, Sirtex. KK: advisory board participation, invited speaker or conference honoraria from: Merck, Sanofi, MSD, Glycotope, Roche, Novartis and BMS. BH: employment and shares with Glycotope GmbH. ZA: employment with Glycotope GmbH. GE: research: Novartis/Roche/Thermo Fisher/AstraZeneca/Taiho/BeiGene; consultant/advisor: Roche/Genentech–F.Hoffmann/La Roche–Ellipses Pharma–Neomed Therapeutics 1 Inc.–Boehringer Ingelheim–Janssen Global Services–SeaGen–TFS–Alkermes–Thermo Fisher–BMS–MabDiscovery–Anaveon; speakers bureau: MSD/Roche/Thermo Fisher/Lilly; clinical trials PI or Co-PI (Institution): Affimed GmbH–Amgen SA–Anaveon AG–AstraZeneca AB–BioNTech GmbH–CatalYm GmbH–Cytomx–F. Hoffmann La Roche Ltd–F-Star Beta Limited–Genentech Inc.–Genmab B.V.–Hutchison Medipharma Limited–Icon–Imcheck Therapeutics–Immunocore Ltd–Janssen-Cilag SA–Medimmune LLC–Merck KGaA–Novartis Farmacéutica, S.A.–Peptomyc–Ribon Therapeutics–Roche Farma SA–Seattle Genetics Inc.–Symphogen A/S–Taiho Pharma USA Inc. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Hollingsworth M.A., Swanson B.J. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4(1):45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 2.Nath S., Mukherjee P. MUC1: a multifaceted oncoprotein with a key role in cancer progression. Trends Mol Med. 2014;20(6):332–342. doi: 10.1016/j.molmed.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bozkaya G., Korhan P., Cokaklı M., et al. Cooperative interaction of MUC1 with the HGF/c-Met pathway during hepatocarcinogenesis. Mol Cancer. 2012;11:64. doi: 10.1186/1476-4598-11-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh P.K., Hollingsworth M.A. Cell surface-associated mucins in signal transduction. Trends Cell Biol. 2006;16:467–476. doi: 10.1016/j.tcb.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Dharmaraj N., Engel B.J., Carson D.D. Activated EGFR stimulates MUC1 expression in human uterine and pancreatic cancer cell lines. J Cell Biochem. 2013;114:2314–2322. doi: 10.1002/jcb.24580. [DOI] [PubMed] [Google Scholar]
- 6.Bitler B.G., Goverdhan A., Schroeder J.A. MUC1 regulates nuclear localization and function of the epidermal growth factor receptor. J Cell Sci. 2010;123:1716–1723. doi: 10.1242/jcs.062661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engel B.J., Bowser J.L., Broaddus R.R., Carson D.D. MUC1 stimulates EGFR expression and function in endometrial cancer. Oncotarget. 2016;7:32796–32809. doi: 10.18632/oncotarget.8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Boer H.R., Pool M., Joosten E., et al. Quantitative proteomics analysis identifies MUC1 as an effect sensor of EGFR inhibition. Oncogene. 2019;38(9):1477–1488. doi: 10.1038/s41388-018-0522-7. [DOI] [PubMed] [Google Scholar]
- 9.Danielczyk A., Stahn R., Faulstich D., et al. PankoMab: a potent new generation anti-tumour MUC1 antibody. Cancer Immunol Immunother. 2006;55:1337–1347. doi: 10.1007/s00262-006-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiedler W., DeDosso S., Cresta S., et al. A phase I study of PankoMab-GEX, a humanised glyco-optimised monoclonal antibody to a novel tumour-specific MUC1 glycopeptide epitope in patients with advanced carcinomas. Eur J Cancer. 2016;63:55–63. doi: 10.1016/j.ejca.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Fiedler W., Cresta S., Schulze-Bergkamen H., et al. Phase I study of tomuzotuximab, a glycoengineered therapeutic antibody against the epidermal growth factor receptor, in patients with advanced carcinomas. ESMO Open. 2018;3(2) doi: 10.1136/esmoopen-2017-000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shields R.L., Lai J., Keck R., et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277(30):26733–26740. doi: 10.1074/jbc.M202069200. [DOI] [PubMed] [Google Scholar]
- 13.Kellner C., Otte A., Cappuzzello E., Klausz K., Peipp M. Modulating cytotoxic effector functions by Fc engineering to improve cancer therapy. Transfus Med Hemother. 2017;44(5):327–336. doi: 10.1159/000479980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mauri G., Pizzutilo E.G., Amatu A., et al. Retreatment with anti-EGFR monoclonal antibodies in metastatic colorectal cancer: Systematic review of different strategies. Cancer Treat Rev. 2019;73:41–53. doi: 10.1016/j.ctrv.2018.12.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.