Abstract
A 70-year-old woman with a bioprosthetic aortic valve replacement for aortic valve endocarditis complicated by recurrent endocarditis and requiring homograft aortic root replacement 10 years earlier had presented at 1 month after her admission for pseudomonal bacteremia with right-sided chest pain. An aortic pseudoaneurysm, identified on computed tomography, was treated with an ascending aorta thoracic endovascular aortic repair using two overlapping abdominal aortic stent grafts in the ascending aorta. Postoperative and follow-up imaging demonstrated exclusion of the pseudoaneurysm with stable positioning of the stent grafts. Ascending aorta thoracic endovascular aortic repair can be performed safely with good short-term results in patients presenting with infected pseudoaneurysms of the ascending aorta.
Keywords: Ascending aorta, Infected pseudoaneurysm, TEVAR
Infected pseudoaneurysm of the ascending aorta is a rare, complex, and frequently fatal complication of cardiac surgery. Traditional management has required open sternotomy. For patients unfit for open repair, stent grafting of the ascending aorta is a viable option. The present patient provided written informed consent for the report of her case details and imaging studies.
Case report
A 70-year-old woman with a history of Streptococcus pneumoniae aortic valve endocarditis had been treated with a bioprosthetic porcine aortic valve replacement in 2011, which had been complicated by recurrent S. pneumoniae endocarditis requiring aortic root replacement with a homograft in 2011. She was prescribed rivaroxaban for atrial fibrillation. One month before presentation, she had been admitted with pseudomonal bacteremia of unknown origin, which was treated with cefepime and outpatient levofloxacin and was completed before her representation.
She had presented in April 2021 with 2 weeks of worsening right-sided chest pain and a growing pulsatile mass on the right sternal border. A computed tomography angiogram (CTA) demonstrated a 2.5 × 2.0-cm proximal thoracic aortic pseudoaneurysm with an anterior mediastinal hematoma. The initial medications for blood pressure control included labetalol and esmolol. Prothrombin complex concentrate was given to reverse the rivaroxaban, and she received vancomycin and levofloxacin. A CTA 7 hours later demonstrated growth of the pseudoaneurysm to 3.4 × 2.1 cm, with a neck 0.6 cm wide and location 1.4 cm superior to the right coronary artery ostium (Fig 1). The distance from the right coronary ostium to the innominate artery was 60.5 mm. Given her history of endocarditis, recent bacteremia, and the location of the pseudoaneurysm, the lesion was thought to be infectious. Because of her two prior sternotomies and substernal pulsatile mass, she was deemed at very high risk for another sternotomy. After discussion, she provided consent for endovascular stent graft placement.
Fig 1.
Preoperative computed tomography angiogram (CTA; Left) and computed tomography reconstruction (Right) showing a pseudoaneurysm from the proximal ascending aorta.
The procedure was performed in a hybrid operating room with the patient under general anesthesia. A transvenous pacer was advanced into the right ventricle through right femoral access. The left axillary artery was accessed via an infraclavicular incision. The axillary artery was punctured under direct visualization, and a 0.035 Glidewire (Terumo Medical, Somerset, NJ) was advanced into the ascending aorta. An 8F sheath was placed in the axillary artery. A GLIDECATH (Terumo Medical) was advanced into the left ventricle, and the wire was exchanged for a double-curved Lunderquist wire (Cook Medical Inc, Bloomington, IN). A pigtail catheter was advanced to the level of the aortic valve through a left femoral 5F sheath.
A 36 × 50-mm Zenith abdominal extension stent graft (Cook Medical Inc) was advanced via axillary access into the left ventricular outflow tract. Rapid pacing was initiated and the device deployed. Angiography demonstrated continued pseudoaneurysm filling, with the stent graft angled along the lesser curve of the aorta (Fig 2). Therefore, another 36 × 50-mm Zenith stent (Cook Medical Inc) was deployed proximally along the greater curve of the aorta using the first stent for support. Completion angiography demonstrated patency of both coronary arteries with exclusion of the pseudoaneurysm.
Fig 2.
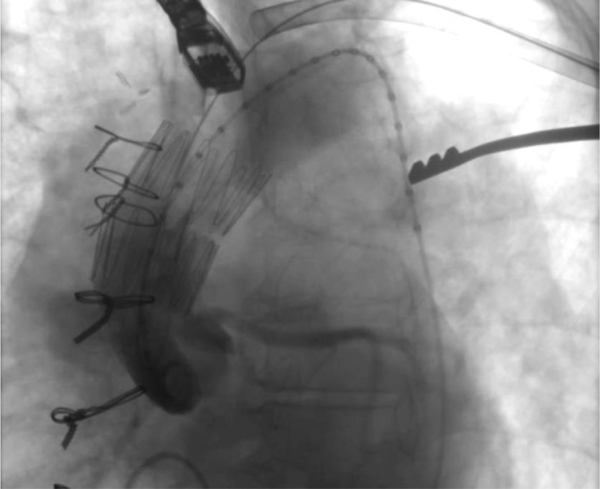
Angiogram after deployment of the first stent graft demonstrating continued filling of the pseudoaneurysm.
The patient was transferred to the critical care unit postoperatively. Her antibiotic therapy was changed to tobramycin and meropenem, because her admission blood cultures had grown Pseudomonas aeruginosa. During her admission, she received aspirin and prophylactic subcutaneous heparin. Her rivaroxaban was discontinued, and her heart rate was controlled with metoprolol. The predischarge computed tomography scan demonstrated exclusion of the pseudoaneurysm (Fig 3). She was discharged to home on postoperative day 10 with home infusions to complete 2 weeks of tobramycin and 6 weeks of meropenem via a peripherally inserted central catheter, followed by lifelong suppressive therapy with oral levofloxacin. Follow-up CTAs at 4 and 6 months demonstrated exclusion of the pseudoaneurysm with a decreased size of the mediastinal hematoma, stable positioning of the stent grafts, and resolution of all symptoms (Figs 4 and 5).
Fig 3.
Predischarge computed tomography angiogram (CTA; Left) and computed tomography reconstruction (Right) showing resolution of pseudoaneurysm after stent graft placement.
Fig 4.
Follow-up computed tomography angiogram (CTA; Left) and computed tomography reconstruction (Right) at 4 months demonstrating a decreased mediastinal hematoma, stable stent graft positioning, and no evidence of endoleak.
Fig 5.
Follow-up computed tomography angiogram (CTA; Left) and computed tomography reconstruction (Right) at 6 months demonstrating a decreased mediastinal hematoma, stable stent graft positioning, and no evidence of endoleak.
Discussion
Our patient with an aortic root replacement had had an infected pseudoaneurysm located at the homograft to transverse aortic arch anastomosis. Infected pseudoaneurysms often enlarge rapidly and rupture, rendering nonoperative management likely fatal.1 The incidence of infected aneurysms is difficult to determine; however, estimates have ranged from 0.7% to 2.6%.2,3 Infected pseudoaneurysms have generally been treated with open surgery.4 For patients very high risk for open surgery endovascular intervention has been performed successfully, with low mortality or the need for reintervention.5, 6, 7, 8, 9, 10 The most common complications have been sepsis or reinfection, usually within the first year.11,12 Pseudomonas is a rare cause of arterial infection, and, owing to the high risk of the implanted stent graft to become infected if the patient cannot undergo explantation, the patient will require lifelong suppressive antibiotic therapy.13
Developing endovascular solutions for treatment in the ascending aorta has been challenging owing to the short landing zones, high shear stress, left ventricular outflow tract forces, curvature of the aorta, and proximity to the coronary artery ostia and arch vessels. Hybrid debranching thoracic endovascular aortic repair (TEVAR) and endovascular coiling have been reported.14,15 However, endovascular coiling or plugging would likely have failed to embolize the high-flow pseudoaneurysm in our patient. Patient and device selection are of paramount importance for ascending aorta TEVAR (AA-TEVAR) to avoid complications such as stroke and myocardial infarction. The anatomic criteria for ascending aorta interventions have been suggested as a sinotubular junction diameter of ≤38 mm and a proximal landing zone of ≥10 mm.16 Although no endovascular devices dedicated to the ascending aorta have been approved by the Food and Drug Administration, studies have reported successful procedures using thoracic and abdominal aortic cuffs.17, 18, 19 Early data with ascending aortic devices from trials are promising, including grafts from W.L. Gore & Associates (Flagstaff, AZ), Cook Medical Inc, and Bolton Medical (Sunrise, FL).20,21
Some groups have used thoracic cuffs, which have the advantage of easier fitting to the curvature of the aortic arch and the ability to use femoral access; however, these can only be used when the ascending aorta has an adequate length to support these devices.22 Our patient had a limited length of the ascending aorta with only 14 mm of clearance above the ostia of the right coronary artery; thus, we chose an abdominal cuff. The graft used was a completely covered stent, with no bare metal component, which is attractive when in proximity to the aortic valve. Owing to the length of the delivery system and ease of deployment, a left axillary access was chosen. Some groups have favored a transapical or transcarotid approach.23,24 Our approach avoided the increased stroke risk associated with transcarotid access and the risk of cardiac or valvular complications with transapical approaches. The graft diameter was oversized in accordance with the device's instructions for use. Rapid ventricular pacing was used to limit cardiac output and motion at the landing zone. For the present patient, extra care was taken to avoid covering the coronary arteries, because conversion to an open procedure would likely have been fatal. As such, the first device had landed more distally than intended and had failed to exclude the pseudoaneurysm. The position of the first device became advantageous, because it provided a scaffold to push the second device against the greater curve of the aorta, allowing exclusion of the pseudoaneurysm without coverage of the coronary ostia.
Conclusions
The present case of AA-TEVAR using overlapping abdominal extension grafts to exclude an infected pseudoaneurysm has provided evidence that AA-TEVAR can be performed successfully with good short-term results. Longer follow-up is needed to determine the durability and complications of such repairs. Developing guidelines for patient and device selection will aid in further advances for this procedure. The future development of devices for AA-TEVAR could involve branched grafts to extend the seal or devices that can be unsheathed and resheathed to aid in precise deployment. At present, for patients with a short proximal landing zone, AA-TEVAR can be performed using a second stent graft to help correctly angle another stent graft without covering the coronary arteries.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Yano M., Hayase T., Furukawa K., Nakamura K. Mycotic pseudoaneurysm of the ascending aorta caused by Escherichia coli. Interact Cardiovasc Thorac Surg. 2013;16:81–83. doi: 10.1093/icvts/ivs376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkhurst G.F., Decker J.P. Bacterial aortitis and mycotic aneurysm of the aorta: a report of twelve cases. Am J Pathol. 1955;31:821–835. [PMC free article] [PubMed] [Google Scholar]
- 3.Oderich G.S., Panneton J.M., Bower T.C., Cherry K.J., Rowland C.M., Noel A.A., et al. Infected aortic aneurysms: aggressive presentation, complicated early outcome, but durable results. J Vasc Surg. 2001;34:900–908. doi: 10.1067/mva.2001.118084. [DOI] [PubMed] [Google Scholar]
- 4.Haidar G.M., Hicks T.D., Strosberg D.S., El-Sayed H.F., Davies M.G. “In situ” endografting in the treatment of arterial and graft infections. J Vasc Surg. 2017;65:1824–1829. doi: 10.1016/j.jvs.2016.12.134. [DOI] [PubMed] [Google Scholar]
- 5.Gelpi G., Cagnoni G., Vanelli P., Antona C. Endovascular repair of ascending aortic pseudoaneurysm in a high-risk patient. Interact Cardiovasc Thorac Surg. 2012;14:494–496. doi: 10.1093/icvts/ivr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rayan S.S., Vega J.D., Shanewise J.S., Kong L.S., Chaikof E.L., Milner R. Repair of mycotic aortic pseudoaneurysm with a stent graft using transesophageal echocardiography. J Vasc Surg. 2004;40:567–570. doi: 10.1016/j.jvs.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Plichta R.P., Hughes G.C. Thoracic endovascular aortic repair for the ascending aorta: experience and pitfalls. J Vis Surg. 2018;4:92. doi: 10.21037/jovs.2018.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heye S., Daenens K., Maleux G., Nevelsteen A. Stent-graft repair of a mycotic ascending aortic pseudoaneurysm. J Vasc Interv Radiol. 2006;17:1821–1825. doi: 10.1097/01.RVI.0000244834.71601.65. [DOI] [PubMed] [Google Scholar]
- 9.Vaughan-Huxley E., Hamady M.S., Metcalfe M.J., Adams B., Kashef E., Cheshire N.J.W., et al. Endovascular repair of an acute, mycotic, ascending aortic pseudoaneurysm. Eur J Vasc Endovasc Surg. 2011;41:488–491. doi: 10.1016/j.ejvs.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Piffaretti G., Galli M., Lomazzi C., Franchin M., Castelli P., Mariscalco G., et al. Endograft repair for pseudoaneurysms and penetrating ulcers of the ascending aorta. J Thorac Cardiovasc Surg. 2016;151:1606–1614. doi: 10.1016/j.jtcvs.2015.12.055. [DOI] [PubMed] [Google Scholar]
- 11.Sörelius K., Wanhainen A., Wahlgren C.M., Langenskiöld M., Roos H., Resch T., et al. Nationwide study on treatment of mycotic thoracic aortic aneurysms. Eur J Vasc Endovasc Surg. 2019;57:239–246. doi: 10.1016/j.ejvs.2018.08.052. [DOI] [PubMed] [Google Scholar]
- 12.Sörelius K., Mani K., Bjrck M., Sedivy P., Wahlgren C.M., Taylor P., et al. Endovascular treatment of mycotic aortic aneurysms: a European multicenter study. Circulation. 2014;130:2136–2142. doi: 10.1161/CIRCULATIONAHA.114.009481. [DOI] [PubMed] [Google Scholar]
- 13.Mazzalai F., Ragazzi R., Urilli V., Toniato A., Da Giau G., Ballotta E. Pseudomonas aeruginosa-infected infrarenal abdominal aorta pseudoaneurysm secondary to laparoscopic colorectal surgery: failure of endovascular stent graft treatment after primary open repair failed. Can J Surg. 2009;52:193–194. [PMC free article] [PubMed] [Google Scholar]
- 14.Khanji M.Y., Kumar P., Ionescu A. Ascending aortic pseudo-aneurysm treated with “coil and plug.”. Eur Heart J Cardiovasc Imaging. 2017;18:1299. doi: 10.1093/ehjci/jex179. [DOI] [PubMed] [Google Scholar]
- 15.Sousa J., Oliveira-Pinto J., Soares T., Lachat M., Teixeira J. Symptomatic distal anastomotic pseudo-aneurysm after the bentall procedure successfully treated by supra-aortic trunk debranching and zone 0 thoracic endovascular aneurysm repair. EJVES Short Rep. 2020;47:90–96. doi: 10.1016/j.ejvssr.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moon M.C., Greenberg R.K., Morales J.P., Martin Z., Lu Q., Dowdall J.F., et al. Computed tomography-based anatomic characterization of proximal aortic dissection with consideration for endovascular candidacy. J Vasc Surg. 2011;53:942–949. doi: 10.1016/j.jvs.2010.10.067. [DOI] [PubMed] [Google Scholar]
- 17.Roselli E.E., Idrees J., Greenberg R.K., Johnston D.R., Lytle B.W. Endovascular stent grafting for ascending aorta repair in high-risk patients. J Thorac Cardiovasc Surg. 2015;149:144–154. doi: 10.1016/j.jtcvs.2014.07.109. [DOI] [PubMed] [Google Scholar]
- 18.Gray B.H., Langan E.M., Manos G., Bair L., Lysak S.Z. Technical strategy for the endovascular management of ascending aortic pseudoaneurysm. Ann Vasc Surg. 2012;26:734–738. doi: 10.1016/j.avsg.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Quevedo H.C., Alonso A. Endovascular therapy for ascending aorta pseudoaneurysm. Cardiovasc Revasc Med. 2016;17:586–588. doi: 10.1016/j.carrev.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Tsilimparis N., Debus E.S., Oderich G.S., Haulon S., Terp K.A., Roeder B., et al. International experience with endovascular therapy of the ascending aorta with a dedicated endograft. J Vasc Surg. 2016;63:1476–1482. doi: 10.1016/j.jvs.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 21.Long K.N., Ross M. The current state of branched stent grafts for the aortic arch. Endovasc Today. 2016;15:66–70. [Google Scholar]
- 22.Bernardes R.C., Navarro T.P., Reis F.R., Lima L.C.M., Monteiro E.L., Procopio R.J., et al. Early experience with off-the-shelf endografts using a zone 0 proximal landing site to treat the ascending aorta and arch. J Thorac Cardiovasc Surg. 2014;148:105–112. doi: 10.1016/j.jtcvs.2013.07.049. [DOI] [PubMed] [Google Scholar]
- 23.Muetterties C.E., Menon R., Wheatley G.H. A systematic review of primary endovascular repair of the ascending aorta. J Vasc Surg. 2018;67:332–342. doi: 10.1016/j.jvs.2017.06.099. [DOI] [PubMed] [Google Scholar]
- 24.Vallabhajosyula P., Gottret J.P., Bavaria J.E., Desai N.D., Szeto W.Y. Endovascular repair of the ascending aorta in patients at high risk for open repair. J Thorac Cardiovasc Surg. 2015;149:S144–S150. doi: 10.1016/j.jtcvs.2014.07.063. [DOI] [PubMed] [Google Scholar]






